Influence of high pre-rigor temperature and fast pH fall on muscle proteins and meat quality: a review
Yuan H. Brad Kim A D E , Robyn D. Warner B and Katja Rosenvold CA AgResearch Ltd, Ruakura Research Centre, Hamilton, New Zealand.
B CSIRO Animal, Food and Health Sciences, 671 Sneydes Road, Werribee, Vic. 3030, Australia.
C ANZCO Foods Limited, Stafford Street, Waitara, New Zealand.
D Current address: Muscle Biology Laboratory, Department of Animal Sciences, Purdue University, 915 W. State Street, West Lafayette, IN 47907, USA.
E Corresponding author. Email: bradkim@purdue.edu
Animal Production Science 54(4) 375-395 https://doi.org/10.1071/AN13329
Submitted: 1 August 2013 Accepted: 14 February 2014 Published: 13 March 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
The impacts of accelerated pH decline combined with high muscle temperature on post-mortem muscle metabolism and subsequent meat quality attributes have been extensively studied. Traditionally, this phenomenon has been observed in pork muscles, primarily due to the relatively fast post-mortem glycolysis rate and its relationships to stress susceptibility of pigs before slaughter. However, the protein-denaturing condition of high temperature/rapid pH fall and subsequent PSE (pale, soft and exudative)-like abnormal meat quality characteristics have been observed in muscles from other species such as beef, lamb, venison and even poultry. Various pre-rigor conditions including the application of electrical stimulation, hot-boning, and/or pre-rigor carcass chilling temperatures in various muscles, in conjunction with carcass stretching/hanging methods, can also contribute to muscle-protein denaturation pre-rigor. This review considers the influence of a faster than normal pH fall at a higher than normal pre-rigor temperature on glycolysis, post-mortem muscle proteins and subsequently meat quality attributes. Gaps in current knowledge are identified and recommendations made for additional research.
Additional keywords: chilling, high pre-rigor temperature, meat quality, pH decline, protein denaturation.
Introduction
Consequences of post-mortem muscle energy metabolism are the decline in pH, due to the accumulation of H+ ions (England et al. 2013), and metabolic heat production (Jacob and Hopkins 2014). As the energy reserves decline post-mortem, the muscles in the carcass enter rigor mortis and the muscle temperature drops, due to the cessation of blood supply and the application of chilling. Muscles throughout the carcass vary in their rates of energy metabolism as well as their rates of temperature and pH decline, due to inherent metabolic differences and muscle size (Jacob and Hopkins 2014). The rates of pH and temperature decline during rigor development are probably two of the most important post-mortem factors affecting meat quality in terms of colour, water-holding capacity (WHC) and tenderness (Marsh et al. 1981; Mancini and Hunt 2005; Savell et al. 2005; Thompson et al. 2006; Huff-Lonergan and Lonergan 2007; Kim and Hunt 2011). In particular, an occurrence of abnormally high pre-rigor temperature accompanied by a faster than normal pH decline in muscle (also known as a protein-denaturing condition) has been shown to have detrimental impacts on meat quality attributes (some examples in different species are summarised in Table 1). Adverse effects of the protein-denaturing condition on pork quality (the typical abnormal meat quality known as pale, soft and exudative, PSE) have been extensively studied. PSE in pork is the classic example of the impact of a rapid pH fall at a high muscle temperature on quality. In earlier years, this condition was particularly frequent in pig carcasses due to a high prevalence of the halothane gene (Briskey 1964), which has now been eliminated in most countries (Rosenvold and Andersen 2003). However, beef and lamb processing plants in Australia and in the UK report low pH values in the loin (pH <6.0) when the temperature is still high (35−40°C) (Australian beef: Warner et al. 2014a; Australian sheep: Thompson et al. 2005, Pearce et al. 2010; UK sheep: Matthews 2011), i.e. early post-mortem conditions very similar to those in pig carcasses developing PSE pork.
These protein-denaturing conditions of abnormally high temperature/rapid pH fall and subsequent PSE-like meat quality characteristics have been observed in muscles from beef and sheep carcasses (see above) and also in venison, poultry and turkey (see Table 1 for references). This appears to occur under various pre-rigor conditions, and it can be influenced by the application of electrical stimulation, hot-boning and/or pre-rigor carcass chilling (Jacob and Hopkins 2014).
This review will discuss the influence of accelerated pH fall at a higher than normal pre-rigor temperature on glycolysis, post-mortem muscle proteins and, subsequently, meat quality attributes, particularly meat tenderness, colour and colour stability, and WHC. Because the impacts of protein-denaturing conditions on post-mortem muscle metabolism have been predominantly studied in pork and only limited studies are available for muscles from ruminants, a considerable part of this review will focus on the former; however, its subsequent impact on meat quality attributes of other species will also be covered. Research and development gaps in current knowledge are included in each section to assist in the identification of future research needs on this important topic.
Impacts of accelerated glycolysis at a higher than normal temperature on denaturation of muscle proteins
Accelerated rates of muscle glycolysis early post-mortem result in low muscle pH when the muscle temperature is still high (and often higher than average), causing denaturation of muscle proteins. The rate of chilling of the muscle during rigor development influences glycolytic reaction rates, thereby influencing the rate of pH decline (Marsh et al. 1987; Pike et al. 1993), and muscle temperature directly.
Different muscles within a carcass can exhibit different chilling rates, particularly muscles in the hindleg and chuck of beef carcasses. Due to the high initial muscle temperature caused by metabolic heat production and the low thermal conductivity of muscle (Jacob and Hopkins 2014), post-mortem glycolysis would be completed in deep muscles before refrigeration can lower the temperature in these muscles to <15°C. Consequently, these muscles are more likely to suffer from muscle-protein denaturation, similar to PSE pork (Offer 1991).
Several factors can be cited as evidence for denaturation of muscle proteins pre-rigor:
-
The reduced solubility of the myofibrillar proteins in pork (Wismer-Pedersen 1959; Bendall and Wismer-Petersen 1962).
-
The reduced solubility of the sarcoplasmic proteins in pork (Sayre and Briskey 1963), in lamb (Warner et al. 2014a) and in beef (Hunt and Hedrick 1977b).
-
The reduced activity of myofibrillar ATPase in rabbit (Penny 1967a, 1967b), in pork (Greaser et al. 1969) and in lamb muscle (Warner et al. 2014b), and of myosin ATPase activity in rabbit muscle (Penny 1967a, 1967b).
-
The reduced activity of some sarcoplasmic proteins (Fischer et al. 1979).
-
The precipitation of sarcoplasmic proteins onto myofibrils in pork (Bendall and Wismer-Petersen 1962; Fischer et al. 1977, 1979).
-
Denaturation of the myoglobin (globin moiety) (Zhu and Brewer 1998; Sammel et al. 2002; Zhu and Brewer 2003).
All of these reductions are associated with the accelerated pH fall in musculature post-mortem, producing PSE-like meat.
Myofibrillar proteins and myosin ATPase activity
Myofibrillar proteins constitute 50–55% of the total protein content of skeletal muscle tissue, are soluble in 0.5 m salt solution (Helander 1957), and are defined as those which make up the myofibril. The major myofibrillar proteins can be categorised as the contractile elements myosin (mol. wt 520 kD) and actin (mol. wt 42 kD), the cytoskeletal elements titin (mol. wt 2500 kD), nebulin (mol. wt 800 kD) and α-actinin (mol. wt 190 kD), and the regulatory elements tropomyosin (mol. wt 2 × 33 kD) and the troponins (mol. wt for I, C and T: 21, 18 and 31 kD, respectively) (Bagshaw 1993). These proteins make up 43, 22, 10, 5, 2, 5 and 5%, respectively (Yates and Greaser 1983), with other proteins equalling the remaining 8%.
Accelerated muscle pH decline (pH <6.0 at 45 min post-mortem) when the muscle temperature is still high (>25−30°C) is expected to affect the ATPase active site of myosin and its overall conformation (Jacobson and Henderson 1973). Specific denaturation of the active site is reflected in reduced ATPase activity, whereas general denaturation, which induces overall conformation changes, is reflected in altered solubility (Warner et al. 1997). The two globular heads in myosin are responsible for Ca-ATPase activity (Hultin 1985), and a decrease in Ca-ATPase activity is indicative of denaturation of the myosin heads.
As the ATPase activity of myosin is confined to the head, this suggests that it is the myosin-S1 that is denatured, although Stabursvik et al. (1984) suspected that the myosin tail (LMM) could also be involved. Penny (1967a) suggested that in pre-rigor muscle, myosin (which is bound to actin in the post-rigor myofibril) is more resistant to denaturation by low pH. For actomyosin, deactivation of the active site and changes in overall conformation due to pH occur at 43°C, and appear to involve an irreversible dissociation of the F-actin–myosin complex due to exposure of buried sulfhydryl groups (Jacobson and Henderson 1973). For myosin alone, marked denaturation of the active site may occur at temperatures as low as 32°C, which is at a lower temperature than the changes in overall conformation, which occur at 43°C (Jacobson and Henderson 1973). The core body temperature of grazing and feedlot cattle animals is about 38−39°C (Brown-Brandl et al. 2005; Cafe et al. 2011; Jacob et al. 2014b) but the interfacial seam between the semimembranosus (SM) and semitendinosus (ST) muscle can reach 42−44°C post-mortem, due to metabolic heat production (Jacob et al. 2014a). Thus, it is expected that changes in the overall conformation of myosin can occur during high rigor temperatures conditions, particularly in the deep part of muscles such as the SM.
The susceptibility of myosin to denaturation depends on the myosin isoforms as well as on both the pH and temperature conditions, and these can vary markedly between muscles in one carcass. The myosin from bovine SM muscle (a typically white muscle) at an ultimate pH typical for the muscle of 5.50 is less heat-stable than myosin from bovine vastus intermedius (VI) muscle (a typically red muscle) at a pH typical for the muscle of 6.05 (Vega-Warner and Smith 2001). However, when the heat stability is compared at the same pH, the myosin from the VI is actually less heat-stable. Note that the specific sampling within the SM was not specified by Vega-Warner and Smith (2001), and it is known that the beef SM has more PSE-like qualities (colour, WHC, firmness and exudate, but not pH) in the inner than the outer portion (Hunt and Hedrick 1977b). The percentage of red and white fibres between the outer and inner portions of the SM is relatively consistent (Hunt and Hedrick 1977b) indicating that the difference in quality between the outer and inner portions is most likely driven by the slower rate of cooling in the inner portion. The effects of temperature on the pH decline observed by varying muscle depth are clearly reflected by the denaturation of myosin, as indicated by myofibrillar ATPase activity (Tarrant and Mothersill 1977). Myofibrillar ATPase activity was 29% lower at 8 cm depth in beef adductor (AD) muscle than at 1.5 cm depth. The samples from 8 cm depth in the muscle also had faster pH decline, and examination of these samples at 2 days post-mortem revealed paler, softer and wetter appearance compared with samples from 1.5 cm depth. These beef muscles were described as having developed a mild form of PSE characteristics, which are often observed in pork and previously described in beef (Hunt and Hedrick 1977b). The PSE characteristics increased with depth in the muscle (Tarrant and Mothersill 1977).
Substantially higher levels of myosin (extracted from dark chicken meat) denaturation occur under oxidising conditions at 37°C, as indicated by loss in Ca-ATPase myofibrillar protein activity, relative to non-oxidising or anti-oxidising conditions (Li and King 1996). Low-pH meat is an oxidising environment and muscles at a high temperature (25−40°C) and a low pH (5.4–5.7) post-mortem would suffer damage and denaturation of myosin. Furthermore, the exposed myosin is more susceptible to denaturation than when in the actomyosin complex (Penny 1967a), and thus myosin in pre-rigor muscle undergoing rapid pH fall at high muscle temperatures would be particularly susceptible to denaturation.
Offer (1991) proposed that carcasses undergoing intermediate rates of pH decline might be more susceptible to the PSE condition than carcasses with extremely rapid rates of pH decline. He postulated that the exposure of myosin to a higher pH for a longer time causes more extensive myosin denaturation than exposure to a lower pH for a shorter time pre-rigor. However, once the rigor complex between actin and myosin forms, myosin would be protected from further denaturation (Offer 1991). The studies by Warner et al. (2014b) and Kim et al. (2014) provide evidence to support this hypothesis. They show that muscles with extremely long sarcomeres (and thus less myosin protected by the rigor bond) had more myosin denaturation than muscles with normal sarcomere lengths (e.g. for gluteus medius, 2.71 and 1.91 µm, respectively).
Izumi et al. (1977) reported that the denaturing conditions that occur in PSE muscle caused irreversible binding of actin to myosin, which resulted in a lack of contractility, reduced solubility and absence of the Mg2+-activated myosin ATPase activity. Sung et al. (1977) found that in PSE muscle, some myosin could be extracted from the myofibril that retained ATPase activity but did not exhibit the initial burst of phosphate liberation that normally occurs. They further reported that the myofibrillar ATPase in PSE muscle had a reduced Vmax (maximum velocity of turnover of substrate in enzyme), but that Kapp [dissociation constant of acto-HMM, (heavy meromyosin), which measures the affinity of binding] was unchanged. They concluded that the basic malfunctions in PSE muscle were the irreversible binding of myosin to the actin filaments and functional damage to the myosin ATPase (Sung et al. 1981). Myosin extraction occurs via depolymerisation of the thick filament backbone (promoted by rising ionic strength and presence of pyrophosphate) and dissociation of the myosin heads from actin (promoted by NaCl and pyrophosphate in the presence of Mg2+) (Parsons and Knight 1990). Thus, if myosin is irreversibly bound to actin in PSE muscle (Izumi et al. 1977), it will be difficult to extract. Furthermore, titin and nebulin are degraded more slowly in post-mortem muscles from stress-positive than from stress-negative animals (Boles et al. 1992a), which may also limit extraction of myofibrillar proteins.
Sarcoplasmic proteins
For skeletal muscle tissue, the sarcoplasmic proteins account for ~30–34% of the total protein, are soluble in dilute salt solutions (<0.1 m), are usually classified into four fractions—nuclear, mitochondrial, microsomal and cytoplasmic (Morissey et al. 1987), and consist of 150–200 different proteins. The pre-rigor changes in cytoplasmic proteins appear to be important in PSE muscle. The cytoplasmic proteins include all the enzymes involved in glycolysis and these are the main proteins discussed in this section. The isoelectric points of these proteins are in the range pH 6–7 (Morissey et al. 1987). The influence of accelerated glycolysis at a higher than normal temperature on colour is considered in a later section (see Impacts of fast pH fall at a high muscle temperature on meat colour) and these conditions are known to induce changes to the myoglobin molecule. Proteases, which also reside in the sarcoplasm and are denatured during high rigor temperatures are discussed in the section Evidence for the effects of rapid decline on tenderness.
Bendall and Wismer-Pedersen (1962) first suggested that deposition of denatured sarcoplasmic proteins onto the myofilaments caused reduced extractability of myofibrillar proteins, without necessarily involving the denaturation of these proteins. Fischer et al. (1979) reported that the solubility and activity of the enzymes phosphorylase (mol. wt 180 kD; Scopes 1970) and creatine kinase (CK, mol. wt 42 kD; Scopes 1970) were decreased in PSE muscle and that they were precipitated onto the myofibril. Savage et al. (1990) reported that the concentration of CK and phosphoglycerate kinase (PGK, mol. wt 36 kD; Scopes 1970) in the drip from pork muscle decreases as the amount of drip increases, implying that these enzymes have precipitated and bound onto the myofibrillar proteins. Both CK and PGK have been shown to be particularly susceptible to denaturation and precipitation (Scopes 1964; Fischer et al. 1978). M. Greaser and T. Ishii (pers. comm.) found little evidence for association of any proteins with the myofibrils, except CK and phosphorylase, in PSE muscle. They applied a range of extracting solutions (various combinations of 0.01/0.2 m Na2HPO4, 0.1/0.5/1.1 m Na4P2O7, 0.5/1.1 m NaCl, 1 mm ATP, pH 5.5/8.0) to normal and PSE muscle, and concluded that the poor extractability of myofibrillar proteins in PSE meat was due to myosin denaturation rather than sarcoplasmic proteins coating the myofibrils.
Sayre and Briskey (1963) reported that similar sarcoplasmic protein solubilities were obtained for muscles held at temperatures below or above 35°C, when samples were taken before the onset of rigor. However, for samples taken at the onset of rigor, high muscle temperature reduced the solubility of sarcoplasmic proteins if the pH was <6.0. Muscles that entered rigor >35°C with pH 5.3–5.8 yielded the lowest protein solubility. On the other hand, when muscles entered rigor at a pH >6.0, or had a high ultimate pH, then the protein solubility was not affected by temperature, i.e. they retained high protein solubility. These results emphasise the importance of temperature interaction with the rate of pH decline at rigor onset (Sayre and Briskey 1963).
The effects of temperature on pH decline, as indicated by varying muscle depth, is clearly reflected by the denaturation of sarcoplasmic and myofibrillar proteins (Tarrant and Mothersill 1977). Denaturation of sarcoplasmic proteins is indicated by almost total loss of CK activity during rigor onset at 8 cm in the AD and SM muscles, and faster depletion of ATP at 8 cm than at 1.5 cm depth.
Recommendations
It is not clear whether the proteins, particularly myosin, from different muscles, fibre types and species, vary in the conditions under which they denature.
In addition, the impact of duration of denaturing conditions on the extent of denaturation (and implications for subsequent quality) has not been clearly elucidated, although Offer (1991) provided a hypothesis.
Impacts of rapid pH decline at a high muscle temperature on tenderness
Tenderness is an important meat quality trait, and the biological, structural and physiological mechanisms underlying meat tenderness have been extensively investigated (Dransfield et al. 1981; Koohmaraie 1988; Tornberg 1996; Harper 1999). Essentially, meat tenderness is determined by the amount and solubility of connective tissue, sarcomere shortening during rigor development, and post-mortem proteolysis of myofibrillar and myofibrillar-associated proteins (Koohmaraie and Geesink 2006). The rate of post-mortem energy metabolism is a key determinant of sarcomere shortening and proteolysis post-mortem, and may have an influence on collagen characteristics; thus, these are discussed below.
The evidence for the impacts of rapid pH decline at a higher than normal muscle temperature on tenderness is summarised:
-
In general, exposing pre-rigor muscle to higher than normal temperature (≥35°C), in combination with a rapid pH decline, results in adverse impacts on meat tenderness (so called heat-induced toughening).
-
The incidence of the heat-induced toughening of meat can be attributed to a combined effect of heat-induced additional sarcomere shortening and alterations of proteolytic enzyme activities.
-
In particular, the decrease in ageing potential (either early exhaustion of proteolytic enzyme activities or elevated protein denaturation, which consequently limits the proteolysis) could be a main factor in reduced tenderisation in meat from high pre-rigor temperature carcasses.
-
The increased tenderness seen in unaged meat (at 1–2 days post-slaughter) that has undergone high rigor temperatures may be explained by early activation of the calpain system and possibly changes to the collagen solubility.
This evidence is expanded below.
Evidence for the effect of rapid pH decline on tenderness
Numerous studies have reported the adverse impacts of the accelerated pH decline combined with high temperature on post-mortem meat tenderness development (so called heat-induced toughening) in beef (Hertzman et al. 1993; Devine et al. 1999; Rosenvold et al. 2008; Thomson et al. 2008), lamb (Jaime et al. 1992; Geesink et al. 2000; Devine et al. 2002; Rosenvold and Wiklund 2011), pork (van der Wal et al. 1988; Fernandez et al. 1994; Channon et al. 2000) and poultry (McKee and Sams 1998; Molette et al. 2003; Zhu et al. 2011) (some of which are also summarised in Table 1). In general, meat entering rigor at 15°C produces more tender loin meat, as assessed by both sensory and instrumental tenderness measurement (Warner–Bratzler shear force; WBSF) than meat that has entered rigor at 35−38°C (Devine et al. 1999). Hertzman et al. (1993) determined that high temperatures post-mortem caused increased sensory toughness, but not WBSF toughness, in beef SM and ST, but not in biceps femoris (BF) muscles. In addition, comparing sheep loins entering rigor at 18°C and 35°C, Devine et al. (2002) reported that WBSF was similar at 1 day post-mortem, but after 3 days ageing, the 35°C muscles had higher WBSF and did not age to the same extent. Rosenvold and Wiklund (2011) found that lamb loins (from non-stimulated carcasses) held at 42°C pre-rigor had a significantly higher shear force value than loins held at 15°C pre-rigor (86 N and 48 N, respectively), even after 7 weeks of ageing at –1.5°C, indicating that the extended ageing did not overcome the detrimental effect of high temperature/rapid pH decline on meat tenderness development. PSE meat in pig loin, known to be caused by rapid pH decline at a high muscle temperature, has been found to have acceptable 1-day WBSF values, but fails to age compared with ‘normal’ pork, resulting in non-tenderised pork after 5 days of ageing (Channon et al. 2000).
Some studies show that applying either electrical stimulation or muscle restraining (or wrapping) can override the detrimental effects of heat-induced toughening of meat (Devine et al. 1999, 2002; Rosenvold et al. 2008; Rosenvold and Wiklund 2011), although others found no benefit (Hertzman et al. 1993; Farouk and Swan 1998; Kim et al. 2012). Regardless of the conflicting results (due to different pre-rigor processing, muscle and/or species), in general, most of the studies agree that exposing pre-rigor muscle to high temperature (≥35°C), accompanied by a rapid pH decline, results in a reduced ageing potential, thus adversely affecting meat tenderness development. The phrase ‘heat toughening’ has long been used as a term to describe the reduced ageing effect of muscle undergoing the pre-rigor high temperature condition. However, it has been pointed out that this term does not correctly describe the occurrence, primarily for two reasons (C. Devine, pers. comm.). First, a definition of ‘heat’ is energy transferred from one body to another by thermal interactions. Heat is not a property of a system or body, but instead is always associated with a certain thermal process. This transfer can occur at any temperature above 0 K. Cold refers to the condition or subjective perception of having low temperatures, the opposite of hot. Thus, heat would not be entirely the correct word to use for this circumstance (but ‘hot’ would be more precise in this perspective). Second, ‘toughening’ (i.e. an increase in shear force) does not occur, but the meat does not tenderise as it should, mainly due to reduced ageing potential (i.e. reduced tenderisation or no tenderisation rather than toughing). Therefore, a phrase such as ‘reduced tenderisation at elevated temperature’, or at least ‘heat-induced toughening’, would be a more precise way to describe the incidence.
The exact mechanism by which the abnormally low pH/high temperature condition negatively affects meat tenderness has not been fully elucidated (although ‘heat shortening’ was historically considered to be the major phenomenon causing heat-induced toughening; Locker and Hagyard 1963; Lee and Ashmore 1985; Hertzman et al. 1993). Furthermore, other studies described contrasting results, where accelerated pH decline combined with high muscle temperature actually improved meat tenderness (Locker and Daines 1975; Hwang et al. 2004; Thomson et al. 2008). Lochner et al. (1980) compared the sensory tenderness of beef loins with a 2-h temperature of 27−40°C and found that the loins with a 2-h temperature of 40°C were most tender. Locker and Daines (1975) determined that beef neck muscle (M. sternomandibularis) undergoing pre-rigor holding temperature at 37°C had lower shear force values than the muscle held at 15°C, when measured at 1 day post-mortem. Furthermore, they found that, as the rigor temperatures approached 37°C, the shear force of the muscles decreased, indicating an improvement of tenderness with increasing pre-rigor holding temperatures. Similar positive impacts of high pre-rigor temperatures on meat tenderness of beef M. longissimus (LD) and SM at 36°C (Hwang et al. 2004) and venison LD at 42°C (Bekhit et al. 2007) were also reported. However, most of the studies observing improved tenderness by high pre-rigor temperatures were assessed at 1 day post-mortem, and therefore the confounding effects of ageing in meat tenderness development were not taken into consideration.
Influence of sarcomere (heat) shortening on tenderness
The relation between the rate of glycolysis (pH decline) under different pre-rigor temperatures and the degree of shortening has been well studied (Marsh 1954; Locker and Hagyard 1963; Honikel et al. 1983; Smulders et al. 1990). Locker and Hagyard (1963) determined that shortening was minimal when pre-rigor muscles were held at 15−20°C, whereas substantial shortening was observed in muscles when exposed to pre-rigor temperatures either appreciably above or below this intermediate temperature range (Fig. 1). Particularly, they found more severe shortening (almost 50% of their normal length) at a lower temperature (0°C; cold shortening) than at a higher temperature (about 30% shortening at 42°C; heat shortening or rigor shortening). This observation indicates that variations in the rate of rigor development alter the muscle structure (Dransfield 1994) and that muscle shortening occurring during the development of rigor mortis is temperature-dependent (Locker and Hagyard 1963).
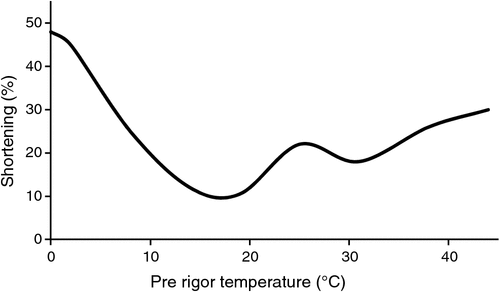
|
Cold shortening caused by rapid temperature decline before the completion of rigor mortis results in an immediate shortening driven by increased cellular calcium through leakage from the sarcoplasmic reticulum (Locker and Hagyard 1963; Bendall 1978; Thompson et al. 2006). Honikel et al. (1983) reported that beef neck muscles incubated at pre-rigor temperature of −1°C resulted in a very rapid and intensive shortening (the muscle shortened by 20 cm within 2 h of incubation), and the onset of rigor was likely to occur at pH 6.85, with high ATP concentration. By contrast, in muscles at pre-rigor temperatures >15°C, shortening did not start at pH values >6.25, indicating that cold shortening can be initiated at any time post-mortem, whereas heat shortening would occur at the onset of rigor rather than before, and at a relatively low ATP concentration (Honikel et al. 1983). Therefore, heat shortening tends to be less severe than cold shortening in the extent of total shortening (%) due to a lack of ATP to stimulate irreversible actomyosin bridge formation (Marsh and Leet 1966; Thompson et al. 2006).
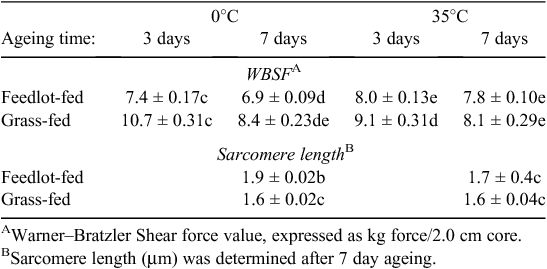
However, despite the different degree of shortening, both cold and heat shortening will cause sarcomere shortening and hence a reduction in the myofibrillar lattice. In general, shortening is recognised as one of major contributors to decreased meat tenderness, where the shorter the sarcomere, the tougher the meat (Marsh and Leet 1966; Herring et al. 1967; Tornberg 1996). Furthermore, the detrimental impact of heat shortening on meat tenderness has been reported (Marsh and Leet 1966; Davey and Gilbert 1973; Bowling et al. 1978; Lee and Ashmore 1985; Hertzman et al. 1993; Geesink et al. 2000). Lee and Ashmore (1985) determined that beef loins from carcass sides held at 35°C for 3 h after slaughter had significantly higher WBSF, lower sensory tenderness and shorter sarcomere length than the loins from carcass sides held at 0°C (see Table 2).
|
Additionally, restraining pre-rigor beef muscles exposed at high temperatures (≥35°C) by wrapping, stretching or hanging seems to overcome heat-induced toughening (Devine et al. 1999; Rosenvold et al. 2008; Kim et al. 2014; Warner et al. 2014b) by minimising shortening, which could indirectly indicate the role of heat shortening in meat tenderness as well. However, several studies (as discussed below) have also identified that occurrence of heat-induced toughening cannot be solely explained by heat shortening when proteolysis and limited ageing effect are taken into consideration.
In fact, a complicated or inconsistent relationship between pre-rigor temperature, sarcomere length and tenderness has been reported. Geesink et al. (2000) found that although a substantial difference in sarcomere length between lamb loins held at 35°C and 5°C pre-rigor was observed (1.5 and 1.9 µm, respectively), it did not result in differences in shear force values at 1 day post-mortem. At 14 days post-mortem, the loins held at 5°C had reduced shear force values, whereas the shear force of the loins held at 35°C was unchanged.
Smulders et al. (1990) concluded that the rate of glycolysis has a significant impact on the correlation between sarcomere length and tenderness. They found a lower impact of sarcomere length on tenderness in muscle that had undergone rapid glycolysis. By contrast, muscle undergoing slow glycolysis (pH >6.3 at 3 h post-mortem) appeared to present a strong correlation between tenderness and sarcomere length (r = +0.84 and –0.80, respectively, for sensory and shear force), suggesting that shortening only influenced tenderness when glycolysis was relatively slow. This could partially explain the much more severe impact of cold shortening (high pH at 3 h post-mortem) on tenderness compared with heat shortening (relatively low pH at 3 h post-mortem), as described in the previous section. Moreover, different muscle fibres differ in their rate of energy (ATP) depletion and pH decline. In particular, oxidative muscle fibres are more susceptible to cold shortening compared with glycolytic muscle (Huff Lonergan et al. 2010).
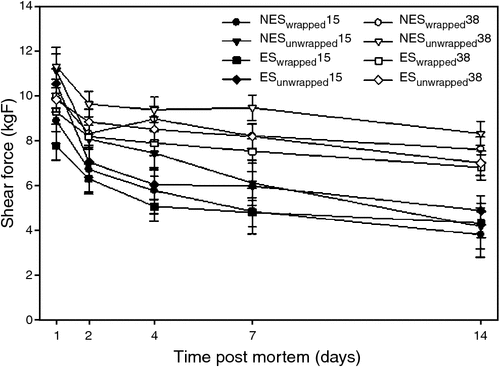
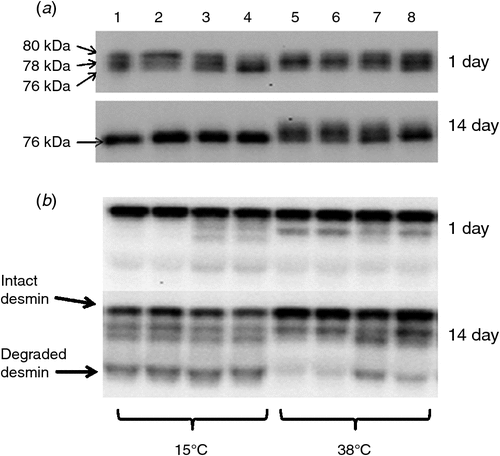
Moreover, other studies found no substantial effects of high pre-rigor temperatures on sarcomere length (Farouk and Swan 1998; Farouk and Lovatt 2000; Devine et al. 2002; Bekhit et al. 2007; Kim et al. 2012). Kim et al. (2012) tested the effects of electrical stimulation and wrapping on beef loins held at 38°C pre-rigor on chemical, physical and biochemical attributes during ageing. This study was conducted because a previous study by Rosenvold et al. (2008) found that applying electrical stimulation and/or wrapping could protect from the detrimental impacts of hot-boned beef loins held at 35°C pre-rigor. However, in contrast to the findings by Rosenvold et al. (2008), holding pre-rigor beef loin at 38°C until rigor resulted in significantly higher shear force values than in the loins held at 15°C for up to 14 days post-mortem, regardless of electrical stimulation and/or wrapping (Fig. 2). Furthermore, no difference in sarcomere length of the loins from the two pre-rigor temperatures (15°C and 38°C) was found, regardless of electrical stimulation and wrapping. The authors concluded that the incidence of heat-induced toughening was likely due to elevated protein denaturation, which subsequently limited the extent of µ-calpain autolysis and desmin degradation, resulting in a limited ageing potential of the meat (Fig. 3).
|
|
Proteolysis and reduced ageing potential in high rigor temperature muscles
Meat tenderness substantially improves with ageing through myofibrillar protein degradation by endogenous proteolytic enzymes in muscle. It is well established that calpains play a significant role in post-mortem protein degradation, and thus influence meat tenderisation (Koohmaraie 1996; Goll et al. 2003; Huff-Lonergan and Lonergan 2005). Pre-rigor processes, particularly different rates of pH and temperature decline at early post-mortem, have a profound impact on proteolytic enzymes (mainly µ-calpain), subsequently affecting the ageing potential for tenderisation of the meat (Dransfield 1994; Hwang and Thompson 2001; Melody et al. 2004).
Numerous studies have reported adverse effect of higher than normal pre-rigor temperature on ageing potential, or limited tenderisation with an extended ageing storage period (see Table 1 for references). Although some studies found improved tenderness (presumably by accelerated proteolytic enzyme activities) at high pre-rigor temperatures (Whipple et al. 1990; Hwang et al. 2004; Thomson et al. 2008), most studies reported that tenderness improvement was only a transient effect, and no further tenderisation took place in meat exposed to high rigor temperatures. In fact, there is general agreement that early activation of µ-calpain by elevated free calcium ions (Hwang et al. 2004), combined with high pre-rigor temperatures, results in faster degradation of myofibrillar proteins. However, it eventually causes an early exhaustion of proteolytic enzyme activity, possibly due to faster autolysis, and thus a decrease in proteolysis with extended ageing (Whipple et al. 1990; Dransfield 1994; Rhee et al. 2000; Rhee and Kim 2001; Thomson et al. 2008; Kim et al. 2013; Warner et al. 2014b, 2014c).
Kim et al. (2010) found less µ-calpain autolysis (based on using whole muscle protein extract, including sarcoplasmic and myofibrillar proteins) and less desmin/troponin-T degradation in the deep portion of the SM muscle compared with the superficial portion of the SM. Furthermore, Kim et al. (2012) also determined that beef loins held at high pre-rigor temperature (38°C) had higher shear force values and a lesser extent of µ-calpain autolysis and desmin degradation (Figs 2 and 3) throughout the entire ageing period (14 days) compared with loins held at 15°C. Although rapid onset of rigor mortis was observed due to the accelerated glycolysis by high temperature, no differences in sarcomere length were found between the two pre-rigor temperatures regardless of electrical stimulation and wrapping (Kim et al. 2012).
Hwang et al. (2004) concluded that optimising ageing potential is a more significant factor affecting the meat tenderisation process during ageing storage, rather than the impact of structural conformation in shortened muscle on meat tenderness. This indicates the importance of maximising ageing potential for meat tenderness. It also suggests a more significant impact of limited ageing potential under high pre-rigor temperature on heat-induced toughening of meat over the incidence of heat shortening. In a recent study, Kim et al. (2013), showed that even moderately high pre-rigor temperature (27°C for 1 h post-mortem) induced early activation of µ-calpain and resulted in less overall tenderisation after 14 days of ageing of lamb loins compared with the counterpart loins from lamb carcasses held at 20°C for 1 h post-mortem.
Influence of rapid pH decline on connective tissue
Data are lacking on the influence of post-mortem metabolism and high muscle temperature on connective tissue and its solubility. Bailey and Light (1989) stated that it is unlikely, considering the nature of connective tissue, particularly its stability and slow turnover, that collagen, elastin or the other constituents of connective tissue are affected by any treatments immediately pre-slaughter or post- slaughter. The general conclusion is that changes in connective tissue post-mortem and during ageing are negligible, and unlikely to contribute to the tenderisation (Bailey and Light 1989). However, PSE pork muscle has been found to have an increased percentage of salt-soluble collagen and heat-labile collagen relative to normal pork muscle (McClain et al. 1969). In addition, Wu et al. (1981) reported the positive impacts of high pre-rigor temperature in bovine muscle on lysosomal enzyme activities and its concomitant positive impact on dissolution of collagen fibres and beef tenderness. These observations in beef and pork may assist in explaining the increased tenderness seen in high rigor temperature muscle at 1 day post-slaughter, but do not explain the lack of tenderisation seen with ageing.
Recommendations
Extensive studies have been undertaken to determine the effects of accelerated pH decline accompanied with higher than normal pre-rigor temperature on meat tenderness, particularly focusing on heat-induced shortening and alteration in the post-mortem meat tenderisation process. These studies have produced quite variable, and sometimes conflicting, results. Thus, there are still gaps in our knowledge, and for these reasons, the following recommendations are made.
-
There is still limited understanding of the underlying mechanism by which endogenous proteolytic systems are influenced by high pre-rigor temperatures Most attention has been given to two major proteolytic enzyme systems, namely cathepsins and calpains, but the possible role for other potential proteolytic systems (i.e. proteasomes and caspases) and involvement of apoptosis, or cell death (Herrera-Mendez et al. 2006), in the meat tenderisation process under high pre-rigor temperature have not been investigated. Recently, Balan et al. (2014) reported a significant correlation between degradation of small heat shock proteins (20 and 27) and myofibrillar protein degradation of bovine muscle, which partially supports a recent hypothesis of the involvement of heat shock proteins (or apoptosis) in meat tenderness (Herrera-Mendez et al. 2006; Pulford et al. 2008, 2009; Lomiwes et al. 2014). It would therefore be worthwhile to consider and further explore our understanding in these potential systems with relation to the consequence of high pre-rigor temperature and meat tenderness.
-
Furthermore, as discussed above, effects of high pre-rigor temperatures on collagen solubility and subsequent meat tenderness development have not been fully investigated.
-
One of the possible reasons for the inconsistent results could be attributed to the fact that most of the studies often overlooked, or did not take into account, the various compounding important intrinsic/extrinsic factors such as animal background (breed, age, sex, castration status, and/or feeding condition), pre-slaughter animal handling (particularly level of animal stress), seasonal (environmental) impact, and variation in different muscle fibre types and location. Obviously, it is not possible for a single study to integrate all of these factors to investigate the effects of accelerated pH decline with higher than normal pre-rigor temperature on meat tenderness. However, meat scientists need to be aware and to take these factors (or at least one) into consideration when designing future studies in this subject area. Future studies need to be expanded from in situ excised pre-rigor muscles under different incubation temperatures to measuring muscles in vivo and in the intact carcass, under commercial conditions.
Impacts of rapid pH decline at a high muscle temperature on meat colour
Colour is one of the most important attributes of meat. It is the primary attribute by which both fresh and cured meats are judged by the consumer before purchase. The desirable colour of meat is usually reddish-pink (or bright cherry red), which indicates to the purchaser that the product is wholesome and edible. Meat colour is usually assessed at grading, at about 24 h, and then on the supermarket shelves. The rate of discoloration on retail shelves is an important consideration, as retailers start to discount product, or remove it from the shelves, once the colour starts to deteriorate. Muscle colour depends on the concentration of pigments, mainly myoglobin, the chemical state of myoglobin and the extent of light scattering. The evidence for impacts of rapid pH decline at a higher than normal muscle temperature on meat colour is summarised as follows:
-
Pre-rigor muscles undergoing higher than normal temperature accompanied by a rapid pH decline result in paler colour and reduced colour stability of meat.
-
The decreased colour stability can be attributed to protein denaturation (particularly myoglobin and/or myofibrillar) as a primary factor, and possibly to altered oxygen consumption by endogenous enzymes and/or metmyoglobin reducing ability.
-
The paler colour can be attributed to light scattering.
These factors are discussed below.
Influence of high rigor temperature on meat colour
The rapid pH decline at high muscle temperature condition has been well known to affect both meat colour at grading and colour stability. In particular, the incidence in pork, recognised as meat being very pale in colour under rapid pH decline in the early pre-rigor period, has been extensively studied (Wismer-Pedersen 1959; Wismer-Pedersen and Briskey 1961; Offer 1991; Fernandez et al. 1994; Warner et al. 1997; Joo et al. 1999). However, the effect of rapid pH decline on the paleness of meat has also been studied in other species, as summarised in Table 1.
Conditions that are PSE-like can vary with depth in beef muscles in the hindquarter. For example, the interior portion of SM cools at a much slower rate than the surface portion of the muscle (ΔT>15°C at 2 h post-mortem), and a much faster pH decline is observed in the interior than the surface (ΔpH >0.5 at 3 h post-mortem) (Sammel et al. 2002). This results in a much lighter colour in the interior portion of the hindquarter than the superficial portion (MacDougall 1982; Renerre 1990; Sammel et al. 2002; Seyfert et al. 2004; Kim et al. 2010).
Furthermore, the rapid glycolysis of beef muscle while temperature is high seems to result in brighter and more red colour at the initial display. In a recent beef experiment, we found that hot-boned beef loins (within 30 min post-mortem) placed in a 38°C water bath during the pre-rigor period appeared to have greater L* (lightness) and a* (redness) values at 1 day post-mortem compared with the loins held at 15°C, regardless of electrical stimulation of the beef carcass (Figs 4 and 5). Furthermore, the greater b* (yellowness) and hue angle (indication of discoloration) values seen in loins held at 38°C pre-rigor compared with loins at 15°C (data not shown) could indirectly indicate a possibility of more rapid discoloration of the loins exposed to the accelerated glycolysis under high temperature condition.
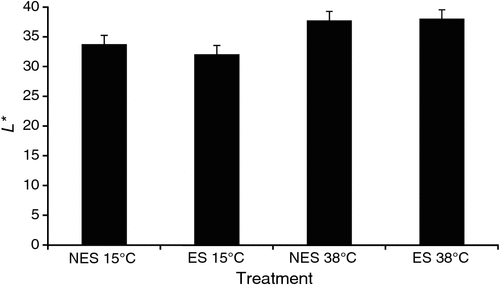
|
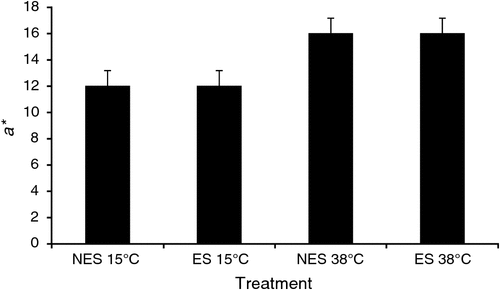
|
In fact, a transient colour improvement (more bright red) due to the PSE-like condition in beef has been reported by many researchers (Ledward 1985; Renerre 1990; Young et al. 1999; Roeber et al. 2000; Sammel et al. 2002), which could be beneficial at times for carcass grading (Roeber et al. 2000). However, decreased colour stability due to greater accumulation of metmyoglobin and rapid discoloration of muscle submitted to the rapid pH decline/high temperature condition has been also reported (Kim et al. 2010; Ledward 1985; Sammel et al. 2002).
The severe discoloration of muscle under rapid pH decline/high temperature has also been found in venison and lamb meat. Bekhit et al. (2007) determined that venison LD held at 42°C pre-rigor temperature had the highest rate of change of redness values during display time compared with other pre-rigor temperatures (0, 15, 25, 30 and 35°C). Rosenvold and Wiklund (2011) found in their study of lamb loins that LD held at 42°C pre-rigor temperature had higher L* and a* values than loins at other pre-rigor temperatures (5, 15 and 25°C) at the initial display time. However, as display time increased, it showed more discoloration (indicated by higher hue angle values) than other loins, confirming lower colour stability of the loins exposed to the protein-denaturing condition.
Paler colour and decreased colour stability of meat undergoing rapid pH decline and high temperatures could be attributed to a significant denaturation of sarcoplasmic (particularly myoglobin) and/or myofibrillar proteins (structural changes influencing WHC), altered oxygen consumption by endogenous enzymes and/or altered metmyoglobin reducing ability, as discussed in the following sections.
Light scattering and meat colour
Light scattering in meat has been postulated to cause the increased paleness observed in meat of rapid pH decline. Meat of rapid pH decline has increased weep/water on the surface, reduced myofilament spacing due to lower pH levels (Swatland 2012) and myosin denaturation (Offer 1991), and also has denatured sarcoplasmic proteins, which aggregate together in the sarcoplasm and also bind to myofibrils (Goldspink and McLoughlin 1964). All of these factors have been postulated to contribute to the paleness and are discussed below.
Weep, exudate or water on the meat surface, such as with PSE-like meat, can cause spectral reflectance to occur. This appears to the eye as a ‘gloss’ on the surface, and causes the surface to reflect more light, thus influencing colour measurements and human perception (Judd and Wyszecki 1975).
Offer and Knight (1988) proposed that sarcoplasmic protein denaturation in PSE meat causes incident light to be scattered to about twice the extent that it is scattered in ‘normal’ muscle, causing light to penetrate to a shallower depth and to be adsorbed to a smaller extent by myoglobin. Because meat is partially translucent, a portion of the incident light is transmitted below the surface and reflected internally. The colour of meat is determined by scattering as well as absorption, and Offer (1991) proposed that the increased light scattering in PSE muscle arises from reduced gaps between myofibrils. The idea that the paleness of PSE meat originates from the increased lateral surface reflectance of myofibrils at low pH was first proposed by Hamm (1960) and has received very little attention, as it is hard to test this theory. Offer and Knight (1988) have also proposed that the pale colour in PSE meat is due to the bands of alternating, super-contracted and stretched myofibrils.
Sarcoplasmic protein denaturation and myoglobin oxidation
The rapid pH decline post-slaughter has an adverse impact on the redox stability of myoglobin, the primary pigment (mol. wt 16 700 D) responsible for meat colour (Kim and Hunt 2011). Myoglobin comprises a prosthetic heme group and a single polypeptide protein or globin molecule, which protects the heme iron group from oxidation by surrounding it as a pocket. Thus, the protein-denaturing condition of rapid pH decline/high temperature can disrupt the globin moiety’s ability to protect the heme, which subsequently leads to the unfolding of globin moiety, thus allowing a greater autoxidation of the pigment and a higher susceptibility to metmyoglobin (oxidised pigment) formation during display (Livingston and Brown 1981; Renerre 1990; Kim and Hunt 2011). Zhu and Brewer (2003) observed that when the damage to the globin moiety occurred, the redox ability of myoglobin decreased, so the extent of reduction of metmyoglobin is substantially decreased. Furthermore, at the low pH values (<6), metmyoglobin-reducing activity is more depressed, which could be attributed to the pH-induced denaturation of the globin moiety (Zhu and Brewer 2003), and the rate of autoxidation increases with decreasing pH and increasing temperature (Ledward 1985). Therefore, faster than normal pH decline combined with higher than normal temperature of pre-rigor muscle can result in myoglobin denaturation (or damage on globin moiety) and subsequent elevated autoxidation (Zhu and Brewer 2003). Hence, maintaining the integrity of the globin molecule by protecting it from physicochemical stress is critical in terms of preserving colour stability of meat. Sammel et al. (2002) found that the interior portion of SM had higher lipid oxidation and metmyoglobin formation than the superficial portion of the muscle. However, when they applied partial hot-boning by exposing the interior portion of the round muscle, it accelerated chilling of muscles in the hindquarter and subsequently facilitated no difference in lipid oxidation and metmyoglobin formation, resulting in more uniform colour between the interior and superficial portion of the muscle (Sammel et al. 2002). This indicates that the chilling rate (or pre-rigor temperature) plays a crucial role in the redox stability of myoglobin, particularly in the deep, interior portion of round muscles, subsequently affecting meat colour and colour stability (Seyfert et al. 2004).
Metmyoglobin-reducing activity and oxygen consumption
The rapid pH decline achieved at higher temperatures could also adversely affect metmyoglobin-reducing ability and thus result in decreasing colour stability of meat. Meat has a limited ability to reduce the oxidised ferric state of myoglobin to the reduced ferrous state via its endogenous reducing system, which includes essential co-factors such as NADH and metmyoglobin reductase (Giddings 1974; Bekhit and Faustman 2005). Several studies report that metmyoglobin reductase is not heat-stable and its activity decreases with increasing temperatures (Hagler et al. 1979; Livingston et al. 1985; Faustman et al. 1988; Mikkelsen et al. 1999; Osborn et al. 2003). Rapid pH decline has also been reported to decrease metmyoglobin reductase enzyme activity (Faustman and Cassens 1990).
Zhu and Brewer (1998) reported lower metmyoglobin reductase activity and greater metmyoglobin accumulation in PSE pork than in normal and DFD (dark, firm and dry) pork. They speculated that the lower metmyoglobin reductase activity of PSE pork could be due to enzyme denaturation by the rapid pH decline/high temperature condition. Sammel et al. (2002) also reported that the interior portion of beef SM could display PSE-like characteristics, with lower metmyoglobin-reducing ability and greater metmyoglobin formation compared with the superficial portion of the same muscle. They further observed that hot-boning of the muscle significantly increased metmyoglobin-reducing activity of the interior portion of the muscle and subsequently increased meat colour stability compared with that of traditionally cold-boned muscles.
In addition, higher temperatures increase the oxygen consumption rate by respiratory enzymes within the muscle tissue (Cheah and Cheah 1971; Bendall and Taylor 1972). Consequently, lower oxygen tension and solubility at the meat surface can be created, which in turn induces increased myoglobin oxidisation (Giddings 1974; Seideman et al. 1984; Faustman and Cassens 1990). Sammel et al. (2002) found significantly higher oxygen consumption rate in the interior portion of beef SM than the superficial portion of the muscle. However, no significant difference in oxygen consumption rate between PSE and normal pork was observed (Zhu and Brewer 1998). Furthermore, high temperature retards oxygen penetration into the meat, which accelerates the expansion of the intermediate metmyoglobin layer towards the surface of meat, inducing more rapid discoloration (Brooks 1935).
Recommendations
-
In general, the impact of high pre-rigor temperature on meat colour and colour stability are well understood. However, the mechanistic reactions governing myoglobin redox stability (e.g. the early exhaustion of reducing potential such as NADH or decreased reductase activity, or a combination of both) and oxygen consumption (e.g. mitochondrial respiratory enzyme activity) under the protein-denaturing condition, and the concomitant impact on meat colour, are not fully understood.
-
Furthermore, the combined impacts of current post-slaughter processing such as electrical stimulation, hot-boning and/or pre-rigor muscle stretching and high pre-rigor temperature on meat colour and colour stability have not been clearly elucidated from the literature, and thus, they warrant further investigation.
-
New methods are required for measuring light scattering in muscle tissue, in order to elucidate its role in muscle colour.
Impacts of fast pH fall at a high muscle temperature on water-holding capacity and juiciness
Water-holding capacity
The WHC is defined as the ability of fresh meat to retain its own water (Pearce et al. 2011). Poor WHC results in high drip and purge loss, which can represent significant loss of weight from carcasses and cuts and may affect the yield and quality of processed meat (Savage et al. 1990). In addition, inferior WHC can negatively affect the appearance of meat, and this can influence consumer willingness to purchase the product.
Several factors are generally known to influence the WHC, as listed below (for a more detailed discussion, refer to Warner 2014):
-
The rate and the extent of pH decline during the conversion of muscle to meat (Bendall and Swatland 1988).
-
Pre-rigor myosin denaturation (Offer 1991).
-
Sarcomere shortening during the rigor period (Honikel et al. 1986; Bertram et al. 2002).
-
Proteolysis and protein oxidation (Huff-Lonergan and Lonergan 2005).
Many papers and reviews have been published on the impacts of rapid pH decline at a high muscle temperature on WHC in pork (for reviews, refer to Bendall and Swatland 1988; Huff-Lonergan and Lonergan 2007; Barbut et al. 2008); however, there are a relatively few studies available on meat from ruminants, and until recently it has not been considered a serious problem in beef (Hamm 1986; Aalhus et al. 1998). This is due to the relatively low proportion of fast-twitch glycolytic muscle fibres in beef compared with pork muscle (Aalhus et al. 1998); hence, few studies have included measurements of WHC when investigating the impact of a rapid pH decline at high muscle temperatures in beef and lamb, and even fewer have included biochemical measurements to understand the underlying mechanism.
When inferior WHC has been found in beef, it is often associated with the deep muscles (or deep parts of muscle) such as in the round (Tarrant and Mothersill 1977; Sammel et al. 2002). Tarrant and Mothersill (1977) concluded that unfavourable post-mortem conditions result in significant denaturation of both sarcoplasmic and myofibrillar proteins, which is in agreement with findings by Monin and Laborde (1985) and Hunt and Hedrick (1977a).
Most data available on the impact of a rapid pH decline at high muscle temperatures on WHC come from studies investigating the effects of electrical stimulation on other meat quality attributes, primarily colour. Both Martin et al. (1983) and Unruh et al. (1986), who achieved pH values <6.0 while the temperature was >35°C in electrically stimulated beef carcasses (i.e. outside the pH/temperatures window used by the Australian beef and sheep meat eating quality program Meat Standards Australia; Thompson 2002), found WHC to be inferior compared with control carcasses that were not electrically stimulated. Similarly, pH values of 6.0 with pre-rigor temperatures >30°C in beef carcasses were also found to result in lower WHC (Hertog-Meischke et al. 1997), and it was concluded that this was due to myosin denaturation, as found in pork in agreement with Tarrant and Mothersill (1977). Hopkins et al. (2014) found purge loss to be increased in the strip loin but not in the cube roll from electrically stimulated beef carcasses that had a rapid pH decline while the muscle temperature was still high. Note that electrical stimulation is not synonymous with high rigor temperature and hence inferior WHC, as exemplified by Hamm (1986), who reviewed ~30 beef and lamb studies and found only minor effects of electrical stimulation on WHC. In most cases, electrical stimulation caused a small, but mostly insignificant, decrease in WHC, regardless of the types of electrical stimulation applied. However, as exemplified in the Australian beef industry, changes in recent years have resulted in substantially larger and fatter cattle that take longer to chill post-mortem, increased grain finishing with associated increased body temperature at the time of slaughter, and increased electrical inputs, which together have increased the incidence of high temperature rigor (Strydom and Rosenvold 2014) and thus caused associated decreases in the WHC of beef meat (Hopkins et al. 2014; Warner et al. 2014a). In both Australia and the UK, high rigor temperature also occurs in lamb carcasses (Thompson et al. 2005; Pearce et al. 2010; Matthews 2011), with proposed detrimental effects on WHC as has been observed in controlled experiments (Kim et al. 2014; Warner et al. 2014b). Data are lacking on the effects of high rigor temperature on WHC in the commercial environment in lamb carcasses.
Other studies have investigated the impact of different post-mortem temperatures by removing the muscles from the carcass early post-mortem while keeping the muscle temperature constant, sometimes in combination with electrical stimulation. Devine et al. (1999) found no differences in WHC when beef muscles were exercised pre-rigor and kept at 15, 20, 25, 30 or 35°C. By contrast, Geesink et al. (2000) reported that WHC measured in lamb loins as drip loss during vacuum-packaged storage decreased with pre-rigor temperatures ≥25°C compared with pre-rigor temperatures of 0, 5, 10, 15 and 20°C. Similarly, Devine et al. (2002) found high cooking loss in lamb loin held at 35°C pre-rigor compared with lamb loins held at18°C pre-rigor. These results were supported by a similar study conducted by Rosenvold et al. (2008), in which variability in time to reach rigor in beef LD was achieved using electrical stimulation and pre-rigor temperatures of 15 and 35°C, respectively. However, as the ageing progressed during storage at 15°C for up to 90 h, the drip of the muscles held at 15°C pre-rigor increased over that of muscles held at 35°C pre-rigor. In venison, purge loss was found to be significantly lower at 25°C than at 42°C, with no differences in drip loss between the other temperatures (0, 15, 30 and 35°C). By contrast, WHC (measured using the filter press method) decreased with increasing rigor temperature, and it was significantly correlated with the solubility of sarcoplasmic and total protein (Bekhit et al. 2007). Similarly, increased protein denaturation at 24 h post-mortem was found in beef loins placed at 38°C pre-rigor temperatures compared with beef loins placed at 15°C pre-rigor (Kim et al. 2012), and was accompanied by inferior WHC as measured by higher purge loss and drip loss. It should be noted, that electrical stimulation, which was also applied in the study, did not result in significant effects on either protein denaturation or WHC.
Aalhus et al. (1998) selected beef and pork carcasses graded as PSE and compared these with carcasses graded to have normal quality from a larger dataset from their previous published work (Aalhus et al. 1994), where variation in pH/temperature decline was achieved using high/low voltage stimulation and different chilling regimes. They found that the beef and pork carcasses graded as PSE had faster glycolysis and significantly higher temperature at 45 min post-mortem. Drip loss in the control pig carcasses were substantially higher than in the control beef carcasses, while the increase in drip associated with the PSE condition in beef was much larger than that in PSE pork (175% vs 28%) (Aalhus et al. 1998). Finally, the role of proteolysis and µ-calpain in WHC of meat is well documented. Limited autolysis of µ-calpain early post-mortem (indicating lower µ-calpain activities) results in a decrease in proteolysis, which induces an increased shrinkage of the muscle cell, creating channels for dripping moisture out of muscle bundles and thus results in greater drip loss (Huff-Lonergan and Lonergan 2007). Moreover, at high pre-rigor temperatures, the impact of rapid glycolysis on µ-calpain activity and autolysis and its subsequent adverse effect on protein degradation, WHC and post-mortem meat tenderisation has been reported for porcine muscle (Claeys et al. 2001; Maddock et al. 2005; Bee et al. 2007).
Juiciness
Juiciness is an eating quality attribute considered to arise from two sources: moisture released by meat after the first bite and during chewing; and moisture from saliva (Winger and Hagyard 1994). It is predominantly determined by two factors: the end-point temperature to which the meat is cooked (Aaslyng 2009); and the intramuscular fat content of the muscle (Savell and Cross 1988).
While intuitively, juiciness would be positively correlated with the WHC of meat, results of studies comparing sensory assessment of juiciness to measures of WHC are contradictory (Winger and Hagyard 1994). These inconsistencies might be a result of the different methodologies applied to measure WHC (Trout 1988) and/or the different cooking methods, as cooking methods specifically affect the result of the sensory analysis (Bejerholm and Aaslyng 2004). Likewise, total water content of the meat and cooking loss cannot explain juiciness of the cooked meat product (Toscas et al. 1999; Safari et al. 2001; Young et al. 2005).
Hence, it is difficult to predict how high pre-rigor temperatures in beef carcasses will affect the juiciness of the resulting meat. In pork, when juiciness of PSE pork was compared with that of DFD and normal pork, PSE pork invariably scored lowest for juiciness if differences were found (Barton-Gade 1996; Aaslyng et al. 2007). However, some studies suggest no differences in juiciness between PSE and normal pork (Winger and Hagyard 1994; Van Oeckel and Warnants 2003). Studies specifically measuring juiciness of beef from high pre-rigor temperature beef are very limited. Warner et al. (2014c) found that juiciness in beef increased with ageing. Whereas the increase was minor in high pre-rigor temperature strip loins, the increase in predicted juiciness score in strip loins with an ‘ideal’ rigor temperature was more than double of the high pre-rigor temperature strip loins. However, these results will have to be confirmed by additional studies to be conclusive, as the influence of high pre-rigor temperature on consumer scores for juiciness may be correlated with the consumer scores for tenderness. In line with the argument by Winger and Hagyard (1994) related to contradictory results on the relationship between WHC and juiciness, Hunt and Hedrick (1977a) found small but significant differences in WHC between PSE, normal and DFD beef, but no differences in juiciness. A study using electrical stimulation as a model for high pre-rigor temperature beef, i.e. lower pH/higher muscle temperature, does not provide substantial information regarding effects of the denaturing condition on juiciness; results of the studies examining the effect of electrical stimulation on meat juiciness are variable (Winger and Hagyard 1994), and no clear conclusion can be drawn. In their comprehensive review on meat juiciness, Winger and Hagyard (1994) concluded that juiciness is a poorly understood aspect of the eating quality of meat, and this appears still to be the case including how juiciness is affected by protein denaturation as a result of a rapid pH decline at high rigor temperatures.
Recommendations
-
Despite the limited number of studies and some inconsistencies in the results, the underlying mechanisms for PSE development in both ruminants and pigs (i.e. low pH in combination with high temperature) appear to be similar. However, this statement cannot be confidently substantiated with biochemical data as only very limited results have been published. It is suggested that measurements of WHC are included as standard measurements in beef and lamb studies focused on the slaughter process to substantiate that the conditions are indeed identical for pigs and ruminants and to identify any adverse effects on meat quality. This is particularly important within a changing system such as in the Australian beef industry.
-
We suggest that research is carried out to understand the underlying biochemical and biophysical aspects of juiciness, including how this is related to WHC. This should include total water losses and a strong focus on what the WHC methods are actually measuring (Trout 1988).
Impacts of rapid pH decline at a high muscle temperature on flavour
The type, quantity and balance of flavour molecules are critical to the acceptability of meat flavour. In its fresh uncooked state, meat has little flavour; it is only as a result of cooking that full flavour develops. During cooking, a complex set of thermally induced reactions occurs between the non-volatile components of lean and fat tissue, which results in the generation of a large number of products. The major precursors of meat flavour are either lipids or water-soluble components, which are subject to two sets of reactions during the cooking process – Maillard reactions between amino acids and reducing sugars, and oxidative degradation of the lipid components (Watkins et al. 2013). Principally, the sugars, amino acids and water-soluble components may be influenced by meat undergoing rapid metabolism and protein denaturation post-mortem, and these are discussed below.
High-pH meat has been reported to have less flavour and to be less preferred than normal pH beef (Young et al. 1993) due to a lack of browning and reduced Maillard reactions (Hunt and Hedrick 1977a). PSE pork is generally reported to be no different in flavour from normal meat (Bennett et al. 1973; Goransson et al. 1992; Flores et al. 1999), whereas, in contrast, the consumer acceptability of the flavour of high-temperature beef strip loin and rump (similar to PSE) is lower than for normal (low rigor temperature) beef muscles (Warner et al. 2014b). In the 1950s, PSE pork meat was reported to have a pronounced sour smell, although the sour smell dissipated when the meat was exposed to air (Ludvigsen 1954. 1960, as cited in Briskey 1964). According to Briskey (1964), the sour smell can be simulated if acid is added to muscle proteins and they are subsequently held at 40°C. Similarly, Locker and Daines (1975) reported that beef muscle held at 37°C developed an unattractive smell, which was not described as ‘sour’, but ‘sweaty’, quite distinct from a putrid smell. As with the sour smell in pork, it seemed superficial, since it was not detectable in fried tenderloin steaks previously held post-rigor for 7 h at 37°C. In addition, Boles et al. (1992b) reported that loins from pig carcasses with a rapid pH decline due to positive stress classification had a less intense pork flavour. This is in agreement with the lower consumer acceptability of the flavour of beef from high pre-rigor temperature (rapid pH decline post-mortem) carcasses (Warner et al. 2014b). Often the result of muscle undergoing high pre-rigor temperature is a lower ultimate pH (~5.3–5.4) of about 0.1–0.2 pH units relative to normal muscle (~5.5) (Warner et al. 1997) and although beef carcasses with lower than average pH are not common, the meat from these animals is usually blander (Calkins and Hodgen 2007).
In summary, there seems to be some variation in the literature with regard to whether there are any effects of fast metabolism at a high temperature post-mortem on the subsequent odour or flavour of cooked meat. It is possible that the variation is due to different methods used for cooking or tasting these products for sensory panellists, and it is suggested that further research is warranted.
Impacts of faster than normal pH decline while at a higher than normal muscle temperature on protein functionality for processed meat
As discussed above in ‘Impacts of accelerated glycolysis at a higher than normal temperature on denaturation of muscle proteins’, myosin extraction occurs via depolymerisation of the thick filament backbone. If myosin is irreversibly bound to actin in PSE muscle, it is difficult to extract (Izumi et al. 1977). As meat processing relies on the application of phosphates and brine solutions in order to solubilise myosin, the denaturation of myosin in PSE-like meat also affects the functionality for processing.
The functional properties of PSE pork have been found to be inferior to those of normal pork in a range of processed meat products (Kauffman et al. 1978; Honkavaara 1988). Severini et al. (1989) stated that PSE muscles produce cured hams of very low quality, in contrast to normal muscles. Muscles from PSE pigs had higher drip loss than normal muscles during chilling, and absorbed more salt during immersion in brine, therefore producing a poor-quality cooked ham (Severini et al. 1989). This may be due to the differences in biochemical and physical properties, whereby myofibrillar proteins are denatured and thus the PSE meat has a poor bind (Torley et al. 2000).
Myofibrillar proteins are very important in meat processing, because they largely provide functionality to the meat system (Acton and Dick 1996). Water-binding phenomena in processed meats depend solely on the extraction of contractile proteins from myofilaments followed by gelation of these proteins during thermal processing (Asghar et al. 1985). Thus, protein denaturation in PSE pork is important because of its direct effects on fresh pork quality and because of its subsequent implications for the functionality of the myofibrillar proteins during processing. Camou and Sebranek (1991) reported that protein from PSE muscle is in a less functional form than protein from ‘normal’ muscle, even after solubilisation with salt. Gel strength of extracted salt-soluble proteins was reduced by 45% from PSE muscle (Camou and Sebranek 1991). The major functional properties of muscle constituents, for which the myofibrillar proteins play a predominant role, include WHC, fat emulsification and protein gelation (particle-to-particle binding ability) (Acton and Dick 1984). Thus, denaturation of the myofibrillar proteins can have a considerable impact on the processing quality of pork.
In summary, there are clearly negative effects of the PSE condition on processed pork products but there is a lack of information on the effect of the PSE-like condition on the quality of beef or lamb processed products.
Conclusion
A faster than normal decline in pH combined with a higher than normal pre-rigor chilling temperature can result in substantial impacts on glycolysis, post-mortem muscle proteins and, subsequently, meat quality attributes. The extensive denaturation of myofibrillar and sarcoplasmic proteins contribute to the increased risk of unacceptable meat quality characteristics, such as in the changes to the colour, WHC, eating quality such as tenderness and/or flavour, and protein functionality during processing. The majority of evidence suggests that muscles entering rigor at a high temperature have reduced ageing potential in terms of sensory tenderness, although this was not always evident when using mechanical measures (WBSF). The effects of high pre-rigor temperature on the muscle colour are sometimes seen as positive, in markets where paler meat is seen as more acceptable. However, the rapid pH decline achieved at higher temperatures will adversely affect myoglobin redox stability and thus subsequently result in decreased colour stability of meat during display. Therefore, identifying optimal pre-rigor chilling temperatures for ruminant carcasses that do not compromise meat colour and colour stability, and yet maximise meat tenderisation, will have considerable impact on future profitability of the meat industry. Developing novel, pre-rigor meat processing technologies based on further utilising the pre-existing techniques such as electrical stimulation, stepwise chilling, hot-boning and/or pre-rigor stretching will result in a substantial improvement in meat quality attributes, as there is no reason to reinvent the wheel. Ample evidence is available for negative effects of high pre-rigor temperature on the WHC (particularly in pork), but still, few studies are available into this aspect for muscles from ruminants. Thus, further study elucidating the underlying mechanism by which rapid pH decline at high muscle temperatures influences WHC of beef and lamb is required. Moreover, the critical pH, temperature and time combinations affecting biochemical/physical modifications of pre-rigor muscle and subsequent meat quality changes have not been clearly understood for beef and lamb from the literature and thus warrant further investigation.
Acknowledgements
The authors greatly appreciate Drs Melvin Hunt and Carrick Devine for their invaluable comments and sincere assistance in preparing a more succinct, accurate and informative review paper.
References
Aalhus JL, Jones SDM, Best DR, Robertson WM, Lutz S (1994) The efficacy of high and low voltage electrical stimulation under different chilling regimes. Canadian Journal of Animal Science 74, 433–442.| The efficacy of high and low voltage electrical stimulation under different chilling regimes.Crossref | GoogleScholarGoogle Scholar |
Aalhus JL, Best DR, Murray AC, Jones SDM (1998) A comparison of the quality characteristics of pale, soft and exudative beef and pork. Journal of Muscle Foods 9, 267–280.
| A comparison of the quality characteristics of pale, soft and exudative beef and pork.Crossref | GoogleScholarGoogle Scholar |
Aaslyng M (2009) Trends in meat consumption and need for improved quality. In ‘Improving the sensory and nutritional quality of fresh meat.’ (Eds J Kerry, D Ledward) pp. 3–18. (Woodhead Publishing: Cambridge, UK)
Aaslyng MD, Oksama M, Olsen EV, Bejerholm C, Baltzer M, Andersen G, Bredie WLP, Byrne DV, Gabrielsen G (2007) The impact of sensory quality of pork on consumer preference. Meat Science 76, 61–73.
| The impact of sensory quality of pork on consumer preference.Crossref | GoogleScholarGoogle Scholar | 22064192PubMed |
Acton J, Dick R (1984) Protein–protein interaction in processed meats. In ‘Reciprocal Meat Conference Proceedings’. No. 37, pp. 36–43. (American Meat Science Association)
Acton J, Dick R (1996) Functional roles of heat induced protein gelation in processed meat. In ‘Food proteins’. (Eds JE Kinsella, WG Soucie) pp. 195–209. (The American Oil Chemists Society: Champaign, IL, USA)
Asghar A, Samejima K, Yasui T (1985) Functionality of muscle proteins in gelation mechanisms of restructured products. CRC Critical Reviews in Food Science and Nutrition 22, 27–106.
| Functionality of muscle proteins in gelation mechanisms of restructured products.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXlsVSgtbo%3D&md5=25b239edddab74c9118caf5a55d6096cCAS | 3899516PubMed |
Bagshaw CR (1993) ‘Muscle contraction.’ 2nd edn. p. 146. (Chapman and Hall: London)
Bailey AJ, Light ND (1989) ‘Connective tissue in meat and meat products.’ pp. 214–215. (Elsevier Applied Science: London)
Balan P, Kim YHB, Blijenburg R (2014) Small heat shock protein degradation could be an indicator of the extent of myofibrillar protein degradation. Meat Science
| Small heat shock protein degradation could be an indicator of the extent of myofibrillar protein degradation.Crossref | GoogleScholarGoogle Scholar | 24583331PubMed | in press.
Barbut S, Sosnicki AA, Lonergan SM, Knapp T, Ciobanu DC, Gatcliffe LJ, Huff-Lonergan E, Wilson EW (2008) Progress in reducing the pale, soft and exudative (PSE) problem in pork and poultry meat. Meat Science 79, 46–63.
| Progress in reducing the pale, soft and exudative (PSE) problem in pork and poultry meat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXitlGmsLo%3D&md5=271fb5f91ea9de2c0e92c86a970471c1CAS | 22062597PubMed |
Barton-Gade PA (1996) Meat quality. In ‘Pig production’. World Animal Science Series. (Eds MR Taverner, AC Dunkin) pp. 17–39. (Elsevier: Amsterdam)
Bee G, Anderson AL, Lonergan SM, Huff-Lonergan E (2007) Rate and extent of pH decline affect proteolysis of cytoskeletal proteins and water-holding capacity in pork. Meat Science 76, 359–365.
| Rate and extent of pH decline affect proteolysis of cytoskeletal proteins and water-holding capacity in pork.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXit12ltro%3D&md5=a10aca66eaf5a12bd3a7b5bcddf29d79CAS | 22064307PubMed |
Bejerholm C, Aaslyng MD (2004) The influence of cooking technique and core temperature on results of a sensory analysis of pork depending on the raw meat quality. Food Quality and Preference 15, 19–30.
| The influence of cooking technique and core temperature on results of a sensory analysis of pork depending on the raw meat quality.Crossref | GoogleScholarGoogle Scholar |
Bekhit AED, Faustman C (2005) Metmyoglobin reducing activity. Meat Science 71, 407–439.
| Metmyoglobin reducing activity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXosVaitbc%3D&md5=aff4a7577f58e01455716fd75a0036ccCAS |
Bekhit AED, Farouk MM, Cassidy L, Gilbert KV (2007) Effects of rigor temperature and electrical stimulation on venison quality. Meat Science 75, 564–574.
| Effects of rigor temperature and electrical stimulation on venison quality.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mbns1amtg%3D%3D&md5=1c089d2e2c53750300287af5f86e53f0CAS |
Bendall JR (1978) Variability in rates of pH fall and of lactate production in the muscles on cooling beef carcasses. Meat Science 2, 91–104.
| Variability in rates of pH fall and of lactate production in the muscles on cooling beef carcasses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1cXkslCgsLc%3D&md5=d4cf116e660d05f3653456c6715ab115CAS | 22054970PubMed |
Bendall JR, Swatland HJ (1988) A review of the relationships of pH with physical aspects of pork quality. Meat Science 24, 85–126.
| A review of the relationships of pH with physical aspects of pork quality.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvFentA%3D%3D&md5=42a9d87f05c73826d8d8cf48f0c59f6fCAS | 22055884PubMed |
Bendall JR, Taylor AA (1972) Consumption of oxygen by the muscles of beef animals and related species. II. Consumption of oxygen by post-rigor muscle. Journal of the Science of Food and Agriculture 23, 707–719.
| Consumption of oxygen by the muscles of beef animals and related species. II. Consumption of oxygen by post-rigor muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE38XkvVKktro%3D&md5=fa8af041587850bb56852b616e6d27cdCAS | 4339650PubMed |
Bendall JR, Wismer-Pedersen J (1962) Some properties of the fibrillar proteins of normal and watery pork muscles. Journal of Food Science 27, 144–159.
| Some properties of the fibrillar proteins of normal and watery pork muscles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2cXhvFaitQ%3D%3D&md5=a99c7acf6b55c139a20c889b21fea461CAS |
Bennett ME, Bramblett V, Aberle ED, Harrington RB (1973) Muscle quality, cooking method and aging vs. palatability of pork loin chops. Journal of Food Science 38, 536–538.
| Muscle quality, cooking method and aging vs. palatability of pork loin chops.Crossref | GoogleScholarGoogle Scholar |
Bertram HC, Purslow PP, Andersen HJ (2002) Relationship between meat structure, water mobility and distribution—A low-field NMR study. Journal of Agricultural and Food Chemistry 50, 824–829.
| Relationship between meat structure, water mobility and distribution—A low-field NMR study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xjt1ShsA%3D%3D&md5=28a276eeba8ead85ca647694e2c71b05CAS | 11829651PubMed |
Boles JA, Parrish FC, Huiatt TW, Robson RM (1992a) Effect of porcine stress syndrome on the solubility and degradation of myofibrillar/cytoskeletal proteins. Journal of Animal Science 70, 454–464.
Boles JA, Parrish FC, Skaggs CL, Christian LL (1992b) Sensory, physical, and chemical properties of pork loin chops from somatotropin-treated pigs of three stress classifications. Journal of Animal Science 70, 3066–3070.
Bowling RA, Smith GC, Dutson TR, Carpenter ZL (1978) Effects of prerigor conditioning treatments on lamb muscle shortening, pH and ATP. Journal of Food Science 43, 502–514.
| Effects of prerigor conditioning treatments on lamb muscle shortening, pH and ATP.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1cXlt1Oqsrs%3D&md5=6032a98fd063d3337e3f9a97d4c10709CAS |
Briskey EJ (1964) Etiological status and associated studies of pale, soft, exudative porcine musculature. Advances in Food Research 13, 89–178.
| Etiological status and associated studies of pale, soft, exudative porcine musculature.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2MXks1KksQ%3D%3D&md5=bfe917d3b223b58f01e2f3c0310dd28eCAS | 14283271PubMed |
Brooks J (1935) The oxidation of hemoglobin to methemoglobin by oxygen. II. The relation between the rate of oxidation and the partial pressure of oxygen. Proceedings of the Royal Society of London. Series B, Biological Sciences 118, 560–577.
| The oxidation of hemoglobin to methemoglobin by oxygen. II. The relation between the rate of oxidation and the partial pressure of oxygen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaA28XotV2i&md5=33cb8cdf0a0800ccbecc1d565da0fe38CAS |
Brown-Brandl TM, Eigenberg RA, Nienaber JA, Hahn GL (2005) Dynamic response indicators of heat stress in shaded and non-shaded feedlot cattle. Part 1: Analyses of indicators. Biosystems Engineering 90, 451–462.
| Dynamic response indicators of heat stress in shaded and non-shaded feedlot cattle. Part 1: Analyses of indicators.Crossref | GoogleScholarGoogle Scholar |
Cafe LM, Robinson DL, Ferguson DM, McIntyre BL, Geesink GH, Greenwood PL (2011) Cattle temperament: persistence of assessments and associations with productivity, efficiency, carcass and meat quality traits. Journal of Animal Science 89, 1452–1465.
| Cattle temperament: persistence of assessments and associations with productivity, efficiency, carcass and meat quality traits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXls1Oiu74%3D&md5=428fb5ad67bc4579d593e39d0492fae8CAS | 21169516PubMed |
Calkins CR, Hodgen JM (2007) A fresh look at meat flavor. Meat Science 77, 63–80.
| A fresh look at meat flavor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmvVOgtrw%3D&md5=8e70dd41211371ea01b47748862c8503CAS | 22061397PubMed |
Camou JP, Sebranek JG (1991) Gelation characteristics of muscle proteins from pale soft exudative (PSE) pork. Meat Science 30, 207–220.
| Gelation characteristics of muscle proteins from pale soft exudative (PSE) pork.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsVGmsw%3D%3D&md5=2b2b7180ea6571325fd5dffe80ecae81CAS | 22061970PubMed |
Channon HA, Payne AM, Warner RD (2000) Halothane genotype, pre-slaughter handling and stunning method all influence pork quality. Meat Science 56, 291–299.
| Halothane genotype, pre-slaughter handling and stunning method all influence pork quality.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsVKntg%3D%3D&md5=f211ced8ded52220201560dad79cb056CAS | 22062081PubMed |
Cheah KS, Cheah AM (1971) Post-mortem changes in structure and function of ox muscle mitochondria. I. Electron microscopic and polarographic investigations. Journal of Bioenergetics 2, 85–92.
| Post-mortem changes in structure and function of ox muscle mitochondria. I. Electron microscopic and polarographic investigations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3sXhtVOjt7w%3D&md5=24cff800a1c32159f6123464f79809d5CAS | 5135876PubMed |
Claeys E, De Smet S, Demeyer D, Geers R, Buys N (2001) Effect of rate of pH decline on muscle enzyme activities in two pig lines. Meat Science 57-, 257–263.
| Effect of rate of pH decline on muscle enzyme activities in two pig lines.Crossref | GoogleScholarGoogle Scholar | 22061500PubMed |
Davey CL, Gilbert KV (1973) The effect of carcass posture on cold, heat and thaw shortening in lamb. International Journal of Food Science & Technology 8, 445–451.
| The effect of carcass posture on cold, heat and thaw shortening in lamb.Crossref | GoogleScholarGoogle Scholar |
Devine CE, Wahlgren NM, Tornberg E (1999) Effect of rigor temperature on muscle shortening and tenderisation of restrained and unrestrained beef M. longissimus thoracicus et lumborum. Meat Science 51, 61–72.
| Effect of rigor temperature on muscle shortening and tenderisation of restrained and unrestrained beef M. longissimus thoracicus et lumborum.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsVWqtA%3D%3D&md5=0fa670a2c0be3f3e48dbef00926751beCAS | 22061537PubMed |
Devine CE, Payne SR, Peachey BM, Lowe TE, Ingram JR, Cook CJ (2002) High and low rigor temperature effects on sheep meat tenderness and ageing. Meat Science 60, 141–146.
| High and low rigor temperature effects on sheep meat tenderness and ageing.Crossref | GoogleScholarGoogle Scholar | 22063237PubMed |
Dransfield E (1994) Modelling post-mortem tenderisation–V: Inactivation of calpains. Meat Science 37, 391–409.
| Modelling post-mortem tenderisation–V: Inactivation of calpains.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXmt12htrg%3D&md5=0f7a088f9aab1db34f9490fe94eb77d9CAS |
Dransfield E, Jones RCD, MacFie HJH (1981) Tenderising in m longissimus dorsi of beef, veal, rabbit, lamb and pork. Meat Science 5, 139–147.
| Tenderising in m longissimus dorsi of beef, veal, rabbit, lamb and pork.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvFCjsQ%3D%3D&md5=9d2723af10abff84a9f9e693ad3eabd8CAS | 22055962PubMed |
Eikelenboom G, Smulders FJM (1986) Effect of electrical stimulation on veal quality. Meat Science 16, 103–112.
| Effect of electrical stimulation on veal quality.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvVKqtg%3D%3D&md5=b213630853dd07f12e037cc7a7d3b0cfCAS | 22054830PubMed |
England EM, Scheffler TL, Kasten SC, Matarneh SK, Gerrard DE (2013) Exploring the unknowns involved in the transformation of muscle to meat. Meat Science 95, 837–843.
| Exploring the unknowns involved in the transformation of muscle to meat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnslCisLw%3D&md5=6df79d4dae2fa2e63be356049ee6edd6CAS | 23673227PubMed |
Farouk MM, Lovatt SJ (2000) Initial chilling rate of pre-rigor beef muscles as an indicator of colour of thawed meat. Meat Science 56, 139–144.
| Initial chilling rate of pre-rigor beef muscles as an indicator of colour of thawed meat.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsVCqsQ%3D%3D&md5=8b799eb55582f730077e64866fbc01caCAS | 22061901PubMed |
Farouk MM, Swan JE (1998) Effect of rigor temperature and frozen storage on functional properties of hot-boned manufacturing beef. Meat Science 49, 233–247.
| Effect of rigor temperature and frozen storage on functional properties of hot-boned manufacturing beef.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsFKmsA%3D%3D&md5=fc92b427663398aa666a4b41eeeeedbfCAS | 22063312PubMed |
Faustman C, Cassens RG (1990) The biochemical basis for discoloration in fresh meat: A review. Journal of Muscle Foods 1, 217–243.
| The biochemical basis for discoloration in fresh meat: A review.Crossref | GoogleScholarGoogle Scholar |
Faustman C, Cassens RG, Greaser ML (1988) Reduction of metmyoglobin by extracts of bovine liver and cardiac muscle. Journal of Food Science 53, 1065–1067.
| Reduction of metmyoglobin by extracts of bovine liver and cardiac muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXlsVyisro%3D&md5=87be36d0bf529d19e8e35d51d618543fCAS |
Fernandez X, Forslid A, Tornberg E (1994) The effect of high post-mortem temperature on the development of pale, soft and exudative pork: Interaction with ultimate pH. Meat Science 37, 133–147.
| The effect of high post-mortem temperature on the development of pale, soft and exudative pork: Interaction with ultimate pH.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mbnt1ymsg%3D%3D&md5=47b331e73cb763577026459022b81d4eCAS | 22059418PubMed |
Fischer C, Hofmann K, Blüchel B (1977) Das elektrophorese-bild von sarkoplasma and myofibrillen bei wäßrigem, blassem rindleisch. Fleischwirtschaft 57, 2050–2051.
Fischer C, Hofmann K, Hamm R (1978) Elektrophoretische untersuchungen der proteine von sarkoplasma und PSE- fleisch beim schween. Fleischwirtschaft 58, 303–306.
Fischer C, Hamm R, Honikel KO (1979) Changes in solubility and enzymic activity of muscle glycogen phosphorylase in PSE-muscles. Meat Science 3, 11–19.
| Changes in solubility and enzymic activity of muscle glycogen phosphorylase in PSE-muscles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXhsFSgurc%3D&md5=d5a3bc668b283e057a81a25b355bab45CAS | 22055196PubMed |
Flores M, Armero E, Aristoy MC, Toldra F (1999) Sensory characteristics of cooked pork loin as affected by nucleotide content and post-mortem meat quality. Meat Science 51, 53–59.
| Sensory characteristics of cooked pork loin as affected by nucleotide content and post-mortem meat quality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjslWltg%3D%3D&md5=c7749d65a415e34b408c22f619a2cc2cCAS | 22061536PubMed |
Geesink GH, Bekhit AD, Bickerstaffe R (2000) Rigor temperature and meat quality characteristics of lamb longissimus muscle. Journal of Animal Science 78, 2842–2848.
Giddings GG (1974) Reduction of ferrimyoglobin in meat. CRC Critical Revews in Food Technology 5, 143–173.
| Reduction of ferrimyoglobin in meat.Crossref | GoogleScholarGoogle Scholar |
Goldspink G, McLoughlin JV (1964) Studies on pig muscle. 3. The effect of temperature on the solubility of the sarcoplasmic proteins in relation to color changes in post-rigor muscle. Israel Journal of Agricultural Research 3, 9–16.
Goll DE, Thompson VF, Li H, Wei W, Cong J (2003) The calpain system. Physiological Reviews 83, 731–801.
Goransson A, Von Seth G, Tornberg E (1992) The influence of intramuscular fat content on the eating quality of pork. In ‘38th International Congress of Meat Science and Technology, 23-28 August, 1992, Clermont Ferrand, France’. pp. 245–248.
Greaser ML, Cassens RG, Briskey EJ, Hoekstra WG (1969) Post-mortem changes in subcellular fractions from normal and pale, soft, exudative porcine muscle. 1. Calcium accumulation and adenosine triphosphatase activities. Journal of Food Science 34, 120–124.
| Post-mortem changes in subcellular fractions from normal and pale, soft, exudative porcine muscle. 1. Calcium accumulation and adenosine triphosphatase activities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1MXkt1SjtLg%3D&md5=ffed94024c9fd905eb08985c32da8a73CAS |
Hagler L, Coppes RI, Herman RH (1979) Metmyoglobin reductase. Identification and purification of a reduced nicotinamide adenine dinucleotide-dependent enzyme from bovine heart which reduces metmyoglobin. The Journal of Biological Chemistry 254, 6505–6514.
Hamm R (1960) Biochemistry of meat hydration. In ‘Advances in Food Research’. pp. 355–463.
Hamm R (1986) Functional properties of the myofibrillar system and their measurement. In ‘Muscle as food’. (Ed. PJ Bechtel) pp. 135–199. (Academic Press: New York)
Harper GS (1999) Trends in skeletal muscle biology and the understanding of toughness in beef. Australian Journal of Agricultural Research 50, 1105–1129.
| Trends in skeletal muscle biology and the understanding of toughness in beef.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXnsVGqu7o%3D&md5=00065077ddf823ba8809f1c9d1a3ca8dCAS |
Hector DA, Brew-Graves C, Hasse N, Ledward DA (1992) Relationship between myosin denaturation and the colour of low-voltage-electrically-stimulated beef. Meat Science 31, 299–307.
| Relationship between myosin denaturation and the colour of low-voltage-electrically-stimulated beef.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XitlCitL0%3D&md5=316069c28363a78ae639d74b19eb2de1CAS | 22059631PubMed |
Helander E (1957) ‘On quantitative muscle protein determination.’ Vol. 40, Suppl. 1. (Acta Physiologica Scandinavica: Gothenburg)
Herrera-Mendez CH, Becila S, Boudjellal A, Ouali A (2006) Meat ageing: Reconsideration of the current concept. Trends in Food Science & Technology 17, 394–405.
| Meat ageing: Reconsideration of the current concept.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XltlKis7s%3D&md5=999eb27f1abfad1fb2e2764799028871CAS |
Herring HK, Cassens RG, Suess GG, Brungardt VH, Briskey EJ (1967) Tenderness and associated characteristics of stretched and contracted bovine muscles. Journal of Food Science 32, 317–323.
| Tenderness and associated characteristics of stretched and contracted bovine muscles.Crossref | GoogleScholarGoogle Scholar |
Hertog-Meischke M, Smulders FJM, van Logtestijn JG, van Knapen F (1997) The effect of electrical stimulation on the water-holding capacity and protein denaturation of two bovine muscles. Journal of Animal Science 75, 118–124.
Hertzman C, Olsson U, Tornberg E (1993) The influence of high temperature, type of muscle and electrical stimulation on the course of rigor, ageing and tenderness of beef muscles. Meat Science 35, 119–141.
| The influence of high temperature, type of muscle and electrical stimulation on the course of rigor, ageing and tenderness of beef muscles.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbntlKjsQ%3D%3D&md5=6f3ca62e08b17e48f991ff29bf19a844CAS | 22060841PubMed |
Honikel KO, Roncalés P, Hamm R (1983) The influence of temperature on shortening and rigor onset in beef muscle. Meat Science 8, 221–241.
| The influence of temperature on shortening and rigor onset in beef muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXhslOlu7k%3D&md5=083bb9d637249c1d6786ea2df6bc160fCAS | 22055561PubMed |
Honikel KO, Kim CJ, Hamm R (1986) Sarcomere shortening of prerigor muscles and its influence on drip loss. Meat Science 16, 267–282.
| Sarcomere shortening of prerigor muscles and its influence on drip loss.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvVyktg%3D%3D&md5=55be2708bf9814dbe60f266c54d49628CAS | 22055082PubMed |
Honkavaara M (1988) Influence of porcine stress on blood composition and early post-mortem meat quality in pigs of different halothane genotypes. Meat Science 24, 21–29.
| Influence of porcine stress on blood composition and early post-mortem meat quality in pigs of different halothane genotypes.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvFeisw%3D%3D&md5=785b950695508c670a49f558b85d3d27CAS | 22055806PubMed |
Hopkins DL, Ponnampalam EN, van de Ven RJ, Warner RD (2014) The effect of pH decline rate on the meat and eating quality of beef carcasses. Animal Production Science 54, 407–413.
| The effect of pH decline rate on the meat and eating quality of beef carcasses.Crossref | GoogleScholarGoogle Scholar |
Huff-Lonergan E, Lonergan SM (2005) Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Science 71, 194–204.
| Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXmt1Wiurc%3D&md5=dad061fff3285009407f273935b16e2bCAS | 22064064PubMed |
Huff-Lonergan E, Lonergan SM (2007) New frontiers in understanding drip loss in pork: recent insights on the role of postmortem muscle biochemistry. Journal of Animal Breeding and Genetics 124, 19–26.
| New frontiers in understanding drip loss in pork: recent insights on the role of postmortem muscle biochemistry.Crossref | GoogleScholarGoogle Scholar | 17988247PubMed |
Huff Lonergan E, Zhang W, Lonergan SM (2010) Biochemistry of postmortem muscle—Lessons on mechanisms of meat tenderization. Meat Science 86, 184–195.
| Biochemistry of postmortem muscle—Lessons on mechanisms of meat tenderization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXos1Cntrw%3D&md5=d9613298f483d21c18070c0eb163b7a1CAS | 20566247PubMed |
Hultin HO (1985) Characteristics of muscle tissue. In ‘Food chemistry’. (Ed. OR Fennema) p. 735. (Dekker: New York)
Hunt MC, Hedrick HB (1977a) Chemical, physical and sensory characteristics of bovine muscle from four quality groups. Journal of Food Science 42, 716–720.
| Chemical, physical and sensory characteristics of bovine muscle from four quality groups.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2sXkt1SktLo%3D&md5=eedc5be2d6d292d4a777d5def4e46273CAS |
Hunt MC, Hedrick HB (1977b) Profile of fiber types and related properties of five bovine muscles. Journal of Food Science 42, 513–517.
| Profile of fiber types and related properties of five bovine muscles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1cXhtlCqt78%3D&md5=81f62de9725dd6437a86798476965719CAS |
Hwang IH, Thompson JM (2001) The interaction between pH and temperature decline early postmortem on the calpain system and objective tenderness in electrically stimulated beef longissimus dorsi muscle. Meat Science 58, 167–174.
| The interaction between pH and temperature decline early postmortem on the calpain system and objective tenderness in electrically stimulated beef longissimus dorsi muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXit1Wgurc%3D&md5=40939faba202cd6c32db4d2d60e5d1ecCAS | 22062112PubMed |
Hwang IH, Park BY, Cho SH, Lee JM (2004) Effects of muscle shortening and proteolysis on Warner–Bratzler shear force in beef longissimus and semitendinosus. Meat Science 68, 497–505.
| Effects of muscle shortening and proteolysis on Warner–Bratzler shear force in beef longissimus and semitendinosus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlsVSmu7o%3D&md5=47c3afc94e61d28200f7ed6c2a3e3db9CAS | 22062419PubMed |
Izumi K, Ito T, Fukazawa T (1977) Isometric tension development of glycerinated fibers prepared from normal and pse porcine muscles and the effect of myosin irrigation on the tension development of ‘ghost’ fibers. Journal of Food Science 42, 113–116.
| Isometric tension development of glycerinated fibers prepared from normal and pse porcine muscles and the effect of myosin irrigation on the tension development of ‘ghost’ fibers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2sXlsVShtg%3D%3D&md5=6cd392b332259de34ed86354f588f4d0CAS |
Jacob RH, Hopkins DL (2014) Techniques to reduce the temperature of beef muscle early in the post mortem period – a review. Animal Production Science 54, 482–493.
| Techniques to reduce the temperature of beef muscle early in the post mortem period – a review.Crossref | GoogleScholarGoogle Scholar |
Jacob RH, Beatty DT, Warner RD (2014a) A preliminary study into the use of ‘heat pipes’ to prevent high rigor temperature in beef carcasses by increasing cooling rate. Animal Production Science 54, 504–509.
| A preliminary study into the use of ‘heat pipes’ to prevent high rigor temperature in beef carcasses by increasing cooling rate.Crossref | GoogleScholarGoogle Scholar |
Jacob RH, Surridge VSM, Beatty DT, Gardner GE, Warner RD (2014b) Grain feeding increases core body temperature of beef cattle. Animal Production Science 54, 444–449.
| Grain feeding increases core body temperature of beef cattle.Crossref | GoogleScholarGoogle Scholar |
Jacobson AL, Henderson J (1973) Temperature sensitivity of Mysosin and actomyosin. Canadian Journal of Biochemistry 51, 71–86.
| Temperature sensitivity of Mysosin and actomyosin.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE3s7ivFaqtA%3D%3D&md5=1b816940312d1c804633bbca91af6d61CAS | 4265981PubMed |
Jaime I, Beltrán JA, Ceña P, López-Lorenzo P, Roncalés P (1992) Tenderisation of lamb meat: Effect of rapid postmortem temperature drop on muscle conditioning and aging. Meat Science 32, 357–366.
| Tenderisation of lamb meat: Effect of rapid postmortem temperature drop on muscle conditioning and aging.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbntlSqtQ%3D%3D&md5=89c3c4e2c797083706a0d3bba594d11dCAS | 22059887PubMed |
Joo ST, Kauffman RG, Kim BC, Park GB (1999) The relationship of sarcoplasmic and myofibrillar protein solubility to colour and water-holding capacity in porcine longissimus muscle. Meat Science 52, 291–297.
| The relationship of sarcoplasmic and myofibrillar protein solubility to colour and water-holding capacity in porcine longissimus muscle.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsFSmug%3D%3D&md5=c318b01bc59399c8d090dcadde2c0d36CAS | 22062578PubMed |
Judd DB, Wyszecki G (1975) ‘Colour in business, science and industry.’ (John Wiley & Sons: New York)
Kauffman RG, Wachholz D, Henderson D, Lochner JV (1978) Shrinkage of PSE, normal and DFD hams during transit and processing. Journal of Animal Science 46, 1236–1240.
Kim YHB, Hunt MC (2011) Advance technology to improve meat color. In ‘Control of meat quality.’ (Ed. ST Joo) pp. 31–60. (Research Signpost: Trivandrum, Kerala, India)
Kim YH, Lonergan SM, Huff-Lonergan E (2010) Protein denaturing conditions in beef deep semimembranosus muscle results in limited µ-calpain activation and protein degradation. Meat Science 86, 883–887.
| Protein denaturing conditions in beef deep semimembranosus muscle results in limited µ-calpain activation and protein degradation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFCrurnE&md5=4becf077926d7fe23677f72d367918a4CAS | 20674187PubMed |
Kim YHB, Stuart A, Nygaard G, Rosenvold K (2012) High pre rigor temperature limits the ageing potential of beef that is not completely overcome by electrical stimulation and muscle restraining. Meat Science 91, 62–68.
| High pre rigor temperature limits the ageing potential of beef that is not completely overcome by electrical stimulation and muscle restraining.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtVCmsLg%3D&md5=1f902cbe6afe74ff48f6d5b2ac116177CAS |
Kim YHB, Luc G, Rosenvold K (2013) Pre rigor processing, ageing and freezing on tenderness and colour stability of lamb loins. Meat Science 95, 412–418.
| Pre rigor processing, ageing and freezing on tenderness and colour stability of lamb loins.Crossref | GoogleScholarGoogle Scholar |
Kim YHB, Kerr M, Geesink G, Warner RD (2014) Impacts of hanging method and high pre-rigor temperature and duration on quality attributes of ovine muscles. Animal Production Science 54, 414–421.
| Impacts of hanging method and high pre-rigor temperature and duration on quality attributes of ovine muscles.Crossref | GoogleScholarGoogle Scholar |
Koohmaraie M (1988). The role of endogenous proteases in meat tenderness. In ‘Reciprocal Meat Conference Proceedings’. No. 41, pp. 89−100. (American Meat Science Association: Chicago, IL)
Koohmaraie M (1996) Biochemical factors regulating the toughening and tenderization processes of meat. Meat Science 43, 193–201.
| Biochemical factors regulating the toughening and tenderization processes of meat.Crossref | GoogleScholarGoogle Scholar |
Koohmaraie M, Geesink GH (2006) Contribution of post-mortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system. Meat Science 74, 34–43.
| Contribution of post-mortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xms1Grsbk%3D&md5=1e6bab8be15345f5680d47f98eb09e37CAS | 22062714PubMed |
Ledward DA (1985) Post-slaughter influences on the formation of metmyoglobin in beef muscles. Meat Science 15, 149–171.
| Post-slaughter influences on the formation of metmyoglobin in beef muscles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28XmslOjsw%3D%3D&md5=c5791c119f84ad5193c4463226eef27fCAS | 22054503PubMed |
Lee YB, Ashmore CR (1985) Effect of early postmortem temperature on beef tenderness. Journal of Animal Science 60, 1588–1596.
Li SJ, King AJ (1996) Lipid oxidation and myosin denaturation in dark chicken meat. Journal of Agricultural and Food Chemistry 44, 3080–3084.
| Lipid oxidation and myosin denaturation in dark chicken meat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XlvVertbY%3D&md5=4fc35eec67fcdd1f7b79b04c03c56925CAS |
Livingston DJ, Brown WD (1981) The chemistry of myoglobin and its reactions. Food Technology 35, 244–252.
Livingston DJ, Mclachlan SJ, LaMar GN, Brown WD (1985) Myoglobin : cytochrome b5 interaction and the kinetic mechanism of metmyoglobin reductase. The Journal of Biological Chemistry 260, 15699–15707.
Lochner JV, Kauffman RG, Marsh BB (1980) Early-postmortem cooling rate and beef tenderness. Meat Science 4, 227–241.
| Early-postmortem cooling rate and beef tenderness.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvFantg%3D%3D&md5=fc78f413acea1bc88d4d7b4713e993efCAS | 22055702PubMed |
Locker RH, Daines GJ (1975) Rigor mortis in beef sternomandibularis muscle at 37°C. Journal of the Science of Food and Agriculture 26, 1721–1733.
| Rigor mortis in beef sternomandibularis muscle at 37°C.Crossref | GoogleScholarGoogle Scholar |
Locker RH, Hagyard CJ (1963) A cold shortening effect in beef muscles. Journal of the Science of Food and Agriculture 14, 787–793.
| A cold shortening effect in beef muscles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2cXlsFOiug%3D%3D&md5=ebcef65dee72d68932c06451127cd7e1CAS |
Lomiwes D, Farouk MM, Wiklund E, Young OA (2014) Small heat shock proteins and their role in meat tenderness. Meat Science 96, 26–40.
| Small heat shock proteins and their role in meat tenderness.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1yhs77F&md5=78ede68be97558232a43d63eabcf2572CAS | 23896134PubMed |
MacDougall DB (1982) Changes in the colour and opacity of meat. Food Chemistry 9, 75–88.
| Changes in the colour and opacity of meat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38Xls1Wisrk%3D&md5=5df65721491a09fdcb31d22030350e99CAS |
Maddock KR, Huff-Lonergan E, Rowe LJ, Lonergan SM (2005) Effect of pH and ionic strength on µ- and m-calpain inhibition by calpastatin. Journal of Animal Science 83, 1370–1376.
Mancini RA, Hunt MC (2005) Current research in meat color. Meat Science 71, 100–121.
| Current research in meat color.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXmt1Wiurg%3D&md5=e86e0e5a69b530aaad1ca1f4bc76de2cCAS | 22064056PubMed |
Marsh BB (1954) Rigor mortis in beef. Journal of the Science of Food and Agriculture 5, 70–75.
| Rigor mortis in beef.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG2cXjtlKqsQ%3D%3D&md5=20c13aeeb6fdd9ec8cb0cb2d4e1acd6dCAS |
Marsh BB, Leet NG (1966) Studies in meat tenderness. III. The effects of cold shortening on tenderness. Journal of Food Science 31, 450–459.
| Studies in meat tenderness. III. The effects of cold shortening on tenderness.Crossref | GoogleScholarGoogle Scholar |
Marsh BB, Lochner JV, Takahashi G, Kragness DD (1981) Effects of early post-mortem pH and temperature on beef tenderness. Meat Science 5, 479–483.
| Effects of early post-mortem pH and temperature on beef tenderness.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvVGmsg%3D%3D&md5=79ed9b14424f73b0dd7be364e1a8c99dCAS | 22054608PubMed |
Marsh BB, Ringkob TP, Russell RL, Swartz DR, Pagel LA (1987) Effects of early-postmortem glycolytic rate on beef tenderness. Meat Science 21, 241–248.
| Effects of early-postmortem glycolytic rate on beef tenderness.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvVymtA%3D%3D&md5=21c2e1f7a8e8a703443160d9a9db41daCAS | 22055055PubMed |
Martin AH, Murray AC, Jeremiah LE, Dutson PJ (1983) Electrical stimulation and carcass aging effects on beef carcasses in relation to postmortem glycolytic rates. Journal of Animal Science 57, 1456–1462.
Matthews K (2011) ‘Evaluation of the pH fall in English lamb abattoirs against the Meat Standards Australia pH/temperature window. Report to EBLEX. Available at http://www.eblex.org.uk/wp/wp-content/uploads/2013/04/evaluationofthephfallinenglishlambabattoirs_271011-final-report.pdf [Verified 19 February 2014]
McClain PE, Creed GJ, Wiley ER, Hornstein I (1969) Connective tissues from normal and PSE porcine muscle 1. Chemical characterization. Journal of Food Science 34, 115–119.
| Connective tissues from normal and PSE porcine muscle 1. Chemical characterization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1MXkt1SjtLs%3D&md5=a9e015b62eaa89e847934f59202b820cCAS |
McKee SR, Sams AR (1998) Rigor mortis development at elevated temperatures induces pale exudative turkey meat characteristics. Poultry Science 77, 169–174.
| Rigor mortis development at elevated temperatures induces pale exudative turkey meat characteristics.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c7ivFKksw%3D%3D&md5=562da4209ac2ea741d6d2753196a8218CAS | 9469769PubMed |
Melody JL, Lonergan SM, Rowe LJ, Huiatt TW, Mayes MS, Huff-Lonergan E (2004) Early postmortem biochemical factors influence tenderness and water-holding capacity of three porcine muscles. Journal of Animal Science 82, 1195–1205.
Mikkelsen A, Juncher D, Skibsted LH (1999) Metmyoglobin reductase activity in porcine m. longissimus dorsi muscle. Meat Science 51, 155
| Metmyoglobin reductase activity in porcine m. longissimus dorsi muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjslWksQ%3D%3D&md5=4e82bf9859c47d7688ba2e8a6bfcd8c8CAS | 22061700PubMed |
Molette C, Rémignon H, Babilé R (2003) Maintaining muscles at a high post-mortem temperature induces PSE-like meat in turkey. Meat Science 63, 525–532.
| Maintaining muscles at a high post-mortem temperature induces PSE-like meat in turkey.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsFSjuw%3D%3D&md5=6d680bdd0389154c3bd157ccb2a7c038CAS | 22062523PubMed |
Monin G, Laborde D (1985) Note—Water holding capacity of pig muscle proteins: interaction between the myofibrillar proteins and sarcoplasmic compounds. Sciences des Aliments 5, 341–345.
Morissey PA, Mulvihill DM, O’Neill EM (1987) Functional properties of muscle proteins. In ‘Developments in food proteins – 5’. (Ed. BJF Hudson) pp. 195–256. (Elsevier Applied Science: New York)
Offer G (1991) Modelling of the formation of pale, soft and exudative meat: Effects of chilling regime and rate and extent of glycolysis. Meat Science 30, 157–184.
| Modelling of the formation of pale, soft and exudative meat: Effects of chilling regime and rate and extent of glycolysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXmslygu7Y%3D&md5=ab78c2132e3b69fa280d2bb8d1e81744CAS | 22061833PubMed |
Offer G, Knight P (1988) The structural basis of water-holding in meat. Part 1: General principles and water uptake in processing. In ‘Developments in meat science – 4’. (Ed. RA Lawrie) pp. 63–171. (Elsevier Science: London)
Osborn HM, Brown H, Adams JB, Ledward DA (2003) High temperature reduction of metmyoglobin in aqueous muscle extracts. Meat Science 65, 631–637.
| High temperature reduction of metmyoglobin in aqueous muscle extracts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjt1Oiu70%3D&md5=4154fe4e23f72c4b19beae5bdb6103e9CAS | 22063258PubMed |
Parsons N, Knight P (1990) Origin of variable extraction of myosin from myofibrils treated with salt and pyrophosphate. Journal of the Science of Food and Agriculture 51, 71–90.
| Origin of variable extraction of myosin from myofibrils treated with salt and pyrophosphate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXhslOiurg%3D&md5=b4319bc87a9533215ce9b72c3e4af01bCAS |
Pearce KL, Van De Ven R, Mudford CR, Warner RD, Hocking-Edwards JE, Pethick DW, Hopkins DL (2010) Case studies demonstrating the optimisation of medium voltage electrical stimulation of lamb carcases. Animal Production Science 50, 1107–1114.
| Case studies demonstrating the optimisation of medium voltage electrical stimulation of lamb carcases.Crossref | GoogleScholarGoogle Scholar |
Pearce K, Rosenvold K, Andersen HJ, Hopkins D (2011) Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes - a review. Meat Science 89, 111–124.
| Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes - a review.Crossref | GoogleScholarGoogle Scholar | 21592675PubMed |
Penny IF (1967a) The effect of post-mortem conditions on the extractability and adenosine triphosphatase activity of myofibrillar proteins of rabbit muscle. International Journal of Food Science & Technology 2, 325–338.
| The effect of post-mortem conditions on the extractability and adenosine triphosphatase activity of myofibrillar proteins of rabbit muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1cXosVentQ%3D%3D&md5=897c3f56fe169e8c87413f7414169e62CAS |
Penny IF (1967b) The influence of pH and temperature on the properties of myosin. Biochemical Journal 104, 609–615.
Pike MM, Ringkob TP, Beekman DD, Koh YO, Gerthoffer WT (1993) Quadratic relationship between early-post-mortem glycolytic rate and beef tenderness. Meat Science 34, 13–26.
| Quadratic relationship between early-post-mortem glycolytic rate and beef tenderness.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXisFeltrk%3D&md5=4e8f2f89c9df9b4b2b21d0dec19eddf1CAS | 22060264PubMed |
Pulford DJ, Fraga Vazquez S, Frost DF (2008) The intracellular distribution of small heat shock proteins in post-mortem beef is determined by ultimate pH. Meat Science 79, 623–630.
| The intracellular distribution of small heat shock proteins in post-mortem beef is determined by ultimate pH.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnsF2qt7w%3D&md5=902013a25c1a49f1b52787fb7098ec3dCAS | 22063023PubMed |
Pulford DJ, Dobbie P, Fraga Vazquez S (2009) Variation in bull beef quality due to ultimate muscle pH is correlated to endopeptidase and small heat shock protein levels. Meat Science 83, 1–9.
| Variation in bull beef quality due to ultimate muscle pH is correlated to endopeptidase and small heat shock protein levels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmvFGhtL4%3D&md5=d775526adfaa54c84633244ef51e7b09CAS | 20416615PubMed |
Renerre M (1990) Factors involved in the discoloration of beef meat. International Journal of Food Science & Technology 25, 613–630.
| Factors involved in the discoloration of beef meat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXhsVWlu7w%3D&md5=5af244a77842cf8f4c84807542aea917CAS |
Rhee MS, Kim BC (2001) Effect of low voltage electrical stimulation and temperature conditioning on postmortem changes in glycolysis and calpains activities of Korean native cattle (Hanwoo). Meat Science 58, 231–237.
| Effect of low voltage electrical stimulation and temperature conditioning on postmortem changes in glycolysis and calpains activities of Korean native cattle (Hanwoo).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXisVWis70%3D&md5=e2703ced7c73b9aa7f06b299c76b2b10CAS | 22062250PubMed |
Rhee MS, Ryu YC, Imm JY, Kim BC (2000) Combination of low voltage electrical stimulation and early postmortem temperature conditioning on degradation of myofibrillar proteins in Korean native cattle (Hanwoo). Meat Science 55, 391–396.
| Combination of low voltage electrical stimulation and early postmortem temperature conditioning on degradation of myofibrillar proteins in Korean native cattle (Hanwoo).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXksVGjtb8%3D&md5=46710b1ec96e4e247c3b91478c0ec2e2CAS | 22061570PubMed |
Roeber DL, Cannell RC, Belk KE, Tatum JD, Smith GC (2000) Effects of a unique application of electrical stimulation on tenderness, color, and quality attributes of the beef longissimus muscle. Journal of Animal Science 78, 1504–1509.
Rosenvold K, Andersen HJ (2003) Factors of significance for pork quality—a review. Meat Science 64, 219–237.
| Factors of significance for pork quality—a review.Crossref | GoogleScholarGoogle Scholar | 22063008PubMed |
Rosenvold K, Wiklund E (2011) Retail colour display life of chilled lamb as affected by processing conditions and storage temperature. Meat Science 88, 354–360.
| Retail colour display life of chilled lamb as affected by processing conditions and storage temperature.Crossref | GoogleScholarGoogle Scholar | 21316158PubMed |
Rosenvold K, North M, Devine C, Micklander E, Hansen P, Dobbie P, Wells R (2008) The protective effect of electrical stimulation and wrapping on beef tenderness at high pre rigor temperatures. Meat Science 79, 299–306.
| The protective effect of electrical stimulation and wrapping on beef tenderness at high pre rigor temperatures.Crossref | GoogleScholarGoogle Scholar | 22062758PubMed |
Safari E, Fogarty NM, Ferrier GR, Hopkins LD, Gilmour A (2001) Diverse lamb genotypes. 3. Eating quality and the relationship between its objective measurement and sensory assessment. Meat Science 57, 153–159.
| Diverse lamb genotypes. 3. Eating quality and the relationship between its objective measurement and sensory assessment.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsVSmsw%3D%3D&md5=56d9005067c62f2d3fa80c5d577c6968CAS | 22061358PubMed |
Sammel LM, Hunt MC, Kropf DH, Hachmeister KA, Kastner CL, Johnson DE (2002) Influence of chemical characteristics of beef inside and outside semimembranosus on color traits. Journal of Food Science 67, 1323–1330.
| Influence of chemical characteristics of beef inside and outside semimembranosus on color traits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XkvVyqsb4%3D&md5=0704891bf821bf0a99289d18ec1a063fCAS |
Savage AW, Warriss PD, Jolley PD (1990) The amount and composition of the proteins in drip from stored pig meat. Meat Science 27, 289–303.
| The amount and composition of the proteins in drip from stored pig meat.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvFSmtg%3D%3D&md5=a119b5bcd8566ca1322be299203a3645CAS | 22055365PubMed |
Savell JW, Cross HR (1988) The role of fat in the palatability of beef, pork, and lamb. In ‘Designing foods: Animal product options in the marketplace’. (Eds NRC Committee on Technological Options to Improve the Nutritional Attributes of Animal Products) pp. 345–355. (National Academy Press: Washington, DC)
Savell JW, Mueller SL, Baird BE (2005) The chilling of carcasses. Meat Science 70, 449–459.
| The chilling of carcasses.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mbns1Sisg%3D%3D&md5=9ca9d80413e7acb630edeb637dd809bfCAS | 22063744PubMed |
Sayre RN, Briskey EJ (1963) Protein solubility as influenced by physiological conditions in the muscle. Journal of Food Science 28, 675–679.
| Protein solubility as influenced by physiological conditions in the muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2cXkt1Wis78%3D&md5=ec5b00c413983b65142f8c7792c63235CAS |
Scopes RK (1964) The influence of post-mortem conditions on the solubilities of muscle proteins. Biochemical Journal 91, 201–207.
Scopes RK (1970) Characterization and study of sarcoplasmic proteins. In ‘The physiology and biochemistry of muscle as food, 2’. (Eds EJ Briskey, RG Cassens, BB Marsh) pp. 471–492. (University of Wisconsin Press: Madison, WI)
Seideman SC, Cross HR, Smith GC, Durland PR (1984) Factors associated with fresh meat color: A review. Journal of Food Quality 6, 211–237.
| Factors associated with fresh meat color: A review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXkvVyrtbc%3D&md5=1bc1f870d53a7a41bb3ad0353d627ec6CAS |
Severini M, Vizzani M, Cenci G (1989) Problems associated with processing PSE pigment for aged cured hams and loins. Italian Journal of Food Science 1, 65–68.
Seyfert M, Hunt MC, Mancini RA, Hachmeister KA, Kropf DH, Unruh JA (2004) Accelerated chilling and modified atmosphere packaging affect colour and colour stability of injection-enhanced beef round muscles. Meat Science 68, 209–219.
| Accelerated chilling and modified atmosphere packaging affect colour and colour stability of injection-enhanced beef round muscles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXltVOhtLs%3D&md5=364cfc3faaa8eccb9f71935ab412d40fCAS | 22062230PubMed |
Shaw FD, Baud SR, Richards I, Pethick DW, Walker PJ, Thompson JM (2005) New electrical stimulation technologies for sheep carcasses. Australian Journal of Experimental Agriculture 45, 575–583.
| New electrical stimulation technologies for sheep carcasses.Crossref | GoogleScholarGoogle Scholar |
Smulders FJM, Marsh BB, Swartz DR, Russell RL, Hoenecke ME (1990) Beef tenderness and sarcomere length. Meat Science 28, 349–363.
| Beef tenderness and sarcomere length.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvFagsQ%3D%3D&md5=7e4dc6cb5c35de1a3948efe9fc27fddeCAS |
Stabursvik E, Fretheim K, Frøystein T (1984) Myosin denaturation in pale, soft, and exudative (PSE) porcine muscle tissue as studied by differential scanning calorimetry. Journal of the Science of Food and Agriculture 35, 240–244.
| Myosin denaturation in pale, soft, and exudative (PSE) porcine muscle tissue as studied by differential scanning calorimetry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXhtVWku7c%3D&md5=607641ae8d98613f93425abfeb09d63dCAS | 6708468PubMed |
Strydom PE, Rosenvold K (2014) Muscle metabolism in sheep and cattle in relation to high rigor temperature – overview and perspective. Animal Production Science 54, 510–518.
| Muscle metabolism in sheep and cattle in relation to high rigor temperature – overview and perspective.Crossref | GoogleScholarGoogle Scholar |
Sung SK, Izumi K, Ito T, Fukazawa T (1977) Contractility of porcine ‘ghost’ myofibril irrigated with myosin from normal and pse porcine muscle. Agricultural and Biological Chemistry 41, 1087–1089.
| Contractility of porcine ‘ghost’ myofibril irrigated with myosin from normal and pse porcine muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2sXkvVams74%3D&md5=b4b9a6f2908b0fdbc76037a6bd88fbbbCAS |
Sung SK, Ito T, Izumi K (1981) Myosin ATPase and acto-heavy meromyosin ATPase in normal and in pale, soft and exudative (PSE) porcine muscle. Agricultural and Biological Chemistry 45, 953–957.
| Myosin ATPase and acto-heavy meromyosin ATPase in normal and in pale, soft and exudative (PSE) porcine muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXitVOhur4%3D&md5=ca36fd01fbb896326221172b621aa371CAS |
Swatland HJ (2012) Optical properties of meat. In ‘Proceedings of the 65th Reciprocal Meat Conference, 17–20 June 2012’. pp. 1–7. (North Dakota State University: Fargo, ND)
Takahashi G, Lochmer JV, Marsh BB (1984) Effects of low-frequency electrical stimulation on beef tenderness. Meat Science 11, 207–225.
| Effects of low-frequency electrical stimulation on beef tenderness.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXhtFWks78%3D&md5=b4c34f811045ddf890318e7258f239e6CAS | 22054858PubMed |
Tarrant PV, Mothersill C (1977) Glycolysis and associated changes in beef carcasses. Journal of the Science of Food and Agriculture 28, 739–749.
| Glycolysis and associated changes in beef carcasses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2sXls1Kitbg%3D&md5=03dc1ca58578fb9c995d87844c8b3af0CAS |
Thompson J (2002) Managing meat tenderness. Meat Science 62, 295–308.
| Managing meat tenderness.Crossref | GoogleScholarGoogle Scholar | 22061606PubMed |
Thompson JM, Hopkins DL, D’Souza DN, Walker PJ, Baud SR, Pethick DW (2005) The impact of processing on sensory and objective measurements of sheep meat eating quality. Australian Journal of Experimental Agriculture 45, 561–573.
| The impact of processing on sensory and objective measurements of sheep meat eating quality.Crossref | GoogleScholarGoogle Scholar |
Thompson JM, Perry D, Daly B, Gardner GE, Johnston DJ, Pethick DW (2006) Genetic and environmental effects on the muscle structure response post-mortem. Meat Science 74, 59–65.
| Genetic and environmental effects on the muscle structure response post-mortem.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xms1GrsbY%3D&md5=3d30dde2c61a1d825d8a38f9663b2007CAS | 22062716PubMed |
Thomson KL, Gardner GE, Simmons N, Thompson JM (2008) Length of exposure to high post-rigor temperatures affects the tenderisation of the beef M. longissmus dorsi. Australian Journal of Experimental Agriculture 48, 1442–1450.
| Length of exposure to high post-rigor temperatures affects the tenderisation of the beef M. longissmus dorsi.Crossref | GoogleScholarGoogle Scholar |
Torley PJ, D’Arcy BR, Trout GR (2000) The effect of ionic strength, polyphosphates type, pH, cooking temperature and preblending on the functional properties of normal and pale, soft, exudative (PSE) pork. Meat Science 55, 451–462.
| The effect of ionic strength, polyphosphates type, pH, cooking temperature and preblending on the functional properties of normal and pale, soft, exudative (PSE) pork.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsVagtA%3D%3D&md5=d678fb95d72da79cee7502c62c8d7e23CAS | 22061578PubMed |
Tornberg E (1996) Biophysical aspects of meat tenderness. Meat Science 43, 175–191.
| Biophysical aspects of meat tenderness.Crossref | GoogleScholarGoogle Scholar |
Toscas PJ, Shaw FD, Beilken SL (1999) Partial least squares (PLS) regression for the analysis of instrument measurement and sensory meat quality data. Meat Science 52, 173–178.
| Partial least squares (PLS) regression for the analysis of instrument measurement and sensory meat quality data.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsVyksQ%3D%3D&md5=63167498a0e0d7214904b56f60e54dccCAS | 22062369PubMed |
Trout GR (1988) Techniques for measuring water-binding capacity in muscle foods—a review of methodology. Meat Science 23, 235–252.
| Techniques for measuring water-binding capacity in muscle foods—a review of methodology.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvFaltA%3D%3D&md5=be3ff9538d9be446ebff359724839006CAS | 22055740PubMed |
Unruh JA, Kastner CL, Kropf DH, Dikeman ME, Hunt MC (1986) Effects of low-voltage electrical stimulation during exsanguination on meat quality and display colour stability. Meat Science 18, 281–293.
| Effects of low-voltage electrical stimulation during exsanguination on meat quality and display colour stability.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvFalsA%3D%3D&md5=a8aa37a737e6f2adf88ed75b5a983df2CAS | 22055733PubMed |
van der Wal PG, Bolink AH, Merkus GSM (1988) Differences in quality characteristics of normal, PSE and DFD pork. Meat Science 24, 79–84.
| Differences in quality characteristics of normal, PSE and DFD pork.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvFeitA%3D%3D&md5=65b37e28f3097c54d117c89fe7d6f602CAS | 22055811PubMed |
Van Oeckel MJ, Warnants N (2003) Variation of the sensory quality within the m. longissimus thoracis et lumborum of PSE and normal pork. Meat Science 63, 293–299.
| Variation of the sensory quality within the m. longissimus thoracis et lumborum of PSE and normal pork.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsVyrsg%3D%3D&md5=672d96c93d7096cbc8ec88a8dfa5539cCAS | 22062380PubMed |
Vega-Warner V, Smith DM (2001) Denaturation and aggregation of myosin from two bovine muscle types. Journal of Agricultural and Food Chemistry 49, 906–912.
| Denaturation and aggregation of myosin from two bovine muscle types.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhtVWrsA%3D%3D&md5=af6de3500429bffd21381776fcc9af31CAS | 11262048PubMed |
Warner RD (2014). Measurements of water-holding capacity and color – objective and subjective. In ‘Encyclopedia of meat science’. 2nd edn. (Eds ME Dikeman, C. Devine) (Elsevier Publishing: London)
Warner RD, Kauffman RG, Greaser ML (1997) Muscle protein changes post mortem in relation to pork quality traits. Meat Science 45, 339–352.
| Muscle protein changes post mortem in relation to pork quality traits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXisFyrtrY%3D&md5=7a2e794968870801cd62ddf945b9145dCAS | 22061472PubMed |
Warner RD, Dunshea FR, Gutzke D, Lau J, Kearney G (2014a) Factors influencing the incidence of high rigor temperature in beef carcasses in Australia. Animal Production Science 54, 363–374.
| Factors influencing the incidence of high rigor temperature in beef carcasses in Australia.Crossref | GoogleScholarGoogle Scholar |
Warner RD, Kerr M, Kim YHB, Geesink G (2014b) Pre-rigor carcass stretching counteracts the negative effects of high rigor temperature on tenderness and water-holding capacity – using lamb muscles as a model. Animal Production Science 54, 494–503.
| Pre-rigor carcass stretching counteracts the negative effects of high rigor temperature on tenderness and water-holding capacity – using lamb muscles as a model.Crossref | GoogleScholarGoogle Scholar |
Warner RD, Thompson JM, Polkinghorne R, Gutzke D, Kearney GA (2014c) A consumer sensory study of the influence of rigor temperature on eating quality and ageing potential of beef striploin and rump. Animal Production Science 54, 396–406.
| A consumer sensory study of the influence of rigor temperature on eating quality and ageing potential of beef striploin and rump.Crossref | GoogleScholarGoogle Scholar |
Warriss PD, Brown SN, Nute GR, Knowles TG, Edwards JE, Perry AM, Johnson SP (1995) Potential interactions between the effects of preslaughter stress and post mortem electrical stimulation of the carcasses on meat quality in pigs. Meat Science 41, 55–68.
| Potential interactions between the effects of preslaughter stress and post mortem electrical stimulation of the carcasses on meat quality in pigs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mbntlagug%3D%3D&md5=a759d59b112cb5b7462b1ae4dc32570eCAS | 22060113PubMed |
Watkins PJ, Frank D, Singh TK, Young OA, Warner RD (2013) The effect of diet on sheepmeat flavor. Journal of Agricultural and Food Chemistry 61, 3561–3579.
| The effect of diet on sheepmeat flavor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjvFWiu74%3D&md5=fa0d8000daae86db3520f5587f5f66c3CAS | 23488874PubMed |
Whipple G, Koohmaraie M, Dikeman ME, Crouse JD (1990) Effects of high-temperature conditioning on enzymatic activity and tenderness of Bos indicus longissimus muscle. Journal of Animal Science 68, 3654–3662.
Winger RJ, Hagyard CJ (1994) Juiciness – Its importance and some contributing factors. In ‘Quality attributes and their measurement in meat, poultry and fish products’. (Eds AM Pearson, TR Dutson) pp. 94–124. (Springer: New York)
Wismer-Pedersen J (1959) Quality of pork in relation to rate of pH change post mortem. Journal of Food Science 24, 711–727.
| Quality of pork in relation to rate of pH change post mortem.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF3cXktVSnuw%3D%3D&md5=6196c808e37308ce5a3ea2b7b0a8b73fCAS |
Wismer-Pedersen J, Briskey EJ (1961) Rate of anaerobic glycolysis versus structure in pork muscle. Nature 189, 318–320.
| Rate of anaerobic glycolysis versus structure in pork muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF3MXkvFKjsQ%3D%3D&md5=4ae0a202c5bec9960fb45fd4fd2e6928CAS | 13786016PubMed |
Wu JJ, Dutson TR, Carpenter ZL (1981) Effect of postmortem time and temperature on the release of lysosomal enzymes and their possible effect on bovine connective tissue components of muscle. Journal of Food Science 46, 1132–1135.
| Effect of postmortem time and temperature on the release of lysosomal enzymes and their possible effect on bovine connective tissue components of muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXkslyltLs%3D&md5=1ae88437bf07c95727ec744063c9f07aCAS |
Yates LD, Greaser ML (1983) Quanitative determination of myosin and actin in rabbit skeletal muscle. Journal of Molecular Biology 168, 123–141.
| Quanitative determination of myosin and actin in rabbit skeletal muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXltVWqs74%3D&md5=2835fcd983225e0e1ce99daa4887c745CAS | 6876172PubMed |
Young OA, Reid D, Scales G (1993) Effect of breed and utlimate pH on the odour and flavour of sheep meat. New Zealand Journal of Agricultural Research 36, 363–370.
| Effect of breed and utlimate pH on the odour and flavour of sheep meat.Crossref | GoogleScholarGoogle Scholar |
Young OA, Priolo A, Simmons NJ, West J (1999) Effects of rigor attainment temperature on meat blooming and colour on display. Meat Science 52, 47–56.
| Effects of rigor attainment temperature on meat blooming and colour on display.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsVKrtQ%3D%3D&md5=d1de6dac70818dd6b018f515b7b1e176CAS | 22062142PubMed |
Young OA, Hopkins DL, Pethick DW (2005) Critical control points for meat quality in the Australian sheep meat supply chain. Australian Journal of Experimental Agriculture 45, 593–601.
| Critical control points for meat quality in the Australian sheep meat supply chain.Crossref | GoogleScholarGoogle Scholar |
Zhu LG, Brewer MS (1998) Metmyoglobin reducing capacity of fresh normal, PSE, and DFD pork during retail display. Journal of Food Science 63, 390–393.
| Metmyoglobin reducing capacity of fresh normal, PSE, and DFD pork during retail display.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXksVWisLY%3D&md5=05f85081f4c80127c8f018a4fed1a29eCAS |
Zhu LG, Brewer MS (2003) Effects of urea denaturation and ph on the ability of porcine myoglobin to undergo reduction. Meat Science 63, 427–432.
| Effects of urea denaturation and ph on the ability of porcine myoglobin to undergo reduction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XptlWjtrg%3D&md5=d02f4e768217a57c7479014a115b85bcCAS | 22062511PubMed |
Zhu X, Ruusunen M, Gusella M, Zhou G, Puolanne E (2011) High post-mortem temperature combined with rapid glycolysis induces phosphorylase denaturation and produces pale and exudative characteristics in broiler Pectoralis major muscles. Meat Science 89, 181–188.
| High post-mortem temperature combined with rapid glycolysis induces phosphorylase denaturation and produces pale and exudative characteristics in broiler Pectoralis major muscles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXntlGhs7w%3D&md5=53ce76c33e7f688828bcfa02121d875fCAS | 21663805PubMed |



