The 3rd Molecular Materials Meeting (M3) @ Singapore
Yun Zong A C and T. S. Andy Hor A B CA Institute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), 3 Research Link, 117602 Singapore.
B Department of Chemistry, National University of Singapore, 3 Science Drive 3, 117543 Singapore.
C Corresponding authors. Email: y-zong@imre.a-star.edu.sg; andyhor@imre.a-star.edu.sg

Yun Zong is the Manager of Molecular Materials Lab, the Manager of Advanced Energy Storage Lab and Deputy Head of Synthesis and Integration Capability Group at the Institute of Materials Research and Engineering of A*STAR, Singapore. He received his Ph.D. in Chemistry from Johannes-Gutenberg University of Mainz, and was a postdoctoral research fellow of National University of Singapore, a visiting scholar of Stanford University, and a technical advisor of Int.-Mat. Technologies. He chaired the Scientific Committee of the 2nd and the 3rd Molecular Materials Meeting (M3) @ Singapore, and chairs the Management Board of Molecular Materials Thematic Scholarship funded by A*STAR Graduate Academy. His current research focuses on nanostructured porous materials, bifunctional catalysts and ionic liquids for metal-air rechargeable batteries applications. |

Professor Hor is the Executive Director of the Institute of Materials Research and Engineering of A*STAR of Singapore, Professor of Chemistry at the National University of Singapore, President of the Singapore National Institute of Chemistry, and President of the Federation of Asian Chemical Societies. His main research interests are organometallic complexes and catalytic materials. He has delivered numerous plenary, keynote, and invited lectures in international conferences. He is an Associate Editor (Commissioning) of Aust. J. Chem. and on the international advisory board of Dalton Trans. (RSC), Inorg. Chim. Acta (Elsevier), and Chem. Asian J. (VCH-Wiley). He has published more than 310 international papers with annual citations of about 500. He obtained his B.Sc.(Hon) from Imperial College, D.Phil. from Oxford University, D. Sc. from University of London, and was a postdoctoral associate of Yale University. |
Australian Journal of Chemistry 66(9) 993-996 https://doi.org/10.1071/CH13430
Published: 11 September 2013
On 14–16 January 2013, the 3rd Molecular Materials Meeting (M3) @ Singapore was successfully held at Biopolis, a biomedical hub of Singapore hosting key research institutes of A*STAR and research and development laboratories of multinational corporations. With the theme of ‘Frontiers in Materials Science, Chemistry and Physics’, the conference featured six thematic sessions, namely New Catalysts and Catalytic Science, Soft Materials for Cosmetic and Health Care, Electrochemical Energy Conversion, Storage Materials and Technology, Materials for Advanced Sensors and Transducers, Lanthanide-doped Luminescence Nanomaterials, and Interface Engineering for Devices. The 3-day conference enabled lively discussions geared at cross-discipline collaborations in molecular materials (Fig. 1).
This year, the M3 @ Singapore follows the past practice to publish a commemorative edition in the Australian Journal of Chemistry on selected papers presented at the conference. In the first issue [Aust. J. Chem. 2011, 64 (9)], the volume covered diverse topics that reflected the contemporary interests in the broad field of molecular materials, e.g. from temperature-driven molecular self-organisation[1] to atom-transfer radical polymerisation based functional amphiphilic polymers synthesis,[2] from dye-sensitised solar cells[3] to nanoparticle-catalysed water-splitting,[4] from metal-organic frameworks[5] to aggregation-induced emission bioimaging,[6] etc. The second edition [Aust. J. Chem. 2012, 65 (9)] is however relatively focussed. It featured mainly on contributions in research of nanoparticles and organic energy materials.[7] The nanoparticle papers typified the harness of nanotechnology to enable new applications, e.g. using electro-responsive core-shell nanoparticles for rheology tuning,[8] carbon nanotube-based materials as catalysts in fuel cells,[9] using chitosan-functionalised Au/Pd alloy nanoparticles/nanoclusters for catalysis of aerobic oxidative homocoupling reactions,[10] and CdTe based hybrid fibres enabled low-voltage-driven electroluminescence devices,[11] etc; papers on organic semiconductor research focussed on the structure–property relationship that leads to design and synthesis of high performance molecular materials, e.g. heterocyclic dyes for efficient dye-sensitised solar cells,[12] phenyl-1H-pyrrole end-capped thiophenes for organic field-effect transistors,[13] and pyridine incorporated dihexylquaterthiophene as blue emitter in organic light emitting diode applications.[14]
In this commemorative edition, seven papers are selected as highlights of the meeting. Bai et al.[15] (Institute of Materials Research and Engineering, Singapore) introduced a new dinuclear Cu(II) complex (Fig. 2) with 1,2,3-triazoles of a chelate-bridging mode. Using rare azole-bridged ferromagnetically coupled dinuclear Cu(II) complexes as examples, the paper demonstrates the potential of using heterocyclic triazoles to support magnetically active dinuclear Cu(II) complexes. The finding marks a step closer to the establishment of magneto-structural relationship and understanding of magnetic exchange mechanism through 1,2,3-triazoles. To give a computational perspective, Manzhos[16] (National University of Singapore) presented a modified molecular dynamics algorithm (Quantised Energy Molecular Dynamics) for the choice of substituted groups on polythiophene, a common building block for many polymer donors in organic solar cells (e.g. P3HT). It shows that a significant broadening of absorption spectra achievable at elevated temperature or through deuteration is due to the broadening of energy levels of frontier orbitals which are critical for energy level matching with the acceptor and electrolyte species. The latter is a result of nuclear dynamics that can be predicted by Quantised Energy Molecular Dynamics. The knowledge of the effects of nuclear vibrations on the energetic and energy level matching, as described in the paper, empowers rational molecular and material design for organic photovoltaics related applications.

|
In nanocomposite materials system, Ye and Loh[17] (Institute of Materials Research and Engineering, Singapore) reviewed the recent research and development on the combined use of hydrogels and nanoparticles as a single entity in applications such as catalysis, Surface Enhanced Raman scattering Spectroscopy (SERS), biosensors, biomedical therapy, and drug delivery. Apart from a critical review of the current literatures, the authors also gave their perspective on the future of this emerging field. Xu et al[18] (Institute of Materials Research and Engineering, Singapore) reported polyurethane/clay nanocomposites with high mechanical strength and improved thermal conductivity. The markedly high mechanical strength of such nanocomposites, as concluded in the paper, comes from the high molecular weight polymers prepared via chain extension of the designed pre-polymers, and better dispersion of clay that was possible if lower molecular weight pre-polymers are used at the stage of dispersion. For materials system with well defined structure, Dinda et al.[19] (Siksha O Anushandan University, India) constructed substrate-supported Au nanoparticle-cluster arrays through directed self-assembly of block copolymers, and examined the SERS sensitivity of such arrays in trace analyte detection and quantification. The uniform and high density of hot-spots formed due to inter-particle and intercluster gaps of sub-10 nm across the surface pushed the limit of detection to as low as 10 nM, with well defined intensity-concentration dependence in the concentration range of 0.01–1.0 μM (Fig. 3). Such highly sensitive SERS substrates with low fabrication cost can be useful in real-life analytical applications.
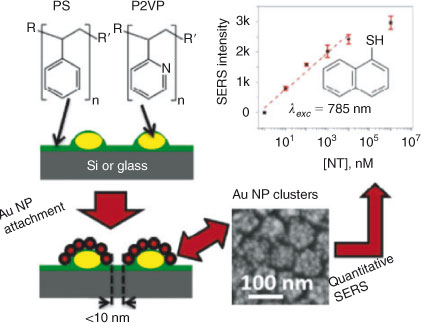
|
As a demonstration of integrating molecular materials into functional devices, Xu et al[20] (Institute of Materials Research and Engineering, Singapore) constructed electrochromic devices from thin films of a series of random π-conjugated azulene-containing electrochromic copolymers that were synthesised from Suzuki cross-coupling reactions. Electron acceptors and azulene units were used to tune the optical and electrochemical properties of the polymers so that the optical bandgaps can match the electrochemical bandgaps. With incorporation of suitable electron acceptor, the azulene-containing conjugated polymers showed improved redox stability. A colour change from yellowish green to greyish brown with optical contrast of up to 17 % in the near infrared (NIR) region was obtained upon oxidation via application of a positive bias (1.2 V) (Fig. 4). The process is reversible if a reduction reaction takes place upon application of a negative bias (–2.0 V). Such tunability of NIR transmission could be used for heat management which is of interest to green-building related applications.
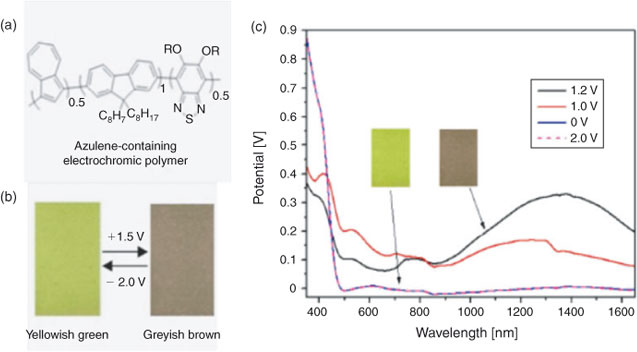
|
A more comprehensive demonstration is to combine the design of molecular materials with physical architecture (e.g. repeated strips with layered structures) for waveguide applications. Tan et al[21] (Singapore University of Technology and Design) presented a mini-review that captured the rationale for the design and synthesis of luminescent photonic nanocomposite systems, a continuous polymeric matrix with homogeneously dispersed rare-earth doped particulates. It provided a panoramic view that covers nanostructured photonic composites design, structure–property relationship examination, performance evaluation criteria, strategies for the rational design of solvothermal processes, examples of luminescent nanocomposite systems fabricated for optical waveguides (Fig. 5) and biomedical imaging, etc. It is an excellent review in a field of translational research in molecular materials.
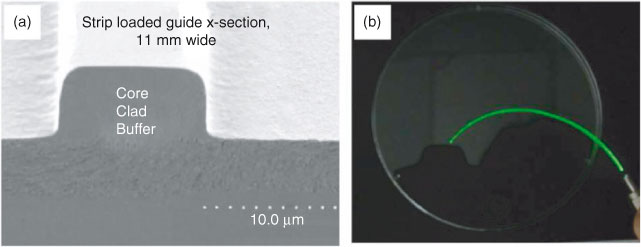
|
The beauty of Molecular Materials is beyond its fanciful term. It is a universal tool for materials scientists in the modern era to tackle real and major engineering and technological challenges using molecular scientific concepts. The three biggest challenges facing the world today are energy, environment, and healthcare – three areas that molecular materials have the strongest influence. This is the time for molecular materials scientists to rise to our challenges. The conferences provides a forum for new ideas to be discussed and harnessed, cutting across areas such as organic solar cells, biomass conversion, nanomaterials, biosensors, nanomedicine, consumer care products, and others. The limit of Molecular materials is our creativity and ambition.
The Molecular Materials Meeting (M3) @ Singapore also provides a unified platform for scientists and engineers to exchange ideas, foster collaboration, and develop new partnership. Singapore, known for its education focus, research investment and economic affluence, is the meeting point between east and west, as well as south and north. It is also a place that uses its compactness to its advantage. Through M3 @ Singapore, we open a door for cross-institutional interdisciplinary research; through science, we create new partnership and knowledge; through collaboration, we make a difference to humankind.
The 4th M3@Singapore will take place in Singapore in 14–16 January 2014 (http://www.imre.a-star.edu.sg/). We welcome all students, researchers, and innovators to join us in this expedition that springs delightful surprises in almost every turn.
References
[1] J. M. MacLeod, F. Rosei, Aust. J. Chem. 2011, 64, 1299.| Crossref | GoogleScholarGoogle Scholar |
[2] H. Hussain, E. Amado, J. Kressler, Aust. J. Chem. 2011, 64, 1183.
| Crossref | GoogleScholarGoogle Scholar |
[3] W. Zhang, R. Zhu, B. Liu, S. Ramakrishna, Aust. J. Chem. 2011, 64, 1282.
| Crossref | GoogleScholarGoogle Scholar |
[4] K. J. Young, Y. Gao, G. W. Brudvig, Aust. J. Chem. 2011, 64, 1221.
| Crossref | GoogleScholarGoogle Scholar | 22140273PubMed |
[5] R. P. Davies, P. D. Lickiss, K. Robertson, A. J. P. White, Aust. J. Chem. 2011, 64, 1239.
| Crossref | GoogleScholarGoogle Scholar |
[6] Y. Hong, J. W. Y. Lam, S. Chen, B. Z. Tang, Aust. J. Chem. 2011, 64, 1203.
| Crossref | GoogleScholarGoogle Scholar |
[7] Y. Zong, T. S. A. Hor, Aust. J. Chem. 2012, 65, 1191.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWksb7I&md5=add1bde714f67138c439efbcbdd511adCAS |
[8] Y. D. Liu, K. Zhang, W. L. Zhang, H. J. Choi, Aust. J. Chem. 2012, 65, 1195.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWksbzM&md5=0922cb65f43aad49850070363679f4d0CAS |
[9] J. Liu, L. Lai, N. G. Sahoo, W. Zhou, Z. Shen, S. H. Chan, Aust. J. Chem. 2012, 65, 1213.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWksbzJ&md5=12004b55b78156ddb3d0efc12c8fb567CAS |
[10] O. Sophiphun, J. Wittayakun, R. N. Dhital, S. Haesuwannakij, A. Murugadoss, H. Sakurai, Aust. J. Chem. 2012, 65, 1238.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWksb%2FJ&md5=6be08489c9b2647a18a2358ef5247f4cCAS |
[11] M. Ando, C. Hosokawa, P. Yang, N. Murase, Aust. J. Chem. 2012, 65, 1257.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWksbzP&md5=e3edf7f59a9aefaf035870817f134e63CAS |
[12] Q. Li, Z. Jiang, J. Qin, Z. Li, Aust. J. Chem. 2012, 65, 1203.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWksbzF&md5=a3502776c56bb4efd5256e221ad14763CAS |
[13] Y. Liu, J. Zhang, Y. Liu, G. Yu, Z. Ge, Aust. J. Chem. 2012, 65, 1252.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWksb3N&md5=3d68c4061bade1151d9fff6973064e70CAS |
[14] P. Sonar, S. G. Santamaria, T. T. Lin, A. Sellinger, H. Bolink, Aust. J. Chem. 2012, 65, 1244.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWksbzN&md5=08ea085d0859ca039c59ca771392d85aCAS |
[15] S. Q. Bai, L. Jiang, J. L. Zuo, C. H. Yan, T. S. A. Hor, Aust. J. Chem. 2013, 66, 1029.
| Crossref | GoogleScholarGoogle Scholar |
[16] S. Manzhos, Aust. J. Chem. 2013, 66, 1021.
| Crossref | GoogleScholarGoogle Scholar |
[17] E. Ye, X. J. Loh, Aust. J. Chem. 2013, 66, 997.
| Crossref | GoogleScholarGoogle Scholar |
[18] S. L. Sin, J. N. Kumar, H. R. Tan, C. He, Y. Liu, J. Xu, Aust. J. Chem. 2013, 66, 1039.
| Crossref | GoogleScholarGoogle Scholar |
[19] S. Dinda, F. L. Yap, V. Suresh, R. K. Gupta, D. Das, S. Krishnamoorthy, Aust. J. Chem. 2013, 66, 1034.
| Crossref | GoogleScholarGoogle Scholar |
[20] S. Z. H. Lim, W. T. Neo, C. M. Cho, X. Wang, A. Y. X. Tan, H. S. O. Chan, J. Xu, Aust. J. Chem. 2013, 66, 1048.
| Crossref | GoogleScholarGoogle Scholar |
[21] M. C. Tan, D. J. Naczynski, P. V. Moghe, R. E. Riman, Aust. J. Chem. 2013, 66, 1008.
| Crossref | GoogleScholarGoogle Scholar |



