Some Products from C=O Condensations of Quinacridones
Anthony S. R. Chesman A B C and Andris J. Liepa A
A B C and Andris J. Liepa A
A CSIRO Manufacturing, Jerry Price Laboratory, Clayton, Vic. 3168, Australia.
B Melbourne Centre of Nanofabrication, Clayton, Vic. 3168, Australia.
C Corresponding author. Email: anthony.chesman@csiro.au
Australian Journal of Chemistry 74(2) 111-124 https://doi.org/10.1071/CH20109
Submitted: 7 April 2020 Accepted: 6 May 2020 Published: 25 August 2020
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
Quinacridone chemistry has been developed which provides a source of new compounds that have potential application in organic photovoltaic (OPV) devices. Phosphoryl chloride is used for the conversion of carbonyl groups in N,N′-dialkylquinacridones, generating reactive intermediates that enable selective condensation with either one or two nucleophilic molecules under mild conditions.
Introduction
With anthropogenic climate change potentially affecting all elements of the biosphere, efforts to mitigate the most significant of its effects by minimising carbon emissions are essential. Photovoltaic (PV) devices are a source of renewable energy that are critical to achieving this objective. Among the various PV device types that are being actively explored, solar cells based on organic materials demonstrate significant potential, due to their low production costs, high power-to-weight ratios, and the power conversion efficiencies (PCEs) of single junction devices now exceeding 18 %.
In particular, organic photovoltaics (OPVs) using soluble small molecules have attracted attention[1] due to their numerous favourable attributes, such as high purity, scalable synthesis, tuneable optoelectronic properties through molecular structural variation, and excellent stability.[2,3] As a result, a wide variety of planar small organic molecules have been evaluated for prospective utility in OPV devices. Both electron donor and electron acceptor molecules have been of interest, and while substantial progress has been made in the area of suitable donor molecules, promising acceptor molecules have been rather less abundant.
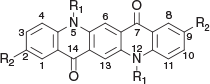
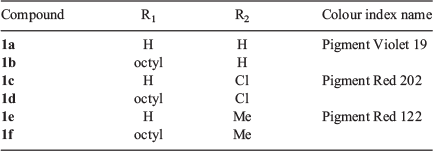
Fullerene-based compounds have long been among the most effective and popular single molecule electron acceptors since their first application. Gradual improvements have led to devices using this material giving PCEs in excess of 14 %.[4] Nevertheless, cost and other shortcomings have encouraged a continuing search for novel acceptor materials as alternatives for fullerenes. Several classes of well known commercial pigments, such as perylene diimides, phthalocyanines, and others, have shown some promise as n-type materials. The quinacridone (QA) nucleus (Fig. 1, Table 1, compounds 1a, 1c, 1f), which has previously been extensively utilised in the preparation of light fast pigments, has also been investigated for photovoltaic utility, both in a role as an acceptor or donor molecule.[5]
|
|
Substantial efforts have been devoted to the synthesis and characterisation of various QA derivatives, however, somewhat less attention has been paid to specifically modifying its optoelectronic properties. One route to modification is via condensation of the relatively unreactive carbonyl groups at C-7 and C-14 to afford molecules with extended conjugation and possibly other desirable electronic properties.[6] Some success has been achieved in condensing QAs with arylamines in the presence of titanium tetrachloride to form N,N′-dialkyl quinacridoneimines. Photophysical and electrochemical properties and temperature dependent geometrical isomerism in alkyl quinacridoneimines have been documented,[7] but these products had relatively low efficacy in solar cell trials when tested as p-type materials.
While Wang and others have been able to condense the carbonyl groups of N,N′-dialkylquinacridones with selected highly activated methylene groups in compounds such as malononitrile in a Knoevenagel-type condensation under prolonged reflux in acetic anhydride, the synthetic diversity available by this route has been limited.[8,9] Subsequent developments employing TiCl4[10,11] to enable condensations have somewhat improved the accessibility of some condensed QAs, increasing the potential for producing candidate compounds for photovoltaic applications. Trials of a bis N-octylated dicyanoethylenated QA as an acceptor material in a solar cell with poly3-hexylthiophene (P3HT) as a donor gave encouraging conversion efficiencies up to 1.57 %.
While the power conversion efficiency achieved was relatively moderate, the study nevertheless confirmed the potential of a QA core, suitably condensed at the C=O groups, to provide a template worth exploring more extensively for designing improved acceptor molecules.[12] To this end, it was of interest to develop an alternative, more versatile, methodology for creating a wider variety of condensation products from diverse starting materials under mild conditions. In addition, if needed, several potentially useful QA variants bearing nuclear substituents such as 5,12-dichloro- (1c) and 5,12-dimethyl- (1e) are readily available commercially.
Results and Discussion
During a search for reagents to effect activation of an N-alkylated QA with relatively unreactive carbonyl groups into a more active species, thereby rendering the molecule more amenable to condensations, an excess of POCl3 was added to an orange suspension of QA (1a) in 1,2-dichloroethane (DCE). When the mixture was stirred at 80°C for 1 h it darkened, and the suspended material dissolved to form an emerald green solution which did not change with further heating. When a portion of this reaction mixture was stirred with an excess of water it recovered its original orange colouration and TLC indicated only starting material was present.
The nature of the compound contained in the green solution was examined more closely. With deuterochloroform as the reaction medium (containing a few drops of DMF as catalyst), a similar green solution was formed at room temperature over five days. The change from a symmetrical substitution pattern in the starting material (Fig. 1, Table 1, Fig. S1 (Supplementary Material), compound 1b) to an unsymmetrical reaction product (Scheme 1, Fig. S2 (Supplementary Material), Intermediate A) was inferred from a doubling in the number of resonances observed in the 1H NMR spectrum. Particularly significant was the large downfield shift shown by one of the N–CH2 groups from δ 4.44 (in the starting QA 1b) to δ 5.48, which was accompanied by a downfield shift of several of the 10 aromatic proton signals. This suggested a possible transformation to a quinacridinium salt (14-chloro-5,12-dimethyl-7-oxo-7,12-dihydroquinolino[2,3-b]acridin-5-ium chloride) (Intermediate A, Scheme 1). This inference is consistent with reactions observed subsequently with nucleophiles in the presence of an organic base. The absence of any indication of P–O–C(14) coupling in the 13C NMR spectrum suggests that while a phosphate may have formed initially, it was readily converted into a reactive 14-chloro species in a manner resembling a vinylogous Vilsmeier reaction (Scheme 1).
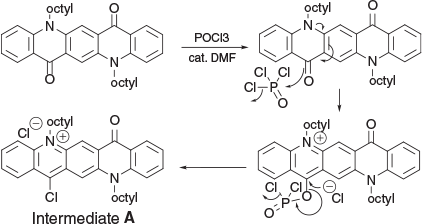
|
From a consideration of the 1H NMR spectrum of intermediate A, it seems likely that phosphoryl chloride (POCl3) initially forms a mono quaternary salt which, as a consequence, now renders the molecule significantly more electron deficient and inhibits a similar conversion of the second carbonyl group. When the positive charge is discharged by condensation with a suitable nucleophile the ability to generate a reactive quaternary species is restored. This leads to a second activation step, and the second C=O is converted into a species amenable to further condensation and the formation of a bis-condensed product. Thus, even with a large excess of POCl3 present it was feasible, by limiting the reaction time, to synthesise a mainly mono-condensed product which could be isolated, the second carbonyl group activated, and condensation effected with a different nucleophile.
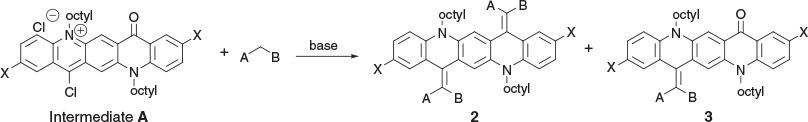
Condensations with Activated –CH2– Groups
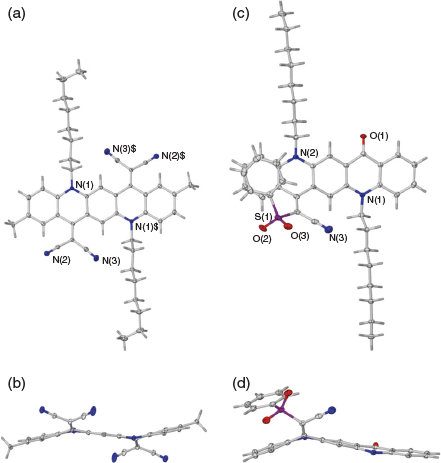
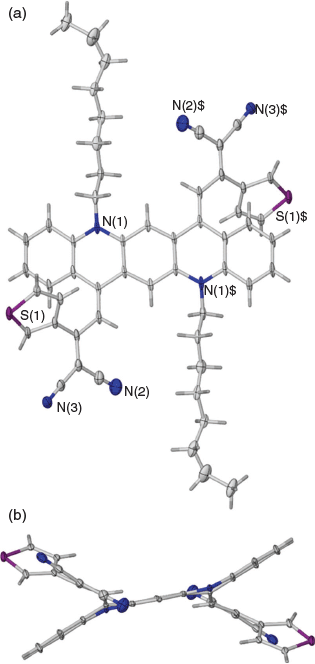
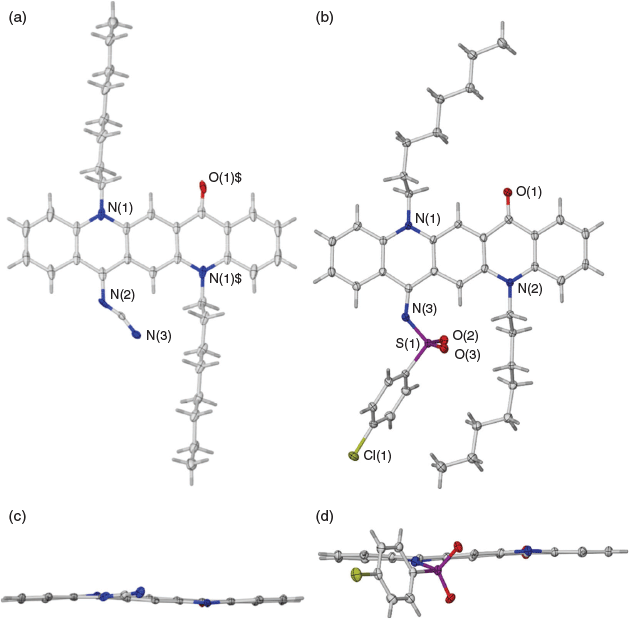
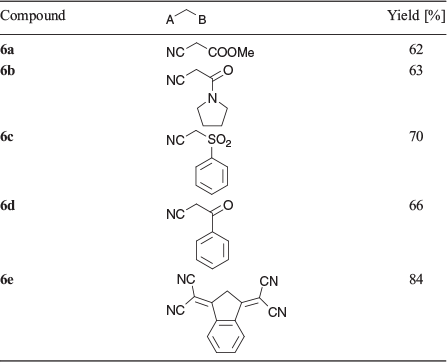
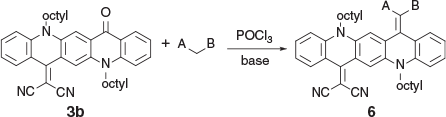
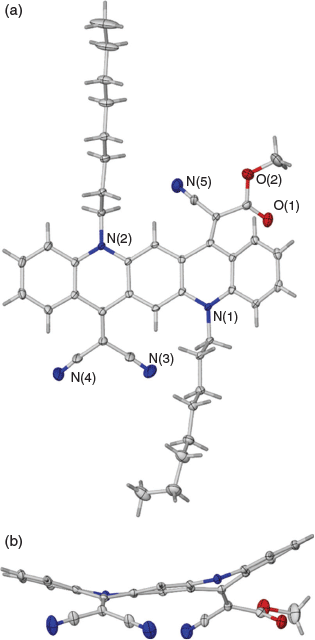
When a reaction was carried out with the green solution described above with an excess of methyl cyanoacetate at room temperature overnight in the presence of 2,4,6-collidine the bis product 2a was formed in 74 % yield. Several similar products (Scheme 2, Table 2) were obtained under comparable conditions with a variety of compounds, with the principal requirement for condensation being a methylene group sufficiently activated by flanking electron-withdrawing groups. An example of the conformation adopted by a representative of these bis-condensed products, 2b, in the solid state as determined by X-ray crystallography is shown in Fig. 2a, b. A monocondensed product 3d is shown in Fig 2c, d.
|

|
|
Alternatively, in order to favour mono-condensation, the green solution was cooled to 0°C and treated with an excess of methyl cyanoacetate followed by triethylamine. After 2 h at room temperature the mixture was quenched with ethyl acetate and water. Chromatography (silica gel/DCM) of the material contained in the organic phase yielded a blue mono adduct (3a, 74 %). Similarly, a brief reaction with an excess of malononitrile also gave a mono-condensed product (3b, 85 %). Several organic bases, such as triethylamine, diisopropylethylamine, and 2,4,6-collidine, were effective in promoting these condensations, with 2,4,6-collidine being the most effective. In all cases the presence of a substantial excess of both POCl3 and the nucleophilic component was tolerated and formation of the bis-condensation product could be enhanced by extending the reaction time or by gently warming the reaction mixture. Whether the routine addition of an excess of the nucleophilic component had any significant effect on the yield or rate of formation of the products was not investigated.
Surprisingly, it was subsequently found that it was unnecessary to preprepare a solution of the green reactive intermediate before reaction with a nucleophile, and indeed the condensations proceeded quite readily when a mixture containing the QA, an excess of POCl3, the nucleophilic component, and an organic base was stirred at room temperature. In all reactions, progress was conveniently monitored by examining the TLC plate of a quenched aliquot, which showed the initial emergence of a major blue or violet component accompanied by a minor green spot that represented the bis-adduct, which gradually increased in relative intensity at the expense of the mono product. In general, the condensations were surprisingly tolerant of the number of reaction equivalents of the reacting compounds. Thus, with one equivalent of QA or mono-condensed QA, satisfactory condensation products were isolated from reaction mixtures containing from 3 to 5 equivalents of POCl3 and > 1 equivalent of the nucleophilic coupling component. Progress of the condensation was readily monitored by quenching a small sample from the reaction solution and observing the elution of distinctive coloured spots, which were easily identifiable as starting material, mono-condensed, and biscondensed entities. As expected, prolonged reaction favoured formation of bis-products, especially in the presence of 2,4,6-collidine as a base (Table 2).
Condensations with Activated –CH3 Groups
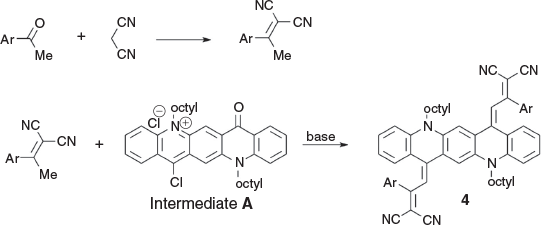
Condensation of the reactive green QA intermediate could also be affected with activated methyl groups. Substrates sufficiently activated for such reactions were obtained by condensing acetophenones with malononitrile[13] to generate a product of the type ArC(CH3)=C(CN2), in which the CH3 group was sufficiently reactive for condensation to occur (Scheme 3). Several compounds bearing this moiety were successfully condensed (Scheme 3, Table 3). This procedure may be of some potential value to produce products with enhanced pi conjugation due to the greater planarity that could result from relatively less congestion at the newly formed methylene group. An example of the conformation of a representative of this group 4c as determined by X-ray crystallography is shown in Fig. 3.
|
|
|
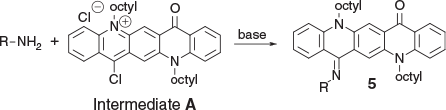
Condensations with R–NH2 Groups
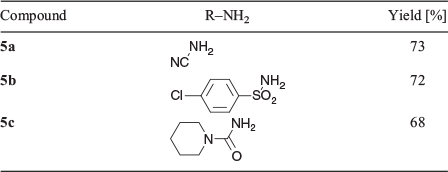
In addition, it was also found that primary amino groups reacted readily (Scheme 4, Fig. 4). This synthesis was readily extended to affect successful condensations with amides (–CONH2), 1,1-disubstituted ureas (R1R2NCONH2), as well as an aryl sulfonamide (ArSO2NH2) (Table 4).
|
|
|
Owing to their industrial applications as versatile pigments, several ring substituted QAs are commercially available. With the intention of evaluating the effect that a change in nuclear substitution might have on the photovoltaic properties of derivatives of the type previously described, a sample of Pigment Red 202 (1c, Colour Index 73907) was purchased and duly alkylated as received with n-octyl bromide. Pigment Red 202 is usually assumed to be the 2,9-dichloro derivative of QA. Rather surprisingly, the product from alkylation of this commercial sample was found to consist of two dioctylated species which were formed in comparable quantities and consisted of a mixture of 5,12-dioctyl-2,9-dichloroquinacridone (1d) and 5,12-dioctyl-2,9-dimethylquinacridone (1f). Evidently the QA sample as supplied was not a single entity as expected but in fact consisted of a blend of Pigment Red 202 (1c) and Pigment Red 122 (1e) in similar proportions. After separation, each of these analogues, in the presence of POCl3, readily gave products analogous to those observed with the unsubstituted parent (Table 5). Although the mixture as supplied (after N-alkylation) readily condensed with nucleophiles in the presence of POCl3 to give the expected products as a mixture, it was more convenient to simplify subsequent purifications by carrying out the condensations with the previously separated individual QAs.
|
|
Condensation with a Different Second Component
Mono-condensed products, such as the initial product 3b obtained from malononitrile, were readily prepared and isolated from reactions employing short reaction times. Hence it became possible to activate the remaining C=O as a separate second step initiated by a second charge of POCl3. This enabled a stepwise introduction of a second substituent to yield a molecule with asymmetric electron flow characteristics, potentially enabling graduated tuning of various electronic properties. Notably, in an example of a product of this reaction that was characterised by X-ray crystallography, 6a, the QA moiety adopted a concave morphology (Fig. 5), which was in direct contrast to the planar or armchair morphologies observed for the products of the condensations with R-NH2 groups (Fig. 4) and the bis-condensation with malononitrile (Fig. 3), respectively.
|
UV-Visible Spectra
The spectra of representative compounds of structural type 2–6 were determined (Fig. 6). Significantly, each of each of these groups showed intense absorption in the range 650 to 750 nm, an energy rich range of the visible spectrum over which the popular n-type fullerene acceptors have low absorption. The newly synthesised QAs of structural type 4 showed particularly strong absorption in this region.
Conclusions
A new method has been developed which enables dialkylated QAs to be transformed under mild conditions to reactive intermediates which readily condense with a variety of nucleophilic components to yield condensed products. The reaction conditions can be modified to select either bis-condensed products utilising both carbonyl groups at once or mono-condensed products in which the second carbonyl group can be activated and condensed with a second, different, nucleophile. This work provides a pathway to a variety of highly substituted quinacridone derivatives, allowing for customisation of their optoelectronic and physical properties, which is potentially useful in the fabrication of bulk heterojunction solar cells.
Experimental
Methods and Materials
QA was purchased from Tokyo Kasei Kogyo Co. and a QA described as CI 202 was purchased from Winchem Industrial Co Ltd, China. Both compounds were used without further purification. N-(n-Octyl)-N′-(n-octyl)quinacridone was prepared by the literature method[14] with minor modifications.
Analytical TLC was performed on Merck Kiesegel 60 F254 silica aluminium backed sheets, and was visualised under visible or UV light. Column chromatography was performed on Merck Kiesegel (particle size: 0.04–0.063 mm) silica gel.
NMR spectra were recorded with a Bruker UltraShield Avance III 400 MHz Spectrometer running the TopSpin 2.1 software package at 293 K. CDCl3 and DMSO-d6 were used as solvents and as an internal lock. Chemical shifts are measured in ppm. 1H NMR chemical shifts were referenced to δ 7.26 for CDCl3 and δ 2.50 for DMSO-d6. Spectra were run routinely as a solution in deuterochloroform unless otherwise stated. 13C NMR were run in CDCl3 at 100.6 MHz unless otherwise stated; chemical shifts were referenced to δ 77.0 for CDCl3 and δ 39.52 for DMSO-d6. Spectroscopic data were reported using the following format: chemical shift (ppm), integration, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, dd = doublet of doublets, m = multiplet, br = broad), J-coupling constant (Hz), assignment. Dichloromethane is abbreviated as DCM and 1,2-dichloroethane as DCE.
Melting points were determined using a Gallenkamp hotstage microscope and are uncorrected.
Atmospheric pressure chemical ionisation (APCI)/atmospheric sample analysis probe (ASAP) mass spectrometry, including high resolution experiments, were carried out on a Thermo Scientific Q Exactive FTMS employing ASAP/APCI probes.
Synthesis
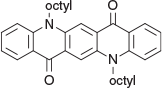
5,12-Di(n-octyl)quinacridone (1b) (Chart 1)
|
n-Octyl bromide (19.3 g, 100 mmol) was added to a suspension of QA (10.41 g, 30 mmol) in a mixture of hexamethylphosphoramide (80 mL) and benzene (120 mL) followed by addition of sodium hydride (4.5 g, 60 % in oil) over 6 h. The blue mixture was stirred for 7 d at room temperature and the excess of hydride was destroyed with 50 % aq. iPrOH (5 mL). The benzene was evaporated and the mixture was diluted with a mixture of methanol (150 mL) and water (5 mL). The precipitate was filtered off the next day and purified by chromatography over alumina. Elution with dichloromethane containing ethyl acetate (up to 10 %) gave the product (14.7 g, 64 %) as orange needles, sufficiently pure for subsequent reactions. δH 8.66 (2H, s, 2 × ArH), 8.50 (2H, m, 2 × ArH), 7.7 (2H, m, 2 × ArH), 7.43 (2H, d, J 8.8, 2 × ArH), 4.44 (4H, m, 2 × CH2N), 1.95 (4H, m, 2 × CH2), 1.60 (4H, m, 2 × CH2), 1.45 (4H, m, 2 × CH2), 1.30 (12H, m, 6 × CH2), 0.88 (6H, m, 2 × CH3).
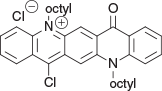
14-Chloro-5,12-dioctyl-7-oxo-7,12-dihydroquinolino[2,3-b]acridin-5-ium chloride (Intermediate A) (Chart 2)
|
δH (CDCl3 + DMF) 9.53 (1H, s, ArH), 8.82 (1H, m, ArH), 8.73 (1H, s, ArH), 8.58 (1H, m, ArH), 8.58 (1H, m, ArH), 8.44 (1H, m, ArH), 8.0 (1H, m, ArH), 7.89 (1H, m, ArH), 7.59 (1H, d, J 8.8, ArH), 7.36 (1H, m, ArH), 5.48 (2H, m, +NCH2), 4.54 (2H, m, NCH2), 2.23 (2H, m, CH2), 2.08 (2H, m, CH2), 1.2–1.8 (20H, m, 10 × CH2), 0.87 (6H, m, 2 × CH3). δC (CDCl3 + DMF) 177.2, 153.8, 142.5, 141.0, 140.3, 140.0, 136.7, 133.8, 130.3, 129.7, 128.5, 127.9, 127.2, 126.0, 122.8, 121.1, 118.7, 118.6, 115.2, 110.8, 52.1, 47.1, 31.7, 31.6, 29.6, 29.3, 29.2, 29.1, 29.0, 26.9, 26.2, 22.6, 22.5, 14.1, 14.0.
2,9-Dichloro-5,12-dioctylquinolino[2,3-b]acridine-7,14(5H,12H)-dione (1d) and 2,9-Dimethyl-5,12-dioctylquinolino[2,3-b]acridine-7,14(5H,12H)-dione (1f) (Chart 3)
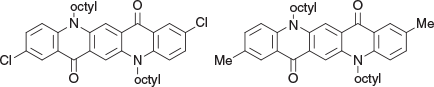
|
The mixture of QAs supplied as Colour Index Pigment Red 202 (18 g) and n-octyl bromide (30 g, 155 mmol) in a mixture of hexamethylphosphoramide (300 mL) and benzene (600 mL) was boiled down to a volume of ~360 mL and tetrabutylammonium bromide (2 g) was added. The mixture was cooled and a portion of sodium hydride (2 g of 8.4 g, 60 % in oil) added. After stirring for 5 h at 40°C and then for 2 d at room temperature more sodium hydride (1 g) was added at 50°C, the rest of the hydride was then added over 4 h. After 48 h the mixture was cooled to room temperature and quenched by the slow addition of aqueous methanol (90 %, 100 mL). Filtration and washing of the precipitate with methanol gave a mixture of the crude products as red needles (28.7 g). A portion (1 g) of the above material was chromatographed over silica gel and 2,9-dichloro-5,12-dioctylquinolino[2,3-b]acridine-7,14(5H,12H)-dione (1d, 0.32 g), mp 260–262°C, was eluted with DCM/5 % ethyl acetate. δH 8.48 (2H, s, 2 × ArH), 8.28 (2H, s, 2 × ArH), 7.57 (2H, dd, J 8.8, 2.0, 2 × ArH), 7.30 (2H, d, J 9.3, 2 × ArH), 4.37 (4H, m, 2 × CH2N), 1.88 (4H, m, 2 × CH2), 1.59 (8H, m, 4 × CH2), 1.2–1.6 (16H, m, 8 × CH2), 1.88 (6H, m, 2 × CH3). δC (125.7 MHz,) 176.3, 140.1, 135.0, 134.5, 126.7(2C), 125.4, 121.1, 116.6, 113.4, 46.7, 32.0, 29.6, 29.5, 27.2(2C), 22.85, 14.3. m/z 604.2618; calcd for C36H42Cl2N2O2 604.2623.
This was followed by the 2,9-dimethyl product (1f, 0.44 g) which was recrystallised from acetic acid to give red needles, mp 234–236°C. δH 8.62 (2H, s, 2 × ArH), 8.24 (2H, br s, 2 × ArH), 7.48 (2H, dd, J 8.8, 2.0, 2 × ArH), 7.55 (2H, dd, J 8.8, 2.0, 2 × ArH), 4.45 (4H, t, J 8.0, 2 × CH2N), 2.42 (6H, s, 2 × ArCH3), 1.90 (4H, m, 2 × CH2), 1.60 (4H, m, 2 × CH2), 1.45 (4H, m, 2 × CH2), 1.35 (12H, m, 6 × CH2), 0.86 (6H, m, 2 × CH3). δC 179.4, 141.9, 137.5, 136.9, 131.8, 128.7, 127.5, 122.3, 116.1, 114.8, 47.8, 33.35, 31.0, 30.9, 29.7, 28.6, 24.2, 22.1, 15.65. m/z 564.3710; calcd for C38H48N2O2 564.3716.
General QA Condensation Methods
Method A (for Bis-condensed Products)
5,12-Dioctylquinacridone (1b) (0.54 g, 1 mmol) was suspended in DCE (15 ml) containing DMF (2 drops) and POCl3 (3 mmol), and the mixture was stirred at 60°C for 2 h. The substrate (4 mmol) and 2,4,6-collidine (8 mmol) were added to the clear green solution at room temperature and the reaction was continued for 48 h. The mixture was added to aqueous citric acid (15 %, 100 mL), stirred for 20 min, and extracted with ethyl acetate (2 × 50 mL). The organic phase was separated, the volatile components removed by evaporation, and the residue was chromatographed over silica gel.
Method B (for Mono-condensed Products)
The green reactive intermediate was prepared from the quinacridone (1 mmol) as in Method A above. The substrate (2 mmol) and 2,4,6-collidine (8 mmol) were added to the clear green solution at room temperature and the reaction was continued for 2 h. The mixture was worked up as in Method A.
Method C (Malononitrile Mono-condensation Product 3b Coupling with a Second Component)
A mixture of the malononitrile monocondensation product (3b, 0.58 g, 1 mmol), DCE (15 ml), DMF (2 drops), and POCl3 (0.45 g, 3 mmol) was stirred at 60°C for 2 h and then cooled to room temperature. The nucleophilic component (2 mmol) was added followed by triethylamine (6 mmol) and the mixture was stirred for 24 h at room temperature. The mixture was worked up as in Method A.
Method D (All Components Combined at the Start of Reaction)
A stirred mixture of the 5,12-dioctylquinacridone (1 mmol), DCM (20 ml), and POCl3 (0.6 g, 4 mmol) at 0°C was treated with the nucleophilic component (4 mmol) added in one portion followed by disopropylethylamine or 2,4,6-collidine (10 mmol), and allowed to react overnight at room temperature. The next day the mixture was worked up as in Method A.
Adducts Using an Activated –CH2–
Dimethyl 2,2′-(5,12-dioctylquinolino[2,3-b]acridine-7,14(5H,12H)-diylidene)bis(2-cyanoacetate) (2a) (Chart 4)
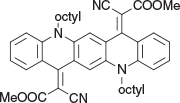
|
Method A: Methyl cyanoacetate (0.40 g, 4 mmol) was reacted with 5,12-dioctylquinacridone (0.54 g, 1 mmol). The material which was eluted from silica gel with light petroleum/DCM (1 : 4) was collected and crystallised from DCM/methanol to afford 2a as long blue needles (0.52 g, 74 % based on 1 mmol 5,12-dioctylquinacridone), mp 171–173°C. δH 8.49 (2H, br s, 2 × ArH), 7.78 (2H, m, 2 × ArH) 7.60 (2H, m, 2 × ArH), 7.38 (2H, m, 2 × ArH), 7.14 (2H, m, 2 × ArH), 4.33 (4H, m, 2 × CH2N), 3.81 (6H, s, 2 × OCH3), 2.0 (4H, m, 2 × CH2), 1.24–1.67 (20H, m, 10CH2), 0.90 (6H, m, 2 × CH3). δC 166.9, 153.65, 141.2, 135.2, 134.4, 130.6, 125.0, 122.1, 121.4, 119.65, 115.5, 112.7, 93.6, 54.3. 50.1, 33.3, 30.84, 30.82, 28.44. 28.31, 24.2, 15.7. m/z 698.3827; calcd for C44H50N4O4 698.3832.
2,2′-(2,9-Dimethyl-5,12-dioctylquinolino[2,3-b]acridine-7,14(5H,12H)-diylidene)dimalononitrile (2b) (Chart 5)
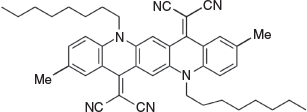
|
Method A: 2,9-Dimethyl-5,12-dioctylquinacridone (1d) (0.56 g, 1mmol) was reacted with malononitrile (0.25 g, 4 mmol). The residue was chromatographed over silica and the product was eluted with DCM/light petroleum (3 : 1) and crystallised from ethyl acetate to give dark green needles of 2b (0.43 g, 66 % based on 1 mmol of 2,9-dimethylquinacridone), mp 200–202°C.
Method D: 2,9-Dimethyl-5,12-dioctylquinacridone (1d) (0.56 g, 1 mmol) was reacted with malononitrile (0.25 g, 4 mmol) and POCl3 (0.52 g, 4 mmol) to give 0.37 g (56 %) of 2b.
δH (500 Mz) 8.7 (2H, s, 2 × ArH), 8.35 (2H, s, 2 × ArH), 7.60 (2H, m, 2 × ArH), 7.40 (2H, m, 2 × ArH), 4.45 (4H, t, J 7.8, 2 × CH2N), 2.46 (6H, s, 2 × ArCH3), 198 (4H, m, 2 × CH2), 1.40–1.60 (20H, m, 10 × CH2), 0.85 (6H, m, 2 × CH3). δC 154.3, 137.0, 135.0, 133.0, 131.4, 126.2, 122.3, 117.4, 116.7, 116.7, 114.0, 111.1, 69.4, 48.7, 31.4, 28.9, 26.7, 26.4, 22.29, 20.4, 13.7. m/z 660.3935; Calcd for C44H48N6 660.3940.
2,2′-(2,9-Dichloro-5,12-dioctylquinolino[2,3-b]acridine-7,14(5H,12H)-diylidene)dimalononitrile (2c) (Chart 6)
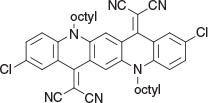
|
Method A: 2,9-Dichloroquinacridone (0.64 g, 1 mmol) and malononitrile (0.25 g, 4 mmol) were treated as above and the crude product was applied to a silica column and eluted with 1 : 1 DCM/light petroleum and crystallised from DCM/ethanol to give the product 2c (0.41 g, 58 % based on the QA) as green granules, mp 220–222°C. δH 8.58 (2H, s, 2 × ArH), 8.52 (2H, m, 2 × ArH), 7.69 (2H, dd, J 9.2, 2.3, 2 × ArH), 7.47 (2H, m, 2 × ArH), 4.45 (4H, m, 2 × CH2N), 2.0 (4H, m, 2 × CH2), 1.50 (4H, m, 2 × CH2), 1.25–1.48 (12H, m, 6 × CH2), 1.35 (6H, t, J 8.1, 2 × CH3). δC 155.13, 139.34, 135.94, 135.21, 129.04, 127.74, 124.29, 119.39, 118.61, 117.70, 117.61, 113.36, 72.91, 50.96, 33.30, 30.79, 30.77, 28.56, 28.32, 24.22, 15.69. m/z 700.2843; calcd for C42H42Cl2N6 700.2848.
2,2′-(5,12-Dioctylquinolino[2,3-b]acridine-7,14(5H,12H)-diylidene)bis(3-oxo-3-(pyrrolidin-1-yl)propanenitrile) (2d) (Chart 7)
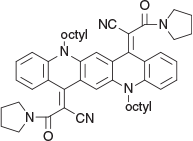
|
Method A: Compound 2d was prepared from 3-oxo-3-(pyrrolidin-1-yl)propanenitrile (0.52 g, 4 mmol) and the QA (1b, 1 mmol) followed by work up and chromatography over silica gel and eluted with 1 : 1 DCM/light petroleum and crystallised from DCM/ethanol to give blue needles (0.50 g, 66 % based on the QA), mp 183–185°C. δH 8.19 (2H, s, 2 × ArH), 8.36 (2H, m, 2 × ArH), 7.47 (2H, m, 2 × ArH), 7.25 (2H, m, 2 × ArH), 7.03 (2H, m, 2 × ArH), 4.24 (4H, m, 2 × CH2N), 3.50 (4H, m, 2 × CH2NCH2), 2.87 (4H, br m, 2 × CH2NCH2), 1.2–1.95 (32H, m 16 × CH2), 0.85 (6H, m, 2 × CH3). δC 162.8, 144.8, 138.9, 133.8, 131.4, 126.7, 122.45, 120.3, 118.85, 117.7, 113.25, 110.67, 98.44, 37.45, 46.44, 45.68, 31.41, 29.0, 28.9, 26.6, 26.0, 25.0, 24.0, 22.3, 13.7. m/z 776.4772; calcd for C50H60N6O2 776.4778.
Methyl 2-Cyano-2-(5,12-dioctyl-14-oxoquinolino[2,3-b]acridin-7(5H,12H,14H)-ylidene)acetate (3a) (Chart 8)
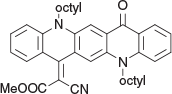
|
Method B: Methyl cyanoacetate (0.20 g, 2 mmol) was reacted with 1b (0.54 g, 1 mmol). After work up the material contained in the organic phase was purified (SiO2/DCM) and crystallised from methanol to afford 3a (0.46 g, 74 %) as purple, woolly needles, mp 151–153°C. δH 8.50 (3H, m, 3 × ArH), 7.03–7.83 (7H, m, 7 × ArH), 4.37 (2H, m, CH2N), 4.28 (2H, m, CH2N), 3.28 (3H, s, OCH3), 1.93 (4H, m, 2 × CH2), 1.2–1.6 (24H, m, 12 × CH2), 0.66 (6H, m, 2 × CH3). δC 178.8, 166.6, 153.8, 143.6, 141.4, 137.4, 136.2, 135.2, 134.4, 130.3, 129.5, 122.7, 122.6, 122.0, 121.0, 119.5, 116.2, 115.5, 115.4, 114.5, 112.9, 54.35, 54.3, 49.3, 48.6, 33.4, 33.3, 30.9, 30.88, 30.86, 30.83, 28.85, 28.6, 28.5, 28.0, 24.2, 15.68, 15.66. m/z 617.3613; calcd for C40H47N3O3 617.3617.
2-(5,12-Dioctyl-14-oxoquinolino[2,3-b]acridin-7(5H,12H,14H)-ylidene)malononitrile (3b) (Chart 9)
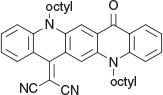
|
Method B: Malononitrile (0.13 g, 2 mmol) was reacted with QA 1b (0.54 g, 1 mmol). After work up the residue was crystallised from methanol (15 mL) to give 3b (0.50 g, 85.3 % based on 1b), mp 155–157°C. δH 8.67 (2H, s, 2 × ArH), 8.54 (2H, m, 2 × ArH), 7.73 (2H, m, ArH), 7.51 (1H, d, J 8.8, ArH), 7.47 (1H, d, J 8.4 Hz, ArH), 7.30 (2H, m, 2 × ArH), 4.45 (4H, m, 2 × CH2N), 2.04 (4H, m, 2 × CH2) 1.25–1.68 (20H, m, 10 × CH2), 0.89 (6H, m, 2 × CH3). δC 177.4, 155.8, 142.3, 139.4, 136.1, 135.0, 134.2, 133.1, 128.1, 127.3, 125.6, 123.1, 121.5, 121.4, 121.3, 117.7, 117.0, 116.9, 114.8, 114.25, 112.5, 112.4, 70.25, 48.1, 47.3, 31.83, 31.76, 29.5, 29.30, 29.28, 27.2, 27.0, 26.9, 26.7, 22.7, 22.65, 14.14, 14.11. m/z 584.3510; calcd for C39H44N4O 584.3515.
2-(2,9-Dimethyl-5,12-dioctyl-14-oxoquinolino[2,3-b]acridin-7(5H,12H,14H)-ylidene)malononitrile (3c) (Chart 10)
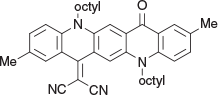
|
Method B: 2,9-Dimethylquinacridone (1f) (0.56 g, 1 mmol) was reacted with malononitrile (0.12 g, 2 mmol).The residue was chromatographed over silica and 3c was eluted with DCM/light petroleum (5 : 1) and crystallised as stout needles (0.32 g, 52 % based on 1f) from DCM/ethanol, mp 154–156°C. δH 8.64 (2H, s, 2 × ArH), 8.30 (2H, m, 2 × ArH), 7.59 (2H, m, 2 × ArH), 7.41 (2H, m, 2 × ArH), 4.46 (4H, m, 2 × CH2N), 2.46, 2.46 (3H, 3H, s, s, 2 × ArCH3), 1.98 (4H, m, 2 × CH2), 1.3–1.7 (20H, m, 10 × CH2), 1.38 (6H, m, 2 × CH3), 0.9 (6H, m, 2 × CH3). δC (125.7 MHz) 178.6, 156.8, 142.0, 138.1, 137.3, 134.4, 132.8, 132.6, 128.7, 128.2, 126.7, 124.5, 122.4, 119.5, 118.9, 118.4, 116.4, 115.8, 114.0, 113.7, 70.6, 49.5, 48.7, 33.4, 33.3, 30.9, 30.8 (2 × C), 28.8, 28.6, 28.5, 28.3, 22.9. 22.8, 20.9, 20.75, 14.3, 14.2. m/z 612.3823; calcd for C41H48N4O 612.3828.
2-(5,12-Dioctyl-14-oxoquinolino[2,3-b]acridin-7(5H,12H,14H)-ylidene)-2-(phenylsulfonyl)acetonitrile (3d) (Chart 11)
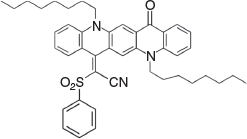
|
Method B: 2-(Phenylsulfonyl)acetonitrile (0.36 g, 2 mmol) was reacted with 1b (0.54 g, 1 mmol). The product was eluted from silica gel with 20 % light petroleum in DCM and crystallised from DCM/methanol to give 3d (0.52 g, 74 % based on 1b) as large, chunky needles, mp > 250°C. δH 8.76 (1H, s, ArH), 8.60 (1H, s, ArH), 8.59 (1H, m, ArH), 8.41 (1H, s, ArH), 7.2–7.9 (11H, m, 11ArH), 4.50 (2H, m, CH2N), 4.85 (2H, m, CH2N), 2.0 (4H, m, 2 × CH2), 1.2–1.7 (8H, m, 4 × CH2), 0.89 (6H, m, 2 × CH3). δC (125.7 MHz, CDCl3) 177.5, 152.0, 142.2, 139.7, 139.3, 135.8, 134.6, 133.9, 133.4, 133.3, 129.6, 128.8, 128.6, 128.0, 125.0, 123.9, 121.2, 121.0, 120.7, 117.6, 117.5, 114.7, 113.3, 111.2, 47.6, 46.9, 31.8, 31.7, 29.3, 29.2, 27.3, 27.0, 26.8, 26.5, 22.7, 22.6, 14.1, 14.0. m/z 699.3489; calcd for C44H49N3O3S 699.3495.
5-(5,12-Dioctyl-14-oxoquinolino[2,3-b]acridin-7(5H,12H,14H)-ylidene)-4-methyl-2,6-dioxo-1-phenyl-1,2,5,6-tetrahydropyridine-3-carbonitrile (3e) (Chart 12)
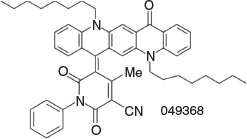
|
Method B: The QA 1b (1.0 g, 1.9mmol) and the dioxopyridine[15] (1 g, 4.5 mmol) were reacted and worked up in the usual way. The organic phase was separated, the solvent removed by evaporation, and the residue was simmered with methanol (25 mL) for 5 min and cooled. The next day the solid was filtered off and purified by elution from a silica column with 5 % isopropyl alcohol/DCM and crystallised from DCM/ethanol to give 3e (1.13 g, 81.3 % based on 1b) as fine green needles, mp > 250°C. δH 9.40 (1H, s, ArH), 8.52 (3H, m, 3 × ArH), 8.20 (2H, br s, 2 × ArH), 7.87 (1H, m, ArH), 7.76 (1H, m, ArH), 7.55 (1H, d, J 8.8, ArH), 7.2–7.4 (6H, m, 6 × ArH), 5.24 (2H, t, J 7.1, CH2N), 4.30 (2H, t, J 8.1, CH2N), 2.20 (2H, m, CH2), 1.94 (3H, s, =C–CH3), 1.95 (2H, m, CH2), 1.2–1.7 (16H, m, 8 × CH2), 0.85 (6H, m, 2 × CH3). δC (125.6 MHz, CDCl3) 177.7, 163.9, 162.9, 161.9, 153.5, 142.8, 140.8, 139.3, 138.9, 136.7, 136.4, 133.4, 132.1, 130.4, 129.6, 128.9, 128.8, 128.7, 128.4, 127.5, 127.2, 122.2, 121.0, 119.4, 116.9, 116.8, 115.0, 102.3, 84.0, 50.5, 47.0, 31.7, 31.6, 29.5, 29.2, 29.1, 29.1, 27.2, 27.0, 26.8, 22.6, 22.5, 22.5, 20.1, 14.1, 14.0. m/z 744.4034; calcd for C49H52N4O3 744.4039.
Adducts Using an Activated –CH3
2,2′-(5,12-Dioctylquinolino[2,3-b]acridine-7,14(5H,12H)-diylidene)bis(1-(3,4-dichlorophenyl)ethane-2,1-diylidene))dimalononitrile (4a) (Chart 13)
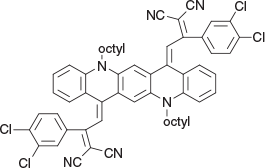
|
Method D: To dioctylquinacridone 1b (0.54 g, 1 mmol) in DCM (15 mL), was added POCl3 (0.60 g, 4mmol) and 2,4,6-collidine (0.96 g, 8mmol) at 0°C followed by the nitrile[16] (0.72 g, 3 mmol). The components were mixed and then allowed to react for 48 h before the mixture was worked up and purified by chromatography (SiO2/DCM), affording deep purple needles of 4a from DCM/EtOAc (0.66 g, 82.6 % based on 1b), mp > 250°C. δH 7.63 (4H, br s, 2 × ArH and 2 × C=CH), 6.6–7.4 (16H, m, 16 × ArH), 4.20 (4H, m, 2 × CH2N), 1.9 (4H, m, 2 × CH2), 1.3–1.6 (20H, m, 10 × CH2), 0.9 (6H, m, 2 × CH3). δC (125.8 MHz) 167.1, 146.9, 139.35, 135.2, 134.55, 133.3, 133.0, 132.4, 131.2, 130.9, 130.7, 128.8, 126.4, 121.45, 120.0, 115.6, 115.0, 114.9, 113.7, 109.8, 78.7, 47.4, 32.1, 32.00, 29,5, 29.4, 27.2, 26.9, 22.85, 14.3. m/z 972.3002; calcd for C58H52Cl4N6 972.3008.
2,2′-(5,12-Dioctylquinolino[2,3-b]acridine-7,14(5H,12H)-diylidene)bis(1-(9H-fluoren-2-yl)ethane-2,1-diylidene))dimalononitrile (4b) (Chart 14)
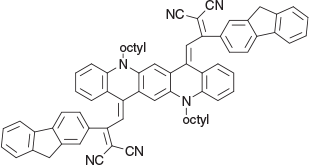
|
Method A: The fluorene[17] (0.76 g, 3 mmol) and the QA 1b were reacted. The mixture was worked up the next day and 4b was eluted from silica (25 % light petroleum/DCM) and recrystallised from DCM/ethanol to give dark green prisms (0.62 g, 55 % from 1b), mp 234−236°C. δH 7.48 (2H, br s, 2 × =CH–), 7.0–7.5 (24H, m, 24 × ArH), 4.18 (4H, br s, 2 × CH2N), 3.61 (4H, s, 2 × ArCH2Ar), 1.85–1.22 (24H, m, 12 × CH2), 0.88 (6H, m, 2 × CH3). δC 170.0, 144.4, 143.3, 142.6, 139.8, 138.6, 131.0, 138.8, 128.2, 127.5, 126.7, 125.7, 124.7, 120.1, 119.3, 115.8, 115.2, 115.1. 46.7, 36.2, 31.4, 29.0, 28.9, 26.6, 26.2, 22.3, 13.7. m/z 1012.5187; calcd for C72H64N61012.5192.
2,2′-(5,12-Dioctylquinolino[2,3-b]acridine-7,14(5H,12H)-diylidene)bis(1-(thiophen-3-yl)ethane-2,1-diylidene))dimalononitrile (4c) (Chart 15)
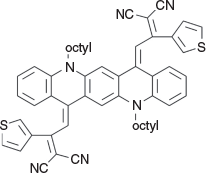
|
Method D: 2-(1-(Thiophen-3-yl)ethylidene)malononitrile[18] (0.68 g, 4 mmol) was reacted with QA 1b (0.54 g, 1 mmol). The mixture was worked up and 4c was eluted from silica with 30 % light petroleum/DCM, and recrystallised from EtOH to give dendrites (0.40 g, 36 % based on 1b), mp 156–158°C. δH 7.60 (4H, br s, 4 × ArH), 7.36 (4H, m, 2 × ArH), 7.17 (2H, m, 2 × ArH), 6.94–7.17 (8H, m, 8 × ArH), 6.81 (2H, s, 2 × C=CH), 4.18 (4H, m, 2 × CH2N), 1.88 (4H, m, 2 × CH2), 1.30–1.60 (16H, m, 8 × CH2), 0.90 (6H, m, 2 × CH3). δC 163.9, 145.5, 139.1, 134.6, 134.2, 131.5, 130.4, 127.7, 126.4, 121.0, 115.4, 115.2, 115.1, 113.3, 47.4, 31.7, 29.3, 29.2, 26.9, 26.5, 22.6, 14.1 m/z 848.3689; calcd for C54H52N6S2 848.3695.
Adducts Using R–NH2
N-(5,12-dioctyl-14-oxoquinolino[2,3-b]acridin-7(5H,12H,14H)-ylidene)cyanamide (5a) (Chart 16)
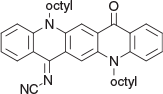
|
Method B: The reaction was carried out with cyanamide (0.08 g, 2 mmol) and the QA 1b (0.54 g, 1 mmol) and worked up in the usual way, purified on silica (DCM) and the residue was crystallised from DCM/ethanol to give 5a as lustrous needles (0.41 g, 73 % based on 1b), mp 168–170°C. δH 9.10 (2H, br m, 2 × ArH), 8.68 (1H, s, ArH), 8.37 (1H, dd J 1.5, 8, ArH), 7.78 (1H, m, ArH), 7.69 (1H, m, ArH), 7.54 (1H, d, J 8.8, ArH), 7.45 (2H, d, J 8.8, 2 × ArH), 7.26 (1H, m, ArH), 7.13 (1H, m, ArH), 4.56 (2H, t, J 7.9, CH2N), 4.44 (2H, t, J 7.9, CH2N), 1.97 (4H, m, 2 × CH2), 1.67 (4H, m, 2 × CH2), 1.2–1.5 (16H, m, 8 × CH2), 0.90 (6H, m, 2 × CH3). δC (125.7 MHz, CDCl3) 177.7, 164.2, 142.4, 140.55, 136.05, 135.5, 135.1, 133.8, 128.0, 127.7, 126.7, 124.7, 122.0, 121.2, 121.1, 120.1, 118.0, 115.25, 115.0, 113.95, 112.8, 47.6, 46.8, 32.0, 32.0, 29.6, 29.6, 29.45, 27.45, 27.2 (2 × C), 27.0, 22.9, 22.8, 14.3 (2 × C). m/z 560.3510; calcd for C37H44N4O 560.3515.
4-Chloro-N-(5,12-dioctyl-14-oxoquinolino[2,3-b]acridin-7(5H,12H,14H)-ylidene)benzenesulfonamide (5b) (Chart 17)
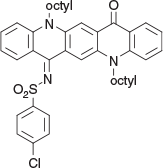
|
Method D: 4-Chlorobenzenesulfonamide (0.29 g, 1.5 mmol) was reacted with QA 1b (0.54 g, 1 mmol) in the usual way and after work up and chromatography (silica/chloroform) gave 5b as purple flat needles from DCM/ethanol (0.51 g, 72 % based on 1b), mp 193–195°C. δH 9.18 (1H, s, ArH), 8.77 (1H, s, ArH), 8.72 (1H, dd, J 8.4, 1.5, ArH), 8.48 (1H, dd, J 8.0, 1.7, ArH), 8.07 (2H, m, 2 × ArH), 7.70 (2H, m, 2 × ArH), 7.48 (4H, m, 4 × ArH), 7.23 (2H, m, ArH), 4.58 (2H, t, J 8.2, NCH2), 4.42 (2H, t, J 8.2, NCH2), 1.95 (4H, m, 2 × CH2), 1.2–1.7 (20H, m, 10 × CH2), 0.88 (6H, m, 2 × CH3). δC 179.3, 162.7, 145.3, 144.0, 142.8, 139.1, 137.6, 136.7, 136.5, 136.2, 131.6, 130.4, 129.55, 129.2, 128.2, 125.3, 123.5, 122.6, 122.5, 121.3, 118.2, 116.3, 116.15, 114.9, 49.2, 48.4, 33.4, 33.3, 30.94, 30.88, 30.87, 30.85, 28.9, 28.6, 28.4, 28.3, 24.2, 24.2, 15.7, 14.7. m/z 709.3099; calcd for C42H48ClN3O3S 709.3105.
N-(5,12-Dioctyl-14-oxoquinolino[2,3-b]acridin-7(5H,12H,14H)-ylidene)piperidine-1-carboxamide (5c) (Chart 18)
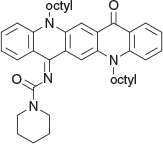
|
Method B: A reaction between piperidine-1-carboxamide (0.26 g, 2 mmol) and QA 1b (0.54 g, 1 mmol) was followed by chromatography and recrystallisation from methanol and gave 5c as red plates (0.44 g, 68 % based on 1b), mp 153–155°C. δH 8.68 (1H, s, ArH), 8.50 (1H, dd, J 1.7, 8.0, ArH), 8.49 (1H, s, ArH), 8.17 (1H, dd, J 1.6, 8.2, ArH), 7.63 (1H, m, ArH), 7.54 (1H, m, ArH), 7.47 (1H, d, J 8.7, ArH), 7.31 (1H, d, J 8.6, ArH), 7.18 (1H, m, ArH), 7.09 (1H, m, ArH), 4.41 (2H, t, J 8.1, CH2N), 4.28 (2H, t, J 8.2, CH2N), 3.73 (2H, t, J 5.4, CH2NCH2), 3.28 (2H, t, J 5.4, CH2NCH2), 1.92 (4H, m, 2 × CH2), 1.2–1.7 (20H, m, 10 × CH2), 0.86 (6H, m, 2 × CH3). δC (125.7 MHz, CDCl3) 178.05, 164.2, 152.85, 142.3, 141.25, 136.1, 135.1, 134.5, 133.2, 128.5, 128.2, 126.2, 125.4, 121.4, 120.9, 120.8, 118.1, 114.8, 114.6, 113.8, 112.2, 47.0, 46.53, 46.50, 43.7, 32.00. 31.98, 29.65, 29.6 (2 × C), 29.5, 27.4, 27.23, 27.20, 26.8, 26.1, 25.9, 24.85, 22.83 (2 × C), 14.3 (2 × C). m/z 646.4241; calcd for C42H54N4O2 646.4247.
Condensations of QA Malononitrile Mono-Condensation Product 3b with a Second Reactant
Methyl 2-Cyano-2-(14-(dicyanomethylene)-5,12-dioctylquinolino[2,3-b]acridin-7(5H,12H,14H)-ylidene)acetate (6a) (Chart 19)
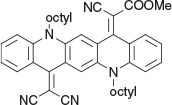
|
Method C: Methyl cyanoacetate (0.20 g, 2 mmol) was reacted with the QA 3b (0.58 g, 1 mmol). Compound 6a was eluted with DCM containing up to 20 % light petroleum and crystallised from DCM/methanol to give green needles (0.41 g, 62 % based on 3b), mp 172–174°C. δH 8.59 (1H, d, J 8.0, ArH), 8.51 (1H, s, ArH), 7.74 (1H, m, ArH), 7.63 (1H, m, ArH), 7.49 (1H, d, J 8.7, ArH), 7.38 (1H, d, J 8.6, ArH), 7.31 (1H, m, ArH), 7.17 (1H, m, ArH), 4.45 (2H, t, J 6.4, CH2N), 4.38 (2H, t, J 8.1, CH2N), 3.85 (3H, s, CH3O), 2.03 (4H, 2 × CH2), 1.65 (4H, m, 4 × ArH), 1.50 (4H, m, 4 × ArH), 1.3–1.4 (12H, m, 6 × CH2), 0.93 (6H, m, 2 × CH3). δC (125.7 MHz) 166.5, 156.7, 141.2, 140.7, 135.6, 134.8, 134.6, 128.8, 123.2, 123.0, 122.4, 121.0, 119.4, 118.9, 118.7, 116.0, 115.6, 112.1, 70.2, 54.4, 50.5, 50.3, 33.3, 33.3, 30.8, 30.8, 28.6, 28.4, 28.2, 24.2 15.7. m/z 665.3724; calcd for C43H47N5O2 665.3730.
2-(14-(1-Cyano-2-oxo-2-(pyrrolidin-1-yl)ethylidene)-5,12-dioctylquinolino[2,3-b]acridin-7(5H,12H,14H)-ylidene)malononitrile (6b) (Chart 20)
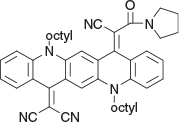
|
Method C: 3-Oxo-3-(pyrrolidin-1-yl)propanenitrile[19] (0.28 g, 2mmol) was reacted with QA 3b (0.58 g, 1mmol). Compound 6b was eluted with DCM containing up to 2 % ethyl acetate and crystallised from DCM/cyclohexane as granules (0.44 g, 62.4 % from 3b), mp 155–157°C. δH (2 % trifluoroacetic acid in CDCl3) 8.59 (H, dd, J 1.3, 8.4, ArH), 8.53 (H, s, ArH), 8.48 (H, s, ArH), 7.75 (2H, m, 2 × ArH), 7.57 (2H, m, 2 × ArH), 7.36 (2H, m, 2 × ArH), 7.14 (H, t, J 7.1, ArH), 4.47 (2H, m, CH2N). 4.31 (2H, m, CH2N), 3.57 (2H, m, CH2NCO (restricted rotation)), 2.88 (2H, m, CH2N(CO)H2C (restricted rotation)), 2.05 (2H, m, CH2), 1.94 (2H, m, CH2), 1.79 (2H, m, CH2), 1.70 (2H, m, CH2), 1.25–1.50 (20H, m, 10 × CH2), 0.88 (6H, m, 2 × CH3). δC (125.7 MHz,) 177.8, 159.9, 155.1, 146.7, 142.3, 141.35, 136.25, 135.0, 134.7, 134.3, 128.1, 127.95, 126.4, 125.3, 124.8, 121.6, 121.2 (2 × C), 116.6, 115.7, 114.8, 113.6 (2 × C), 111.9. 109.2, 107.2, 66.0, 47.4, 46.4, 32.0, 31.85, 29.6, 29.52, 29.46 (2 × C), 27.3, 27.1, 27.1 (2 × C), 22.8 (2 × C), 14.28, 14.27, 13.8. m/z 704.4197, calcd for C46H52N6O 704.4203.
2-(14-(Cyano(phenylsulfonyl)methylene)-5,12-dioctylquinolino[2,3-b]acridin-7(5H,12H,14H)-ylidene)malononitrile (6c) (Chart 21)
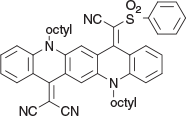
|
Method C: 2-(Phenylsulfonyl)acetonitrile[20] (0.36 g, 2 mmol) was reacted with QA 3b (0.58 g, 1 mmol). Compound 6c was eluted with DCM and crystallised from methanol to give dark flocs (0.52 g, 70 % based on 3b), mp 170–172°C. δH 8.62 (H, s, ArH), 8.59 (H d, J 8.3, ArH), 8.42 (H, s, ArH), 8.40 (H, d, J 8.3, ArH), 7.30–7.80 (12H, m, 12 × ArH), 4.45, 4.34 (2H, 2H, m, m, 2 × CH2N), 1.95 (4H, m, 2 × CH2), 1.6 (4H, m, 2 × CH2), 1.23–1.5 (20H, m, 10 × CH2), 0.88 (6H, m, 2 × CH3). δC 154.9, 150.8, 139.1, 138.9, 138.5, 133.9, 133.8, 133.4, 133.2, 132.7, 128.5, 128.3, 126.8, 121.5, 121.3, 120.8, 117.4, 117.05, 116.8, 116.7, 114.1, 113.2, 109.8, 48.4, 48.3, 31.4, 28.9, 28.85, 26.65, 26.5, 26.4, 26.4, 22.31, 22.29, 13.75. m/z 747.3601; calcd for C47H49N5O2S 747.3607.
2-(14-(1-Cyano-2-oxo-2-phenylethylidene)-5,12-dioctylquinolino[2,3-b]acridin-7(5H,12H,14H)-ylidene)malononitrile (6d) (Chart 22)
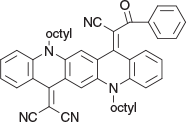
|
Method C: Benzoylacetonitrile (0.29 g, 2 mmol) was reacted with QA 3b (0.58 g, 1 mmol). Compound 6d was eluted with 5 % ethyl acetate/DCM and crystallised from ethanol to give blue needles (0.44 g, 66 % from 3b), mp 172–174°C. δH 8.77 (H, s, ArH), 8.71 (H, s, ArH), 8.52 (H, d, J 8.4, ArH), 7.85 (H, d, J 8.3, ArH), 7.2–7.7 (11H, m, 11 × ArH), 4.50 (2H, m, CH2N), 4.40 (2H, m, 2 × CH2N), 2.0 (4H, m, 2 × CH2), 1.2–1.7 (20H, m, 10 × CH2), 0.87 (6H, m, 2 × CH3). δC (125.7 MHz) 170.2, 155.1, 152.7, 139.7, 139.3, 134.6, 134.5, 134.34, 134.29, 133.7, 132.9, 130.0, 129.1, 129.0, 127.2, 125.5, 123.3, 122.9, 122.1, 120.2, 199.9, 117.4, 117.2, 116.6, 115.2, 114.61, 114.57, 112.4, 111.9, 77.2, 49.2, 49.1, 31.8, 31.7, 29.24, 29.21, 29.18, 27.36, 27.0, 26.9, 26.8, 22.7, 22.6, 14.1 (2 × C). 192.1, 155.4, 149.0, 139.7, 139.1, 135.55, 134.4, 134.2, 134.05, 133.7, 132.8, 129.2, 128.95, 128.8, 127.3, 125.6, 121.8, 121.5, 121.4, 119.3, 118.8, 118.1, 117.5, 117.4, 114.6, 113.8, 112.5, 110.7, 102.2, 68.6, 49.0, 48.5, 31.95, 29.45 (2 × C), 29.4 (2 × C), 27.3, 27.0 (2 × C), 27.0, 26.7, 22.85, 22.8, 14.3 (2 × C). m/z 711.3932; calcd for C48H49N5O 711.3937.
2,2′-(2-(14-(Dicyanomethylene)-5,12-dioctylquinolino[2,3-b]acridin-7(5H,12H,14H)-ylidene)-1H-indene-1,3(2H)-diylidene)dimalononitrile (6e) (Chart 23)
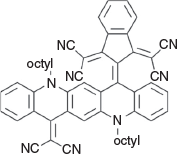
|
Method D: 2,2′-(1H-Indene-1,3(2H)-diylidene)dimalononitrile[21] (0.48 g, 2 mmol) was reacted with QA 3b (0.58 g, 1 mmol). Compound 6e was eluted with DCM containing up to 2 % ethyl acetate and crystallised from DCM/ethanol to afford a dark powder (0.68 g, 84.1 % from 3b), mp > 250°C. δH 9.50 (1H, s, ArH), 8.51 (3H, m, 3 × ArH), 8.28 (3H, m, 3 × ArH), 8.11 (1H, s, ArH), 7.84 (2H, m, 2 × ArH), 7.55 (2H, m, 2 × ArH), 7.48 (1H, d, J 8.6, ArH), 7.42 (1H, m, ArH), 5.30 (2H, t, J 8.2, CH2N), 4.24 (2H, t, J 8.6, CH2N), 2.30 (2H, m, CH2), 1.91 (2H, m, CH2), 1.75 (2H, m, CH2), 1.53 (2H, m, CH2), 1.05–1.45 (16H, m, 8 × CH2), 0.92 (6H, m, 2 × CH3). δC (125.7 MHz) 156.4, 156.0, 154.9, 139.9, 139.2, 139.1, 137.1, 136.5, 135.3, 132.6, 130.8, 130.5, 130.3, 130.2, 128.4, 128.0, 127.25, 123.1, 122.6, 117.3, 117.0, 116,4, 116.3, 116.0, 115.9, 114.9, 114.1, 111.2, 103.8, 77.0, 54.2, 51.1, 48.8, 31.3, 31.2, 29.1, 28.9, 28.80, 28.69, 26.7, 26.6, 25.3, 22.3, 22.25, 13.8, 13.7. m/z 808.3996; calcd for C54H48N8 808.4002.
X-Ray Crystallography
Crystals were mounted on fine glass fibres using viscous hydrocarbon oil. Data were collected on a Bruker X8 Apex II CCD Diffractometer (3d, 4c, 5a, 6a) (Mo Kα radiation, λ 0.71073 Å, 123 K) or on the MX1: Macromolecular Crystallography beamline at the Australian Synchrotron (2b, 5b) (λ 0.710900 Å, 100 K). Data collection temperatures were maintained using an open flow N2 cryostream. Solutions were obtained by direct methods or Patterson synthesis using SHELXS 97[22] followed by successive refinements using full matrix least-squares method against F2 using SHELXL 97.[22] The data collection and integration at the Australian Synchrotron were performed within Blu-Ice[23] and XDS software programs.[24] The program X-Seed was used as a graphical SHELX interface.[25] All datasets were treated for the effects of absorption. CCDC 1847848 (2b), CCDC 1847849 (3d), CCDC 1847850 (4c), CCDC 1847851 (5a), CCDC 1847852 (5b), CCDC 1874853 (6a) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre at www.ccdc.cam.ac.uk/data_request/cif.
Crystal data for 2b: C22H24N3, M 330.44, red block, 0.1 × 0.1 × 0.1 mm3, triclinic, space group P-1, V 894.4(4) Å3, Z 2, Dc 1.227 g cm−3, F000 354, 2θmax 55.0°, 7599 reflections collected, 3731 unique (Rint 0.0315). Final GooF 1.055, R1 0.0510, wR2 0.1376, R indices based on 3134 reflections with I > 2σ(I) (refinement on F2), 228 parameters, 0 restraints.
Crystal data for 3d: C44H49N3O3S, M 699.92, red plate, 0.5 × 0.4 × 0.2 mm3, monoclinic, space group P21/n, V 3603.9(3) Å3, Z 4, Dc 1.290 g cm−3, F000 1496, 2θmax 55.0°, 41054 reflections collected, 8263 unique (Rint 0.0210). Final GooF 1.058, R1 0.0460, wR2 0.1200, R indices based on 7405 reflections with I > 2σ(I) (refinement on F2), 462 parameters, 0 restraints.
Crystal data for 4c: C27H26N3S, M 424.57, green flat needle, 0.4 × 0.2 × 0.1 mm3, monoclinic, space group P21/n, V 2237.4(2) Å3, Z 4, Dc 1.260 g cm−3, F000 900, 2θmax 55.0°, 17398 reflections collected, 5099 unique (Rint 0.0257). Final GooF 1.035, R1 0.0618, wR2 0.1632, R indices based on 3414 reflections with I > 2σ(I) (refinement on F2), 309 parameters, 0 restraints. A portion of the octyl group is disordered over two positions, with the site occupancies refined against each other (53 : 44).
Crystal data for 5a: C37H44N4O, M 560.76, red plate, 0.3 × 0.3 × 0.0 mm3, triclinic, space group P-1, V 771.02(10) Å3, Z 1, Dc 1.208 g cm−3, F000 302, 2θmax 55.0°, 5730 reflections collected, 3500 unique (Rint 0.0195). Final GooF 1.024, R1 0.0606, wR2 0.1508, R indices based on 2395 reflections with I > 2σ(I) (refinement on F2), 209 parameters, 0 restraints.
Crystal data for 5b: C42H48ClN3O3S, M 710.34, red block, 0.1 × 0.1 × 0.1 mm3, triclinic, space group P-1, V 1790.0(6) Å3, Z 2, Dc 1.318 g cm−3, F000 756, 2θmax 55.0°, 15078 reflections collected, 7451 unique (Rint 0.0634). Final GooF 0.965, R1 0.0606, wR2 0.1501, R indices based on 4844 reflections with I > 2σ(I) (refinement on F2), 453 parameters, 0 restraints.
Crystal data for 6a: C43H47N5O2, M 665.85, blue plate, 0.8 × 0.8 × 0.1 mm3, monoclinic, space group C2/c, V 7387.8(8) Å3, Z 8, Dc 1.197 g cm−3, F000 2848, 2θmax 55.0°, 25415 reflections collected, 8432 unique (Rint 0.0521). Final GooF 1.024, R1 0.0642, wR2 0.1517, R indices based on 5073 reflections with I > 2σ(I) (refinement on F2), 465 parameters, 0 restraints. A portion of an octyl group is disordered over two positions, with the site occupancies refined against each other (72 : 28).
Supplementary Material
1H and 13C NMR spectra of characterised materials are available on the Journal’s website.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
This research was undertaken on the MX1 beamline at the Australian Synchrotron, part of ANSTO. The authors thank Dr Roger Mulder and Dr Jo Cosgriff for assistance with NMR spectroscopy, and Mr Carl Braybrook and Dr Jo Cosgriff for mass spectrometry. This research did not receive any specific funding.
References
[1] P. M. Beaujuge, J. M. J. Frechet, J. Am. Chem. Soc. 2011, 133, 20009.| Crossref | GoogleScholarGoogle Scholar | 21999757PubMed |
[2] M. Lloyd, J. Anthony, G. Malliaras, Mater. Today 2007, 10, 34.
| Crossref | GoogleScholarGoogle Scholar |
[3] J. Roncali, Acc. Chem. Res. 2009, 42, 1719.
| Crossref | GoogleScholarGoogle Scholar | 19580313PubMed |
[4] Z. He, B. Xiao, F. Liu, H. Wu, Y. Yang, S. Xiao, C. Wang, T. P. Russell, Y. Cao, Nat. Photonics 2015, 9, 174.
| Crossref | GoogleScholarGoogle Scholar |
[5] J. J. Chen, T. L. Chen, B. Kim, D. A. Poulsen, J. L. Mynar, J. M. Frechet, B. Ma, ACS Appl. Mater. Interfaces 2010, 2, 2679.
| Crossref | GoogleScholarGoogle Scholar | 20804141PubMed |
[6] I. Javed, A. Khurshid, M. N. Arshad, Y. Wang, New J. Chem. 2014, 38, 752.
| Crossref | GoogleScholarGoogle Scholar |
[7] S. De Feyter, A. Gesquière, F. C. De Schryver, U. Keller, K. Müllen, Chem. Mater. 2002, 14, 989.
| Crossref | GoogleScholarGoogle Scholar |
[8] T. Zhou, T. Jia, B. Kang, F. Li, M. Fahlman, Y. Wang, Adv. Energy Mater. 2011, 1, 431.
| Crossref | GoogleScholarGoogle Scholar |
[9] T. Takeda, H. Sugihara, Y. Suzuki, J. Kawamata, T. Akutagawa, J. Org. Chem. 2014, 79, 9669.
| Crossref | GoogleScholarGoogle Scholar | 25254634PubMed |
[10] W. Chen, L. Weiping, J. Lv, J. Han, Y. Chen, T. Jia, L. Tao, Y. Wang, J. Mater. Chem. A 2016, 4, 2169.
| Crossref | GoogleScholarGoogle Scholar |
[11] I. Javed, T. Zhou, F. Muhammad, J. Guo, H. Zhang, Y. Wang, Langmuir 2012, 28, 1439.
| Crossref | GoogleScholarGoogle Scholar | 22149176PubMed |
[12] W. Chen, K. Tian, X. Song, Z. Zhang, K. Ye, G. Yu, Y. Wang, Org. Lett. 2015, 17, 6146.
| Crossref | GoogleScholarGoogle Scholar | 26605431PubMed |
[13] D. T. Mowry, J. Am. Chem. Soc. 1945, 67, 1050.
| Crossref | GoogleScholarGoogle Scholar |
[14] J. Jia, Y. Li, W. Wang, C. Luo, L. Han, Y. Li, J. Gao, Dyes Pigm. 2017, 146, 251.
| Crossref | GoogleScholarGoogle Scholar |
[15] R. M. Fekry, H. A. El-Sayed, M. G. Assy, A. Shalby, A. S. Mohamed, Organic Chem. Curr. Res. 2016, 5, 171.
| Crossref | GoogleScholarGoogle Scholar |
[16] Y. Zhu, L. Yi, M. Q. Yao, X. Li, Org. Chem. Front. 2016, 3, 709.
| Crossref | GoogleScholarGoogle Scholar |
[17] V. Kundi, P. P. Thankachan, J. Phys. Chem. A 2016, 120, 2757.
| Crossref | GoogleScholarGoogle Scholar | 27054876PubMed |
[18] A. M. Asiri, Appl. Organomet. Chem. 2001, 15, 907.
| Crossref | GoogleScholarGoogle Scholar |
[19] F. Schwartz, L. F. Worrell, J. N. Delgado, J. Pharm. Sci. 1967, 56, 80.
| Crossref | GoogleScholarGoogle Scholar |
[20] A. Bello, L. Cheng, J. Griffiths, J. Chem. Soc., Perkin Trans. 2 1987, 6, 815.
| Crossref | GoogleScholarGoogle Scholar |
[21] J. B. Dorsch, S. M. McElvain, J. Am. Chem. Soc. 1932, 54, 2960.
| Crossref | GoogleScholarGoogle Scholar |
[22] G. M. Sheldrick, Acta Crystallogr. 2008, A64, 112.
| Crossref | GoogleScholarGoogle Scholar |
[23] T. M. McPhillips, S. E. McPhillips, H. J. Chiu, A. E. Cohen, A. M. Deacon, P. J. Ellis, E. Garman, A. Gonzalez, N. K. Sauter, R. P. Phizackerley, S. M. Soltis, P. Kuhn, J. Synchrotron Radiat. 2002, 9, 401.
| Crossref | GoogleScholarGoogle Scholar | 12409628PubMed |
[24] W. Kabsch, J. Appl. Cryst. 1993, 26, 795.
| Crossref | GoogleScholarGoogle Scholar |
[25] L. J. Barbour, J. Supramol. Chem. 2001, 1, 189.
| Crossref | GoogleScholarGoogle Scholar |



