Supplementation of reduced protein diets with l-arginine and l-citrulline for broilers challenged with subclinical necrotic enteritis. 1. Growth, carcass yield, and intestinal lesion scores
Hiep Thi Dao A B , Nishchal K. Sharma A , Ali Daneshmand A , Alip Kumar A , Emma J. Bradbury C , Shu-Biao Wu
A B , Nishchal K. Sharma A , Ali Daneshmand A , Alip Kumar A , Emma J. Bradbury C , Shu-Biao Wu  A and Robert A. Swick
A and Robert A. Swick  A *
A *
A School of Environmental and Rural Science, Faculty of Science, Agriculture, Business and Law, University of New England, Armidale, NSW 2351, Australia.
B Faculty of Animal Science, Vietnam National University of Agriculture, Trau Quy Town, Gia Lam District, Hanoi 100000, Vietnam.
C Ridley AgriProducts, Melbourne, Vic. 3000, Australia.
Animal Production Science 62(13) 1236-1249 https://doi.org/10.1071/AN21393
Submitted: 28 July 2021 Accepted: 6 February 2022 Published: 4 March 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Improving immune status through nutritional adjustments may be part of an effective strategy to reduce reliance on antibiotic growth promoters for controlling necrotic enteritis (NE) in broiler chickens.
Aims: This study examined the effect of dietary protein level and the replacement of crystalline l-arginine (Arg) with l-citrulline (Cit) in the reduced-protein diet on the performance of broilers challenged with subclinical NE.
Methods: Ross 308 cockerels (n = 720) were randomly allocated to six dietary treatments, with eight replicates of 15 birds per pen, during a 35-day feeding experiment. The treatments were as follows: standard protein without NE challenge (SP−); standard protein with NE challenge (SP+); reduced protein (two percentage points lower crude protein) without NE challenge (RP−); reduced protein with NE challenge (RP+); RP+ plus added Arg (103% of RP, RPA+) and RPC+ where supplemental Arg in RPA+ was replaced with Cit. The first four treatments were considered as a 2 × 2 factorial arrangement, with factors being NE (− or +) and protein level (SP or RP). Treatments SP+, RP+, RPA+, and RPC+ were analysed by one-way ANOVA.
Key results: Subclinical NE challenge reduced feed intake (FI), reduced body weight gain (BWG) and increased feed to gain ratio (FCR) from Day 0 to Day 35, increased intestinal lesion scores on Day 16, and reduced relative breast yield on Day 35 (P < 0.05). Feeding RP diets increased FI (P < 0.001), increased BWG (P < 0.01) and reduced FCR (P < 0.01) during the grower phase compared with SP diets when birds were challenged with NE. Birds in the RPC+ treatment had a lower overall FCR than did those in the SP+ treatment (P < 0.001). Birds in the RPA+ treatment had similar FI, BWG and FCR to those in the RP+ treatment (P > 0.05).
Conclusions: Collectively, the results showed protective effects of replacing the supplemental Arg with Cit against NE in RP diets, as indicated by higher performance during and after the challenge.
Implications: Feeding the RP diets supplemented with Cit may be part of an effective strategy to reduce reliance on antibiotic growth promoters for controlling NE in broiler chickens.
Keywords: arginine, carcass yield, citrulline, liver, low protein, meat chicken, necrotic enteritis, uric acid.
Introduction
Economic losses from necrotic enteritis (NE) have increased in the broiler industry with the removal of in-feed antibiotic growth promoters (Widyaratne 2012). The disease is caused by Clostridium (C.) perfringens, a Gram-positive, rod-shaped, spore-forming, and non-motile bacteria (Wilson et al. 2005). In clinical form, C. perfringens may overgrow in the small intestine, causing mucosal necrosis, acute diarrhoea, and high mortality rates (up to 40%, Ross 1999). Coccidiosis predisposes birds to NE (Rodgers et al. 2015). The subclinical form of NE is more economically devastating than is the acute form as it reduces growth performance in a greater number of birds, and increases carcass condemnation at slaughter (Løvland and Kaldhusdal 1999; Lovland and Kaldhusdal 2001). Subclinical NE may also increase feed cost as birds are kept for a longer time to achieve their target bodyweight (BW). Losses in the global broiler industry due to NE have been estimated to be USD6 billion annually (Wade and Keyburn 2015). Furthermore, the presence of C. perfringens in poultry meat has raised public health concerns as it is considered a factor in causing food-born infections (Immerseel et al. 2004). Incorporating antibiotics in broiler diets has been used as a common approach to control NE in birds (Widyaratne 2012). However, a ban on in-feed antibiotic growth promoters (AGP) in animal production in several countries with increasing consumer demand for AGP-free poultry products has resulted in an increased incidence of NE worldwide (Kaldhusdal and Lovland 2000). Mwangi et al. (2019) recently reported that confounding factors such as bird health before the proliferation of virulent C. perfringens are crucial for NE development in broiler chickens. Thus, improving immune status through nutritional adjustments may be part of an effective strategy to reduce reliance on AGP for controlling NE in broiler chickens.
Dietary protein concentration and amino acid (AA) balance play a critical role in the development of C. perfringens in the gastrointestinal tract (Drew et al. 2004). Diets with a high protein level, particularly animal-derived proteins, have been shown to facilitate the proliferation of C. perfringens and are considered a predisposing factor for NE (Drew et al. 2004; Wilkie et al. 2005; Liu et al. 2017). A high concentration of protein-bound AA from high crude protein (CP) diets and the use of poorly digested protein sources increase undigested protein reaching the hindgut (Hilliar et al. 2019). This material can then provide a substrate for the proliferation of gut-specific pathogens, including C. perfringens, that may reduce gut health and growth performance (Lan et al. 2004; Mcdevitt et al. 2006) and increase losses from NE. Thus, the use of reduced-protein diets with greater protein digestibility may be beneficial for chickens subjected to NE challenge.
Arginine (Arg) has been known to have a direct immunomodulatory effect through its metabolic pathways involving the production of ornithine and nitric oxide (NO, Le Floc’h et al. 2004). It has been hypothesised that the demand for Arg would increase in birds exposed to inflammation or disease stress (Kidd 2004; Le Floc’h et al. 2004; Li et al. 2007). Furthermore, Arg has been demonstrated to effectively compensate for reduced growth performance in birds inoculated with infectious bronchitis virus (Lee et al. 2002) and alleviate gut injury and normalise ileal microbiota population in C. perfringens-challenged birds (Zhang et al. 2018). Citrulline (Cit), a metabolite of Arg, can be recycled to Arg and has been reported to be more effective than dietary Arg in increasing blood Arg concentrations and NO production in mammals (Schwedhelm et al. 2008; Lassala et al. 2009; Wijnands et al. 2012). There are few if any reports on Arg and/or Cit supplementation in broilers fed reduced-protein (RP) diets. Thus, this study was designed to investigate the effects of Arg and Cit supplementation to RP diets on growth performance, carcass traits, internal organ weights, serum uric acid, and intestinal lesion score of broilers growing under NE challenge. The results of this study may provide valuable information to control and/or mitigate the effects of NE in AGP-free poultry production.
Materials and methods
Experimental design and diets
The study was implemented at the Centre of Animal Research and Teaching at the University of New England, Armidale, New South Wales, Australia, approved by its Animal Ethics Committee (Approval number: AEC19-119), and met the requirements of the Australian code of practice to care and use of animals for scientific purposes (NHMRC 2013). Day-old Ross 308 cockerels (n = 720) were assigned to 48 equal-sized floor pens (120 × 80 cm), with 15 birds per pen and eight replicates per treatment. Starting pen weights were similar across treatments. Birds were grown to mimic commercial conditions with hardwood shavings as bedding material in environmentally controlled rooms. Feed and water were provided ad libitum throughout the 35-day feeding study. The temperature, lighting and ventilation conditions followed Ross 308 recommendations (Aviagen 2014a). Six treatments were used in this study, with eight replicate pens per treatment. Feed was provided as crumbles for starter (Days 0–10), and pellets for grower (Days 10–24) and finisher (Days 24–35) phases. Feed was pelleted at a temperature of 65°C. The treatments were as follows: standard protein diet without NE challenge (SP−); SP with NE challenge (SP+); reduced protein balanced with crystalline AA without NE challenge (RP−); RP with NE challenge (RP+); RP diet supplemented with additional Arg to 103% of the requirement (equal to 15% additional supplemental crystalline Arg) with NE challenge (RPA+); and RP with Cit replacing all supplemental Arg in previous treatment with NE challenge (RPC+). The levels of essential AA in the RP diet were equivalent to those in the SP diet and in accordance with Ross 308 broiler nutrition specifications (Aviagen 2014b). Concentrations of added crystalline Arg in the RP treatments in starter, grower and finisher phases were 0.217%, 0.213% and 0.212% respectively. Concentrations of added crystalline Arg in the RPA+ treatment in starter, grower and finisher phases were 0.249%, 0.245%, and 0.244% respectively. Concentrations of Cit in the RPC+ treatment were equivalent to Arg concentrations in the RPA+ treatment. Details on diet composition and nutrient contents are presented in Tables 1 and 2. Arg and Cit were supplemented in the RP diets at the expense of wheat. The nutritional compositions of wheat, sorghum and soybean meal were analysed before diet formulation. Crude protein, crude fat, dry matter and ash content of ingredients were measured using AOAC methods (AOAC 1994) and metabolisable energy, and total and digestible AA were estimated using near-infrared reflectance spectroscopy (Foss NIR 6500, Denmark) and standardised with the Evonik AMINONIR® Advanced calibration. There was a two percentage point difference in crude protein content between SP and RP diets for all feeding phases.

|

|
Necrotic enteritis challenge
Subclinical NE was established following procedures previously described by Rodgers et al. (2015). On Day 9, birds in RPA+ and RPC+ treatments and half of the birds in SP and RP treatments (challenged group) were orally inoculated with 1 mL of sterile phosphate-buffered solution (PBS) containing a vaccine strain of Eimeria with 5000 sporulated oocysts of Eimeria acervulina, 5000 sporulated oocysts of Eimeria maxima, and 2500 sporulated oocysts of Eimeria brunetti (Eimeria Pty Ltd, Ringwood, Victoria, Australia). The remaining birds in SP and RP treatments (eight replicates for each) were given 1 mL of sterile PBS as a sham treatment on Day 9 (unchallenged control groups). On Day 14, challenged birds were orally inoculated with 1 mL of C. perfringens with an approximate concentration of 108 CFU (EHE-NE18 strain, Commonwealth Scientific and Industrial Research Organization, Geelong, Victoria, Australia) in a starch thioglycollate broth. Birds in the unchallenged groups were orally inoculated with 1 mL of sterile thioglycollate broth media as a sham treatment. Necropsy was performed on the victims to determine the cause of death.
Data collection
Bodyweight and feed consumption were measured per pen on Days 8, 10, 14, 16, 24 and 35 of the study. Bodyweight gain (BWG) and feed conversion ratio (expressed as feed:gain, FCR) were then calculated accordingly. The FCR was corrected for mortality by adding the weight gain of dead birds to live birds for each period. Feed intake (FI) was calculated as the corrected FCR multiplied by BWG. On Day 16, three birds per pen were randomly collected, weighed, electrically stunned (MEFE CAT 44N, Mitchell Engineering Food Equipment, Clontarf, Queensland, Australia), and euthanised by decapitation for collection of blood (from a jugular vein) and small intestine for serum uric acid measurement and lesion scoring respectively. Blood samples were collected in vacutainers (Becton, Dickinson UK Ltd, Plymouth, UK) containing spray-coated silica and a polymer gel and centrifuged at 3000g at 4°C for 10 min to separate the serum. Serum samples were stored at −20°C until further analysis. Serum uric acid concentration was quantified using an integrated chemistry analyser (Siemens Dimension Xpand Plus, Siemens Healthcare, Newark, NJ, USA) following the manufacturer’s instructions (URCA Uric Acid, reference number: DF77). Lesion scoring (scored from 0 to 6 on lesion severity) was performed in the duodenum, jejunum and ileum samples on Day 16 by experienced personnel blind to the experimental design following criteria described by Keyburn et al. (2006). Weights of internal organs (liver, spleen, bursa of Fabricius) were also collected on Day 16. On Day 35, two birds per pen were randomly selected and euthanised using similar procedures as described for Day 16 sampling. After birds were dissected, weights of different carcass cuts (breast, thigh and drumstick, abdominal fat) and internal organs (liver, spleen, bursa of Fabricius) were determined. Weights of breast, thigh and drumstick, abdominal fat, and internal organs on Day 16 and Day 35 were expressed as relative weights per unit of live BW.
Feed analysis
The nutrient composition including CP, dry matter, crude fiber, ash contents, and AA profiles of diets were analysed by standard methods (AOAC 1994). Added Cit in RPC diets were quantified using the Waters AccQTag amino acid analysis methodology (Cohen 2001), but adapted to run on an ultra-performance liquid chromatography system as described by Wheat et al. (2008). Specifically, samples (100–130 mg) were weighed in duplicate into hydrolysis vials and 5 mL of 20% HCl was added. The samples were then incubated at 110°C for 24 h. After hydrolysis, the samples were derivatised using AccQTag reagents (Waters Corporation, Milford, MA, USA). Then, samples were analysed using a high-resolution reversed-phase column (BEH C18, 2.1 × 100 mm; 1.7 μm) on an ultra-performance liquid chromatography system with a 12-min run time. The column temperature, detection wavelength, and flow rate employed were 57°C, 260 nm and 0.55 mL/min respectively.
Data analyses
R Commander (version 3.3.1, R Foundation for Statistical Computing, Vienna, Austria) was used to analyse data. All data were tested for normality and variance homogeneity before analysis. First, two-way ANOVA was used to test the interaction between NE challenge (no or yes) and protein level (SP or RP) in four treatments, including SP−, SP+, RP− and RP+ (2 × 2 factorial arrangement of treatments). The results were used to evaluate the successful implementation of the NE model and the interactions between NE and protein levels. Then, one-way ANOVA was used to test statistical differences between the four NE-challenged treatments (SP+, RP+, RPA+, and RPC+) that were then employed to evaluate the effects of Arg and Cit supplementation to the RP diets during the NE challenge. Tukey’s post hoc test was used to identify pairwise differences between the treatments from significant ANOVA results. As data on livability and intestinal lesion scores were found to be not normally distributed, they were tested for significance using the Kruskal–Wallis non-parametric test and were not subjected to two-way ANOVA. The P-value of <0.05 was considered significant, and a tendency was considered at 0.05 ≤ P ≤ 0.10.
Results
Diet and growth performance
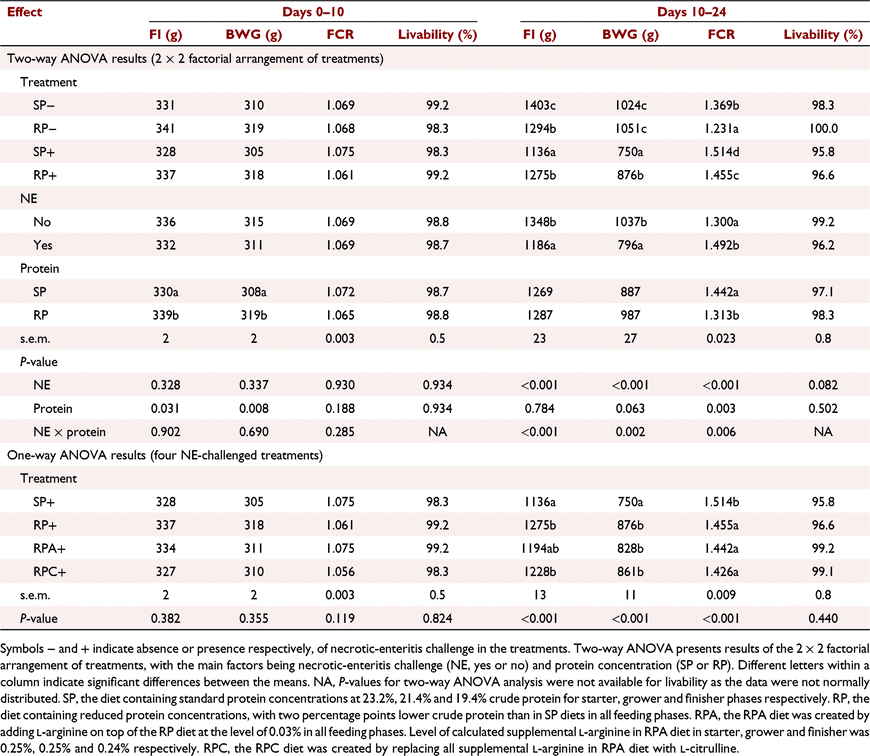
Generally, the final diets satisfied formulation objectives in terms of achieving reduced CP diets with lower CP concentrations than in the SP diets. The analysed CP concentrations of all starter diets were higher than expected, with only a one-percentage point difference between the SP and RP diet; however, those of grower and finisher diets were as expected, with a two-percentage point difference between the SP and RP diets. The analysed AA contents of the diets were generally consistent with the calculated values (Tables 1, 2). The analysed lysine concentrations of the RP diets were lower than the recommended total lysine concentrations for Ross 308 broilers (Aviagen 2014b), but similar to those of the SP diets.
Performance results are presented in Tables 3 and 4. The NE × protein interactions were not observed for performance parameters from Day 0 to Day 10 (P > 0.05; Table 3). From Day 10 to Day 24, NE × protein interactions were detected for FI (P < 0.001), BWG (P < 0.01) and FCR (P < 0.01), indicating higher performance with the RP diet than with the SP diet in NE-challenged birds (Table 3). Under NE-challenge conditions, birds fed the RP had higher FI (P < 0.001), higher BWG (P < 0.001) and lower FCR (P < 0.001) than did those fed the SP diet from Day 10 to Day 24. The NE × protein interactions were also observed for FI (P < 0.05) and FCR (P < 0.001) in the overall period (Days 0–35; Table 4). Birds fed the RP diet had similar FI (P > 0.05) but lower FCR (P < 0.001) than did those fed the SP diet only when the birds were not challenged with NE from Day 0 to Day 35. In NE-challenged birds, FI and FCR were not different between birds fed the RP and SP diets from 0 to 35 days (P > 0.05). NE challenge reduced FI in birds fed the SP diets but not in birds fed the RP diets from Day 0 to Day 35.
|

|
Necrotic-enteritis challenge as the main effect did not affect growth performance during the starter phase (Days 0–10) as expected, as Eimeria gavaging was not applied until Day 9, but the challenge decreased (P < 0.05) FI and BWG and increased (P < 0.05) FCR during the grower (Days 10–24), finisher (Days 24–35), and overall periods (Tables 3, 4). Birds challenged with NE had 12% lower FI (1186 g vs 1348 g), 23% lower BWG (796 g vs 1037 g), and 19 points higher FCR (1.492 vs 1.300) than did unchallenged birds during the grower phase (P < 0.001; Table 3). Birds fed the RP diet had a higher FI and BWG than did those offered the SP diet during the starter phase (P < 0.05), as shown by the main effect of protein concentration. Protein concentration as the main effect did not affect FI, BWG and FCR during the finisher phase (P > 0.05). Neither NE challenge nor protein concentration affected livability (P > 0.05; Tables 3, 4).
Birds in the RP+, RPA+ and RPC+ treatments had a higher BWG and lower FCR than did those in the SP+ treatment in the grower phase (P < 0.001; Table 3). Additionally, birds in the RP+ and RPC+ treatments had a higher FI than did those in the SP+ treatment in the grower phase (P < 0.001; Table 3). During the finisher phase, FI, BWG and FCR were not different among the SP+, RP+, RPA+ and RPC+ treatments (P > 0.05; Table 4). Birds in the RPA+ treatment had 7% lower FI (3107 g vs 3348 g, P = 0.01), 7% lower BWG (2088 g vs 2247 g, P < 0.01), but similar FCR (P > 0.05) to those in the RP+ treatment from Day 0 to Day 35. Birds in the RPC+ treatment had lower FCR (approximately three points) than did birds in the SP+ treatment from Day 0 to Day 35 (1.469 vs 1.497, P < 0.05).
Serum uric acid concentration and intestinal lesion score
A NE × protein interaction was detected for serum uric acid concentration on Day 16 (P < 0.01; Table 5), indicating that serum uric acid was decreased in birds fed the SP diet when they were challenged with NE. The one-way ANOVA results showed that birds in the RP+, RPA+ and RPC+ treatments had lower serum uric acid concentration on Day 16 than did those in the SP+ treatment (P < 0.01; Table 5). Necrotic-enteritis challenge as the main effect increased lesion scores of both duodenum (P < 0.01), jejunum (P < 0.001) and ileum (P < 0.001) on Day 16 (Fig. 1). Intestinal lesion scores on Day 16 were not different between birds fed SP or RP diets (P > 0.05; Fig. 1). The one-way ANOVA results showed that intestinal lesion scores on Day 16 were not different among the SP+, RP+, RPA+ and RPC+ treatments (P > 0.05; Fig. 1).

|
Carcass traits and internal organ weights
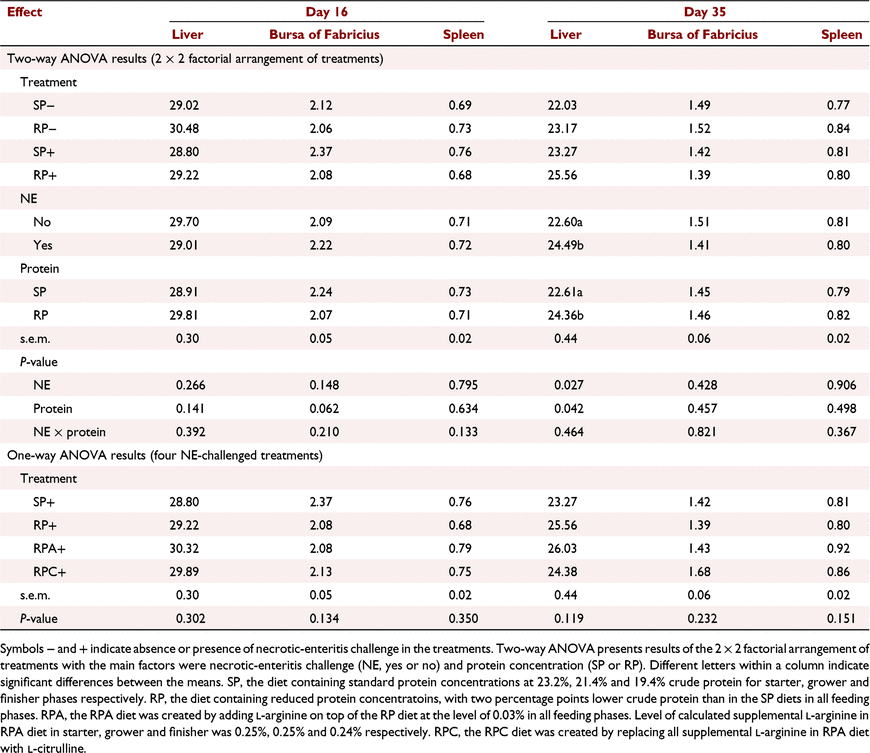
Results on relative weights of the carcass on Day 35 as well as relative weights of liver, spleen and bursa of Fabricius on Day 16 and Day 35 are shown in Tables 5 and 6. No NE × protein interactions were observed for relative weights of breast, thigh and drumstick, and fat pad on Day 35 (P > 0.05; Table 5). Necrotic-enteritis challenge as the main effect reduced relative weights of the breast (P < 0.01), increased relative weight of thigh and drumstick (P < 0.05), and tended to increase relative fat pad weight on Day 35 (P = 0.079; Table 5). Protein concentration as the main effect did not affect relative weights of breast, thigh and drumstick, and fat pad on Day 35 (P > 0.05; Table 5). The relative breast weight of birds in the RPC+ treatment was higher than that of SP+ birds on Day 35 (P < 0.001; Table 5).
|
No NE × protein interactions were detected for relative weights of the liver, spleen, and bursa of Fabricius on day 16 and day 35 (P > 0.05; Table 6). Necrotic enteritis challenge as the main effect increased relative weight of the liver on day 35 (P < 0.05; Table 6). Birds fed the RP diet tended to have lower relative bursa of Fabricius weight on day 16 (P = 0.062), and higher relative liver weight on day 35 (P < 0.05) compared to those of SP fed birds regardless of the NE challenge. Arginine or Cit supplementation to the RP+ diet did not affect relative weights of liver, spleen, and bursa of Fabricus in the respective groups on day 16 and day 35 (P > 0.05; Table 6).
Discussion
The NE challenge established in the current study was effective in decreasing FI and BWG and increasing FCR in grower, finisher and overall growth periods without affecting livability. These results are in agreement with previous reports (Sharma et al. 2018; Hilliar et al. 2020; Zanu et al. 2020a). Damaged intestinal epithelial cells, reduced nutrient absorption, and loss of appetite have been considered as the main reasons for reduced growth performance in NE-challenged birds (Cooper et al. 2013; Amerah and Ravindran 2015; Kraieski et al. 2017). This was confirmed in the current study, as shown by increased intestinal lesion scores in the grower phase due to the NE challenge. Furthermore, the results of the current study showed that NE challenge increased the relative weight of liver on Day 35. A similar result was observed by Ugwuoke and Pewan (2020) in Eimeria-challenged birds. The increased relative liver weight in NE-challenged birds in the current study might be attributed to an increase in metabolic activities as a result of bacterial infection (Ogbe et al. 2008; Mustapha et al. 2017). This process needs a certain amount of time that might explain the absence of difference in liver weight between NE-challenged and non-challenged groups on Day 16 in the current study. All changes observed in NE-challenged birds suggest that subclinical NE was successfully established in the current study.
Subclinical NE can cause more economic problems to poultry producers than does the acute form as it reduces the meat yield in a greater number of birds and increases carcass condemnation at slaughter (Løvland and Kaldhusdal 1999; Lovland and Kaldhusdal 2001). In the current study, the subclinical NE challenge reduced the relative weights of the breast, increased the relative weight of thigh and drumstick, and tended to increase tje relative fat pad weight on Day 35. As relative carcass weights were calculated by dividing the absolute weights by the BW and the absolute weights of both breast, thigh and drumstick in NE-challenged birds were lower than those in the unchallenged birds (P < 0.001, data no presented), these results reflect that the weight loss might occur more severely and rapidly in the breast and other parts of the body than the in the thigh and drumstick in the NE-challenged birds. Xue et al. (2017) reported reduced relative weights of breast, thigh and drumstick but no difference in relative fat pad weight as a result of NE challenge compared with unchallenged controls. A reduction in feed consumption results in a decreasing glucose production that may induce skeletal muscle to catabolise gluconeogenic AA to provide energy for maintenance and this may reduce muscle protein accretion (Wu et al. 1991). The latter phenomena were observed in the current study, as illustrated by the reduced FI, with the subsequent effects on reduced BWG, and relative breast yield in NE-challenged birds. Additionally, literature evidence has shown that the fat accumulation in the liver and adipose tissues is regulated by the concentration of serum lipids such as cholesterol and triglyceride and lipoproteins (Mossab et al. 2002; Afsharmanesh et al. 2013). Thus, lowering total cholesterol and triglyceride concentrations has been considered the most effective way to decrease fat accumulation (Yang et al. 2014). Previous studies have shown that NE challenge increases concentrations of total cholesterol and low-density lipoprotein cholesterol and downregulates gene expression of lipoprotein lipase and peroxisome proliferator-activated receptor γ that mediates adipocyte differentiation and maturation, resulting in an increased rate of abdominal fat in the respective group (Zhou et al. 2016; Qing et al. 2017). These facts may explain the increased relative fat pad weight in NE-challenged birds compared with the unchallenged group in the current study.
The NE × protein interactions observed in the current study indicated the beneficial effects of dietary protein reductions on FI, BWG and FCR in birds challenged with NE. Feeding the RP diets increased FI and BWG during the starter phase and better prepared birds for NE challenge, with increased FI, increased BWG, and lower FCR being observed during the main course of NE challenge (grower phase), than in those fed the higher protein levels. These findings confirmed the tested hypothesis in the present study. Broiler diets with high protein concentrations have been shown to facilitate the proliferation of C. perfringens, the causative agent of NE (Drew et al. 2004; Wilkie et al. 2005; Liu et al. 2017). High-CP diets are likely to be less digestible than are reduced-protein diets, as the latter have higher additions of crystalline AA (Hilliar et al. 2019; Dao et al. 2021a, 2021b). Undigested AA from the small intestine may accumulate in the hindgut, providing nutrients for the proliferation of pathogenic microbes such as C. perfringens that may, consequently, influence gut health and growth performance in birds (Lan et al. 2004; McDevitt et al. 2006). As C. perfringens lack the genes responsible for the synthesis of Arg, lysine, methionine, threonine, serine, histidine and branched-chain AA (Shimizu et al. 2002), they are highly dependent on feed materials for these nutrients. In contrast to the current findings, Hilliar et al. (2020) found a reduced growth rate in NE-challenged birds fed RP diets, with 4.5 and 4 percentage points lower CP concentrations in the grower and finisher phases respectively, than in those offered SP diets. In the current study, CP concentrations of the RP diets were only two percentage points lower than were thos of the SP diets in all feeding phases. The difference in protein reductions best explains these dichotomous results between the studies. The NE × protein interaction on serum uric acid in the current study showed that serum uric acid was decreased in birds fed the SP diet only when they were challenged with NE. The decrease in FI in the challenged SP fed birds compared to the unchallenged SP group might reduce substrate for uric acid production, then serum uric acid concentration as a consequence. Furthermore, it may be worth noting that NE-challenged birds fed RP diet or RP diet supplemented with Arg or Cit had lower serum uric acid concentrations than did those offered the SP diet in the current study. As serum uric acid is a product of AA degradation (Namroud et al. 2008); this finding may re-affirm the higher protein utilisation efficiency in birds fed the RP diet than in those fed the SP diet. Besides, the lower relative bursa of Fabricius weight observed on Day 16 and the higher relative liver weight on Day 35 in RP-fed birds than in those offered the SP diet in the current study may suggest differential effects of dietary protein level on internal organ weights in birds.
The main objective of the current study was to determine whether feeding RP diets supplemented with Arg or Cit would improve the growth performance and gut health of birds experimentally induced with subclinical NE, compared with the birds fed SP diets. Dietary Arg supplementation has been shown to reduce growth loss and alleviate gut damage in chickens challenged with C. perfringens, infectious bronchitis virus and Eimeria (Tan et al. 2014; Laika and Jahanian 2017; Zhang et al. 2018). The results of the current study showed that Cit supplementation to the RP diet further increased the beneficial effects of the RP diet on the growth performance and promoted recovery in NE-challenged birds. The same supplementation with Arg had only minor effects. During the experimental period, feeding-challenged birds with the RPC diet reduced the FCR compared with those fed the SP diets. Additional supplementation of Arg to the RP diet reduced FI and BWG in the challenged birds by 7%, compared with challenged birds fed the RP diet from Day 0 to Day 35. No differences in FI, BWG and FCR were observed by Hilliar et al. (2020) when a reduced-CP diet with either 115% essential AA, 100%, or 115% of both essential and non-essential AA were fed to NE-challenged birds. Thus, the lack of response to additional supplementation of Arg in the current study might be attributed to reduced digestion and absorption capacity in NE-challenged birds, and/or low levels of Arg supplementation in the RPA diet. Subclinical NE is likely to alter the requirement for Arg (and other essential AA) relative to breeder company recommendations targeted at healthy flocks. Kidd and Tillman (2016) pointed out that the threonine requirement was greatly increased in broilers with subclinical enteric infection. Deficiencies of aspartate, asparagine and alanine have been reported to increase the requirements of Arg, choline, methionine, threonine and branched-chain AA in NE-challenged birds in an attempt to maintain muscle accretion (Wu 2014). Supplemental Arg is known to be degraded by arginase in the intestinal mucosa, thereby limiting its presence in plasma while Cit can escape this degradation and, once absorbed, Cit may be converted to Arg in the kidney, thereby increasing plasma Arg concentration (McCarty 2010). Studies on mammals have shown that dietary Cit supplementation results in a higher plasma Arg and NO production than does supplementation with Arg at the same dose (Schwedhelm et al. 2008; Lassala et al. 2009; Wijnands et al. 2012). These facts may explain the positive effects of Cit over Arg supplementation on the growth performance in challenged birds in the current study.
It has been reported that supplementation of either meat and bone meal, antibiotic, phytase, or phytogenic feed additives does not affect relative weights of the breast, leg, and fat pad in NE-challenged birds (Cho et al. 2014; Zanu et al. 2020b). In the current study, Cit supplementation in the RP diet was effective in increasing breast meat yield in NE-challenged birds. This information may be important to the poultry producers who may want to reduce the economic loss caused by subclinical NE infection in the flock where the disease symptoms are not easy to diagnose. Besides, dietary Arg supplementation has been reported to reduce C. perfringens count, intestinal lesion scores, and mucosal damage in C. perfringens challenged birds by modulating innate immune responses, enhancing gut integrity, and promote NO production (Zhang et al. 2017, 2018). However, the beneficial effects of either Arg or Cit supplementation on gut NE lesion scores were not observed in the current study. The differences in experimental design, diet composition, dosage use, and chicken age may explain the dichotomous results between the studies.
Conclusions
An advantage of Cit over Arg supplementation was demonstrated and it is likely to be attributed to its escape from degradation by arginase present in enterocytes. It can be concluded that feeding the RP diet supplemented with Cit was beneficial in promoting gut health and recovery from the NE challenge. Further work is warranted to determine optimal levels of Cit to support growth and immune status in birds subjected to disease stress such as the NE challenge.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Declaration of funding
The authors thank Poultry Hub Australia for their financial support for this study (grant number: 18-414).
Acknowledgements
We thank Australian Proteome Analysis Facility, University of Macquarie, Australia, for feed analysis. We thank Evonik (South East Asia) Pte Ltd (Singapore) for NIRS feed analysis. Also, the authors thank Mr Jonathon Clay studying at School of Environmental and Rural Science and the Poultry Research and Teaching Unit, the University of New England, Australia, for their help during the experiment and laboratory analysis.
References
Afsharmanesh M, Sadaghi B, Silversides FG (2013) Influence of supplementation of prebiotic, probiotic, and antibiotic to wet-fed wheat-based diets on growth, ileal nutrient digestibility, blood parameters, and gastrointestinal characteristics of broiler chickens. Comparative Clinical Pathology 22, 245–251.| Influence of supplementation of prebiotic, probiotic, and antibiotic to wet-fed wheat-based diets on growth, ileal nutrient digestibility, blood parameters, and gastrointestinal characteristics of broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Amerah AM, Ravindran V (2015) Effect of coccidia challenge and natural betaine supplementation on performance, nutrient utilization, and intestinal lesion scores of broiler chickens fed suboptimal level of dietary methionine. Poultry Science 94, 673–680.
| Effect of coccidia challenge and natural betaine supplementation on performance, nutrient utilization, and intestinal lesion scores of broiler chickens fed suboptimal level of dietary methionine.Crossref | GoogleScholarGoogle Scholar | 25691757PubMed |
AOAC (1994) ‘Official methods of analysis.’ (Association of Official Analytical Chemists: Washington DC, USA)
Aviagen (2014a) ‘Ross 308 broiler management handbook.’ (Ross Breeders Limited: Newbridge, Midlothian, Scotland)
Aviagen (2014b) ‘Ross 308 broiler nutrition specification.’ (Ross Breeders Limited: Newbridge, Midlothian, Scotland)
Cho JH, Kim HJ, Kim IH (2014) Effects of phytogenic feed additive on growth performance, digestibility, blood metabolites, intestinal microbiota, meat color and relative organ weight after oral challenge with Clostridium perfringens in broilers. Livestock Science 160, 82–88.
| Effects of phytogenic feed additive on growth performance, digestibility, blood metabolites, intestinal microbiota, meat color and relative organ weight after oral challenge with Clostridium perfringens in broilers.Crossref | GoogleScholarGoogle Scholar |
Cohen SA (2001) Amino acid analysis using precolumn derivatisation with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate. In ‘Methods in molecular biology’. (Eds C Cooper, N Packer, K Williams) pp. 39–47. (Humana Press: Totowa, NJ, USA)
Cooper KK, Songer JG, Uzal FA (2013) Diagnosing clostridial enteric disease in poultry. Journal of Veterinary Diagnostic Investigation 25, 314–327.
| Diagnosing clostridial enteric disease in poultry.Crossref | GoogleScholarGoogle Scholar | 23572451PubMed |
Dao HT, Sharma NK, Bradbury EJ, Swick RA (2021a) Response of meat chickens to different sources of arginine in low-protein diets. Journal of Animal Physiology and Animal Nutrition 105, 731–746.
| Response of meat chickens to different sources of arginine in low-protein diets.Crossref | GoogleScholarGoogle Scholar | 33410556PubMed |
Dao HT, Sharma NK, Bradbury EJ, Swick RA (2021b) Effects of l-arginine and l-citrulline supplementation in reduced protein diets for broilers under normal and cyclic warm temperature. Animal Nutrition 7, 927–938.
| Effects of l-arginine and l-citrulline supplementation in reduced protein diets for broilers under normal and cyclic warm temperature.Crossref | GoogleScholarGoogle Scholar | 34703910PubMed |
Drew MD, Syed NA, Goldade BG, Laarveld B, Van Kessel AG (2004) Effects of dietary protein source and level on intestinal populations of Clostridium perfringens in broiler chickens. Poultry Science 83, 414–420.
| Effects of dietary protein source and level on intestinal populations of Clostridium perfringens in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 15049494PubMed |
Hilliar M, Huyen N, Girish CK, Barekatain R, Wu S, Swick RA (2019) Supplementing glycine, serine, and threonine in low protein diets for meat type chickens. Poultry Science 98, 6857–6865.
| Supplementing glycine, serine, and threonine in low protein diets for meat type chickens.Crossref | GoogleScholarGoogle Scholar | 31433853PubMed |
Hilliar M, Keerqin C, Girish CK, Barekatain R, Wu S-B, Swick RA (2020) Reducing protein and supplementing crystalline amino acids, to alter dietary amino acid profiles in birds challenged for subclinical necrotic enteritis. Poultry Science 99, 2048–2060.
| Reducing protein and supplementing crystalline amino acids, to alter dietary amino acid profiles in birds challenged for subclinical necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 32241490PubMed |
Immerseel FV, Buck JD, Pasmans F, Huyghebaert G, Haesebrouck F, Ducatelle R (2004) Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathology 33, 537–549.
| Clostridium perfringens in poultry: an emerging threat for animal and public health.Crossref | GoogleScholarGoogle Scholar |
Kaldhusdal M, Lovland A (2000) The economical impact of Clostridium perfringens is greater than anticipated. World Poultry 16, 50–51.
Keyburn AL, Sheedy SA, Ford ME, Williamson MM, Awad MM, Rood JI, Moore RJ (2006) Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infection and Immunity 74, 6496–6500.
| Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens.Crossref | GoogleScholarGoogle Scholar | 16923791PubMed |
Kidd MT (2004) Nutritional modulation of immune function in broilers. Poultry Science 83, 650–657.
| Nutritional modulation of immune function in broilers.Crossref | GoogleScholarGoogle Scholar | 15109062PubMed |
Kidd MT, Tillman PB (2016) Key principles concerning dietary amino acid responses in broilers. Animal Feed Science and Technology 221, 314–322.
| Key principles concerning dietary amino acid responses in broilers.Crossref | GoogleScholarGoogle Scholar |
Kraieski AL, Hayashi RM, Sanches A, Almeida GC, Santin E (2017) Effect of aflatoxin experimental ingestion and Eimeira vaccine challenges on intestinal histopathology and immune cellular dynamic of broilers: applying an Intestinal Health Index. Poultry Science 96, 1078–1087.
| Effect of aflatoxin experimental ingestion and Eimeira vaccine challenges on intestinal histopathology and immune cellular dynamic of broilers: applying an Intestinal Health Index.Crossref | GoogleScholarGoogle Scholar | 27794052PubMed |
Laika M, Jahanian R (2017) Increase in dietary arginine level could ameliorate detrimental impacts of coccidial infection in broiler chickens. Livestock Science 195, 38–44.
| Increase in dietary arginine level could ameliorate detrimental impacts of coccidial infection in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Lan Y, Xun S, Tamminga S, Williams BA, Verstegen MWA, Erdi G (2004) Real time based detection of lactic acid bacteria in caecal contents of E. tenella infected broilers fed soybean oligosaccharides and soluble soybean polysaccharides. Poultry Science 83, 1696–1702.
| Real time based detection of lactic acid bacteria in caecal contents of E. tenella infected broilers fed soybean oligosaccharides and soluble soybean polysaccharides.Crossref | GoogleScholarGoogle Scholar | 15510555PubMed |
Lassala A, Bazer FW, Cudd TA, Li P, Li X, Satterfield MC, Spencer TE, Wu G (2009) Intravenous administration of l-citrulline to pregnant ewes is more effective than l-arginine for increasing arginine availability in the fetus. The Journal of Nutrition 139, 660–665.
| Intravenous administration of l-citrulline to pregnant ewes is more effective than l-arginine for increasing arginine availability in the fetus.Crossref | GoogleScholarGoogle Scholar | 19225132PubMed |
Le Floc’h N, Melchior D, Obled C (2004) Modifications of protein and amino acid metabolism during inflammation and immune system activation. Livestock Production Science 87, 37–45.
| Modifications of protein and amino acid metabolism during inflammation and immune system activation.Crossref | GoogleScholarGoogle Scholar |
Lee JE, Austic RE, Naqi SA, Golemboski KA, Dietert RR (2002) Dietary arginine intake alters avian leukocyte population distribution during infectious bronchitis challenge. Poultry Science 81, 793–798.
| Dietary arginine intake alters avian leukocyte population distribution during infectious bronchitis challenge.Crossref | GoogleScholarGoogle Scholar | 12079045PubMed |
Li P, Yin Y-L, Li D, Kim SW, Wu G (2007) Amino acids and immune function. British Journal of Nutrition 98, 237–252.
| Amino acids and immune function.Crossref | GoogleScholarGoogle Scholar |
Liu N, Wang JQ, Gu KT, Deng QQ, Wang JP (2017) Effects of dietary protein levels and multienzyme supplementation on growth performance and markers of gut health of broilers fed a miscellaneous meal based diet. Animal Feed Science and Technology 234, 110–117.
| Effects of dietary protein levels and multienzyme supplementation on growth performance and markers of gut health of broilers fed a miscellaneous meal based diet.Crossref | GoogleScholarGoogle Scholar |
Løvland A, Kaldhusdal M (1999) Liver lesions seen at slaughter as an indicator of necrotic enteritis in broiler flocks. FEMS Immunology and Medical Microbiology 24, 345–351.
| Liver lesions seen at slaughter as an indicator of necrotic enteritis in broiler flocks.Crossref | GoogleScholarGoogle Scholar | 10397321PubMed |
Lovland A, Kaldhusdal M (2001) Severely impaired production performance in broiler flocks with high incidence of Clostridium perfringens-associated hepatitis. Avian Pathology 30, 73–81.
| Severely impaired production performance in broiler flocks with high incidence of Clostridium perfringens-associated hepatitis.Crossref | GoogleScholarGoogle Scholar | 19184877PubMed |
McCarty MF (2010) Potential utility of full-spectrum antioxidant therapy, citrulline, and dietary nitrate in the management of sickle cell disease. Medical Hypotheses 74, 1055–1058.
| Potential utility of full-spectrum antioxidant therapy, citrulline, and dietary nitrate in the management of sickle cell disease.Crossref | GoogleScholarGoogle Scholar | 20089363PubMed |
Mcdevitt RM, Brooker JD, Acamovic T, Sparks NHC (2006) Necrotic enteritis; a continuing challenge for the poultry industry. World’s Poultry Science Journal 62, 221–247.
| Necrotic enteritis; a continuing challenge for the poultry industry.Crossref | GoogleScholarGoogle Scholar |
Mossab A, Lessire M, Guillaumin S, Kouba M, Mourot J, Peiniau P, Hermier D (2002) Effect of dietary fats on hepatic lipid metabolism in the growing turkey. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 132, 473–483.
| Effect of dietary fats on hepatic lipid metabolism in the growing turkey.Crossref | GoogleScholarGoogle Scholar |
Mustapha GG, Igwebuike JU, Adamu SB, Kwari ID (2017) The effect of replacement levels of boiled and fermented castor seed (Ricinus cummunis) meal on the productive performance, carcass evaluation and cost effectiveness in cockerels. International Journal of Agriculture and Biosciences 6, 129–135.
Mwangi S, Timmons J, Fitz-Coy S, Parveen S (2019) Characterization of Clostridium perfringens recovered from broiler chicken affected by necrotic enteritis. Poultry Science 98, 128–135.
| Characterization of Clostridium perfringens recovered from broiler chicken affected by necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 30053181PubMed |
Namroud NF, Shivazad M, Zaghari M (2008) Effects of fortifying low crude protein diet with crystalline amino acids on performance, blood ammonia level, and excreta characteristics of broiler chicks. Poultry Science 87, 2250–2258.
| Effects of fortifying low crude protein diet with crystalline amino acids on performance, blood ammonia level, and excreta characteristics of broiler chicks.Crossref | GoogleScholarGoogle Scholar | 18931175PubMed |
NHMRC (2013) ‘Australian code of practice for the care and use of animals for scientific purposes’, 8th edn. (The National Health and Medical Research Council: Australia)
Ogbe AO, Mgbojikwe LO, Abdu PA, Atawodi SE (2008) Organ and carcass weight variation and histopathological changes in Eimeria tenella infected broiler chickens treated with aqueous extract of a wild mushroom (Ganoderma lucidum). Electronic Journal of Environmental, Agricultural and Food Chemistry 7, 2906–2913.
Qing X, Zeng D, Wang H, Ni X, Liu L, Lai J, Khalique A, Pan K, Jing B (2017) Preventing subclinical necrotic enteritis through Lactobacillus johnsonii BS15 by ameliorating lipid metabolism and intestinal microflora in broiler chickens. AMB Express 7, 373
| Preventing subclinical necrotic enteritis through Lactobacillus johnsonii BS15 by ameliorating lipid metabolism and intestinal microflora in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Rodgers NJ, Swick RA, Geier MS, Moore RJ, Choct M, Wu S-B (2015) A multifactorial analysis of the extent to which Eimeria and fishmeal predispose broiler chickens to necrotic enteritis. Avian Diseases 59, 38–45.
| A multifactorial analysis of the extent to which Eimeria and fishmeal predispose broiler chickens to necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 26292532PubMed |
Ross T (1999) Necrotic enteritis and associated conditions in broiler chickens. World Poultry 15, 44–47.
Schwedhelm E, Maas R, Freese R, Jung D, Lukacs Z, Jambrecina A, Spickler W, Schulze F, Böger RH (2008) Pharmacokinetic and pharmacodynamic properties of oral l-citrulline and l-arginine: impact on nitric oxide metabolism. British Journal of Clinical Pharmacology 65, 51–59.
| Pharmacokinetic and pharmacodynamic properties of oral l-citrulline and l-arginine: impact on nitric oxide metabolism.Crossref | GoogleScholarGoogle Scholar | 17662090PubMed |
Sharma NK, Choct M, Wu S-B, Swick RA (2018) Necrotic enteritis challenge and high dietary sodium level affect odorant composition or emission from broilers. Poultry Science 97, 39–46.
| Necrotic enteritis challenge and high dietary sodium level affect odorant composition or emission from broilers.Crossref | GoogleScholarGoogle Scholar | 29077885PubMed |
Shimizu T, Ohtani K, Hirakawa H, Ohshima K, Yamashita A, Shiba T, Ogasawara N, Hattori M, Kuhara S, Hayashi H (2002) Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proceedings of the National Academy of Sciences of the United States of America 99, 996–1001.
| Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater.Crossref | GoogleScholarGoogle Scholar | 11792842PubMed |
Tan J, Applegate TJ, Liu S, Guo Y, Eicher SD (2014) Supplemental dietary l-arginine attenuates intestinal mucosal disruption during a coccidial vaccine challenge in broiler chickens. British Journal of Nutrition 112, 1098–1109.
| Supplemental dietary l-arginine attenuates intestinal mucosal disruption during a coccidial vaccine challenge in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Ugwuoke GM, Pewan S (2020) Effect of methanol extract of Parkia biglobosa root bark on organ and carcass weight and histopathological changes in Eimeria tenella infected broiler chickens. Animal Research International 17, 3587–3595.
Wade B, Keyburn A (2015) The true cost of necrotic enteritis. World Poultry 31, 16–17.
Wheat TE, Grumbach ES, Mazzeo JR (2008) ‘UPLC amino acid analysis solution.’ Application Note No. 720001683en. (Waters Corporation: Milford, MA, USA)
Widyaratne GP (2012) The role of protein and amino acid nutrition in controlling Clostridium perfringens in the gastrointestinal tract of broiler chickens. PhD thesis, University of Saskatchewan, Saskatoon, Saskatchewan, Canada.
Wijnands KAP, Vink H, Briedé JJ, van Faassen EE, Lamers WH, Buurman WA, Poeze M (2012) Citrulline a more suitable substrate than arginine to restore NO production and the microcirculation during endotoxemia. PloS ONE 7, e37439
| Citrulline a more suitable substrate than arginine to restore NO production and the microcirculation during endotoxemia.Crossref | GoogleScholarGoogle Scholar |
Wilkie DC, Van Kessel AG, White LJ, Laarveld B, Drew MD (2005) Dietary amino acids affect intestinal Clostridium perfringens populations in broiler chickens. Canadian Journal of Animal Science 85, 185–193.
| Dietary amino acids affect intestinal Clostridium perfringens populations in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Wilson J, Tice G, Brash ML, Hilaire SS (2005) Manifestations of Clostridium perfringens and related bacterial enteritides in broiler chickens. World’s Poultry Science Journal 61, 435–449.
| Manifestations of Clostridium perfringens and related bacterial enteritides in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Wu G (2014) Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. Journal of Animal Science and Biotechnology 5, 1–12.
| Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition.Crossref | GoogleScholarGoogle Scholar |
Wu G, Thompson JR, Baracos VE (1991) Glutamine metabolism in skeletal muscles from the broiler chick (Gallus domesticus) and the laboratory rat (Rattus norvegicus). Biochemical Journal 274, 769–774.
| Glutamine metabolism in skeletal muscles from the broiler chick (Gallus domesticus) and the laboratory rat (Rattus norvegicus).Crossref | GoogleScholarGoogle Scholar |
Xue GD, Wu SB, Choct M, Pastor A, Steiner T, Swick RA (2017) Impact of a Macleaya cordata-derived alkaloid extract on necrotic enteritis in broilers. Poultry Science 96, 3581–3585.
| Impact of a Macleaya cordata-derived alkaloid extract on necrotic enteritis in broilers.Crossref | GoogleScholarGoogle Scholar | 28637224PubMed |
Yang K-T, Lin C, Liu C-W, Chen Y-C (2014) Effects of chicken-liver hydrolysates on lipid metabolism in a high-fat diet. Food Chemistry 160, 148–156.
| Effects of chicken-liver hydrolysates on lipid metabolism in a high-fat diet.Crossref | GoogleScholarGoogle Scholar | 24799221PubMed |
Zanu HK, Kheravii SK, Morgan NK, Bedford MR, Swick RA (2020a) Over-processed meat and bone meal and phytase effects on broilers challenged with subclinical necrotic enteritis: Part 1. Performance, intestinal lesions and pH, bacterial counts and apparent ileal digestibility. Animal Nutrition 6, 313–324.
| Over-processed meat and bone meal and phytase effects on broilers challenged with subclinical necrotic enteritis: Part 1. Performance, intestinal lesions and pH, bacterial counts and apparent ileal digestibility.Crossref | GoogleScholarGoogle Scholar | 33005765PubMed |
Zanu HK, Keerqin C, Kheravii SK, Morgan N, Wu S-B, Bedford MR, Swick RA (2020b) Influence of meat and bone meal, phytase, and antibiotics on broiler chickens challenged with subclinical necrotic enteritis: 2. intestinal permeability, organ weights, hematology, intestinal morphology, and jejunal gene expression. Poultry Science 99, 2581–2594.
| Influence of meat and bone meal, phytase, and antibiotics on broiler chickens challenged with subclinical necrotic enteritis: 2. intestinal permeability, organ weights, hematology, intestinal morphology, and jejunal gene expression.Crossref | GoogleScholarGoogle Scholar | 32359594PubMed |
Zhang B, Lv Z, Li H, Guo S, Liu D, Guo Y (2017) Dietary l-arginine inhibits intestinal Clostridium perfringens colonisation and attenuates intestinal mucosal injury in broiler chickens. British Journal of Nutrition 118, 321–332.
| Dietary l-arginine inhibits intestinal Clostridium perfringens colonisation and attenuates intestinal mucosal injury in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Zhang B, Lv Z, Li Z, Wang W, Li G, Guo Y (2018) Dietary l-arginine supplementation alleviates the intestinal injury and modulates the gut microbiota in broiler chickens challenged by Clostridium perfringens. Frontiers in Microbiology 9, 1716
| Dietary l-arginine supplementation alleviates the intestinal injury and modulates the gut microbiota in broiler chickens challenged by Clostridium perfringens.Crossref | GoogleScholarGoogle Scholar | 30108569PubMed |
Zhou M, Zeng D, Ni X, Tu T, Yin Z, Pan K, Jing B (2016) Effects of Bacillus licheniformis on the growth performance and expression of lipid metabolism-related genes in broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Lipids in Health and Disease 15, 1–10.
| Effects of Bacillus licheniformis on the growth performance and expression of lipid metabolism-related genes in broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis.Crossref | GoogleScholarGoogle Scholar |


