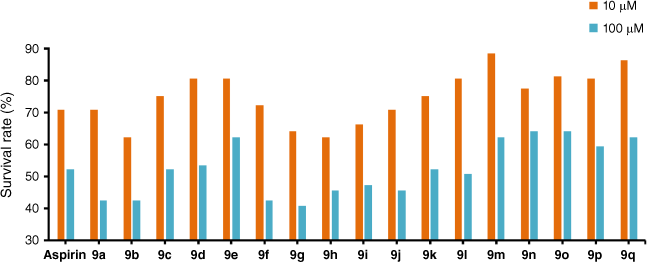Synthesis of 12-quinoline substituted andrographolide derivatives and their preliminary evaluation as anti-aggregation drugs
Xue Li A * , Jiafeng Yu A , Xianhao Wu A , Cui Hu A and Xiaoqing Wang A
A * , Jiafeng Yu A , Xianhao Wu A , Cui Hu A and Xiaoqing Wang A
A Drug Research Center, Jiangxi Provincial Institute of Traditional Chinese Medicine, Nanchang 330046, PR China.
Australian Journal of Chemistry 76(2) 100-114 https://doi.org/10.1071/CH22248
Submitted: 28 November 2022 Accepted: 6 February 2023 Published: 28 February 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC)
Abstract
Based on the structure of the natural product andrographolide, a series of novel 12-quinoline substituted derivatives 9 were designed and synthesized. In preliminary biological evaluation, these synthesized compounds showed prominent anti-platelet aggregation activities in response to thrombin and adenosine diphosphate (ADP) agonists. Among them, compound 9o (inhibition rate 55.73%, IC50 0.36 µM/L) had the highest anti-platelet aggregation activity induced by ADP. Compound 9q (inhibition rate 54.31%, IC50 0.30 µM/L) showed the highest anti-platelet aggregation activity induced by thrombin. Most of the derivatives had no significant cytotoxicity. Our research results provide a novel candidate drug structure for anti-platelet aggregation and enrich the scope of application of andrographolide derivatives.
Keywords: 12-quinoline substituted andrographolide derivatives, ADP, andrographolide, anti-platelet aggregation, design, Inhibition rate, synthesis, Thrombin.
Introduction
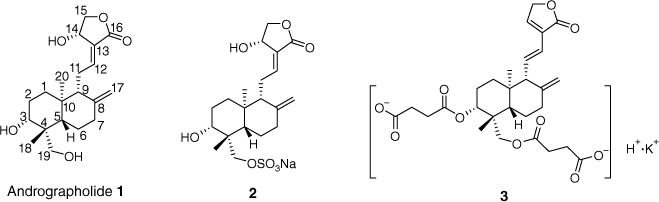
Natural products are endowed with various interesting pharmacophores and show various pharmacological activities.[1–3] Therefore, natural products are the source of many lead compounds in drug development. Andrographolide 1 (Fig. 1), a natural product from the aerial parts of Andrographis paniculati Nees with up to 2.0%,[4] shows rich biological activity such as anti-influenza virus,[5] anti-hepatotoxic,[6] anti-HIV,[7] anti-cancer,[8] anti-inflammatory[9] and anti-hyperglycaemic activities.[10] It is worth mentioning that, in our previous work, we reported firstly that andrographolide showed low anti-platelet aggregation activity due to its poor aqueous solubility. Owing to its high aqueous solubility, its sulfonate 2 (medicinal composition of Xiyanping) and succinate 3 (medicinal composition of Chuanhuning) (Fig. 1) had better anti-platelet aggregation activity, when adenosine diphosphate (ADP) was employed as inducer.[11–13] Quinoline derivatives also exhibit a wide spectrum of biological activities such as anti-malarial,[14] anti-bacterial,[15] anti-cancer,[16] anti-oxidant,[17] anti-tuberculous,[18] anti-parasitic,[19] and anti-platelet aggregation (methyl liensinine, rhynchophylline, berberine) activities.[20–22] Both andrographolide and quinoline are readily available pharmacophores and have been subjects in the search for new biologically active compounds. At the same time, there are practically no literature reports on anti-thrombotic or anti-platelet aggregation properties of hybrid derivatives with a combination of andrographolide and quinoline structures.
Thrombotic disease is a common and typical disease of cardiovascular disease, which seriously threatens human health and life quality.[23] Inhibition of platelet aggregation is one of the most direct and effective strategies for the treatment of thrombosis.[24–27] At present, there are quite a few kinds of anti-platelet aggregation drugs such as thromboxane A2 (TXA2) inhibitors (aspirin),[28] ADP induction inhibitors (clopidogrel),[29] phosphodiesterase (PDE) inhibitors (cilostazol),[30] 5-hydroxytryptamine receptor antagonists (sarpogrelate),[31] Ca2+ channel antagonists (flunarizine),[32–34] platelet membrane glycoproteins (GP) Ⅱb/Ⅲa receptor inhibitors (tirofiban),[35] and the protease active receptor-1 (PAR-1) antagonist vorapaxar sulfate.[36,37] However, these drugs present many drawbacks in clinical treatment to some extent, such as excessive bleeding, allergic reactions, nausea, dyspnea, neutropenia, aplastic anaemia and thrombocytopenia.[38–43] Therefore, it has been a research focus to develop new skeleton compounds for inhibition of platelet aggregation in recent years.
The purpose of this work was to develop accessible and effective methods to construct compounds with novel high activity and no obvious cell toxicity platelet aggregation from andrographolide and quinolines.
Results and discussion
Synthesis
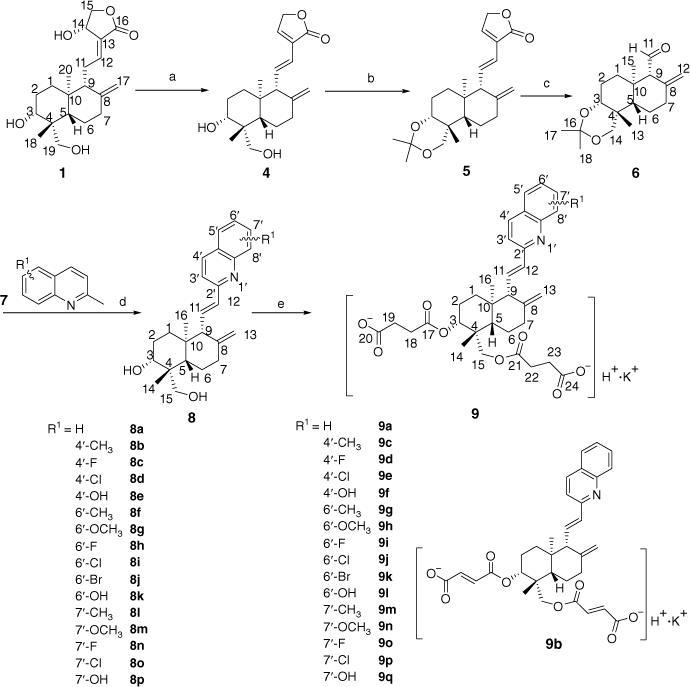
We successfully designed the synthetic route for the new skeleton compounds 9 with a combination of andrographolide and quinolines, shown in Scheme 1. Initially, andrographolide 1 underwent dehydration with activated aluminium oxide (neutral) to transform into dehydroandrographolide 4.[44] Afterwards, the 3,19-OH groups of 4 were protected as isopropylidenes with 2,2-dimethoxypropane with pyridinium p-toluenesulfonate (PPTS) to yield 5.[45] Compound 5 was degraded to key intermediate 6 by selective oxidation of the C-12,13 olefin bond with KMnO4.[46] Compounds 8 were synthesized from key intermediate 6 by reacting with 2-methylquinolines 7 in the presence of Fe(OAc)2/trifluoroacetic acid (TFA) and deprotection with AcOH.[47] To increase water solubility, two molecules of carboxylate were introduced into intermediates 8 to prepare a series of target derivatives 9 (12-quinoline substituted andrographolide derivatives).
Biological properties
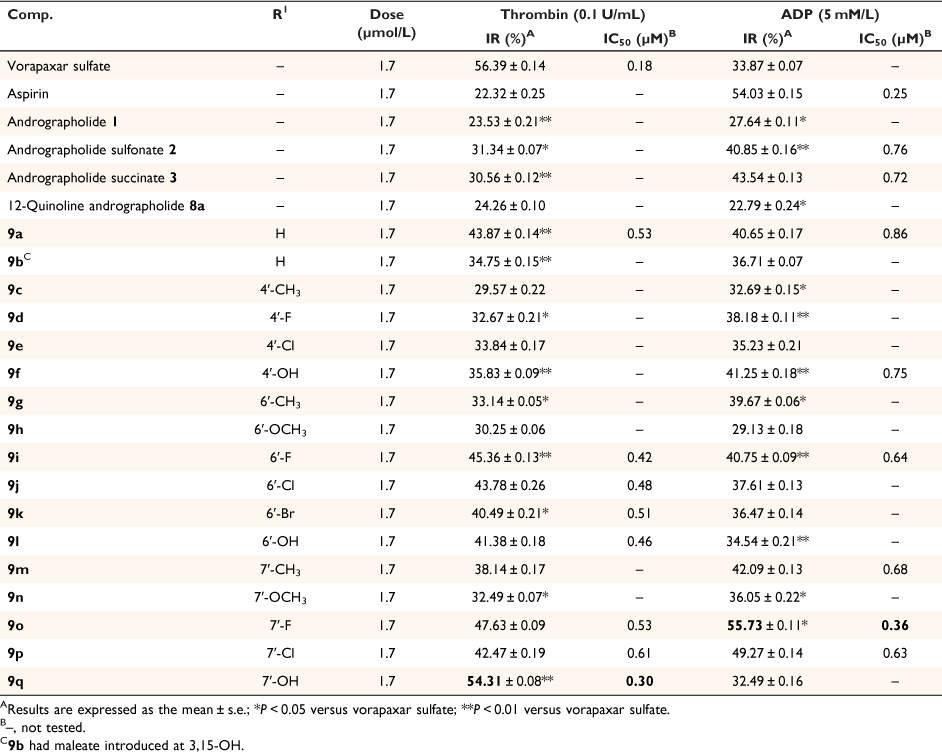
In preliminary screening, the 12-quinoline substituted andrographolide derivatives showed pronounced anti-platelet aggregation activities in vitro by turbidimetric test.[48] Thrombin and ADP were used as inducers for platelet aggregation. Vorapaxar sulfate and aspirin were selected as positive controls. The inhibition rate (IR) and IC50 of compounds 9 in vitro were calculated and are summarized in Table 1.
|
As shown in Table 1, compared with the positive control drugs vorapaxar sulfate and aspirin, both andrographolide (IR 23.53% for thrombin; IR 27.64% for ADP) and compound 8a (IR 24.26% for thrombin; IR 22.79% for ADP) displayed low activities. However, compared with andrographolide and compound 8a, compounds 2, 3, 9a and 9b, which contain sulfonate, succinate or maleate, exhibited better activities. The inhibition rate increased by 10–20%. This revealed that enhancing water solubility is beneficial to improving activity. Comparing compound 2 with 3, or comparing 9a with 9b, we could confirm that succinic acid salinization could be the most beneficial to increase the activity of the derivatives. Therefore, in our current work, the derivatives were all succinated at 3,19-OH, shown in Scheme 1.
For thrombin as inducer, the IR of 4′-substituted quinoline derivates (9c–9f) was significantly lower than the 6′-substituted (9g–9l) and 7′-substituted (9m–9q) quinoline derivates, as well as for ADP as inducer. Comparing 9g–9l with 9m–9q, the activities of latter were higher than the former. For 9m–9q, the order of inhibition rate for thrombin was 9q (7′-OH) > 9o (7′-F) > 9p (7′-Cl) > 9m (7′-CH3) > 9n (7′-OCH3). Anti-platelet aggregation activities were higher slightly when electron-withdrawing groups (F, Cl) and hydroxyl were introduced. Among these, 9o and 9q showed superior platelet aggregation activities induced by thrombin. Especially, 9q (IR 54.31%, IC50 0.30 μM/L) was equivalent to the positive control drug vorapaxar sulfate. The order of IR for ADP was 9o (7′-F) > 9p (7′-Cl) > 9m (7′-CH3) > 9n (7′-OCH3) > 9q (7′-OH). Also, 9o and 9p, which possess electron-withdrawing groups at 7′-C, showed superior platelet aggregation activities. Of these, 9o (IR 55.73%, IC50 0.36 μM/L) was equivalent to the positive control drug aspirin.
Cytotoxicity assay in vitro
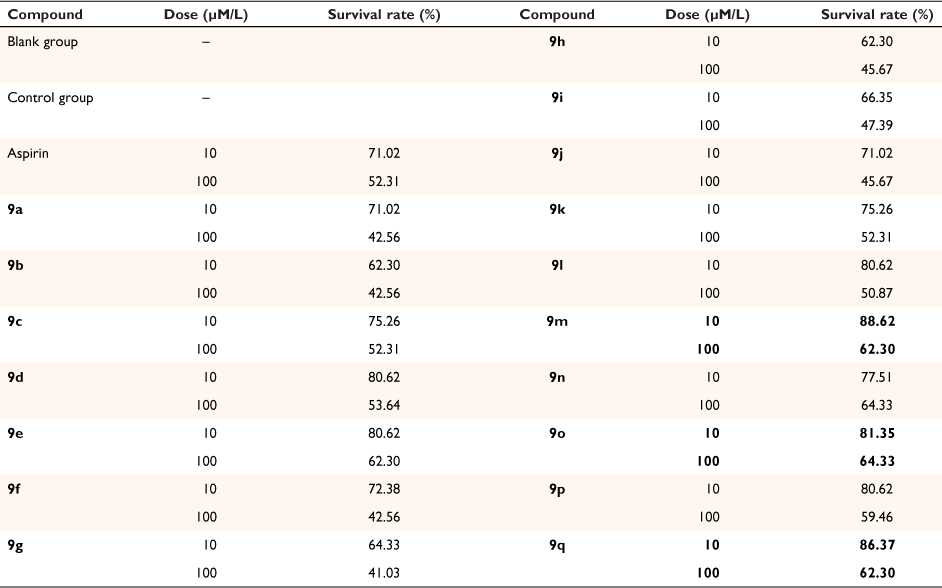
Mouse fibroblast cells (L929) were used to evaluate the cell toxicity of target derivatives 9. The relative survival rate is shown in Table 2 and Fig. 2. The results revealed that most compounds had no significant cytotoxicity. Among them, the survival rate of 9m, 9o and 9q was higher than of aspirin at a dose of 10 μM/L.
|
Conclusion
Through our efforts, based on the structure of the natural product andrographolide, a series of 12-quinoline substituted derivatives were designed and synthesized. In preliminary biological evaluation, these compounds showed strong anti-platelet aggregation activities in response to thrombin and ADP agonists and no significant cytotoxicity. Among them, compound 9o (IR 55.73%, IC50 0.36 µM/L) had the highest anti-platelet aggregation activity induced by ADP. Compound 9q (IR 54.31%, IC50 0.30 µM/L) showed the highest anti-platelet aggregation activity induced by thrombin. In addition, both 9o and 9q had low cell toxicities at doses of 10 and 100 µM/L. The most active compounds were selected to be prepared for further studies such as bleeding time and mechanisms of action.
Experimental
General chemistry experimental
Infrared spectra were recorded on a Nicolet Avatar-370 spectrometer in KBr (ν in cm−1). Melting points were measured on a Büchi B-540 capillary melting point apparatus and are uncorrected. Mass spectra (electrospray ionization mass spectrometry, ESI-MS) were obtained on a Thermo Finnigan LCQ-Advantage. High-resolution mass spectra (ESI-HRMS) were obtained on an Agilent 6210 time-of-flight (TOF) instrument. 1H NMR and 13C NMR spectra were recorded on a Varian Mercury Plus-400 spectrometer (400 and 100 MHz) (δ in parts per million, J in hertz), using TMS as internal standard. Platelet aggregation rates were measured on an LG-PABER platelet aggregation apparatus (Beijing Shidi Scientific Instrument Co. Ltd, Beijing). All commercially available reagents and solvents were of analytical reagent grade and were used directly without further purification.
Synthesis of derivatives
Compound 4: andrographolide 1 (100.00 g, 285.55 mmol) was dissolved in anhydrous pyridine (100 mL) and then activated alumina (neutral) (23.43 g, 228.42 mmol) was added. The mixture stirred at 100°C for ~12 h (reaction complete by TLC analysis). After cooling to room temperature, the reaction mixture was filtered and washed with CH2Cl2 (2 × 200 mL). The combined organic filtrate was concentrated under vacuum. The residue was purified by column chromatography (CH2Cl2/MeOH 20:1) and afforded product 4 dehydroandrographolide (69.82 g, 92.01%) as a white solid. Mp 203–204°C (lit.[49] 204–205°C). IR (KBr) νmax 3526, 3480, 2973, 2935, 2855, 1805, 1635, 1447, 1386, 1346, 1271, 1222, 1102, 1036, 974, 891 cm−1. 1H NMR (600 MHz, [D6]DMSO) δ 7.67 (1H, s, 14-H), 6.73 (1H, dd, J = 6.6 Hz, J = 15.8 Hz, 11-H), 6.13 (1H, d, J = 15.8 Hz, 12-H), 5.06 (1H, brs, 3-OH), 4.90 (2H, s, 15-H), 4.74 (1H, s, 17-Ha), 4.43 (1H, s, 17-Hb), 4.17 (1H, brs, 19-OH), 4.11 (1H, d, J = 11.8 Hz, 19-Ha), 3.54 (1H, d, J = 11.8 Hz, 19-Hb), 3.26–3.24 (1H, m, 3-H), 2.49 (1H, d, J = 6.6 Hz, 9-H), 2.05–1.22 (9H, m, 1,2,5,6,7-H), 1.10 (3H, s, 18-H), 0.87 (3H, s, 20-H). 13C NMR (100 MHz, [D6]DMSO) δ 173.2, 148.5, 138.8, 135.9, 131.5, 120.5, 110.1, 78.5, 71.0, 65.8, 61.2, 48.4, 43.2, 39.8, 36.8, 33.5, 28.3, 24.6, 19.6, 15.3 ppm.
Compound 5: dehydroandrographolide 4 (66.00 g, 198.68 mmol) was dissolved in a mixed solution of toluene and dimethyl sulfoxide (200 mL, 7:3) and then 2,2-dimethoxypropane (62.03 g, 595.85 mmol) and PPTS (cat.) (5.02 g, 20.05 mmol) were added. The mixture stirred at reflux for ~3–5 h (reaction complete by TLC analysis). After cooling to room temperature, water (200 mL) was added and the organic phase separated. Then, the aqueous phase was extracted with ethyl acetate (3 × 300 mL). The combined organic extract was washed with brine (3 × 200 mL) and dried over anhydrous Na2SO4, filtered and concentrated under vacuum. The residue was washed with ether (2 × 100 mL) at 5°C and crystallized from ethanol to give compound 5 (3-((E)-2-((4aR,6aR,7R,10aS,10bR)-3,3,6a,10b-tetramethyl-8-methylenedecahydro-1H-naphtho[2,1-d][1,3]dioxin-7-yl)vinyl)furan-2(5H)-one) as a light yellow solid (65.82 g, yield 89.01%). Mp 167–168°C. IR (KBr) νmax 2875,1808, 1645, 1449, 1385, 1377, 1343, 1274, 1234, 1113, 1023, 971, 892 cm−1. 1H NMR (600 MHz, [D6]DMSO) δ 7.64 (1H, s, 14-H), 6.53 (1H, dd, J = 6.6 Hz, J = 15.7 Hz, 11-H), 6.16 (1H, d, J = 15.7 Hz, 12-H), 4.93 (2H, s, 15-H), 4.84 (1H, s, 17-Ha), 4.46 (1H, s, 17-Hb), 4.10 (1H, d, J = 11.7 Hz, 19-Ha), 3.52 (1H, d, J = 11.7 Hz, 19-Hb), 3.28–3.25 (1H, m, 3-H), 2.53 (1H, d, J = 6.6 Hz, 9-H), 2.07–1.28 (9H, m, 1,2,5,6,7-H), 1.23–1.22 (6H, m, 23,24-H), 1.09 (3H, s, 18-H), 0.91 (3H, s, 20-H). 13C NMR (100 MHz, [D6]DMSO) δ 171.2, 140.2, 138.4, 136.2, 131.6, 120.2, 115.9, 110.0, 77.5, 71.2, 65.6, 61.3, 48.5, 43.1, 39.6, 36.7, 33.3, 31.3, 26.6, 26.5, 24.3, 16.6, 13.3 ppm. MS (ESI) m/z (%) 373.2 ([M + H]+, 100%). HRMS (ESI) calcd for C23H33O4 [M + H]+ 373.2301, found 373.2309.
Compound 6: compound 5 (62.00 g, 166.56 mmol) was dissolved in tetrahydrofuran (THF) (300 mL) and then KMnO4 (52.60 g, 333.20 mmol) was added slowly at −5°C and the mixture stirred for 5 h until total consumption of compound 5. Then, the mixture was stirred at reflux for ~3–5 h (reaction complete by TLC analysis). After reaction, the mixture was filtered and the solvent was removed under vacuum. Then, CH2Cl2 (3 × 200 mL) was poured into the residue and stirred strongly for 30 min. The combined CH2Cl2 extract was washed with brine (3 × 100 mL) and dried over anhydrous Na2SO4, filtered and concentrated under vacuum. The residue was purified by column chromatography (light petroleum/ethyl acetate 2:1) and afforded product 6 ((4aR,6aR,7R,10aS,10bR)-3,3,6a,10b-tetramethyl-8-methylenedecahydro-1H-naphtho[2,1-d][1,3]dioxine-7-carbaldehyde) (38.98 g, 80.10%) as a white solid. Mp 134 −135°C. IR (KBr) νmax 2975, 2825, 2720, 1748, 1645, 1449, 1385, 1378, 1343, 1269, 1234, 1123, 1023, 971, 892 cm−1. 1H NMR (600 MHz, [D6]DMSO) δ 9.72 (1H, d, J = 2.4 Hz, 11-H), 5.25 (1H, s, 12-Ha), 5.14 (1H, s, 12-Hb), 4.10 (1H, d, J = 11.9 Hz, 14-Ha), 3.52 (1H, d, J = 11.9 Hz, 14-Hb), 3.07–3.05 (1H, m, 3-H), 2.93 (1H, d, J = 2.4 Hz, 9-H), 2.07–1.28 (9H, m, 1,2,5,6,7-H), 1.22–1.21 (6H, m, 17,18-H), 1.09 (3H, s, 13-H), 0.91 (3H, s, 15-H). 13C NMR (100 MHz, [D6]DMSO) δ 205.1, 145.0, 115.8, 109.2, 77.6, 68.2, 65.6, 48.5, 43.1, 39.5, 36.7, 33.1, 31.3, 26.5, 26.4, 24.3, 15.6, 14.3 ppm. MS (ESI) m/z (%) 293.2 ([M + H]+, 100%). HRMS (ESI) calcd for C18H29O3 [M + H]+ 293.2038, found 293.2048.
Compound 8 (8a is selected as an example): compound 6 (2.00 g, 6.84 mmol) was dissolved in dry toluene (20 mL) and then Fe(OAc)2 (0.06 g, 0.34 mmol), 2-methylquinoline (1.16 g, 8.20 mmol) and TFA (0.08 g, 0.68 mmol) were added under N2 at room temperature. The mixture was stirred at 100°C for 24 h until total consumption of compound 6. The mixture was then cooled to room temperature and the solvent was removed under reduced pressure. The residue was dissolved in a mixture of AcOH and H2O (15 mL, 3:1) at room temperature for ~2 h. After reaction, sodium bicarbonate solution was added dropwise to neutralize the mixture. CH2Cl2 (3 × 40 mL) was poured into the mixture. The combined CH2Cl2 extract was washed with brine (3 × 30 mL) and dried over anhydrous Na2SO4, filtered and concentrated under vacuum. The residue was purified by column chromatography (CH2Cl2/MeOH 15:1) and afforded product 8a ((1R,2R,4aR,5R,8aS)-1-(hydroxymethyl)-1,4a-dimethyl-6-methylene-5-((E)-2-(quinolin-2-yl)vinyl)decahydronaphthalen-2-ol) (77.52%, 2.00 g) as a pale yellow solid. Mp 220–221°C. IR (KBr) νmax 3527, 3470, 3256, 3102, 2983, 2945, 2825, 1635, 1447, 1396, 1348, 1271, 1232, 1112, 1036, 975, 886 cm−1. 1H NMR (600 MHz, [D6]DMSO) δ 8.22–8.07 (1H, m, 4′-H), 8.08–7.83 (3H, m, 5′,7′,8′-H), 7.62–7.60 (1H, m, 6′-H), 7.37 (1H, d, J = 8.3 Hz, 3′-H), 6.68 (1H, dd, J = 6.8 Hz, J = 15.8 Hz, 11-H), 6.54 (1H, d, J = 15.8 Hz, 12-H), 5.03 (1H, s, 13-Ha), 4.87 (1H, s, 13-Hb), 3.68 (1H, brs, 15-OH), 3.59 (1H, brs, 3-OH), 3.50 (1H, d, J = 11.8 Hz, 15-Ha), 3.31 (1H, d, J = 11.8 Hz, 15-Hb), 3.17–3.14 (1H, m, 3-H), 2.76 (1H, d, J = 6.8 Hz, 9-H), 2.10–1.20 (9H, m, 1,2,5,6,7-H), 1.11 (3H, s, 14-H), 0.83 (3H, s, 16-H). 13C NMR (100 MHz, [D6]DMSO) δ 156.4, 148.8, 148.1, 136.5, 130.5, 128.8, 128.0, 126.9, 126.0, 125.8, 125.1, 118.5, 110.1, 78.5, 65.8, 61.9, 47.4, 43.3, 39.9, 36.7, 33.3, 27.9, 24.7, 19.4, 15.0 ppm. MS (ESI) m/z (%) 378.2 ([M + H]+, 100%). HRMS (ESI) calcd for C25H32NO2 [M + H]+ 378.2433, found 378.2445.
(1R,2R,4aR,5R,8aS)-1-(Hydroxymethyl)-1,4a-dimethyl-6-methylene-5-((E)-2-(4-methylquinolin-2-yl)vinyl)decahydronaphthalen-2-ol 8b (pale yellow solid, 74.25%, 1.99 g); mp 190–191°C. IR (KBr): νmax 3527, 3453, 3104, 2973, 2945, 2925, 1635, 1447, 1363, 1343, 1276, 1230, 1037, 973, 893 cm−1. 1H NMR (600 MHz, [D6]DMSO) δ 8.10–7.74 (3H, m, 5′,7′,8′-H), 7.62–7.58 (1H, m, 6′-H), 7.17 (1H, s, 3′-H), 6.68 (1H, dd, J = 6.2 Hz, J = 15.7 Hz, 11-H), 6.55 (1H, d, J = 15.7 Hz, 12-H), 5.05 (1H, s, 13-Ha), 4.88 (1H, s, 13-Hb), 3.67 (1H, brs, 15-OH), 3.58 (1H, brs, 3-OH), 3.49 (1H, d, J = 11.8 Hz, 15-Ha), 3.32 (1H, d, J = 11.8 Hz, 15-Hb), 3.17–3.13 (1H, m, 3-H), 2.76 (1H, d, J = 6.2 Hz, 9-H), 2.61 (3H, s, 4′-CH3), 2.10–1.18 (9H, m, 1,2,5,6,7-H), 1.08 (3H, s, 14-H), 0.80 (3H, s, 16-H). 13C NMR (100 MHz, [D6]DMSO) δ 157.4, 148.9, 146.8, 144.1, 130.5, 128.7, 128.2, 126.7, 126.0, 125.8, 125.2, 118.3, 109.1, 78.5, 65.8, 61.9, 47.4, 43.2, 39.8, 36.6, 33.2, 27.8, 24.8, 20.2, 19.6, 15.1 ppm. MS (ESI) m/z (%) 392.3 ([M + H]+, 100%). HRMS (ESI) calcd for C26H34NO2 [M + H]+ 392.2589, found 392.2596.
(1R,2R,4aR,5R,8aS)-5-((E)-2-(4-Fluoroquinolin-2-yl)vinyl)-1-(hydroxymethyl)-1,4a-dimethyl-6-methylenedecahydronaphthalen-2-ol 8c (yellow solid, 71.48%, 1.93 g); mp 210–211°C. IR (KBr) νmax 3525, 3456, 3106, 2957, 2948, 2925, 1655, 1446, 1382, 1346, 1277, 1233, 1032, 975, 885 cm−1. 1H NMR (600 MHz, [D6]DMSO) δ 8.07–7.74 (4H, m, 5′,6′,7′,8′-H), 7.07–7.00 (1H, m, 3′-H), 6.65 (1H, dd, J = 6.2 Hz, J = 15.7 Hz, 11-H), 6.54 (1H, d, J = 15.7 Hz, 12-H), 5.05 (1H, s, 13-Ha), 4.87 (1H, s, 13-Hb), 3.65 (1H, brs, 15-OH), 3.54 (1H, brs, 3-OH), 3.43 (1H, d, J = 11.8 Hz, 15-Ha), 3.32 (1H, d, J = 11.8 Hz, 15-Hb), 3.15–3.11 (1H, m, 3-H), 2.78 (1H, d, J = 6.2 Hz, 9-H), 2.13–1.13 (9H, m, 1,2,5,6,7-H), 1.04 (3H, s, 14-H), 0.84 (3H, s, 16-H). 13C NMR (100 MHz, [D6]DMSO) δ 167.2 (1JCF = 246.2 Hz), 156.4, 148.9, 148.5, 131.5, 129.0, 127.2, 125.7, 125.0, 120.8, 118.3 (2JCF = 22.0 Hz), 109.1, 101.8 (2JCF = 22.6 Hz), 78.7, 65.5, 61.6, 47.2, 43.3, 39.5, 36.3, 33.4, 27.3, 24.5, 19.1, 15.0 ppm. MS (ESI) m/z (%) 396.2 ([M + H]+, 100%). HRMS (ESI) calcd for C25H31FNO2 [M + H]+ 396.2339, found 396.2348.
(1R,2R,4aR,5R,8aS)-5-((E)-2-(4-Chloroquinolin-2-yl)vinyl)-1-(hydroxymethyl)-1,4a-dimethyl-6-methylenedecahydronaphthalen-2-ol 8d (pale yellow solid, 69.75%, 1.96 g); mp 215–216°C. IR (KBr) νmax 3527, 3446, 3105, 2977, 2945, 2927, 1638, 1445, 1384, 1347, 1274, 1233, 1033, 974, 885 cm−1. 1H NMR (600 MHz, [D6]DMSO) δ 8.62–8.58 (1H, m, 5′-H), 8.10–7.74 (3H, m, 6′,7′,8′-H), 7.57 (1H, s, 3′-H), 6.64 (1H, dd, J = 6.2 Hz, J = 15.7 Hz, 11-H), 6.54 (1H, d, J = 15.7 Hz, 12-H), 5.05 (1H, s, 13-Ha), 4.84 (1H, s, 13-Hb), 3.63 (1H, brs, 15-OH), 3.55 (1H, brs, 3-OH), 3.42 (1H, d, J = 11.8 Hz, 15-Ha), 3.32 (1H, d, J = 11.8 Hz, 15-Hb), 3.16–3.10 (1H, m, 3-H), 2.79 (1H, d, J = 6.2 Hz, 9-H), 2.13–1.14 (9H, m, 1,2,5,6,7-H), 1.02 (3H, s, 14-H), 0.82 (3H, s, 16-H). 13C NMR (100 MHz, [D6]DMSO) δ 156.4, 148.9, 147.8, 143.4, 133.4, 129.1, 127.2, 126.7, 125.6, 125.1, 124.2, 118.1, 109.0, 78.8, 65.5, 62.3, 47.3, 43.3, 39.5, 36.4, 33.3, 27.4, 24.5, 19.3, 15.1 ppm. MS (ESI) m/z (%) 412.2 ([M + H]+, C25H3135ClNO2, 100%), 414.2 ([M + H]+, C25H3137ClNO2, 33%). HRMS (ESI) calcd for C25H3135ClNO2 [M + H]+ 412.2043, found 412.2055; for C25H3137ClNO2 [M + H]+ 414.2014, found 414.2023.
2-((E)-2-((1R,4aS,5R,6R,8aR)-6-Hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)vinyl)quinolin-4-ol 8e (light yellow solid, 64.68%, 1.74 g); mp 195–196°C. IR (KBr) νmax 3525, 3456, 3257, 3108, 2977, 2948, 2923, 1637, 1444, 1386, 1347, 1273, 1235, 1033, 975, 893 cm−1. 1H NMR (600 MHz, [D6]DMSO) δ 8.30–7.80 (3H, m, 5′,7′,8′-H), 7.72–7.59 (1H, m, 6′-H), 6.80 (1H, s, 3′-H), 6.65 (1H, dd, J = 6.2 Hz, J = 15.7 Hz, 11-H), 6.53 (1H, d, J = 15.7 Hz, 12-H), 5.35 (1H, brs, 4′-OH), 5.04 (1H, s, 13-Ha), 4.83 (1H, s, 13-Hb), 3.64 (1H, brs, 15-OH), 3.52 (1H, brs, 3-OH), 3.41 (1H, d, J = 11.8 Hz, 15-Ha), 3.34 (1H, d, J = 11.8 Hz, 15-Hb), 3.17–3.12 (1H, m, 3-H), 2.78 (1H, d, J = 6.2 Hz, 9-H), 2.12–1.19 (9H, m, 1,2,5,6,7-H), 1.06 (3H, s, 14-H), 0.90 (3H, s, 16-H). 13C NMR (100 MHz, [D6]DMSO) δ 166.3, 157.4, 149.2, 148.8, 130.8, 128.5, 127.1, 125.7, 125.2, 121.3, 117.3, 109.2, 102.4, 78.8, 65.4, 61.8, 47.6, 43.3, 39.4, 36.6, 33.3, 27.4, 24.4, 19.6, 15.0 ppm. MS (ESI) m/z (%) 394.2 ([M + H]+, 100%). HRMS (ESI) calcd for C25H32NO3 [M + H]+ 394.2382, found 394.2392.
(1R,2R,4aR,5R,8aS)-1-(Hydroxymethyl)-1,4a-dimethyl-6-methylene-5-((E)-2-(6-methylquinolin-2-yl)vinyl)decahydronaphthalen-2-ol 8f (yellow solid, 74.62%, 2.00 g); mp 201–202°C. IR (KBr) νmax 3528, 3453, 3107, 2948, 2935, 1645, 1457, 1387, 1349, 1274, 1233, 1034, 973, 885 cm−1. 1H NMR (600 MHz, [D6]DMSO) δ 8.21 (1H, d, J = 8.3 Hz, 4′-H), 7.92 (1H, d, J = 8.3 Hz, 8′-H) 7.62–7.58 (2H, m, 5′,7′-H), 7.17 (1H, dd, J = 8.3 Hz, J = 4.3 Hz, 3′-H), 6.70 (1H, dd, J = 6.2 Hz, J = 15.7 Hz, 11-H), 6.55 (1H, d, J = 15.7 Hz, 12-H), 5.07 (1H, s, 13-Ha), 4.85 (1H, s, 13-Hb), 3.69 (1H, brs, 15-OH), 3.59 (1H, brs, 3-OH), 3.51 (1H, d, J = 11.8 Hz, 15-Ha), 3.31 (1H, d, J = 11.8 Hz, 15-Hb), 3.18–3.15 (1H, m, 3-H), 2.70 (1H, d, J = 6.2 Hz, 9-H), 2.34 (3H, s, 6′-CH3), 2.13–1.18 (9H, m, 1,2,5,6,7-H), 1.12 (3H, s, 14-H), 0.86 (3H, s, 16-H). 13C NMR (100 MHz, [D6]DMSO) δ 155.4, 148.7, 146.2, 137.1, 135.8, 130.5, 128.2, 126.7, 126.2, 125.8, 125.2, 118.4, 109.3, 78.7, 65.5, 61.6, 47.5, 43.4, 39.6, 36.7, 33.3, 27.4, 24.6, 20.3, 19.7, 15.3 ppm. MS (ESI) m/z (%) 392.3 ([M + H]+, 100%). HRMS (ESI) calcd for C26H34NO2 [M + H]+ 392.2589, found 392.2597.
(1R,2R,4aR,5R,8aS)-1-(Hydroxymethyl)-5-((E)-2-(6-methoxyquinolin-2-yl)vinyl)-1,4a-dimethyl-6-methylenedecahydronaphthalen-2-ol 8g (pale yellow solid, 76.34%, 2.13 g); mp 206–207°C. IR (KBr) νmax 3529, 3452, 3103, 2943, 2822, 1643, 1459, 1384, 1344, 1277, 1233, 1032, 972, 896 cm−1. 1H NMR (600 MHz, [D6]DMSO) δ 8.20–8.16 (1H, m, 4′-H), 7.78 (1H, d, J = 8.3 Hz, 8′-H), 7.39–7.32 (2H, m, 3′,7′-H), 7.17–7.09 (1H, m, 5′-H), 6.69 (1H, dd, J = 6.2 Hz, J = 15.7 Hz, 11-H), 6.54 (1H, d, J = 15.7 Hz, 12-H), 5.05 (1H, s, 13-Ha), 4.85 (1H, s, 13-Hb), 3.83 (3H, s, 6′-OCH3), 3.67 (1H, brs, 15-OH), 3.58 (1H, brs, 3-OH), 3.50 (1H, d, J = 11.8 Hz, 15-Ha), 3.30 (1H, d, J = 11.8 Hz, 15-Hb), 3.17–3.14 (1H, m, 3-H), 2.77 (1H, d, J = 6.2 Hz, 9-H), 2.11–1.16 (9H, m, 1,2,5,6,7-H), 1.04 (3H, s, 14-H), 0.90 (3H, s, 16-H). 13C NMR (100 MHz, [D6]DMSO) δ 157.4, 154.7, 148.2, 143.1, 135.3, 130.4, 129.2, 125.5, 125.0, 122.5, 119.4, 109.2, 105.4, 78.9, 65.6, 61.3, 55.3, 47.4, 43.3, 39.3, 36.5, 33.3, 27.5, 24.7, 19.5, 15.1 ppm. MS (ESI) m/z (%) 408.3 ([M + H]+, 100%). HRMS (ESI) calcd for C26H34NO3 [M + H]+ 408.2540, found 408.2549.
(1R,2R,4aR,5R,8aS)-5-((E)-2-(6-Fluoroquinolin-2-yl)vinyl)-1-(hydroxymethyl)-1,4a-dimethyl-6-methylenedecahydronaphthalen-2-ol 8h (yellow solid, 66.66%, 1.80 g); mp 216–217°C. IR (KBr) νmax 3535, 3458, 3107, 2943, 2922, 1644, 1454, 1384, 1345, 1273, 1235, 1033, 972, 883 cm−1. 1H NMR (600 MHz, [D6]DMSO) δ 8.26–8.20 (1H, m, 4′-H), 7.93 (1H, d, J = 8.3 Hz, 8′-H), 7.39–7.32 (3H, m, 3′,5′,7′-H), 6.67 (1H, dd, J = 6.2 Hz, J = 15.7 Hz, 11-H), 6.53 (1H, d, J = 15.7 Hz, 12-H), 5.03 (1H, s, 13-Ha), 4.84 (1H, s, 13-Hb), 3.63 (1H, brs, 15-OH), 3.51 (1H, brs, 3-OH), 3.52 (1H, d, J = 11.8 Hz, 15-Ha), 3.32 (1H, d, J = 11.8 Hz, 15-Hb), 3.15–3.12 (1H, m, 3-H), 2.79 (1H, d, J = 6.2 Hz, 9-H), 2.20–1.13 (9H, m, 1,2,5,6,7-H), 1.03 (3H, s, 14-H), 0.91 (3H, s, 16-H). 13C NMR (100 MHz, [D6]DMSO) δ 160.3 (1JCF = 245.0 Hz), 155.4, 148.6, 147.1, 145.3, 135.9, 130.2, 125.6, 125.1, 121.5 (2JCF = 21.2 Hz), 119.3, 109.5 (2JCF = 21.3 Hz), 109.0, 78.7, 65.4, 62.3, 47.5, 43.1, 40.3, 36.2, 33.1, 27.2, 24.3, 19.3, 14.8 ppm. MS (ESI) m/z (%) 396.2 ([M + H]+, 100%). HRMS (ESI) calcd for C25H31FNO2 [M + H]+ 396.2339, found 396.2349.
(1R,2R,4aR,5R,8aS)-5-((E)-2-(6-Chloroquinolin-2-yl)vinyl)-1-(hydroxymethyl)-1,4a-dimethyl-6-methylenedecahydronaphthalen-2-ol 8i (yellow solid, 70.32%, 1.99 g); mp 218–219°C. IR (KBr) νmax 3515, 3458, 3102, 2942, 2921, 1646, 1454, 1385, 1346, 1273, 1234, 1035, 994, 883 cm−1. 1H NMR (600 MHz, [D6]DMSO) δ 8.26–8.20 (1H, m, 4′-H), 8.10 (1H, d, J = 8.3 Hz, 8′-H), 7.82 (1H, d, J = 8.3 Hz, 7′-H), 7.39 (1H, d, J = 8.3 Hz, 3′-H), 7.17–7.08 (1H, m, 5′-H), 6.64 (1H, dd, J = 6.2 Hz, J = 15.7 Hz, 11-H), 6.53 (1H, d, J = 15.7 Hz, 12-H), 5.02 (1H, s, 13-Ha), 4.82 (1H, s, 13-Hb), 3.66 (1H, brs, 15-OH), 3.54 (1H, brs, 3-OH), 3.46 (1H, d, J = 11.8 Hz, 15-Ha), 3.25 (1H, d, J = 11.8 Hz, 15-Hb), 3.16–3.10 (1H, m, 3-H), 2.82 (1H, d, J = 6.2 Hz, 9-H), 2.13–1.14 (9H, m, 1,2,5,6,7-H), 1.02 (3H, s, 14-H), 0.91 (3H, s, 16-H). 13C NMR (100 MHz, [D6]DMSO) δ 156.4, 148.4, 146.1, 135.4, 132.0, 131.4, 130.2, 128.7, 125.6, 125.1, 123.5, 119.8, 109.3, 78.8, 65.5, 61.3, 47.4, 43.6, 39.4, 36.2, 33.3, 27.2, 24.5, 19.2, 15.0 ppm. MS (ESI) m/z (%) 412.2 ([M + H]+, C25H3135ClNO2, 100%), 414.2 ([M + H]+, C25H3137ClNO2, 33%). HRMS (ESI) calcd for C25H3135ClNO2 [M + H]+: 412.2043, found 412.2057; for C25H3137ClNO2[M + H]+ 414.2014, found 414.2022.
(1R,2R,4aR,5R,8aS)-5-((E)-2-(6-Bromoquinolin-2-yl)vinyl)-1-(hydroxymethyl)-1,4a-dimethyl-6-methylenedecahydronaphthalen-2-ol 8j (yellow solid, 65.18%, 2.04 g); mp 241–242°C. IR (KBr) νmax 3534, 3437, 3101, 2945, 2926, 1646, 1457, 1383, 1346, 1276, 1231, 1031, 974, 882 cm−1. 1H NMR (600 MHz, [D6]DMSO) δ 8.28–8.01 (4H, m, 4′,5′,7′,8′-H), 7.44 (1H, d, J = 8.3 Hz, 3′-H), 6.68 (1H, dd, J = 6.2 Hz, J = 15.7 Hz, 11-H), 6.54 (1H, d, J = 15.7 Hz, 12-H), 5.04 (1H, s, 13-Ha), 4.85 (1H, s, 13-Hb), 3.65 (1H, brs, 15-OH), 3.56 (1H, brs, 3-OH), 3.50 (1H, d, J = 11.8 Hz, 15-Ha), 3.31 (1H, d, J = 11.8 Hz, 15-Hb), 3.18–3.12 (1H, m, 3-H), 2.83 (1H, d, J = 6.2 Hz, 9-H), 2.14–1.15 (9H, m, 1,2,5,6,7-H), 1.03 (3H, s, 14-H), 0.90 (3H, s, 16-H). 13C NMR (100 MHz, [D6]DMSO) δ 156.1, 148.8, 148.4, 135.3, 133.1, 132.4, 129.8, 128.7, 125.5, 125.1, 123.5, 120.8, 109.3, 78.7, 65.3, 61.2, 48.4, 43.7, 40.4, 36.7, 33.4, 27.6, 24.4, 19.7, 15.2 ppm. MS (ESI) m/z (%) 456.2 ([M + H]+, C25H3179BrNO2, 100%), 458.2 ([M + H]+, C25H3181BrNO2, 97%). HRMS (ESI) calcd for C25H3179BrNO2 [M + H]+: 456.1538, found 456.1547; for C25H3181BrNO2 [M + H]+ 458.1518, found 458.1527.
2-((E)-2-((1R,4aS,5R,6R,8aR)-6-Hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)vinyl)quinolin-6-ol 8k (pale yellow solid, 57.62%, 1.55 g); mp 220–221°C. IR (KBr) νmax 3532, 3464, 3105, 2946, 2924, 1643, 1452, 1385, 1346, 1273, 1235, 1032, 972, 885 cm−1. 1H NMR (600 MHz, [D6]DMSO) δ 8.06–8.00 (1H, m, 4′-H), 7.81 (1H, d, J = 8.3 Hz, 8′-H), 7.42–7.17 (3H, m, 3′,5′,7′-H), 6.68 (1H, dd, J = 6.2 Hz, J = 15.7 Hz, 11-H), 6.55 (1H, d, J = 15.7 Hz, 12-H), 5.35 (1H, s, 6′-OH), 5.05 (1H, s, 13-Ha), 4.84 (1H, s, 13-Hb), 3.64 (1H, brs, 15-OH), 3.55 (1H, brs, 3-OH), 3.43 (1H, d, J = 11.8 Hz, 15-Ha), 3.26 (1H, d, J = 11.8 Hz, 15-Hb), 3.17–3.13 (1H, m, 3-H), 2.84 (1H, d, J = 6.2 Hz, 9-H), 2.15–1.13 (9H, m, 1,2,5,6,7-H), 1.05 (3H, s, 14-H), 0.84 (3H, s, 16-H). 13C NMR (100 MHz, [D6]DMSO) δ 155.4, 154.1, 148.8, 143.1, 134.8, 130.2, 128.9, 125.6, 125.4, 125.0, 119.2, 111.3, 109.3, 78.7, 65.3, 61.7, 47.5, 43.6, 39.7, 36.5, 33.3, 27.4, 24.5, 19.3, 15.3 ppm. MS (ESI) m/z (%) 394.2 ([M + H]+, 100%). HRMS (ESI) calcd for C25H32NO3 [M + H]+ 394.2382, found 394.2393.
(1R,2R,4aR,5R,8aS)-1-(Hydroxymethyl)-1,4a-dimethyl-6-methylene-5-((E)-2-(7-methylquinolin-2-yl)vinyl)decahydronaphthalen-2-ol 8l (yellow solid, 75.37%, 2.02 g); mp 213–214°C. IR (KBr) νmax 3538, 3446, 3107, 2952, 2942, 1654, 1453, 1386, 1350, 1264, 1230, 1044, 978, 887 cm−1. 1H NMR (600 MHz, [D6]DMSO) δ 8.21 (1H, d, J = 8.2 Hz, 4′-H), 7.92–7.58 (3H, m, 5′,6′,8′-H), 7.33 (1H, dd, J = 8.2 Hz, J = 4.3 Hz, 3′-H), 6.66 (1H, dd, J = 6.2 Hz, J = 15.7 Hz, 11-H), 6.53 (1H, d, J = 15.7 Hz, 12-H), 5.02 (1H, s, 13-Ha), 4.88 (1H, s, 13-Hb), 3.65 (1H, brs, 15-OH), 3.59 (1H, brs, 3-OH), 3.50 (1H, d, J = 11.6 Hz, 15-Ha), 3.31 (1H, d, J = 11.6 Hz, 15-Hb), 3.16–3.11 (1H, m, 3-H), 2.80 (1H, d, J = 6.2 Hz, 9-H), 2.34 (3H, s, 7′-CH3), 2.12–1.17 (9H, m, 1,2,5,6,7-H), 1.09 (3H, s, 14-H), 0.92 (3H, s, 16-H). 13C NMR (100 MHz, [D6]DMSO) δ 156.2, 148.8, 146.4, 139.1, 136.0, 130.5, 128.2, 127.1, 126.2, 125.5, 125.0, 117.6, 109.2, 78.5, 65.3, 62.1, 46.5, 43.1, 40.3, 36.7, 33.1, 27.7, 24.6, 21.3, 19.5, 15.1 ppm. MS (ESI) m/z (%) 392.3 ([M + H]+, 100%). HRMS (ESI) calcd for C26H34NO2 [M + H]+ 392.2589, found 392.2598.
(1R,2R,4aR,5R,8aS)-1-(Hydroxymethyl)-5-((E)-2-(7-methoxyquinolin-2-yl)vinyl)-1,4a-dimethyl-6-methylenedecahydronaphthalen-2-ol 8m (pale yellow solid, 71.33%, 1.99 g); mp 232–233°C. IR (KBr) νmax 3528, 3464, 3102, 2945, 2821, 1651, 1451, 1384, 1350, 1261, 1227, 1041, 975, 892 cm−1. 1H NMR (600 MHz, [D6]DMSO) δ 8.23 (1H, d, J = 8.2 Hz, 4′-H), 7.99–7.90 (1H, m, 5′-H), 7.35–7.22 (3H, m, 3′,6′,8′-H), 6.68 (1H, dd, J = 6.2 Hz, J = 15.7 Hz, 11-H), 6.54 (1H, d, J = 15.7 Hz, 12-H), 5.03 (1H, s, 13-Ha), 4.86 (1H, s, 13-Hb), 3.83 (1H, s, 7′-OCH3), 3.64 (1H, brs, 15-OH), 3.58 (1H, brs, 3-OH), 3.47 (1H, d, J = 11.6 Hz, 15-Ha), 3.33 (1H, d, J = 11.6 Hz, 15-Hb), 3.14–3.10 (1H, m, 3-H), 2.83 (1H, d, J = 6.2 Hz, 9-H), 2.10–1.15 (9H, m, 1,2,5,6,7-H), 1.10 (3H, s, 14-H), 0.90 (3H, s, 16-H). 13C NMR (100 MHz, [D6]DMSO) δ 156.9, 151.2, 148.6, 147.4, 136.2, 129.5, 128.2, 125.5, 125.0, 117.6, 116.5, 109.2, 107.2, 78.7, 65.4, 62.5, 55.8, 46.5, 43.3, 40.3, 36.5, 33.4, 27.4, 24.3, 19.6, 15.0 ppm. MS (ESI) m/z (%) 408.2 ([M + H]+, 100%). HRMS (ESI) calcd for C26H34NO3 [M + H]+ 408.2538, found 408.2548.
(1R,2R,4aR,5R,8aS)-5-((E)-2-(7-Fluoroquinolin-2-yl)vinyl)-1-(hydroxymethyl)-1,4a-dimethyl-6-methylenedecahydronaphthalen-2-ol 8n (yellow solid, 67.77%, 1.83 g); mp 209–210°C. IR (KBr) νmax 3531, 3462, 3111, 2912, 1658, 1454, 1389, 1353, 1265, 1232, 1042, 973, 893 cm−1. 1H NMR (600 MHz, [D6]DMSO) δ 8.23–8.05 (1H, m, 4′-H), 8.03–7.95 (1H, m, 5′-H), 7.76–7.69 (1H, m, 8′-H), 7.37 (1H, d, J = 8.3 Hz, 3′-H), 7.13–7.03 (1H, m, 6′-H), 6.68 (1H, dd, J = 6.2 Hz, J = 15.7 Hz, 11-H), 6.54 (1H, d, J = 15.7 Hz, 12-H), 5.04 (1H, s, 13-Ha), 4.88 (1H, s, 13-Hb), 3.63 (1H, brs, 15-OH), 3.57 (1H, brs, 3-OH), 3.46 (1H, d, J = 11.6 Hz, 15-Ha), 3.31 (1H, d, J = 11.6 Hz, 15-Hb), 3.15–3.10 (1H, m, 3-H), 2.83 (1H, d, J = 6.2 Hz, 9-H), 2.10–1.19 (9H, m, 1,2,5,6,7-H), 1.04 (3H, s, 14-H), 0.90 (3H, s, 16-H). 13C NMR (100 MHz, [D6]DMSO) δ 163.6 (1JCF = 245.2 Hz), 157.2, 148.6, 147.4, 136.6, 130.5, 125.5, 125.0, 118.6, 116.8 (2JCF = 21.2 Hz), 112.8 (2JCF = 21.3 Hz), 109.1, 78.7, 65.4, 62.2, 47.5, 43.2, 40.4, 36.5, 33.3, 27.6, 24.5, 21.2, 19.3, 15.0 ppm. MS (ESI) m/z (%) 396.2 ([M + H]+, 100%). HRMS (ESI) calcd for C25H31FNO2 [M + H]+ 396.2339, found 396.2351.
(1R,2R,4aR,5R,8aS)-5-((E)-2-(7-Chloroquinolin-2-yl)vinyl)-1-(hydroxymethyl)-1,4a-dimethyl-6-methylenedecahydronaphthalen-2-ol 8o (pale yellow solid, 64.31%, 1.82 g); mp 196–197°C. IR (KBr) νmax 3526, 3468, 3112, 2923, 1650, 1455, 1383, 1354, 1263, 1232, 1043, 975, 885 cm−1. 1H NMR (600 MHz, [D6]DMSO) δ 8.24–8.15 (2H, m, 4′,5′-H), 7.96–7.69 (2H, m, 6′,8′-H), 7.36 (1H, d, J = 8.3 Hz, 3′-H), 6.66 (1H, dd, J = 6.2 Hz, J = 15.7 Hz, 11-H), 6.56 (1H, d, J = 15.7 Hz, 12-H), 5.05 (1H, s, 13-Ha), 4.85 (1H, s, 13-Hb), 3.66 (1H, brs, 15-OH), 3.57 (1H, brs, 3-OH), 3.43 (1H, d, J = 11.6 Hz, 15-Ha), 3.33 (1H, d, J = 11.6 Hz, 15-Hb), 3.14–3.10 (1H, m, 3-H), 2.83 (1H, d, J = 6.2 Hz, 9-H), 2.10–1.15 (9H, m, 1,2,5,6,7-H), 1.02 (3H, s, 14-H), 0.85 (3H, s, 16-H). 13C NMR (100 MHz, [D6]DMSO) δ 157.5, 148.8, 147.2, 136.3, 135.4, 129.5, 128.4, 126.1, 125.5, 125.3, 125.0, 118.9, 109.2, 78.8, 65.5, 62.3, 47.4, 43.3, 40.0, 36.4, 33.2, 27.7, 24.2, 19.3, 15.0 ppm. MS (ESI) m/z (%) 412.2 ([M + H]+, C25H3135ClNO2, 100%), 414.2 ([M + H]+, C25H3137ClNO2, 33%). HRMS (ESI) calcd for C25H3135ClNO2 [M + H]+ 412.2043, found 412.2058; for C25H3137ClNO2 [M + H]+ 414.2014, found 414.2026.
2-((E)-2-((1R,4aS,5R,6R,8aR)-6-Hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)vinyl)quinolin-7-ol 8p (pale yellow solid, 58.74%, 1.58 g); mp 224–225°C. IR (KBr) νmax 3535, 3446, 3254, 2914, 1651, 1455, 1381, 1354, 1261, 1235, 1042, 976, 888 cm−1. 1H NMR (600 MHz, [D6]DMSO) δ 8.24–8.13 (1H, m, 4′-H), 8.04–7.98 (1H, m, 5′-H), 7.33–7.12 (3H, m, 3′,6′,8′-H), 6.67 (1H, dd, J = 6.2 Hz, J = 15.7 Hz, 11-H), 6.55 (1H, d, J = 15.7 Hz, 12-H), 5.35 (1H, brs, 7′-OH), 5.02 (1H, s, 13-Ha), 4.87 (1H, s, 13-Hb), 3.66 (1H, brs, 15-OH), 3.57 (1H, brs, 3-OH), 3.50 (1H, d, J = 11.6 Hz, 15-Ha), 3.31 (1H, d, J = 11.6 Hz, 15-Hb), 3.17–3.11 (1H, m, 3-H), 2.82 (1H, d, J = 6.2 Hz, 9-H), 2.04–1.12 (9H, m, 1,2,5,6,7-H), 1.05 (3H, s, 14-H), 0.92 (3H, s, 16-H). 13C NMR (100 MHz, [D6]DMSO) δ 158.3, 156.8, 151.1, 148.6, 136.2, 130.0, 125.5, 125.0, 123.3, 118.6, 116.3, 110.8, 109.2, 78.8, 65.5, 62.4, 47.4, 43.3, 40.1, 36.8, 33.5, 27.4, 24.7, 19.6, 15.1 ppm. MS (ESI) m/z (%) 394.2 ([M + H]+, 100%). HRMS (ESI) calcd for C25H32NO3 [M + H]+ 394.2382, found 394.2393.
Compound 9 (9a is selected as an example): compound 8a (1.50 g, 3.98 mmol) and succinic anhydride (1.19 g, 15.9 mmol) (for 9b, maleic anhydride 1.56 g, 15.9 mmol) were dissolved in CH2Cl2 (30 mL). Then, DMAP (N,N-4-dimethylaminopyridine, 0.53 g, 4.38 mmol) and Et3N (0.60 g, 5.97 mmol) were added under stirring. The mixture was stirred at room temperature overnight until no compound 8a was detected by TLC. After reaction, hydrochloric acid (4 M) was added dropwise to neutralize the mixture. CH2Cl2 (3 × 30 mL) was poured into the mixture. The combined CH2Cl2 extract was washed with brine (3 × 30 mL) and dried over anhydrous Na2SO4, filtered and concentrated under vacuum. The residue was dissolved in ethanol (10 mL) and then KHCO3 saturated solution (1.1 equiv.) was slowly added, producing a large number of bubbles. The mixture changed gradually from clear to milky white and stirring was continued for 1 h until bubbling ceased. Last, the mixture was filtered, dried and crystallized from acetone to give potassium 4-(((1R,2R,4aR,5R,8aS)-2-(3-carboxypropanoyloxy)-1,4a-dimethyl-6-methylene-5-((E)-2-(quinolin-2-yl)vinyl)decahydronaphthalen-1-yl)methoxy)-4-oxobutanoate 9a (1.54 g, yield 62.86%, purity 96.2%) as an off white solid. IR (KBr) νmax 3412, 3080, 2938, 2851, 1753, 1720, 1636, 1575, 1447, 1384, 1346, 1277, 1235, 1114, 1037, 973, 892 cm−1. 1H NMR (600 MHz, D2O) δ 8.24–8.17 (1H, m, 4′-H), 8.06 -7.80 (3H, m, 5′,7′,8′-H), 7.61–7.55 (1H, m, 6′-H), 7.34 (1H, d, J = 8.3 Hz, 3′-H), 6.67 (1H, dd, J = 6.2 Hz, J = 15.4 Hz, 11-H), 6.54 (1H, d, J = 15.4 Hz, 12-H), 5.05 (1H, s, 13-Ha), 4.86 (1H, s, 13-Hb), 4.15 (1H, d, J = 11.8 Hz, 15-Ha), 4.01–3.95 (1H, m, 3-H), 3.83 (1H, d, J = 11.8 Hz, 15-Hb), 2.86–2.75 (9H, m, 9,18,19,22,23-H), 2.13–1.45 (9H, m, 1,2,5,6,7-H), 1.01 (3H, s, 14-H), 0.93 (3H, s, 16-H). 13C NMR (100 MHz, D2O) δ 176.5, 176.1, 173.5, 173.1, 156.4, 148.8, 148.1, 136.5, 130.5, 128.8, 128.0, 126.9, 126.0, 125.8, 125.1, 118.5, 110.1, 75.5, 65.8, 61.9, 47.4, 43.3, 39.9, 36.7, 33.3, 32.6, 32.3, 32.0, 30.2, 27.9, 24.7, 21.9, 15.0 ppm. MS (ESI) m/z (%) 616.3 ([M + H]+, 100%). HRMS (ESI) calcd for C33H39NO8K [M + H]+ 616.3659, found 616.3667.
Potassium (E)-4-(((1R,2R,4aR,5R,8aS)-2-((E)-3-carboxyacryloyloxy)-1,4a-dimethyl-6-methylene-5-((E)-2-(quinolin-2-yl)vinyl)decahydronaphthalen-1-yl)methoxy)-4-oxobut-2-enoate 9b: creamy white solid, 1.58 g, yield 65.02%, purity 96.8%. IR (KBr) νmax 3422, 3084, 2934, 2853, 1753, 1634, 1575, 1446, 1383, 1343, 1275, 1233, 1114, 1032, 972, 891 cm−1. 1H NMR (600 MHz, D2O) δ 8.25–8.19 (1H, m, 4′-H), 8.07–7.82 (3H, m, 5′,7′,8′-H), 7.63–7.52 (1H, m, 6′-H), 7.37–7.24 (3H, m, 19,23,3′-H), 6.65 (1H, dd, J = 6.2 Hz, J = 15.4 Hz, 11-H), 6.53 (1H, d, J = 15.4 Hz, 12-H), 6.21–6.09 (2H, m, 18,22-H), 5.05 (1H, s, 13-Ha), 4.85 (1H, s, 13-Hb), 4.15 (1H, d, J = 11.8 Hz, 15-Ha), 4.00–3.94 (1H, m, 3-H), 3.82 (1H, d, J = 11.8 Hz, 15-Hb), 2.83–2.73 (1H, m, 9-H), 2.16–1.45 (9H, m, 1,2,5,6,7-H), 1.04 (3H, s, 14-H), 0.91 (3H, s, 16-H). 13C NMR (100 MHz, D2O) δ 167.7, 167.1, 166.6, 166.1, 156.7, 148.6, 148.4, 139.7, 139.0, 136.4, 131.2, 131.1, 130.5, 128.6, 128.0, 126.7, 126.0, 125.4, 125.0, 118.6, 110.0, 75.7, 65.5, 62.3, 47.6, 43.3, 39.2, 36.4, 33.5, 27.6, 24.4, 21.7, 15.2 ppm. MS (ESI) m/z (%) 612.3 ([M + H]+, 100%). HRMS (ESI) calcd for C34H41NO8K [M + H]+ 612.3346, found 616.3359.
Potassium 4-(((1R,2R,4aR,5R,8aS)-2-(3-carboxypropanoyloxy)-1,4a-dimethyl-6-methylene-5-((E)-2-(4-methylquinolin-2-yl)vinyl)decahydronaphthalen-1-yl)methoxy)-4-oxobutanoate 9c: creamy white solid, 1.58 g, yield 65.00%, purity 95.9%. IR (KBr) νmax 3422, 3084, 3124, 2938, 2856, 1755, 1723, 1636, 1573, 1443, 1386, 1348, 1274, 1237, 1117, 1033, 973, 894 cm−1. 1H NMR (600 MHz, D2O) δ 8.20–7.84 (3H, m, 5′,7′,8′-H), 7.62–7.57 (1H, m, 6′-H), 7.15 (1H, s, 3′-H), 6.69 (1H, dd, J = 6.2 Hz, J = 15.7 Hz, 11-H), 6.65 (1H, d, J = 15.7 Hz, 12-H), 5.05 (1H, s, 13-Ha), 4.87 (1H, s, 13-Hb), 4.14 (1H, d, J = 11.8 Hz, 15-Ha), 4.00–3.93 (1H, m, 3-H), 3.85 (1H, d, J = 11.8 Hz, 15-Hb), 2.86–2.74 (9H, m, 9,18,19,22,23-H), 2.61 (3H, s, 4′-CH3), 2.17–1.49 (9H, m, 1,2,5,6,7-H), 1.04 (3H, s, 14-H), 0.90 (3H, s, 16-H). 13C NMR (100 MHz, D2O) δ 176.2, 176.0, 173.6, 173.4, 157.5, 148.6, 146.7, 144.6, 130.7, 128.4, 128.5, 126.3, 126.0, 125.5, 125.1, 118.2, 109.1, 75.5, 65.9, 61.4, 47.3, 43.4, 39.5, 36.3, 33.2, 32.3, 32.0, 31.8, 30.1, 27.2, 24.5, 21.7, 20.3, 15.1 ppm. MS (ESI) m/z (%) 630.2 ([M + H]+, 100%). HRMS (ESI) calcd for C24H41NO8K[M + H]+ 630.2469, found 630.2483.
Potassium 4-(((1R,2R,4aR,5R,8aS)-2-(3-carboxypropanoyloxy)-5-((E)-2-(4-fluoroquinolin-2-yl)vinyl)-1,4a-dimethyl-6-methylenedecahydronaphthalen-1-yl)methoxy)-4-oxobutanoate 9d: off white solid, 1.58 g, yield 62.70%, purity 96.8%. IR (KBr) νmax 3418, 3081, 2933, 2854, 1757, 1722, 1634, 1576, 1444, 1387, 1346, 1274, 1237, 1114, 1036, 994, 886 cm−1. 1H NMR (600 MHz, D2O) δ 8.17–7.85 (4H, m, 5′,6′,7′,8′-H), 7.14 (1H, d, J = 4.3 Hz, 3′-H), 6.68 (1H, dd, J = 6.2 Hz, J = 15.4 Hz, 11-H), 6.55 (1H, d, J = 15.4 Hz, 12-H), 5.04 (1H, s, 13-Ha), 4.84 (1H, s, 13-Hb), 4.16 (1H, d, J = 11.8 Hz, 15-Ha), 4.07–3.99 (1H, m, 3-H), 3.84 (1H, d, J = 11.8 Hz, 15-Hb), 2.83–2.72 (9H, m, 9,18,19,22,23-H), 2.15–1.46 (9H, m, 1,2,5,6,7-H), 1.04 (3H, s, 14-H), 0.87 (3H, s, 16-H). 13C NMR (100 MHz, D2O) δ 176.6, 176.2, 173.4, 173.0, 167.3 (1JCF = 245.2 Hz), 156.1, 148.8, 148.3, 131.7, 129.2, 127.3, 125.5, 125.0, 120.3, 118.4 (2JCF = 23.0 Hz), 109.5, 101.4 (2JCF = 22.5 Hz), 75.7, 65.4, 61.7, 47.4, 43.4, 39.6, 36.6, 33.3, 32.4, 32.0, 31.6, 30.2, 27.4, 24.4, 21.9, 15.2 ppm. MS (ESI) m/z (%) 634.2 ([M + H]+, 100%). HRMS (ESI) calcd for C33H38FNO8K [M + H]+ 634.2218, found 634.2229.
Potassium 4-(((1R,2R,4aR,5R,8aS)-2-(3-carboxypropanoyloxy)-5-((E)-2-(4-chloroquinolin-2-yl)vinyl)-1,4a-dimethyl-6-methylenedecahydronaphthalen-1-yl)methoxy)-4-oxobutanoate 9e: off white solid, 1.55 g, yield 59.85%, purity 96.3%. IR (KBr) νmax 3419, 3085, 2933, 2856, 1759, 1725, 1631, 1572, 1443, 1389, 1341, 1273, 1234, 1112, 1036, 973, 892 cm−1. 1H NMR (600 MHz, D2O) δ 8.64–8.55 (1H, m, 5′-H), 8.12–7.74 (3H, m, 6′,7′,8′-H), 7.54 (1H, s, 3′-H), 6.65 (1H, dd, J = 6.2 Hz, J = 15.4 Hz, 11-H), 6.56 (1H, d, J = 15.4 Hz, 12-H), 5.04 (1H, s, 13-Ha), 4.86 (1H, s, 13-Hb), 4.16 (1H, d, J = 11.8 Hz, 15-Ha), 4.01–3.95 (1H, m, 3-H), 3.83 (1H, d, J = 11.8 Hz, 15-Hb), 2.82–2.75 (9H, m, 9,18,19,22,23-H), 2.13–1.45 (9H, m, 1,2,5,6,7-H), 1.03 (3H, s, 14-H), 0.89 (3H, s, 16-H). 13C NMR (100 MHz, D2O) δ 176.5, 176.1, 173.5, 173.1, 156.4, 148.8, 148.1, 136.5, 130.5, 128.8, 128.0, 126.9, 126.0, 125.8, 125.1, 118.5, 110.1, 75.5, 65.8, 61.9, 47.4, 43.3, 39.9, 36.7, 33.3, 32.6, 32.3, 32.0, 30.2, 27.9, 24.7, 21.9, 15.0 ppm. MS (ESI) m/z (%) 650.2 ([M + H]+, C33H3835ClNO8K, 100%), 652.2 ([M + H]+, C33H3837ClNO8K, 33%). HRMS (ESI) calcd for C33H3835ClNO8K [M + H]+ 650.1970, found 650.1978; for C33H3837ClNO8K [M + H]+ 652.1941, found 652.1951.
Potassium 4-(((1R,2R,4aR,5R,8aS)-2-(3-carboxypropanoyloxy)-5-((E)-2-(4-hydroxyquinolin-2-yl)vinyl)-1,4a-dimethyl-6-methylenedecahydronaphthalen-1-yl)methoxy)-4-oxobutanoate 9f: creamy white solid, 1.36 g, yield 54.18%, purity 95.8%. IR (KBr) νmax 3478, 3392, 3082, 2948, 2854, 1756, 1723, 1638, 1572, 1442, 1386, 1342, 1272, 1232, 1116, 1034, 976, 893 cm−1. 1H NMR (600 MHz, D2O) δ 8.33–7.80 (3H, m, 5′,7′,8′-H), 7.72–7.54 (1H, m, 6′-H), 6.83 (1H, s, 3′-H), 6.64 (1H, dd, J = 6.2 Hz, J = 15.4 Hz, 11-H), 6.53 (1H, d, J = 15.4 Hz, 12-H), 5.34 (1H, brs, 4′-OH), 5.04 (1H, s, 13-Ha), 4.85 (1H, s, 13-Hb), 4.16 (1H, d, J = 11.8 Hz, 15-Ha), 4.03–3.95 (1H, m, 3-H), 3.85 (1H, d, J = 11.8 Hz, 15-Hb), 2.82–2.73 (9H, m, 9,18,19,22,23-H), 2.16–1.42 (9H, m, 1,2,5,6,7-H), 1.04 (3H, s, 14-H), 0.85 (3H, s, 16-H). 13C NMR (100 MHz, D2O) δ 176.6, 176.2, 173.7, 173.2, 166.8, 157.5, 149.1, 148.8, 130.3, 128.8, 127.2, 125.7, 125.1, 121.6, 117.6, 109.3, 102.6, 75.8, 65.6, 61.6, 47.4, 43.6, 39.7, 36.8, 33.3, 32.7, 32.3, 32.0, 30.6, 27.4, 24.4, 20.6, 15.4 ppm. MS (ESI) m/z (%) 632.2 ([M + H]+, 100%). HRMS (ESI) calcd for C33H39NO9K [M + H]+ 632.2310, found 632.2320.
Potassium 4-(((1R,2R,4aR,5R,8aS)-2-(3-carboxypropanoyloxy)-1,4a-dimethyl-6-methylene-5-((E)-2-(6-methylquinolin-2-yl)vinyl)decahydronaphthalen-1-yl)methoxy)-4-oxobutanoate 9g: white solid, 1.60 g, yield 64.05%, purity 96.6%. IR (KBr) νmax 3432, 3084, 2932, 2854, 1752, 1725, 1632, 1572, 1441, 1385, 1342, 1274, 1237, 1117, 1033, 973, 892 cm−1. 1H NMR (600 MHz, D2O) δ 8.23 (1H, d, J = 8.3 Hz, 4′-H), 7.96 (1H, d, J = 8.3 Hz, 8′-H) 7.62–7.53 (2H, m, 5′,7′-H), 7.14 (1H, dd, J = 8.3 Hz, J = 4.3 Hz, 3′-H), 6.68 (1H, dd, J = 6.2 Hz, J = 15.4 Hz, 11-H), 6.58 (1H, d, J = 15.4 Hz, 12-H), 5.04 (1H, s, 13-Ha), 4.85 (1H, s, 13-Hb), 4.16 (1H, d, J = 11.8 Hz, 15-Ha), 4.01–3.96 (1H, m, 3-H), 3.85 (1H, d, J = 11.8 Hz, 15-Hb), 2.84–2.75 (9H, m, 9,18,19,22,23-H), 2.35 (3H, s, 6′-CH3), 2.15–1.45 (9H, m, 1,2,5,6,7-H), 1.05 (3H, s, 14-H), 0.96 (3H, s, 16-H). 13C NMR (100 MHz, D2O) δ 176.7, 176.1, 173.5, 173.0, 155.3, 148.8, 146.4, 137.3, 135.5, 130.5, 128.6, 126.4, 126.2, 125.6, 125.2, 118.7, 109.1, 75.7, 65.5, 61.6, 47.6, 43.3, 39.6, 36.5, 33.4, 32.6, 32.3, 31.6, 30.2, 27.5, 24.6, 21.5, 20.4, 15.3 ppm. MS (ESI) m/z (%) 630.2 ([M + H]+, 100%). HRMS (ESI) calcd for C34H41NO8K [M + H]+ 630.2469, found 630.2482.
Potassium 4-(((1R,2R,4aR,5R,8aS)-2-(3-carboxypropanoyloxy)-5-((E)-2-(6-methoxyquinolin-2-yl)vinyl)-1,4a-dimethyl-6-methylenedecahydronaphthalen-1-yl)methoxy)-4-oxobutanoate 9h: white solid, 1.71 g, yield 64.04%, purity 96.2%. IR (KBr) νmax 3432, 3080, 2938, 2823, 1754, 1723, 1646, 1574, 1447, 1384, 1346, 1273, 1235, 1116, 1037, 973, 892 cm−1. 1H NMR (600 MHz, D2O) δ 8.20–8.16 (1H, m, 4′-H), 7.75 (1H, d, J = 8.3 Hz, 8′-H), 7.39–7.35 (2H, m, 3′,7′-H), 7.17–7.09 (1H, m, 5′-H), 6.66 (1H, dd, J = 6.2 Hz, J = 15.4 Hz, 11-H), 6.56 (1H, d, J = 15.4 Hz, 12-H), 5.03 (1H, s, 13-Ha), 4.86 (1H, s, 13-Hb), 4.15 (1H, d, J = 11.8 Hz, 15-Ha), 4.05–3.95 (1H, m, 3-H), 3.89 (3H, s, 6′-OCH3), 3.83 (1H, d, J = 11.8 Hz, 15-Hb), 2.88–2.75 (9H, m, 9,18,19,22,23-H), 2.13–1.46 (9H, m, 1,2,5,6,7-H), 1.06 (3H, s, 14-H), 0.90 (3H, s, 16-H). 13C NMR (100 MHz, D2O) δ 176.7, 176.4, 173.4, 173.1, 157.4, 154.4, 148.6, 143.1, 135.4, 130.7, 129.4, 125.5, 125.1, 122.5, 119.4, 109.3, 105.4, 75.9, 65.6, 61.7, 55.3, 47.4, 43.3, 39.4, 36.5, 33.6, 32.6, 32.4, 32.1, 30.2, 27.5, 24.7, 21.0, 15.1 ppm. MS (ESI) m/z (%) 646.2 ([M + H]+, 100%). HRMS (ESI) calcd for C34H41NO9K [M + H]+ 646.2418, found 646.2432.
Potassium 4-(((1R,2R,4aR,5R,8aS)-2-(3-carboxypropanoyloxy)-5-((E)-2-(6-fluoroquinolin-2-yl)vinyl)-1,4a-dimethyl-6-methylenedecahydronaphthalen-1-yl)methoxy)-4-oxobutanoate 9i: off white solid, 1.31 g, yield 51.98%, purity 97.2%. IR (KBr) νmax 3417, 3084, 2938, 2856, 1753, 1724, 1636, 1578, 1447, 1384, 1346, 1277, 1235, 1114, 1034, 975, 892 cm−1. 1H NMR (600 MHz, D2O) δ 8.27–8.22 (1H, m, 4′-H), 7.96 (1H, d, J = 8.3 Hz, 8′-H), 7.39–7.37 (3H, m, 3′,5′,7′-H), 6.66 (1H, dd, J = 6.2 Hz, J = 15.4 Hz, 11-H), 6.55 (1H, d, J = 15.4 Hz, 12-H), 5.05 (1H, s, 13-Ha), 4.84 (1H, s, 13-Hb), 4.15 (1H, d, J = 11.8 Hz, 15-Ha), 4.06–3.95 (1H, m, 3-H), 3.86 (1H, d, J = 11.8 Hz, 15-Hb), 2.82–2.78 (9H, m, 9,18,19,22,23-H), 2.16–1.45 (9H, m, 1,2,5,6,7-H), 1.04 (3H, s, 14-H), 0.87 (3H, s, 16-H). 13C NMR (100 MHz, D2O) δ 176.6, 176.2, 173.5, 173.2, 160.6 (1JCF = 244.8 Hz), 155.8, 148.6, 147.2, 145.3, 135.4, 130.2, 125.6, 125.3, 121.5 (2JCF = 24.2 Hz), 119.3, 109.5 (2JCF = 21.5 Hz), 109.1, 75.7, 65.4, 62.3, 47.7, 43.1, 40.3, 36.2, 33.6, 32.5, 32.3, 32.0, 30.3, 27.5, 24.3, 21.3, 14.8 ppm. MS (ESI) m/z (%) 634.2 ([M + H]+, 100%). HRMS (ESI) calcd for C33H38FNO8K [M + H]+ 634.2218, found 634.2231.
Potassium 4-(((1R,2R,4aR,5R,8aS)-2-(3-carboxypropanoyloxy)-5-((E)-2-(6-chloroquinolin-2-yl)vinyl)-1,4a-dimethyl-6-methylenedecahydronaphthalen-1-yl)methoxy)-4-oxobutanoate 9j: offwhite solid, 1.47 g, yield 56.98%, purity 95.3%. IR (KBr) νmax 3422, 3084, 2933, 2854, 1756, 1725, 1634, 1576, 1445, 1384, 1346, 1277, 1237, 1114, 1034, 973, 894 cm−1. 1H NMR (600 MHz, D2O) δ 8.27–8.20 (1H, m, 4′-H), 8.12 (1H, d, J = 8.3 Hz, 8′-H), 7.83 (1H, d, J = 8.3 Hz, 7′-H), 7.36 (1H, d, J = 8.3 Hz, 3′-H), 7.14–7.07 (1H, m, 5′-H), 6.68 (1H, dd, J = 6.2 Hz, J = 15.4 Hz, 11-H), 6.52 (1H, d, J = 15.4 Hz, 12-H), 5.04 (1H, s, 13-Ha), 4.86 (1H, s, 13-Hb), 4.15 (1H, d, J = 11.8 Hz, 15-Ha), 4.05–3.92 (1H, m, 3-H), 3.82 (1H, d, J = 11.8 Hz, 15-Hb), 2.82–2.75 (9H, m, 9,18,19,22,23-H), 2.15–1.45 (9H, m, 1,2,5,6,7-H), 1.05 (3H, s, 14-H), 0.83 (3H, s, 16-H). 13C NMR (100 MHz, D2O) δ 176.6, 176.2, 173.6, 173.2, 156.4, 148.6, 146.3, 135.4, 132.2, 131.4, 130.1, 128.7, 125.6, 125.3, 123.5, 119.2, 109.3, 75.8, 65.2, 61.7, 47.4, 43.3, 39.4, 36.1, 33.5, 32.6, 32.2, 32.0, 30.3, 27.2, 24.5, 21.2, 15.1 ppm. MS (ESI) m/z (%) 650.2 ([M + H]+, C33H3835ClNO8K, 100%), 652.2 ([M + H]+, C33H3837ClNO8K, 33%). HRMS (ESI) calcd for C33H3835ClNO8K [M + H]+ 650.1970, found 650.1978; for C33H3837ClNO8K [M + H]+ 652.1941, found 652.1951.
Potassium 4-(((1R,2R,4aR,5R,8aS)-5-((E)-2-(6-bromoquinolin-2-yl)vinyl)-2-(3-carboxypropanoyloxy)-1,4a-dimethyl-6-methylenedecahydronaphthalen-1-yl)methoxy)-4-oxobutanoate 9k: off white solid, 1.41 g, yield 52.03%, purity 96.8%. IR (KBr) νmax 3416, 3078, 2934, 2855, 1755, 1723, 1636, 1574, 1447, 1385, 1346, 1273, 1235, 1114, 1035, 973, 886 cm−1. 1H NMR (600 MHz, D2O) δ 8.28–8.05 (4H, m, 4′,5′,7′,8′-H), 7.46 (1H, d, J = 8.3 Hz, 3′-H), 6.63 (1H, dd, J = 6.2 Hz, J = 15.4 Hz, 11-H), 6.51 (1H, d, J = 15.4 Hz, 12-H), 5.04 (1H, s, 13-Ha), 4.86 (1H, s, 13-Hb), 4.15 (1H, d, J = 11.8 Hz, 15-Ha), 4.06–3.95 (1H, m, 3-H), 3.83 (1H, d, J = 11.8 Hz, 15-Hb), 2.87–2.74 (9H, m, 9,18,19,22,23-H), 2.16–1.45 (9H, m, 1,2,5,6,7-H), 1.06 (3H, s, 14-H), 0.94 (3H, s, 16-H). 13C NMR (100 MHz, D2O) δ 176.7, 176.3, 173.6, 173.3, 156.4, 148.5, 148.1, 135.3, 133.4, 132.1, 129.7, 128.5, 125.5, 125.2, 123.4, 120.3, 109.3, 75.7, 65.3, 61.2, 48.4, 43.7, 40.6, 36.7, 33.4, 32.7, 32.5, 32.0, 30.5, 27.6, 24.4, 20.7, 15.0 ppm. MS (ESI) m/z (%) 694.1 ([M + H]+, C33H3879BrNO8K, 100%), 696.1 ([M + H]+, C33H3881BrNO8K, 97%). HRMS (ESI) calcd for C33H3879BrNO8K [M + H]+ 694.1465, found 694.1475; for C33H3881BrNO8K [M + H]+ 696.1408, found 696.1418.
Potassium 4-(((1R,2R,4aR,5R,8aS)-2-(3-carboxypropanoyloxy)-5-((E)-2-(6-hydroxyquinolin-2-yl)vinyl)-1,4a-dimethyl-6-methylenedecahydronaphthalen-1-yl)methoxy)-4-oxobutanoate 9l: creamy white solid, 1.36 g, yield 54.18%, purity 95.9%. IR (KBr) νmax 3416, 3084, 2935, 2853, 1757, 1724, 1633, 1572, 1445, 1386, 1346, 1277, 1237, 1114, 1034, 973, 894 cm−1. 1H NMR (600 MHz, D2O) δ 8.08–8.02 (1H, m, 4′-H), 7.84 (1H, d, J = 8.3 Hz, 8′-H), 7.45–7.13 (3H, m, 3′,5′,7′-H), 6.68 (1H, dd, J = 6.2 Hz, J = 15.4 Hz, 11-H), 6.55 (1H, d, J = 15.4 Hz, 12-H), 5.36 (1H, s, 6′-OH), 5.04 (1H, s, 13-Ha), 4.87 (1H, s, 13-Hb), 4.17 (1H, d, J = 11.8 Hz, 15-Ha), 4.07–3.95 (1H, m, 3-H), 3.85 (1H, d, J = 11.8 Hz, 15-Hb), 2.87–2.77 (9H, m, 9,18,19,22,23-H), 2.17–1.45 (9H, m, 1,2,5,6,7-H), 1.03 (3H, s, 14-H), 0.91 (3H, s, 16-H). 13C NMR (100 MHz, D2O) δ 176.6, 176.4, 173.5, 173.3, 155.6, 154.2, 148.6, 143.2, 134.5, 130.6, 128.6, 125.6, 125.3, 125.0, 119.2, 111.4, 109.1, 75.7, 65.3, 61.5, 47.5, 43.2, 39.5, 36.5, 33.5, 32.6, 32.2, 32.0, 30.1, 27.4, 24.6, 20.3, 15.2 ppm. MS (ESI) m/z (%) 632.2 ([M + H]+, 100%). HRMS (ESI) calcd for C33H39NO9K [M + H]+ 632.2310, found 632.2322.
Potassium 4-(((1R,2R,4aR,5R,8aS)-2-(3-carboxypropanoyloxy)-1,4a-dimethyl-6-methylene-5-((E)-2-(7-methylquinolin-2-yl)vinyl)decahydronaphthalen-1-yl)methoxy)-4-oxobutanoate 9m: white solid, 1.59 g, yield 63.60%, purity 97.9%. IR (KBr) νmax 3421, 3084, 2933, 2852, 1753, 1722, 1633, 1572, 1444, 1385, 1344, 1274, 1233, 1116, 1035, 973, 892 cm−1. 1H NMR (600 MHz, D2O) δ 8.23 (1H, d, J = 8.2 Hz, 4′-H), 7.90–7.58 (3H, m, 5′,6′,8′-H), 7.35 (1H, dd, J = 8.2 Hz, J = 4.3 Hz, 3′-H), 6.64 (1H, dd, J = 6.2 Hz, J = 15.4 Hz, 11-H), 6.52 (1H, d, J = 15.4 Hz, 12-H), 5.04 (1H, s, 13-Ha), 4.85 (1H, s, 13-Hb), 4.17 (1H, d, J = 11.8 Hz, 15-Ha), 4.04–3.95 (1H, m, 3-H), 3.83 (1H, d, J = 11.8 Hz, 15-Hb), 2.83–2.75 (9H, m, 9,18,19,22,23-H), 2.35 (3H, s, 7′-CH3), 2.17–1.46 (9H, m, 1,2,5,6,7-H), 1.04 (3H, s, 14-H), 0.87 (3H, s, 16-H). 13C NMR (100 MHz, D2O) δ 176.7, 176.1, 173.4, 173.1, 156.5, 148.7, 146.4, 139.4, 136.2, 130.5, 128.3, 127.1, 126.4, 125.6, 125.2, 117.4, 109.1, 75.5, 65.3, 62.4, 46.6, 43.4, 40.2, 36.3, 33.6, 32.6, 32.2, 32.0, 30.2, 27.3, 24.6, 21.3, 20.5, 15.1 ppm. MS (ESI) m/z (%) 630.2 ([M + H]+, 100%). HRMS (ESI) calcd for C24H41NO8K [M + H]+ 630.2469, found 630.2480.
Potassium 4-(((1R,2R,4aR,5R,8aS)-2-(3-carboxypropanoyloxy)-5-((E)-2-(7-methoxyquinolin-2-yl)vinyl)-1,4a-dimethyl-6-methylenedecahydronaphthalen-1-yl)methoxy)-4-oxobutanoate 9n: creamy white solid, 1.66 g, yield 66.00%, purity 96.7%. IR (KBr) νmax 3419, 3086, 2933, 2854, 1756, 1723, 1635, 1575, 1447, 1386, 1342, 1273, 1235, 1116, 1034, 971, 892 cm−1. 1H NMR (600 MHz, D2O) δ 8.26 (1H, d, J = 8.2 Hz, 4′-H), 7.95–7.90 (1H, m, 5′-H), 7.38–7.26 (3H, m, 3′,6′,8′-H), 6.67 (1H, dd, J = 6.2 Hz, J = 15.4 Hz, 11-H), 6.55 (1H, d, J = 15.4 Hz, 12-H), 5.05 (1H, s, 13-Ha), 4.85 (1H, s, 13-Hb), 4.17 (1H, d, J = 11.8 Hz, 15-Ha), 4.06–3.95 (1H, m, 3-H), 3.86 (1H, s, 7′-OCH3), 3.82 (1H, d, J = 11.8 Hz, 15-Hb), 2.88–2.75 (9H, m, 9,18,19,22,23-H), 2.16–1.45 (9H, m, 1,2,5,6,7-H), 1.04 (3H, s, 14-H), 0.93 (3H, s, 16-H). 13C NMR (100 MHz, D2O) δ 176.5, 176.0, 173.2, 173.0, 156.5, 151.6, 148.3, 147.4, 136.5, 129.5, 128.7, 125.5, 125.1, 117.6, 116.5, 109.1, 107.4, 75.7, 65.4, 62.2, 55.4, 46.5, 43.3, 40.2, 36.6, 33.5, 32.7, 32.5, 32.0, 30.5, 27.4, 24.3, 20.6, 15.3 ppm. MS (ESI) m/z (%) 646.2 ([M + H]+, 100%). HRMS (ESI) calcd for C34H41NO9K [M + H]+ 646.2418, found 646.2429.
Potassium 4-(((1R,2R,4aR,5R,8aS)-2-(3-carboxypropanoyloxy)-5-((E)-2-(7-fluoroquinolin-2-yl)vinyl)-1,4a-dimethyl-6-methylenedecahydronaphthalen-1-yl)methoxy)-4-oxobutanoate 9o: off white solid, 1.34 g, yield 53.17%, purity 96.7%. IR (KBr) νmax 3432, 3084, 2938, 2854, 1753, 1725, 1633, 1575, 1444, 1384, 1346, 1275, 1235, 1114, 1036, 973, 892 cm−1. 1H NMR (600 MHz, D2O) δ 8.25–8.12 (1H, m, 4′-H), 8.07–7.98 (1H, m, 5′-H), 7.76–7.64 (1H, m, 8′-H), 7.35 (1H, d, J = 8.3 Hz, 3′-H), 7.16–7.08 (1H, m, 6′-H), 6.63 (1H, dd, J = 6.2 Hz, J = 15.4 Hz, 11-H), 6.51 (1H, d, J = 15.4 Hz, 12-H), 5.05 (1H, s, 13-Ha), 4.86 (1H, s, 13-Hb), 4.17 (1H, d, J = 11.8 Hz, 15-Ha), 4.05–3.95 (1H, m, 3-H), 3.86 (1H, d, J = 11.8 Hz, 15-Hb), 2.86–2.75 (9H, m, 9,18,19,22,23-H), 2.15–1.45 (9H, m, 1,2,5,6,7-H), 1.04 (3H, s, 14-H), 0.91 (3H, s, 16-H). 13C NMR (100 MHz, D2O) δ 176.7, 176.2, 173.5, 173.0, 163.6 (1JCF = 245.5 Hz), 157.2, 148.3, 147.4, 136.2, 130.3, 125.5, 125.1, 118.6, 116.3 (2JCF = 22.2 Hz), 112.8 (2JCF = 21.5 Hz), 109.5, 78.7, 65.4, 62.4, 47.5, 43.5, 40.4, 36.2, 33.3, 32.6, 32.3, 32.0, 30.1, 27.2, 24.5, 21.2, 20.1, 15.1 ppm. MS (ESI) m/z (%) 634.2 ([M + H]+, 100%). HRMS (ESI) calcd for C33H38FNO8K [M + H]+ 634.2218, found 634.2232.
Potassium 4-(((1R,2R,4aR,5R,8aS)-2-(3-carboxypropanoyloxy)-5-((E)-2-(7-chloroquinolin-2-yl)vinyl)-1,4a-dimethyl-6-methylenedecahydronaphthalen-1-yl)methoxy)-4-oxobutanoate 9p: off white solid, 1.42 g, yield 55.04%, purity 96.1%. IR (KBr) νmax 3432, 3083, 2934, 2853, 1755, 1721, 1633, 1572, 1444, 1384, 1346, 1275, 1235, 1116, 1034, 973, 890 cm−1. 1H NMR (600 MHz, D2O) δ 8.26–8.13 (2H, m, 4′,5′-H), 7.90–7.62 (2H, m, 6′,8′-H), 7.34 (1H, d, J = 8.3 Hz, 3′-H), 6.67 (1H, dd, J = 6.2 Hz, J = 15.4 Hz, 11-H), 6.54 (1H, d, J = 15.4 Hz, 12-H), 5.05 (1H, s, 13-Ha), 4.85 (1H, s, 13-Hb), 4.17 (1H, d, J = 11.8 Hz, 15-Ha), 4.03–3.95 (1H, m, 3-H), 3.83 (1H, d, J = 11.8 Hz, 15-Hb), 2.84–2.75 (9H, m, 9,18,19,22,23-H), 2.13–1.45 (9H, m, 1,2,5,6,7-H), 1.03 (3H, s, 14-H), 0.91 (3H, s, 16-H). 13C NMR (100 MHz, D2O) δ 177.6, 176.4, 173.5, 173.1, 157.6, 148.8, 147.2, 136.1, 135.4, 129.5, 128.4, 126.4, 125.5, 125.1, 124.8, 118.5, 109.1, 75.8, 65.5, 62.3, 47.4, 43.3, 40.0, 36.4, 33.2, 32.7, 32.4, 32.1, 30.2, 27.6, 24.2, 21.3, 15.1 ppm. MS (ESI) m/z (%) 650.2 ([M + H]+, C33H3835ClNO8K, 100%), 652.2 ([M + H]+, C33H3837ClNO8K, 33%). HRMS (ESI) calcd for C33H3835ClNO8K [M + H]+ 650.1970, found 650.1979; for C33H3837ClNO8K [M + H]+ 652.1941, found 652.1953.
Potassium 4-(((1R,2R,4aR,5R,8aS)-2-(3-carboxypropanoyloxy)-5-((E)-2-(7-hydroxyquinolin-2-yl)vinyl)-1,4a-dimethyl-6-methylenedecahydronaphthalen-1-yl)methoxy)-4-oxobutanoate 9q: white solid, 1.46 g, yield 58.17%, purity 95.7%. IR (KBr) νmax 3421, 3083, 2934, 2855, 1756, 1723, 1636, 1575, 1444, 1384, 1346, 1274, 1235, 1114, 1033, 973, 892 cm−1. 1H NMR (600 MHz, D2O) δ 8.26–8.15 (1H, m, 4′-H), 8.04–7.96 (1H, m, 5′-H), 7.36–7.18 (3H, m, 3′,6′,8′-H), 6.67 (1H, dd, J = 6.2 Hz, J = 15.4 Hz, 11-H), 6.55 (1H, d, J = 15.4 Hz, 12-H), 5.35 (1H, brs, 7′-OH), 5.06 (1H, s, 13-Ha), 4.86 (1H, s, 13-Hb), 4.17 (1H, d, J = 11.8 Hz, 15-Ha), 4.01–3.95 (1H, m, 3-H), 3.86 (1H, d, J = 11.8 Hz, 15-Hb), 2.84–2.75 (9H, m, 9,18,19,22,23-H), 2.15–1.43 (9H, m, 1,2,5,6,7-H), 1.03 (3H, s, 14-H), 0.91 (3H, s, 16-H). 13C NMR (100 MHz, D2O) δ 176.6, 176.1, 173.7, 173.1, 158.5, 156.8, 151.5, 148.6, 136.2, 130.6, 125.5, 125.1, 123.3, 118.6, 116.4, 110.8, 109.1, 75.8, 65.5, 62.4, 47.4, 43.3, 40.3, 36.8, 33.5, 32.5, 32.3, 32.0, 30.3, 27.4, 24.7, 20.6, 15.1 ppm. MS (ESI) m/z (%) 632.2 ([M + H]+, 100%). HRMS (ESI) calcd for C33H39NO9K [M + H]+ 632.2310, found 632.2324.
Biological assays
The anti-platelet aggregation activities of 8a and 9a–9q were assessed using SD (Sprague Dawley) male rat arterial blood in vitro. Thrombin and ADP were used to induce platelet aggregation. Vorapaxar sulfate and aspirin were selected as positive controls. Fresh arterial blood was taken from the groin of SD male rats (180–220 g per rat) with 3.8% sodium citrate as anticoagulant (9:1 by volume). Then, whole blood samples were centrifuged at 1000 rpm/min for 10 min at room temperature to give platelet-rich plasma (PRP-1). The residue continued to be centrifuged at 3000 rpm/min for 10 min at room temperature to prepare platelet-poor plasma (PPP). PPP was used as the blank control. The measurement range of the platelet aggregation apparatus was set according to the number of platelets of human blood. The number of platelets of human blood is (100–300) × 109 L−1, but the number of platelets of rats is (600–1000) × 109 L−1. In order to measure the platelet aggregation rate accurately, PPP was added to PRP to dilute the number of rat platelets to ~150 × 109 L−1 (PRP-2).[50] The sample group solution (5 µL), with the target compound dissolved in normal saline (1.7 μmol/L) in advance, was added into PRP-2 (200 µL) and the mixture was incubated for 2 min, as well as positive controls. Normal saline (5 µL) was used as negative control with the procedure as above. Afterwards, adding 20 µL ADP (5 mM/L) or thrombin (0.1 U/mL) respectively induced platelet aggregation. IR was calculated with the formula IR = [1 − (SG/NC)] × 100% where SG and NC represent the platelet aggregation rates of sample group and negative control respectively.
Cytotoxicity assay in vitro
Mouse fibroblast cells (L929) were used to evaluate the cell toxicity of target derivatives 9 with Cell Counting Kit-8 (CCK-8) assays.[51] First, test compounds were dissolved and diluted to 10 and 100 μM/L with DMSO. Cells were added to 96-well microplates (1 × 104 cell/well) and then cultivated at 37°C in a humidified atmosphere of CO2 (5%) for 24 h. Second, test compounds were added into the cells and incubation was continued at 37°C for 48 h. Third, the medium was removed and 100 µL of fresh complete medium of RPMI-1640 was added. CCK-8 solution was added to the microplates at 10 μL per well. Last, the absorbance of the test solution was measured at 450 nm on a Bio-Tek Flx800 fluorescence microplate reader. According to the formula to calculate relative survival rate: relative survival rate (%) = {[Abs(test cells) − Abs(blank cells)]/[Abs(controlled cells) − Abs(blank cells)]} × 100% (Abs, absorbance).
Statistical analysis
Results are presented as the means ± s.e. (SEM). Data were analyzed with one-way ANOVA (SPSS software) to measure statistical significance of the differences. The level of significance was considered at P < 0.05 and P < 0.01.
Data availability
The data that support this study are available in the article.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This work was funded by Natural Science Foundation of Jiangxi Province grant no. 20212BAB206073 and earmarked fund for China Agriculture Research System of MOF and MARA Grant CARS-21.
References
[1] LP Köse, İ Gülçin, AC Gören, J Namiesnik, AL Martinez-Ayala, S Gorinstein, LC-MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind Crop Prod 2015, 74, 712.| LC-MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes.Crossref | GoogleScholarGoogle Scholar |
[2] E Bursal, İ Gülçin, Polyphenol contents and in vitro antioxidant activities of lyophilised aqueous extract of kiwifruit (Actinidia deliciosa). Food Res Int 2011, 44, 1482.
| Polyphenol contents and in vitro antioxidant activities of lyophilised aqueous extract of kiwifruit (Actinidia deliciosa).Crossref | GoogleScholarGoogle Scholar |
[3] İ Gülçin, E Bursal, MH Şehitoğlu, M Bilsel, AC Gören, Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem Toxicol 2010, 48, 2227.
| Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey.Crossref | GoogleScholarGoogle Scholar |
[4] A Sharma, K Lal, SS Handa, Standardization of the indian crude drug kalmegh by high pressure liquid chromatographic determination of andrographolide. Phytochem Anal 1992, 3, 129.
| Standardization of the indian crude drug kalmegh by high pressure liquid chromatographic determination of andrographolide.Crossref | GoogleScholarGoogle Scholar |
[5] A Paemanee, A Hitakarun, P Wintachai, S Roytrakul, DR Smith, A proteomic analysis of the anti-dengue virus activity of andrographolide. Biomed Pharmacother 2019, 109, 322.
| A proteomic analysis of the anti-dengue virus activity of andrographolide.Crossref | GoogleScholarGoogle Scholar |
[6] J Lu, Y Ma, J Wu, H Huang, X Wang, Z Chen, J Chen, H He, C Huang, A review for the neuroprotective effects of andrographolide in the central nervous system. Biomed Pharmacother 2019, 117, 109078.
| A review for the neuroprotective effects of andrographolide in the central nervous system.Crossref | GoogleScholarGoogle Scholar |
[7] C Tang, Y Liu, B Wang, G Gu, L Yang, Y Zheng, H Qian, W Huang, Synthesis and biological evaluation of andrographolide derivatives as potent anti-HIV agents. Arch Pharm 2012, 345, 647.
| Synthesis and biological evaluation of andrographolide derivatives as potent anti-HIV agents.Crossref | GoogleScholarGoogle Scholar |
[8] J Li, F Li, F Tang, J Zhang, R Li, D Sheng, SM-Y Lee, G-C Zhou, GP-H Leung, AGS-30, an andrographolide derivative, suppresses tumor angiogenesis and growth in vitro and in vivo. Biochem Pharmacol 2020, 171, 113694.
| AGS-30, an andrographolide derivative, suppresses tumor angiogenesis and growth in vitro and in vivo.Crossref | GoogleScholarGoogle Scholar |
[9] H Xia, J Xue, H Xu, M Lin, M Shi, Q Sun, T Xiao, X Dai, L Wu, J Li, Q Xiang, H Tang, Q Bian, Q Liu, Andrographolide antagonizes the cigarette smoke-induced epithelial–mesenchymal transition and pulmonary dysfunction through anti-inflammatory inhibiting HOTAIR. Toxicology 2019, 422, 84.
| Andrographolide antagonizes the cigarette smoke-induced epithelial–mesenchymal transition and pulmonary dysfunction through anti-inflammatory inhibiting HOTAIR.Crossref | GoogleScholarGoogle Scholar |
[10] R Husen, AHL Pihie, M Nallappan, Screening for antihyperglycaemic activity in several local herbs of Malaysia. J Ethanopharmacol 2004, 95, 205.
| Screening for antihyperglycaemic activity in several local herbs of Malaysia.Crossref | GoogleScholarGoogle Scholar |
[11] SY Zhao, W Wei, XM Yin, XQ Wang, H Xie, DF Wu, The platelet aggregation activity of Xiyanping induced by ADP. Pract Clin Res Chin Med 2016, 16, 81.
[12] X-M Yin, S-Y Zhao, X-Q Wang, H Xie, D-F Wu, The platelet aggregation activity of Chuanhuning induced by ADP. Pract Clin Res Chin Med 2017, 5, 48.
[13] S-L Duan, W Wei, X-M Yin, H Xie, D-F Wu, S-Y Zhao, The platelet aggregation activity of andrographolide and its sulfonate. Pract Clin Res Chin Med 2016, 16, 83.
[14] S Kapishnikov, T Staalsø, Y Yang, J Lee, AJ Pérez-Berná, E Pereiro, Y Yang, S Werner, P Guttmann, L Leiserowitz, J Als-Nielsen, Mode of action of quinoline antimalarial drugs in red blood cells infected by Plasmodium falciparum revealed in vivo. Proc Natl Acad Sci U S A 2019, 116, 22946.
| Mode of action of quinoline antimalarial drugs in red blood cells infected by Plasmodium falciparum revealed in vivo.Crossref | GoogleScholarGoogle Scholar |
[15] M-G Kayirere, A Mahamoud, J Chevalier, J-C Soyfer, A Crémieux, J Barbe, Synthesis and antibacterial activity of new 4-alkoxy, 4-aminoalkyl and 4-alkylthioquinoline derivatives. Eur J Med Chem 1998, 33, 55.
| Synthesis and antibacterial activity of new 4-alkoxy, 4-aminoalkyl and 4-alkylthioquinoline derivatives.Crossref | GoogleScholarGoogle Scholar |
[16] V Gayam, S Ravi, GMVNAR Ravikumar, A Thangamani, Synthesis, anticancer activity and molecular docking studies of some novel quinoline hydrazide derivatives of substituted benzaldehydes. Rasayan J Chem 2019, 12, 880.
| Synthesis, anticancer activity and molecular docking studies of some novel quinoline hydrazide derivatives of substituted benzaldehydes.Crossref | GoogleScholarGoogle Scholar |
[17] A Ouchi, M Nakano, S Nagaoka, K Mukai, Kinetic study of the antioxidant activity of pyrroloquinolinequinol (PQQH2, a reduced form of pyrroloquinolinequinone) in micellar solution. J Agric Food Chem 2009, 57, 450.
| Kinetic study of the antioxidant activity of pyrroloquinolinequinol (PQQH2, a reduced form of pyrroloquinolinequinone) in micellar solution.Crossref | GoogleScholarGoogle Scholar |
[18] J Ramprasad, V Kumar Sthalam, R Linga Murthy Thampunuri, S Bhukya, R Ummanni, S Balasubramanian, S Pabbaraja, Synthesis and evaluation of a novel quinoline-triazole analogs for antitubercular properties via molecular hybridization approach. Bioorg Med Chem Lett 2019, 29, 126671.
| Synthesis and evaluation of a novel quinoline-triazole analogs for antitubercular properties via molecular hybridization approach.Crossref | GoogleScholarGoogle Scholar |
[19] VV Kouznetsov, LY Vargas Méndez, CE Puerto Galvis, MC Ortiz Villamizar, The direct C–H alkenylation of quinoline N-oxides as a suitable strategy for the synthesis of promising antiparasitic drugs. New J Chem 2020, 44, 12.
| The direct C–H alkenylation of quinoline N-oxides as a suitable strategy for the synthesis of promising antiparasitic drugs.Crossref | GoogleScholarGoogle Scholar |
[20] J Yu, WS Hu, Effects of neferine on platelet aggregation in rabbits. Acta Pharm Sin 1997, 32, 1. [PMID: 11243209]
[21] RM Jin, CX Chen, YK Li, PK Xu, Effect of rhyncophylline on platelet aggregation and experimental thrombosis. Acta Pharm Sin 1991, 26, 246.
[22] CG Huang, ZL Chu, ZM Yang, Effects of berberine on synthesis of platelet TXA2 and plasma PGI2 in rabbits. Acta Pharm Sin 1991, 12, 526.
[23] G De Luca, S Savonitto, AWJ van’t Hof, H Suryapranata, Platelet GP IIb-IIIa Receptor Antagonists in Primary Angioplasty: Back to the Future. Drugs 2015, 11, 1229.
| Platelet GP IIb-IIIa Receptor Antagonists in Primary Angioplasty: Back to the Future.Crossref | GoogleScholarGoogle Scholar |
[24] FCF Brito, AE Kummerle, C Lugnier, CAM Fraga, EJ Barreiro, ALP Miranda, Novel thienylacylhydrazone derivatives inhibit platelet aggregation through cyclic nucleotides modulation and thromboxane A2 synthesis inhibition. Eur J Pharmacol 2010, 638, 5.
| Novel thienylacylhydrazone derivatives inhibit platelet aggregation through cyclic nucleotides modulation and thromboxane A2 synthesis inhibition.Crossref | GoogleScholarGoogle Scholar |
[25] Z Eskandariyan, M Esfahani Zadeh, K Haj Mohammad Ebrahim Tehrani, V Mashayekhi, F Kobarfard, Synthesis of thioether derivatives of quinazoline-4-one-2-thione and evaluation of their antiplatelet aggregation activity. Arch Pharm Res 2014, 37, 332.
| Synthesis of thioether derivatives of quinazoline-4-one-2-thione and evaluation of their antiplatelet aggregation activity.Crossref | GoogleScholarGoogle Scholar |
[26] AS Go, D Mozaffarian, VL Roger, EJ Benjamin, JD Berry, MJ Blaha, S Dai, ES Ford, CS Fox, S Franco, HJ Fullerton, C Gillespie, SM Hailpern, JA Heit, VJ Howard, MD Huffman, SE Judd, BM Kissela, SJ Kittner, DT Lackland, JH Lichtman, LD Lisabeth, RH Mackey, DJ Magid, GM Marcus, A Marelli, DB Matchar, DK McGuire, ER Mohler, CS Moy, ME Mussolino, RW Neumar, G Nichol, DK Pandey, NP Paynter, MJ Reeves, PD Sorlie, J Stein, A Towfighi, TN Turan, SS Virani, ND Wong, D Woo, MB Turner, Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2014, 129, 399.
| Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association.Crossref | GoogleScholarGoogle Scholar |
[27] DJ Angiolillo, D Capodanno, S Goto, Platelet thrombin receptor antagonism and atherothrombosis. Eur Heart J 2010, 31, 17.
| Platelet thrombin receptor antagonism and atherothrombosis.Crossref | GoogleScholarGoogle Scholar |
[28] M Berrettini, M De Cunto, P Parise, S Grasselli, GG Nenci, “In vitro” and “ex vivo” effects of picotamide, a combined thromboxane A2-synthase inhibitor and -receptor antagonist, on human platelets. Eur J Clin Pharmacol 1990, 39, 495.
| “In vitro” and “ex vivo” effects of picotamide, a combined thromboxane A2-synthase inhibitor and -receptor antagonist, on human platelets.Crossref | GoogleScholarGoogle Scholar |
[29] S Ray, Clopidogrel resistance: the way forward. Indian Heart J 2014, 66, 530.
| Clopidogrel resistance: the way forward.Crossref | GoogleScholarGoogle Scholar |
[30] WR Hiatt, SR Money, EP Brass, Long-term safety of cilostazol in patients with peripheral artery disease: the CASTLE study (Cilostazol: A Study in Long-term Effects). J Vasc Surg 2008, 47, 330.
| Long-term safety of cilostazol in patients with peripheral artery disease: the CASTLE study (Cilostazol: A Study in Long-term Effects).Crossref | GoogleScholarGoogle Scholar |
[31] I Kajiwara, H Soejima, S Miyamoto, H Ogawa, Effects of additional treatment of sarpogrelate to aspirin therapy on platelet aggregation and plasma plasminogen activator inhibitor activity in patients with stable effort angina. Thromb Res 2011, 128, 547.
| Effects of additional treatment of sarpogrelate to aspirin therapy on platelet aggregation and plasma plasminogen activator inhibitor activity in patients with stable effort angina.Crossref | GoogleScholarGoogle Scholar |
[32] P Piccini, A Nuti, AM Paoletti, A Napolitano, GB Melis, U Bonuccelli, Possible involvement of dopaminergic mechanisms in the antimigraine action of flunarizine. Cephalalgia 1990, 10, 3.
| Possible involvement of dopaminergic mechanisms in the antimigraine action of flunarizine.Crossref | GoogleScholarGoogle Scholar |
[33] JE Belforte, C Magariños-Azcone, I Armando, W Buño, JH Pazo, Pharmacological involvement of the calcium channel blocker flunarizine in dopamine transmission at the striatum. Parkinsonism Relat Disord 2001, 8, 33.
| Pharmacological involvement of the calcium channel blocker flunarizine in dopamine transmission at the striatum.Crossref | GoogleScholarGoogle Scholar |
[34] P Cortelli, M Santucci, F Righetti, P Pirazzoli, F Albani, A Baruzzi, T Sacquegna, E Cacciari, Neuroendocrinological evidence of an anti-dopaminergic effect of flunarizine. Acta Neurol Scand 1988, 77, 289.
| Neuroendocrinological evidence of an anti-dopaminergic effect of flunarizine.Crossref | GoogleScholarGoogle Scholar |
[35] MH Namazi, M Safi, H Vakili, H Saadat, E Karimi, RK Bagheri, Comparison between intracoronary abciximab and intravenous eptifibatide administration during primary percutaneous coronary intervention of acute ST-segment elevation myocardial infarction. J Tehran Heart Cent 2013, 8, 132.
[36] DJ Whellan, P Tricoci, E Chen, Z Huang, D Leibowitz, P Vranckx, GD Marhefka, C Held, JC Nicolau, RF Storey, W Ruzyllo, K Huber, P Sinnaeve, AT Weiss, J-P Dery, DJ Moliterno, F Van de Werf, PE Aylward, HD White, PW Armstrong, L Wallentin, J Strony, RA Harrington, KW Mahaffey, Vorapaxar in acute coronary syndrome patients undergoing coronary artery bypass graft surgery: subgroup analysis from the TRACER trial (Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome). J Am Coll Cardiol 2014, 63, 1048.
| Vorapaxar in acute coronary syndrome patients undergoing coronary artery bypass graft surgery: subgroup analysis from the TRACER trial (Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome).Crossref | GoogleScholarGoogle Scholar |
[37] C Held, P Tricoci, Z Huang, F Van de Werf, HD White, PW Armstrong, G Ambrosio, PE Aylward, DJ Moliterno, L Wallentin, E Chen, A Erkan, L Jiang, J Strony, RA Harrington, KW Mahaffey, Vorapaxar, a platelet thrombin-receptor antagonist, in medically managed patients with non-ST-segment elevation acute coronary syndrome: results from the TRACER trial. Eur Heart J Acute Cardiovasc Care 2014, 3, 246.
| Vorapaxar, a platelet thrombin-receptor antagonist, in medically managed patients with non-ST-segment elevation acute coronary syndrome: results from the TRACER trial.Crossref | GoogleScholarGoogle Scholar |
[38] AA Kei, M Florentin, DP Mikhailidis, MS Elisaf, EN Liberopoulos, Review: antiplatelet drugs: what comes next? Clin Appl Thromb Hemost 2011, 17, 9.
| Review: antiplatelet drugs: what comes next?Crossref | GoogleScholarGoogle Scholar |
[39] R Guthrie, Review and management of side effects associated with antiplatelet therapy for prevention of recurrent cerebrovascular events. Adv Ther 2011, 28, 473.
| Review and management of side effects associated with antiplatelet therapy for prevention of recurrent cerebrovascular events.Crossref | GoogleScholarGoogle Scholar |
[40] NE Barrett, L Holbrook, S Jones, WJ Kaiser, LA Moraes, R Rana, T Sage, RG Stanley, KL Tucker, B Wright, JM Gibbins, Future innovations in anti-platelet therapies. Br J Pharmacol 2008, 154, 918.
| Future innovations in anti-platelet therapies.Crossref | GoogleScholarGoogle Scholar |
[41] T Kosoglou, P Statkevich, B Kumar, F Xuan, JE Schiller, AO Johnson-Levonas, S Young, DL Cutler, The effect of multiple doses of ketoconazole or rifampin on the single- and multiple-dose pharmacokinetics of vorapaxar. J Clin Pharmacol 2013, 53, 540.
| The effect of multiple doses of ketoconazole or rifampin on the single- and multiple-dose pharmacokinetics of vorapaxar.Crossref | GoogleScholarGoogle Scholar |
[42] BM Scirica, MP Bonaca, E Braunwald, GM De Ferrari, D Isaza, BS Lewis, F Mehrhof, PA Merlini, SA Murphy, MS Sabatine, M Tendera, F Van de Werf, R Wilcox, DA Morrow, Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2°P-TIMI 50 trial. Lancet 2012, 380, 1317.
| Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2°P-TIMI 50 trial.Crossref | GoogleScholarGoogle Scholar |
[43] MP Bonaca, BM Scirica, MA Creager, J Olin, H Bounameaux, M Dellborg, JM Lamp, SA Murphy, E Braunwald, DA Morrow, Vorapaxar in Patients With Peripheral Artery Disease: Results From TRA2°P-TIMI 50. Circulation 2013, 127, 1522.
| Vorapaxar in Patients With Peripheral Artery Disease: Results From TRA2°P-TIMI 50.Crossref | GoogleScholarGoogle Scholar |
[44] HW Xu, GF Dai, GZ Liu, JF Wang, HM Liu, Synthesis of andrographolide derivatives: A new family of α-glucosidase inhibitors. Bioorg Med Chem 2007, 15, 4247.
| Synthesis of andrographolide derivatives: A new family of α-glucosidase inhibitors.Crossref | GoogleScholarGoogle Scholar |
[45] Y Luo, K Wang, M-h Zhang, D-y Zhang, Y-c Wu, X-m Wu, W-y Hua, Synthesis of new ent-labdane diterpene derivatives from andrographolide and evaluation on cytotoxic activities. Bioorg Med Chem Lett 2015, 25, 2421.
| Synthesis of new ent-labdane diterpene derivatives from andrographolide and evaluation on cytotoxic activities.Crossref | GoogleScholarGoogle Scholar |
[46] S Nanduri, VK Nyavanandi, SSR Thunuguntla, M Velisoju, S Kasu, S Rajagopal, RA Kumar, R Rajagopalan, J Iqbal, Novel routes for the generation of structurally diverse labdane diterpenes from andrographolide. Tetrahedron Lett 2004, 45, 4883.
| Novel routes for the generation of structurally diverse labdane diterpenes from andrographolide.Crossref | GoogleScholarGoogle Scholar |
[47] D Pi, K Jiang, H Zhou, Y Sui, Y Uozumi, K Zou, Iron-catalyzed C(sp3)–H functionalization of methyl azaarenes: a green approach to azaarene-substituted α- or β-hydroxy carboxylic derivatives and 2-alkenylazaarenes. RSC Adv 2014, 4, 57875.
| Iron-catalyzed C(sp3)–H functionalization of methyl azaarenes: a green approach to azaarene-substituted α- or β-hydroxy carboxylic derivatives and 2-alkenylazaarenes.Crossref | GoogleScholarGoogle Scholar |
[48] GVR Born, Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 1962, 194, 927.
| Aggregation of blood platelets by adenosine diphosphate and its reversal.Crossref | GoogleScholarGoogle Scholar |
[49] XJ He, JK Li, H Gao, F Qiu, X Cui, X Yao, Six new andrographolide metabolites in rats. Chem Pharm Bull 2003, 51, 586.
| Six new andrographolide metabolites in rats.Crossref | GoogleScholarGoogle Scholar |
[50] XM Yin, SY Zhao, DM Peng, LH Rao, Platelet aggregation test in SD rats. J Nanchang Univ (Med Sci) 2013, 53, 6.
[51] BJ Kim, YK Jung, Calpeptin suppresses tumor necrosis factor-α-induced death and accumulation of p53 in L929 mouse sarcoma cells. Apoptosis 2002, 7, 115.
| Calpeptin suppresses tumor necrosis factor-α-induced death and accumulation of p53 in L929 mouse sarcoma cells.Crossref | GoogleScholarGoogle Scholar |