Substance Abuse: Carbon Tetrachloride and the Ozone Layer*
Ian D. RaeSchool of Chemistry, University of Melbourne, Melbourne, Vic. 3010, Australia. Email: iandrae@bigpond.com

Ian Rae is an organic chemist who moved from a career in university teaching, research, and administration to become an advisor to Australian governments and the United Nations Environment Program on the health and environmental impacts of chemicals. For a decade, he was co-chair of a technical advisory committee for the Montreal Protocol on substances that deplete the ozone layer. An honorary professorial fellow at the University of Melbourne, he is a past president of the Royal Australian Chemical Institute and recipient of its Leighton Medal in 2015. |
Australian Journal of Chemistry 69(12) 1375-1379 https://doi.org/10.1071/CH16451
Submitted: 2 August 2016 Accepted: 18 August 2016 Published: 2 September 2016
Abstract
Each year, ~200000 tonnes of carbon tetrachloride are produced and consumed as a solvent, a starting material for synthesis, or in standard methods of analysis. Because it is an ozone-depleting substance, this information is reported on an annual basis to the Montreal Protocol, under which it is a ‘controlled’ substance. Replacing emissive uses of carbon tetrachloride with ozone-friendly chemicals is proceeding slowly. One example is the use of tetrachloroethylene as a replacement for carbon tetrachloride in oil and grease analysis by infrared spectroscopy. Overall, however, there is more carbon tetrachloride in the upper atmosphere than can be accounted for in terms of known uses and emissions. The discrepancy is the subject of intensive and repeated investigation by atmospheric scientists.
Montreal Protocol
As a consequence of scientific observations of the thinning of the ozone layer that protects the Earth from hard UV radiation, especially over the Antarctic, an intergovernmental agreement – the Vienna Convention for the Protection of the Ozone Layer – was developed under the auspices of the United Nations Environment Program (UNEP) and signed in March 1985. The scientists credited with the identification of this global environmental problem, Paul Crutzen, Mario Molina, and F. Sherwood Rowland, were awarded the 1995 Nobel Prize in Chemistry but action to curb ozone depletion was well under way by then. To resort to corporate language, the Convention is the strategic plan for the rescue of the ozone layer. While waiting for it to come into force, which happened in September 1988 after it was ratified by at least 20 states, the Parties (countries that had signed the Convention) got moving with the implementation plan, the Montreal Protocol on Substances That Deplete the Ozone Layer. The Protocol was agreed in September 1987 and came into force in January 1989. Both instruments have since been ratified by all 197 states.[1]
The Montreal Protocol contained a list of substances that were known or thought to deliver chlorine and bromine atoms to the lower stratosphere, 20–30 km above Earth, where they could be responsible for enhanced destruction of ozone molecules. Parties were required to report production and consumption of these ozone-depleting substances (ODSs) on an annual basis, and act in accordance with decisions taken under the Protocol to restrict or eliminate their use. Among the substances of concern were chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), halons (bromine-containing molecules used as fire retardants), methyl bromide (fumigant), and solvents such as 1,1,1-trichlorethane (methyl chloroform), bromochloromethane, and carbon tetrachloride. Control measures were introduced under the Montreal Protocol to phase out the use of substances in all of these categories. Longer times were allowed for developing countries and a multilateral fund was established to assist them in making the necessary changes in chemical use. Several technical advisory panels were created to help the Parties in their work.
The Montreal Protocol has been a huge success. With greatly reduced emissions of ODS, degradation of the ozone layer has been arrested and it is beginning to recover. As many of the ODSs have serious global warming potential, actions taken under the Montreal Protocol have also made a substantial contribution to slowing global warming and climate change.
Carbon Tetrachloride and the Montreal Protocol
As organic chemicals go, carbon tetrachloride (CTC) is a venerable one, coming to the attention of chemists in the early nineteenth century and still in use today despite Montreal Protocol strictures against it. Michael Faraday was probably the first to encounter it when he explored the reactions of one- and two-carbon compounds with chorine in the presence of sunlight,[2] but the formation of clearly identified carbon tetrachloride is generally attributed to the French chemist Victor Regnault.[3]
Although production and consumption of most ODSs are banned or subject to decreasing quotas leading to eventual phase-out, there are several categories of exemption, and carbon tetrachloride features prominently in each of them. The ozone-depleting potential (ODP) of carbon tetrachloride is 1.1, comparable with those of the simplest CFCs, CFC-11 (CFCl3) and CFC-12 (CF2Cl2), which are taken as 1.0, so its widespread use is a matter of concern. It is produced by chlorination of one- and two-carbon substrates, in quantities of ~200 ktonne year–1. The number is the same when expressed in the internationally used unit that may be unfamiliar to chemists, the gigagram (Gg).
Some uses of ODSs are permitted in a special category known as Process Agents. These are uses that commenced before 1996 and could not be easily or economically replaced because of the special nature of the application. Carbon tetrachloride as solvent is the most common Process Agent and although the number of approved applications has diminished owing to loss of market for the product, or end-of-life of the plant, a few still remain. Emissions from such uses are reported annually to the Ozone Secretariat and, provided those figures are accurate, they do not constitute a major source of carbon tetrachloride in the atmosphere, a subject dealt with the final section of the present review.
Emissive uses of ODSs are proscribed under the Protocol but feedstock uses (also reported annually) are permitted because the ODS is thereby destroyed and cannot contribute to ozone depletion. Carbon tetrachloride is used as starting material for the production of a range of substances. These include fluorinated compounds for which it is often the precursor to a CF3– group, and also the pyrethroid insecticide Permethrin, in which a dichloromethylene group in the Cl2C=CH– moiety is derived from carbon tetrachloride:
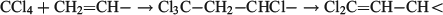
At meetings of the Parties to the Montreal Protocol, some delegates, notably representatives of western European countries, have opposed the continuing uses of ODSs as feedstocks, on the grounds that fugitive and accidental emissions can damage the ozone layer. They have argued that the best way to avoid these emissions is to stop the uses. Such pressures have generally not met with success because feedstocks are carefully selected on the basis of cost and availability as well as suitability for the proposed chemistry. While a product made from carbon tetrachloride, for example, continues to find good markets and is not itself the subject of local, regional or international bans, the producing companies are unlikely to seek alternatives.
Turning to operations on a smaller scale, there are many laboratory and analytical uses of ODSs, especially of carbon tetrachloride, to which exemptions apply under the Montreal Protocol. These exemptions are constantly reviewed and technical committees report that most of them can be replaced, but the cost of replacing simple analytical techniques in developing countries with more expensive procedures has been accepted as a reason for prolonging the exemptions.
Laboratory and Analytical Uses of Carbon Tetrachloride
Alternatives to the use of carbon tetrachloride in iodometric titrations, and as a solvent for 1H NMR and column chromatography are fairly easily found. More difficulty has been experienced in finding a symmetrical molecule to serve as solvent in Raman spectroscopy, and as a solvent for reactions of N-bromosuccinimide. Benzotrifluoride (α,α,α-trifluorotoluene, C6H5CF3) has been touted as a replacement for carbon tetrachloride in this application[4] but it has not been widely adopted. Another use of carbon tetrachloride that is hard to substitute is its use in very small quantities as a liver toxicant in studies of liver function and alcoholism.
Carbon tetrachloride is in widespread use, especially in developing countries, in standard methods of analysis. Here, the search for an alternative solvent encounters difficulties of a different kind, because standard methods of analysis play important roles in quality assurance and in many cases, their use is supported by production managers and by customers who are concerned with the reliable quality of a product. When environmental monitoring is being conducted, the use of standard methods is required by company management and government agencies. New standard methods can be slow to emerge and there is always a danger that a new method may require instrumentation that is too expensive for users in developing countries. Without new, inexpensive methods, the task of persuading users to change to methods that have not been developed through the rigorous processes of the standards organizations is a difficult one. Two examples of successful substitution are presented here.
Examples of Successful Replacement
Surface Area Determination of Activated Carbon
Activated carbon is widely used to remove impurities from gases and liquids, including drinking water. One of the common sources is the char produced from coconut shells. Activation of the char by steam treatment can produce activated carbons with surface areas, mainly in the form of micropores, of ~1000 m2 g–1. Coconut shell activated carbon is produced in several developing countries, notably Sri Lanka, India, Malaysia, and the Philippines. As the source of the carbon is the fruit of a long-lived tree, and production techniques involve only modest levels of chemical technology, this is a sustainable industry that makes an important contribution to the economies of these countries.
The surface area of the activated carbon is measured by physical adsorption of gases and interpretation of the adsorption isotherm in terms of theory developed by Brunauer, Emmett, and Teller (BET),[5] which in turn has its roots in the work of Irving Langmuir.[6] The gas most used for estimating the surface area of activated carbons is the vapour of carbon tetrachloride, the results being used for quality control and marketing. Standard Methods have been devised by the American Society for Testing Methods (ASTM) and, despite pressure from the Montreal Protocol to eliminate the use of ODSs such as carbon tetrachloride from laboratory and analytical procedures, the ASTM methods have continued to be revised and promulgated.[7] However, the relevant working committee of ASTM has also developed two test methods that avoid the use of ODSs and are based instead on the adsorption of butane. The first of these measures the butane activity and also provides for correlation of the results obtained by the new method with those obtained using carbon tetrachloride, so traditional activity numbers can be maintained.[8] The second measures the ability of the activated carbon to adsorb and desorb butane from dry air.[9]
When these alternative methods were brought to the attention of Montreal Protocol Parties in the 2013 report of the Technology and Economic Advisory Committee, they were quickly adopted by industry chemists in Sri Lanka. There was considerable technical capacity there, both in the industry and in the Coconut Research Institute, founded in 1929 to promote research into genetics, chemistry, and soils that would foster the industry. A mark of the prowess of Sri Lankan experts was that they found that liquefied petroleum gas (LPG), a commercial mixture consisting mainly of propane and butane that is more widely available and considerable less expensive than pure butane, could be used in the test. The information about this modification has been conveyed to other producers in the south Asian and Asia–Pacific region.
Hydrocarbon Residues
Several analytical methods have been used for the analysis of hydrocarbon (oil and grease) contamination of water and soil and for mists in gas streams from which the oil could be trapped by a filter. The simplest method involves extraction of the hydrocarbons into a solvent such as hexane or cyclohexane, drying, evaporation of the solvent, and weighing the residue. All substances less volatile than the organic solvent would be lost in the evaporation step and so the analysis may under-report the hydrocarbon content. These volatiles are likely to have been lost from environmental samples so this inexpensive analytical method has found widespread application and I have seen it also used to estimate the yield of ‘biodiesel’ formed by transesterification of glycerides.
Solvent extraction is also used in an elegant analysis that retains the volatiles and estimates the hydrocarbon content by measurement of the infrared absorption at 2930 cm–1 (3.4 μm). As spectrometers became more common in laboratories, manufacturers encouraged their use by developing and publishing methods for analysis. Both Beckman (1968) and PerkinElmer (1972) published application notes describing the use of carbon tetrachloride in infrared analysis of hydrocarbon residues. Methods were also published by the American Petroleum Institute, the American Public Health Association, and other organizations concerned with environmental analyses. A standard method was developed by ASTM (ASTM D3921–85) and was widely adopted. Eventually, concerns about the toxicity of carbon tetrachloride caused the method to be withdrawn in the mid-1990s and replaced with one using a less toxic but still ozone-depleting substance, 1,1,2-trichloro-1,2,2-trifluoroethane (CFC-113). The use of this fluorocarbon had been recommended some years before after it was shown to be just as effective as carbon tetrachloride in the analysis and much less toxic.[10] As production and consumption of CFCs was phased out under the Montreal Protocol – by January 1996 in developed countries and 2010 in developing countries, although there were some exemptions for laboratory and analytical uses – this standard was, in turn, withdrawn.[11]
Instrument manufacturers introduced the use of another CFC, a mixture of the dimer (probably a cyclobutane) and trimer of chlorotrifluoroethylene marketed as S-316.[12] It was some years before ASTM introduced a spectroscopic method based on the use of this solvent.[13] This was criticized heavily by delegates to the Montreal Protocol Meeting of the Parties in 2012 and, perhaps also because the solvent was very expensive, it has not been widely adopted. The most recent standard method[14] involves extraction of the hydrocarbon into a cyclic aliphatic hydrocarbon such as cyclohexane and, after clean-up with an absorbent such as Florisil, quantitation using peaks in the 1370–1380 cm–1 (7.25–7.30 μm) region of the infrared spectrum where the solvent does not absorb. The most sophisticated method for assessing hydrocarbon content, described in US Environmental Protection Authority (USEPA) Method 8260B ‘Volatile Organic Compounds by Gas Chromatography–Mass Spectrometry (GC/MS)’, gives a more detailed picture of the hydrocarbons but involves expensive instrumentation. A full account of modern methods for oil analyses has been published.[15]
The criteria to be met by an alternative to carbon tetrachloride in the infrared method for oil residue analysis were considered by the Montreal Protocol Chemicals Technical Options Committee as they developed the advice they could provide to Parties. As well as being non-ozone-depleting and, for good measure, of low global warming potential, the solvent must have no hydrogens, so that the region of the infrared spectrum near 3000 cm–1 was not obscured, and also be acceptable on health and amenity grounds. Carbon disulfide met the first criteria but was judged to be too volatile and definitely too smelly for this kind of use. However, tetrachloroethylene (perchloroethylene) seemed suitable. Its toxicity was under scrutiny, especially where it was used in large quantities in dry-cleaning of garments, or in industrial degreasing, from which it had in any case largely been phased out, but it was not worse that carbon tetrachloride and in a laboratory setting, its use should be acceptable. It is also included by many jurisdictions in a list of hazardous organic air pollutants. A literature search showed that others had identified this substance as a suitable replacement. Tetrachloroethylene had been used as a degreasing solvent by America’s National Aeronautics and Space Administration (NASA) and the hydrocarbons removed from the metal surfaces were quantified by infrared spectroscopy.[16] Some Greek analysts had explored its use and reported satisfactory analytical results.[17] Tetrachloroethylene was also recommended by Rintoul,[12] who observed that ‘it is important to use spectrophotographic-grade perchloroethylene that has not been stabilised with a hydrocarbon compound’. The literature also included an application note from spectrometer manufacturer PerkinElmer, describing the use of tetrachloroethylene as extracting solvent and measurement of the peak area in the 2945–2915-cm–1 region of the infrared spectrum.[18]
An opportunity to trial the use of tetrachloroethylene was afforded in 2008 in Chile, where the government, with support from the United Nations Development Program, had embarked on a program of finding alternatives to the use of ODSs, especially carbon tetrachloride. A first step was to persuade the major supplier that selling of this ODS was in contravention of the company’s environmental responsibility and ethics statement. Attention then turned to chemists at the national petroleum refining company, Empresa Nacional del Petróleo, who were using carbon tetrachloride as solvent in their infrared assay for hydrocarbons in waste water, as part of their compliance with local environmental regulations. The petroleum company agreed to replace carbon tetrachloride with tetrachloroethylene, and after a one-year trial, they reported satisfactory results. Environmental researchers at Universidad de Concepción, working with environment samples containing traces of hydrocarbons, found that the presence of low levels of (unspecified) impurities in the tetrachloroethylene reduced the accuracy of their results.
There the matter rested until this year, when the Ministry of Environment Protection in China, where many chemists use the infrared method, announced that it would adopt the use of tetrachloroethylene in a new standard method to be issued in 2017 and implemented in 2018. The Ministry is investigating methods for purification of the solvent to ensure the accuracy of determinations.[19]
The Great Atmospheric Carbon Tetrachloride Mystery
It had been known since the late 1990s that there is a discrepancy between estimates of the emissions of carbon tetrachloride derived from reported production figures and reported or estimated emission rates (‘bottom–up’ estimates) and estimates based on observed tropospheric and stratospheric concentrations and calculated lifetimes of carbon tetrachloride in the atmosphere (‘top–down’ estimates). Working with figures for production and consumption in 2002 (the latest available to them in 2005), the Chemicals Technical Options Committee calculated that 35–47 ktonne would have been emitted. Results from atmospheric scientists suggested that emissions of 70 ± 6 ktonne were required to produce the observed atmospheric concentrations. A review of the calculations conducted by a task force established by the Technology and Economic Assessment Panel and the Science Assessment Panel, using data for 2006, was reported in 2008. Global production of CTC in 2006 was 200 ktonne, of which 161 ktonne were used as feedstock. However, to maintain the measured 2006 atmospheric concentration, it was still found necessary to assume annual emissions of 70 ktonne. It was inconceivable that such a large proportion of the known production (34 %) could have been emitted.
Year by year, there were successive attempts to refine the ‘bottom–up’ estimates by getting better data on production and consumption, by considering possible legacy sources such as landfills, and to refine ‘top–down’ estimates by adjusting the atmospheric lifetime of carbon tetrachloride. Estimated annual production remained at ~200 ktonne, and emissions were estimated to be 24 ktonne, with a possible further 7.5 ktonne lost during storage and transport. A substantial gap remained.
A clue to the existence of diffuse legacy sources of carbon tetrachloride emissions was provided by Australian and other researchers in 2014,[20] using data from the CSIRO and Bureau of Meteorology monitoring site at Cape Grim, Tasmania. Whereas global ‘bottom–up’ estimates of emissions varied widely, prompting the remark that ‘the quality of reported UNEP production and consumption data could be improved’, there was a consistent trend in ‘top–down’ estimates, which fell from 130–140 ktonne in the 1980s to 70 ± 7 ktonne in 2002–06 and 59 ± 9 ktonne by 2011. Emissions from the Melbourne region for 1996, monitored at Cape Grim, were 28 ± 8 tonne, allowing a total for Australia to be scaled to 150 ± 45 tonne. Contaminated soils and toxic waste-treatment facilities were suggested as likely sources of the emissions, with possible contributions from chlor-alkali plants but negligible quantities from swimming pool chlorination. If Australia accounts for 1 ± 0.5 % of global emissions, the global total would then be 10–30 ktonne year–1, approximately half of the gap between ‘bottom–up’ and ‘top–down’ estimates.
A recent study of American air space for the period 2008–12 has identified industrial regions as sources of carbon tetrachloride emissions that are two orders of magnitude greater than emissions reported to the US EPA Toxics Release Inventory.[21]
In late 2015, a technical group was brought together by the World Climate Research Program – Stratosphere–Troposphere Processes and Their Role in Climate (SPARC)[22] – to study the discrepancy. Making a major change in the atmospheric lifetime to 44 (36–58) years, and consequent change in total (air, land, water) lifetime from 26 to 33 (28–41) years has enabled recalculation of aggregated ‘top–down’ emissions as 35 ± 16 ktonne year–1. ‘Bottom–up’ emissions are estimated at 20 ± 5 ktonne year–1, meaning that ‘these new emissions estimates reconcile the CCl4 budget discrepancy when considered at the edges of their uncertainties’. The authors comment that consideration of fugitive emissions alone ‘is not adequate for estimating total global CCl4 emissions and that, in agreement with the findings of Hu et al.,[21] most CCl4 emissions originate from chemical industrial regions – not from major population centres’. They recommend several avenues for further research and the reader is free to assume that full resolution of the mystery cannot be expected for several years. The SPARC report will be considered at Montreal Protocol meetings in the second half of 2016.
Conclusion
The carbon tetrachloride story is an exemplar of the factors in play during the implementation of an international agreement. Although there have been dramatic reductions in production and consumption of ‘headline’ substances such as CFCs and HCFCs, chemists and chemical industry have found it hard to replace carbon tetrachloride as feedstock and as solvent for chemical reactions and analytical procedures. This despite the efforts of chemists, chemical engineers, atmospheric scientists, economists, and, not least, the delegates who represent their countries and strive to make the Montreal Protocol work.
It is reasonable to conclude that large quantities of carbon tetrachloride will continue to be used as long as the chemical industry finds it useful, but that it will gradually disappear from use in analytical chemistry. Looming over them all is the great discrepancy between what we can reasonably estimate is being released to the atmosphere and what the science tells us is apparently being released. The latest study of the gap between ‘top–down’ and ‘bottom–up’ releases of carbon tetrachloride to the atmosphere has narrowed the gap but it is only closed if the substantial error limits in the estimates are taken into account, and even then, only just closed. It would seem that the Great Atmospheric Carbon Tetrachloride Mystery is not really solved yet.
* The author is the winner of the 2015 RACI Leighton Medal.
References
[1] The Montreal Protocol: Celebrating 20 Years of Environmental Progress (Ed. D. Kaniaru) 2007 (UNEP/Earthprint: London).[2] M. Faraday, Phil. Trans. R. Soc. Lond. 1821, 111, 47.
| Crossref | GoogleScholarGoogle Scholar |
[3] V. Regnault, Ann. Chim. Phys. 1839, 70, 104.
[4] R. Thapa, Regioselectivity in Free Radical Bromination of Unsymmetrical Dimethylated Pyridines 2009, M.Sc. thesis, Miami University, Oxford, OH. Available at: https://etd.ohiolink.edu/rws_etd/document/get/miami1263340046/inline (accessed June 2016).
[5] (a) S. Brunauer, P. H. Emmett, E. Teller, J. Am. Chem. Soc. 1938, 60, 309.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaA1cXivFaruw%3D%3D&md5=21fc518f10ad5fa12df6bdd21faac98cCAS |
(b) S. Brunauer, Physical Adsorption of Gases and Vapors 1943 (Princeton University Press: Princeton, NJ).
[6] I. Langmuir, J. Am. Chem. Soc. 1916, 38, 2221.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaC28Xhs1egsQ%3D%3D&md5=ce04282fd0d07b3040805da38410a512CAS |
[7] Standard Test Method for Carbon Tetrachloride Activity of Activated Carbon, ASTM D3467–99 (1999), D3467–04 (2014). Available at: www.astm.org/Standards/D3467.htm (accessed May 2016).
[8] Standard Test Method for Determination of Butane Activity of Activated Carbon, ASTM D5742–95 (2015). Available at: www.astm.org/Standards/D5742-95.htm (accessed May 2016).
[9] Standard Test Method for Determination of Butane Working Capacity of Activated Carbon, D5228–92 (2015). Available at: www.astm.org/Standards/D5228.htm (accessed June 2016).
[10] M. Gruenfeld, Environ. Sci. Technol. 1973, 7, 636.
| Crossref | GoogleScholarGoogle Scholar |
[11] ASTM D3921–96 (2011), Standard Test Method for Oil and Grease and Petroleum Hydrocarbons in Water (Withdrawn 2013). Available at: www.astm.org/Standards/D3921.htm (accessed May 2016).
[12] S. Rintoul, American Laboratory, 1 September 2005. Available at: www.americanlaboratory.com/914-Application-Notes/36172-On-Site-Wastewater-Analysis-Using-a-TOG/TPH-Analyzer/ (accessed May 2016).
[13] ASTM D7066–04, Standard Test Method for Dimer/Trimer of Chlorotrifluoroethylene (S-316) Recoverable Oil and Grease and Non-Polar Material by Infrared Determination. Available at: www.astm.org/Standards/D7066.htm (accessed May 2016).
[14] ASTM D7678–11, Standard Test Method for Total Petroleum Hydrocarbons (TPH) in Water and Wastewater with Solvent Extraction Using Mid-IR Laser Spectroscopy. Available at: https://www.astm.org/Standards/D7678.htm (accessed June 2016).
[15] B. Brkić, N. France, S. Taylor, Anal. Chem. 2011, 83, 6230.
| Crossref | GoogleScholarGoogle Scholar | 21718034PubMed |
[16] Surface Cleanliness of Fluid Systems, Specification for. NASA Report KSC-C-123H, 25 September 1995. Available at: http://ntrs.nasa.gov/archive/nasa/casi.ntrs.gov/19960007875.pdf (accessed May 2016).
[17] E. Farmaki, T. Kaloudis, K. Dimitrou, N. Thanasoulias, L. Koursouris, F. Tzoumerkas, Desalination 2007, 210, 52.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXltFygsbg%3D&md5=673c02d661079c5f4eae25718ef8b1fcCAS |
[18] A. Pisal, Determination of Oil and Grease in Water with a Mid-Infrared Spectrometer. Available at: https://www.perkinelmer.com/lab-solutions/resources/docs/APP-OilandGreaseinWaterbyMid-Infrared.pdf (accessed June 2016).
[19] Progress Report of the UNEP Technology and Economic Assessment Panel, June 2016, Volume 1. Available at: http://ozone.unep.org/en/assessment-panels/technology-and-economic-assessment-panel (accessed June 2016).
[20] P. J. Fraser, B. L. Dunse, A. J. Manning, S. Walsh, R. H. J. Wang, P. B. Krummel, L. P. Steele, L. W. Porter, C. Allison, S. O’Doherty, P. G. Simmonds, J. Mühle, R. F. Weiss, R. G. Prinn, Environ. Chem. 2014, 11, 77.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXjtFSiur8%3D&md5=605f090fc55790ebe1a45b4b994d5100CAS |
[21] L. Hu, S. A. Montzka, B. R. Miller, A. E. Andrews, J. B. Miller, S. J. Lehman, C. Sweeney, S. Miller, K. Thoning, C. Siso, E. Atlas, D. Blake, J. A. de Gouw, J. B. Gilman, G. Dutton, J. W. Elkins, B. D. Hall, H. Chen, M. L. Fischer, M. Mountain, T. Nehrkorn, S. C. Biraud, F. Moore, P. P. Tans, Proc. Natl. Acad. Sci. USA 2016, 113, 2880.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28Xjt12hsr8%3D&md5=7fcad747ce64a17838d4d5980dd86382CAS | 26929368PubMed |
[22] SPARC Report on the Mystery of Carbon Tetrachloride. Available at: http://www.sparc-climate.org/publications/sparc-reports/sparc-report-no7/ (accessed July 2016).


