Chemical constituents of lawang root oil
Neanne Alnafta A , Marlowe Graham A , Nicholas W. Proschogo A * and Christopher S. P. McErlean
A * and Christopher S. P. McErlean  A *
A *
A School of Chemistry, The University of Sydney, Sydney, NSW 2006, Australia.
Handling Editor: Craig Hutton
Australian Journal of Chemistry 76(3) 169-172 https://doi.org/10.1071/CH23013
Submitted: 20 January 2023 Accepted: 14 February 2023 Published: 28 April 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Spectroscopic analysis of lawang root oil identified methyl salicylate as the major component. This result contrasts with the known composition of bark-derived lawang oil, in which eugenol is the major component. The validity of the compositional analysis was confirmed by extraction of an authentic sample of plant root tissue, and comparison with the commercially available essential oil.
Keywords: chemical composition, culitlawan oil, essential oils, lawang root oil, mass spectrometry, methyl salicylate, paramao oil, structure elucidation.
The United Nation’s Comtrade database reported that the global market for essential oils was valued at almost US$35 billion in 2021.[1] One of the major challenges facing this international industry is quality assurance. For both regulatory purposes and customer satisfaction, a detailed knowledge of the chemical constituents of essential oils is necessary. The process for ensuring that essential oils are free from adulteration is called authentication, and employs many analytical techniques.[2] To be effective, these comparative analysis methods require knowledge of the chemical constituents of a pristine sample of the essential oil in question.[3–7] In this communication, we report for the first time the chemical composition of lawang root oil.
Lawang oil (also called paramao oil and culitlawan oil) is used in traditional Indonesian medicine for the topical treatment of pain associated with swollen joints. In recent years, it has found increasing use in Australia as a natural remedy for muscle pain, and is available from a number of commercial sources. To the best of our knowledge, only one pharmacological study of lawang oil has been published. In 1968, McMillan reported the use of lawang oil as a topical treatment for lymphatic filariasis, resulting from nematode infection.[8]
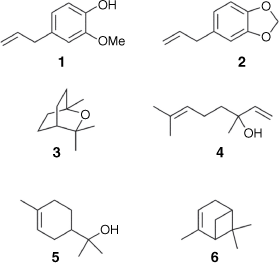
Lawang oil is traditionally isolated by steam distillation of the bark of Cinnamomum culitlawan (Lauraceae) that occurs in East Java.[9] Initially there was some confusion between lawang oil and massoi oil, but Meijer conclusively demonstrated that these two essential oils were distinct.[10] Meijer noted that lawang oil smelled of cloves as the result of its high eugenol concentration, whereas massoi oil contained no eugenol. Indeed, recent analysis of lawang oil by Arifin and co-workers revealed that the major components were eugenol (1) (38%), followed by safrole (2) (18%), eucalyptol (7%), linalool (3) (5%), α-terpineol (4) (4%) and α-pinene (5) (1%) (Fig. 1).[11] In contrast to the work on bark-derived lawang oil, until now there have been no investigations into the chemical composition of lawang root oil. Despite this, root-derived lawang oil is also used as a topical analgesic for the relief of muscle pain. Given that secondary metabolites are unequally distributed throughout plant tissues,[12] we thought that an investigation into the chemical constituents of lawang root oil was warranted.
|
Our investigation began with CGMS analysis of commercial lawang root oil, and a commercial lawang root oil infusion (in which the plant tissue is present). As shown in Fig. 2, both essential oils contained one major component. Comparison with an authentic sample of eugenol (1) conclusively demonstrated that lawang root oil does not contain this compound. Comparison of the mass spectrum fragmentation pattern of the major component against an MS library suggested that the compound was in fact methyl salicylate (7).
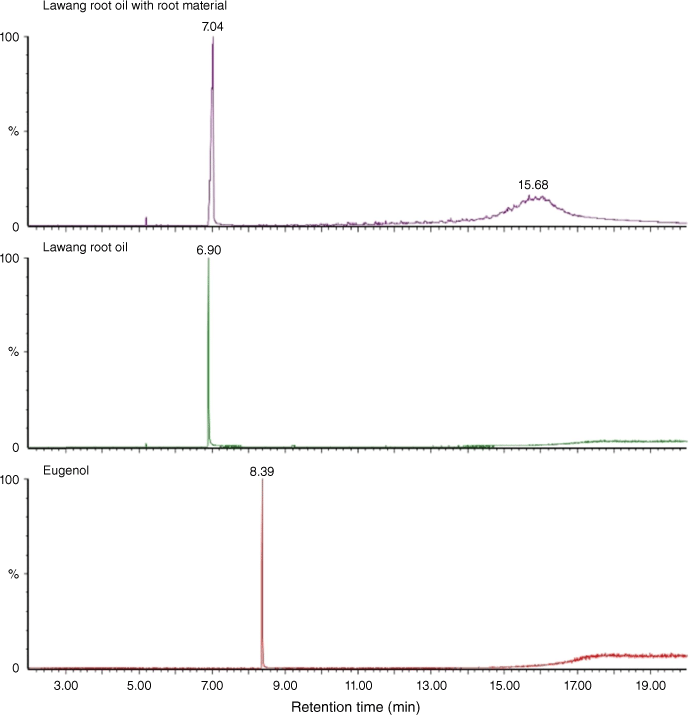
|
The relative homogeneity of the lawang root oil facilitated detailed NMR analysis. The 1H NMR spectrum featured a 3 H singlet at 3.95 ppm indicative of a methyl ester, and four resonances in the aromatic region that indicated an ortho-substituted benzene ring. 13C NMR spectroscopy confirmed the presence of a methyl benzoate with a carbonyl resonance at 170 ppm and a methyl resonance at 52 ppm. The identity of the ortho-substituent was ascertained as a phenol on the basis of the signal at 162 ppm in the 13C NMR spectrum. Together, these supported the assignment of the major component of lawang root oil as methyl salicylate (7) (Fig. 3).
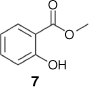
|
Comparative GCMS analysis of the two samples of lawang root oil against an authentic sample of methyl benzoate (7) unambiguously confirmed that 7 was the major component of this essential oil (Fig. 4).
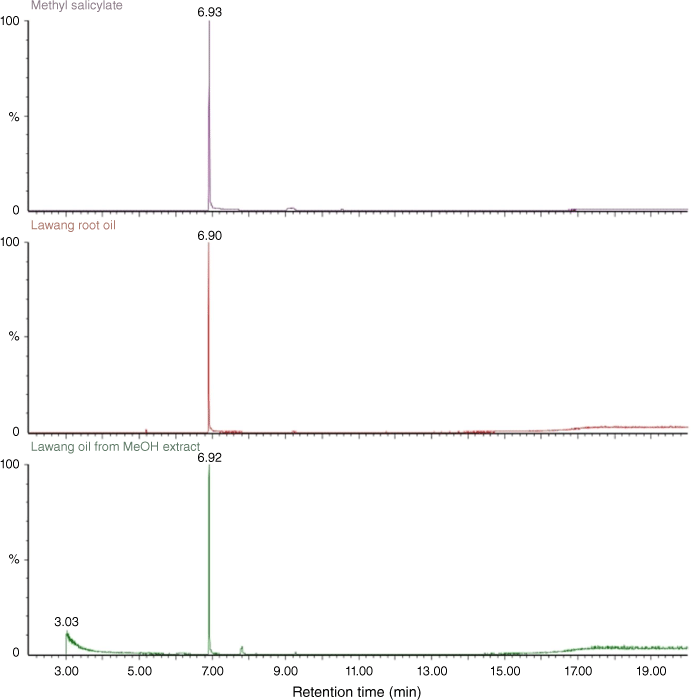
|
Given the previous uncertainties surrounding lawang oil, we investigated the chemical extracts from a sample of the plant material that is used in the commercial preparation. The plant material was generously donated by J.G. Davidson and A.G Holland (Directors of Gahn Elements). The root material was subjected to continuous extraction with methanol. GCMS comparison of the extract with the commercial lawang root oil confirmed that the methyl salicylate (7) originated from the root tissues.
Finally, although the commercial lawang oil consisted predominantly of methyl salicylate (7), both extraction of the commercial lawang root oil infusion and the extracted plant tissue also resulted in the isolation of waxy components that resisted separation and characterisation (see Supplementary Material).
In summary, we report the first investigation into the chemical composition of lawang root oil. In contrast to the bark-derived material, which contains eugenol, the major component of the root material is methyl salicylate. Given the long history of methyl salicylate (oil of wintergreen) as a treatment for muscular pain and its efficacy in the treatment of acute pain,[13] it is likely that this compound is responsible for the reported beneficial effects of topical lawang root oil application.
Experimental
All solvents and reagents were used as received from commercial sources. Lawang root oil (paramao root oil) was generously donated by Gahn Elements. Infrared spectra were acquired neat on a Bruker Alpha-E ATR spectrometer. 1H and 13C NMR spectra were recorded on a Bruker AVANCE DPX300 (1H frequency 300 MHz; 13C frequency 75 MHz). 1H chemical shifts are expressed as parts per million (ppm) with residual chloroform (δ 7.26) as reference and are reported as chemical shift (δΗ); relative integral; multiplicity (s, singlet; t, triplet; dd, doublet of doublets; td, triplet of doublets) and coupling constants (J) reported in hertz. 13C NMR chemical shifts are expressed as parts per million (ppm) with residual chloroform (δ 77.1) as internal reference and are reported as chemical shift (δC); multiplicity (assigned from DEPT experiments). GCMS spectra were recorded on a Perkin Elmer Clarus 680 with Clarus SQ 8 C electron ionisation (EI) quadrupole mass spectrometer, using a Perkin Elmer DB5 stationary phase (30 m × 250 μm × 25 μm film thickness). Injection port: 250°C; carrier gas: helium, 1.3 mL/min; split injection with 100:1; oven program 50°C for 1 min, then 15°C/min to 290°C, hold for 2 min; MS transfer line: 200°C; MS temperature: 150°C; EI voltage: 70 eV; MS solvent delay: 4 min; scan range: 45–600 m/z. High resolution mass spectra were recorded on a Thermo Velos Pro Orbitrap using the standard electrospray ionisation source and inbuilt syringe pump.
Sample preparation
A sample (0.5 μL) of lawang root oil, lawang root oil infusion, eugenol (1) or methyl salicylate (7) was diluted to 1:1000 with hexane and subjected to GCMS analysis.
A root sample of Cinnamomum culitlawan (1.10 g) was placed in a Soxhlet apparatus and continuously extracted with methanol overnight. The solvent was evaporated and the crude residue was subjected to column chromatography, eluting with pentane, to give methyl salicylate (7), 10 mg, 1% w/w as a colourless oil, and wax, 96 mg, 8.7% w/w.
Methyl salicylate (7)
1H NMR (300 MHz, CDCl3): 3.95 (3H, s, Me), 6.88 (1H, t, J = 8.1 Hz, ArH), 6.98 (1H, d, J = 8.7 Hz, ArH), 7.45 (1H, td, J = 8.7, 1.8 Hz, ArH), 7.83 (1H, dd, J = 8.1, 1.8 Hz, ArH) 10.75 (1H, s, OH); 13C NMR (75 MHz, CDCl3): 52.3 (CH3), 112.5 (C),117.7 (CH), 119.3 (CH), 130.0 (CH), 135.8 (CH), 161.7 (C), 170.7 (C); MS (EI, 70 eV) m/z (%) 152 [M+], 121, 120 (100), 92, 65; HRMS: m/z [M + H+] calcd for C8H9O3: 153.05462; found: 153.05451.
Supplementary material
1H, 13C NMR spectra and MS. Supplementary material is available online.
Data availability
The data that support this study are available in the article and accompanying online Supplementary Material.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
N. A. and M. G. gratefully acknowledge receipt of Australian Government Research Training Program Scholarships. The authors are grateful to Ms Jenny Davidson and Mr Adam Holland for their advice and assistance. This research was facilitated by access to Sydney Analytical, a core research facility at the University of Sydney.
References
[1] United Nations (2022) UN Comtrade Database. Free access to detailed global trade data. http://comtrade.un.org[2] TKT Do, F Hadji-Minaglou, S Antoniotti, X Fernandez, Authenticity of essential oils. Trends Analyt Chem 2015, 66, 146.
| Authenticity of essential oils.Crossref | GoogleScholarGoogle Scholar |
[3] R Amorati, MC Foti, L Valgimigli, Antioxidant activity of essential oils. J Agric Food Chem 2013, 61, 10835.
| Antioxidant activity of essential oils.Crossref | GoogleScholarGoogle Scholar |
[4] A Bouzabata, J Casanova, A Bighelli, C Cavaleiro, L Salgueiro, F Tomi, The genus Myrtus L. in Algeria: composition and biological aspects of essential oils from M. communis and M. nivellei: a review. Chem Biodivers 2016, 13, 672.
| The genus Myrtus L. in Algeria: composition and biological aspects of essential oils from M. communis and M. nivellei: a review.Crossref | GoogleScholarGoogle Scholar |
[5] L Jing, Z Lei, L Li, R Xie, W Xi, Y Guan, LW Sumner, Z Zhou, Antifungal activity of citrus essential oils. J Agric Food Chem 2014, 62, 3011.
| Antifungal activity of citrus essential oils.Crossref | GoogleScholarGoogle Scholar |
[6] EJ Lenardão, L Savegnago, RG Jacob, FN Victoria, DM Martinez, Antinociceptive effect of essential oils and their constituents: an update review. J Braz Chem Soc 2016, 27, 435.
| Antinociceptive effect of essential oils and their constituents: an update review.Crossref | GoogleScholarGoogle Scholar |
[7] HAE Shaaban, AH El-Ghorab, T Shibamoto, Bioactivity of essential oils and their volatile aroma components: review. J Essent Oil Res 2012, 24, 203.
| Bioactivity of essential oils and their volatile aroma components: review.Crossref | GoogleScholarGoogle Scholar |
[8] B McMillan, Lawang bark as a rubefacient in the treatment of filarial lymphangitis in New Guinea. Med J Aust 1968, 2, 63.
| Lawang bark as a rubefacient in the treatment of filarial lymphangitis in New Guinea.Crossref | GoogleScholarGoogle Scholar |
[9] Quattrocchi U. CRC World Dictionary of Medicinal and Poisonous Plants: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology. CRC Press; 2012. p. 956.
[10] TM Meijer, The essential oil of Massoi bark. Recl Trav Chim Pays-Bas 1940, 59, 191.
| The essential oil of Massoi bark.Crossref | GoogleScholarGoogle Scholar |
[11] B Arifin, DF Tang, SS Achmadi, Transformation of eugenol and safrole into hydroxychavicol. Indones J Chem 2015, 15, 77.
| Transformation of eugenol and safrole into hydroxychavicol.Crossref | GoogleScholarGoogle Scholar |
[12] B Li, C Knudsen, NK Hansen, K Jørgensen, R Kannangara, S Bak, A Takos, F Rook, SH Hansen, BL Møller, C Janfelt, N Bjarnholt, Visualizing metabolite distribution and enzymatic conversion in plant tissues by desorption electrospray ionization mass spectrometry imaging. Plant J 2013, 74, 1059.
| Visualizing metabolite distribution and enzymatic conversion in plant tissues by desorption electrospray ionization mass spectrometry imaging.Crossref | GoogleScholarGoogle Scholar |
[13] L Mason, RA Moore, JE Edwards, HJ McQuay, S Derry, PJ Wiffen, Systematic review of efficacy of topical rubefacients containing salicylates for the treatment of acute and chronic pain. BMJ 2004, 328, 995.


