Effects of stimulation on tenderness of lamb with a focus on protein degradation
K. M. Martin A B E , D. L. Hopkins A C , G. E. Gardner A D and J. M. Thompson A BA Australian Sheep Industry Cooperative Research Centre, Chiswick, New England Highway, Armidale, NSW 2350, Australia.
B School of Rural Sciences and Natural Resources, University of New England, Armidale, NSW 2351, Australia.
C NSW Department of Primary Industries, Centre for Sheep Meat Development, Cowra, NSW 2794, Australia.
D School of Veterinary and Biomedical Sciences, Murdoch University, Murdoch, WA 6150, Australia.
E Corresponding author. Email: kirstie.martin@une.edu.au
Australian Journal of Experimental Agriculture 46(7) 891-896 https://doi.org/10.1071/EA06010
Submitted: 26 November 2005 Accepted: 8 May 2006 Published: 8 June 2006
Abstract
Past studies have identified that electrical stimulation systems not only affect the pH–temperature decline of lamb carcasses, but also affect the tenderness of the product. It is unknown whether these differences are due to the effects of an optimal pH–temperature decline path by which the occurrence of shortening is decreased and proteolytic enzyme activity is increased, or by another mechanism, such as disruption of the muscle myofibres. This study attempted to elucidate this by placing control and mid-voltage electrically stimulated samples of the M. longissimus thoracis et lumborum in a water bath to prevent shortening by imposing an even pH–temperature decline on all samples. In order to obtain a complete understanding of the effects of stimulation on the muscle, samples were taken for indicators of tenderness, myofibre degradation and protein breakdown. No effect of stimulation was seen on tenderness and structural degradation measures aside from the expected rapid lowering of muscle pH. Interesting relationships were observed, however, between the different methods of meat tenderness assessment, with muscle structural protein degradation, shear force and muscle fibre and myofibre breakdown comparisons. These results suggest that the effects of electrical stimulation seen in mid-voltage systems as applied in this study are due only to the prevention of shortening conditions.
Additional keywords: fibre breaks, lamb, myofibrillar protein, tenderness.
Introduction
It is generally believed that the effect of electrical stimulation is mediated through changes in the pH–temperature relationship during rigor. However, recent work indicates that when accelerated rigor onset at high temperatures is removed from comparisons between stimulated and non-stimulated muscle, there is evidence that stimulation per se improves tenderness (Devine et al. 2002a). It is unknown by which mechanism an effect of stimulation per se on tenderness is mediated independent of a change in the pH–temperature relationship, although it has been suggested that physical disruption of the sarcomere may occur (Hwang et al. 2003).
This study was designed to examine macro (myofibre) and micro (myofibre proteins) degradation in response to electrical stimulation independent of pH and temperature interactions. The proteins considered of interest in this experiment were: desmin, an interfilament protein partially responsible for maintaining the structural integrity of the muscle fibre by linking adjacent myofibrils together, which has been shown to be an indicator of calpain-mediated degradation (Lametsch et al. 2004); and troponin-T, a protein involved in the regulation of muscle fibre contraction. Whilst troponin-T itself is not responsible for maintaining the structural integrity of the muscle fibres, it can be used as an indicator protein for protein degradation and has been shown to be correlated to shear force (Steen et al. 1997). At the macro level, there is potential for breaks in fibres to be a useful indicator of degradation (Taylor and Frylinck 2003) and this paper will describe the application of this approach in the context of describing the effect of electrical stimulation.
Traditionally, high voltage stimulation systems used on sheep carcasses have applied a fixed voltage averaged across all carcasses being stimulated (Devine et al. 2004). Rubbing bars have been used effectively to apply high voltage stimulation to lamb and sheep carcasses at the completion of the dressing procedure (Morton et al. 1999; Toohey and Hopkins 2004), but this process poses concerns for working safety. In Australia, a new approach has been developed whereby each carcass is stimulated individually using segmented electrodes to ensure that each segment only contacts 1 carcass at a time (Devine et al. 2004). This allows computer-controlled electronics to give a precise, but adjustable electrical input to each carcass to match the requirements of a particular carcass type while maintaining the delivery of a predetermined level of current. In effect, a feedback system that detects the level of resistance is used. This approach also reduces the installation costs with respect to occupational health and safety because the power levels and pulse widths used eliminate the need for isolation of the unit, a requirement of high voltage systems. An experiment was conducted using this new approach to examine whether this less aggressive form of electrical stimulation (lower current and voltage) would result in an effect on tenderness as reported by Devine et al. (2002a).
Materials and methods
Animals and management
The 24 mixed-sex lambs used in this experiment equally represented 2 types: Poll Dorset (growth) × Merino and Poll Dorset (muscling) × Merino. Sires of the lambs were selected based on LAMBPLAN estimated breeding values (Banks 1994) for either growth or muscling and there were 4 different sires represented in each type with 3 progeny/sire. These lambs were bred as part of the Australian Sheep Industry CRC experimental flock at the Centre for Sheepmeat Development, Cowra, NSW.
The lambs were weaned at 4 months of age in November 2003, and 1 week later they were transported730 km to Werribee (Victoria) where they were held for 6 days before the first group was slaughtered. The lambs were fed lucerne hay at pasture.
Slaughter procedures
Lambs were randomly allocated to 1 of 4 slaughter days (6 lambs/day). This randomisation was balanced across sires and types. Lambs were yarded at 1600 hours the night before each slaughter day and held in lairage with water available but no food, except on the last slaughter day where the lambs were yarded on the morning of slaughter. The order of animals slaughtered on any day was random. All animals were electrically stunned (head only) and after dressing, the carcass was split down the backbone with a hand-operated saw. Carcasses were trimmed according to the specifications of AUS-MEAT (Anon. 1992).
A section of loin consisting of the M. longissimus thoracis et lumborum (LL) and overlaying subcutaneous fat from the ninth rib to the lumbar–sacral junction was removed from the left side of each carcass. The right side was stimulated at a constant current (400 mA) by varying the voltage (about 300 V peak), for 35 s at 14 pulses/s and a pulse width of 1.0 ms. This type of stimulation (described as mid-voltage) was selected because it is similar to that used in some commercial sheep abattoirs (Hopkins et al. 2005). After stimulation, the loin section from the ninth rib to the lumbar–sacral junction was removed.
Sampling and meat quality measurements
The pH was measured in the cranial end of the LL of each removed section using a WPS meter with temperature compensation (TPS, WP-80, PTS, Brisbane, Qld) and a polypropylene spear-type gel electrode (Ionode IJ 44, Tennyson, Qld) calibrated at ambient temperature. A sample of LL (about 5 g) was taken from the unstimulated loin section and frozen in liquid nitrogen for examination by SDS PAGE (2 g) and iodoacetate pH (1 g) determination and held at –80°C. The sections of removed loin were weighed and a temperature logger (Thermochron ibutton, Dallas Semiconductor Corp., Dallas, Texas) was inserted into the cranial end of each loin section and the loin was then wrapped in cling wrap (Devine et al. 2002b), which was held firmly by tape. Wrapped sections of loin were placed in plastic bags and submerged in 130 L of water. Water temperature was maintained at 15°C with a thermoregulator and an immersion cooler (Ratek Instruments, Boronia, Vic.).
At regular intervals the entire loin section was removed from the water and the pH measured in the cranial end. When the LL reached a pH of 6.0, samples of muscle were taken for SDS PAGE (2 g) and pH (1 g) determination and frozen in liquid nitrogen and this was repeated when the pH dropped to 5.8. At this time a portion of the LL (cranial, medial, caudal) was removed from the loin depending on prior randomisation and a thin slice of LL was taken from the lateral sides of the muscle for determination of myofibrillar fragmentation index (MFI) and frozen at –20°C. Additionally, samples were prepared into 65-g blocks for subsequent shear testing and frozen (–20°C). The remainder of the loin was returned to the water and maintained at 15°C until 1 day post-mortem samples were removed. Samples of muscle were taken from the section removed at 1 day post-mortem for SDS PAGE (2 g), pH (1 g) and free calcium (2 g) determination and frozen in liquid nitrogen. A sample was also taken for determination of MFI and sarcomere length and frozen at –20°C, both from the lateral sides of the muscle. Samples were prepared into 65-g blocks for subsequent shear testing and frozen (–20°C). A small sample (1 g) was taken from the lateral side of the muscle and fixed in 2.5% glutaraldehyde in 0.1 mol/L cacodylate buffer. The remaining portion was prepared into a 65-g block and frozen (–20°C). After a further 3 days, remaining samples were taken from the water and prepared as for day 1 samples, except no samples were kept for sarcomere length or free calcium determination.
Measurement of shear force, sarcomere length and myofibrillar fragmentation index
The 65-g LL samples (pH 5.8, 1 and 4 days) were cooked from the frozen state for 35 min in plastic bags at 70°C in an 80-L water bath for determination of shear force measured as Newtons (N) as described by Hopkins and Thompson (2001a). Determination of sarcomere length on 1 day post-mortem samples was performed as described by Hopkins and Thompson (2001a). A thin slice of frozen muscle from each portion of the LL was used for determination of myofibrillar fragmentation index (MFI) as described by Hopkins et al. (2004) and Martin et al. (2004).
Free calcium concentration and myofibrillar protein degradation
Muscle samples (2 g) taken at 1 day post-mortem and held at –70°C were used for determination of free calcium concentration. The calcium concentration was determined using a Ca2+ electrode (Cole-Parmer, Niles Illinois, IL) as described by Hopkins and Thompson (2001b) with the samples centrifuged 16 500 rpm.
The method for determining fibre breaks was adapted from that reported by Taylor and Frylinck (2003). This involved the fixing, embedding and staining of muscle samples. Digital images were collected at 40× magnification using a Leica DMR microscope and Nikon DXM1200F digital camera. Fibre detachment and breaks across the fibres were quantified for 40 fibres per sample.
Gel electrophoresis
SDS PAGE (12% acrylamide) was performed to separate muscle proteins using homogenised (buffer, 20 mmol/L MOPS, 0.3 mol/L sucrose, pH 7.0, 4°C) and denatured muscle samples obtained ‘at death’ (pH 6.0 and 5.8) and then at 1 and 4 days post-mortem. The proteins were then transferred onto a nitrocellulose membrane using the Western blotting technique. Troponin-T and desmin were identified using monoclonal antibodies (JLT-12 and DE-U-10, respectively; Sigma) followed by a secondary antibody (total mouse IgG; Sigma). An alkaline phosphatase reaction was used to identify the protein bands in conjunction with a prestained molecular marker. The relative intensity, measured as Gaussian volume (GV), of the bands on Western blots was measured using Phoretix 1D software. Undegraded desmin and troponin-T bands were identified at 48 and 36 kDa, respectively. Four main subunits of degraded troponin-T were identified at 30, 28, 14 and 6 kDa. The sum of these band intensities was referred to the total troponin-T degradation products.
Statistical analysis
Desmin and troponin-T decline rate and total troponin-T degradation product accumulation rate were calculated by transformation of the decline data points which followed an exponential decline over time into a natural log, with rate being the slope of this linear line. A linear mixed effects model (SAS, SAS Institute, Cary, NC) was used to test for differences between treatment groups (control, stimulation). Shear force, cooking loss, MFI, percentage of fibre breaks and percentage of detached fibres, undegraded desmin and troponin-T and total troponin-T degradation products were analysed using a REML procedure (Genstat 7.1, Lawes Agricultural Trust, Hemel Hempstead, UK, and SAS), which contained fixed effects for stimulation (stimulation, control), aging period (pH 5.8, 1 day, 4 days) and significant interactions. Slaughter days (1–4) was included as a random effect. Regression was used to derive relationships between MFI, shear force and percentage of fibre breaks.
Results
Effect of stimulation on pH fall
The mean time between death and stimulation of the right side was 37.6 min and the time between death and sample being placed in the water at 15°C was 43.5 and 45.8 min for control and stimulated loins, respectively. The initial mean pH measured after removal of the loin from the carcass was significantly (P<0.001) higher for control loins at 6.73 compared with stimulated loins at 6.52. Stimulated carcasses had a faster rate of pH decline, reaching pH 6.0 at 3.95 h after death; this was significantly (P<0.001) faster than control muscle, which took 4.60 h to reach pH 6.0. At the pH 5.8 sampling, the times were 6.30 and 6.78 h for stimulated and control muscle, respectively, and again the differences were significant (P<0.001). Muscle temperature at these sampling times was not different between treatment groups at 16.5°C.
Relationship between protein degradation and indicators of myofibre degradation
At pH 5.8, shear force significantly (P<0.01) interacted with the amount of undegraded desmin protein remaining in the sample (Fig. 1), where the muscles with more undegraded desmin had higher shear forces than those with less undegraded desmin. No other significant interactions were seen between the desmin and troponin-T levels of degradation and MFI, shear force, fibre breaks and detachment or pH.
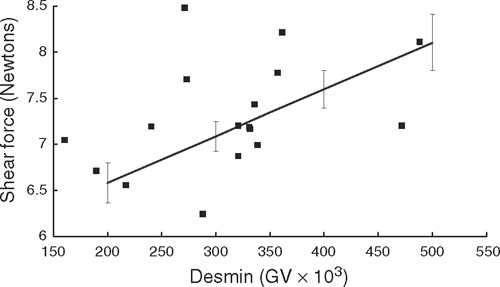
|
Relationship between electrical stimulation and indicators of myofibre degradation
Modelling the relationship between MFI and the percentage of fibre breaks produced the following non linear model:
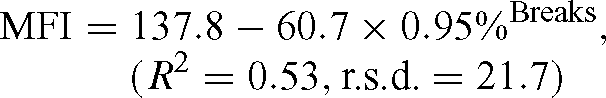
This same type of nonlinear regression model was also used to derive the relationship between shear force (Newtons) and the percentage of fibre breaks:
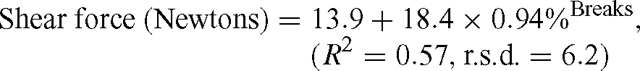
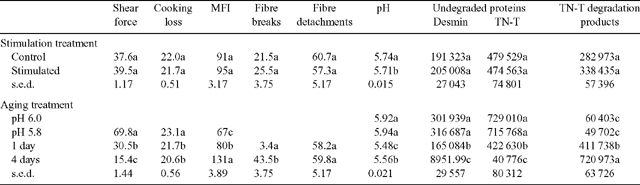
The effects of treatment (control and stimulation) and aging (pH 5.8, 1 day and 4 days) on shear force, cooking loss, MFI, fibre breaks and detachment, pH and protein degradation are shown in Table 1. Data for protein degradation at pH 6.0 is also shown in Table 1. Shear force was measured on samples at pH 5.8 to ensure early changes in tenderness were detected before the full onset of rigor mortis. There was no effect of electrical stimulation on any of the parameters measured except for the expected significant (P<0.05) lowering of muscle pH in stimulated samples. Aging did affect most parameters, with little difference seen between pH 5.8 and pH 6.0 samples when measuring protein degradation of the individual proteins, followed by significant (P<0.05) increases in myofibre degradation as the muscles aged (day 1 cf. day 4). Shear force was reduced to 25% of the starting value, MFI doubled and the percentage of fibre breaks from days 1 to 4 increased 10-fold. The exception to this was the percentage of fibre detachments where little difference was seen between days 1 to 4. There was no interaction between stimulation treatment and aging. As expected, pH decreased over time, as did the levels of desmin and troponin-T in the muscle. The total troponin-T degradation products increased considerably over time.
|
The use of histological images to assess myofibre degradation is a relatively new technique and may become more popular as its applications are expanded. The images obtained (Fig. 2) are very useful in observing the locations in the muscle myofibre where degradation occurs and how this links with results from other measures.
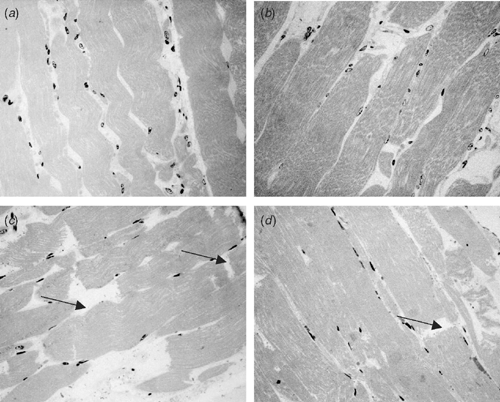
|
Discussion
The wrapping of the removed muscle prevented cold-induced shortening as demonstrated previously by Devine et al. (2002b) and this ensured that no confounding of results occurred due to premature shortening. The temperature of the water into which the samples were placed was considered to be optimal for muscle to enter rigor (Devine et al. 2002b) and this strategy was adopted in the current study to eliminate the confounding which occurs when comparing stimulated and unstimulated meat under conventional chilling. Unlike Devine et al. (2002a), we did not find any difference in shear force between treatments at the various sampling points, but as expected there was a large reduction in shear force with aging. Devine et al. (2002a) did use high voltage stimulation which would have resulted in a faster decline in pH than found in the current study and this may partially explain the differences. However, if effects are mediated by the interaction between pH and temperature, it should be stressed that the temperature of incubation in the current study was similar to that used by Devine et al. (2002a). This suggests that in the study of Devine et al. (2002a), the time between stimulation and incubation may have been a causative factor given the muscle would have experienced a rapid drop in pH at elevated temperature prior to being cooled to 15°C. By comparison, in the current study the differences were more subtle. Additionally, it should be noted that in the study reported by Hopkins et al. (2005), the same stimulation system as used in the current study produced a much faster rate of pH decline, yet there was no significant impact on shear force compared with unstimulated meat.
This work has extended the work of Devine et al. (2002a) with indicators of protein degradation being used to obtain a clearer understanding of the changes at the myofibril level which would be expected to impact on tenderness. The results of the histology indicate that fibre detachment occurs faster than breaks in the fibres during aging, but that by 4 days of aging there were significant breaks in the fibres. Taylor and Frylinck (2003) suggested that when using bovine muscle, detachment of fibres gives rise to early post-mortem improvements in tenderness while fibre breaks give rise to later improvements in tenderness. Given the results reported here then there is no support for the view that stimulation per se as applied in this study causes physical fibre disruption as postulated by Hwang et al. (2003) and that this in itself contributes to tenderisation. Clearly there is a strong relationship between degradation of myofibrils measured using MFIs and fibre breaks and therefore a strong relationship between shear force and fibre breaks.
Due to the role of desmin as a structural protein, holding myofibres together, it was surprising that no significant interactions were seen between MFI and desmin, while at pH 5.8 the positive correlation between undegraded desmin and shear force was quite strong. This relationship could be explained by the reduced rigidity of the myofibres when more desmin has degraded allowing the myofibres to be cut more easily. The lack of difference seen in troponin-T may be attributed to the low level of electrical stimulation used in this study, compared to others where the more intense levels of myofibre contractions may have led to increased mechanical breakdown of troponin-T.
Throughout the literature there are numerous examples (Lee et al. 2000) of electrical stimulation systems increasing the degradation of structural muscle proteins. This study has been one of the few that removed the interaction of changes in pH–temperature declines and electrical stimulation in an attempt to identify other potential effects of electrical stimulation on the muscle that may be causing this increased tenderness. The results suggest that with this particular stimulation system, the hypothesised muscle fibre mass-contraction leading to structural damage has not occurred. Recently, it has been demonstrated that a faster rate of pH decline can be achieved with the stimulation system used in this study by use of a different combination of electrical parameters, but any impact on shear force is yet to be established (Pearce et al. 2006). Additionally, the lack of difference in the degradation rate of the 2 studied proteins, desmin and troponin-T, suggests that when temperature at rigor is standardised, protein degradation does not differ according to stimulation treatment.
Acknowledgments
The technical assistance of David Stanley and Leonie Martin (NSW DPI), and Sheridon Moll (UNE) is gratefully acknowledged. The study was funded by NSW Department of Primary Industries, Meat and Livestock Australia, the Australian Sheep Industry Cooperative Centre and UNE. The assistance of Paul Weston (DPI, Vic.) in the slaughter and processing of the lambs at Werribee is noted with appreciation. Development of the histology method has been undertaken under the guidance of Steve Parkinson, School of Anatomy and Human Biology, University of Western Australia and the work of his group in preparing images is gratefully acknowledged.
Devine CE,
Payne SR,
Peachey BM,
Lowe TE,
Ingram JR, Cook CJ
(2002b) High and low rigor temperature effects on sheep meat tenderness and ageing. Meat Science 60, 141–146.
| Crossref | GoogleScholarGoogle Scholar |

Hopkins DL, Thompson JM
(2001a) The relationship between tenderness, proteolysis, muscle contraction and dissociation of actomyosin. Meat Science 57, 1–12.
| Crossref | GoogleScholarGoogle Scholar |

Hopkins DL, Thompson JM
(2001b) Inhibition of protease activity. 2. Degradation of myofibrillar proteins, myofibril examination and determination of free calcium levels. Meat Science 59, 199–209.
| Crossref | GoogleScholarGoogle Scholar |

Hopkins DL,
Martin L, Gilmour AR
(2004) The impact of homogenizer type and speed on the determination of myofibrillar fragmentation. Meat Science 67, 705–710.
| Crossref | GoogleScholarGoogle Scholar |

Hopkins DL,
Shaw FD,
Baud S, Walker PJ
(2005) Effects of level of current during lamb carcase electrical stimulation on post-mortem muscle changes and meat quality. Proceedings of the New Zealand Society of Animal Production 65, 247–251.

Hwang IH,
Devine CE, Hopkins DL
(2003) The biochemical and physical effects of electrical stimulation on beef and sheep meat tenderness – a review. Meat Science 65, 677–691.
| Crossref | GoogleScholarGoogle Scholar |

Lametsch R,
Roepstorff P,
Møller HS, Bendixen E
(2004) Identification of myofibrillar substrates for μ-calpain. Meat Science 68, 515–521.
| Crossref | GoogleScholarGoogle Scholar |

Lee S,
Polidori P,
Kauffman RG, Kim BC
(2000) Low-voltage electrical stimulation effects on proteolysis and lamb tenderness. Journal of Food Science: Food Chemistry and Toxicology 10, 786–790.

Martin LC,
Hopkins DL, Morgan JE
(2004) Streamlining the determination of myofibrillar fragmentation index. Animal Production in Australia 25, 279.

Morton JD,
Bickerstaffe R,
Kent MP,
Dransfield E, Keeley GM
(1999) Calpain-calpastatin and toughness in M. longissimus from electrically stimulated lamb and beef carcasses. Meat Science 52, 71–79.
| Crossref | GoogleScholarGoogle Scholar |

Pearce KL,
Hopkins DL,
Toohey ES,
Pethick DW, Richards I
(2006) Quantifying the rate of pH and temperature decline in lamb carcasses using medium voltage electrical stimulation in an Australian abattoir. Australian Journal of Experimental Agriculture 46, 869–874.

Steen D,
Claeys E,
Uytterhaegen L,
De Smet D, Demeyer D
(1997) Early post-mortem conditions and the calpain/calpastatin system in relation to tenderness of double-muscled beef. Meat Science 45, 307–319.
| Crossref | GoogleScholarGoogle Scholar |

Toohey ES, Hopkins DL
(2004) Preliminary data on the rate of glycolysis in ovine carcasses under commercial processing. Animal Production in Australia 25, 330.



