Influencing the future: interactions of skeleton, energy, protein and calcium during late gestation and early lactation
Ian J. Lean A B E , Peter J. DeGaris C , Pietro Celi B , David M. McNeill D , Rachael M. Rodney A B and David R. Fraser BA SBScibus, Camden, NSW 2570, Australia.
B Faculty of Veterinary Science, The University of Sydney, Camden, NSW 2570, Australia.
C Tarwin Veterinary Group, Leongatha, Vic. 3953, Australia.
D School of Veterinary Science, University of Queensland, Gatton, Qld 4072, Australia.
E Corresponding author. Email: IanL@sbscibus.com.au
Animal Production Science 54(9) 1177-1189 https://doi.org/10.1071/AN14479
Submitted: 4 April 2014 Accepted: 5 June 2014 Published: 10 July 2014
Abstract
Marked improvements in milk production, health and reproduction have resulted from manipulations of the pre-calving diet. An understanding of the underlying physiological changes resulting from manipulation of late gestational diets is needed in order to refine and enhance these responses. The physiology of late gestation and early lactation of the dairy cow is examined in the context of exploring the hypothesis that changes in physiology occur not only through homeostatic, but also homeorhetic change. Studies in mice and man have identified a pivotal role for skeleton, particularly through production of active forms of osteocalcin, in integrating energy metabolism. Skeleton appears to particularly influence lipid metabolism and vice versa. Further insights into the factors influencing skeletal function and calcium (Ca) metabolism are emerging, including the potential for negative dietary cation anion difference (DCAD) diets to upregulate the responses of the skeleton in metabolism through increased bone mobilisation and in enhancing responses to parathyroid hormone. The rumen appears to be an important site of absorption of Ca, but physiological mechanisms influencing this uptake are not clear. We provide quantitative evidence of the magnitude of responses that reflect relationships linking Ca metabolism, skeleton and production, using meta-analytic methods. Negative DCAD diets increase milk production in multiparous cattle, but not in heifers. Further, examination of concentrations of metabolites related to energy metabolism obtained from cattle exposed to a negative DCAD diet over calving identified a dominant role for Ca concentrations, which were associated with blood-free fatty acids (NEFA), blood 3-hydroxybutyrate, glucose and cholesterol. These relationships were homeostatic, occurring on the same day, but also homeorhetic with concentrations of Ca and NEFA being significantly associated over 21 days. The findings in cattle are consistent with those in the murine models. However, Ca and the skeleton are not the only significant factors in the transition period influencing future performance as hormonal treatments, metabolic demands and sex of the conceptus, and inflammation and the factors controlling this play a role in future performance. Homeorhetic, longer-term, adaptive responses are critical to achieving orchestrated longer-term adaptive responses to calving and lactation. We consider that the teleological question ‘why would a bone-specific hormone (osteocalcin) regulate energy metabolism?’ is answered by the specific needs for integrated metabolism to address the extreme metabolic demands of lactation in many species.
Additional keywords: calcium, health, metabolism, osteocalcin.
Introduction
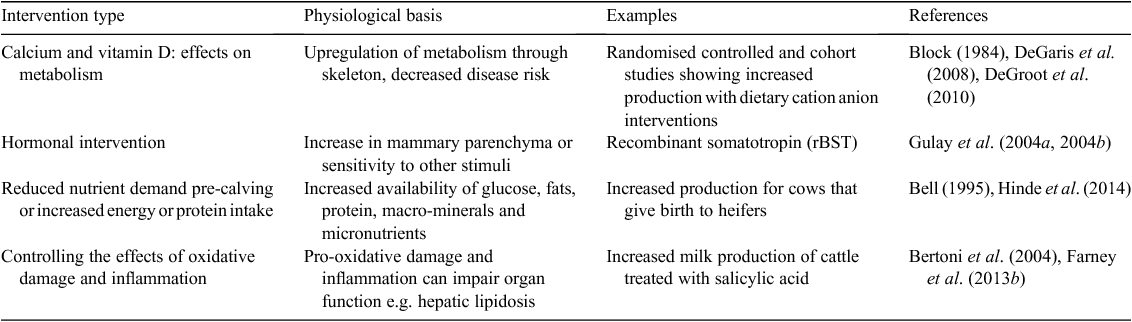
Lee et al. (2007) published a series of studies in mice presenting evidence of metabolic effects, orchestrated by osteocalcin (OC) produced by mature osteoblasts (OB), which influence regulation of energy metabolism. They raised the teleological question, ‘why would a bone-specific hormone regulate energy metabolism?’ We suggest in this paper, that the role of the skeleton in energy metabolism in dairy cattle reflects an essential need to integrate the homeorhetic changes that are required to upregulate metabolism in response to the demands of lactation. Homeorhetic changes are defined as the ‘coordinated changes in metabolism of body tissues necessary to support a physiological state’ (Bauman and Currie 1980). Evidence from basic and applied studies is provided to support the concept of homeorhesis. The period of most pronounced change in cattle from non-lactating to lactating occurs from 3 to 4 weeks before calving to 3 to 4 weeks after calving and is called the ‘transition’ period. Apart from examining the role of skeleton, we briefly examine other influences affecting the transition period that stimulated long-term metabolic change as indicated by increased milk production: specifically hormonal manipulation, factors that may change the nutrient demands of the conceptus, and the effects of oxidative damage and inflammation.
Evidence is provided from published papers and new data to support the hypothesis that the skeleton plays an important role in homeorhetic adaptation to lactation and that this relationship may be influenced by nutrition during the transition period. Recent developments in understanding of the role of the skeleton in the regulation of glucose homeostasis are discussed in the context of the role of calcium (Ca) and vitamin D in metabolism. The effects of pre-calving nutritional interventions are reviewed, using quantitative methods, to provide estimates of milk production responses. These positive, prolonged responses are considered in the context of the potential role of the skeleton in metabolism.
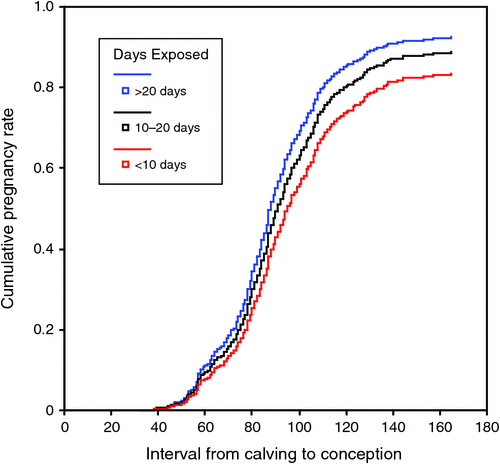
The modern dairy cow represents an extreme example of lactation, with many cows now producing more than 1.5 kg of each of milk fat, milk protein and milk lactose per day in early lactation. Cattle in early lactation need a highly integrated metabolism to ensure that production is sustained, health is optimal and that conception can occur when optimal for profit. The underlying physiology of the peri-parturient cow (Bell 1995), vulnerability to disease (Curtis et al. 1983, 1985) and methods of providing environments that result in better production and health have been extensively reviewed (Lean et al. 2003). Prospective studies of dairy cattle exposed to transition diets found marked increases in milk production (DeGaris et al. 2008; DeGroot et al. 2010) and improved reproductive performance (DeGaris et al. 2010a). The transition diets described in DeGaris et al. (2008) provided increased dry matter (DM) intake, estimated energy and metabolisable protein balances that were positive, macro- and micro-elements that were formulated to meet or exceed National Research Council (2001) requirements and a negative dietary cation anion difference (DCAD) balance. Increasing exposure to the pre-partum transition diets increased milk, milk fat and protein yield. The increase in milk production for optimal days of exposure to the transition diet (22 days for milk yield and 25 days for milk protein yield) against minimal exposure (3 days or less) was 3.75 L of 4.0% fat and 3.2% protein-corrected milk per day and 100 g of milk protein per day up to Day 150 of lactation. DeGaris et al. (2010a) also found that exposure to the transition diet increased risk of conception by 1.2% per day on the transition diet. Figure 1 shows the cumulative pregnancy rate for cows exposed to the diet for less than 10 days, to those exposed for 10–20 days and those cows exposed for more than 20 days. The cohort studies provide strong evidence that exposure to transition diets has positive effects on cattle, but does not provide evidence that any particular component of the diet caused such benefit. Notwithstanding the impressive body of information on the underlying physiology of the transition period, the longer-term responses, as expressed in milk production or fertility, require further evaluation. In particular, the roles of Ca metabolism, lipid metabolism and factors influencing gluconeogenesis, including control of insulin release, tissue sensitivity to insulin and the integration of these processes with other metabolism requires further consideration. Table 1 provides a list of transition interventions that could result in prolonged elevation of milk production and mechanisms that may influence these.
|
|
Detailed examination of evidence pertaining to particular aspects of diet is warranted, particularly in the context of recent understandings of the role of bone in the regulation of metabolism in mice models that should equally apply to the cow (McNeill and Anderson 2012). In order to investigate the mechanisms by which an intervention, limited to the period immediately before calving can influence future production and reproduction, we evaluated the effects of Ca and vitamin D on bone and the integration of energy metabolism; examined the effects of pre-calving DCAD and macro-mineral content of the diet on milk production and undertook further analysis of data collected by DeGaris et al. (2010b).
Calcium and vitamin D: interactions with energy metabolism
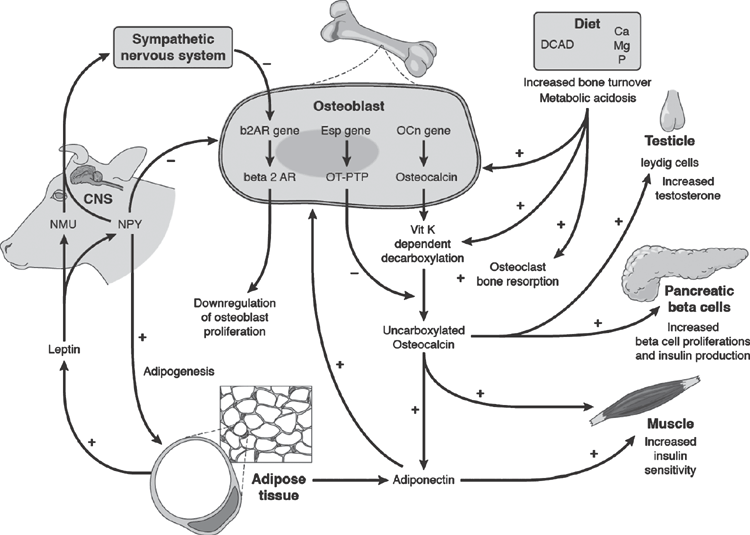
The possibility that the skeleton was involved in energy homeostasis and homeorhesis was first postulated when it was observed that obesity reduced the risk of osteoporosis in humans (Felson et al. 1993). Ducy et al. (2000) proposed that bone and energy metabolism may be regulated by the same hormones. An extensive series of Murine studies identified actions of OC produced by mature OB that completed a negative feedback loop between bone and energy metabolism, that is the hallmark of homeostatic regulation (Lee et al. 2007). The carboxylated form of OC has a high affinity for bone and is considered biologically inactive, whereas the uncarboxylated form (uOC) is biologically active and promotes β-cell proliferation, insulin secretion and independently increases peripheral tissue insulin sensitivity and stimulates adiponectin secretion by adipose cells (Lee et al. 2007). Adiponectin increases OB proliferation and differentiation (Berner et al. 2004) and increases bone deposition (Kanazawa et al. 2007). Further, adiponectin increases glucose uptake by skeletal muscle and may suppress hepatic gluconeogenesis (Yamauchi et al. 2002). Figure 2 shows understandings of interactions between skeleton, energy and protein metabolism and the influence of these effects on metabolism in general.
|
Insulin acts to directly inhibit OB activity, thereby enhancing bone resorption (Lee et al. 2007). As a result of these actions, the extracellular environment within the absorption lacunae is acidified and increases the vitamin K-dependent decarboxylation of OC released from the bone matrix, thus although OC is produced by OB, decarboxylation of OC to the active form is determined by osteoclast activity. This may indicate that bone resorption is the key link between bone and energy metabolism. Changes in the concentrations of markers of bone metabolism in the plasma and urine, suggest that the most likely time to replenish bone is mid–late lactation, and to mobilise bone is late pregnancy and early lactation (McNeill et al. 2002; Bhanugopan et al. 2010).
Adipose tissue also influences bone metabolism (Fig. 2). Leptin, produced by adipocytes, acts to inhibit bone mass accrual through the action of the sympathetic nervous system on osteoblast β2-AR receptors after central processing involving neuropeptide Y (NPY) and neuromedian U to inhibit osteoblast activity (Takeda et al. 2002). NPY acts directly to inhibit OB and stimulate adipogenesis through non-hypothalamic Y1 receptors (Baldock et al. 2007). Leptin also has a potent anorexigenic effect and enhances reproduction possibly through increased expression of Kiss1 and resultant increase in gonadotrophin releasing hormone (GnRH) release (Popa et al. 2008). Leptin stimulates LH release in fasted cows and ewes (Nagatani et al. 1998; Williams et al. 2002). In contrast NPY is orexigenic and decreases release of GnRH and luteinising hormone (LH) and interestingly, oestrogen has been shown to both increase NYP production, but also be anorexogenic (Bonavera et al. 1994).
There are limited data to evaluate the role of bone in energy metabolism in cattle; however, there are findings that support the hypothesis that these are linked. Heuer et al. (1999) found that obese cows [body condition score (BCS) >4.5/5] were at greater risk of milk fever and DeGaris et al. (2010b) found a positive relationship between BCS and area under the curve for blood Ca concentrations after calving when BCS at calving was less than 3.0/5. Martinez et al. (2014) induced sub-clinical hypocalcaemia (SCH) by infusion of 5% ethylene glycol tetra-acetic acid, a selective ionised Ca chelator in non-lactating Holstein cows. These SCH cattle had lower DM intake, higher blood glucose concentrations and higher plasma non-esterified fatty acids (NEFA) than the cows infused with saline and fed 43 g of Ca as sulfate and chlorides. Physiological concentrations of Ca in blood may be necessary for glucose stimulation of insulin secretion by β-cells (Capen and Rosol 1989); Martinez et al. (2014) found lower insulin in the infused SCH cows.
Adiponectin concentrations were relatively low in early lactation and pre-partum and decreased in response to diets designed to induce a negative energy balance, whereas there was little change in adiponectin concentrations induced in mid or late lactation by diets creating a similar energy deficit (Singh et al. 2014). Singh et al. (2014) also observed a negative correlation between BCS and adiponectin and a positive correlation between plasma insulin and adiponectin. The insensitivity of dairy cattle in early lactation to insulin is well recognised. Bigner et al. (1996) found an increase in insulin resistance in cows fed low DCAD rations with a difference of >500 meq/kg to a high DCAD ration group after calving, although Grunberg et al. (2011) found no such association in a study conducted in groups of cows with a difference in DCAD of ~200 meq/kg and –100 meq/kg in the negative DCAD group. The role of bone and Ca metabolism in mediating the effects of body tissue reserves, lipid and protein, and energy and protein fluxes obtained from feed in the peri-parturient period clearly needs further exploration in cattle.
Support for a possible role for Ca in the increased productive and reproductive performance can be derived from several studies. Clinical milk fever was associated with increased days to first service, conception and number of services per conception (Borsberry and Dobson 1989) and increased risk of reproductive disorders (Erb et al. 1985). SCH was associated with increases in risk of uterine diseases in dairy cows (Martinez et al. 2012). In New Zealand, McKay (1994) supplemented pasture-fed cows with Ca-enriched molasses and found a 12% increase in first service conception rate for cows of parity 3 and greater, compared with controls and Stevenson et al. (1999) found a marked reduction in time from planned start of mating to conception for cows treated with Ca chloride in one of three herds studied. In the US, it has been reported that Wang et al. (1991) found a 17% increase in conception rates in 510 cows in a randomised controlled study of DCAD intervention before calving. The results of inducing SCH extend beyond the reduced DM intake and impaired energy metabolism to altered immune function as indicated by reduced phagocytic and killing activities of neutrophils (Martinez et al. 2014).
In addition to elucidating the homeostatic regulatory pathways acting between bone and energy metabolism, Lee et al. (2007) demonstrated a homeorhetic relationship between energy and bone metabolism; β-cell proliferation increased by between 60% and 300% over controls by 30 days of age in mice selected for a high level of OC endocrine activity. While the concept of homeorhesis is well established (Bauman and Currie 1980), it has rarely been specifically tested in cattle. The time series cross-correlation analysis of Lean et al. (1992) demonstrated for the first time temporal relationships between 3-hydroxybutyrate (BHB), glucose, cholesterol and free fatty acids (FFA), over a periods as long as 9 days, that were consistent with homeorhetic change in response to increasing milk production and decreasing estimated net energy balance.
Effect of pre-calving DCAD on milk production: a meta-analysis of milk responses
Negative DCAD diets have been used to reduce the risk of hypocalcaemia in periparturient cattle (Block 1984; Lean et al. 2006). Apart from the effects in reducing the risk of hypocalcaemia, negative DCAD diets increase mobilisation of bone as indicated by bone markers (Block 1984; Goff and Horst 1997; Leclerc and Block 1989). Other effects of lowering the DCAD of pre-calving diets include induction of metabolic acidosis in goats (Fredeen et al. 1988a, 1988b) and cattle (Gaynor et al. 1989), an action consistent with the strong ion theory of acid-base balance. There is decreased renal sensitivity to parathyroid hormone (PTH) in cows fed a strongly positive DCAD pre-calving diet (Gaynor et al. 1989; Goff et al. 1991) and evidence of increased Ca homeostatic responses to PTH injection in cows fed a negative DCAD diet (Goff et al. 2014) leading those authors to conclude that hypocalcaemia and milk fever are, in part, a diet induced pseudo-hypoparathyroidism. Further, there is enhanced renal production of 1,25(OH) vitamin D3 in response to a low DCAD pre-calving diet (Gaynor et al. 1989; Goff et al. 1991), increased responsiveness of target tissues to 1,25(OH) vitamin D3 associated with increased Ca absorption from the intestinal tract (Allsop and Pauli 1985) and increased plasma ionised Ca concentrations (Oetzel et al. 1991; Phillippo et al. 1994). Critically, the overall effect of negative DCAD diets is to increase Ca turnover through increased gastrointestinal tract (GIT) absorption and increased sensitivity of target tissues to homeostatic signals such as PTH, rather than an improvement in overall Ca balance. The effects of a negative DCAD diet may well include an enhanced sensitivity to the actions of skeleton in integrated metabolism (Fig. 2), and allow increased milk production in treated cattle.
In order to further evaluate the positive milk production responses observed to negative DCAD diets in some studies (Block 1984; DeGaris et al. 2008; DeGroot et al. 2010), the studies included in Lean et al. (2006) were examined to identify studies that included the effects of pre-calving diet on milk production. Further literature searches were conducted using PubMed, Google Scholar and Thompson ISI to ensure that recent studies were identified using the following keywords DCAD or DCAB or CAB or cation anion difference or cation anion balance and milk production or yield. A total of 15 studies and 34 comparisons were found and analysed (Stata 13.0, Statacorp, TX, USA) using a random effects model (DerSimonian and Laird 1986), with the Hartung and Knapp (2001) method used to provide estimates of effect size and confidence intervals for cows and heifers. A weighted mean difference between treated and control, with the weighting reflecting the inverse of the variance of the studies was calculated according to the nostandard method (Stata 13.0, Statacorp College Station Texas, USA).
Figure 3 shows the effect size estimates for all studies for which milk production and measures of dispersion were available as a Forest plot. Information on the parity of the cattle in the studies and diets obtained from the papers were entered into CPMDairy (Version 3.08, Miner Institute, Cornell, PA, USA) and meta-regression analysis was conducted to identify whether parity of the cattle, difference in DCAD between treatment and the control diet and estimated energy density, crude protein, crude fat, neutral detergent fibre (NDF), non-fibre carbohydrate, DCAD, Ca, magnesium, phosphorus, or potassium content of the control diet influenced responses. Only the parity (P = 0.003) and NDF of the control diet (P = 0.02) significantly influenced milk production responses, which increased in multiparous cows (P = 0.01), but not in heifers (P = 0.11). The effect size response was lower with higher NDF diets when tested on all studies.

|
The average ± s.e. days of exposure to a transition diet for cows was 29 ± 3 and the DCAD of controls and treated cows 232 ± 37 and –27 ± 33 meq/kg, respectively. The effect size estimate for milk production of studies in cows estimated using the Hartung and Knapp (2001) method was positive 0.671 (95% confidence interval 0.178–1.164). The I2 was 77.1 indicating considerable variability in responses. In studies that estimated responses in milk or fat-corrected milk over the first 65 ± 14 days of lactation, the weighted mean difference was 1.153 (95% confidence interval 0.335 to 1.971) L per day. In heifers milk production responses were negative with an effect size –1.225 (95% confidence interval –2.807 to 0.357) and I2 87.2 indicating considerable variability in responses. The weighted mean difference was –1.482 (95% confidence interval –1.872 to –1.093) L per day. The responses in heifers were strongly influenced by two trials in which DCAD changes were confounded with Ca concentrations in the diet. The lower response to DCAD in high NDF studies may indicate a role for ruminal outputs to influence acid–base status. Critically, other sources of variation, as indicated by the high I2, were not identified, despite the large number of covariates tested.
These studies provide strong evidence that the physiological effects of negative DCAD diets, potentially mediated, in part, through mechanisms described above and by others in mice and man (Lee et al. 2007; Wolf 2008), are to increase milk production independent of most other dietary factors characterised using a contemporary feeding evaluation system, CPMDairy. These findings derived from randomised controlled studies are supported by epidemiological studies indicating that SCH before and after calving was associated with lower milk production (Chapinal et al. 2012). It is very likely that responses to DCAD diets differ between heifers and multiparous cows, suggesting that prior lactation or age substantially influence responses to DCAD diets day and indicates a need to understand differences in Ca metabolism between heifers and multiparous cows, that might include sensitivity to PTH, differences in labile Ca reserves and the absorptive surface area of bone.
Evaluation of the role of Ca in integrated metabolism
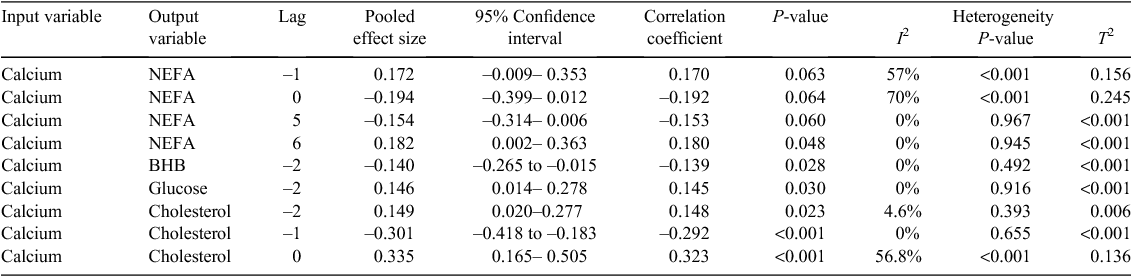
We explored the interactions between metabolites over time, in order to further understand the potential for integration of metabolism of peri-parturient cattle exposed to pre-calving diets to extend beyond homeostatic responses. In brief, data from blood samples collected every 3.5 ± 0.5 days from ~25 days before calving to 30 days after calving were extracted for a range of metabolites from 32 individual cattle (DeGaris et al. 2010b); the data series for each metabolite were de-trended to produce approximately stationary series and cross-correlated with other metabolites. Cross-correlations for each cow were treated as separate studies and a random effects, pooled effect of estimate was produced using meta-analytic methods described by Hedges and Vevea (1998) after Fisher’s transformation of each cross-correlation coefficient. Only the results relating to Ca are provided here (Table 2). The results show the random effects average response as effect size, 95% confidence interval, and measures of heterogeneity or variance among cows as assessed by I2 and τ2. Calcium concentrations 3.5 days before were positively correlated with NEFA and negatively correlated on the same day, but there was significant heterogeneity in these results. There were homogeneous correlations between NEFA concentrations 17.5 and 21 days before and Ca. For 3 hydroxybutyrate and glucose, blood concentrations were negatively, and positively associated respectively, with Ca concentrations 7 days before and these results were homogenous. Calcium concentrations 7 days before were positively associated with cholesterol and Ca concentrations 3.5 days before were more strongly negatively associated with cholesterol concentrations. Both findings were homogenous, indicating that results were consistent among the cattle. Calcium concentrations on the same day were positively associated with cholesterol, but results were significantly heterogenous indicating more variability in these results. The pattern of positive and negative associations over time, particularly between Ca and NEFA, indicates negative feedback mechanisms and this feedback pattern was very evident in individual cows (Fig. 4).
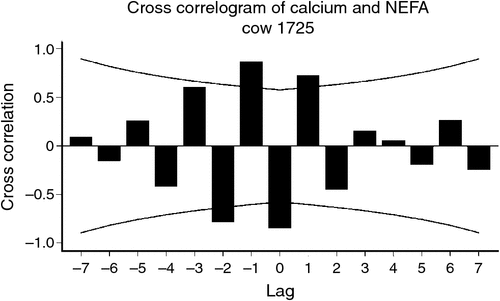
|
These observations, indicating strong associations between serum Ca and NEFA, BHB, glucose and cholesterol in 32 peri-parturient cows, can be considered in the context of relationships between bone function and energy metabolism. They support a positive homeorhetic and homeostatic effect of Ca on energy metabolism with higher NEFA, glucose, and lower BHB subsequent to increased Ca concentrations. In most cases, Ca concentrations up to 7 days before and on the same day were associated with these metabolites, indicating that changes in Ca concentrations preceded those in NEFA, glucose and BHB. The relationships with NEFA and Ca 17–21 days later raise the possibility of lipid mobilisation and body lipid reserves influencing Ca metabolism and the negative correlation between Ca and NEFA on the same day is consistent with the findings of Martinez et al. (2014). The relationships identified using the time series methods are consistent with the relationships identified in mouse models and man (Lee et al. 2007; Wolf 2008; Confavreux 2011), given that acidifying conditions increase Ca concentrations and uOC production. The strong association between Ca and cholesterol may also be consistent with an overall positive effect of Ca metabolism on energy balance, given that energy balance and cholesterol concentrations are positively associated (Lean et al. 1992). Blood cholesterol concentrations have positive associations with reproductive performance in dairy cattle in studies (Westwood et al. 2002).
A potential role for the skeleton in reproduction has also been postulated based on increased cases of osteoporosis in hypogonadal human patients (Confavreux 2011). Recently, uOC has been shown to enhance male fertility via increased production of testosterone in Leydig cells (Oury et al. 2011) but not the ovary. However, a porcine study found OC levels increased with increasing oestradiol and markers of bone resorption showed a cyclical fluctuation during the oestrus cycle with highest concentrations found around oestrus and the lowest concentrations during the luteal phase (Babel 2007). Adipose tissue has also recently been identified as a site of both OC and uOC production under the influence of androgens (Foresta et al. 2010).
While studies investigating the relationship between uOC and energy metabolism are lacking in dairy cattle, there is a good body of evidence that OC is a reliable marker of bone formation (Liesegang et al. 1998). In the cow, OC is elevated in the plasma of younger animals, especially fetuses, declines with age, before the first lactation, is lower in multiparous than primiparous cows, declines in late pregnancy and at the time of calving and rises in the days to weeks thereafter (Sato et al. 2011). Taylor et al. (2008) similarly found lower OC concentrations in cows >2 lactations than second-lactation cows. Kim et al. (2011) administered an exogenous injection of 1,25-hydroxy-vitamin D3 to stimulate bone formation in non-pregnant non-lactating cows, and found that the injection elevated plasma Ca, ‘total OC and uOC within days’. However, an earlier study (Taylor et al. 2008) found no increase in total OC concentrations following treatment with 25-hydroxy-vitamin D3 or vitamin D3.
The role of vitamin D in metabolism requires consideration in the context of the role of skeleton in metabolism. While the role of vitamin D in Ca homeostasis has been well established in monogastric species, including the human (Fraser 1995), there are questions remaining about ruminant metabolism. When there is an increased demand for Ca as in growth or lactation, the ionised concentration of Ca (Ca2+) in blood tends to fall. This initiates an increased secretion of PTH, which acts in both the kidney and bone to enhance the supply of Ca2+ to the extracellular fluid. In the kidney, PTH induces an increased secretion of the vitamin D hormone, 1,25(OH)2D, which then induces increased active Ca transport in the small intestine. The molecular basis for this active transport involves an entry ion channel across the microvillous membrane (the transient receptor potential vanilloid channel type 6 or TRPV6), which facilitates Ca entry from the intestinal lumen into the mucosal cell (Peng et al. 1999). The internalised Ca2+ is bound to a cytoplasmic specific Ca-binding protein, calbindin-D9k (Bronner 1987). A basolateral membrane Ca pump then transfers the absorbed Ca2+ into the submucosal extracellular fluid.
In ruminants, however, there is also considerable evidence that Ca2+ is absorbed across the rumen mucosa (Höller et al. 1988; Beardsworth et al. 1989; Wadhwa and Care 2000) and that this is mediated by a regulated active transport process. The rumen is the main site for magnesium absorption in ruminants (Tomas and Potter 1976; Care et al. 1984) and it is possible that the rumen is a major site also for the absorption of Ca. In sheep, when the blood concentration of 1,25(OH)2D is raised, there is not only an increase in the absorption capacity for Ca2+ in the small intestine but also an increase in the transport capacity for Ca2+ across the ruminal mucosa in vivo (Hyde and Fraser in press). Therefore, in comparison to monogastrics, ruminants have two distinct regions of the alimentary tract where there are regulated, active absorption processes, which can increase the supply of Ca2+ when there is an increased demand, as at the onset of lactation.
The transport process for Ca2+ from the rumen in sheep is enhanced when the level of 1,25(OH)2D in the circulation is raised, thus suggesting that the rumen mucosa has the same Ca transport mechanism, regulated by the vitamin D hormone as in the small intestine. However, no effect of vitamin D was detected in vitro in sheep on the active transport of Ca across the ruminal mucosa of sheep (Schröder et al. 2001). Furthermore, Wilkens et al. (2009, 2011) found that the components of the Ca transport mechanism that were readily demonstrated in the small intestinal mucosa, were either undetectable or were expressed in very small amounts in the rumen mucosa of sheep. Hence, there is a paradox in understanding the Ca homeostasis processes of the alimentary tract in ruminants. Studies in vivo have found that increased circulating levels of 1,25(OH)2D are associated with increased absorption capacities for Ca in both the rumen and small intestine (Hyde and Fraser in press), yet the rumen mucosa does not have the molecular machinery, present in the small intestinal mucosal cells, for the active transport of Ca2+. An additional puzzle in this paradox is that the receptor protein for 1,25(OH)2D that mediates the specific action of this hormone on the expression of the components of Ca transport, is at a very low concentration in the rumen mucosa cells compared with those of the small intestine. Therefore, although it appears that increased secretion of 1,25(OH)2D by the kidney would lead to increased absorption of Ca in both the small intestine and the rumen, this is unlikely to be by direct action of 1,25(OH)2D on the rumen mucosa cells. This suggests that some other signal, activated by 1,25(OH)2D, is upregulating the absorption capacity for Ca in the rumen. The identification of this postulated secondary regulating factor might help to explain part of the mechanism for the development of clinical parturient hypocalcaemia in some dairy cows at the start of lactation.
Hormonal intervention
Bovine somatotropin (rBST) has been used to increase milk production in lactation and studies with rBST administered in the pre-calving period have resulted in increased milk production in lactation. Putnam et al. (1999) treated cows with rBST every 14 days from 28 days before anticipated calving until parturition in a factorial study with protein treatments. Milk production responses to the rBST intervention were substantial, 3.3 kg/day more milk to Day 42 of lactation. Gulay et al. (2004a, 2004b) similarly, found increases in milk production to Day 70 of lactation in treated cattle, but these did not persist to Day 100, suggesting the influence of rBST alone administered before calving can be moderately effective in triggering longer-term milk responses and raising the possibility of the somatotropic axis being involved in responses to pre-calving diets. Studies to identify cattle treated with rBST have found that OC concentrations are elevated following treatment (Ludwig et al. 2012), as are OC concentrations and bone turnover indicators in humans treated with human growth hormone (Wallace et al. 2000), thereby providing a link between the somatotropic axis and bone. Some caution should be applied to these findings as the links identified may not indicate a potential to influence milk production through the skeletal axis. Studies in rats (Ammann et al. 2010) highlight an interaction between protein intake and bone responses to bovine growth hormone, because rats with low protein intake increased osteoclast surface area, but had lower bone mineral density and strength. The loss of bone mass in humans associated with low protein diets (Ammann et al. 2010) may indicate the potential for protein loss in cattle to be a factor in the susceptibility of older cattle to hypocalcemia.
Reduced nutrient demand pre-calving or increased energy or protein intake
Wolfenson et al. (1988) found that increased calf birthweight was associated with higher milk production, suggesting that effects of female progeny of increasing milk production in the next lactation identified by Hinde et al. (2014) may not be mediated through calf weight alone. This result may be consistent with the lack of significant effect in a meta-analysis of increased pre-calving crude protein intake on milk production (Lean et al. 2013). The latter finding is unsurprising because estimated metabolisable protein balance is a more meaningful measure of protein nutrition and stores of labile protein will vary among cattle (Belyea et al. 1978) and could influence responses to diet. Greater DM intake has increased milk production significantly after calving as demonstrated by forced feeding periparturient cows through a ruminal fistula (Bertics et al. 1992). Cows that received more feed had less hepatic lipid accumulation. The higher milk production after calving, resulted from greater post-calving feed intake and a highly significant positive correlation between pre- and post-calving feed intake was identified (Bertics et al. 1992). However, more work is needed to examine the effects of mineral, carbohydrate, lipid and protein nutrition in the pre-calving period, sire of calf and calf birthweight on milk production in a subsequent lactation to understand, isolate and potentially manipulate the magnitude of effects arising from the metabolic demands of the fetus for energy, amino acids and other nutrients (Bell 1995) including those required for skeletal development as opposed to those of hormonal or other influences of the fetus on future performance.
Controlling the effects of oxidative damage and inflammation
As consequence of rapid fetal growth during the last trimester of pregnancy and production of large amounts of colostrum and milk at the beginning of lactation, both maternal and fetal metabolism is increased due to augmented mitochondrial activity in maternal tissues and the conceptus (Aurousseau et al. 2006). This activity results in an increase in the production of reactive oxygen species during late gestation (Castillo et al. 2005) and early lactation (Pedernera et al. 2010) and increased requirements for micronutrients, including antioxidants. A pro-inflammatory state may be an essential part of the homeorhetic adaptations to lactation (Farney et al. 2013a). The potential to control the effects of oxidative damage through manipulation of micronutrients, other antioxidants or mediators of inflammation should be considered in the context of the exposure of dairy cows to oxidative stress and inflammatory events (Miller et al. 1993; Sordillo and Raphael 2013). Moreover the cow, and the calf, may compete for nutrients (energy, protein, minerals and antioxidants) during the transition period (Curtis 1997). A lowered antioxidant status has been associated with disease and metabolic disorders including mastitis, metritis, retained placenta, acidosis, ketosis and milk fever (Miller et al. 1993; Celi 2011). The most compelling support for the importance of mediation of inflammation in the peri-parturient period comes from the intervention studies with anti-inflammatory therapeutics of Bertoni et al. (2004), Shwartz et al. (2009) and Farney et al. (2013b) who either injected acetyl-salicylic acid for 5 days after calving (Bertoni et al. 2004) or fed sodium salicylate in water for 7 days (Farney et al. 2013b) or injected flunixin (Shwartz et al. 2009). In both the salicylate studies, milk production increased significantly, but did not increase in the flunixin study.
The mechanisms for the increased milk production were not elucidated; however, both antioxidant defence and reactions catalysed by steroidogenic enzymes require reducing equivalents provided by nicotinamide adenine dinucleotide (NADPH) (Liebler 1993). Excessive consumption of reducing equivalents by oxidative stress can lower NADPH2 and increase NADP concentrations despite elevated activity of the monophosphate shunt, which generates the reduced form (Kehrer and Lund 1994). Consumption of reducing equivalents by oxidative stress reactions can diminish the supply of NADPH available for important physiological processes. The induction of the monophosphate shunt by an impaired oxidant/antioxidant balance would divert glucose from other pathways. This possibility assumes greater importance when the requirement for glucose and the quantity available in the ruminant are considered and may be a factor influencing the production responses to the interventions (Bertoni et al. 2004; Farney et al. 2013a, 2013b). The positive milk production responses in these studies (Bertoni et al. 2004; Farney et al. 2013b) appeared to be independent of effects of treatment on disease, although this hypothesis was not specifically tested in either study.
While adequate antioxidant concentrations, including selenium and vitamin E, are important for the maintenance of health and performance of cows and calves (Lacetera et al. 1996), responses to antioxidant supplementation have resulted in conflicting reports. For example, vitamin E and selenium supplementation at doses greater than the National Research Council (2001) recommendations did not result in improved daily gain or morbidity in feedlot cattle (Cusack et al. 2009), nor did use of an organic selenium improve reproductive performance (Cerri et al. 2009). Such variable results are to be anticipated from the extensive interactions between antioxidants (Curtis 1997) and the pro-oxidative stressors of the metabolism of cattle per se and exerted through the environment. There is considerable capacity for interactions with other dietary substrates and environmental conditions including heat stress (Dunshea et al. 2013). It is possible that more sophisticated understandings of the optimal means of controlling oxidative stress and inflammation around calving will improve reproduction and health.
Conclusions
There are several major pathways through which improved production, reproduction and health may be achieved by manipulation of the transition environment and probably more to be identified. Lee et al. (2007) demonstrated that OC, produced by mature OB, can regulate fat metabolism in mice, thus completing the homeostatic loop between the neuro-endocrine regulation of energy and bone metabolism. They also demonstrated homeorhetic responses in glucose metabolism mediated by OC and characterised by pancreatic β-cell proliferation extending over 45–60 days.
Negative DCAD diets stimulated milk production in cows, but not in heifers and these effects were not modified by dietary factors evaluated, other than the NDF of the diet. Negative DCAD diets stimulate bone turnover, acidify the blood, thereby potentially enhancing the vitamin K-mediated decarboxylation of OC to uOC and increasing hormone release from the skeleton. This could enhance the effect of uOC on energy and protein metabolism and possibly explain some of the very long-term effects of negative DCAD diets on metabolism.
We found that nutritional interventions, including a negative DCAD diet, applied only in the pre-calving period markedly increase milk production, improved reproduction and reduce the risks of metabolic disease. These interventions associated with negative DCAD diets fed before calving also influenced concentrations of glucose and BHB. When examined by time series methods, correlations were found for blood Ca concentrations with blood concentrations of glucose, FFA, BHB and cholesterol taken on the same day and on up to 21 days later. This provides evidence to support a homeorhetic role for Ca metabolism in cattle. Treatment with rBST had a moderate, but positive effect on production and has links to bone and protein metabolism. Studies have found prolonged differences in production with weight and sex of the conceptus indicating the importance of considering the role of conceptus in nutrient demand and hormonal signalling. Inflammation plays an important role in lactation and health. Two studies showed benefits of anti-inflammatory treatments administered for a few days near calving to increase milk production.
While we have provided information to support such a hypothesis, it remains to be specifically determined if the homeostatic and homeorhetic pathways demonstrated in murine models exist in cattle. The potential for manipulation of both energy metabolism and bone metabolism in a coordinated fashion increases production, and may have the potential to modify the risk of disease in early lactation. If these effects are demonstrated to be clinically significant, it may require a reconsideration of current recommendations for dietary Ca, phosphorus and magnesium balance, particularly during the transition period and early lactation. Such recommendations may be modified according to body tissue reserves of the cattle. We have provided background on Ca and vitamin D metabolism highlighting new information on the absorption of Ca that may lead to better dietary recommendations for intake. The quantitative importance of bone resorption to health, production and reproductive performance needs to be determined, however, there is sufficient evidence to merit such studies.
Acknowledgement
Andrew and Jan Troutt for assistance with the presentation of figures.
References
Allsop TF, Pauli JV (1985) Failure of 25-hydroxycholecalciferol to prevent milk fever in dairy cows. New Zealand Veterinary Journal 13, 19–22.Ammann P, Brennan TC, Mekraldi S, Aubert ML, Rizzoli R (2010) Administration of growth hormone in selectively protein-deprived rats decreases BMD and bone strength. Bone 46, 1574–1581.
| Administration of growth hormone in selectively protein-deprived rats decreases BMD and bone strength.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmsVShu7k%3D&md5=4f169bb308701cab27ce36e326cb53edCAS | 20178866PubMed |
Aurousseau B, Gruffat D, Durand D (2006) Gestation linked radical oxygen species fluxes and vitamins and trace mineral deficiencies in the ruminant. Reproduction, Nutrition, Development 46, 601–620.
| Gestation linked radical oxygen species fluxes and vitamins and trace mineral deficiencies in the ruminant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1SjsLc%3D&md5=98b56989ec3d9608f941d8700f0b8000CAS | 17169308PubMed |
Babel B (2007) Investigations on bone metabolism in intact and ovariohysterectomised miniature pigs. PhD Thesis. Institut für Physiologie, physiologische Chemie und Tierernährung der Tierärztlichen Fakultät der Ludwig-Maximilians-Universität, München, Geschäftsführender Vorstand, München, Germany.
Baldock PA, Allison SJ, Lundberg P, Lee NJ, Slack K, Lin EJ, Enriquez DRF, McDonald MM, Zhang L, During MJ, Little DG, Eisman JA, Gardiner EM, Yulyaningsih E, Lin S, Sainsbury A, Herzog H (2007) Novel role of Y1 receptors in the coordinated regulation of bone and energy homeostasis. The Journal of Biological Chemistry 282, 19 092–19 102.
| Novel role of Y1 receptors in the coordinated regulation of bone and energy homeostasis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmvVart7s%3D&md5=a6bf9587f535ce229aac56dac6d3325bCAS |
Bauman DE, Currie WB (1980) Partitioning of nutrients during pregnancy and lactation: a review of mechanism involving homeostasis and homeorhesis. Journal of Dairy Science 63, 1514–1529.
| Partitioning of nutrients during pregnancy and lactation: a review of mechanism involving homeostasis and homeorhesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXmtFygu7s%3D&md5=c19f766652cfef1e62946e1077b9d7aeCAS | 7000867PubMed |
Beardsworth L, Beardsworth P, Care AD (1989) Calcium fluxes across the wall of the ovine reticulorumen in vivo. Research in Veterinary Science 47, 404–405.
Bell AW (1995) Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. Journal of Animal Science 73, 2804–2819.
Belyea RL, Frost GR, Martz FA, Clark JL, Forkner LG (1978) Body composition of dairy cattle by potassium-40 scintillation detection. Journal of Dairy Science 61, 206–211.
| Body composition of dairy cattle by potassium-40 scintillation detection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1cXhtFygsL8%3D&md5=ca59a90860fa012806e842e356e62489CAS |
Berner HS, Lyngstadass SP, Spahr A, Monjo M, Thommesen L, Drevon CA, Syversen U, Reseland JE (2004) Adiponectin and its receptors are expressed in bone-forming cells. Bone 35, 842–849.
| Adiponectin and its receptors are expressed in bone-forming cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXnvV2rtLo%3D&md5=d894fd86996388e103212cc147714910CAS | 15454091PubMed |
Bertics SJ, Grummer RR, Cadorniga-Valino C, Stoddard EE (1992) Effect of prepartum dry matter intake on liver triglyceride concentration and early lactation. Journal of Dairy Science 75, 1914–1922.
| Effect of prepartum dry matter intake on liver triglyceride concentration and early lactation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XltlSgsbs%3D&md5=a6ecfbad0718a1e40325bdf082de0488CAS | 1500587PubMed |
Bertoni G, Trevisi E, Piccioli-Cappelli F (2004) Effects of acetyl-salicylate used in post-calving of dairy cows. Veterinary Research Communications 28, 217–219.
| Effects of acetyl-salicylate used in post-calving of dairy cows.Crossref | GoogleScholarGoogle Scholar | 15372961PubMed |
Bhanugopan MS, Fulkerson W, Hyde ML, Fraser DR, McNeill DM (2010) Carryover effects of potassium supplementation on calcium metabolism in dairy cows at parturition. Journal of Dairy Science 93, 2119–2129.
| Carryover effects of potassium supplementation on calcium metabolism in dairy cows at parturition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnvVansrk%3D&md5=c25a1b0e5d842f7b7c7fd695e4cdd6cbCAS | 20412927PubMed |
Bigner DR, Goff JP, Faust MA, Burton JL, Tyler HD, Horst RL (1996) Acidosis effects on insulin response during glucose tolerance tests in jersey cows. Journal of Dairy Science 79, 2182–2188.
| Acidosis effects on insulin response during glucose tolerance tests in jersey cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXls1GjsA%3D%3D&md5=2a19a0b7bdbf6cd8fc42fd9fd5fa33c3CAS | 9029356PubMed |
Block E (1984) Manipulating dietary anions and cations for prepartum dairy cows to reduce incidence of milk fever. Journal of Dairy Science 67, 2939–2948.
| Manipulating dietary anions and cations for prepartum dairy cows to reduce incidence of milk fever.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXhtFWqtrk%3D&md5=79b5b1ff2c64bfb5e0f45190288850c4CAS | 6530489PubMed |
Bonavera JJ, Dube MG, Kalra PS, Kalra SP (1994) Anorectic effects of estrogen may be mediated by decreased neuropeptide-Y release in the hypothalamic paraventricular nucleus. Endocrinology 134, 2367–2370.
Borsberry S, Dobson H (1989) Periparturient diseases and their effect on reproductive performance in five dairy herds. The Veterinary Record 124, 217–219.
| Periparturient diseases and their effect on reproductive performance in five dairy herds.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1M7ovVemsw%3D%3D&md5=6a8b44eb83aba7a558eddfd13316cebdCAS | 2929110PubMed |
Bronner F (1987) Intestinal calcium absorption mechanisms and applications. The Journal of Nutrition 117, 51–58.
Capen CC, Rosol TJ (1989) Calcium-regulating hormones and disease of abnormal mineral metabolism. In ‘Clinical biochemistry of domestic animals’. 4th edn. (Ed. JJ Kaneko) pp. 678–752. (Academic Press: San Diego, CA)
Care AD, Brown RC, Farrar AR, Pickard DW (1984) Magnesium absorption from the digestive tract of sheep. Experimental Physiology 69, 577–587.
Castillo C, Hernandez J, Bravo A, Lopez-Alonso M, Pereira V, Benedito JL (2005) Oxidative status during late pregnancy and early lactation in dairy cows. Veterinary Journal (London, England) 169, 286–292.
| Oxidative status during late pregnancy and early lactation in dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhsVOitr0%3D&md5=1fcd49a1073b9ac08e086454ef50434fCAS |
Celi P (2011) Oxidative stress in ruminants. In ‘Studies on veterinary medicine. Vol. 5’. (Eds L Mandelker, P Vajdovich) pp. 191–231. (Humana Press: New York)
Cerri RLA, Rutigliano HM, Lima FS, Araújo DB, Santos JEP (2009) Effect of source of supplemental selenium on uterine health and embryo quality in high-producing dairy cows. Theriogenology 71, 1127–1137.
| Effect of source of supplemental selenium on uterine health and embryo quality in high-producing dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjsV2qs74%3D&md5=0d55312af47271261e352138b8adfaa8CAS |
Chapinal N, Carson ME, LeBlanc SJ, Leslie KE, Godden S, Capel M, Santos JEP, Overton MW, Duffield TF (2012) The association of serum metabolites in the transition period with milk production and early-lactation reproductive performance. Journal of Dairy Science 95, 1301–1309.
| The association of serum metabolites in the transition period with milk production and early-lactation reproductive performance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtFWgsbg%3D&md5=068c16ada4f92f384722cafcc0357fcfCAS | 22365212PubMed |
Confavreux CB (2011) Bone: from a reservoir of minerals to a regulator of energy metabolism. Kidney International 79, S14–S19.
| Bone: from a reservoir of minerals to a regulator of energy metabolism.Crossref | GoogleScholarGoogle Scholar |
Curtis MA (1997) Epidemiology of uterine infections in dairy cows. Antioxidant and metabolic investigations. PhD Thesis, University of Sydney.
Curtis CR, Erb EH, Sniffen CJ (1983) Association of parturient hypocalcemia with eight periparturient disorders in Holstein cows. Journal of the American Veterinary Medical Association 183, 559-561
Curtis CR, Erb EH, Sniffen CJ, Smith RD, Kronfeld DS (1985) Path analysis of dry period nutrition, postpartum metabolic and reproductive disorders, and mastitis in Holstein cows. Journal of Dairy Science 68, 2347–2360.
| Path analysis of dry period nutrition, postpartum metabolic and reproductive disorders, and mastitis in Holstein cows.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL28%2FmtVaqtQ%3D%3D&md5=0a916fdc08417d3cd25a593124c55c4cCAS | 4067048PubMed |
Cusack PMV, McMeniman NP, Rabiee AR, Lean IJ (2009) Assessment of the effects of supplementation with vitamin E on health and production of feedlot cattle using meta-analysis. Preventive Veterinary Medicine 88, 229–246.
| Assessment of the effects of supplementation with vitamin E on health and production of feedlot cattle using meta-analysis.Crossref | GoogleScholarGoogle Scholar |
DeGaris PJ, Lean IJ, Rabiee AR, Heuer C (2008) Effects of increasing days of exposure to pre-partum transition diets on milk production and milk composition in dairy cows. Australian Veterinary Journal 86, 341–351.
| Effects of increasing days of exposure to pre-partum transition diets on milk production and milk composition in dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1ShtrvM&md5=6423c6d2bffabd51914c002cacfca304CAS | 18782415PubMed |
DeGaris PJ, Lean IJ, Rabiee AR, Heuer C (2010a) Effects of increasing days of exposure to pre-partum diets on reproduction and health in dairy cows. Australian Veterinary Journal 88, 84–92.
| Effects of increasing days of exposure to pre-partum diets on reproduction and health in dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3c3ntlOhsA%3D%3D&md5=2cc3050de23bfe305ce6a281311101e9CAS | 20402690PubMed |
DeGaris PJ, Lean IJ, Rabiee AR, Stevenson MA (2010b) Effects of increasing days of exposure to a pre-partum diet on the concentration of certain blood metabolites in dairy cows. Australian Veterinary Journal 88, 137–145.
| Effects of increasing days of exposure to a pre-partum diet on the concentration of certain blood metabolites in dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3c3ntlOgsg%3D%3D&md5=8788d515a63a195a7f5d47b768720cedCAS | 20402701PubMed |
DeGroot MA, Block E, French PD (2010) Effect of prepartum anionic supplementation on periparturient feed intake, health and milk production. Journal of Dairy Science 93, 5268–5279.
| Effect of prepartum anionic supplementation on periparturient feed intake, health and milk production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnsl2ruw%3D%3D&md5=fa8e6f2ece8ae01aa5203bf5a1d2bf40CAS | 20965343PubMed |
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Controlled Clinical Trials 7, 177–188.
| Meta-analysis in clinical trials.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2s7gsVamtA%3D%3D&md5=5eaf75c05b3ae7eaaf816f60a6785ef4CAS | 3802833PubMed |
Ducy P, Amling M, Takeda S, Priemel M, Schilling A, Beil F, Shen J, Vinson C, Rueger J, Karsenty G (2000) Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100, 197–207.
| Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXotFChsw%3D%3D&md5=10f77b652999a909b6190df893832adcCAS | 10660043PubMed |
Dunshea FR, Leury BJ, Fahri F, DiGiacomo K, Hung A, Chauhan S, Clarke IJ, Collier R, Little S, Baumgard L (2013) Amelioration of thermal stress impacts in dairy cows. Animal Production Science 53, 965–975.
Erb HN, Smith RD, Oltenacu PA, Guard CL, Hillman RB, Powers PA, Smith MC, White ME (1985) Path model of reproductive disorders and performance, milk fever, mastitis, milk yield and culling in holstein cows. Journal of Dairy Science 68, 3337–3349.
| Path model of reproductive disorders and performance, milk fever, mastitis, milk yield and culling in holstein cows.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL287ktFWgtg%3D%3D&md5=81206e23a97c91042140344014f85cd0CAS | 4093528PubMed |
Farney JK, Mamedova LK, Coetzee JF, KuKanich B, Sordillo LM, Stoakes SK, Minton JE, Hollis LC, Bradford BJ (2013a) Anti-inflammatory salicylate treatment alters the metabolic adaptations to lactation in dairy cattle. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 305, R110–R117.
| Anti-inflammatory salicylate treatment alters the metabolic adaptations to lactation in dairy cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht1OmtrrJ&md5=cf1d6208456d03fdcf575c1511c35e00CAS | 23678026PubMed |
Farney JK, Mamedova LK, Coetzee JF, Minton JE, Hollis LC, Bradford BJ (2013b) Sodium salicylate treatment in early lactation increases whole-lactation milk and milk fat yield in mature dairy cows. Journal of Dairy Science 96, 7709–7718.
| Sodium salicylate treatment in early lactation increases whole-lactation milk and milk fat yield in mature dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1CrurvL&md5=99a84dc05ec588ffc135a9c788bb059fCAS | 24140330PubMed |
Felson DT, Zhang Y, Hannan MT, Anderson JJ (1993) Effects of weight and body mass index on bone mineral density in men and women: the Framington study. Journal of Bone and Mineral Research 8, 567–573.
| Effects of weight and body mass index on bone mineral density in men and women: the Framington study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3s3ovFymug%3D%3D&md5=805d9c38a828e6380ea8062cccccc419CAS | 8511983PubMed |
Foresta C, Strapazzon G, De Toni L, Gianesello L, Calcagno A, Pilon C, Plebani M, Vettor R (2010) Evidence for osteocalcin production by adipose tissue and its role in human metabolism. The Journal of Clinical Endocrinology and Metabolism 95, 3502–3506.
| Evidence for osteocalcin production by adipose tissue and its role in human metabolism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXps12rtbo%3D&md5=5c1c16ac32e68abf8f0a812563abec25CAS | 20410230PubMed |
Fraser DR (1995) Vitamin D. Lancet 345, 104–107.
| Vitamin D.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2M7hsFCmtw%3D%3D&md5=c73970f33616c06245f45d392de097b4CAS | 7815853PubMed |
Fredeen AH, DePeeters EJ, Baldwin RL (1988a) Characterisation of acid-base disturbances on calcium and phosphorus balances of dietary fixed ions in pregnant or lactating does. Journal of Animal Science 66, 157–173.
Fredeen AH, DePeeters EJ, Baldwin RL (1988b) Effects of acid-base disturbances caused by differences in dietary fixed ion balance on kinetics of calcium metabolism in ruminants with high calcium demand. Journal of Animal Science 66, 174–184.
Gaynor PJ, Mueller FJ, Miller JK, Ramsey N, Goff JP, Horst RL (1989) Parturient hypocalcemia in jersey cows fed alfalfa haylage-based diets with different cation to anion ratios. Journal of Dairy Science 72, 2525–2531.
| Parturient hypocalcemia in jersey cows fed alfalfa haylage-based diets with different cation to anion ratios.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3c%2FpsVOkuw%3D%3D&md5=e9ba110c797d70bc8088f3b6a8f58f90CAS | 2600220PubMed |
Goff JP, Horst RL (1997) Effects of the addition of potassium or sodium but not calcium to prepartum rations on milk fever in dairy cows. Journal of Dairy Science 80, 176–186.
| Effects of the addition of potassium or sodium but not calcium to prepartum rations on milk fever in dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXpvFamtw%3D%3D&md5=db2957f13272ab1caa8ac917d6eb8abbCAS | 9120088PubMed |
Goff JP, Horst RL, Mueller FJ, Miller JK, Kiess GA, Dowlen HH (1991) Addition of chloride to a prepartal diet high in cations increases 1,25-dihydroxyvitamin D response to hypocalcemia preventing milk fever. Journal of Dairy Science 74, 3863–3871.
| Addition of chloride to a prepartal diet high in cations increases 1,25-dihydroxyvitamin D response to hypocalcemia preventing milk fever.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XitVSrug%3D%3D&md5=d6ffb21b3f1d688a7f920d4c2b00803aCAS | 1757627PubMed |
Goff JP, Liesegang A, Horst RL (2014) Diet-induced pseudohypoparathyroidism: a hypocalcemia and milk fever risk factor. Journal of Dairy Science 97, 1520–1528.
| Diet-induced pseudohypoparathyroidism: a hypocalcemia and milk fever risk factor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtFWit7s%3D&md5=652a277c0c53ddee2bb4c486e7d7a84eCAS | 24418271PubMed |
Grünberg W, Donkin SS, Constable PD (2011) Periparturient effects of feeding a low dietary cation-anion difference diet on acid-base, calcium and phosphorus homeostasis and on intravenous glucose tolerance test in high-producing dairy cows. Journal of Dairy Science 94, 727–745.
| Periparturient effects of feeding a low dietary cation-anion difference diet on acid-base, calcium and phosphorus homeostasis and on intravenous glucose tolerance test in high-producing dairy cows.Crossref | GoogleScholarGoogle Scholar | 21257041PubMed |
Gulay MS, Garcia AN, Hayen MJ, Wilcox CJ, Head HH (2004a) Responses of Holstein cows to different bovine somatotropin (bST) treatments during the transition period and early lactation. Asian–Australasian Journal of Animal Sciences 17, 784–793.
Gulay MS, Hayen MJ, Liboni M, Belloso TI, Wilcox CJ, Head HH (2004b) Low doses of bovine somatotropin during the transition period and early lactation improves milk yield, efficiency of production, and other physiological responses of Holstein cows. Journal of Dairy Science 87, 948–960.
| Low doses of bovine somatotropin during the transition period and early lactation improves milk yield, efficiency of production, and other physiological responses of Holstein cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXivFyru7s%3D&md5=616a71ce423e5081dab0d8fa229508a4CAS | 15259229PubMed |
Hartung J, Knapp G (2001) A refined method for the meta analysis of controlled clinical trials with binary outcome. Statistics in Medicine 20, 3875–3889.
| A refined method for the meta analysis of controlled clinical trials with binary outcome.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38%2Fls1Slsw%3D%3D&md5=4cdb378f064619a2caf528b6dcc2f1b8CAS | 11782040PubMed |
Hedges LV, Vevea JL (1998) Fixed- and random-effects models in meta-analysis. Psychological Methods 3, 486–504.
| Fixed- and random-effects models in meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Heuer C, Schukken YH, Dobbleaar P (1999) Postpartum body condition score and results form the first test day milk as predictors of disease, fertility, yield and culling in commercial dairy herds. Journal of Dairy Science 82, 295–304.
| Postpartum body condition score and results form the first test day milk as predictors of disease, fertility, yield and culling in commercial dairy herds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXhvVWhsb0%3D&md5=ee06ea8ffcc5b3b3d8a57a6185def1d7CAS | 10068951PubMed |
Hinde K, Carpenter AJ, Clay JC, Bradford BJ (2014) Holsteins favor heifers, not bulls: biased milk production programmed during pregnancy as a function of fetal sex. PLoS ONE 9, e86169
| Holsteins favor heifers, not bulls: biased milk production programmed during pregnancy as a function of fetal sex.Crossref | GoogleScholarGoogle Scholar | 24498270PubMed |
Höller H, Breves G, Gerdes H, Kocabatmaz M (1988) Flux of calcium across the sheep rumen wall in vivo and in vitro. Experimental Physiology 73, 609–618.
Hyde ML, Fraser DR (2014) Measurement in vivo of the absorption of strontium from the rumen and intestines of sheep as an index of calcium absorption capacity. British Journal of Nutrition in press.
Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Yamamoto M, Sugimoto T (2007) Adiponectin and AMP kinase activator stimulate proliferation, differentiation, and mineralization of osteoblastic MC3T3-E1 cells. BMC Cell Biology 8, 51
| Adiponectin and AMP kinase activator stimulate proliferation, differentiation, and mineralization of osteoblastic MC3T3-E1 cells.Crossref | GoogleScholarGoogle Scholar | 18047638PubMed |
Kehrer JP, Lund LG (1994) Cellular reducing equivalents and oxidative stress. Free Radical Biology & Medicine 17, 65–75.
| Cellular reducing equivalents and oxidative stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXhvFejtA%3D%3D&md5=18f408593f4eb74bf5d547ccc424b50fCAS |
Kim D, Kawakami Y, Yamagishi N, Abe I, Furuhama K, Devkota B, Okura N, Sato S, Ohashi S (2011) Response of plasma bone markers to a single intramuscular administration of calcitriol in dairy cows. Research in Veterinary Science 90, 124–126.
| Response of plasma bone markers to a single intramuscular administration of calcitriol in dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsVKltw%3D%3D&md5=23e8e67fb46de8e66c52a8c4f8c968d0CAS | 20553702PubMed |
Lacetera N, Bernabucci U, Ronchi B, Nardone A (1996) Effects of selenium and vitamin E administration during a late stage of pregnancy on colostrum and milk production in dairy cows, and on passive immunity and growth of their offspring. American Journal of Veterinary Research 57, 1776–1780.
Lean IJ, Farver TB, Trout HF, Bruss ML, Galland JC, Baldwin RL, Holmberg CA, Weaver LD (1992) Time series cross-correlation analysis of postparturient relationships among serum metabolites and yield variables in Holstein cows. Journal of Dairy Science 75, 1891–1900.
| Time series cross-correlation analysis of postparturient relationships among serum metabolites and yield variables in Holstein cows.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK38zmsVWgtA%3D%3D&md5=13ef2c18e8ff0de4210654637da5e8cfCAS | 1500586PubMed |
Lean IJ, DeGaris PJ, Wade LK, Rajczyk ZK (2003) Transition Management of Dairy Cows: 2003. In ‘Australian and New Zealand combined dairy veterinarians’ conference’. (Ed T Parkinson) pp. 221–248. (Foundation for Continuing Education of the NZ Veterinary Association: Taupo, New Zealand)
Lean IJ, DeGaris PJ, McNeil DM, Block E (2006) Hypocalcemia in dairy cows: meta analysis and dietary cation anion difference theory revisited. Journal of Dairy Science 89, 669–684.
| Hypocalcemia in dairy cows: meta analysis and dietary cation anion difference theory revisited.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtlWrtL4%3D&md5=2ab59e0987266c7ff66313c78a1d32b9CAS | 16428636PubMed |
Lean IJ, Van Saun R, DeGaris PJ (2013) Energy and protein nutrition management of transition dairy cows. The Veterinary Clinics of North America. Food Animal Practice 29, 337–366.
| Energy and protein nutrition management of transition dairy cows.Crossref | GoogleScholarGoogle Scholar | 23809895PubMed |
Leclerc H, Block E (1989) Effects of reducing dietary cation-anion balance for prepartum dairy cows with specific reference to hypocalcemic parturient paresis. Canadian Journal of Animal Science 69, 411–423.
| Effects of reducing dietary cation-anion balance for prepartum dairy cows with specific reference to hypocalcemic parturient paresis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXltlejsrY%3D&md5=e2f648d5a119092198a6e964f7116282CAS |
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G (2007) Endocrine regulation of energy metabolism by the skeleton. Cell 130, 456–469.
| Endocrine regulation of energy metabolism by the skeleton.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXptlyntbs%3D&md5=c1bd62a5b77193f2e909e14bd8873291CAS | 17693256PubMed |
Liebler DC (1993) The role of metabolism in the antioxidant function of vitamin E. CRC Critical Reviews in Toxicology 23, 147–169.
| The role of metabolism in the antioxidant function of vitamin E.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXltVyht78%3D&md5=fc1f90ff9071def31820ca2f1cb02799CAS | 8329114PubMed |
Liesegang A, Sassi ML, Risteli J, Eicher R, Wanner M, Riond JL (1998) Comparison of bone resorption markers during hypocalcemia in dairy cows. Journal of Dairy Science 81, 2614–2622.
| Comparison of bone resorption markers during hypocalcemia in dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXntF2rtrY%3D&md5=e9f058e0a70b76350df166a4609aee6fCAS | 9812267PubMed |
Ludwig SKJ, Smits NGE, van der Veer G, Bremer MGEG, Nielen MWF (2012) Multiple protein biomarker assessment for recombinant bovine somatotropin (rbST) abuse in cattle. PLoS ONE 7, e52917
| Multiple protein biomarker assessment for recombinant bovine somatotropin (rbST) abuse in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnsVKnsg%3D%3D&md5=d717c7843beb7386e2d831cee1eb24b8CAS |
Martinez N, Risco CA, Lima FS, Bisinotto RS, Greco LF, Riberio ES, Maunsell F, Galvao KN, Santos JEP (2012) Evaluation of peripartal calcium status, energetic profile and neutrophil function in dairy cows at low or high risk of developing uterine disease. Journal of Dairy Science 95, 7158–7172.
| Evaluation of peripartal calcium status, energetic profile and neutrophil function in dairy cows at low or high risk of developing uterine disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs12gtb7P&md5=ac1808e8fe030d737db0d2a856496954CAS | 23021755PubMed |
Martinez N, Sinedino LDP, Bisinotto RS, Ribeiro ES, Gomes GS, Lima FS, Greco LF, Risco CA, Galvão KN, Taylor-Rodriguez D (2014) Effect of induced subclinical hypocalcemia on physiological responses and neutrophil function in dairy cows. Journal of Dairy Science 97, 874–887.
| Effect of induced subclinical hypocalcemia on physiological responses and neutrophil function in dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvFOhtL3L&md5=dd4a5ad7c28f8de9f88653fcf65700e7CAS | 24359833PubMed |
McKay B (1994) Subclinical hypocalcaemia: a possible effect on fertility. In ‘Proceedings of the 11th seminar of The Society of Dairy Cattle Veterinarians of the New Zealand Veterinary Association’. (Ed. W Webber) pp. 89–98. (Dairy Cattle Society of the New Zealand Veterinary Association: Queenstown, New Zealand)
McNeill DM, Anderson ST (2012) Bone as an endocrine organ and the mineral nutrition of the dairy cow. In ‘International conference on livestock production and veterinary technology’. (Eds E Wina, L Hardi Prasetyo, I Inounu, A Priyanti, A Angraeni, D Yulistiani, A Sinurat, P Situmorang, A Wardhana, NLP Indi Dharmayanti, N Ilham, P James, Z Asnan) pp. 220–225. (Indonesian Centre for Animal Research and Development (ICARD): Bogor, Indonesia)
McNeill DM, Roche JR, McLachlan BP, Stockdale CR (2002) Nutritional strategies for the prevention of hypocalcaemia at calving for dairy cows in pasture-based systems. Australian Journal of Agricultural Research 53, 755–770.
| Nutritional strategies for the prevention of hypocalcaemia at calving for dairy cows in pasture-based systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xmt1GmtL0%3D&md5=453039c1dd39e6362e6f88332a9705fcCAS |
Miller JK, Brzezinska-Slebodzinska E, Madsen FC (1993) Oxidative stress, antioxidants, and animal function. Journal of Dairy Science 76, 2812–2823.
| Oxidative stress, antioxidants, and animal function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXms1Olt7g%3D&md5=f75b60253ed0bb84395c3207ba209488CAS | 8227685PubMed |
Nagatani S, Guthikonda P, Thompson RC, Tsukamura H, Maeda KI, Foster DL (1998) Evidence for GnRH regulation by leptin: leptin administration prevents reduced pulsatile LH secretion during fasting. Neuroendocrinology 67, 370–376.
| Evidence for GnRH regulation by leptin: leptin administration prevents reduced pulsatile LH secretion during fasting.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXktVKmurY%3D&md5=6ae085902a2d2d3689a406aad001957cCAS | 9662716PubMed |
(a) National Research Council (2001) ‘Nutrient requirements of dairy cattle.’ 7th revised edn. (The National Academies Press: Washington, DC)
Oetzel GR, Fettemn MJ, Hamar DW (1991) Screening of anionic salts for palatability, effects on acid-base status, and urinary calcium excretion in dairy cows. Journal of Dairy Science 74, 965–971.
| Screening of anionic salts for palatability, effects on acid-base status, and urinary calcium excretion in dairy cows.Crossref | GoogleScholarGoogle Scholar |
Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, Hermo L, Suarez S, Roth BL, Ducy P (2011) Endocrine regulation of male fertility by the skeleton. Cell 144, 796–809.
| Endocrine regulation of male fertility by the skeleton.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsFeqt7k%3D&md5=07ea3d53913b0b6a3c5ee0f56fc68281CAS | 21333348PubMed |
Pedernera M, Celi P, Garcia SC, Salvin HE, Barchia I, Fulkerson WJ (2010) Effect of diet, energy balance and milk production on oxidative stress in early-lactating dairy cows grazing pasture. Veterinary Journal (London, England) 186, 352–357.
| Effect of diet, energy balance and milk production on oxidative stress in early-lactating dairy cows grazing pasture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFWqtrbN&md5=6ae39d44238b7f17280233a304cfc0b9CAS |
Peng JB, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA (1999) Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. The Journal of Biological Chemistry 274, 22 739–22 746.
| Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlt1Cnt7o%3D&md5=9bb9dc2ad5e58e97a9f43d851e828021CAS |
Phillippo M, Reid GW, Nevison IM (1994) Parturient hypocalcaemia in dairy cows: effects of dietary acidity on plasma mineral and calciotropic hormones. Research in Veterinary Science 56, 303–309.
| Parturient hypocalcaemia in dairy cows: effects of dietary acidity on plasma mineral and calciotropic hormones.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXmvF2ktb4%3D&md5=7476dcd66af357c51c67082817beff27CAS | 8073181PubMed |
Popa SM, Clifton DK, Steiner RA (2008) The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Annual Review of Physiology 70, 213–238.
| The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXkt1eqtro%3D&md5=978b76ed5a0d37bd15d6f3e726422cbfCAS | 17988212PubMed |
Putnam DE, Varga GA, Dann HM (1999) Metabolic and production responses to dietary protein and exogenous somatotropin in late gestation dairy cows. Journal of Dairy Science 82, 982–995.
| Metabolic and production responses to dietary protein and exogenous somatotropin in late gestation dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjt1yksL0%3D&md5=d01e6715db3e19138a5c47b1c6714704CAS | 10342237PubMed |
Sato R, Onda K, Ochiai H, Iriki T, Yamazaki Y, Wada Y (2011) Serum osteocalcin in dairy cows: age-related changes and periparturient variation. Research in Veterinary Science 91, 196–198.
| Serum osteocalcin in dairy cows: age-related changes and periparturient variation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFShtLjE&md5=486dafe2709fc3a5dbb0d1b0b25c85daCAS | 21300389PubMed |
Schröder B, Goebel W, Huber K, Breves G (2001) No effect of vitamin D3 treatment on active calcium absorption across ruminal epithelium of sheep. Journal Veterinary Medicine Series A 48, 353–363.
| No effect of vitamin D3 treatment on active calcium absorption across ruminal epithelium of sheep.Crossref | GoogleScholarGoogle Scholar |
Shwartz G, Hill KL, VanBaale MJ, Baumgard LH (2009) Effects of flunixin meglumine on pyrexia and bioenergetic variables in postparturient dairy cows. Journal of Dairy Science 92, 1963–1970.
| Effects of flunixin meglumine on pyrexia and bioenergetic variables in postparturient dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlsFynu7o%3D&md5=17beea19dd49278a21d90249395d4100CAS | 19389953PubMed |
Singh SP, Haussler S, Gross JJ, Schwarz FJ, Bruckmaier RM, Sauerwein H (2014) Short communication: Circulating and milk adiponectin change differently during energy deficiency at different stages of lactation in dairy cows. Journal of Dairy Science 97, 1535–1542.
| Short communication: Circulating and milk adiponectin change differently during energy deficiency at different stages of lactation in dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsV2jt7g%3D&md5=cc7c25199ab28db789ae11a9a24129d7CAS | 24472130PubMed |
Sordillo LM, Raphael W (2013) Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. The Veterinary Clinics of North America. Food Animal Practice 29, 267–278.
| Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders.Crossref | GoogleScholarGoogle Scholar | 23809891PubMed |
Stevenson MA, Williamson NB, Hanlon DW (1999) The effects of calcium supplementation of dairy cattle after calving on milk, milk fat and protein production, and fertility. New Zealand Veterinary Journal 47, 53–60.
| The effects of calcium supplementation of dairy cattle after calving on milk, milk fat and protein production, and fertility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjs1ansrw%3D&md5=cb3e3c8e5634f7e0718306cedcefe987CAS | 16032071PubMed |
Takeda S, Elefteriou F, Levasseur R, Liu X, Shao L, Parker KL, Armstrong D, Ducy P, Karsenty G (2002) Leptin regulates bone formation via the sympathetic nervous system. Cell 111, 305–317.
| Leptin regulates bone formation via the sympathetic nervous system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XovVakurk%3D&md5=d85dae7bce30f9373db6d598e002a638CAS | 12419242PubMed |
Taylor MS, Knowlton KF, McGilliard ML, Seymour WM, Herbein JH (2008) Blood mineral, hormone, and osteocalcin responses of multiparous Jersey cows to an oral dose of 25-hydroxyvitamin D3 or Vitamin D3 before parturition. Journal of Dairy Science 91, 2408–2416.
| Blood mineral, hormone, and osteocalcin responses of multiparous Jersey cows to an oral dose of 25-hydroxyvitamin D3 or Vitamin D3 before parturition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmsVWmtbs%3D&md5=59892320cdb8470bd6ba52d1c24a2c7cCAS | 18487663PubMed |
Tomas FM, Potter BJ (1976) The site of magnesium absorption from the ruminant stomach. The British Journal of Nutrition 36, 37–45.
| The site of magnesium absorption from the ruminant stomach.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28XkvFSqt7s%3D&md5=a9bf7fc0b72988e7a01ea2e5c121fb93CAS | 949467PubMed |
Wadhwa DR, Care AD (2000) Effects of strontium on the absorption of calcium, magnesium and phosphate ions from the ovine reticulo-rumen. Journal of Comparative Physiology. B, Biochemical, Systemic, and Environmental Physiology 170, 225–229.
| Effects of strontium on the absorption of calcium, magnesium and phosphate ions from the ovine reticulo-rumen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXkt1Gju7Y%3D&md5=a9e870f31c644ebbe7467f14010df6b5CAS | 10841263PubMed |
Wallace JD, Cuneo RC, Lundberg PA, Rosén T, Jørgensen JOL, Longobardi S, Keay N, Sacca L, Christiansen JS, Bengtsson B-Å, Sönksen PH (2000) Responses of markers of bone and collagen turnover to exercise, growth hormone (GH) administration, and GH withdrawal in trained adult males. The Journal of Clinical Endocrinology and Metabolism 85, 124–133.
Wang C, Beede DK, Donovan GA, Archbald LF, DeLorenzo MA, Sanchez WK (1991) Effects of dietary negative cation-anion difference and high calcium content prepartum on calcium metabolism, health. Lactational and reproductive performance of Holstein cows. Journal of Dairy Science 74, 275
Westwood CT, Lean IJ, Garvin JK (2002) Factors influencing fertility of Holstein dairy cows: a multivariate description. Journal of Dairy Science 85, 3225–3237.
| Factors influencing fertility of Holstein dairy cows: a multivariate description.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXht1aruw%3D%3D&md5=df07d8123824ebd07a9c5fa2fa6ee579CAS | 12512596PubMed |
Wilkens MR, Kunert-Keil C, Brinkmeier H, Schröder B (2009) Expression of calcium channel TRPV6 in ovine epithelial tissue. Veterinary Journal (London, England) 182, 294–300.
| Expression of calcium channel TRPV6 in ovine epithelial tissue.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFWqur%2FE&md5=4ea5048134011ef4e788fddf8fcf5218CAS |
Wilkens MR, Mrochen N, Breves G, Schröder B (2011) Gastrointestinal calcium absorption in sheep is mostly insensitive to an alimentary induced challenge of calcium homeostasis. Comparative Biochemistry and Physiology. B, Comparative Biochemistry 158, 199–207.
| Gastrointestinal calcium absorption in sheep is mostly insensitive to an alimentary induced challenge of calcium homeostasis.Crossref | GoogleScholarGoogle Scholar |
Williams GL, Amstalden M, Garcia MR, Stanko RL, Nizielski SE, Morrison CD, Keisler DH (2002) Leptin and its role in the central regulation of reproduction in cattle. Domestic Animal Endocrinology 23, 339–349.
| Leptin and its role in the central regulation of reproduction in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xls1Wkurw%3D&md5=a1af4d93e1638fcaba6204e16aedbba3CAS | 12142250PubMed |
Wolf G (2008) Energy regulation by the skeleton. Nutrition Reviews 66, 229–233.
| Energy regulation by the skeleton.Crossref | GoogleScholarGoogle Scholar | 18366536PubMed |
Wolfenson D, Flamenbaum I, Berman A (1988) Dry period heat stress relief effects on prepartum progesterone, calf birth weight, and milk production. Journal of Dairy Science 71, 809–818.
| Dry period heat stress relief effects on prepartum progesterone, calf birth weight, and milk production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXhvVCgsb0%3D&md5=88c5575c4064c060a457d754ab7108a6CAS | 3372821PubMed |
Yamauchi T, Kamon J, Minokoshi YA, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K (2002) Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nature Medicine 8, 1288–1295.
| Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XotlWlsrg%3D&md5=dc51ef61858d8e99b992496786b1e22eCAS | 12368907PubMed |