n-Octyl (Thio)glycosides as Potential Cryoprotectants: Glass Transition Behaviour, Membrane Permeability, and Ice Recrystallization Inhibition Studies*
Rekha Raju A , Theresa Merl B , Madeleine K. Adam C , Emiliyan Staykov C , Robert N. Ben C , Gary Bryant A D and Brendan L. Wilkinson B DA Centre for Molecular and Nanoscale Physics, School of Science, RMIT University, Melbourne, Vic. 3000, Australia.
B School of Science and Technology, University of New England, Armidale, NSW 2351, Australia.
C Department of Chemistry and Biomolecular Sciences, University of Ottawa, Ottawa, ON K1N 6N5, Canada.
D Corresponding authors. Email: gary.bryant@rmit.edu.au; brendan.wilkinson@une.edu.au
Australian Journal of Chemistry 72(8) 637-643 https://doi.org/10.1071/CH19159
Submitted: 10 April 2019 Accepted: 25 July 2019 Published: 14 August 2019
Abstract
A series of eight n-octyl (thio)glycosides (1α, β–4α, β) with d-glucose or d-galactose-configured head groups and varying anomeric configuration were synthesized and evaluated for glass transition behaviour, membrane permeability, and ice recrystallization inhibition (IRI) activity. Of these, n-octyl β-d-glucopyranoside (2β) exhibited a high glass transition temperatures (Tg), both as a neat sample and 20 wt-% aqueous solution. Membrane permeability studies of this compound revealed cellular uptake to concentrations relevant to the inhibition of intracellular ice formation, thus presenting a promising lead candidate for further biophysical and cryopreservation studies. Compounds were also evaluated as ice recrystallization inhibitors; however, no detectable activity was observed for the newly tested compounds.
Cryopreservation is a technique that utilizes ultralow temperatures for the long-term preservation and subsequent re-use of structurally intact and biochemically viable cells.[1] However, unprotected freezing is almost universally lethal and requires the use of chemical additives, or cryoprotective agents (CPAs), to preserve life at the very low temperatures required— ideally well below −100°C.[2] Combined with carefully optimized cooling (and warming) rates, CPAs make it possible to achieve these low temperatures without the damaging effects of ice formation and excessive freeze-induced cell dehydration. By increasing the overall solute concentration, CPAs can act intracellularly or extracellularly (or both) by several known mechanisms, including restoration of osmotic balance to avoid freeze-induced dehydration, promotion of a glassy state (vitrification), and the elimination of intracellular ice formation (IIF) – a major cause of cryoinjury.[3]
Since the first use of glycerol as a CPA in 1949,[4] cryopreservation technologies utilizing CPAs have evolved to the point where it is now possible to successfully preserve animal and plant cells and tissues, including red and white blood cells, plant seeds and leaf tips, some stem cells, and cancer cells.[5] However, despite decades of research, serious problems associated with the toxicity of conventional CPAs persist, which has limited their routine use to all but a few cell and tissue types. Widely used cell-penetrating CPAs such as dimethyl sulfoxide (DMSO) and glycerol are toxic to many cell types and can disrupt lipid membranes at high concentrations.[2,5] Successful freezing protocols using CPAs are highly cell-dependent and require a careful balance between cooling rates and CPA concentration and exposure time. The toxicity of commonly used CPAs also requires rapid thawing followed by time-consuming and expensive removal before re-use of the sample, which is less than ideal for critical applications including reproductive technologies, stem cell therapies, organ and tissue transplantation, and preservation of endangered plant and animal species. Most conventional CPAs also do not inhibit damaging ice recrystallization (the growth of larger ice crystals at the expense of smaller ones) that often occurs during thawing cycles. New, non-toxic CPAs that can penetrate and access all cellular compartments, promote vitrification, and which are capable of inhibiting ice recrystallization are highly sought after in several research fields and industries including biotechnology, regenerative medicine, food technology, and conservation. However, despite decades of research and the clear benefit of these CPAs to humankind, no such breakthrough has eventuated.
Many cold-tolerant and freeze-avoiding organisms residing in the Earth’s temperate and polar regions produce natural CPAs and macromolecular antifreezes as key adaptations that enable survival in these harsh climates.[6] For example, soluble sugars such as sucrose, trehalose, and raffinose play essential roles in reducing cold stress and improving cold tolerance in several plant, fungal, and insect species.[7] These polyhydric molecules also restore osmotic equilibrium during freezing, thus alleviating freeze-induced dehydration and cell death, and stabilize lipid membranes during freeze-induced dehydration by retaining additional water, and hindering the close approach of membranes.[8,9] Glycolipids of natural and synthetic origin have been shown to promote thermal hysteresis activity in several cold-tolerant organisms,[10] as well as stabilize proteins[11] and artificial vesicles during freeze-drying, presumably through hydration of phospholipid head groups within the lipid bilayer.[12] Owing to their low toxicity, promising ice growth inhibition activity, and high glass transition temperatures in experimental systems, carbohydrates have emerged as attractive candidates for the development of new CPAs. Unfortunately, owing to their high polarity, soluble sugars alone are poor cryoprotectants as they are generally not membrane permeable, and so are incapable of inhibiting IIF.
An alternative, and extremely promising, approach aimed at improving the membrane permeability of soluble sugars is to enhance lipophilicity by modifying the carbohydrate scaffold with hydrophobic groups.[13–15] In their previous work, Toner and coworkers reported the synthesis and membrane permeability properties of some modified trehalose derivatives possessing enzyme-cleavable acetate esters.[15] Increasing the intracellular concentration of soluble sugars has also been shown to enhance the survival and viability of mammalian cells post thaw.[16] Recent work from our laboratory, along with Ben and colleagues, have also demonstrated the significant ice recrystallization inhibition (IRI) activity of amphiphilic carbohydrate derivatives including surfactants and hydrogelators,[17] azobenzene analogues,[18,19] and aryl glycosides.[20] N-octyl (thio)glycosides are non-ionic surfactants that have emerged as viable alternatives to ionic surfactants from petrochemical origin due to their clinical mildness, low toxicity, and performance. As such, they have seen widespread use in pharmaceutical and consumer health care industries, as well as in biochemical research.[21] Non-ionic, carbohydrate-based surfactants have emerged as promising CPAs given their remarkably high, sub-zero glass transition temperatures and ability to inhibit eutectic formation in NaCl/H2O mixtures.[22] Despite this, their viability as a new class of penetrating CPAs is yet to be fully explored. Based on these earlier observations and inspired by the natural antifreeze and cryoprotective activity of glycolipids, we were interested in exploring the potential of n-octyl (thio)glycosides as a novel and accessible class of penetrating CPA. We reasoned that, given their low toxicity, promising thermal properties, and hydrophobicity, these molecules could induce glass transitions at relatively high temperatures and display sufficient membrane permeability to access intracellular compartments and eliminate IIF. Herein, we report the glass transition properties, membrane permeability, and IRI activity of eight n-octyl (thio)glycosides (1α, β–4α, β). These (thio)glycosides possess a variable monosaccharide head group (glucose or galactose) linked to an n-octyl tail group via an oxygen or sulfur atom with either α or β anomeric configuration. We demonstrate the high dependence of the glass transition behaviour on the molecular structure of these surfactants, membrane permeability, and IRI activity, which are preliminary steps in their overall examination as potential cryoprotectants.
In order to understand the effect of the monosaccharide head group and anomeric linkage on the cryoprotective properties of the surfactants, our studies commenced with the synthesis of eight n-octyl (thio)glycosides 1α, β–4α, β, each possessing a glucose or galactose head group linked to an n-octyl tail group via an oxygen or sulfur atom at the anomeric centre (Fig. 1). Optically pure surfactants 1α, β–4α, β were synthesized using known and adapted literature procedures employing Lewis acid (BF3.EtO2)-promoted glycosylation of 1-octanol or 1-octanethiol with commercial β-d-galactose pentaacetate or β-d-glucose pentaacetate as donors (see Supplementary Material for details).[23,24] Protected (thio)glycosides were afforded in good yields as anomeric mixtures (2 : 1 to 2 : 3 α/β selectivity), which were resolved using flash silica gel chromatography. Optically pure per-O-acetylated n-octyl (thio)glycosides were then smoothly deprotected under standard Zemplén conditions to give target compounds in quantitative yields.
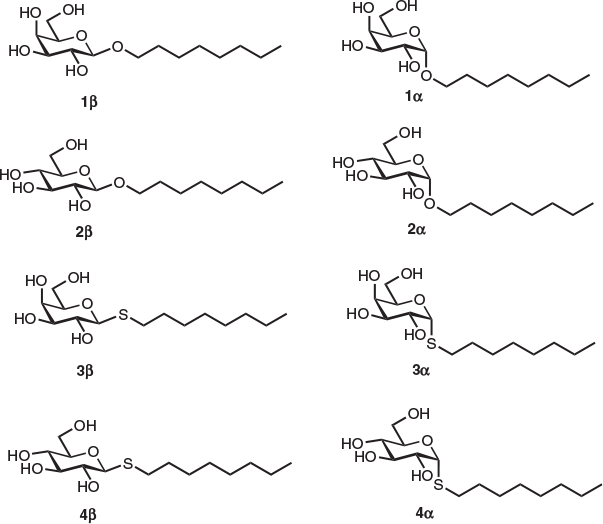
|
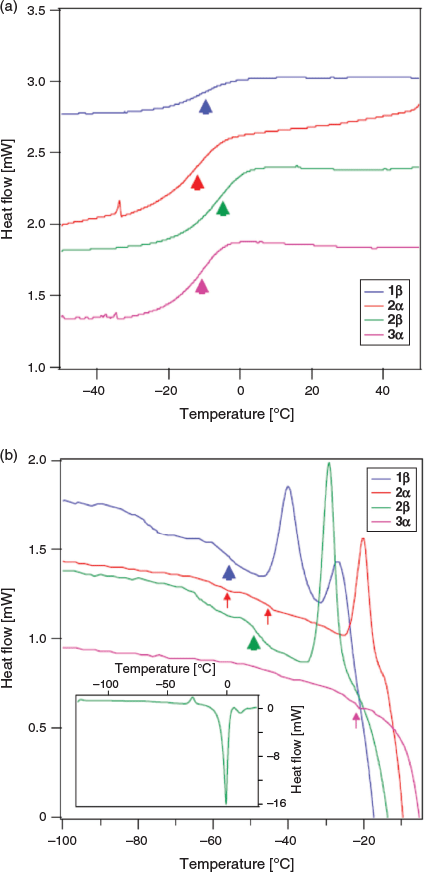
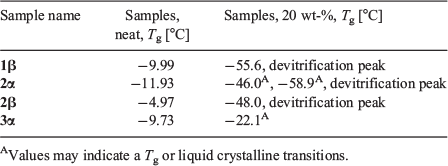
We next investigated the vitrification behaviour of these n-octyl (thio)glycosides using differential scanning calorimetry (DSC), both as neat samples and as 20 wt-% aqueous solutions (0.68 M for 1α, β and 2α, β; 0.65 M for 3 α, β and 4 α, β) relevant to cryopreservation. The glass transition behaviour (Tg) of octyl β-d-glucopyranoside (2β) and octyl β-d-thioglucopyranoside (4β) has been previously investigated by Ogawa and coworkers, with relatively high Tg values observed on heating and no ice formation observed at concentrations above ~80 wt-% in water.[22] We were thus interested in observing the glass transition temperatures of surfactants 1–4 possessing alternative head groups and anomeric configuration at concentrations and temperatures most relevant to cryopreservation. For DSC thermograms of neat surfactants, samples (5–10 mg) were hermetically sealed in aluminium sample pans and cycled between +150°C and −50°C at a rate of 10°C min−1, with 1-min equilibration times at the endpoints (Fig. 2a). The thermal values for neat surfactants are summarized in Table 1. Of the eight samples studied, only thioglucosides 4α and 4β did not show any glass transition behaviour as anhydrous solids on warming from −100°C to 25°C (see Fig. S1, Supplementary Material). The remaining soluble samples showed Tg values between 0°C and −15°C, which are clearly visible as step-like inflections in the thermograms, as previously reported for some n-octyl glycosides.[25,26] However, the Tg values for the neat samples are slightly lower than that previously reported, which may be attributable to the adsorption of trace water and the possibility of hemihydrate crystal formation.[27]
|
|
Galactoside 1α, thiogalactoside 3β, and thioglucosides 4α, β unfortunately were only partially soluble and so could not be assessed as a 20 wt-% binary system with water. DSC thermograms of the four soluble surfactants (1β, 2α, 2β, and 3α) were recorded by warming samples from −120°C to 25°C at a rate of 5°C min−1, with 1-min equilibration time, and the results are shown in Fig. 2b. On cooling, large exothermic peaks could be seen between −15°C and −25°C, thus indicating ice crystallization (see Fig. S2, Supplementary Material). Following glass melting in the warming run, the supercooled solution then underwent freezing, as shown by a positive peak. The ice subsequently melted as it reached the freezing point, as indicated by the large endothermic peaks. The melting temperature, Tm, can thus be defined at the end of ice melting for most of the solutions studied. Encouragingly, all soluble n-octyl (thio)glycosides displayed Tg values above −60°C, which are higher than the commonly used CPA, DMSO (Tg approximately −130°C; see Fig. 2). Of these, n-octyl β-d-glucoside 2β showed particular promise, with high Tg values, both as a neat sample (Tg −4.97°C) and in solution (Tg −48°C), which is in good agreement with previous thermal studies on this compound.[28] For 2α and 3α, there were small changes in the baseline that may correspond to Tg, but could also represent liquid crystalline transitions that could not be confirmed based on current data. They are indicated by the small arrows (Fig. 2b) and values with superscript letter ‘A’ in Table 1. In the case of 2α, the fact that there is a clear devitrification peak indicates that glass melting must have occurred; however, the value of Tg is unclear. The positive peaks at temperatures just below ice melting could be due to ice recrystallization, but may also be attributed to crystallization of synthesized glycosides, as recently shown by Ogawa and coworkers.[29] For the purpose of the present study, we are only interested in the glass transition properties, and so this was not pursued further.
Clear devitrification peaks are observed for 1β, 2α, and 2β, which must be preceded by glass melting. The glass transitions are clear for 1β and 2β, but indistinct for 2α, where two possible weak transitions were identified (marked by superscript letter ‘A’). 3α shows no devitrification peak, with a weak possible glass transition just before melting (marked by superscript letter ‘A’). The thermal values for aqueous surfactants are summarized in Table 1.
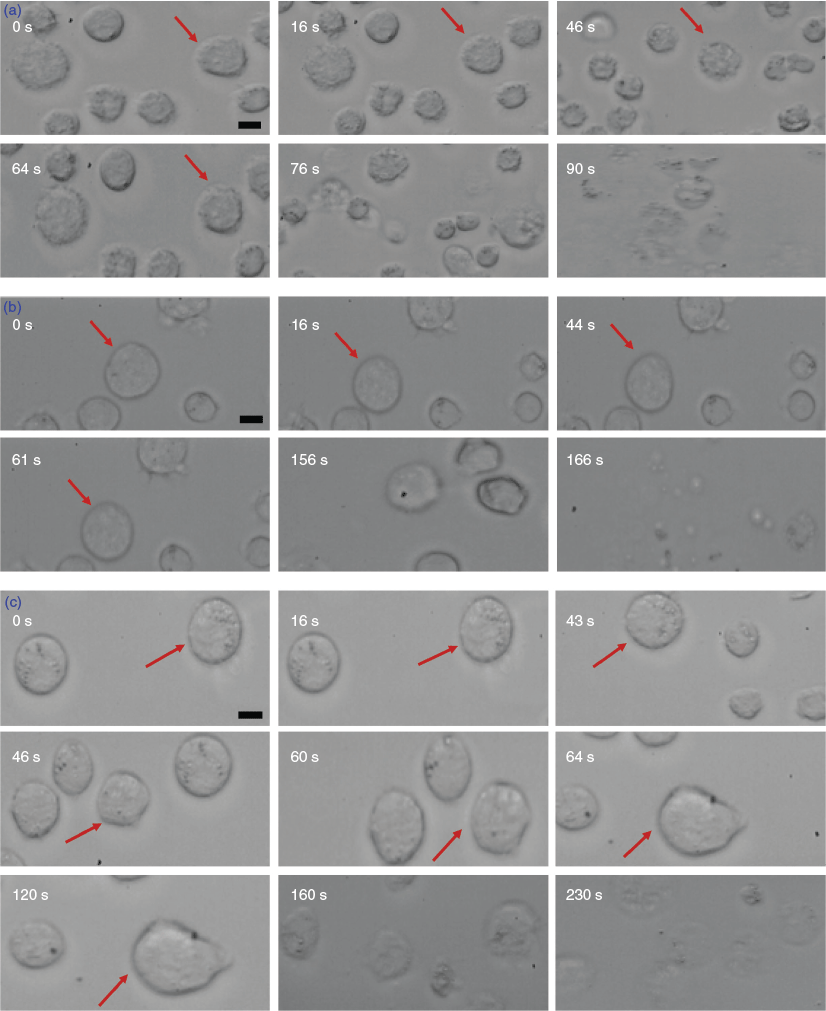
Having established the thermal properties of the n-octyl (thio)glycosides, the next stage of our study focussed on the cell permeability of the four molecules possessing promising Tg values and high solubility in water and RPMI cell media (1β, 2β, 2α, 3α). For the most commonly used CPA, DMSO, the cryobiologically relevant concentrations are 5 and 10 % by volume, which is equivalent to 0.65 and 1.3 M respectively. A cell perfusion permeability study was conducted using 0.65 M solutions of each compound using the THP-1 human leukemia monocytic cell line. Although this method can provide detailed concentration-dependent cell permeability data with high temporal sensitivity, it does have intrinsic limitations based on compatible cell types (spherical only), as well as difficulties associated with maintaining the concentration of the CPA used and the position of the settled cell(s) during perfusion within the conventional well plate. As a result, all experiments were repeated several times to obtain consistent results. At this concentration, α-glucoside 2α and α-thiogalactoside 3α were not permeable and displayed some cytotoxicity (cells started bulging towards the sides with lysis occurring within 1 min). In contrast, β-d-octyl galactoside 1β and glucoside 2β showed good shrink–swell behaviour, which indicates membrane permeability (Fig. 3). Unfortunately, these compounds also resulted in some cell damage at the end of the incubation period (~1 min) as shown from visible cell lysis in the micrographs (Fig. 3a). However, it is not at all surprising to see some cell membrane damage after prolonged exposure given the strong surfactant properties of these molecules, particularly at concentrations well above their reported critical micelle concentration (CMC) values.[30] To test the concentration dependence of this cytotoxicity, the cell perfusion assay was then repeated at 0.33 M. Encouragingly, at this lower concentration, good shrink–swell behaviour was observed for both n-octyl β-d-glycosides 1β and 2β, similar to that observed at 0.65 M concentration but with little observed membrane damage, particularly for the glucoside 2β. Perfusion of glucoside 2β resulted in total cell lysis only after ~230 s of incubation, with evidence of membrane damage occurring from ~160 s of contact time, as shown from the blurring of the cell image in Fig. 3c. Interestingly, in contrast to the glucoside, β-d-galactoside 1β showed evidence of cell damage within 1 min of incubation, with total cell lysis observed within ~166 s (Fig. 3b). Unlike the β-d-glycosides 1β and 2β, the corresponding α-glucoside 2α and thiogalactoside 3α were not membrane-permeable and displayed significant cytotoxicity at both 0.65 and 0.33 M concentrations.
Fig. 4 shows the normalized cell volume as a function of time for 1β and 2β (at 0.33 M). For both samples, the normalized volume decreases to ~80 % of the initial volume (V0) after ~45 s. For comparison, in the presence of 0.65 M DMSO, the cells shrink to ~65 % V0 in ~25 s. Overall, provided that long-term contact with cells is avoided, β-d-glycosides 1β and 2β show promising membrane permeability at concentrations relevant to cryopreservation.
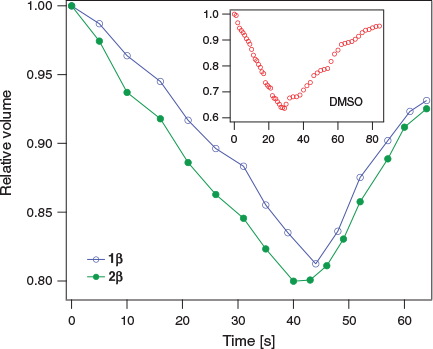
|
Ice recrystallization is a thermodynamic phenomenon whereby larger ice crystals grow at the expense of smaller ones and is a significant cause of cell damage during cryopreservation.[31] The IRI activity of carbohydrate-based surfactants has been previously determined at concentrations up to 44 mM, with the glucoside 2β displaying weak to moderate IRI activity, whereas the galactoside 1β exhibited potent IRI activity.[17] The IRI activity of amphiphilic carbohydrates is highly sensitive to molecular structure, with the polarity, hydration, and absolute configuration of the carbohydrate head group, as well as tail group hydrophobicity and substitution all regarded as important determinants for inducing potent IRI activity.[17,19,32] Previous studies have also demonstrated the importance of tail-group hydrophobicity of amino acid derivatives[33] and amphiphilic block copolymers[34] for tuning potent IRI activity. Interestingly, although earlier work has evaluated the effect of changing the structure of the head and tail groups of carbohydrate surfactants on IRI activity, an investigation into the effect of the anomeric configuration and linkage type (e.g. O- and S-glycoside) for carbohydrate-based surfactants has yet to be conducted.
In order to evaluate the effect of substituting the oxygen atom at the anomeric centre with a more hydrophobic sulfur atom, the IRI activity of thioglycosides 3α, β and 4α, β was determined using an established splat-cooling assay up to 5.5 mM concentration, which represents the upper solubility limit for these compounds in PBS buffer (see Fig. S3, Supplementary Material).[35] Unfortunately, unlike the n-octyl glycosides previously reported, the analogous thioglycosides used in the present study did not display any significant IRI activity. Curiously, although the O-glycoside 1β displayed weak to moderate IRI activity at 1 mM concentration (~80 % mean grain size relative to PBS),[17] replacement with a sulfur atom to give thioglycoside 3β resulted in complete loss of activity (100 % mean grain size relative to PBS), albeit at a lower concentration (0.5 mM). This loss in IRI activity compared with previously described O-glycosides is perplexing; however, it may be due to freeze-induced crystallization or precipitation of compounds within the assay. Alternatively, substitution of oxygen for sulfur may have resulted in a decrease in head group polarity, with a concomitant decrease in its hydration index, thus lowering IRI activity.[36] Regardless of mechanism, these compounds were not IRI-active and this mode of action cannot account for their potential cryoprotective effect.
In conclusion, we have reported the glass transition behaviour, membrane permeability, and IRI activity of a series of n-octyl (thio)glycosides (1α, β–4α, β). Of all soluble samples studied, n-octyl β-d-glucopyranoside 2β exhibited high sub-zero Tg values from the DSC thermograms, both as a neat sample and as a 20 wt-% aqueous solution. Using a cell perfusion permeability assay against human THP-1 cells, glucoside 2β (0.33 M) also exhibited favourable shrink–swell behaviour similar to DMSO. Encouragingly, we observed promising cell permeability with little cytotoxicity, provided the exposure time is kept within ~160s, a reasonable timeframe for exposure before cryopreservation. The IRI activity of thioglycosides 3α, β and 4α, β was also evaluated and compared with previously published O-glycosides 1β and 2β. Substitution of the anomeric oxygen with a sulfur atom resulted in significantly lower IRI activity, which may be a result of the increased hydrophobicity and decreased hydration of the carbohydrate head group in thioglycosides. In addition to the vitrification and permeability data presented herein, glucoside 2β has also previously been shown to display moderate IRI activity and therefore represents a promising lead compound for further biophysical and cryopreservation studies. In future work, we will investigate the mechanisms of cellular uptake for these surfactants, as well as the rational development of new, hydrophobic carbohydrate analogues as potential CPAs capable of promoting vitrification, eliminating IIF, and inhibiting ice recrystallization.
Supplementary Material
General experimental information, supplementary DSC and IRI data, and NMR data are available on the Journal’s website.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
B.W. and G.B. acknowledge funding from the Australian Research Council (ARC) grant DP190101010. G. B. acknowledges funding by the ARC grants LP140100993 and LP160101496. R.R. acknowledges the support of an Australian Government Research Training Program Scholarship. M. K. A. acknowledges the Natural Sciences and Engineering Research Council of Canada (NSERC) for a Canada Graduate Scholarship (CGS D).
References
[1] J. G. Baust, D. Gao, J. M. Baust, Organogenesis 2009, 5, 90.| Crossref | GoogleScholarGoogle Scholar | 20046670PubMed |
[2] G. D. Elliott, S. Wang, B. J. Fuller, Cryobiology 2017, 76, 74.
| Crossref | GoogleScholarGoogle Scholar | 28428046PubMed |
[3] K. R. Diller, E. G. Cravalho, Cryobiology 1971, 7, 191.
| Crossref | GoogleScholarGoogle Scholar |
[4] C. Polge, A. U. Smith, A. S. Parkes, Nature 1949, 164, 666.
| Crossref | GoogleScholarGoogle Scholar | 18143360PubMed |
[5] (a) B. J. Fuller, Cryo Lett. 2004, 25, 375.
(b) R. Streczynski, H. Clark, L. M. Whelehan, S.-T. Ang, L. K. Hardstaff, B. Funnekotter, E. Bunn, C. A. Offord, K. D. Sommerville, R. L. Mancera, Aust. J. Bot. 2019, 67, 1.
| Crossref | GoogleScholarGoogle Scholar |
[6] K. B. Storey, J. M. Storey, Annu. Rev. Ecol. Syst. 1996, 27, 365.
| Crossref | GoogleScholarGoogle Scholar |
[7] L. P. Tarkowski, W. Van den Ende, Front. Plant Sci. 2015, 6, 203.
| Crossref | GoogleScholarGoogle Scholar | 25926837PubMed |
[8] B. Kent, T. Hauß, B. Demé, V. Cristiglio, T. Darwish, T. Hunt, G. Bryant, C. J. Garvey, Langmuir 2015, 31, 9134.
| Crossref | GoogleScholarGoogle Scholar | 26225718PubMed |
[9] T. Lenné, G. Bryant, R. Holcomb, K. L. Koster, Biochim. Biophys. Acta Biomembr. 2007, 1768, 1019.
| Crossref | GoogleScholarGoogle Scholar |
[10] K. R. Walters, A. S. Serianni, Y. Voituron, T. Sformo, B. M. Barnes, J. G. Duman, J. Comp. Physiol. B 2011, 181, 631.
| Crossref | GoogleScholarGoogle Scholar | 21279720PubMed |
[11] K. Imamura, K. Murrai, T. Korehisa, N. Shimizu, R. Yamahira, T. Matsuura, H. Tada, H. Imanaka, N. Ishida, K. Nakashini, J. Pharm. Sci. 2014, 103, 1628.
| Crossref | GoogleScholarGoogle Scholar | 24797557PubMed |
[12] G. Bendas, F. Wilhelm, W. Ritcher, P. Nuhn, Eur. J. Pharm. Sci. 1996, 4, 211.
| Crossref | GoogleScholarGoogle Scholar |
[13] M. F. Ross, T. Da Ros, F. H. Blaikie, T. A. Prime, C. M. Porteous, I. I. Serevina, V. P. Skulachev, H. G. Kjaergaard, R. A. J. Smith, M. P. Murphy, Biochem. J. 2006, 400, 199.
| Crossref | GoogleScholarGoogle Scholar | 16948637PubMed |
[14] M. P. Murphy, R. J. Murphy, R. J. Smith, Annu. Rev. Pharmacol. Toxicol. 2007, 47, 629.
| Crossref | GoogleScholarGoogle Scholar | 17014364PubMed |
[15] A. Abazari, L. G. Meimetis, G. Budin, S. S. Bale, R. Weissleder, M. Toner, PLoS One 2015, 10, e0130323.
| Crossref | GoogleScholarGoogle Scholar | 26115179PubMed |
[16] A. Eroglu, M. J. Russo, R. Bieganski, A. Fowler, S. Cheley, H. Bayley, M. Toner, Nat. Biotechnol. 2000, 18, 163.
| Crossref | GoogleScholarGoogle Scholar | 10657121PubMed |
[17] C. J. Capicciotti, M. Leclère, F. A. Perras, D. L. Bryce, H. Paulin, J. Harden, Y. Liuc, R. N. Ben, Chem. Sci. 2012, 3, 1408.
| Crossref | GoogleScholarGoogle Scholar |
[18] M. K. Adam, Y. Hu, J. S. Poisson, M. J. Pottage, R. N. Ben, B. L. Wilkinson, Carbohydr. Res. 2017, 439, 1.
| Crossref | GoogleScholarGoogle Scholar | 28011438PubMed |
[19] M. K. Adam, J. S. Poisson, Y. Hu, G. Prasannakumar, M. J. Pottage, R. N. Ben, B. L. Wilkinson, RSC Adv. 2016, 6, 39240.
| Crossref | GoogleScholarGoogle Scholar |
[20] C. J. Capicciotti, J. D. R. Kurach, T. R. Turner, R. S. Mancini, J. P. Acker, R. N. Ben, Sci. Rep. 2015, 5, 9692.
| Crossref | GoogleScholarGoogle Scholar | 25851700PubMed |
[21] C. J. Drummond, C. Fong, I. Krodkiewska, B. J. Boyd, I. J. A. Baker, in Novel Surfactants (Ed. K. Holmberg) 2003, Ch. 3, pp. 95–128 (Marcel Dekker: New York, NY).
[22] (a) S. Ogawa, K. Asakura, S. Osanai, Carbohydr. Res. 2010, 345, 2534.
| Crossref | GoogleScholarGoogle Scholar | 20889145PubMed |
(b) S. Ogawa, S. Osanai, Cryobiology 2007, 54, 173.
| Crossref | GoogleScholarGoogle Scholar |
[23] J. Schmidt-Lassen, T. K. Lindhorst, MedChemComm 2014, 5, 1218.
| Crossref | GoogleScholarGoogle Scholar |
[24] H. A. van Doren, R. van der Geest, R. M. Kellogg, H. Wynberg, Carbohydr. Res. 1989, 194, 71.
| Crossref | GoogleScholarGoogle Scholar |
[25] B. Hoffmann, W. Milius, G. Voss, M. Wunschel, S. van Smaalen, S. Diele, G. Platz, Carbohydr. Res. 1999, 323, 192.
| Crossref | GoogleScholarGoogle Scholar |
[26] S. Ogawa, S. Osanai, in Supercooling (Ed. P. Wilson) 2012, pp. 29–54 (IntechOpen: Rijeka, Croatia).
[27] S. Ogawa, Y. Ozaki, I. Takahashi, ChemPhysChem 2016, 17, 2808.
| Crossref | GoogleScholarGoogle Scholar | 27304203PubMed |
[28] S. Ogawa, K. Asakura, S. Osanai, Phys. Chem. Chem. Phys. 2012, 14, 16312.
| Crossref | GoogleScholarGoogle Scholar | 23133837PubMed |
[29] S. Ogawa, I. Takahashi, M. Koga, K. Asakura, S. Osanai, J. Oleo Sci. 2018, 67, 627.
| Crossref | GoogleScholarGoogle Scholar | 29628491PubMed |
[30] R. M. Garavito, S. Ferguson-Miller, J. Biol. Chem. 2001, 276, 32403.
| Crossref | GoogleScholarGoogle Scholar | 11432878PubMed |
[31] (a) P. Mazur, Am. J. Physiol. Cell Physiol. 1984, 247, C125.
| Crossref | GoogleScholarGoogle Scholar |
(b) J.-H. Wang, Cryobiology 2000, 41, 1.
| Crossref | GoogleScholarGoogle Scholar |
[32] C. J. Capicciotti, R. S. Mancini, T. R. Turner, T. Koyama, M. G. Alteen, M. Doshi, T. Inada, J. P. Acker, R. N. Ben, ACS Omega 2016, 1, 14656.
[33] A. K. Balcerzak, M. Febbraroa, R. N. Ben, RSC Adv. 2013, 3, 3232.
| Crossref | GoogleScholarGoogle Scholar |
[34] C. Stubbs, J. Lipecki, M. Gibson, Biomacromolecules 2017, 18, 295.
| Crossref | GoogleScholarGoogle Scholar | 27936601PubMed |
[35] M. K. Adam, C. Jarrett-Wilkins, M. Beards, E. Staykov, L. R. MacFarlane, T. D. M. Bell, J. M. Matthews, I. Manners, C. F. J. Faul, P. D. J. Moens, R. N. Ben, B. L. Wilkinson, Chem. – Eur. J. 2018, 24, 7834.
| Crossref | GoogleScholarGoogle Scholar | 29644728PubMed |
[36] R. Y. Tam, S. S. Ferreira, P. Czechura, J. L. Chaytor, R. N. Ben, J. Am. Chem. Soc. 2008, 130, 17494.
| Crossref | GoogleScholarGoogle Scholar | 19053462PubMed |
* Brendan L. Wilkinson is the winner of the 2017 RACI Athel Beckwith Award.