Structures of 4-Iminopyrido[1,2-a]pyrimidines, Pyrido[1,2-a]pyrimidin-4-ones, Pyridopyrimidinium Olates, and Thiazolo[3,2-a]pyrimidine Analogues
Paul V. Bernhardt A and Curt Wentrup A BA School of Chemistry and Molecular Biosciences, The University of Queensland, Brisbane, Qld 4072, Australia.
B Corresponding author. Email: wentrup@uq.edu.au
Australian Journal of Chemistry 65(4) 371-375 https://doi.org/10.1071/CH12040
Submitted: 24 January 2012 Accepted: 1 March 2012 Published: 2 April 2012
Abstract
The Structure-Correlation Principle of Bürgi and Dunitz is invoked in an analysis of the structures of 2-chloro-8-methyl-4-(2-(4-picolinyl)imino-4H-pyrido[1,2-a]pyrimidine 8, 7-chloro-5-(2-thiazolyl)imino-5H-thiazolo[3,2-a]pyrimidine 9, 2-methylamino-4H-pyrido[1,2-a]pyrimidin-4-one 10, 7-methylthio-5H-thiazolo[3,2-a]pyrimidin-5-one 11, 2,3-dihydro-7-methylthio-5H-thiazolo[3,2-a]pyrimidin-5-one 12, and 1-methyl-2-[(o-tert-butylphenyl)imino]-1,2-dihydropyrido[1,2-a]pyrimidin-1-ium-4-olate 13, which have been determined by X-ray crystallography. The most notable structural peculiarities are the long ‘amidine’ and ‘amide’ C–N bonds (1.40–1.50 Å) and the tilting of the ‘amidine’ C=N and ‘amide’ C=O groups towards a ring nitrogen atom (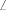 NCX = 114–118°). Also the ‘amidine’ C=N (1.28 Å) and ‘amide’ C=O bonds (1.22–1.24 Å) are long, i.e. in the normal range for resonance-stabilized amidines and amides in spite of the lack of such resonance in these compounds. These features mimic the transition states for ring opening to ketenes. The long amidine and amide C–N bonds and acute NCX angles are in accord with the observed thermal ring opening to ketenimines and ketenes, respectively.
NCX = 114–118°). Also the ‘amidine’ C=N (1.28 Å) and ‘amide’ C=O bonds (1.22–1.24 Å) are long, i.e. in the normal range for resonance-stabilized amidines and amides in spite of the lack of such resonance in these compounds. These features mimic the transition states for ring opening to ketenes. The long amidine and amide C–N bonds and acute NCX angles are in accord with the observed thermal ring opening to ketenimines and ketenes, respectively.
References
[1] H. G. Andersen, D. Kvaskoff, C. Wentrup, Aust. J. Chem. 2012, in press.| Crossref | GoogleScholarGoogle Scholar |
[2] M. Shtaiwi, C. Wentrup, J. Org. Chem. 2002, 67, 8558.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XosVWgsr4%3D&md5=f8ffd7e5329f9e6bc5384f1b7b45c26dCAS |
[3] C. Plüg, B. Wallfisch, H. G. Andersen, P. V. Bernhardt, L.-J. Baker, G. R. Clark, M. W. Wong, C. Wentrup, J. Chem. Soc., Perkin Trans. 2 2000, 2096.
| Crossref | GoogleScholarGoogle Scholar |
[4] C. Plüg, W. Frank, C. Wentrup, J. Chem. Soc., Perkin Trans. 2 1999, 1087.
[5] H. G. Andersen, U. Mitschke, C. Wentrup, J. Chem. Soc., Perkin Trans. 2 2001, 602.
| Crossref | GoogleScholarGoogle Scholar |
[6] (a) H. B. Bürgi, J. D. Dunitz, Acc. Chem. Res. 1983, 16, 153.
| Crossref | GoogleScholarGoogle Scholar |
(b) B. R. Pool, J. M. White, Org. Lett. 2000, 2, 3505.
| Crossref | GoogleScholarGoogle Scholar |
(c) D. Birney, T. K. Lim, J. H. P. Koh, B. R. Pool, J. M. White, J. Am. Chem. Soc. 2002, 124, 5091.
| Crossref | GoogleScholarGoogle Scholar |
(d) G. R. Unruh, D. M. Birney, J. Am. Chem. Soc. 2003, 125, 8529.
| Crossref | GoogleScholarGoogle Scholar |
(e) H.-X. Wei, C. Zhou, S. Ham, J. M. White, D. M. Birney, Org. Lett. 2004, 6, 4289.
| Crossref | GoogleScholarGoogle Scholar |
(f) Y. W. Goh, S. M. Danczak, T. K. Lim, J. M. White, J. Org. Chem. 2007, 72, 2929.
| Crossref | GoogleScholarGoogle Scholar |
[7] W. E. Thiessen, H. Hope, J. Am. Chem. Soc. 1967, 89, 5977.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1cXhtVyktbo%3D&md5=0e43a0d9257e59bb7c0fcbbe3b90e64dCAS |
[8] P. Bernhardt, D. Kvaskoff, R. N. Veedu, C. Wentrup, Aust. J. Chem. 2012, 65,
| Crossref | GoogleScholarGoogle Scholar |
[9] H. G. Andersen, C. Wentrup, Aust. J. Chem. 2012, 65, 105.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XisFaqtL0%3D&md5=d8818640ae8cbb8807979c2c8a946201CAS |
[10] H. Bibas, D. W. J. Moloney, R. Neumann, M. Shtaiwi, P. V. Bernhardt, C. Wentrup, J. Org. Chem. 2002, 67, 2619.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhvFymtrY%3D&md5=992080d3f8f3dbddc13b501d9df5dd4aCAS |
[11] M. Di Braccio, G. Roma, G. C. Grossi, G. Ciarello, J. Heterocycl. Chem. 1992, 29, 25.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XksVyntbg%3D&md5=fc9c6f5ab3c0cd27a2b3e951d0d67607CAS |
[12] E. J. Masters, M. T. Bogert, J. Am. Chem. Soc. 1942, 64, 2712.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaH3sXjs1am&md5=4ae2131ee9c15d7199e64930f409c71aCAS |
[13] E. Ziegler, H. Biemann, Monatsh. Chem. 1962, 93, 34.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF38XktlKgsbY%3D&md5=bfe96cf47ca4339cdaba75146f2929f3CAS |
[14] L. J. Farrugia, J. Appl. Cryst. 1999, 32, 837.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlsVSlurk%3D&md5=a99bb00e596a61480b8be4159291b3beCAS |
[15] G. M. Sheldrick, Acta Crystallogr. A 2008, 64, 112.
| Crossref | GoogleScholarGoogle Scholar |
[16] L. J. Farrugia, J. Appl. Cryst. 1997, 30, 565.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXnt1KgsLg%3D&md5=631f2e5f27cc94401b67d1297e3db297CAS |


