Ergovaline, an endophytic alkaloid. 1. Animal physiology and metabolism
J. L. Klotz A C and A. M. Nicol BA USDA–ARS, Forage–Animal Production Research Unit, Lexington, KY 40546, USA.
B AMN Consulting, 1248 Old West Coast Road, RD 1, Christchurch 7671, New Zealand.
C Corresponding author. Email: james.klotz@ars.usda.gov
Animal Production Science 56(11) 1761-1774 https://doi.org/10.1071/AN14962
Submitted: 26 November 2014 Accepted: 28 June 2016 Published: 19 August 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Ergovaline is an ergot alkaloid found in some endophyte-infected ryegrasses and it has been implicated in the expression of ergotism-like symptoms of grazing livestock, as well as in the protection of the plant against invertebrate predation and abiotic stresses. These selection pressures have resulted in a conflict between the needs of the pasture for persistence and the needs of the animal for production. Ergovaline has not been well studied in terms of animal physiology until recently. There are several putative mechanisms that limit the bioavailability of ergovaline, ranging from microbial biotransformation to post-absorptive hepatic detoxification. Although there are mechanisms that protect the animal from ergovaline exposure, tissues are very sensitive to ergovaline, indicating that ergovaline is very potent and that small quantities have the potential to cause noticeable physiological effects. The range of physiological effects, including decreased circulating prolactin, vasoconstriction and increased susceptibility to heat stress are all linked to the interaction of ergovaline with biogenic amine receptors found throughout the body. This review will focus on understanding the variation of ergovaline concentration in terms of bioavailability, the myriad of hurdles a molecule of ergovaline must overcome to cause an effect, what the ergovaline-induced effects are in New Zealand livestock and how this relates to the potency of ergovaline.
Additional keywords: ergot alkaloids, livestock, perennial ryegrass, tall fescue.
Introduction
As food demands for expanding global populations continue to rise and there is an increased emphasis by the public for a safe and secure sources of sustainable meat and milk products, the role of pasture-based animal production in agriculture is increasing in importance. As grazing animal systems increase in importance, so to does the impact of mycotoxins on food production (Bryden 2012). One tool that has evolved with a high degree of success in New Zealand is the manipulation of fungal endophytes to minimise the impact of insect herbivory and abiotic stresses and, thus, extend the productive life of pastures. This is accomplished through the fungal production of an array of alkaloids that promote plant persistence. The main alkaloids produced by the wild-type endophyte (Neotyphodium lolii) associated with perennial ryegrass (Lolium perenne) that can affect livestock are lolitrem B (an indole diterpene), peramine (a pyrrolopyrazine alkaloid), paxilline (an indole diterpene) and ergovaline (an ergopeptine ergot alkaloid).
Ergovaline is an ergot alkaloid that is at the centre of fescue toxicosis syndrome in the United States. It is also produced by an endophyte (Neotyphodium coenophialum) in tall fescue (Lolium arundinaceum) commonly found in the south-eastern USA. The negative impacts of ergovaline are not as evident in New Zealand. This is due to the different host plant, the presence of other alkaloids, and a different climate compared with the United States. Lolitrem B, paxilline, and epoxy-janthitrems are associated with the neuromuscular disorder; ryegrass staggers (Fletcher and Harvey 1981; Tapper and Lane 2004). Livestock are not affected by peramine (Popay and Latch 1993), which is a deterrent of the Argentine stem weevil (Rowan et al. 1990).
Removal of indole diterpinoid alkaloids (lolitrem B, paxilline and epoxy-janthitrems) results in the cessation of ryegrass staggers, but early commercial grass–endophyte releases (e.g. Endosafe) successful in alleviating ryegrass staggers produced signs similar to fescue toxicosis (heat stress, lameness, decrease in live-weight gain). Ergovaline is produced by the plant endophyte to discourage herbivory, and had not been considered in the initial plant breeding selection processes (Fletcher 2012). Due to the higher concentrations of lolitrem B than ergovaline in wild type-infected ryegrass, animals would always develop staggers, and this, presumably, concealed or reduced the intake of ergovaline (Tor-Agbidye et al. 2001). Situations in which livestock consume ergovaline in ryegrass, at concentrations that exceeds a threshold, may impair animal performance and lead to the development of fescue toxicosis-like symptoms. As a result of this, ergovaline was added to the list of alkaloids evaluated when considering new ryegrass–endophyte associations (Fletcher 2012).
Because of a widespread adoption of novel endophyte use in ryegrass pastures, New Zealand agriculture has had a seemingly dichotomous relationship with ergovaline. On one hand, consumption of ergovaline in sufficient quantities by livestock can result in a decrease in animal performance that results from the negative effects of ergovaline. On the other hand, pastures containing ergovaline-free symbiota show increased susceptibility to pests such as the black beetle (Ball et al. 1997) and root aphid (Popay and Gerard 2007), which translates to reduced production and persistence of the ryegrass stand. Thus, there are incentives to identify an adequate level of ergovaline in perennial ryegrass to confer resistance to invertebrate pests, but yet have a negligible effect on the performance of livestock. The challenge is in understanding the levels produced by the fungus, external factors that manipulate the concentration of ergovaline in the grass, and the internal factors that affect the intake, release, breakdown, absorption and elimination of ergovaline in the herbivore. The purpose of the present review is to examine the complex animal–plant interaction and discuss the impact and role that ergovaline has on animal physiology and production in terms of New Zealand pastoral agriculture.
Ergovaline fundamentals
What is ergovaline?
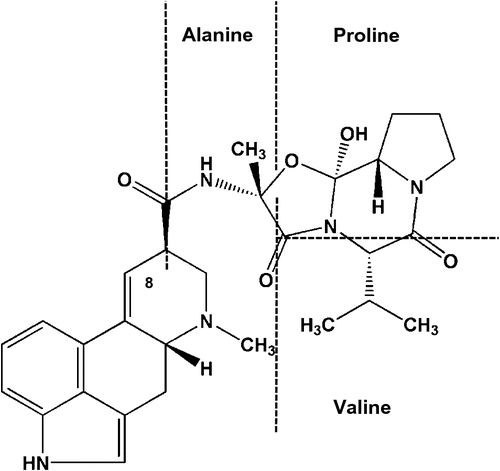
Ergovaline is a secondary metabolite that is considered a hallmark of a fungal endophyte as it is produced only in small quantities by external fungi such as Claviceps purpurea (e.g. 4% of total alkaloid content from a Claviceps sp. source (Brunner et al. 1979) compared with 84–97% of total alkaloid content from a Neotyphodium coenophialum source (Lyons et al. 1986)). Ergovaline is part of a group of ergot alkaloids referred to as ergopeptide alkaloids. This particular class of ergot alkaloids is identifiable by the tricyclic peptide moiety attached to the 8-carbon of the tetracyclic ergoline ring or lysergic acid structure (Fig. 1). The structural similarity of this ergoline-ring component to the structures of biogenic amines (e.g. serotonin, norepinephrine and dopamine) allows ergopeptide alkaloids to interact with an array of different receptors (Berde 1980). It is thought that the variations in the structure of the peptide associated with the ergoline ring is what affects the potency and efficacy of the ergopeptide alkaloid (Klotz et al. 2010). In the case of ergovaline, this consists of alanine, proline and valine. In solution and during isolation, ergovaline can undergo a spontaneous epimerisation at the 8-carbon on the ergoline ring to yield a pharmacologically inactive form of ergovalinine (Smith and Shappell 2002). This formation of the -inine isomer is an equilibrium-driven process, but biological processes and compartments, such as crossing a cell barrier, can influence it. This was demonstrated by Mulac et al. (2012) with a primary porcine brain endothelial-cell transport system and ergocristine and ergocristinine. Only ergocristine crossed the cell barrier, whereas biologically inactive ergocristinine accumulated within the cells. It is unclear whether this happens in the rumen environment and is an aspect that needs additional study, as it could be exploited to reduce animal uptake of ergot alkaloids, such as ergovaline.
|
Total in vitro synthesis of ergovaline was reported by Stadler et al. (1964). Synthesis of ergovaline starts with a molecule of tryptophan that is converted to various clavine and lysergic acid derivatives before the ergopeptide ergovaline (Garner et al. 1993; Bush and Fannin 2009). Complete descriptions of the biosynthesis of ergovaline by the Neotyphodium or Eplichloë spp. such as N. lolii are described in detail elsewhere (Schardl et al. 2013; Young et al. 2013). While ergovaline synthesis by the endophytes in tall fescue (Lyons et al. 1986) and perennial ryegrass (Rowan and Shaw 1987) seem to predominate other ergopeptides, it is not the only ergopeptide produced. In addition to being the first to detect ergovaline in endophyte-infected ryegrass, Rowan and Shaw (1987) reported that ergovaline made up 40% of the ergot alkaloids detected with ergotamine, ergosine, ergoptine, ergocryptine and ergocornine, making up the majority of the remaining percentages. These percentages will likely vary in the make-up of the total alkaloid content present, depending on cultivar, environment and time of year interactions. These other alkaloids are likely to contribute to the variable responses reported in the New Zealand literature, as ergovaline is the only ergot alkaloid that has been consistently evaluated and reported. Additionally, the percentage of ergovaline reported by Rowan and Shaw (1987) was substantially lower than what has been reported for tall fescue (Yates et al. 1985; Lyons et al. 1986) and this may be one explanation for the differences between the USA reports and those from New Zealand concerning ergovaline.
The interface of ergovaline and herbivores
One of the major challenges when trying to interpret data across ergovaline-related experiments and across laboratories is the variability in the concentration of ergovaline in ryegrass used as a treatment (this topic is described in greater detail in the accompanying paper, where Nicol and Klotz (2016) review the intake of ergovaline). Aside from the obvious effect of the different endophyte biotypes, these concentrations can be affected by the host–plant genotype (Easton et al. 2002), the reproductive status of the plant (Lane et al. 1997) and tissue position and age (Spiering et al. 2005). This has resulted in a diverse array of outcomes and conclusions, all pertaining to the overlapping aspect of ergovaline exposure in livestock. In addition to the aforementioned internal variation of ergovaline concentration within ryegrass (which can be affected by the type of sampling), there are also external factors that affect production of ergovaline in the plant (e.g. seasonal variation and extreme environmental effects). Furthermore, individual research objectives (e.g. interface between animal and pasture may be of interest compared with a basic understanding of biological mechanisms of ergovaline metabolism) may drive researchers to use different vehicles (e.g. grazing compared with seed dosing) for delivering ergovaline in research studies. Also, there is no ‘standard’ ergovaline concentration in use in animal studies, due, in large part, to the challenges of defining an animal threshold for ergovaline. The interpretation of the broad range of ergovaline concentrations and correlation with results and impacts on animal performance is tenuous and will be discussed in detail by Nicol and Klotz (2016). Consequently, the subsequent sections will describe what is currently known about ergovaline once consumed by the animal and possible post-prandial outcomes.
Ergovaline delivery
Frequently, research concerned with ergovaline reports the concentration or amount offered to the experimental animal (mg ergovaline/kg DM) and, less often, how much ergovaline was administered to, or consumed by, the experimental animals (mg ergovaline/kg liveweight (LW)0.75). Furthermore, the proportion of ergovaline freed from the plant tissue and absorbed also determines the true physiological dose. A further complication is that ergovaline has been administered in a variety of forms from grazing (Bluett et al. 2005; Aiken et al. 2011b; Thom et al. 2013), feeding whole seed (Gadberry et al. 2003; Aiken et al. 2009), ground seed (Koontz et al. 2012; Koontz et al. 2013), or administration of a purified form of ergovaline (Koontz et al. 2012; Foote et al. 2013). McDowell et al. (2013) evaluated whether using ground seed compared with whole seed had an effect on ergovaline-induced vasoconstriction in horses and reported that horses fed ground seed had greater reductions in artery lumen diameter, circumference and area than did horses fed whole seed. As the delivery of ergovaline becomes more refined (e.g. ground seed or seed extracts), the physiological dose can be more precisely controlled and the effects more defined, but the obvious compromise is the departure from what the grazing animal will actually encounter.
Siegel et al. (1984) investigated whether cattle can disseminate endophyte-infected tall fescue seeds and reported numerous whole seeds recovered in faeces. They also reported that these seeds germinated at 50% of the non-fed control-seed germination. Although, ergovaline concentrations were not reported by Siegel et al. (1984), it is plausible that an animal that has excreted an intact seed capable of germination has also excreted the ergovaline associated with that seed, effectively reducing the actual amount of ergovaline exposure when compared with what was consumed. While it is probable for some seeds to escape digestion in traditional settings, Siegel et al. (1984) dosed a steer through a ruminal cannula with 5.5 kg of seeds over 3 days. The odds of this occurring naturally are not likely and this volume of seeds is likely to have overwhelmed the normal processes of the digestive tract, but still serves as an example that the likelihood of the animal absorbing 100% of the ergovaline fed or dosed is low. In terms of New Zealand agriculture, it is not likely that grazing dairy cattle will consume high ergovaline-containing ryegrass seed heads, but grazing sheep and beef on dryland farms may have opportunities to graze seed heads.
Bioavailability
Literature on the release of ergovaline and the potential for biotransformation by rumen microbial communities before absorption suggests that the majority of ergovaline fed to ruminants is at least freed from the plant tissue before excretion. There are no data describing the bioavailability of ergovaline from ryegrass leaf or seed head. In vitro fermentation studies with tall fescue have demonstrated that feeding rats a whole seed that was previously incubated with rumen fluid is less toxic than feeding a seed not incubated, which suggests that rumen microflora reduced the concentration of toxic compounds (Westendorf et al. 1992). Using in vitro fermentation of rumen fluid containing added pyrrolizidine alkaloids, Westendorf et al. (1993) reported that pyrrolizidine alkaloids were degraded by rumen fermentation. Also using in vitro fermentation, Moyer et al. (1993) demonstrated a linear decline in ergovaline in the soluble fraction, but an apparent increase in ergovaline associated with the insoluble fraction over a 48-h incubation. This demonstrated that, at least in the liquid phase, there is microbial degradation of ergovaline or metabolism and conversion to another compound. The maturity level of the seed head will also affect bioavailability. In a study on the extent that alkaloids are released from developing tall fescue seedheads (beef cattle have been shown to preferentially graze emerging seed heads), Goff et al. (2012) reported that 100% of ergovaline was released from an immature seed but this dropped to 95.9% from a mature seed (undigested seed-head concentration compared with digested residue). In that study (Goff et al. 2012), the rates of ergovaline released from vegetative tissues would closely approximate reported dosage rates. In another in vitro rumen-fermentation study, only 60% of total ergot alkaloids were released (Stuedemann et al. 1998) and an in vivo study with sheep reported a 64% (De Lorme et al. 2007) apparent release of ergovaline from digested plant tissues. Differences between the in vitro studies of Goff et al. (2012) and Stuedemann et al. (1998) may related to differences in in vitro digestion methodology. The difference between the in vivo study of De Lorme et al. (2007) and the study reported by Goff et al. (2012) may be attributed to differences and particle sizes between in vivo and in vitro, and the smaller size achieved when grinding substrate for in vitro fermentation than with mastication and comminution by the animal (De Lorme et al. (2007) fed a mixture of endophyte-infected straw (0.35 mg/kg DM ergovaline) and ground seed (3.30 mg/kg DM) formulated to achieve 0.610 mg/kg DM ergovaline in the diet) that lead to a greater release of ergovaline during digestion. This further indicates that ergovaline release from an intact seed may not be as complete as that from a ground seed.
Another variable to be considered once the alkaloid is freed from the feedstuff is the potential for biotransformation or conversion of ergovaline to another metabolite such as lysergic acid before absorption. Hill et al. (2003) demonstrated a decrease in ergovaline from 9 μg/kg to 1 μg/kg in rumen fluid after 6 h, and an increase in lysergic acid from 20 μg/kg to 240 μg/kg in 48 h.
A series of in vivo alkaloid-balance studies all came to similar conclusions regarding the conversion of ergovaline to lysergic acid in geldings (Schultz et al. 2006), wethers (De Lorme et al. 2007) and steers (Merrill et al. 2007). Specifically, these studies all reported intake concentrations of ergovaline and lysergic acid and concentrations of these compounds in the urine and faeces. In all three studies, there was less ergovaline and more lysergic acid excreted than consumed. Reported recoveries of ergovaline were 58.3%, 35.4% and 55% for Schultz et al. (2006), De Lorme et al. (2007) and Merrill et al. (2007) respectively, whereas recoveries for lysergic acid in all three studies were >200% of intake. All ergovaline recovered was in the faeces, as Stuedemann et al. (1998) reported that excretion of ergovaline occurs via the biliary system, whereas the majority of lysergic acid was recovered in urine. Gooneratne et al. (2011) reported a similar phenomenon in ewe lambs fed ryegrass seed containing ergovaline, where excretion of lysergol in the urine increased with an increasing exposure to ergovaline.
While these ergot alkaloid-balance studies all suggest that there is at least some conversion of ergovaline to lysergic acid, they do not differentiate between biotransformation by the gut microbes and detoxification by the liver occurring after ergovaline has been absorbed. However, the ruminal conversion of ergovaline to lysergic acid before absorption clearly affects how much ergovaline is available to the animal. The area of biotransformation needs additional research for not just endophyte-infected ryegrass, but for all ergot alkaloid-containing feedstuffs. Determining what type of ruminal environment (e.g. pH) and population of microbes maximises the biotransformation of ergovaline is another possible avenue to further reduce ergovaline uptake by ruminant livestock.
Ergovaline absorption
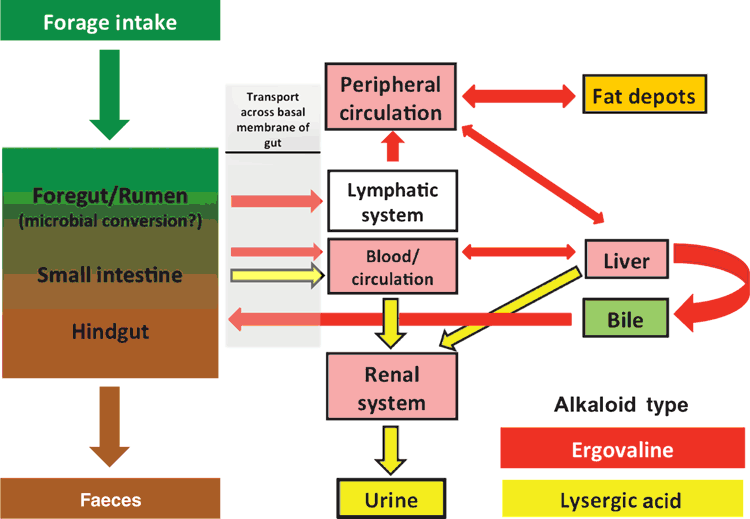
Potential paths of ergovaline once consumed by a ruminant are outlined in Fig. 2. Because of an absence of a commercial source of ergovaline and a lack of necessary analytical methods, there are limited data that provide direct evidence that ergovaline is absorbed intact and there are currently no in vivo data that confirm this occurrence. Looking at total ergot alkaloids, Westendorf et al. (1993) reported that 50–60% of fed ergot alkaloids (tall fescue-derived) were recovered in the abomasal contents, indicating a significant reduction of alkaloids in the foregut. Westendorf et al. (1993) went on to report only a 6–7% recovery of fed ergot alkaloids in faecal excretion. This led to two major conclusions, namely that (1) there was a significant alkaloid reduction in the foregut and (2) the majority of the remainder appeared to be absorbed or metabolised in the intestines. Although Westendorf et al. (1993), did not measure ergovaline directly, their study was unique because it looked at the ergot alkaloid concentrations leaving the foregut and entering the mid-gut (at the abomasum). Subsequent research has focussed more directly on the role that the foregut plays in ergovaline absorption. Using ruminal, reticular and omasal epithelia mounted in parabiotic chambers, Hill et al. (2001) evaluated the transport of ergoline (e.g. lysergic acid) and ergopeptine alkaloids (e.g. ergovaline). Although Hill et al. (2001) did not evaluate ergovaline directly, they reported that the major alkaloid to cross the tissue barrier was lysergic acid and that ergopeptine absorbance was negligible when compared with the ergoline alkaloids. In a review of ergot alkaloid absorption in ruminants, Hill (2004) concluded that the rumen is the primary site of ergot-alkaloid absorption. By corollary, the toxin responsible for fescue toxicosis in the USA may not be ergovaline, but rather the ergoline alkaloids, given their significantly greater absorption rates (Hill et al. 2001). These conclusions were contradicted by a report by Shappell and Smith (2005), who reported a flux rate of ergovaline (22 μM) across a Caco-2 cell (human intestinal cell) monolayer of 7.5 ng/min.cm. Although Hill et al. (2001) did not evaluate ergovaline directly, they did evaluate ergotamine, which differs from ergovaline by one amino acid in the tricyclic peptide moiety (the valine residue in ergovaline (Fig. 1) is replaced with phenylalanine to create ergotamine). The flux of ergovaline was comparable to the calculated flux of 7.0 ng/min.cm for ergotamine (30.5 μM) across sheep omasum reported by Hill et al. (2001). Thus, if ergovaline is not absorbed from the rumen, then the epithelial cells lining the intestine are capable of transporting ergovaline intact to the basal compartment and, conceivably, into the blood stream.
More recent data have directly evaluated the transport of ergovaline and indicated that ergovaline is capable of crossing the rumen epithelium intact. Foote et al. (2013) conducted a washed-rumen experiment in Holstein steers that demonstrated a marked reduction of volatile fatty acid (VFA) flux across the rumen epithelium in steers dosed with 0.015 mg ergovaline/kg LW. Concerns were raised that this decrease in VFA crossing the epithelium could be independent of blood flow and possibly due to an alteration of the barrier function. Using Ussing chamber technology, Foote et al. (2014) evaluated ergovaline flux, barrier function, and total, facilitated and passive VFA flux across rumen epithelia that were exposed to either 50 or 250 ng of ergovaline/mL. This acute exposure of rumen epithelium did not affect total, facilitated or passive butyrate flux and ergovaline did not alter barrier function. Foote et al. (2014) did report ergovaline in the serosal buffer in the 250 ng of ergovaline/mL treatment and estimated an ergovaline flux of 0.25–0.44 ng/cm2.h. Currently, these findings are the most definitive description of ergovaline transport across the rumen epithelium and the implications are that ergovaline is absorbed by the rumen epithelium, although at a slow rate, approximating only 1% of the daily dose administered.
If all of the aforementioned results regarding ergovaline, namely, rumen availability, biotransformation to lysergic acid by rumen microbes, and low flux rate across the rumen epithelium, are true, how is ergovaline absorbed in sufficient quantities to cause an effect on the animal at all? These findings suggest that the small intestine may play an important role in ergovaline absorption. Ergot-alkaloid absorption has been studied in the intestines of monogastrics (reviewed by Eckert et al. 1978) and these studies have been conducted predominantly with radiolabelled ergotamine (Meszaros et al. 1975). Toxin-absorption models for ergovaline in the small intestine of ruminants are non-existent. Intact ergovaline reaching the small intestine of a ruminant would have at least three potential routes (Fig. 2). The first route is continuing on unabsorbed, with possible metabolism by hindgut microbes and eventual excretion via the faeces. The second route is absorption and vascular transport via the mesenteric veins to the liver through the portal circulation, for likely hepatic detoxification (Zanzalari et al. 1989), most likely by cytochrome P450 (Moubarak and Rosenkrans 2000), or excretion via the biliary system back into the gut (Stuedemann et al. 1998). A third route is an alternate path of absorption. Ergovaline could be absorbed in the small intestine and transported via the lymphatic system, through the thoracic duct and enter venous circulation at the subclavian vein. This last route would bypass a first-pass detoxification of ergovaline by the liver and, instead, direct the absorbed ergovaline to the heart for systemic distribution via arterial blood supply before detoxification by the liver. Franz and Vonderscher (1981) reported that intestinal absorption of ergopeptine alkaloids in the rat was enhanced when the alkaloids were administered in micellar solutions. As lipids are commonly absorbed from the intestinal lumen in the form of micelles through a lacteal leading to the lymphatic system and given the lipophilic nature of ergovaline and the other ergopeptine alkaloids, the lymphatic route of intestinal absorption is likely in ruminants as well.
There is little available literature addressing the fate of an intact molecule of ergovaline that makes it into systemic circulation, and, to the knowledge of the authors, there is none in cattle. Jaussaud et al. (1998); Durix et al. (1999) and Bony et al. (2001) looked at direct infusion of purified ergovaline in sheep, milk goats and geldings respectively. Jaussaud et al. (1998) administered 17 μg ergovaline/kg LW and looked at plasma ergovaline concentrations. Durix et al. (1999) admininstered 32 μg ergovaline/kg LW and looked at plasma and milk concentrations. Bony et al. (2001) administered 15 μg ergovaline/kg BW in four geldings. In all three species, the plasma half-life of ergovaline was very short (~24 min in sheep, ~32 min in goats and ~56 min in the horse; Jaussaud et al. 1998; Durix et al. 1999; Bony et al. 2001). In the lactating goat, ergovaline was detected in milk, but not beyond 8 h post-dosing (Durix et al. 1999). The short plasma half-life of ergovaline in these reports would suggest that there is also a short biological half-life. In the case of ergovaline and other ergopeptine alkaloids, this could also be a consequence of rapid tissue accumulation (from the circulation) and not necessarily equate to excretion of ergovaline. This rapid tissue distribution could result in an extended biological half-life (or bioaccumulation) of ergovaline that could manifest as vasoconstriction or as an extended recovery period after removal of livestock from the source of ergovaline.
Bioaccumulation and excretion of ergovaline
What happens to ergovaline once it leaves circulation, but is not excreted, is an area not fully understood. Dyer (1993), Schöning et al. (2001), Klotz et al. (2007) and Pesqueira et al. (2014) all demonstrated a very strong ligand–receptor complex for ergovaline in different vascular bioassays. This lengthy receptor occupation by ergovaline, and the like, has led to a hypothesis that ergopeptides such as ergovaline that manage to be liberated from a feedstuff, evade biotransformation by the gut microbes, succeed in traversing the epithelial basal membrane barrier of the gut and avoid hepatic detoxification or reintroduction into the gut via the biliary system can enter the peripheral tissues of the body where the ergovaline molecule can interact with any number of receptors, and because of the extended receptor binding, there is an accumulation. Klotz et al. (2009) demonstrated that bovine lateral saphenous veins directly exposed to 1 × 10−7 M (0.053 mg/kg) ergovaline for a total of eight consecutive times resulted in corresponding increases in contractile response as well as the amount of ergovaline that was extracted from the same vessels. This suggested that, with each exposure, more ergovaline was interacting with receptors on the blood vessel and increasing the cumulative contractile response observed.
Mulac and Humpf (2011) demonstrated an accumulation of ergopeptide alkaloids, in particular ergocristine (a primary alkaloid produced by Claviceps purpurea, the source of ergotism in cereal grains) in primary cultures of cells. The authors speculated that this could result in the formation of a ‘toxin-depot’ within cells that could cause a prolonged toxin release from the body. Schumann et al. (2007, 2009) attempted to quantify C. purpurea-produced ergot alkaloids in animal tissues that are consumed by humans (muscle, fat, and milk), but neither study could demonstrate ergot alkaloid carry-over into edible tissues. Realini et al. (2005) reported higher concentrations of ergot alkaloids in subcutaneous fat collected from cattle that grazed wild type-infected tall fescue than the concentrations in subcutaneous fat collected from cattle that grazed AR542-infected tall fescue. However, these data reported only total ergot alkaloid (that contained the ergoline ring structure; Fig. 1). There is no published evidence that demonstrates an accumulation of ergovaline in edible animal tissues in the tall fescue or perennial ryegrass literature. Some studies have clearly demonstrated the presence of lolitrem B and epoxy-janthitrems in lambs (Finch et al. 2012) and cattle (Miyazaki et al. 2004) adipose tissue. The report by EFSA (2012) suggested a tolerable daily intake of ergot alkaloids at 0.60 μg/kg BW.day. The maximum recommended oral therapeutic ergotamine dose for treatment of migraines in adults is 8 μg/kg LW.day over 30 days, so as to avoid peripheral vasoconstriction (EFSA 2012). Schumann et al. (2007) fed a high dose of ergot alkaloids to bulls at 8.56 μg/kg LW.day and reported no accumulation in evaluated tissue samples. Further research that evaluates animal tissues for the presence of ergovaline following grazing exposure that titrates active concentrations of ergovaline and utilises more sensitive analytical technologies (e.g. liquid chromatography coupled to mass spectrometry) is warranted.
The hypothesis that ergovaline can bioaccumulate in animal tissues has complicated the understanding of how ergovaline is excreted and has led to speculation about how animals will recover following an exposure to ergovaline. If ergovaline is still being released as tissues turn over, then this will extend the time it takes for an animal to recover (as ergovaline is re-released) and clear ergovaline from their system. Alkaloid concentrations in ruminal fluid have been reported to decrease within 24 h of removal from endophyte-infected tall fescue pasture, and decrease to zero within 3 days after removal (Ayers et al. 2009). Aiken et al. (2011b) detected alkaloids (lysergyl) in urine collected from lambs still 4 days after switching them from an AR6 to an endophyte-free perennial ryegrass pasture, suggesting that the alkaloids are coming from a source other than rumen contents. Using cattle that were exposed to ergovaline through grazing tall fescue, Aiken et al. (2013) evaluated the recovery of steers following removal from a tall fescue pasture. When comparing the ergovaline-exposed steers to controls, serum prolactin concentrations were similar by 10–15 days after removal from treatment, rectal temperatures were similar between 15 and 30 days after removal from treatment, and the luminal area of the caudal artery never returned to control values during the experimental periods (30 days for Year 1 and 21 days for Year 2). This outlines both the manner in which animals recover from an exposure to ergovaline (indicating that one measure alone may not be fully indicative of the actual recovery or onset of toxicosis) and how long in can take for animals to fully recover. Bussard (2012) demonstrated in a similar experiment that evaluated vasoconstriction in cattle after removal from an ergovaline-containing pasture that vascular recovery can take up to 5–6 weeks. Thus, when conducting research evaluating ergot alkaloids such as ergovaline, it is important that the researchers consider the dietary history of all experimental animals.
Consumption of ergovaline-containing feeds such as ryegrass silage (1.6 µg/g) or tall fescue hay (up to 0.782 µg/g) has been associated with decreased milk production (Lean 2001) and altered milk composition in dairy cows (Kim et al. 2007) respectively; however, do lactating animals have an additional route of ergovaline excretion (that may alter amounts that bioaccumulate)? Durix et al. (1999) administered ergovaline intravenously to milk goats. Ergovaline was initially detected at a concentration of 0.71 ng/mL in milk, but was undetectable 8 h following the ergovaline infusion. These authors concluded that milk is a minor route of excretion for intact ergovaline. In a separate study, ergovaline-containing perennial ryegrass hay (0.851 µg/g) was fed to lactating ewes for 28 days and ergovaline was detected in milk collected from some (but not all) of the exposed ewes, but all detectable concentrations were below the concentrations of quantitation (Zbib et al. 2015). Schumann et al. (2009) fed cows an ergot alkaloid-contaminated diet and did not detect any ergot alkaloids in the subsequent milk samples evaluated. Although milk does not appear to be a major excretory route for ergovaline, in the rare instances where animals encounter intake concentrations high enough for ergovaline to reach detectable concentrations in milk, the available data indicate that these concentrations of ergovaline are far below published tolerable limits for human consumption (EFSA 2012). In terms of the pasture-based dairy industries of New Zealand, the relationship between ergovaline intake and concentrations found in milk has yet to be thoroughly investigated and reported.
Effects of ergovaline
Because ergot alkaloids such as ergovaline have the ability to bind to serotonergic, adrenergic and dopaminergic receptors (Eckert et al. 1978), the physiologic effects that these compounds can elicit are diverse. This, coupled with their ability to assume multiple roles as an agonist, partial agonist and antagonist, may cause several disruptions that manifest as decreases in serum prolactin, vasoconstriction, effects on gastrointestinal activity, and endocrine effects that cause decreases in reproductive performance. The effects of ergot alkaloids are so multifaceted that it is beyond the scope of the present review to cover them all and the reader is referred to Oliver (1997), Pertz and Eckart (1999) and Strickland et al. (2009a, 2009b, 2011) for further detail. The effects discussed below were chosen specifically to represent aspects that could have a direct impact on livestock production in terms of the individual animal.
Ergovaline–receptor interaction
Because ergopeptine alkaloids such as ergovaline have a low receptor specificity and selectivity, the responses observed in a given anatomical area of study are in large part due to the population of biogenic amine receptors present. The majority of these receptors are members of the G protein-coupled receptor superfamily. These are transmembrane-bound receptors that transmit intracellular effects by using guanine nucleotide-binding proteins (G-proteins). Therefore, the following sections will briefly describe the different categories of receptors that ergovaline has had a demonstrated interaction with, followed up by a more detailed review of the associated physiological responses.
Dopaminergic receptor involvement
At present, there are five recognised isoforms of dopamine receptors that are divided up into D1-like (D1 and D5) and D2-like (D2, D3, and D4) subfamilies on the basis of their biochemical and structural properties (Sibley and Monsma 1992). The interaction of ergovaline with dopamine receptors is best represented by the suppression of circulating prolactin (Elsasser and Bolt 1987). In vitro studies (Strickland et al. 1994; Larson et al. 1995) have specifically shown that ergovaline is a D2 dopamine-receptor agonist. Specifically, Larson et al. (1995) showed that when ergovaline binds D2 receptors, a second messenger response is triggered that is similar to that of dopamine.
Because the D2-derived suppression of prolactin by ergopeptine alkaloids such as ergovaline is so sensitive and consistent, it has probably detracted focus from further ergovaline influences on other dopaminergic receptors. Strickland et al. (1993) suggested that ergopeptine alkaloid interactions with dopaminergic receptors might have an influence on gut motility and feed intake. This was based on the involvement of dopamine in these functions. In terms of ergovaline, these aspects are as yet unproven in livestock.
α-Adrenergic receptor involvement
There are two subfamilies of α-adrenergic receptors, namely α1 and α2, with each having its own subtypes (α1A, α1B and α1D; α2A, α2B, α2C and α2D). Stimulation of both types of receptors on blood vessels causes vasoconstriction. There have been reports of greater populations of α1 receptors in arteries and α2 receptors in veins (Oliver 1997). McPherson and Beart (1983) reported a general selectivity for α2 receptors by ergot derivatives. Oliver et al. (1998) evaluated α1 and α2 receptors in the lateral saphenous veins collected from cattle that had grazed endophyte-free and endophyte-infected tall fescue (ergovaline concentrations not provided). Using selective agonists, the α1-adrenergic response was not affected by pasture treatment, whereas the α2-adrenergic receptor responses where enhanced in veins collected from the endophyte-infected group of cattle. Two studies have looked at the interaction of α-adrenergic receptors and ergovaline directly. Dyer (1993) reported that non-selective α1-adrenergic receptor antagonists did not prevent ergovaline-induced contractions in bovine uterine arteries. Using a rat thoracic aorta bioassay, Schöning et al. (2001) reported that when interacting with α1-adrenergic receptors, ergovaline was a low-efficacy partial agonist. It is likely that α-adrenergic receptors are involved in the negative vascular effects caused by ergovaline, but the level of their involvement will be dependent on the anatomical location of the vasculature being evaluated.
In addition to vasoconstriction, activation of α2-adrenergic receptor has been shown to result in bronchoconstriction (Nolan et al. 1986). Respiratory distress has been reported as a complicating factor in cattle grazing endophyte-infected tall fescue (Oliver 1997) and this is exacerbated in heat-stress situations (Koontz et al. 2012). This has also been reported in sheep with ryegrass staggers (Fletcher 1993).
Serotonergic receptor involvement
Serotonin (or 5-hydroxytryptamine; 5HT) receptors are located throughout the mammalian body and consist of seven structurally defined groups (collectively made up of 14 subtypes) and six of these are membrane-bound G protein-coupled receptors (Barnes and Sharp 1999). The interaction of ergot alkaloids, and, in particular, ergopeptine alkaloids and serotonergic receptors, has been well researched for some time (Müller-Schweinitzer and Weidmann 1978). Using a bovine uterine artery, Dyer (1993) was the first to directly demonstrate that 5HT2 receptors are involved in ergovaline-derived vasoconstriction. Schöning et al. (2001) used isolated arterial preparations from rat and guinea pig to demonstrate that the vasoconstrictive effect associated with ergovaline is due to activation of vascular 5HT2A and 5HT1B/1D receptors. Klotz et al. (2012) further defined serotonergic receptor involvement by reporting that lateral saphenous vein responses to 5HT2A and 5HT7 agonists were altered in cattle that grazed endophyte-infected tall fescue. Using this same bovine bioassay, Klotz et al. (2013) found that antagonism of the 5HT2A receptor not only suppresses the contractile responses of ergovaline, ergotamine and ergocornine, but that cattle that had grazed endophyte-infected tall fescue pastures were more sensitive to lower concentrations of these compounds.
Where much of the aforementioned research addressing ergovaline and serotonergic receptors has been directed at vasoconstriction, the functions of the serotonin receptors are as numerous as their locations throughout the body. Recent research has shown that steers that are exposed to tall fescue seed have suppressed gene expression of 5HT2A and 5HT4 receptors in the smooth muscle of the foregut, small intestine, large intestine and caecum (Klotz et al. 2014). This may be either a direct or indirect effect of the ergovaline exposure, but these receptors have direct involvement in gut motility functions. A decrease in receptor expression could be related to altered gut motility reported in other studies. Serotonin receptors have been recently identified in bovine sweat glands (Hamzaoui et al. 2014). This has not been evaluated in terms of ergovaline, but the potential exists for this as an avenue for the ergovaline–serotonin receptor complex to play a role in the hyperthermia associated with ergovaline exposure.
Physiological responses to ergovaline
Prolactin
The suppression of prolactin release from the anterior pituitary is a hallmark of exposure to ergopeptine alkaloids such as ergovaline (Schillo et al. 1988). Ergot alkaloids such as ergovaline bind the D2 dopamine receptors, which elicits a secondary messenger response that mimics the binding of dopamine (Aldrich et al. 1993) and this causes the decrease in prolactin concentrations. The decrease in serum prolactin appears to be very closely related to the exposure to ergovaline and occurs within 2–3 days after exposure from ryegrass (Gooneratne et al. 2011) or tall fescue (Thompson and Stuedemann 1993). Because the reduction in prolactin appears so quickly after an ergot-alkaloid exposure, it has frequently been used as an indicator of ergovaline exposure and little else. However, it does not indicate the concentration of ergovaline that an animal was exposed to, for example, and does not indicate a complete recovery from the exposure either. Aiken et al. (2013) reported that prolactin concentrations of steers stabilised in less than 2 weeks following removal from an endophyte-infected tall fescue pasture, whereas ergovaline-induced vasoconstriction was not alleviated after 30 days.
Prolactin has not been implicated as a causal factor for any of the associated endophyte toxicities. Other than an indication of ergot-alkaloid exposure, what does the suppression of prolactin mean for the animal? The involvement of prolactin on the initiation of lactation has been well documented (Houdebine et al. 1985), but prolactin has also been implicated in numerous other processes throughout the body (Strickland et al. 1993). Looking at the hair-coat growth of cattle that had grazed tall fescue, Aiken et al. (2011a) speculated that prolactin concentrations were too low to initiate shedding of the winter hair coat and too low to prevent the onset of the summer hair-coat growth (prolactin receptors are located in the wool follicles of sheep; Nixon et al. 2002). The involvement of prolactin in circadian rhythms of processes such as hepatic lipogenesis to immunomodulation (Strickland et al. 1993) suggests a much broader possible involvement of prolactin, beyond merely being an indicator. These functions could be involved in decreased LW gain or impaired immune function associated with animals and alkaloid toxicities. The role that low prolactin concentrations have in the observed effects of ergovaline exposure is an area where further research is needed.
Vasoconstriction
A major effect of ergovaline consumption is constriction of vasculature that results in several clinical symptoms. The aspect of vasoconstriction as a result of exposure to ergot alkaloid has been reviewed in detail (Strickland et al. 2009a, 2009b, 2011) in regard to tall fescue consumption. In general, animals that are exposed to high concentrations of ergot alkaloids lose their ability to dissipate heat (excessive panting, spending excessive time in shade and standing in ponds), they lose their ability to maintain peripheral body temperature in cold climates, which can result in frostbite, and the exposure may culminate in complete death of peripheral tissues, which results in loss of hooves, tail switches and ears; all effects are likely to be due to a the resultant decrease in blood flow (Jacobson et al. 1963). Vasoconstriction has also been implicated as a cause for observed decreases in milk production in dairy cattle associated with ergovaline intake (Lean 2001). The functional changes of vasoconstriction are caused by morphological changes in the affected vasculature that appears as a thickened vascular wall (smooth muscle) and by corollary small lumens that are frequently accompanied by perivascular oedema (Williams et al. 1975). These vascular effects are mediated through stimulation of various biogenic amine receptors (Berde 1980). Vasoactivity of ergovaline has been shown in the bovine uterine and umbilical arteries (Dyer 1993) and in rat-tail artery and guinea-pig iliac artery (Schöning et al. 2001). Klotz et al. (2007, 2008) made a direct link between ergovaline and bovine peripheral vasoconstriction using the bovine lateral saphenous-vein bioassay. More recently, Foote et al. (2011) demonstrated that ergovaline is vasoactive in the bovine right ruminal artery and vein and, further, went on to demonstrate an ergovaline-induced reduction of rumen epithelial blood flow by using in vivo washed-rumen technique (Foote et al. 2013). Egert et al. (2014) demonstrated vasoactivity of ergovaline in the bovine mesenteric artery and vein. These findings could have implications that relate to nutrient uptake from the digestive tract and the productive efficiency of livestock, although this has not been directly demonstrated.
To give a sense of the amount of ergovaline required to cause an effect, the contractility data presented by Klotz et al. (2008), Foote et al. (2011) and Egert et al. (2014) generated concentration response curves that permitted calculation of the half maximal effective concentration (EC50), or the concentration of a drug or toxin required to 50% of the maximum possible response. This is commonly used as a measure of a compound potency, or concentration of a compound required to give an effect. The potency of a compound varies inversely with the numerical value of the EC50 (the lower the value, the more potent the compound). In the case of pure ergovaline (synthesised), Foote et al. (2011) reported EC50 of 5.6 × 10−6 M (2.99 mg/kg) and 2.0 × 10−6 M (1.06 mg/kg) for the right ruminal artery and vein respectively and Foote et al. (2012) reported an EC50 of 2.4 × 10−6 M (1.28 mg/kg) for the bovine lateral saphenous vein. Using a tall fescue seed extract, Foote et al. (2012) reported EC50 3.3 × 10−7 M (0.176 mg/kg), 2.04 × 10−6 M (1.09 mg/kg) and 1.02 × 10−7 M (0.054 mg/kg) for the lateral saphenous vein, right ruminal artery and right ruminal vein respectively. Also, using the same tall fescue seed extract, Egert et al. (2014) reported even lower EC50 of 7.2 × 10−8 M (0.038 mg/kg) and 8.1 × 10−8 M (0.043 mg/kg) for mesenteric artery and vein respectively. It should be noted that the extract EC50 data cannot be directly compared with the pure ergovaline EC50 data, as the response curves were generated using different ranges of concentrations (i.e. the maximum extract concentration was 1 × 10−6 M, whereas pure ergovaline concentration was 1 × 10−4 M). Although these studies were all conducted in vitro and involved a direct exposure of the blood vessel to ergovaline, they demonstrated the sensitivity of different types of blood vessel to ergovaline and the diversity of responses that can result simply by selecting vasculature from different regions of the body. Interestingly, the efficacy (a term used to characterise the level of maximal response) of ergovaline was much greater for the peripheral lateral saphenous vein (Klotz et al. 2007; Foote et al. 2012) than for any of the visceral blood vessels evaluated (Foote et al. 2011, 2012; Egert et al. 2014). Thus, the mesenteric vasculature in particular seem much more sensitive to very low concentrations of ergovaline, but peripheral vasculature such as the lateral saphenous vein seem to have a much greater contractile response.
Up to this point in this section, the data presented have been generated using tall fescue research. The only report of vasoconstriction induced by livestock consuming ergovaline-containing perennial ryegrass was published by Aiken et al. (2011b), using colour Doppler ultrasonography to monitor the cross-sectional area of the auricular and carotid arteries in lambs grazing AR6 novel (1.0 mg ergovaline/kg DM), wild-type endophyte (0.55 mg ergovaline/kg DM) or endophyte-free pastures (0.0 mg ergovaline/kg DM). Lambs were on these pasture treatments for 19 days prior to subsequent phases of the trial. During this time, Aiken et al. (2011b) measured the luminal area in the carotid artery and saw only a tendency for the endophyte-free lambs to have a greater area did the AR6 and wild-type lambs (which did not differ). The auricular artery was affected by pasture treatment, with the lambs grazing endophyte-free pastures having a greater luminal area than did lambs grazing AR6 or wild-type endophyte-infected ryegrass (which did not differ). The study of Aiken et al. (2011b) clearly demonstrated vasoconstriction at the auricular artery, but the different concentrations of ergovaline reported in AR6 and wild type-infected ryegrass pastures did not affect the magnitude of vasoconstrictive response (in terms of a clear dose response).
Gut motility and feed intake
Another aspect of livestock consuming ergovaline-containing feed is a decrease in DM intake. (This aspect of the influence that ergovaline can have on livestock can be exacerbated by heat stress.) Some investigations looking into this aspect have focussed on ergovaline affecting gut motility as a possible explanation. Changes in rumen motility may affect feed digestion, passage rate and nutrient availability. The aforementioned constriction that occurs in blood vessels is likely to be driven by the interaction of ergovaline with biogenic amine receptors (e.g. serotonergic, adrenergic and dopaminergic) that in turn affects the associated smooth muscle. This could also be occurring with the smooth muscle associated with motility or peristalsis of the gastrointestinal tract. McLeay and Smith (2006) reported increases in the baseline tonus of the reticulum and rumen, as well as the amplitude of the reticular contractions following an intravenous infusion of ergovaline, whereas the frequency of reticular and ruminal contractions decreased in sheep. The authors concluded that disruption of digestion might occur in livestock grazing endophyte-infected pastures with a high ergovaline content. On the basis of similar results from a separate study, Poole et al. (2009) speculated that the effects of ergovaline on amplitude and tonus are due to an agonistic action on the 5HT4 serotonin receptor. The effects of ergovaline on the smooth muscle of the small intestine have yet to be evaluated; however, Dalziel et al. (2013) evaluated the effect of ergotamine (and related them to ergovaline) on preparations of isolated distal rat colon and demonstrated an increased contractile tension and momentary increase in contractile frequency. Differences in the observations between the foregut and hindgut responses to ergovaline and ergotamine may be a result of different receptor populations at these different locations of the gastrointestinal tract.
The above evaluations into gut motility (McLeay and Smith 2006; Poole et al. 2009; Dalziel et al. 2013) all suggest that there should be an effect of ergovaline on passage rate and one could infer that this could drive changes in DM intake and LW gain in exposed livestock. Unfortunately, this area of ergovaline research is similar to many others, with there being an array of differing published results regarding passage rate. Early reports of passage rates reported it to increase (Hannah et al. 1990), decrease (Goetsch et al. 1987) or remain unchanged (Forcherio et al. 1995) in relationship to consumption of endophyte-infected tall fescue (there are no published data evaluating ergovaline in perennial rye grass on passage in livestock). The challenge in comparing these reports is differences in diets (between control and treatment), intakes (some held intakes equal, whereas other did not) and the concomitant differences in ergovaline intake by the animals. There was also a difference in the delivery of ergovaline via seed versus hay or straw. One implication of an altered passage rate is gut fill. Effects that cause an increase in rumen fill can reduce intake. In separate studies using steers, Koontz et al. (2013) and Foote et al. (2013) controlled intake such that it was equal across endophyte-free and infected treatments of ruminally dosed seed at 0.051 mg ergovaline/kg0.75 LW.day (Koontz et al. 2013) or at 0.059 mg ergovaline/kg0.75 LW.day (Foote et al. 2013). In both studies, there was a significant increase in ruminal DM mass that could not be explained by a different DM intake, suggesting that ruminal motility was affected, altering passage rate in the steers exposed to ergovaline. Conversely, Emile et al. (2000) fed lambs endophyte-free or infected hay at 0.058 mg ergovaline/kg0.75 LW.day and reported a lower total ruminal content (it was not clear whether this was wet or dry), but no difference in DM intake between the two hay treatments. The authors made a strong case that the decrease in gut fill was responsible for the decreased LW gain observed in the lambs fed the endophyte-infected hay. Obviously, there is an effect of ergovaline in the smooth muscle of the digestive tract, but how this translates into an effect on gut fill and passage rate and LW gain has yet to be clearly delineated.
Increased core temperature and susceptibility to heat stress
Elevated body temperatures and heat stress of livestock are of concern in terms of animal welfare. Ergovaline has been considered the causative agent for heat stress in livestock grazing endophyte-infected ryegrass (McLeay et al. 2002). The summer-syndrome component of fescue toxicosis in the south-eastern United States is in large part a heat stress caused or intensified by consumption of ergovaline. Many of the signs of summer syndrome are heat-stress symptoms that include high rectal temperatures, excessive salivation, high respiration rates, panting and shade or water seeking. These all contribute to production losses. Because ergovaline has been detected in some endophyte-infected ryegrasses, there are assumptions that similar ‘summer syndromes’ could occur in New Zealand. However, the incidence of heat stress in dairy cattle has yet to be associated with consumption of ergovaline-producing ryegrass endophytes in New Zealand (Bluett et al. 2005; Thom et al. 2013). Many of the heat stress-related symptoms can be related back to ergovaline interactions (mentioned earlier in the article) with biogenic amine receptors, causing vasoconstriction in pulmonary vasculature that results in a decrease in heat transfer (elevated body temperature and increased respiration), as well as interaction with adrenergic and serotonergic receptors in the periphery that results in a decrease in heat transfer (elevated body temperature) and interaction with dopaminergic receptors in the higher neural centers responsible for thermoregulation (Faichney and Barry 1986). Animals consuming ergovaline-containing feedstuffs appear to have a reduced capacity to maintain a thermoneutral body temperature with rapid changes in ambient temperature (Spiers et al. 2012). The main challenge in separating or relating the effects of heat stress to ergovaline intake are the commonly measured variables of body temperature, respiration rate and feed intake. Many times, both stressors affect all three variables and this confounds the interpretation of the causal stressor. This aspect of ergovaline in ryegrass deserves to be studied in greater detail.
Conclusions
As a secondary metabolite produced by some fungal endophytes in perennial ryegrass, ergovaline can have a significant impact on the physiology of livestock consuming it. The scope of this physiologic impact is largely dictated by variations in ergovaline concentration in the consumed plant material, liberation from the plant material either through digestion or fermentation, microbial interactions and modifications of bioavailable ergovaline, absorptive efficiency and route of absorption from the digestive tract into the blood stream, hepatic detoxification and degrees of tissue deposition or excretion of ergovaline. This is further complicated by the ability of ergot alkaloids such as ergovaline to interact with the functionally diverse class of biogenic amine receptors that are ubiquitously distributed throughout the body. The physiologic effects of ergovaline described in this review are not always going to be observed in production settings, but should, nevertheless, not be ignored as cumulative decreases in animal productivity may be occurring. In addition to highlighting the range of ergovaline-related effects, the present review has demonstrated the ability of the ruminant to tolerate (breakdown and excrete) a certain amount of ergovaline. The key issue to New Zealand producers and those around the world is keeping the concentration of ergovaline low enough not to exceed the ability of livestock to metabolise it, but also have concentrations that are sufficient to impart some of the positive benefits provided by ergovaline that improve pasture persistence. The challenge that remains is to define these concentrations for a productive animal on a productive pasture.
References
Aiken GE, Strickland JR, Looper ML, Bush LP, Schrick FN (2009) Hemodynamics are altered in the caudal artery of beef heifers fed different ergot alkaloid concentrations. Journal of Animal Science 87, 2142–2150.| Hemodynamics are altered in the caudal artery of beef heifers fed different ergot alkaloid concentrations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXms1eis7w%3D&md5=764982dde57b02491ad80c179246bfb5CAS | 19251925PubMed |
Aiken GE, Klotz JL, Looper ML, Tabler SF, Schrick FN (2011a) Disrupted hair follicle activity in cattle grazing endophyte-infected tall fescue in the summer insulates core body temperatures. The Professional Animal Scientist 27, 336–343.
| Disrupted hair follicle activity in cattle grazing endophyte-infected tall fescue in the summer insulates core body temperatures.Crossref | GoogleScholarGoogle Scholar |
Aiken GE, Sutherland BL, Fletcher LR (2011b) Haemodynamics of lambs grazing perennial ryegrass (Lolium perenne L.) either infected with AR6 novel, wild-type endophyte, or endophyte-free. New Zealand Veterinary Journal 59, 179–184.
| Haemodynamics of lambs grazing perennial ryegrass (Lolium perenne L.) either infected with AR6 novel, wild-type endophyte, or endophyte-free.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mrpt1Cktg%3D%3D&md5=35430e244168e5ebf4851a1c1cfb86a0CAS | 21660847PubMed |
Aiken GE, Klotz JL, Johnson JM, Strickland JR, Schrick FN (2013) Postgraze assessment of toxicosis symptoms for steers grazed on toxic endophyte-infected tall fescue pasture. Journal of Animal Science 91, 5878–5884.
| Postgraze assessment of toxicosis symptoms for steers grazed on toxic endophyte-infected tall fescue pasture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvFOrur3I&md5=4dfda04f5f4b8adaecd283be4c053b63CAS | 24126272PubMed |
Aldrich CG, Paterson JA, Tate JL, Kerley MS (1993) The effects of endophyte-infected tall fescue consumption on diet utilization and thermal regulation in cattle. Journal of Animal Science 71, 164–170.
Ayers AW, Hill NS, Rottinghaus GE, Stuedemann JA, Thompson FN, Purinton PT, Seman DH, Dawe DL, Parks AH, Ensley D (2009) Ruminal metabolism and transport of tall fescue ergot alkaloids. Crop Science 49, 2309–2316.
| Ruminal metabolism and transport of tall fescue ergot alkaloids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFWjsrrP&md5=ad6f2570611e83e143786c56fca23f63CAS |
Ball OJP, Miles CO, Prestidge RA (1997) Ergopeptine alkaloids and Neotyphodium lolii-mediated resistance in perennial ryegrass against adult Heteronychus arator (Coleoptera: Scarabaeidae). Journal of Economic Entomology 90, 1382–1391.
| Ergopeptine alkaloids and Neotyphodium lolii-mediated resistance in perennial ryegrass against adult Heteronychus arator (Coleoptera: Scarabaeidae).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXns1ynsb4%3D&md5=5ea3f7632ac6c3927bdb8b58a6fa2aabCAS |
Barnes NM, Sharp T (1999) A review of central 5-HT receptors and their function. Neuropharmacology 38, 1083–1152.
| A review of central 5-HT receptors and their function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXltVCgsrg%3D&md5=d0dab11710d67439a58ee54167c2e940CAS | 10462127PubMed |
Berde B (1980) Ergot compounds: a synopsis. In ‘Ergot compounds and brain function – neuro-endocrine and neuropsychiatric aspects’. (Eds ALM Goldstein, DB Calne, MO Thorner) pp. 3–24. (Raven Press: New York)
Bluett SJ, Thom ER, Clark DA, Macdonald KA, Minneé EMK (2005) Effects of perennial ryegrass infected with either AR1 or wild endophyte on dairy production in the Waikato. New Zealand Journal of Agricultural Research 48, 197–212.
| Effects of perennial ryegrass infected with either AR1 or wild endophyte on dairy production in the Waikato.Crossref | GoogleScholarGoogle Scholar |
Bony S, Durix A, Leblond A, Jaussaud P (2001) Toxicokinetics of ergovaline in the horse after an intravenous administration. Veterinary Research 32, 509–513.
| Toxicokinetics of ergovaline in the horse after an intravenous administration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXnslKmsrc%3D&md5=987c062904a5f62ffb37a08f7b57a046CAS | 11592620PubMed |
Brunner R, Stütz PL, Tscherter H, Stadler PA (1979) Isolation of ergovaline, ergoptine, and ergonine, new alkaloids of the peptide type, from ergot sclerotia. Canadian Journal of Chemistry 57, 1638–1641.
| Isolation of ergovaline, ergoptine, and ergonine, new alkaloids of the peptide type, from ergot sclerotia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXltVGhsLg%3D&md5=fa9ae87b61019a097d65968b509dd13eCAS |
Bryden WL (2012) Food and feed, mycotoxins and the perpetual pentagram in a changing animal production environment. Animal Production Science 52, 383–397.
| Food and feed, mycotoxins and the perpetual pentagram in a changing animal production environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XnvVOhs7w%3D&md5=920886543695388c847c2b596a1d1435CAS |
Bush L, Fannin FF (2009) Alkaloids. In ‘Tall fescue for the twenty-first century’. Agronomy Monograph 53. (Eds HA Fribourg, DB Hannaway, CP West) pp. 229–249. (American Society of Agronomy, Inc., Crop Science Society of America, Inc., Soil Science Society of America, Inc.: Madison, WI)
Bussard JR (2012) Evaluation of vascular changes in cattle relative to time-off endophyte-infected tall fescue. University of Kentucky, Theses and Dissertations--Plant and Soil Sciences, paper 7. Available at http://uknowledge.uky.edu/pss_etds/7 [Verified 2 August 2016]
Dalziel JE, Dunstan KE, Finch SC (2013) Combined effects of fungal alkaloids on intestinal motility in an in vitro rat model. Journal of Animal Science 91, 5177–5182.
| Combined effects of fungal alkaloids on intestinal motility in an in vitro rat model.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslKktrbE&md5=bb1d71c9aa762f17a15575a5fda67ccbCAS | 23989880PubMed |
De Lorme MJ, Lodge-Ivey SL, Craig AM (2007) Physiological and digestive effects of Neotyphodium coenophialum-infected tall fescue fed to lambs. Journal of Animal Science 85, 1199–1206.
| Physiological and digestive effects of Neotyphodium coenophialum-infected tall fescue fed to lambs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXksF2htb8%3D&md5=c56c6913e4680bca0e43f7f1db7dda9cCAS | 17296774PubMed |
Durix A, Jaussaud P, Garcia P, Bonnaire Y, Bony S (1999) Analysis of ergovaline in milk using high-performance liquid chromatography with fluorimetric detection. Journal of Chromatography. B, Biomedical Sciences and Applications 729, 255–263.
| Analysis of ergovaline in milk using high-performance liquid chromatography with fluorimetric detection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXktlaqtrs%3D&md5=47a3262228eb1bb46e89cd9a45a7f06cCAS | 10410950PubMed |
Dyer DC (1993) Evidence that ergovaline acts on serotonin receptors. Life Sciences 53, PL223–PL228.
| Evidence that ergovaline acts on serotonin receptors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXmtF2ntb0%3D&md5=f625b80f77b978345f33dde35cfba9e2CAS | 8371626PubMed |
Easton HS, Latch GC, Tapper BA, Ball OJ (2002) Ryegrass host genetic control of concentrations of endophyte-derived alkaloids. Crop Science 42, 51–57.
| Ryegrass host genetic control of concentrations of endophyte-derived alkaloids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmsVantb8%3D&md5=202d7e91cfc7c81c1bd937e68c290fb3CAS | 11756253PubMed |
Eckert H, Kiechel JR, Rosenthaler J, Schmidt R, Schreier E (1978) Biopharmaceutical aspects. In ‘Ergot alkaloids and related compounds’. (Eds B Berde, HO Schild) pp. 719–803. (Springer-Verlag: Berlin)
EFSA (2012) Scientific opinion on ergot alkaloids in food and feed. EFSA Journal 10, 2798
Egert AM, Kim DH, Schrick FN, Harmon DL, Klotz JL (2014) Dietary exposure to ergot alkaloids decreases contractility of bovine mesenteric vasculature. Journal of Animal Science 92, 1768–1779.
| Dietary exposure to ergot alkaloids decreases contractility of bovine mesenteric vasculature.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2cvhs1KktQ%3D%3D&md5=081ba928b3d00472efbcd154ef29f09aCAS | 24492572PubMed |
Elsasser TH, Bolt DJ (1987) Dopaminergic-like activity in toxic fescue alters prolactin but not growth hormone or thyroid stimulating hormone in ewes. Domestic Animal Endocrinology 4, 259–269.
| Dopaminergic-like activity in toxic fescue alters prolactin but not growth hormone or thyroid stimulating hormone in ewes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXptlagsQ%3D%3D&md5=6c9a8ea4938139a7516ebc8b8291def7CAS | 3507894PubMed |
Emile JC, Bony S, Ghesquiere M (2000) Influence of consumption of endophyte-infested tall fescue hay on performance of heifers and lambs. Journal of Animal Science 78, 358–364.
Faichney GJ, Barry TN (1986) Effects of mild heat exposure and suppression of prolactin secretion on gastro-intestinal tract function and temperature regulation in sheep. Australian Journal of Biological Sciences 39, 85–97.
Finch SC, Fletcher LR, Babu JV (2012) The evaluation of endophyte toxin residues in sheep fat. New Zealand Veterinary Journal 60, 56–60.
| The evaluation of endophyte toxin residues in sheep fat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1GhtL7N&md5=4aaad3768f6e140a26f2d8a2924919c1CAS | 22175431PubMed |
Fletcher LR (1993) ‘Grazing ryegrass/endophyte associations and their effect on animal health and performance. In ‘Proceedings of the 2nd international symposium on Acremonium–grass interactions’, Palmerston North, New Zealand. (Eds DE Hume, GC Latch, HS Easton)
Fletcher LR (2012) Novel endophytes in New Zealand grazing systems: the perfect solution or a compromise? In ‘Epichloae, endophytes of cool season grasses: implications, utilization and biology’. (Eds CA Young, GE Aiken, RL McCulley, JR Strickland, CL Schardl) pp. 5–13. (The Samuel Roberts Notle Foundation: Ardmore, OK)
Fletcher LR, Harvey IC (1981) An association of a Lolium endophyte with ryegrass staggers. New Zealand Veterinary Journal 29, 185–186.
| An association of a Lolium endophyte with ryegrass staggers.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL387kvFemsQ%3D%3D&md5=d83a8de967715274531060e243e5ef59CAS | 6950332PubMed |
Foote AP, Harmon DL, Strickland JR, Bush LP, Klotz JL (2011) Effect of ergot alkaloids on contractility of bovine right ruminal artery and vein. Journal of Animal Science 89, 2944–2949.
| Effect of ergot alkaloids on contractility of bovine right ruminal artery and vein.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFWqtrbL&md5=0c873c9d2469699831295303b205860cCAS | 21512122PubMed |
Foote AP, Harmon DL, Brown KR, Strickland JR, McLeod KR, Bush LP, Klotz JL (2012) Constriction of bovine vasculature caused by endophyte-infected tall fescue seed extract is similar to pure ergovaline. Journal of Animal Science 90, 1603–1609.
| Constriction of bovine vasculature caused by endophyte-infected tall fescue seed extract is similar to pure ergovaline.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xns1eisb0%3D&md5=308ee757a9123c9fa6f2b7be907d3234CAS | 22147482PubMed |
Foote AP, Kristensen NB, Klotz JL, Kim DH, Koontz AF, McLeod KR, Bush LP, Schrick FN, Harmon DL (2013) Ergot alkaloids from endophyte-infected tall fescue decrease reticuloruminal epithelial blood flow and volatile fatty acid absorption from the washed reticulorumen. Journal of Animal Science 91, 5366–5378.
| Ergot alkaloids from endophyte-infected tall fescue decrease reticuloruminal epithelial blood flow and volatile fatty acid absorption from the washed reticulorumen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslKkt7vK&md5=f14644ab61948b34a76ee31f5653bba6CAS | 23989869PubMed |
Foote A, Penner G, Walpole M, Klotz J, Brown K, Bush L, Harmon D (2014) Acute exposure to ergot alkaloids from endophyte-infected tall fescue does not alter absorptive or barrier function of the isolated ruminal epithelium. Animal 8, 1106–1112.
| Acute exposure to ergot alkaloids from endophyte-infected tall fescue does not alter absorptive or barrier function of the isolated ruminal epithelium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXpvVWktr8%3D&md5=0d15eb4c4e267c9e0c6ea9c20e405d0cCAS | 26263028PubMed |
Forcherio JC, Catlett GE, Paterson JA, Kerley MS, Ellersieck MR (1995) Supplemental protein and energy for beef cows consuming endophyte-infected tall fescue. Journal of Animal Science 73, 3427–3436.
Franz JM, Vonderscher JP (1981) Enhancement of the intestinal absorption of ergot peptide alkaloids in the rat by micellar solutions of polyoxyethylene-24-cholesteryl ether. The Journal of Pharmacy and Pharmacology 33, 565–568.
| Enhancement of the intestinal absorption of ergot peptide alkaloids in the rat by micellar solutions of polyoxyethylene-24-cholesteryl ether.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38XlslKhuw%3D%3D&md5=7c0eec8f8391e95b3d19e26bedadfc92CAS | 6117633PubMed |
Gadberry MS, Denard TM, Spiers DE, Piper EL (2003) Effects of feeding ergovaline on lamb performance in a heat stress environment. Journal of Animal Science 81, 1538–1545.
Garner GB, Rottinghaus GE, Cornell CN, Testereci H (1993) Chemistry of compounds associated with endophyte/grass interaction: ergovaline- and ergopeptine-related alkaloids. Agriculture, Ecosystems & Environment 44, 65–80.
| Chemistry of compounds associated with endophyte/grass interaction: ergovaline- and ergopeptine-related alkaloids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXlsFKis74%3D&md5=e8b1aac477a28339f19f72f7da84f2fdCAS |
Goetsch AL, Jones AL, Stokes SR, Beers KW, Piper EL (1987) Intake, digestion, passage rate and serum prolactin in growing dairy steers fed endophyte-infected fescue with noninfected fescue, clover or wheat straw. Journal of Animal Science 64, 1759–1768.
Goff BM, Aiken GE, Witt WW, Sleugh BB, Burch PL (2012) Steer consumption and ergovaline recovery from in vitro digested residues of tall fescue seedheads. Crop Science 52, 1437–1440.
| Steer consumption and ergovaline recovery from in vitro digested residues of tall fescue seedheads.Crossref | GoogleScholarGoogle Scholar |
Gooneratne SR, Scannell M, Wellby M, Fletcher L (2011) Changes in concentrations of lysergol in urine and prolactin in plasma, rectal temperature and respiration rate in sheep selected for resistance or susceptibility to ryegrass staggers and fed ergovaline. New Zealand Veterinary Journal 59, 233–238.
| Changes in concentrations of lysergol in urine and prolactin in plasma, rectal temperature and respiration rate in sheep selected for resistance or susceptibility to ryegrass staggers and fed ergovaline.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlGju7zI&md5=8b8dbc93b173ac09e1883f17c40b5a2eCAS | 21851300PubMed |
Hamzaoui S, Collier JL, Collier RJ (2014) Serotonin (5-HT) receptor expression in bovine apocrine sweat gland epithelial cells isolated from cow skin. Journal of Animal Science 92, 255
Hannah SM, Paterson JA, Williams JE, Kerley MS, Miner JL (1990) Effects of increasing dietary levels of endophyte-infected tall fescue seed on diet digestibility and ruminal kinetics in sheep. Journal of Animal Science 68, 1693–1701.
Hill NS (2004) Absorption of ergot alkaloids in the ruminant. In ‘Neotyphodium in cool-season grasses’. (Eds CA Roberts, CP West, DE Spiers) pp. 271–290. (Blackwell Publishing: Oxford, UK)
Hill NS, Thompson FN, Stuedemann JA, Rottinghaus GW, Ju HJ, Dawe DL, Hiatt EE (2001) Ergot alkaloid transport across ruminant gastric tissues. Journal of Animal Science 79, 542–549.
Hill NS, Ayer AW, Stuedemann JA, Thompson FN, Purinton PT, Rottinghaus GE (2003) Metabolism and gastric transport of ergot alkaloids in ruminants grazing endophyte-infected tall fescue. Journal of Animal Science 81, 1
Houdebine LM, Djiane J, Dusanter-Fourt I, Martel P, Kelly PA, Devinoy E, Servely JL (1985) Hormonal action controlling mammary activity. Journal of Dairy Science 68, 489–500.
| Hormonal action controlling mammary activity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXhs1Snurk%3D&md5=d6e414b32b6ffa454d985bf752d91917CAS | 2985667PubMed |
Jacobson DR, Miller WM, Seath DM, Yates SG, Tookey HL, Wolff IA (1963) Nature of fescue toxicity and progress toward identification of the toxic entity. Journal of Dairy Science 46, 416–422.
| Nature of fescue toxicity and progress toward identification of the toxic entity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF3sXktFOqt70%3D&md5=1a2c2eb2704aa40d8a1937293cb0d475CAS |
Jaussaud P, Durix A, Videmann B, Vigie A, Bony S (1998) Rapid analysis of ergovaline in ovine plasma using high-performance liquid chromatography with fluorimetric detection. Journal of Chromatography. A 815, 147–153.
| Rapid analysis of ergovaline in ovine plasma using high-performance liquid chromatography with fluorimetric detection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXlsVersbk%3D&md5=33d194ac9b012b519d5bb162dfbee7f3CAS | 9718715PubMed |
Kim JH, Kim CW, Ahn GC, Park EK, Kim CM, Park KK (2007) Ergovaline levels in tall fescue and its effect on performance of lactating cows. Animal Feed Science and Technology 136, 330–337.
| Ergovaline levels in tall fescue and its effect on performance of lactating cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXnslGhtb8%3D&md5=3b4e40f3f3c719a5132ab9f8d01f92bcCAS |
Klotz JL, Bush LP, Smith DL, Shafer WD, Smith LL, Arrington BC, Strickland JR (2007) Ergovaline-induced vasoconstriction in an isolated bovine lateral saphenous vein bioassay. Journal of Animal Science 85, 2330–2336.
| Ergovaline-induced vasoconstriction in an isolated bovine lateral saphenous vein bioassay.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpsVGlsbw%3D&md5=426c13994296c544268416860e8ca4a2CAS | 17504952PubMed |
Klotz JL, Kirch BH, Aiken GE, Bush LP, Strickland JR (2008) Effects of selected combinations of tall fescue alkaloids on the vasoconstrictive capacity of fescue-naive bovine lateral saphenous veins. Journal of Animal Science 86, 1021–1028.
| Effects of selected combinations of tall fescue alkaloids on the vasoconstrictive capacity of fescue-naive bovine lateral saphenous veins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjvFeru7Y%3D&md5=63dc30a6852ea1784af1e76343145429CAS | 18192563PubMed |
Klotz JL, Kirch BH, Aiken GE, Bush LP, Strickland JR (2009) Bioaccumulation of ergovaline in bovine lateral saphenous veins in vitro. Journal of Animal Science 87, 2437–2447.
| Bioaccumulation of ergovaline in bovine lateral saphenous veins in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnvVCjt7k%3D&md5=d47a79322daaebf0b5a919432bd9bbd0CAS | 19286813PubMed |
Klotz JL, Kirch BH, Aiken GE, Bush LP, Strickland JR (2010) Contractile response of fescue-naive bovine lateral saphenous veins to increasing concentrations of tall fescue alkaloids. Journal of Animal Science 88, 408–415.
| Contractile response of fescue-naive bovine lateral saphenous veins to increasing concentrations of tall fescue alkaloids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXls12jtw%3D%3D&md5=4be4154e57dfd1ef4864b657eb91e3f5CAS | 19783700PubMed |
Klotz JL, Brown KR, Xue Y, Matthews JC, Boling JA, Burris WR, Bush LP, Strickland JR (2012) Alterations in serotonin receptor-induced contractility of bovine lateral saphenous vein in cattle grazing endophyte-infected tall fescue. Journal of Animal Science 90, 682–693.
| Alterations in serotonin receptor-induced contractility of bovine lateral saphenous vein in cattle grazing endophyte-infected tall fescue.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XitVeltbg%3D&md5=ed06e6b69c88d2943f323300269f7702CAS | 22274863PubMed |
Klotz JL, Aiken GE, Johnson JM, Brown KR, Bush LP, Strickland JR (2013) Antagonism of lateral saphenous vein serotonin receptors from steers grazing endophyte-free, wild-type, or novel endophyte-infected tall fescue. Journal of Animal Science 91, 4492–4500.
| Antagonism of lateral saphenous vein serotonin receptors from steers grazing endophyte-free, wild-type, or novel endophyte-infected tall fescue.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVOlsbnI&md5=786805b78a69390cd97adbfb36d60ef4CAS | 23825335PubMed |
Klotz JL, Kim DH, Foote AP, Harmon DL (2014) Effects of ergot alkaloid exposure on serotonin receptor mRNA in the smooth muscle of the bovine gastrointestinal tract. Journal of Animal Science 92, 890–891.
Koontz AF, Bush LP, Klotz JL, McLeod KR, Schrick FN, Harmon DL (2012) Evaluation of a ruminally dosed tall fescue seed extract as a model for fescue toxicosis in steers. Journal of Animal Science 90, 914–921.
| Evaluation of a ruminally dosed tall fescue seed extract as a model for fescue toxicosis in steers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjsFSiurc%3D&md5=ce092223166d4b2b2ceda83d3055c2afCAS | 22064740PubMed |
Koontz AF, Kim DH, Foote AP, Bush LP, Klotz JL, McLeod KR, Harmon DL (2013) Alteration of fasting heat production during fescue toxicosis in Holstein steers. Journal of Animal Science 91, 3881–3888.
| Alteration of fasting heat production during fescue toxicosis in Holstein steers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtlOjsbfK&md5=63571c2562033abd954cf1c6b96abd08CAS | 23908162PubMed |
Lane GA, Ball OJP, Davies E, Davidson C (1997) ‘Ergovaline distribution in perennial ryegrass naturally infected with endophyte.’ In ‘Neotyphodium/grass interactions’. (Eds CW Bacon, NS Hill) pp. 65–67. (Springer: New York)
Larson BT, Samford MD, Camden JM, Piper EL, Kerley MS, Paterson JA, Turner JT (1995) Ergovaline binding and activation of D2 dopamine receptors in GH4ZR7 cells. Journal of Animal Science 73, 1396–1400.
Lean IJ (2001) Association between feeding perennial ryegrass (Lolium perenne cultivar Grasslands impact) containing high concentrations of ergovaline, and health and productivity in a herd of lactating dairy cows. Australian Veterinary Journal 79, 262–264.
| Association between feeding perennial ryegrass (Lolium perenne cultivar Grasslands impact) containing high concentrations of ergovaline, and health and productivity in a herd of lactating dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjtlGqtLo%3D&md5=8078527caf783e99760a34fd228b65b0CAS | 11349413PubMed |
Lyons PC, Plattner RD, Bacon CW (1986) Occurrence of peptide and clavine ergot alkaloids in tall fescue grass. Science 232, 487–489.
| Occurrence of peptide and clavine ergot alkaloids in tall fescue grass.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28XitVCjt7c%3D&md5=ec23ac318b88c053581332979d44c7bbCAS | 3008328PubMed |
McDowell KJ, Moore ES, Parks AG, Bush LP, Horohov DW, Lawrence LM (2013) Vasoconstriction in horses caused by endophyte-infected tall fescue seed is detected with Doppler ultrasonography. Journal of Animal Science 91, 1677–1684.
| Vasoconstriction in horses caused by endophyte-infected tall fescue seed is detected with Doppler ultrasonography.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXntFWrtbs%3D&md5=8823d557d92249ef7a8e279e361bf734CAS | 23449860PubMed |
McLeay LM, Smith BL (2006) Effects of ergotamine and ergovaline on the electromyographic activity of smooth muscle of the reticulum and rumen of sheep. American Journal of Veterinary Research 67, 707–714.
| Effects of ergotamine and ergovaline on the electromyographic activity of smooth muscle of the reticulum and rumen of sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xjslahurw%3D&md5=9babe6240305a312f59c075cedcc7e44CAS | 16579766PubMed |
McLeay LM, Smith BL, Reynolds GW (2002) Cardiovascular, respiratory, and body temperature responses of sheep to the ergopeptides ergotamine and ergovaline. American Journal of Veterinary Research 63, 387–393.
| Cardiovascular, respiratory, and body temperature responses of sheep to the ergopeptides ergotamine and ergovaline.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XisVagtr0%3D&md5=21ff6e04bbc649ea87ec1950f25ea923CAS | 11911573PubMed |
McPherson GA, Beart PM (1983) The selectivity of some ergot derivatives for alpha 1 and alpha 2-adrenoceptors of rat cerebral cortex. European Journal of Pharmacology 91, 363–369.
| The selectivity of some ergot derivatives for alpha 1 and alpha 2-adrenoceptors of rat cerebral cortex.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXltlehu70%3D&md5=92d9186f027fa5bdb58ec1d67354abb8CAS | 6311586PubMed |
Merrill ML, Bohnert DW, Harmon DL, Craig AM, Schrick FN (2007) The ability of a yeast-derived cell wall preparation to minimize the toxic effects of high-ergot alkaloid tall fescue straw in beef cattle. Journal of Animal Science 85, 2596–2605.
| The ability of a yeast-derived cell wall preparation to minimize the toxic effects of high-ergot alkaloid tall fescue straw in beef cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFSjtrjK&md5=768c32e911b1ee762a85362c79d70f50CAS | 17591716PubMed |
Meszaros J, Nimmerfall F, Rosenthaler J, Weber H (1975) Permanent bile duct cannulation in the monkey. A model for studying intestinal absorption. European Journal of Pharmacology 32, 233–242.
| Permanent bile duct cannulation in the monkey. A model for studying intestinal absorption.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28XltlOjtQ%3D%3D&md5=91b57d1312702d17d76aa04d6fb79bc4CAS | 1149809PubMed |
Miyazaki S, Ishizaki I, Ishizaka M, Kanbara T, Ishiguro-Takeda Y (2004) Lolitrem B residue in fat tissues of cattle consuming endophyte-infected perennial ryegrass straw. Journal of Veterinary Diagnostic Investigation 16, 340–342.
| Lolitrem B residue in fat tissues of cattle consuming endophyte-infected perennial ryegrass straw.Crossref | GoogleScholarGoogle Scholar | 15305749PubMed |
Moubarak AS, Rosenkrans CF (2000) Hepatic metabolism of ergot alkaloids in beef cattle by cytochrome P450. Biochemical and Biophysical Research Communications 274, 746–749.
| Hepatic metabolism of ergot alkaloids in beef cattle by cytochrome P450.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXltlertbc%3D&md5=fc9ce25cf382c58cf465d9fa00e84adaCAS | 10924348PubMed |
Moyer JL, Hill NS, Martin SA, Agee CS (1993) Degradation of ergoline alkaloids during in vitro ruminal digestion of tall fescue forage. Crop Science 33, 264–266.
| Degradation of ergoline alkaloids during in vitro ruminal digestion of tall fescue forage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXivFOgur4%3D&md5=81c506f6d77861a226dba53b5f61a0e3CAS |
Mulac D, Humpf HU (2011) Cytotoxicity and accumulation of ergot alkaloids in human primary cells. Toxicology 282, 112–121.
| Cytotoxicity and accumulation of ergot alkaloids in human primary cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsFCksrk%3D&md5=f4036b95fd4d30f7f406c70652cfa1e0CAS | 21295106PubMed |
Mulac D, Huwel S, Galla HJ, Humpf HU (2012) Permeability of ergot alkaloids across the blood-brain barrier in vitro and influence on the barrier integrity. Molecular Nutrition & Food Research 56, 475–485.
| Permeability of ergot alkaloids across the blood-brain barrier in vitro and influence on the barrier integrity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFOitL3E&md5=bd2f716861b5aa9068ca317a18148997CAS |
Müller-Schweinitzer E, Weidmann H (1978) Basic pharmacological properties. In ‘Ergot alkaloids and related compounds’. (Eds B Berde, HO Schild) pp. 87–232. (Springer-Verlag: Berlin)
Nicol AM, Klotz JL (2016) Ergovaline, an endophytic alkaloid. 2. Intake and impact on animal production, with reference to New Zealand. Animal Production Science 56, 1775–1786.
| Ergovaline, an endophytic alkaloid. 2. Intake and impact on animal production, with reference to New Zealand.Crossref | GoogleScholarGoogle Scholar |
Nixon AJ, Ford CA, Wildermoth JE, Craven AJ, Ashby MG, Pearson AJ (2002) Regulation of prolactin receptor expression in ovine skin in relation to circulating prolactin and wool follicle growth status. The Journal of Endocrinology 172, 605–614.
| Regulation of prolactin receptor expression in ovine skin in relation to circulating prolactin and wool follicle growth status.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XisVSht7o%3D&md5=bbb99bb5ebb4d9da8ea3e9f4828e239eCAS | 11874709PubMed |
Nolan A, Livingston A, Waterman A (1986) The effects of alpha 2 adrenoceptor agonists on airway pressure in anaesthetized sheep. Journal of Veterinary Pharmacology and Therapeutics 9, 157–163.
| The effects of alpha 2 adrenoceptor agonists on airway pressure in anaesthetized sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28XktlGmt7w%3D&md5=988c927be13eb60a4da55cd4a1bd22acCAS | 2873256PubMed |
Oliver JW (1997) Physiological manifestations of endophyte toxicosis in ruminant and laboratory species. In ‘Neotyphodium/grass interactions’. (Eds CW Bacon, NS Hill) pp. 311–346. (Springer: New York)
Oliver JW, Strickland JR, Waller JC, Fribourg HA, Linnabary RD, Abney LK (1998) Endophytic fungal toxin effect on adrenergic receptors in lateral saphenous veins (cranial branch) of cattle grazing tall fescue. Journal of Animal Science 76, 2853–2856.
Pertz HH, Eckart E (1999) Ergot alkaloids and their derivatives as ligands for serotoninergic, dopaminergic, and adrenergic receptors. In ‘Ergot’. (Eds V Kren, L Cvak) pp. 441–450. (Harwood Academic Publishers: Amsterdam, The Netherlands)
Pesqueira A, Harmon DL, Branco AF, Klotz JL (2014) Bovine lateral saphenous veins exposed to ergopeptine alkaloids do not relax. Journal of Animal Science 92, 1213–1218.
| Bovine lateral saphenous veins exposed to ergopeptine alkaloids do not relax.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXltVahtLg%3D&md5=0bba5db93cd2a4ecbf175f713716bf1cCAS | 24492541PubMed |
Poole DP, Littler RA, Smith BL, McLeay LM (2009) Effects and mechanisms of action of the ergopeptides ergotamine and ergovaline and the effects of peramine on reticulum motility of sheep. American Journal of Veterinary Research 70, 270–276.
| Effects and mechanisms of action of the ergopeptides ergotamine and ergovaline and the effects of peramine on reticulum motility of sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXivVWgtbY%3D&md5=d1f3bc5380ea1db126dbca97682e1e34CAS | 19231961PubMed |
Popay AJ, Gerard PJ (2007) Cultivar and endophyte effects on a root aphid, Aploneura lentisci, in perennial ryegrass. New Zealand Plant Protection 60, 223–227.
Popay AJ, Latch GC (1993) Prospects for utilising endophytes for grass resistance to insect pests in New Zealand. In ‘6th Australasian conference on grassland invertebrate ecology’. pp. 129–155. (AgResearch: Hamilton, New Zealand)
Realini CE, Duckett SK, Hill NS, Hoveland CS, Lyon BG, Sackmann JR, Gillis MH (2005) Effect of endophyte type on carcass traits, meat quality, and fatty acid composition of beef cattle grazing tall fescue. Journal of Animal Science 83, 430–439.
Rowan DD, Shaw GJ (1987) Detection of ergopeptine alkaloids in endophyte-infected perennial ryegrass by tandem mass spectrometry. New Zealand Veterinary Journal 35, 197–198.
| Detection of ergopeptine alkaloids in endophyte-infected perennial ryegrass by tandem mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2MzntlaltQ%3D%3D&md5=ac6a1f4ec07d30bf96daa436b0205722CAS | 16031349PubMed |
Rowan DD, Dymock JJ, Brimble MA (1990) Effect of fungal metabolite peramine and analogs on feeding and development of argentine stem weevil (Listronotus bonariensis). Journal of Chemical Ecology 16, 1683–1695.
| Effect of fungal metabolite peramine and analogs on feeding and development of argentine stem weevil (Listronotus bonariensis).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXlsVOhsro%3D&md5=a8d12c5e52d53a0b4e22314ed301f535CAS | 24263837PubMed |
Schardl CL, Young CA, Hesse U, Amyotte SG, Andreeva K, Calie PJ, Fleetwood DJ, Haws DC, Moore N, Oeser B, Panaccione DG, Schweri KK, Voisey CR, Farman ML, Jaromczyk JW, Roe BA, O’Sullivan DM, Scott B, Tudzynski P, An Z, Arnaoudova EG, Bullock CT, Charlton ND, Chen L, Cox M, Dinkins RD, Florea S, Glenn AE, Gordon A, Guldener U, Harris DR, Hollin W, Jaromczyk J, Johnson RD, Khan AK, Leistner E, Leuchtmann A, Li C, Liu J, Liu J, Liu M, Mace W, Machado C, Nagabhyru P, Pan J, Schmid J, Sugawara K, Steiner U, Takach JE, Tanaka E, Webb JS, Wilson EV, Wiseman JL, Yoshida R, Zeng Z (2013) Plant-symbiotic fungi as chemical engineers: multi-genome analysis of the clavicipitaceae reveals dynamics of alkaloid loci. PLOS Genetics 9, e1003323
| Plant-symbiotic fungi as chemical engineers: multi-genome analysis of the clavicipitaceae reveals dynamics of alkaloid loci.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXktlSksr0%3D&md5=e219b29648701c68f01b4af88f969081CAS | 23468653PubMed |
Schillo KK, Leshin LS, Boling JA, Gay N (1988) Effects of endophyte-infected fescue on concentrations of prolactin in blood sera and the anterior pituitary and concentrations of dopamine and dopamine metabolites in brains of steers. Journal of Animal Science 66, 713–718.
Schöning C, Flieger M, Pertz HH (2001) Complex interaction of ergovaline with 5-HT2A, 5-HT1B/1D, and alpha1 receptors in isolated arteries of rat and guinea pig. Journal of Animal Science 79, 2202–2209.
Schultz CL, Lodge-Ivey SL, Bush LP, Craig AM, Strickland JR (2006) Effects of initial and extended exposure to an endophyte-infected tall fescue seed diet on faecal and urinary excretion of ergovaline and lysergic acid in mature geldings. New Zealand Veterinary Journal 54, 178–184.
| Effects of initial and extended exposure to an endophyte-infected tall fescue seed diet on faecal and urinary excretion of ergovaline and lysergic acid in mature geldings.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xps1Cnu70%3D&md5=4da540027451ab9a4d612009153e1a55CAS | 16915339PubMed |
Schumann B, Danicke S, Meyer U, Ueberschar KH, Breves G (2007) Effects of different levels of ergot in concentrates on the growing and slaughtering performance of bulls and on carry-over into edible tissue. Archives of Animal Nutrition 61, 357–370.
| Effects of different levels of ergot in concentrates on the growing and slaughtering performance of bulls and on carry-over into edible tissue.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVSku7bJ&md5=d4c6a327d54f52bd75d7d65bad0595e1CAS | 18030918PubMed |
Schumann B, Lebzien P, Ueberschar KH, Danicke S (2009) Effects of the level of feed intake and ergot contaminated concentrate on ergot alkaloid metabolism and carry over into milk. Molecular Nutrition & Food Research 53, 931–938.
| Effects of the level of feed intake and ergot contaminated concentrate on ergot alkaloid metabolism and carry over into milk.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpsV2lu7w%3D&md5=cf2f2d758f9639c59dbf862b2417e834CAS |
Shappell NW, Smith DJ (2005) Ergovaline movement across Caco-2 cells. In Vitro Cellular & Developmental Biology. Animal 41, 245–251.
| Ergovaline movement across Caco-2 cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVymu7g%3D&md5=0904fe0eda30fb24f8c1c07d8bde1c5cCAS |
Sibley DR, Monsma FJ (1992) Molecular biology of dopamine receptors. Trends in Pharmacological Sciences 13, 61–69.
| Molecular biology of dopamine receptors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XhsVCjs74%3D&md5=cb139d737226c1bcf4f9cba702e79effCAS | 1561715PubMed |
Siegel MR, Johnson MC, Varney DR, Nesmith WC, Buckner RC, Bush LP, Burrus PB, Jones TA, Boling JA (1984) A fungal endophyte in tall fescue: incidence and dissemination. Phytopathology 74, 932–937.
| A fungal endophyte in tall fescue: incidence and dissemination.Crossref | GoogleScholarGoogle Scholar |
Smith DJ, Shappell NW (2002) Technical note: epimerization of ergopeptine alkaloids in organic and aqueous solvents. Journal of Animal Science 80, 1616–1622.
Spiering MJ, Lane GA, Christensen MJ, Schmid J (2005) Distribution of the fungal endophyte Neotyphodium lolii is not a major determinant of the distribution of fungal alkaloids in Lolium perenne plants. Phytochemistry 66, 195–202.
| Distribution of the fungal endophyte Neotyphodium lolii is not a major determinant of the distribution of fungal alkaloids in Lolium perenne plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXksVektw%3D%3D&md5=437f3b0d15fb87bb88ae44dcc7ea1e49CAS | 15652576PubMed |
Spiers DE, Eichen PA, Scharf B, Settivari RS, Vellios H, Johnson J, Bryant J, Kishore D (2012) Fescue toxicosis and heat stress: recent advances. In ‘Epichloae, endophytes of cool season grasses: implications, utilization and biology’. (Eds CA Young, GE Aiken, RL McCulley, JR Strickland, CL Schardl) pp. 20–23. (Noble Foundation: Ardmore, OK)
Stadler PA, Frey AJ, Ott H, Hofamann A (1964) Die Synthese des Ergosins und des Valin-analogen der Ergotamin-gruppe. 61. Mitteilung über Mutterkornalkaloide. Helvetica Chimica Acta 47, 1911–1921.
| Die Synthese des Ergosins und des Valin-analogen der Ergotamin-gruppe. 61. Mitteilung über Mutterkornalkaloide.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2MXptFWkug%3D%3D&md5=66d6c5942f32dcb4e7f29d625cfa6088CAS |
Strickland JR, Oliver JW, Cross DL (1993) Fescue toxicosis and its impact on animal agriculture. Veterinary and Human Toxicology 35, 454–464.
Strickland JR, Cross DL, Birrenkott GP, Grimes LW (1994) Effect of ergovaline, loline, and dopamine antagonists on rat pituitary cell prolactin release in vitro. American Journal of Veterinary Research 55, 716–721.
Strickland JR, Aiken GE, Klotz JL (2009a) Ergot alkaloid induced blood vessel dysfunction contributes to fescue toxicosis. Forage and Grazinglands 7,
| Ergot alkaloid induced blood vessel dysfunction contributes to fescue toxicosis.Crossref | GoogleScholarGoogle Scholar |
Strickland JR, Aiken GE, Spiers DE, Fletcher LR, Oliver JW, Fribourg HA, Hannaway DB, West CP (2009b) Physiological basis of fescue toxicosis. In ‘Tall fescue for the twenty-first century’. Agronomy Monograph 53. (Eds HA Fribourg, DB Hannaway, CP West) pp. 203–227. (American Society of Agronomy, Inc., Crop Science Society of America, Inc., Soil Science Society of America, Inc.: Madison, WI)
Strickland JR, Looper ML, Matthews JC, Rosenkrans CF, Flythe MD, Brown KR (2011) Board-invited review: St Anthony’s fire in livestock: causes, mechanisms, and potential solutions. Journal of Animal Science 89, 1603–1626.
| Board-invited review: St Anthony’s fire in livestock: causes, mechanisms, and potential solutions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXls1Ohsrk%3D&md5=7aa0e9e6c93cda5c9df97f89f4ea525dCAS | 21521821PubMed |
Stuedemann JA, Hill NS, Thompson FN, Fayrer-Hosken RA, Hay WP, Dawe DL, Seman DH, Martin SA (1998) Urinary and biliary excretion of ergot alkaloids from steers that grazed endophyte-infected tall fescue. Journal of Animal Science 76, 2146–2154.
Tapper BA, Lane GA (2004) ‘Janthitrems found in a Neothyphodium endophyte of perennial ryegrass. In ‘The 5th international symposium of Neotyphodium/grass interactions’.
Thom ER, Waugh CD, Minnee EM, Waghorn GC (2013) Effects of novel and wild-type endophytes in perennial ryegrass on cow health and production. New Zealand Veterinary Journal 61, 87–97.
| Effects of novel and wild-type endophytes in perennial ryegrass on cow health and production.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38bgsVWnsA%3D%3D&md5=9f5e28c4175c61b4ae346638d81a4fcfCAS | 22913546PubMed |
Thompson FN, Stuedemann JA (1993) Pathophysiology of fescue toxicosis. Agriculture, Ecosystems & Environment 44, 263–281.
| Pathophysiology of fescue toxicosis.Crossref | GoogleScholarGoogle Scholar |
Tor-Agbidye J, Blythe LL, Craig AM (2001) Correlation of endophyte toxins (ergovaline and lolitrem B) with clinical disease: fescue foot and perennial ryegrass staggers. Veterinary and Human Toxicology 43, 140–146.
Westendorf ML, Mitchell GE, Tucker RE (1992) Influence of rumen fermentation on response to endophyte-infected tall fescue seed measured by a rat bioassay. Drug and Chemical Toxicology 15, 351–364.
| Influence of rumen fermentation on response to endophyte-infected tall fescue seed measured by a rat bioassay.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXitFamtw%3D%3D&md5=0f9f55ab83feebfcd510b0409ce84b29CAS | 1459045PubMed |
Westendorf ML, Mitchell GE, Tucker RE, Bush LP, Petroski RJ, Powell RG (1993) In vitro and in vivo ruminal and physiological responses to endophyte-infected tall fescue. Journal of Dairy Science 76, 555–563.
| In vitro and in vivo ruminal and physiological responses to endophyte-infected tall fescue.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXitlamtr0%3D&md5=c3d30c9035f3171b6576736a5b762147CAS | 8445102PubMed |
Williams M, Shaffer SR, Garner GB, Yates SG, Tookey HL, Kintner LD, Nelson SL, McGinity JT (1975) Induction of fescue foot syndrome in cattle by fractionated extracts of toxic fescue hay. American Journal of Veterinary Research 36, 1353–1357.
Yates SG, Plattner RD, Garner GB (1985) Detection of ergopeptine alkaloids in endophyte infected, toxic Ky-31 tall fescue by mass spectrometry/mass spectrometry. Journal of Agricultural and Food Chemistry 33, 719–722.
| Detection of ergopeptine alkaloids in endophyte infected, toxic Ky-31 tall fescue by mass spectrometry/mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXktlCmuro%3D&md5=fc3c0c22ba86b4cce34aa88ed24d2277CAS |
Young CA, Hume DE, McCulley RL (2013) Forages and pastures symposium: fungal endophytes of tall fescue and perennial ryegrass: pasture friend or foe? Journal of Animal Science 91, 2379–2394.
| Forages and pastures symposium: fungal endophytes of tall fescue and perennial ryegrass: pasture friend or foe?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXotl2gu7w%3D&md5=5a77f93eb23c4c90b77c23abcd6bd266CAS | 23307839PubMed |
Zanzalari KP, Heitmann RN, McLaren JB, Fribourg HA (1989) Effects of endophyte-infected fescue and cimetidine on respiration rates, rectal temperatures and hepatic mixed function oxidase activity as measured by hepatic antipyrine metabolism in sheep. Journal of Animal Science 67, 3370–3378.
Zbib N, Repussard C, Tardieu D, Priymenko N, Domange C, Guerre P (2015) Toxicity of endophyte-infected ryegrass hay containing high ergovaline level in lactating ewes. Journal of Animal Science 93, 4098–4109.
| Toxicity of endophyte-infected ryegrass hay containing high ergovaline level in lactating ewes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhsVWktrjK&md5=b92b385f08401f061ac2735ef2efc95fCAS | 26440189PubMed |
1 Mention of trade name, proprietary product, or specified equipment does not constitute a guarantee or warranty by the USDA and does not imply approval to the exclusion of other products that may be suitable.