Element 78 – Platinum
Trevor W. Hambley AA School of Chemistry, The University of Sydney, Camperdown, NSW 2006, Australia. Email: trevor.hambley@sydney.edu.au
Australian Journal of Chemistry 72(9) 649-651 https://doi.org/10.1071/CH19292
Submitted: 26 June 2019 Accepted: 26 June 2019 Published: 16 July 2019
Platinum has been an almost uniquely important element in the history of coordination chemistry with several seminal compounds such as Zeise’s salt, Magnus’ green salt, and Peyrone’s salt (or cisplatin, as it now known). Platinum and its complexes continue to be hugely important in applications ranging from catalytic converters to cancer chemotherapy and is a focus of much work on electrocatalysts for new energy systems.
The defining feature of platinum as a metal is its resistance to corrosion and its consequent uses for decorative purposes date back at least 2000 years to pre-Colombian civilisations in what is now Ecuador. European awareness of platinum derives from samples from Central and Southern America that were sent to Spain and England in the mid-1700s and its name derives from the Spanish platino meaning ‘little silver’. Antonio de Ulloa was one of those who observed the mining of platinum in Colombia and on his return to Spain, he undertook the first systematic studies, establishing its properties and probably its elemental nature. Platinum was included as an element by Lavoisier in his 1789 treatise[1] and was included in his first version of the periodic table published by Mendeleev in 1869.[2] The inert nature of platinum has also resulted in its extensive use as electrodes and until recently, platinum ingots and bars have been used as the standard kilogram and metre.
Currently, the major use of platinum, if measured by weight, is as a catalyst, particularly in catalytic converters in cars. However, it has played a central role in the development of coordination chemistry and especially in the areas of coordination geometry and medicinal inorganic chemistry. The history of the complex now known as cisplatin (cis-[PtCl2(NH3)2], cis-diamminedichloridoplatinum(ii)) provides a useful framing of these roles. Cisplatin was first prepared by Italian chemist Michele Peyrone in 1844, as an unexpected yellow product obtained while synthesising Magnus’ green salt, [Pt(NH3)4][PtCl4], which has the same empirical formula.[3] At the time, Peyrone did not accept the concept of isomerism and speculated that Peyrone’s chloride, as it become known, was the first step on the path to Magnus’ green salt, though he subsequently formed the view that it was an isomer. Some 50 years later, Alfred Werner, the Swiss chemist who won the Nobel prize for work that led to modern coordination chemistry and who was very interested in geometry and isomerism, used his ability to prepare the cis and trans isomers of Peyrone’s chloride to establish that platinum(ii) must have a square planar geometry.[4]
The serendipitous discovery of the anticancer activity of cisplatin by Barnett Rosenberg and his colleagues is one of the great stories of science and is a tribute to the team’s determination in tracking down the compounds responsible for the biological activity. This story has been well documented elsewhere and I particularly recommend Professor Rosenberg’s own description of the story in the book, Cisplatin: Chemistry and Biochemistry of a Leading Anticancer Drug,[5] published to celebrate the 30th anniversary of the 1969 paper in Nature that first reported its biological activity. More recently celebrated was the 40th anniversary of the introduction of cisplatin in the clinic and a special issue of Inorganica Chimica Acta derives from this celebration. Cisplatin had an immediate impact on the treatment of cancer, particularly testicular cancer, which it turned from a disease that killed more than 70 % of its sufferers in the first year to one that is now permanently cured in more than 90 % of cases.[6] There are three platinum drugs in use around the world (cisplatin, carboplatin, and oxaliplatin; Fig. 1) and in Australia these are currently used in nearly 50 % of the treatment regimens of patients undergoing chemotherapy for solid tumours.[7] Similar usage patterns can be found around the world and there are three or four other platinum compounds used in cancer treatments in China, Japan, and Korea. The continued reliance on the platinums in the clinic is a surprise to many, but the use continues to grow. For example, the particularly exciting checkpoint inhibitors, which are so effective in treating melanoma, work most effectively in some other cancers when coupled with a platinum and a carboplatin/checkpoint inhibitor has recently been introduced as a frontline therapy for non-small cell lung cancer.[8] Notable here is that the platinums appear to make the cancer cells more visible to the immune system and that is probably an important part of their mechanism of action, something Rosenberg discussed at some length in the book chapter referred to above.[5]
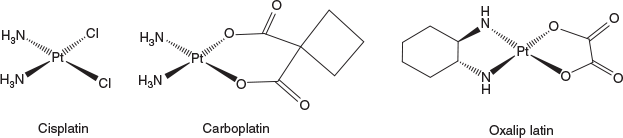
|
Despite the tremendous success of the platinum anticancer agents and the methods that have been developed over the years for ameliorating their toxic side effects, these remain a challenge and work continues apace on developing more efficacious versions of the platinums. Particularly exciting in this regard is the development of targeted platinum(iv) agents,[9] with clinical trials of one or more albumin-targeted compounds currently anticipated.[10]
The anticancer activity of platinum complexes has resulted in a huge amount of coordination chemistry and biological inorganic chemistry. The understanding of the medicinal and biological chemistry of platinum complexes is unmatched for any other exogenous metal and yet there remains ongoing debate and research on the pathways by which they enter cells and their subsequent fate. It is widely accepted that DNA is the critical target,[11] with cisplatin forming adducts of the type shown in Fig. 2.[12] However, it seems unlikely that the numerous other interactions with intracellular molecules do not also impact on the viability of cells. Investigations that relate to use of the platinums in precision oncology will be increasingly important. For instance, there is a correlation between the level of expression of the copper transporter, Ctr 1, and the effectiveness of the platinums in treating various cancers[13] that has generated a lot of interest in the consequences of platinum interactions with this transporter.[14]
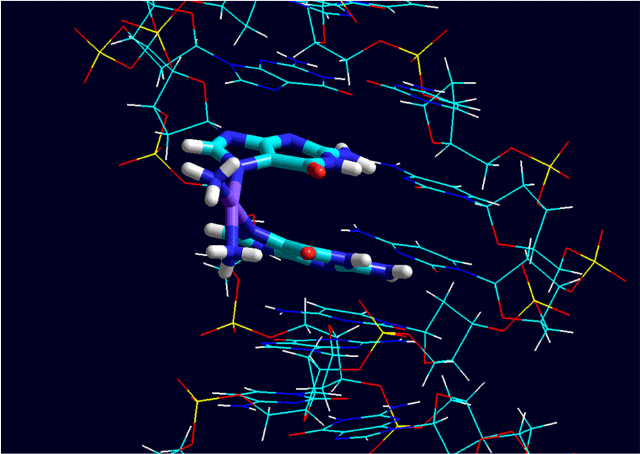
|
Platinum metal is amongst the most effective of heterogeneous catalysts, particularly because of its ability to operate in oxygen-rich conditions at high temperatures and nearly 50 % of all the platinum mined each year is used in catalytic converters in vehicles. First introduced in the 1970s, they are used to convert unreacted hydrocarbons and carbon monoxide to carbon dioxide and to convert NOx compounds to N2. Platinum complexes, particularly organometallic complexes, are also widely used as catalysts.
In addition to the examples referenced above, platinum has a particularly rich coordination chemistry, including an extremely diverse and extensive organometallic chemistry. Indeed, Zeise’s salt, K[PtCl3(ethene)] (Fig. 3),[15] first prepared in 1830, is often credited as being the first organometallic complex,[16] and the first isolatable organometallic complex of a transition metal [Me3PtI]4 was reported by Pope and Peachy in 1907.[16]
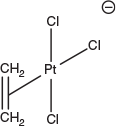
|
The rich chemistry of platinum complexes arises in part because of the ready stabilisation of the five oxidation states ranging from 0 to IV+ with the odd-numbered oxidation states mostly only being stable in dimeric pairs.[17] In addition, polymeric species with formally fractional oxidation states are well known and give rise to materials with metallic character, including high electrical conductivity and bronze and other metallic colours and sheens, arising from intervalence electron transfer, either through direct metal–metal contact or via halide or other bridging atoms. Highly coloured materials such as the platinum blues and platinum greens arise from similar contacts in molecules of defined length. Magnus’ green salt (Fig. 4), mentioned earlier, is related to the polymeric species with Pt ⋯ Pt contacts of 3.25 Å, but has both platinum atoms in the II+ state and is a semiconductor.[18]
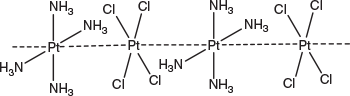
|
One of the quiet achievers in the field of platinum coordination chemistry was Trevor Appleton. Alone and with John Hall, he established many of the important properties of platinum and its complexes, such as its coordination preferences,[19] and he was involved in the work on the multiplatinum complexes that hold so much promise.[20] Many of his most significant contributions were in the area of NMR and the relationships he established between 15N and 195Pt shifts and their chemical environments[21] proved very important in his and other studies of the interaction of cisplatin and related complexes with DNA and other biomolecules.[22] I would like to acknowledge his contributions and dedicate this essay to him.
Conflicts of Interest
The author declares no conflicts of interest.
Acknowledgements
This research did not receive any specific funding.
References
[1] A. Lavoisier, Traité Elémentaire de Chimie 1789 (Paris).[2] D. Mendeleev, Z. Chem. 1869, 12, 405.
[3] G. B. Kauffman, R. Pentimalli, S. Doldi, M. D. Hall, Platin. Met. Rev. 2010, 54, 250.
| Crossref | GoogleScholarGoogle Scholar |
[4] (a) A. Werner, Z. Anorg. Chem 1893, 3, 297.
(b) G. B. Kauffman, Platin. Met. Rev. 1997, 41, 34.
[5] B. Rosenberg, in Cisplatin: Chemistry and Biochemistry of a Leading Anticancer Drug (Ed. B. Lippert) 1999, pp. 3–27 (Verlag Helevetica Chimica Acta and Wiley VCH: Zurich, Switzerland).
[6] L. R. Kelland, Nat. Rev. Cancer 2007, 7, 573.
| Crossref | GoogleScholarGoogle Scholar |
[7] N. J. Wheate, S. Walker, G. E. Craig, R. Oun, Dalton Trans. 2010, 39, 8113.
| Crossref | GoogleScholarGoogle Scholar | 20593091PubMed |
[8] M. Mathew, T. Enzler, C. A. Shu, N. A. Rizvi, Pharmacol. Ther. 2018, 186, 130.
| Crossref | GoogleScholarGoogle Scholar | 29352857PubMed |
[9] T. C. Johnstone, K. Suntharalingam, S. J. Lippard, Chem. Rev. 2016, 116, 3436.
| Crossref | GoogleScholarGoogle Scholar | 26865551PubMed |
[10] T. W. Hambley, J. Biol. Inorg. Chem. 2019, 24, 457.
| Crossref | GoogleScholarGoogle Scholar | 31093745PubMed |
[11] Y. W. Jung, S. J. Lippard, Chem. Rev. 2007, 107, 1387.
| Crossref | GoogleScholarGoogle Scholar |
[12] T. W. Hambley, J. Chem. Soc., Dalton Trans. 2001, 2711.
| Crossref | GoogleScholarGoogle Scholar |
[13] Y. Y. Lee, C. H. Choi, I. G. Do, S. Y. Song, W. Lee, H. S. Park, T. J. Song, M. K. Kim, T.-J. Kim, J.-W. Lee, D.-S. Bae, B.-G. Kim, Gynecol. Oncol. 2011, 122, 361.
| Crossref | GoogleScholarGoogle Scholar | 21570711PubMed |
[14] (a) M. C. Akerfeldt, C. M. N. Tran, C. Shen, T. W. Hambley, E. J. New, J. Biol. Inorg. Chem. 2017, 22, 765.
| Crossref | GoogleScholarGoogle Scholar | 28516214PubMed |
(b) K. M. Bompiani, C. Y. Tsai, F. P. Achatz, J. K. Liebig, S. B. Howell, Metallomics 2016, 8, 951.
| Crossref | GoogleScholarGoogle Scholar |
[15] W. C. Zeise, Ann. Phys. Chem. 1831, 97, 497.
| Crossref | GoogleScholarGoogle Scholar |
[16] L. B. Hunt, Platin. Met. Rev. 1984, 28, 76.
[17] L. M. Rendina, T. W. Hambley, in Comprehensive Coordination Chemistry (Eds J. A. McLeverty, T. J. Meyer) 2004, Vol. 6, pp. 673–745 (Elsevier Science: Amsterdam).
[18] M. Atoji, J. W. Richardson, R. E. Rundle, J. Am. Chem. Soc. 1957, 79, 3017.
| Crossref | GoogleScholarGoogle Scholar |
[19] T. G. Appleton, Coord. Chem. Rev. 1997, 166, 313.
| Crossref | GoogleScholarGoogle Scholar |
[20] N. Farrell, T. G. Appleton, Y. Qu, J. D. Roberts, A. P. Soares Fontes, K. A. Skov, P. Wu, Y. Zou, Biochemistry 1995, 34, 15480.
| Crossref | GoogleScholarGoogle Scholar | 7492537PubMed |
[21] T. G. Appleton, J. R. Hall, S. F. Ralph, Inorg. Chem. 1985, 24, 4685.
| Crossref | GoogleScholarGoogle Scholar |
[22] S. J. Berners-Price, P. J. Sadler, Coord. Chem. Rev. 1996, 151, 1.
| Crossref | GoogleScholarGoogle Scholar |


