A review of environmental contamination and potential health impacts on aquatic life from the active chemicals in sunscreen formulations
Nial J. Wheate A *
A *
A Sydney Pharmacy School, Faculty of Medicine and Health, The University of Sydney, NSW 2006, Australia.

Nial Wheate completed a BSc (Hons I) and PhD in chemistry at the University of New South Wales while serving as an officer in the Royal Australian Navy. In 2005, he was appointed a Senior Fellow at the School of Biomedical and Health Sciences at Western Sydney University, and then in 2007 a Lectureship at the University of Strathclyde’s Institute of Pharmacy and Biomedical Sciences. In 2012 he was appointed a Senior Lecturer, and in 2018 was promoted to Associate Professor, in The University of Sydney (USYD) School of Pharmacy. In 2020 he was awarded a DSc and in 2021 an MBA, both from USYD. |
Australian Journal of Chemistry 75(4) 241-248 https://doi.org/10.1071/CH21236
Submitted: 15 September 2021 Accepted: 17 January 2022 Published: 15 March 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
The active chemicals in sunscreen formulations are released into the environment from human skin, and found in recreational-use waters like seawater, but can also be washed into fresh water from bathing and showering. The level of sunscreen chemicals found in samples varies considerably between regions, time of year (higher in summer months), and time of day. Average typical concentrations are only in the nanograms per litre (ng L−1) range in marine and fresh water systems, and typically, the highest levels are in waste-water sludge because of a concentrating effect during the treatment process. From numerous studies, it is known that the active chemicals in sunscreens can have potential hormonal/oestrogenic activity and non-hormonal effects, including: acting as teratogens, altering gene regulation, inducing changes in antioxidant and free radical production, and inducing coral bleaching. However, the effects of sunscreens on aquatic life under laboratory conditions typically occur only at concentrations (µg or mg L−1) that far exceed (10–10 000-fold) levels found in the environment. As such, when damage does occur to reefs and animal life, there are often other causes that are more likely impacting the aquatic life including changes in water temperature, water turbidity, elevated nutrient levels, and the presence of pesticides and medicines used for human and animal health.
Keywords: aquatic, contamination, coral, damage, environment, ingredients, ocean, sunscreen.
Introduction
Sunscreens are topical pharmaceutical and cosmetic formulations designed to prevent damage to human skin either through the scattering or the absorption of UV light.[1] Within the formulations, the products contain a range of different chemicals including oils, surfactants, fragrances, antimicrobial preservatives and the active pharmaceutical ingredients (APIs). All sunscreens contain one of two types of APIs, either metal oxides (zinc oxide/titanium oxide) or organic UV filtering chemicals.[2,3] Examples of some organic APIs are shown in Fig. 1.
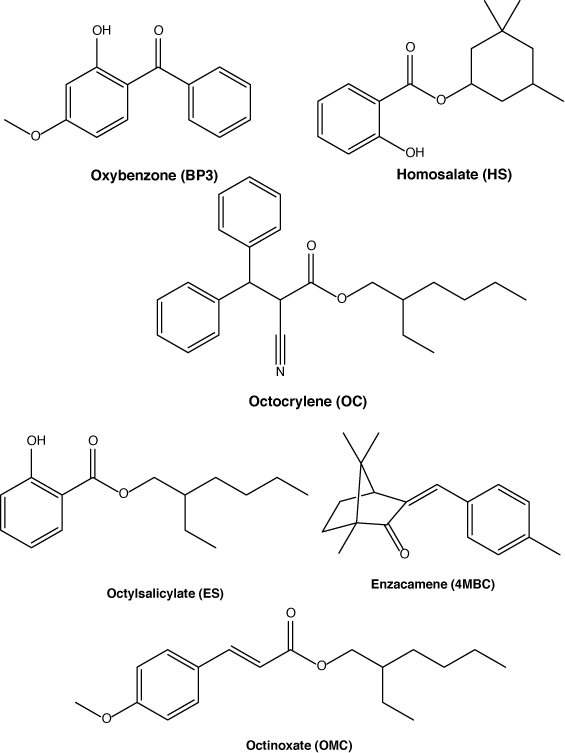
|
As with all consumer cosmetics and personal care products, the use of sunscreens is not risk-free to either humans or the environment. The side effects to humans are expected to be rare, at a rate of less than 1 in 1000;[4] the most common of these include: rashes, skin irritation and photo-allergic contact dermatitis. While some limited research has shown that specific sunscreen ingredients may have endocrine-disrupting effects,[5] government agencies in various nations, including the Therapeutic Goods Administration (TGA) in Australia, have declared them safe for human use.[6]
There is some concern about the human health effects of nanoparticle-containing sunscreens.[7] Specifically, the concerns centre on formulations that contain nanoparticles of titanium oxide or zinc oxide, which researchers have hypothesised are better able to penetrate the skin of humans compared with normal sunscreen formulations, thereby leading to higher levels of the chemicals in the blood stream and organs. These concerns have been demonstrated to be unfounded in numerous studies, including 2018 research that has shown that zinc oxide nanoparticles had no penetration through human skin, or any signs of toxicity.[8]
In addition to the perceived human health threats of sunscreen ingredients, over the last two decades, many researchers have investigated a link between the APIs of different sunscreen formulations and their toxicity to aquatic life. Because they can be found in different environments around the world, it is now common for many research studies to refer to sunscreen ingredients as ‘emerging contaminants’.[9] That APIs from sunscreens can contaminate the environment is not disputed.
While several governments and non-governmental organisations (NGOs) are advocating for a ban on specific sunscreen ingredients, it is unclear how much the evidence supports these positions. Evaluating the evidence is important as further bans on specific products in different regions of the world, including Australia and its states and territories, could have a potentially large negative effect on sunscreen use and leave consumers vulnerable to sun damage (e.g. cancer and the visible signs of aging).
This review evaluates the current evidence regarding the concentrations of the chemicals in various aquatic environments, the ability of the active chemicals in sunscreen formulations to damage aquatic life, and the evidence that their presence is unequivocally affecting aquatic life.
Evidence of sunscreen ingredients in the environment
In order for the APIs in sunscreen formulations to pose a risk to aquatic life, the chemicals must be present in a form that is bioavailable to plants and animals, they must be present in sufficient quantities to have an effect, and they need to persist long enough in the environment to have the effect.
To be functional, the APIs in sunscreens are dependent on their absorption onto human skin and the need to not be washed away easily on contact with water. As such, most sunscreen APIs have a high log octanol–water partition (logP) coefficient, with typical values of between 4 and 8, which means the chemicals have a preference for skin rather than dissolving in water.[9,10] While this means it is unlikely that sunscreen APIs can dissolve at millimolar or higher concentrations in water, the same lipophilic characteristics of the APIs also make it possible for them to associate with, and be absorbed by, lipophilic materials in the environment (e.g. sediments and plant solids).
The ability of sunscreen APIs to persist in the environment is variable across the range of chemicals used owing to differences in their degradation rates by sunlight and metabolism by bacteria. In a laboratory study, 2-ethylhexyl 4-(dimethylamino)benzoate (EH-DPAB) was found to be rapidly degraded, with near 90% metabolism by bacteria in a period of 3 months. In contrast, 4-methyl benzylidene camphor (4MBC) was shown in the same study to be very slowly degraded and was still detectable in samples after a period of 16 months.[11]
Sources of contamination
There are numerous pathways by which sunscreen APIs can enter aquatic environments. Recreational water activity, through direct swimming and using beach-side showering facilities, is a clear source of contamination.[12] A number of studies have shown that the detection of sunscreen ingredients in water samples is highest at locations where beach use is high, and when tested in the same location over extended periods of time, levels are higher during summer when compared with winter.[13]
A large source of sunscreen contamination in both fresh water and marine environments is from sewage wastewater.[14] The chemicals can find their way into the sewerage system after the application of sunscreens as a part of everyday household and business activity, and then showering or bathing.[10] In these instances, the use of soaps and body washes can greatly assist in the removal of the sunscreen ingredients off the skin and into the wastewater.
Contamination of sewage with not just sunscreen APIs, but also many other chemicals found in personal care products, is a problem in wastewater treatment.[15] There are no methods that can reliably remove sunscreen ingredients from water. In a 2015 study of oxybenzone/benzophenone-3 (BP3), its contamination level was the same before and after treatment by coagulation, flocculation, sedimentation, filtration, chlorination and fluoridation.[16] While new methods of treatment are being developed, such as ozone, none have been found to be useful and cost-effective.[17]
Sunscreen APIs have been found to be concentrated in sewage sludge, which is then either disposed of in landfill, or because of its nutrient-rich content, used as agricultural fertiliser.[10] In either case, the sunscreen ingredients can re-enter various surface and ground water environments through water run-off and landfill leachate.[18]
Ultra-violet filtering chemicals that are not used in sunscreens can also be considered a source of contamination. Many consumer products and commodities contain UV filters to protect against bleaching and loss of colour.[9] These products can include plastics, clothing, varnishes and paints for outdoor applications, and outdoor furniture, which leach or volatilise the chemicals.[19] The level of UV filters in the environment from consumer products is an under-studied area, and given the size and quantity of these goods in developed countries, could reflect a source of contamination that is magnitudes greater than any potential risk posed by personal care sunscreen products.
Levels of contamination
The results of numerous studies over the last two decades unconditionally show that sunscreen ingredients can be found in a variety of environments, including: marine and fresh water, soil, sediments, sludge and biota (Table 1).[20]

|
The ability to determine the quantity of sunscreen ingredients in water, sediments, plants and animals is highly dependent on the analysis method used. In sample analysis, the limit of detection (LOD) is defined as a lowest level at which the presence of a chemical can be detected in a sample but its concentration cannot be determined. The limit of quantification (LOQ) is defined as the lowest concentration in a sample at which its presence can be confirmed, and its concentration determined to within a certain limit of accuracy and precision. Values that fall between the LOD and LOQ can give what appears to be an accurate concentration, but the statistical confidence of that value being greater than the LOD is poor, which thus potentially classifies the estimated concentration as a false positive.
For analysis, samples are typically isolated and quantified using a variety of chromatographic techniques (liquid chromatography (LC), gas chromatography (GC), or ultra‐performance liquid chromatography/high‐performance liquid chromatography (UPLC/HPLC) coupled with an appropriate detector (tandem mass spectrometry (MS/MS), high-resolution mass schpectrometry (HRMS), or diode array detection). Columns for LC/HPLC separation are usually C-18, but some other columns (e.g. DB-5) have been used. With these methods, the LOD can be as low as 0.9 ng L−1 depending on the sunscreen ingredient being studied. Similarly, the LOQ for most techniques is as low as 2.9 ng L−1.[18,21,22] The newest analytical techniques support experiments with an LOD as low as 0.6 ng L−1 and an LOQ as low as 2.1 ng L−1.[23] The quantities of sunscreen ingredients found in plants and animals are typically given in units of nanograms of chemical per gram of wet or dried material (ng g−1).
Not all sunscreen ingredients are found in the environment, and of those that have been shown to cause contamination, they do so to differing levels. Contamination levels tend to be highest at coastal locations used for recreational water activity, and there is considerable seasonal variation, with peaks in the summer months and troughs in the winter months.[13] Variations in concentrations in different bodies of water also exist. For example, in a review study of BP3 in different environments, levels of the chemical were found to reach only 125 ng L−1 in freshwater samples but 578 ng L−1 in sea water.[20]
In a specific study that examined the contamination of sea water collected from various Spanish beaches, the eight different sunscreen active ingredients of interest (homosalate, HS; isoamyl 4-methoxycinnamate/amiloxate, IMC; enzacamene, 4MBC; BP3; octyl-methoxycinnamate/octinoxate, OMC; ODPABA/2-ethylhexyl 4-(dimethylamino)benzoate, EDP; octocrylene, OC; and octyl salicylate/2-ethylhexyl salicylate, ES) were found in concentrations between 0 and 880 ng L−1 depending on the location.[22] In another study, researchers undertook sampling of water at six locations along the coast of South Carolina in the USA to look for possible contamination with seven different sunscreen APIs. This included a well-populated beach, a coast guard station, a fishing pier, drainage pipes that feed into the ocean, a park and an inlet to a river. The researchers only found five of the seven chemicals, including the ingredients BP3, butyl-methyloxydibenzylmethane/avobenzone (B-MBM), OC, OMC and ODPABA at average concentrations between 9 and 260 ng L−1.[13] They were unable to find contamination by dioxybenzone or sulisobenzone. Both studies are typical of the results found by other researchers with sunscreen ingredients in water samples where concentrations were, on average, at the nanograms per litre level.
In rare instances, sunscreen ingredients have been found in micrograms per litre or micrograms per gram−1 (µg L−1, µg g−1) or milligrams per litre gram−1 (mg L−1, mg g−1) concentrations. Typically, but not always,[24] these levels are found in concentrated wastewaters, which are not used for recreational water activities, nor in which aquatic life is expected to live. For example, a review article that focussed on research of wastewater treatment plants concluded that BP3 and BP4 are most likely to be found in milligram per litre concentrations in treatment plant wastewater because they are the most hydrophilic. In contrast, EHT, 4-MBC and OC were more likely to be found in microgram per gram concentrations in waste sludge due to their lipophilicity.[10]
However, not all research studies have found evidence of sunscreen contamination. In a study that examined sunscreen levels of marine and inland waters of the Baltic, Black and Mediterranean seas, only 2-phenylbenzimidazole-5-sulfonic acid (PBSA) and benzophenone-4 (BP4) were found. The researchers were unable to detect any contamination with benzophenone-2 (BP2), BP3, BP4, 4-MBC, EHMC, OC, OD-PABA, ethyl-4-aminobenzoate/benzocaine (Et-PABA), or 4-DMB.[18] In another study that examined wastewater treatment in various cities in south-eastern Brazil over a period of 6–12 months, the investigators were able to detect BP3, ES, OMC and OC in the samples, with only OMC and BP3 at or above the LOQ.[16]
Potential for sunscreen ingredients to cause environmental damage
Sunscreen ingredients have been found in a variety of animals, including crabs, prawns and fish,[25] sea urchins, conches, clams and mussels,[21] birds[26] and dolphins.[27] In one study that examined the levels of eight different sunscreen ingredients in fish and prawns, it was found that codfish liver samples contained at least one sunscreen API at detectable levels, with 80% of cod fishlivers containing OC, and 50% of codfish livers and whole prawns containing BP3.[25] In whitefish, OMC was found at a concentration of 200 ng g−1 and at 12 µg g−1 for OC.[25] Not surprisingly, some studies have found higher levels of sunscreen APIs in filter-feeding animals, like mussels, when compared with fish from the same area.[21]
Sunscreen APIs have been shown to have a physiological effects on a range of aquatic animals, including: coral, mussels,[28] sea urchins,[29] small crustacean,[29] fish,[30,31] worms,[32] flies,[33] tadpoles,[34] snails[35] and zebrafish (both embryos and adults).[36] There have been no substantial studies on the effect of sunscreen ingredients on plants, with the exception of algae.[29] Other organisms that have been studied, and shown under laboratory conditions to be affected by sunscreen ingredients include: bacteria, diatoms, dinoflagellates and phytoplankton.[35]
Not all sunscreen ingredients have been studied extensively, and so it is dangerous to make broad generalisations about the APIs in sunscreen formulations and their ability to cause damage to aquatic life. Most scientific studies focus on just four specific chemicals: OC, 4MBC, OMC, and the benzophenones (i.e. BP3 and BP4). Benzophenone-based chemicals have been by far the most studied APIs and are the focus of just under half of all studies. The following active APIs have also been studied, but to a much lesser extent, and some are the focus of just a single research article: 3-benzylidene camphor (3BC), Et-PABA, 2-phenylbenzimidazole-5-sulfonic acid (PBSA), Ethylhexyl Salicylate (EHS), HS, and propyl paraben (PP).
A variety of different chemical sunscreen ingredients can cause hormonal effects, oestrogenic activity and endocrine disruption, which can manifest in a range of ways. With regard to offspring, they can reduce the amount of sperm and eggs produced by fathead minnows and Japanese rice fish,[20,30,37] they can affect the development of gonads in zebra fish,[38] they can reduce the number of successfully hatched rainbow trout eggs,[39] and they can affect embryo-larval development of sea urchins.[40]
Zebrafish feature in a large proportion of toxicology studies owing to their wide acceptance as a model organism for general toxicological studies.[41] Zebrafish share some genetic, cellular, anatomical and physiological similarities with mammals, and so are now routinely used to study the causes and treatments of human diseases, and to examine the effects chemicals may have on normal vertebrate embryonic development.[41] They are widely used because they are easy to grow and cheap, and when in the embryonic phase, have transparent bodies that allow direct visual examination of their developing organs. However, they are not perfect models and toxicological effects cannot always be directly translated to other animals.
It is important to note hormonal/endocrine-disrupting effects have also been reported in laboratory studies on human cells, and all major governmental regulatory bodies, such as the TGA, list the ingredients as safe for human use.[6]
Sunscreen APIs can also affect gene expression and transcription, especially in the brain, liver and gonads.[37] A study where zebrafish were exposed to OC found up- and down-regulation of genes that affected several processes related to embryo development including: organ and blood vessel development, fat cell differentiation and metabolism.[36] In another study, 4MBC, OMC and PP were found to increase mRNA expression in the liver and increase the quantity of oestrogen receptors.[31]
The sunscreen ingredient 4MBC has been found to induce neurotoxicity during zebrafish development. The toxicity manifested as an abnormal curvature of the fish and impaired their mobility. These effects were attributed to a disorganised pattern of slow muscle fibres and reduced acetylcholinesterase activity. From the study, the authors concluded that 4MBC is a potential teratogen that can affect muscular and neuronal development.[42]
As well as affecting whole organs, some sunscreen APIs have been shown to cause direct damage to individual cells. The chemical BP3 was shown in zebrafish to generate free radicals and induce changes in antioxidant levels, including the enzymes catalase, superoxide dismutase and glutathione peroxidase which are involved in cellular redox balance and preventing oxidative stress to the cell.[43]
Of most interest is the potential ability of sunscreen APIs to cause coral bleaching. When sunscreens are present in water, it has been found that they contribute to an increase in the viral load in the water and control bacterial abundance, which affects the virus-to-bacteria ratio. The mechanism by which it is hypothesised they do this is through prophage induction in marine bacterioplankton.[44] The effect is that the viruses infect the zooxanthellae on which the corals depend. When infected, the lytic cycle is induced in the zooxanthellae and as the zooxanthellae die, coral bleaching advances across the reef, which kills them off.[45] Without the zooxanthellae, the corals die and lose their colour.
Sunscreen APIs can also have a photo-toxicant effect on the larval form of coral; their damage is exacerbated by light. When BP3 was tested on coral larvae, the effect was to transform the planulae to a deformed, sessile condition.[24]
Despite all these reported potential adverse effects, not all sunscreen APIs affect all animals. As an example, a 2004 study that examined very high concentrations (1–50 g L−1) of 4MBC on the development of tadpoles found the chemical had no effect at all on their thyroid system, body size, tail length, the metamorphosis process or the sex ratio of offspring.[34]
Concentration dependence of toxicity
While there is compelling evidence that a variety of chemicals in sunscreen formulations can cause damage to aquatic life, this needs to be placed in the context of how the experiments were conducted and the concentrations of the chemicals used.
Except for a few studies, all animal testing was conducted in a laboratory environment. Experiments conducted under these conditions allow the researchers to change one factor (chemical concentration) without the likely influence of other factors such as temperature, water flow, changing concentrations over time, rainfall and predation. While these controlled experiments also allow cross-study comparisons and are critical in developing exposure models, identifying pathways and calculating environmental thresholds, guidelines and criteria concentrations, they are not a perfect model for natural habitats and how animals are exposed to pollutants. For example, it was common in many of the studies to expose the animals to fixed concentrations of sunscreen APIs for long periods of time, up to 60 days.[38] This does not reflect the natural cycling of sunscreen concentrations found in a Spanish 2013 study, in which ingredient concentrations were found to peak during the early to mid-afternoon and go down through the night as recreational water activity tapered off.[46]
The concentration of ingredients being tested was also routinely far above the concentrations found in the natural environment. Typically, the concentrations needed to have an effect on animals are in the microgram per litre to milligram per litre ranges,[9] which are 10–10 000-fold higher than those found on average in the water contamination studies. In a 2015 study examining the effects of BP3 on the sexual development of zebrafish, the animals were exposed to the chemical at a concentration of up to 500 µg L−1.[38] The results indicated that BP3 had a no observed effect concentration (NEOC) of 191 µg L−1 and a lowest observed effect concentration (LEOC) of 388 µg L−1.[38]
In other studies, the LEOC to induce vitellogenin in fish was 3 µg L−1 for benzophenone-2 (BP2), but 1200 µg L−1 for 3BC.[47] In studies with algae, mussels, sea urchins and small crustaceans, the LEOCs were between 30 and 3840 µg L−1 and NEOCs between 15 and 1920 µg L−1.[29] And finally, in studies of the effects on sea snails, the LEOC for OMC was found to be between 0.4 and 10 mg kg−1, with B-MDM and OC showing no negative effects at each the concentrations studied.[35] It should be noted that toxicity thresholds such as a NOEC and LOEC are influenced by the treatment levels tested. Other continuous estimates for toxicity thresholds are via ROBIT tests where effects levels can be estimated at any proportion of population effect (i.e. LC10; LC50; LC90; which are defined as the concentrations at which 10, 50 and 90% of organisms are killed).
Overall, the evidence indicates that sunscreen APIs do have the potential to cause damage to aquatic life, but only at concentrations that are far higher than those found, on average, in the environment, a fact that is acknowledged by many researchers in the field.[39]
Confounding factors and other explanations for damage to aquatic life
Even though sunscreen APIs can be found in the environment, and they have been shown to have a potential damaging effect on a range of organisms and animals, the ingredients are typically found in concentrations well below their predicted NOEC. As such, the current evidence does not support the hypothesis that sunscreen ingredients are the primary cause of significant damage to aquatic life. So if sunscreens are not the most significant danger, then what are the potentially more important factors that may explain times when aquatic life has been damaged in the past? Taking coral bleaching as an example, on their public website, the Australian Museum provides five major factors that lead to damage of this type, namely warming or cooling of waters, excessive or low levels of light, elevated nutrient levels, excessive phosphorous, and shipping accidents;[48] sunscreens were not mentioned.
Likewise, the National Oceanic and Atmospheric Administration of the US Department of Commerce state on their public page that coral bleaching can be caused by changes in temperature (up or down), extreme low tides, overexposure to sunlight and runoff from land following storms effectively diluting the natural salinity of the ocean and in effect poisoning the coral.[49]
Finally, NGOs such as the Australian Marine Conservation Society also state publicly that carbon pollution (either as increased global temperatures or increased ocean acidity from dissolved carbon dioxide) is a cause of coral bleaching.[50,51]
Conclusions
There is clear evidence that sunscreen APIs are found in marine and water systems, and associated sediments and sludge. Reported concentrations of many active sunscreen chemicals are found, on average, to be in the parts per trillion (ng L−1) range in marine and freshwater, but are generally higher when they have accumulated in wastewater, which can then be in the micrograms or milligrams per litre concentration range. While aquatic life in marine ecosystems is likely to be exposed to sunscreen pollution at very low levels, other threats may potentially pose a much greater risk.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
The author is a member of the Standards Australia panel on Sunscreen Agents.
Declaration of funding
This research did not receive any specific funding.
References
[1] MS Latha, J Martis, V Shobha, R Sham Shinde, S Bangera, B Krishnankutty, S Bellary, S Varughese, P Rao, BR Naveen Kumar, J Clin Aesthet Dermatol 2013, 6, 16.| 23320122PubMed |
[2] T Smijs, S Pavel, Nanotechnol Sci Appl 2011, 4, 95.
| Crossref | GoogleScholarGoogle Scholar | 24198489PubMed |
[3] LTN Ngoc, VV Tran, J-Y Moon, M Chae, D Park, Y-C Lee, Cosmetics 2019, 6, 64.
| Crossref | GoogleScholarGoogle Scholar |
[4] Australian Medicines Handbook. Adelaide: Pharmaceutical Society of Australia; 2017.
[5] M Krause, A Klit, M Blomberg Jensen, T Søeborg, H Frederiksen, M Schlumpf, W Lichtensteiger, NE Skakkebaek, KT Drzewiecki, Int J Androl 2012, 35, 424.
| Crossref | GoogleScholarGoogle Scholar | 22612478PubMed |
[6] T. G. Administration, Australian regulatory guidelines for sunscreens. Therapeutic Goods Administration; 2016.
[7] PFA Wright, Med J Aust 2016, 204, 369.
| Crossref | GoogleScholarGoogle Scholar |
[8] YH Mohammed, A Holmes, IN Haridass, WY Sanchez, H Studier, JE Grice, HAE Benson, MS Roberts, J Invest Dermatol 2019, 139, 308.
| Crossref | GoogleScholarGoogle Scholar | 30448212PubMed |
[9] S Rainieri, A Barranco, M Primec, T Langerholc, Food Chem Toxicol 2017, 104, 57.
| Crossref | GoogleScholarGoogle Scholar | 27847220PubMed |
[10] S Ramos, V Homem, A Alves, L Santos, Environ Int 2016, 86, 24.
| Crossref | GoogleScholarGoogle Scholar | 26479831PubMed |
[11] A Volpe, M Pagano, G Mascolo, P Grenni, S Rossetti, Sci Total Environ 2017, 575, 448.
| Crossref | GoogleScholarGoogle Scholar | 27750141PubMed |
[12] D Sánchez-Quiles, A Tovar-Sánchez, Environ Int 2015, 83, 158.
| Crossref | GoogleScholarGoogle Scholar | 26142925PubMed |
[13] S Bratkovics, E Wirth, Y Sapozhnikova, P Pennington, D Sanger, Mar Pollut Bull 2015, 101, 370.
| Crossref | GoogleScholarGoogle Scholar | 26541983PubMed |
[14] S Ramos, V Homem, A Alves, L Santos, Environ Int 2016, 86, 24.
| Crossref | GoogleScholarGoogle Scholar | 26479831PubMed |
[15] ZR Hopkins, L Blaney, Environ Int 2016, 92–93, 301.
| Crossref | GoogleScholarGoogle Scholar | 27128715PubMed |
[16] CP da Silva, ES Emídio, MRR de Marchi, Environ Sci Pollut Res 2015, 22, 19706.
| Crossref | GoogleScholarGoogle Scholar |
[17] Y Guo, Q Lin, B Xu, F Qi, Environ Sci Pollut Res 2016, 23, 7962.
| Crossref | GoogleScholarGoogle Scholar |
[18] A Orlikowska, K Fisch, DE Schulz-Bull, Mar Pollut Bull 2015, 101, 860.
| Crossref | GoogleScholarGoogle Scholar | 26581813PubMed |
[19] C Plagellat, T Kupper, R Furrer, LF De alencastro, D Grandjean, J Tarradellas, Chemosphere 2006, 62, 915.
| Crossref | GoogleScholarGoogle Scholar | 15996716PubMed |
[20] S Kim, K Choi, Environ Int 2014, 70, 143.
| Crossref | GoogleScholarGoogle Scholar | 24934855PubMed |
[21] Z Sang, KS-Y Leung, Sci Total Environ 2016, 566–567, 489.
| Crossref | GoogleScholarGoogle Scholar | 27235899PubMed |
[22] JL Benedé, A Chisvert, A Salvador, D Sánchez-Quiles, A Tovar-Sánchez, Anal Chim Acta 2014, 812, 50.
| Crossref | GoogleScholarGoogle Scholar | 24491764PubMed |
[23] S Montesdeoca-Esponda, Z Sosa-Ferrera, JJ Santana-Rodríguez, Anal Bioanal Chem 2012, 403, 867.
| Crossref | GoogleScholarGoogle Scholar | 22411539PubMed |
[24] CA Downs, E Kramarsky-Winter, R Segal, J Fauth, S Knutson, O Bronstein, FR Ciner, R Jeger, Y Lichtenfeld, CM Woodley, P Pennington, K Cadenas, Arch Environ Contam Toxicol 2016, 70, 265.
| Crossref | GoogleScholarGoogle Scholar | 26487337PubMed |
[25] KH Langford, MJ Reid, E Fjeld, S Øxnevad, KV Thomas, Environ Int 2015, 80, 1.
| Crossref | GoogleScholarGoogle Scholar | 25827264PubMed |
[26] K Fent, A Zenker, M Rapp, Environ Pollut 2010, 158, 1817.
| Crossref | GoogleScholarGoogle Scholar | 20004505PubMed |
[27] P Gago-Ferrero, MB Alonso, CP Bertozzi, J Marigo, L Barbosa, M Cremer, ER Secchi, C Domit, A Azevedo, J Lailson-Brito Jr, JP Torres, O Malm, E Eljarrat, MS Díaz-Cruz, Environ Sci Technol 2013, 47, 5619.
| Crossref | GoogleScholarGoogle Scholar | 23627728PubMed |
[28] E Gomez, M Bachelot, C Boillot, D Munaron, S Chiron, C Casellas, H Fenet, Environ Sci Pollut Res 2012, 19, 2561.
| Crossref | GoogleScholarGoogle Scholar |
[29] E Paredes, S Perez, R Rodil, JB Quintana, R Beiras, Chemosphere 2014, 104, 44.
| Crossref | GoogleScholarGoogle Scholar | 24359924PubMed |
[30] CJ Weisbrod, PY Kunz, AK Zenker, K Fent, Toxicol Appl Pharmacol 2007, 225, 255.
| Crossref | GoogleScholarGoogle Scholar | 17889917PubMed |
[31] M Inui, T Adachi, S Takenaka, H Inui, M Nakazawa, M Ueda, H Watanabe, C Mori, T Iguchi, K Miyatake, Toxicology 2003, 194, 43.
| Crossref | GoogleScholarGoogle Scholar | 14636695PubMed |
[32] C Schmitt, M Oetken, O Dittberner, M Wagner, J Oehlmann, Environ Pollut 2008, 152, 322.
| Crossref | GoogleScholarGoogle Scholar | 17669564PubMed |
[33] I Ozáez, JL Martínez-Guitarte, G Morcillo, Environ Pollut 2014, 192, 19.
| Crossref | GoogleScholarGoogle Scholar | 24878782PubMed |
[34] PY Kunz, HF Galicia, K Fent, Mar Environ Res 2004, 58, 431.
| Crossref | GoogleScholarGoogle Scholar | 15178063PubMed |
[35] D Kaiser, A Sieratowicz, H Zielke, M Oetken, H Hollert, J Oehlmann, Environ Pollut 2012, 163, 84.
| Crossref | GoogleScholarGoogle Scholar | 22325435PubMed |
[36] N Blüthgen, N Meili, G Chew, A Odermatt, K Fent, Sci Total Environ 2014, 476–477, 207.
| Crossref | GoogleScholarGoogle Scholar | 24463256PubMed |
[37] V Christen, S Zucchi, K Fent, Aquat Toxicol 2011, 102, 167.
| Crossref | GoogleScholarGoogle Scholar | 21356179PubMed |
[38] KL Kinnberg, GI Petersen, M Albrekten, M Minghlani, SM Awad, BF Holbech, JW Green, P Bjerregaard, H Holbech, Environ Toxicol Chem 2015, 34, 2833.
| Crossref | GoogleScholarGoogle Scholar | 26118430PubMed |
[39] M Coronado, H De haro, X Deng, MA Rempel, R Lavado, D Schlenk, Aquat Toxicol 2008, 90, 182.
| Crossref | GoogleScholarGoogle Scholar | 18930325PubMed |
[40] C Corinaldesi, E Damiani, F Marcellini, C Falugi, L Tiano, F Brugè, R Danovaro, Sci Rep 2017, 7, 7811.
[41] RL Tanguay, Toxicol Sci 2018, 163, 3.
| Crossref | GoogleScholarGoogle Scholar | 29718442PubMed |
[42] VWT Li, MPM Tsui, X Chen, MNY Hui, L Jin, RHW Lam, RMK Yu, MB Murphy, J Cheng, PKS Lam, SH Cheng, Environ Sci Pollut Res 2016, 23, 8275.
| Crossref | GoogleScholarGoogle Scholar |
[43] G Rodríguez-Fuentes, JJ Sandoval-Gío, A Arroyo-Silva, E Noreña-Barroso, KS Escalante-Herrera, F Olvera-Espinosa, Ecotoxicol Environ Saf 2015, 115, 14.
| Crossref | GoogleScholarGoogle Scholar | 25666732PubMed |
[44] R Danovaro, C Corinaldesi, Microb Ecol 2003, 45, 109.
| Crossref | GoogleScholarGoogle Scholar | 12545312PubMed |
[45] R Danovaro, L Bongiorni, C Corinaldesi, D Giovannelli, E Damiani, P Astolfi, L Greci, A Pusceddu, Environ Health Perspect 2008, 116, 441.
| Crossref | GoogleScholarGoogle Scholar | 18414624PubMed |
[46] A Tovar-Sánchez, D Sánchez-Quiles, G Basterretxea, JL Benedé, A Chisvert, A Salvador, I Moreno-Garrido, J Blasco, PLoS One 2013, 8, e65451.
| Crossref | GoogleScholarGoogle Scholar | 23755233PubMed |
[47] K Fent, PY Kunz, E Gomez, Chimia (Aarau) 2008, 62, 368.
| Crossref | GoogleScholarGoogle Scholar |
[48] Pueschel M. Coral bleaching. Available at https://australianmuseum.net.au/coral-bleaching [Accessed 27 November 2018]
[49] National Oceanic and Atmospheric Administration. What is coral bleaching? Available at https://oceanservice.noaa.gov/facts/coral_bleach.html [Accessed 02 December 2018]
[50] Australian Marine Conservation Society. Coral bleaching. Available at https://www.marineconservation.org.au/coral‐bleaching/ [Accessed 02 December 2018]
[51] AE Douglas, Mar Pollut Bull 2003, 46, 385.
| Crossref | GoogleScholarGoogle Scholar | 12705909PubMed |


