Total evidence analysis of the phylogenetic relationships of Lycosoidea spiders (Araneae, Entelegynae)
Daniele Polotow A B D , Anthea Carmichael A and Charles E. Griswold A CA Arachnology Laboratory, California Academy of Sciences, 55 Music Concourse Drive, San Francisco, CA 94118, USA.
B Present address: Laboratório Especial de Coleções Zoológicas, Instituto Butantan, Av. Vital Brasil, 1500, Butantã, São Paulo, SP, 05503-900.
C Department of Environmental Science, Policy and Management, University of California, Berkeley, CA 94720-3114, USA.
D Corresponding author. Email: danielepolotow@gmail.com
Invertebrate Systematics 29(2) 124-163 https://doi.org/10.1071/IS14041
Submitted: 25 July 2014 Accepted: 23 October 2014 Published: 11 June 2015
Journal Compilation © CSIRO Publishing 2015 Open Access CC BY-NC-ND
Abstract
Phylogenetic relationships within the superfamily Lycosoidea are investigated through the coding and analysis of character data derived from morphology, behaviour and DNA sequences. In total, 61 terminal taxa were studied, representing most of the major groups of the RTA-clade (i.e. spiders that have a retrolateral tibial apophysis on the male palp). Parsimony and model-based approaches were used, and several support values, partitions and implied weighting schemes were explored to assess clade stability. The morphological–behavioural matrix comprised 96 characters, and four gene fragments were used: 28S (~737 base pairs), actin (~371 base pairs), COI (~630 base pairs) and H3 (~354 base pairs). Major conclusions of the phylogenetic analysis include: the concept of Lycosoidea is restricted to seven families: Lycosidae, Pisauridae, Ctenidae, Psechridae, Thomisidae, Oxyopidae (but Ctenidae and Pisauridae are not monophyletic) and also Trechaleidae (not included in the analysis); the monophyly of the ‘Oval Calamistrum clade’ (OC-clade) appears to be unequivocal, with high support, and encompassing the Lycosoidea plus the relimited Zoropsidae and the proposed new family Udubidae (fam. nov.); Zoropsidae is considered as senior synonym of Tengellidae and Zorocratidae (syn. nov.); Viridasiinae (rank nov.) is raised from subfamily to family rank, excluded from the Ctenidae and placed in Dionycha. Our quantitative phylogenetic analysis confirms the synonymy of Halidae with Pisauridae. The grate-shaped tapetum appears independently at least three times and has a complex evolutionary history, with several reversions.
Additional keywords: 28S rRNA, actin, cladistic analysis, COI, Ctenidae, Dionycha, H3, Halidae, Lycosidae, Oxyopidae, Pisauridae, Senoculidae, systematic, taxonomy, Tengellidae, Thomisidae, Udubidae, Zorocratidae, Zoropsidae.
Introduction
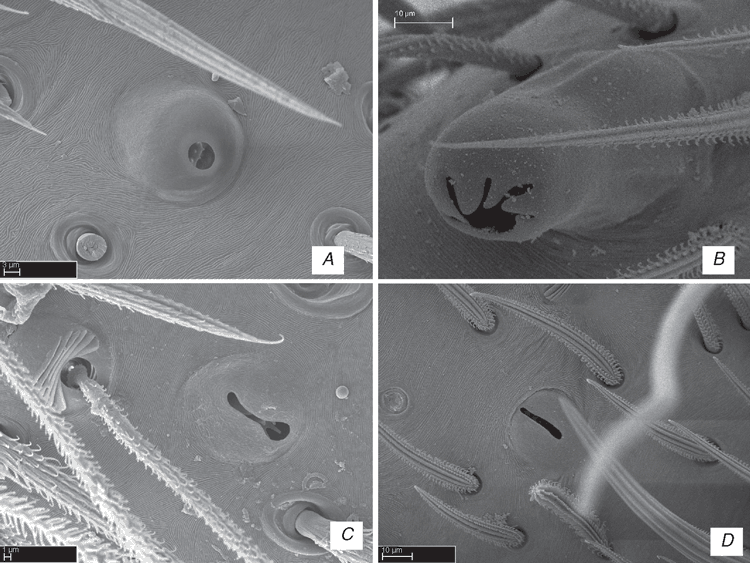
The superfamily Lycosoidea includes, to date, the wolf spiders and their relatives and it was defined by having a grate-shaped tapetum in the secondary eyes (Homann 1971). Most spiders have eight eyes, arranged roughly in two rows, where the first row contains the antero-median and antero-lateral eyes, and the second contains the postero-median and the postero-lateral eyes (Land 1985). The antero-median pair is usually referred to as the principal eyes, which differ from the remaining secondary eyes (Homann 1971; Land 1985). The secondary eyes usually have a tapetum, which consists of several layers of guanine crystals, acting as a colour-selective interference reflector (Land 1972). Its function is probably the same as that of vertebrate tapeta, at least in wandering spiders; that is, to reflect light back through the receptors, giving them a second opportunity to absorb photons (Land 1985), thereby improving the formation of images (Homann 1971). Homann (1971) presented descriptions of the tapeta of several families and classified the eyes according with the arrangement of the crystals layer, although by no means all secondary eyes fit Homann’s classification (Land 1985).
A peculiar kind of tapetum is the grate-shaped type, which has the reflecting material arranged in strips that resembles the grill of an oven (Land 1985: figs 2a, c; Fenk and Schimid 2010: fig. 1). The rhabdomeric portion of each receptor sits on the tapetal strip as though on a chair, with the axon bending round and under the strip before leaving the eye (Baccetti and Bedini 1964). Another distinguished type is the canoe-shaped, which consists of two lateral walls that enclose the rhabdomeric regions of the receptors. At the base there is a slit through which the axons of the receptors penetrate (Land 1985: figs 2b, d). Some spiders present the tapeta as a reflecting sheet with pores in it where the axons of the receptors penetrate (Land 1985).
The grate-shaped tapetum was considered to be a synapomorphy for the superfamily Lycosoidea (Homann 1971; Griswold 1993) and can be found in the wandering spider families Lycosidae, Oxyopidae, Ctenidae, Pisauridae, Senoculidae, Trechaleidae, Psechridae, in some Zoropsidae and Stiphidiidae, in the genera Miturga Thorell, 1870, Argoctenus L. Koch, 1878 and Odo Keyserling, 1887 (Miturgidae) (Homann 1971; Griswold 1993). Several Thomisidae species were reported with a grate-shaped tapetum (Homann 1928; Barth 2002: 132; Ramírez 2014: fig. 12I), but it can only be observed by dissecting the eyes. Recent analyses using molecular data (Agnarsson et al. 2013b) place Thomisidae among the Lycosoidea, and it was also considered a near-optimal solution in the morphological analysis of Ramírez (2014: fig. 215D). Considering some recent cladistic analyses, the superfamily appears to be non-monophyletic and the grate-shaped tapetum probably evolved more than once in the phylogeny of spiders (Silva Dávila 2003; Griswold et al. 2005; Miller et al. 2010; Ramírez 2014: figs 194A, B).
Lycosoidea are placed in the RTA Clade, which also contains Dionycha, dictynoids and amaurobioids and their relatives (Forster 1970), the fused paracribellar clade (also called ‘austral cribellates’) and the oval calamistrum clade (Griswold 1993; Griswold et al. 2005; Spagna and Gillespie 2008; Miller et al. 2010). The RTA Clade is defined by the presence of a unique retrolateral tibial apophysis on the male palp (Coddington and Levi 1991; Griswold et al. 2005). The homology of the palpal elements, including the retrolateral tibial apophysis, of several families is discussed by Sierwald (1990). Lycosoid relationships have been repeatedly studied and with the exception of the results presented by Griswold (1993), all the subsequent phylogenetic analyses indicated that Lycosoidea is non-monophyletic (Griswold et al. 1999, 2005; Silva Dávila 2003; Spagna and Gillespie 2008). There is some evidence that the grate-shaped tapetum has little phylogenetic value and evolved at least three times in the phylogeny of spiders (Silva Dávila 2003; Griswold et al. 2005; Ramírez 2014).
Our main goal here is to test the monophyly and composition of Lycosoidea and propose a total-evidence (genomic, morphological and behavioural data) hypothesis of phylogenetic relationships of Lycosoidea and their kin. On the basis of previous cladistic analyses (Griswold 1993; Griswold et al. 1999, 2005; Silva Dávila 2003; Raven and Stumkat 2005; Spagna and Gillespie 2008; Miller et al. 2010; Agnarsson et al. 2013a, 2013b; Ramírez 2014), we include spiders from the RTA Clade with either a grate- or non-grate-shaped tapetum in the eyes to test the limits of Lycosoidea. Previous phylogenetic analyses of high-level relationships of spiders have included large numbers of cribellate taxa (Griswold et al. 1999, 2005; Spagna and Gillespie 2008; Miller et al. 2010) because these are reasoned to be more likely to straddle the basal nodes of the phylogeny of higher groups than are their relatives that have lost the cribellum (Griswold et al. 2005). In this analysis we include both cribellate and non-cribellate species, because many families assigned to Lycosoidea are entirely ecribellate and because, to date, there is a large literature about the relationships of the basal nodes of spider phylogeny, which permits and encourages the investigation of taxa rarely treated in a molecular phylogeny of higher groups, such as Dionycha and Lycosoidea.
Materials and methods
Taxon sampling
Choice of terminal taxa was based on previous studies, such as the lycosoid phylogeny proposed by Griswold (1993), the ctenoid phylogeny proposed by Silva Dávila (2003), the zoropsoid phylogeny proposed by Raven and Stumkat (2005) and the recent, wide-ranging studies of Agnarsson et al. (2013b) and Ramírez (2014). Our goal was to include representatives of all spider families with the grate-shaped tapetum, a derived condition of the tapetum of the indirect eyes (Fenk and Schmid 2010: fig. 1). All these taxa belong to a group named the ‘RTA-clade’ (Coddington and Levi 1991), an informal group that comprises spiders with retrolateral tibial apophysis (RTA) on the male palp. The taxa used to generate the data for this study are described in Appendix 1. A large proportion of the specimens were used to generate both the molecular and morphological data. Description of the morphological characters can be found in Appendix 2 and the morphological matrix in Table 1.Voucher specimens were provided by the following museums (curator in parentheses): AMNH, American Museum of Natural History, New York (N. Platnick); CAS, California Academy of Sciences, San Francisco (C. Griswold); IBSP, Instituto Butantan, São Paulo (D. M. Barros Battesti); MACN, Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia’, Buenos Aires (C. Scioscia); UFMG, Universidade Federal de Minas Gerais, Belo Horizonte (A. Santos).

|
Morphological and behavioural data
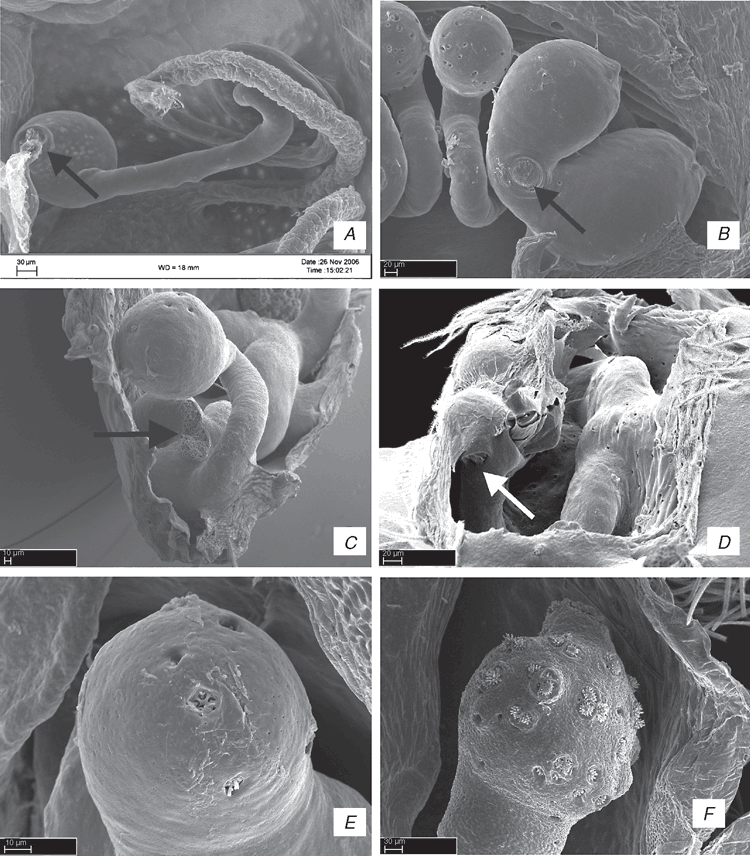
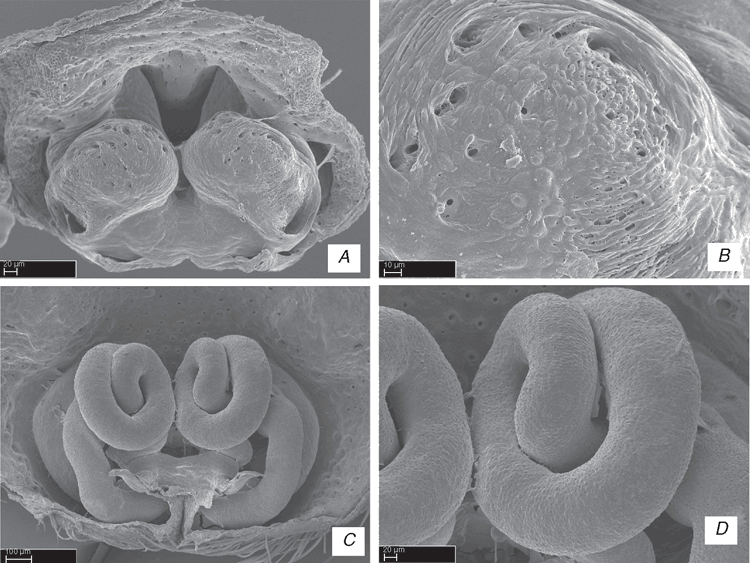
The specimens were preserved in 75–95% ethanol. Morphological observation and illustrations of external structures were made using a Leica MZ12 stereomicroscope with camera lucida. Digital scanning electron microscope (SEM) photographs were taken on a LEO 1450vp SEM at the Entomology Department of California Academy of Sciences, San Francisco, USA. Preparation of specimens for the SEM follows Álvarez-Padilla and Hormiga (2007). For SEM preparation, specimens were cleaned ultrasonically, transferred to 100% ethanol for at least 48 h, submitted to critical-point drying or air-dried. After drying, specimens were mounted on rivets using copper wire, then sputter-coated with gold. Epigyna were excised from the abdomens of adult females and cleaned by either immersion in a trypsin solution, digested with contact lens cleaner (ReNu®) overnight or cleared with clove oil in order to examine internal structures (Sierwald 1989).
The morphological and behavioural dataset included 96 characters, scored for 61 taxa (Appendix 2). Mesquite 2.5 (Maddison and Maddison 2011) was used to build and edit the character matrix. Inapplicable and unknown states are presented as ‘–’ and ‘?’, respectively. All characters were a priori equally weighted and all multistate characters were coded as non-additive (Fitch 1971; Swofford and Maddison 1987). An a posteriori implied weight scheme was applied during some searches with TNT 1.1 (Goloboff et al. 2008a). Ambiguous character optimisations were solved so as to favour reversal or secondary loss over convergence (Farris optimisation or ACCTRAN).
Abbreviations
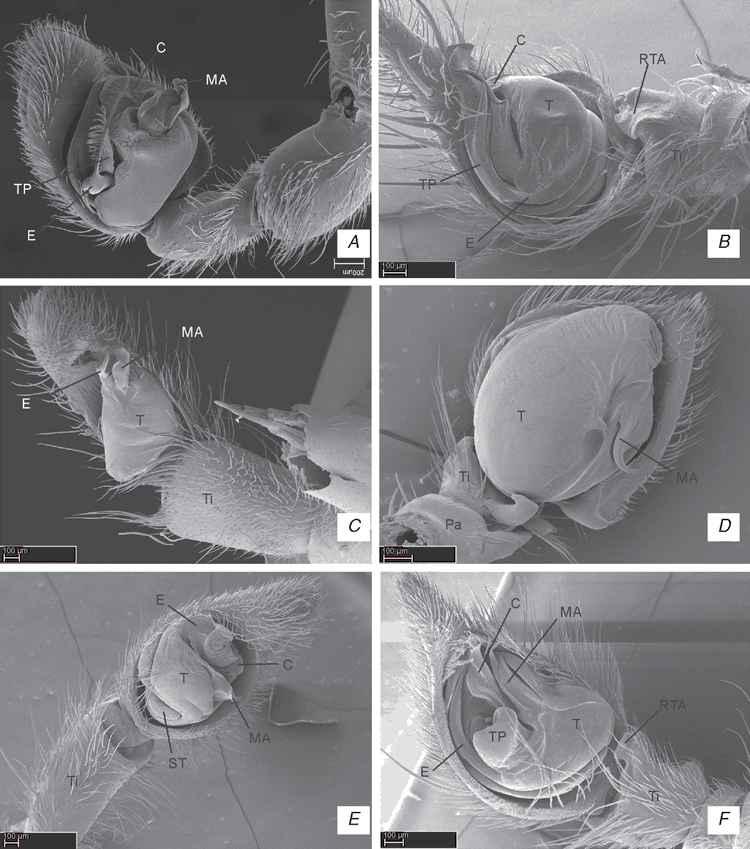
ALE, anterior lateral eyes; AME, anterior median eyes; BS, base of spermathecae; C, conductor; CD, copulatory ducts; Cy, cymbium; E, embolus; ELP, embolus laminar process; FD, fertilization ducts; HS, head of spermathecae; LL, locking lobes; LP, lateral process; LS, lateral sector; MA, median apophysis; MS, median sector; MTA, median tibial apophysis; MTP, membranous tegular process; Pa, patella; PaP, patellar process; PCP, prolateral cymbial process; PLE, posterior lateral eyes; PME, posterior median eyes; PP, pars pendula; RCP, retrolateral cymbial process; RTA, retrolateral tibial apophysis; S, spermathecae; ST, subtegulum; Te, tegulum; Ti, tibia; TP, tegular process; VTA, ventral tibial apophysis; VCP, ventral cymbial process; VTP, ventral tibial process.
References to figures published elsewhere are listed in lower-case type (fig.); references to figures in this paper are capitalised (Fig.).
Molecular protocols
Whole-genomic DNA was extracted from up to four legs with a DNEasy® kit (Qiagen Inc.) following the manufacturer’s protocol for animal tissues. Specimens for molecular work were preferred preserved in 96–100% ethanol. If specimens preserved in 75% ethanol were used, they were no older than 5 years at the time of DNA extraction. All the protocols for DNA amplification, visualisation, purification and sequence reactions were carried out in the Center for Comparative Genomics of California Academy of Sciences.
Four gene fragments were amplified in 25-µL reactions: a ~540-bp region from cytochrome oxidase I (COI), a ~330-bp region of histone H3, a ~770-bp region of 28S rDNA, and a ~330-bp region of Actin. For most of the gene fragments, single amplicons were obtained. For some gene fragments of 28S, combinations of overlapping amplicons were used and the resulting sequences assembled later. These four gene fragments have been shown to be phylogenetically informative in many studies on arachnid systematics and have been reported to evolve at different rates, potentially providing phylogenetic resolution at different taxonomic levels (e.g. Arnedo et al. 2001; Hormiga et al. 2003; Prendini et al. 2003; Spagna and Gillespie 2008; Giribet et al. 2010; Miller et al. 2010). Primers and annealing temperatures for each locus are given in Table 2. Most reactions consisted of 2.5 µL of 10× Apex buffer® (Genesee Scientific, San Diego, USA), 0.42 µL of 10 mm dNTP, 2.4 µL of 25 mm MgCl2, 1 µL each of forward and reverse 10 mm dNTP primers, between 1.5 and 2.5 µL of BSA, 0.3 µL Apex Taq DNA Polymerase® (Genesee Scientific, San Diego, USA), 1–4 µL of template DNA, and water to 25 µL. Reaction conditions included an initial denaturation step at 95°C for 2 min, followed by 35 cycles of 95°C for 30 s (annealing temperatures and times as reported in Table 2) and 72°C for 1 min (H3 and COI) or 1.5 min (Actin and 28S), followed by a final extension at 72°C for 7 min, and a hold at 4°C. The only significant exception to this protocol was for the amplifications of H3. These reactions used 2.5 µL of 10× USB PCR reaction buffer (USB Corporation, Cleveland, USA), 0.5 µL of 10 mm dNTP, 1.5 µL of 25 mm MgCl2, 0.2 µL each of forward and reverse 25 mm primers, 2 µL of BSA, 0.5 µL of HotStart-IT®™ Taq DNA Polymerase (USB Corporation, Cleveland, USA), up to 4 µL template DNA, and water to 25 µL. Reaction conditions followed the protocol of Latiolais et al. (2006). PCR products were purified using Exonuclease 1 (Exo1) and Shrimp Alkaline Phospatase (SAP). For every 1 µL of PCR product, 0.01 µL of Exo1, 0.02 µL of SAP, and 0.11 µL of H2O was added. This mixture was incubated at 37°C for 15 min and 80°C for another 15 min.

|
The ABI BigDye® Terminator kit (ver. 3.1, Applied Biosystems Inc., Foster City, USA) was used to perform 10 µL cycle sequencing reactions using 1.63 µL 5× buffer, 0.5 µL 10 mm primer, and 0.75 µL BigDye® Terminator. Template DNA and water amounts were adjusted on the basis of the concentration of DNA in each sample. Cycle sequencing parameters followed the protocol of Platt et al. (2007) with a variable annealing temperature dependent on the melting temperature of the individual primer. Reaction sequences were obtained from an ABI 3130XL genetic analyser (Applied Biosystems Inc., Foster City, USA). For some 28S rDNA samples, internal primers were used in addition to external primers to provide redundant sequence coverage (see Table 2). Sequence reconciliation, edition and chromatogram evaluation were performed using Geneious 7.1.5 created by Biomatters and available from http://www.geneious.com/. To check for contamination, all edited sequences were BLASTed (Altschul et al. 1997, as implemented by the National Center for Biotechnology Information website http://ncbi.nlm.nih.gov) against the GenBank nucleotide database. GenBank accession numbers for all new sequence data generated for this study are given in Table 3, which also lists the sequence data generated in previous studies, as Megadictyna thilenii Dahl from Blackledge et al. (2009), Coelotes terrestris Blackwall, 1841, Stiphidion facetum Simon, 1912, Tengella radiata (Kulczyn‘ski, 1909) and Textrix denticulata Sundevall, 1833 from Spagna and Gillespie (2008) and Vidole capensis (Pocock, 1900) from Miller et al. (2010). Morphological vouchers are deposited with the California Academy of Sciences (San Francisco, CA, USA) except if noted otherwise (Appendix 1). Extraction and PCR products are vouchered at the Center for Comparative Genomics at the California Academy of Sciences. All terminal taxa represent single exemplar specimens; no ‘chimeras’ or consensus sequences were produced from multiple specimens.

|
Alignment analysis
Alignments were built with MAFFT (MAFFT Multiple alignment program for amino acid or nucleo- tide sequences, version 6, available at http://align.bmr.kyushu-u.ac.jp/mafft/online/server/) (Katoh et al. 2002, 2005; Katoh and Toh 2008). MAFFT is one of the few available alignment programs that have been shown to produce relatively accurate and fast alignments (Golubchik et al. 2007). MAFFT implements three different algorithms, including the Needleman and Wunsch (1970) algorithm. The other algorithms use local alignments with affine gap costs and global alignments with generalised affine gap costs (Berger and Munson 1991; Gotoh 1993; Notredame et al. 2000; Katoh et al. 2002, 2005). We have used the E-INS-i strategy, which is one of the most exhaustive algorithms implemented, because it gives the most accurate results, with the least number of assumptions (MAFFT online documentation). Alignment indel opening penalty was set to a default value of 1.53. Gaps were treated as a fifth state during phylogenetic analyses to account for historical information contained in insertion and deletion events. This treatment maximises independence of characters and logical consistency of phylogenetic analyses, at the expense of upweighting otherwise potentially single events (Giribet and Wheeler 1999). The Actin, CO1 and H3 alignments were further tested by translating the sequences into amino acids and checking for inappropriately placed stop codons.
Phylogeny
Bayesian analysis
Bayesian analysis was performed using MrBayes 3.1.2 (Huelsenbeck and Ronquist 2001; Ronquist and Huelsenbeck 2003). A mixed-model analysis was conducted for each of the four alignments. For the three protein-coding genes, each codon position was modelled independently; the ribosomal gene was modelled independently for a total of eight data partitions. Gaps were treated as missing, not as a fifth character state. Best-fit models for each partition were determined independently according to the non-hierarchical Akaike Information Criterion as implemented in MrModeltest (Nylander 2008). The SYM model was applied to the H3 Position 2 partition; the HKY + G model was applied to the COI Position 3 partition; the GTR + I + C model was applied to the remaining partitions. Parameters (character state frequencies, C-shape parameter, proportion of invariant sites, substitution rates of the GTR model, transition/transversion ratio) were estimated independently for each partition using the following command: unlink statefreq = (all) shape = (all) pinvar = (all) revmat = (all) tratio = (all). Analyses were run on the Phylocluster at the Center for Comparative Genomics, California Academy of Sciences. Tree search proceeded according to MrBayes defaults (two independent analyses consisting of one cold and three heated MCMC chains). Analyses proceeded at least until the deviation of split frequencies fell below 0.01. Trees were sampled every 1000 generations. Chain convergence was evaluated in Tracer (Rambaut and Drummond 2007). At least the first 10% of each search was discarded as ‘burn-in’.
Parsimony analyses
Parsimony analyses were performed with TNT 1.1 (Goloboff et al. 2008a). All characters in this dataset were treated as unordered. Internal branches were considered unsupported and collapsed during searches if they were only supported ambiguously (that is, when some optimisation lacks support, i.e. when the minimum length is zero; Rule 1 of TNT; see discussion in Coddington and Scharff 1994).
Searches consisted of 1000 replicates of random addition sequences, followed by 500 interactions of tree bisection reconnection (TBR) and parsimony ratchet as implemented in TNT (alternating searches and perturbation phases, with periodic rounds of original weights) (Goloboff et al. 2003; program documentation), retaining 10 trees per replication (commands: ratchet: iter 500 equal; mult = ratchet replic 1000 tbr hold 10;). We also analysed the dataset under regimes weighting against homoplasy, using implied weighting (Goloboff 1993). This more-recent method of implied weights was given priority over successive weighting (Farris 1969) because implied weights is not affected by starting points or ambiguities in weights from multiple trees. Analyses under implied weights were conducted with TNT 1.1 (Goloboff et al. 2008b) with values of constant of concavity k = 1, 3, 6, 9, 20, 50, 99, using the same parameters described to perform equal-weight searches.
Sensitivity and stability of clades
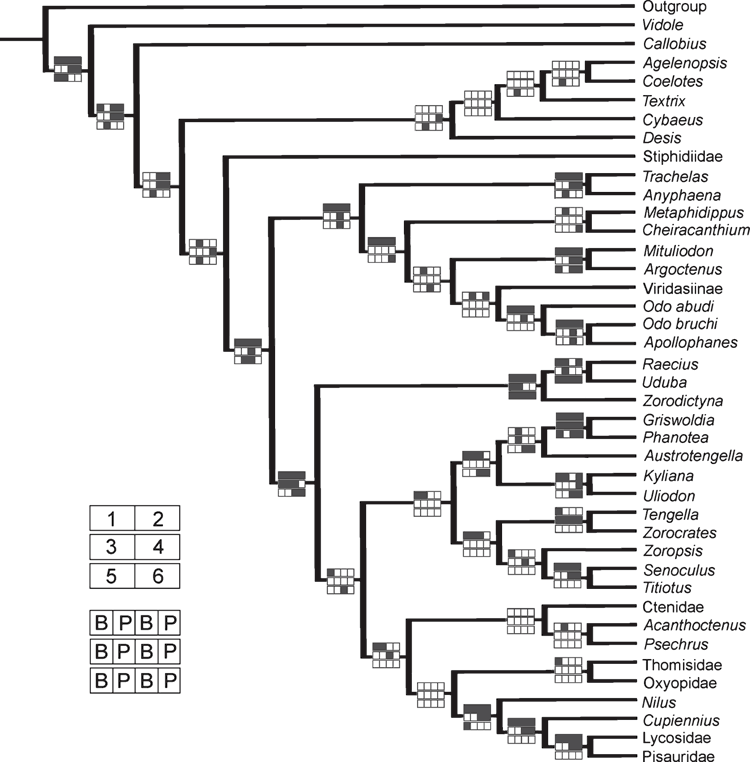
In order to investigate the sensitivity of the data to different partitions schemes, six data partitions were defined: (1) all molecular data (28S, Actin, COI and H3); (2) nuclear gene fragments (28S, Actin and H3); (3) mitochondrial gene fragment (COI); (4) ribosomal gene fragment (28S); (5) morphological and behavioural data; (6) protein-coding genes fragments (Actin, COI and H3). The partitioned data were analysed with the same parameters described for the parsimony and Bayesian analyses and the cladograms obtained were annotated with the results supported under different analysis parameters and represented as ‘Navajo rugs’ (Giribet and Edgecomb 2006).
Ancestral character reconstruction
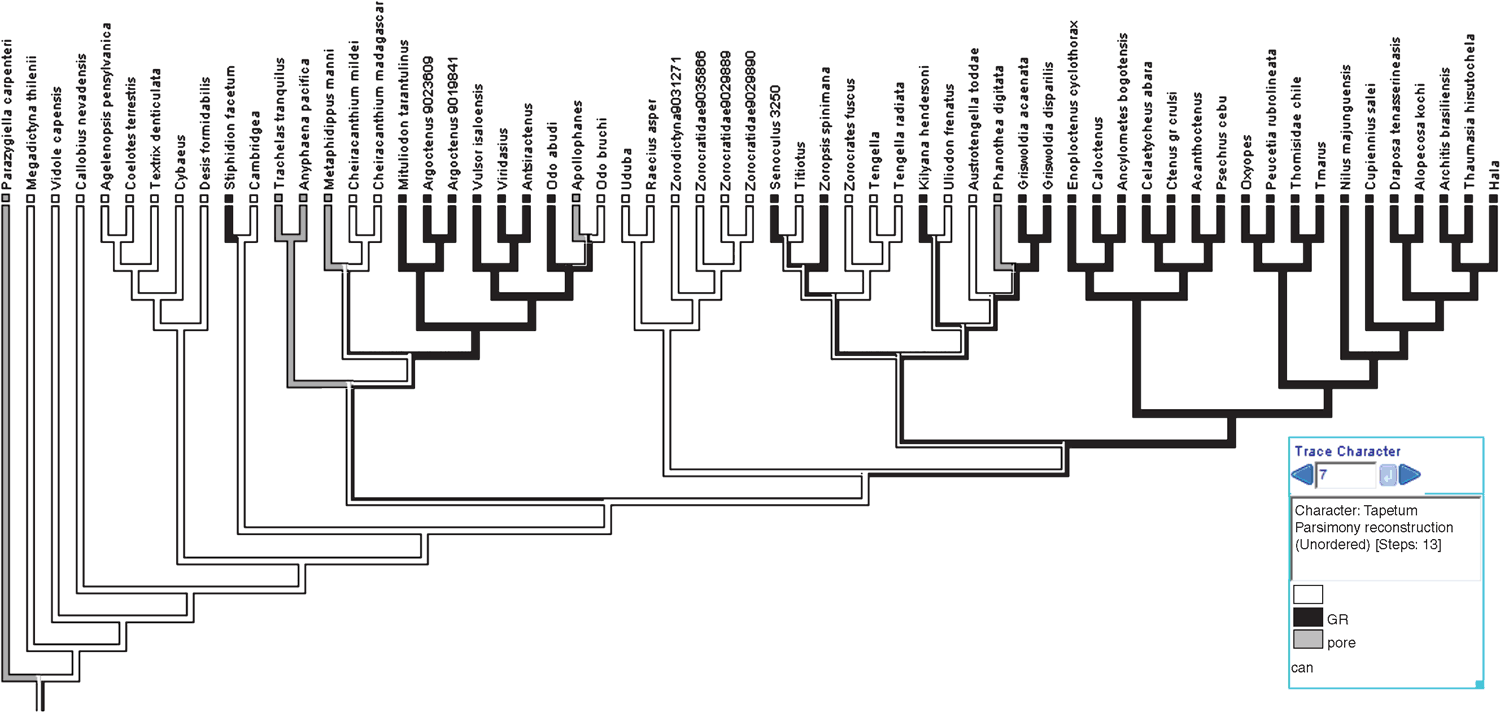
In order to better understand the evolution of the tapetum, an ancestral character reconstruction was performed on this trait. The ancestral character reconstruction was performed using standard parsimony optimisations in Mesquite 2.75 (Maddison and Maddison 2011). The trace character function in Mesquite (Maddison and Maddison 2011) optimises most parsimonious character states at internodes; where multiple equally parsimonious solutions exist, the ancestral character state is equivocal (Fitch 1971; Swofford and Maddison 1987, 1992).
Support values
The following support measures were calculated for the parsimony analyses: absolute Bremer support (BS, Bremer 1994) and bootstrap (Felsenstein 1985). BS was calculated heuristically in TNT searching for suboptimal trees using the optimal trees as a starting point. TBR branch swapping was performed filling the tree-buffer, sequentially increasing the number of steps of suboptimal trees by one (1–2 steps), by five (5–50 steps) and by 10 (60–100 steps), retaining increasing numbers of trees by 3000 (from 2000 to 50 000) and running Bremer support with the saved trees. Support values for groups expressed as Bremer support in units of fit × 100 (BS, bottom) and GC (Group present/Contradicted) frequency differences (top) (Figs 2, 3). GC count the number of occurrences of individual cases of contradiction of a group in the consensus (thus counting cases where the group is unresolved in the consensus as neither favourable nor contradictory) (Goloboff et al. 2003).
Results
This section reports the results of all analyses performed in this study. Names of taxa in quotation marks, e.g. ‘grate-shaped tapetum clade’, refer to informal taxon names based on the preferred hypothesis of relationships (Fig. 3) (see below). Formal taxonomic hypotheses, nomenclatorial actions and diagnosis of groups will be presented in the section ‘Systematics’. Original taxonomic names from previous works or that are formally addressed here are depicted without quotation marks.
Sequence data and alignments
This study produced a final aligned 2092-bp fragment for each taxon, consisting of 737 aligned bp for 28S, 371 aligned bp for Actin, 630 aligned bp for COI, and 354 aligned bp for H3. The analysis of the protein-coding genes was straightforward: Actin and H3 required no gap insertions; COI required a single three-nucleotide deletion in two terminals (Tengella radiata and Tengella sp.), which can be considered a synapomorphy for the genus.
Phylogenetic analysis
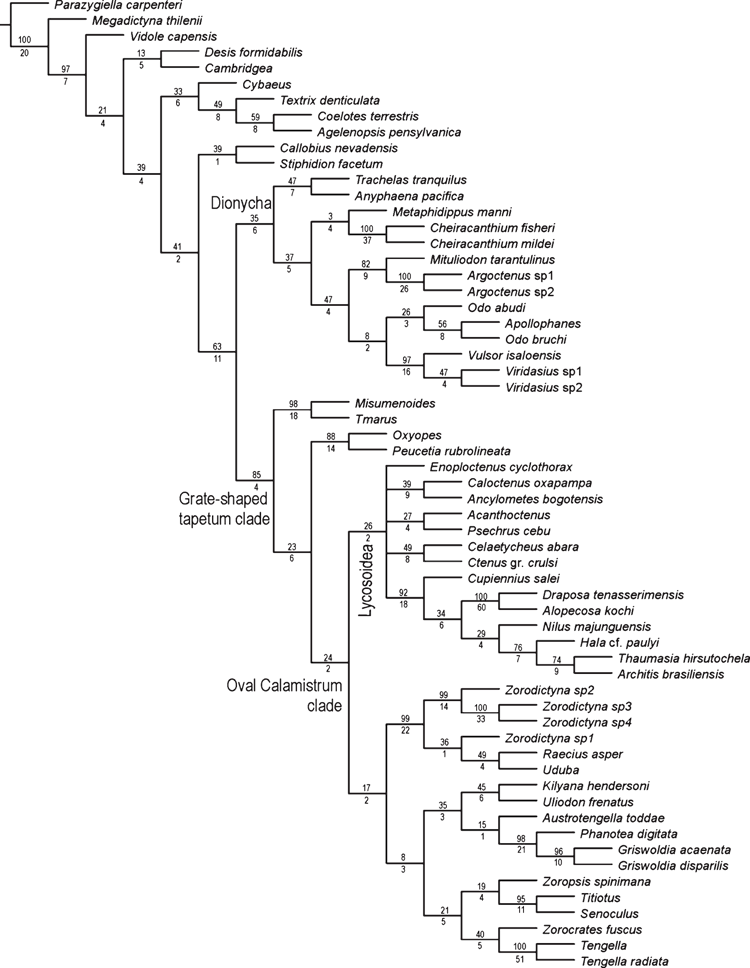
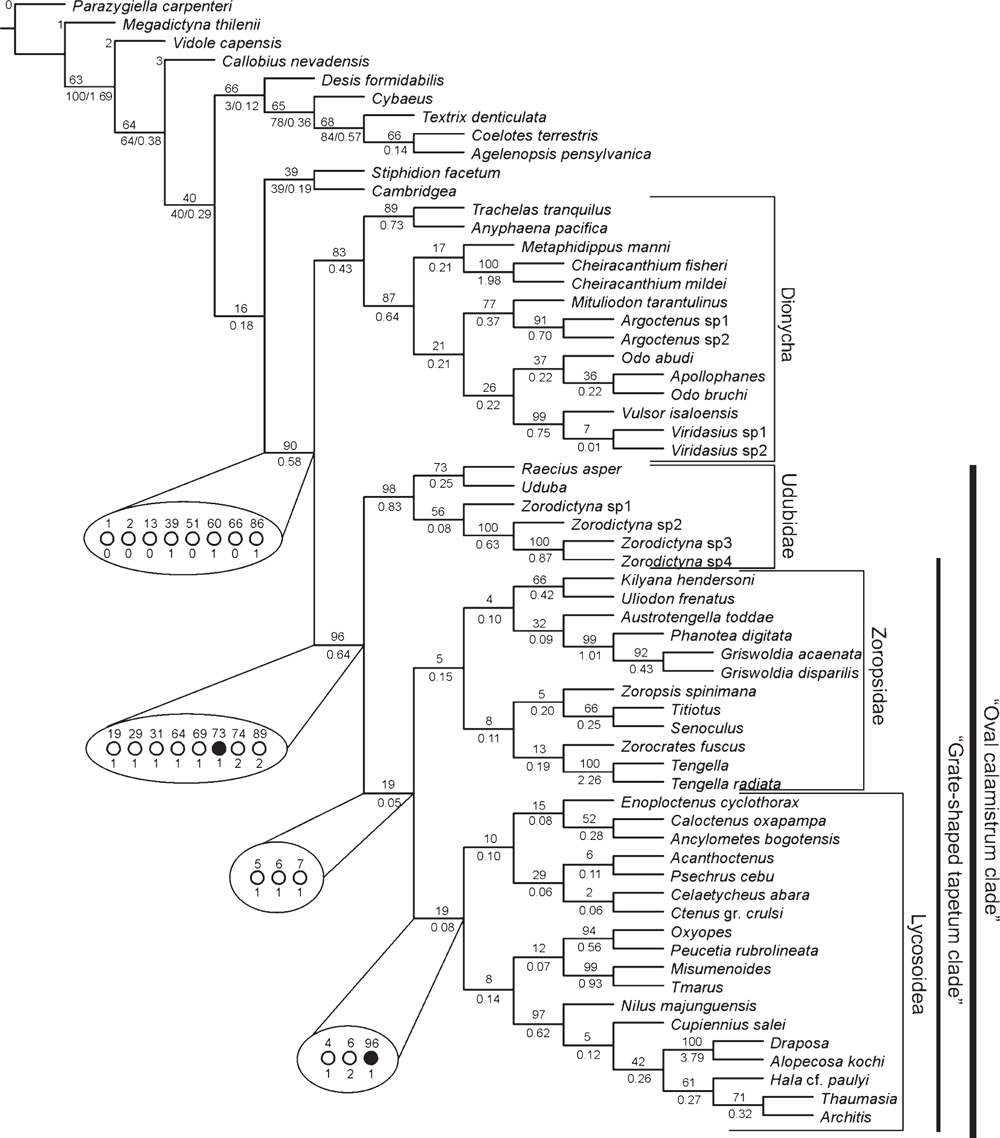
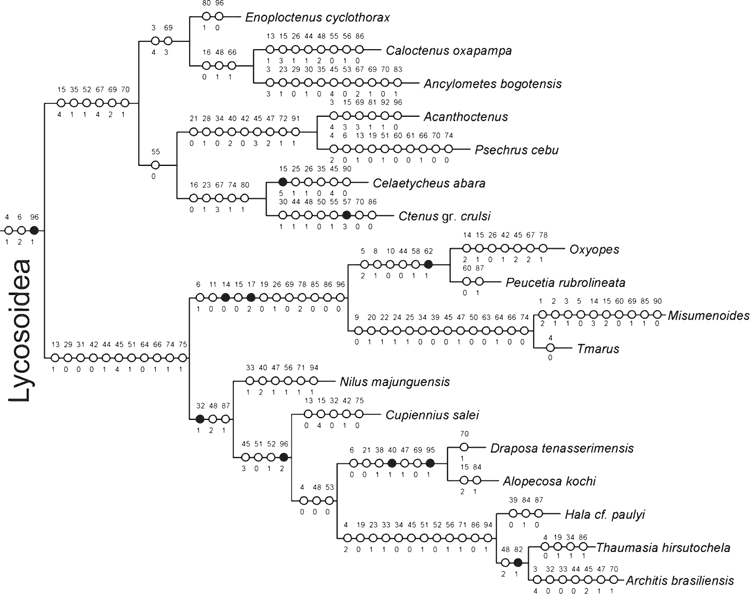
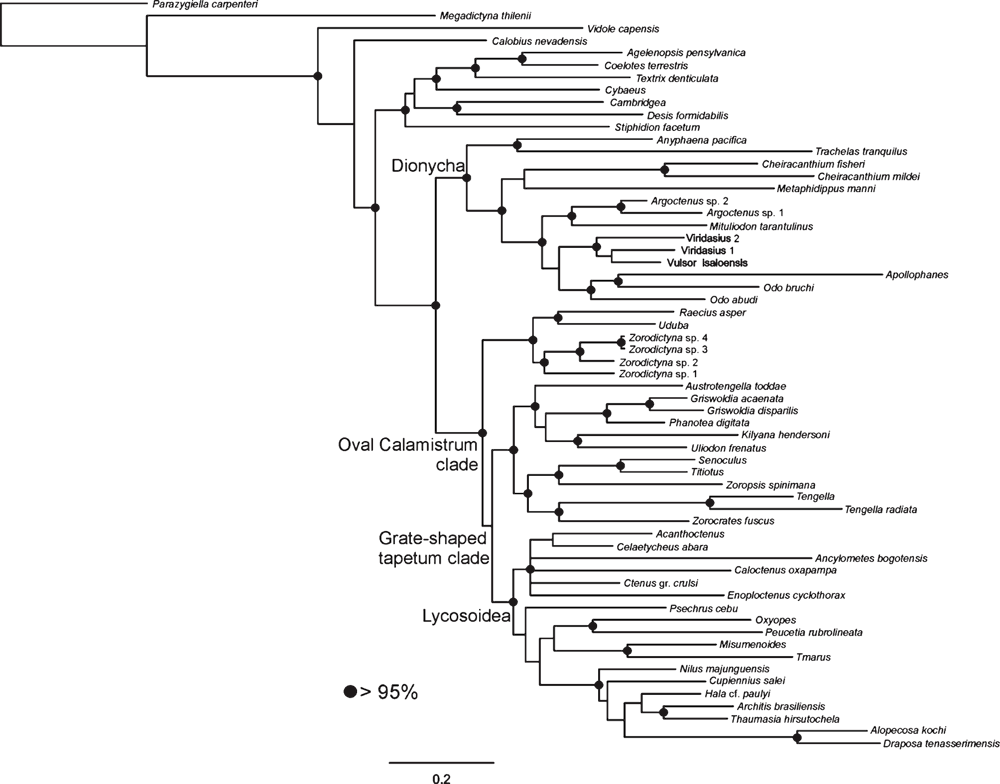
The Bayesian analysis of the total evidence and the six data partitions was allowed to proceed for 50 000 000 generations. Results of the Bayesian analysis are shown in Fig. 1. The parsimony analysis of the total evidence under equal weights resulted in four trees of 8880 steps (CI = 0.23; RI = 0.36). Strict consensus of these parsimony results collapsed three branches and resulted in a tree of 8916 steps (CI = 0.23; RI = 0.36) depicted in Fig. 2. The parsimony analysis of the total evidence with values of the concavity k = 1, 3, 6, 9, 20, 50, 99 resulted in one tree in each of the analyses. The trees obtained by the constant k = 6 and 9 are identical (8906 L; CI = 0.23; RI = 0.36) and the results are selected as the working hypothesis to discuss the character evolution and to diagnose taxa. To facilitate the discussion, we refer to the tree resulting from the concavity value of k = 6 (Figs 3–7). The constant of concavity for the weighting function was also the same as determined in Ramírez (2003) (k = 6). Ramírez (2003) and Goloboff et al. (2008b) found that mild concavity values produced higher topological congruence indices for many morphological and molecular datasets. To explore congruence and sensitivity of different phylogenetic data, we ran analyses of six data partitions under parsimony and Bayesian analyses and summarised the results on the tree of concavity k = 6 as ‘Navajo rugs’ (Giribet and Edgecomb 2006) (Fig. 8). These analyses indicated that no partition reliably predicts the results of the total-evidence analysis. The ancestral character reconstruction of the tapetum shows that the grate-shaped kind evolved at least three times independently, with several reversions to primitive states (total of 13 steps) (Fig. 9).
|
Phylogenetic relationships
Three topologies will be considered for description of the results and further discussion: the tree generated by the Bayesian inference (Fig. 1), the consensus of four trees generated by the equal-weight analysis of parsimony (Fig. 2), and the only tree resulting from the parsimony analysis under implied weights with concavity constant of k = 6 (Figs 3–7). Both parsimony and Bayesian analyses show Lycosoidea to be monophyletic, composed by Lycosidae, Pisauridae, Oxyopidae, Thomisidae, Psechridae and Ctenidae. Pisauridae is monophyletic only in the equal-weight consensus (Fig. 2). Psechridae appears as sister-group of Acanthoctenus in both parsimony analyses (Figs 2, 3), but as sister group to Oxyopidae, Thomisidae, Pisauridae and Lycosidae in the Bayesian inference (Fig. 1). The ‘GST clade’ (grate-shaped tapetum) appears as sister-group of Udubidae (new family), together forming the ‘Oval Calamistrum clade’, in all three analyses. All three analyses show a clade formed with species currently placed in Zoropsidae, Zorocratidae, Tengellidae and Senoculidae. The expanded Zoropsidae appears as sister-group of Lycosoidea in the Bayesian analysis and implied-weights parsimony k = 6 analysis (Figs 1, 3). Inferred phylogenetic trees revealed consistent relationships for most of the groups analysed. A monophyletic Lycosoidea clade, placed as sister-group of our expanded Zoropsidae, was recovered in both the k = 6 parsimony and Bayesian analyses (Figs 1, 3). Thomisidae and Oxyopidae appear as outgroups to the ‘Oval Calamistrum clade’ in the equal-weight parsimony analysis (Fig. 2) and in the analyses with the concavities k = 1, 3, 20, 50, 99, but in the Bayesian analysis and the parsimony analysis of concavity k = 6 and 9, Thomisidae and Oxyopidae appear within the Lycosoidea (Figs 1, 3).
Discussion
We base our following discussion on the results of the total-evidence analyses with parsimony (with implied weights k = 6) (Fig. 3) and Bayesian methods (Fig. 1), unless we state that we discuss a particular partitioned dataset. The morphological character state changes are shown on the tree obtained under concavity k = 6 (Figs 3–7).
Phylogenetic relationships
Outgroups
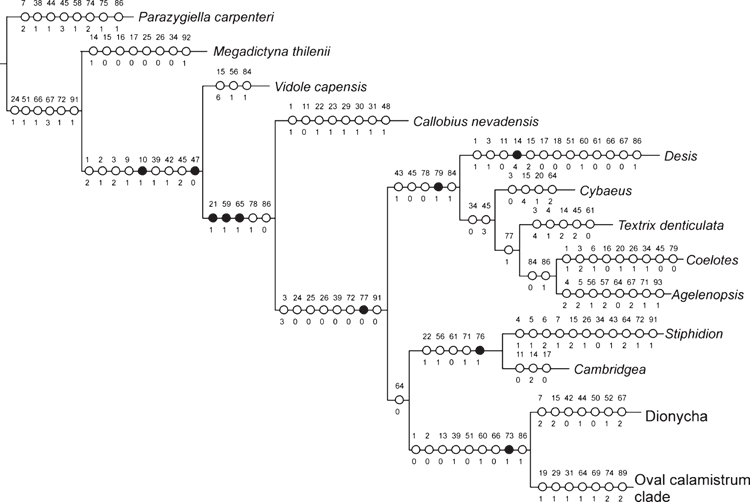
The relationships among the outgroups are not the primary focus of the present study, and accordingly the sample of character data for the outgroups is much less intensive than for the ingroup. Nevertheless, we believe it is worth commenting on the relationships of the basal groups on the cladogram. Following several previous phylogenetic hypotheses (Griswold et al. 1999; Miller et al. 2010), we root our analysis using an Orbicularian taxon (Parazygiella carpenteri Wunderlich). Our results corroborated the basal position of Megadictyna thilenii (Nicodamidae), Vidole capensis (Phyxelididae) and Callobius nevadensis Chamberlin, 1847 (Amaurobiidae) in relation to the remaining taxa used in the analysis (Figs 1–3).
‘Fused Paracribellar clade’ (Austral Cribellate clade)
The ‘Fused Paracribellar clade’ was proposed by Griswold et al. (1999) to include Amphinectidae, Stiphidiidae, Agelenidae (represented by the cribellate Neoramia nana Forster & Wilton, 1973) and Desidae. The name of the clade refers to the fused spigot bases on the posterior median spinnerets, with two to several paracribellar shafts emerging from the same paracribellar base (Griswold et al. 2005: figs 66A, 68B, 73C, 87C). This clade, with the inclusion of cribellate Dictynidae, was corroborated by Spagna and Gillespie (2008) and Miller et al. (2010) as the ‘austral cribellates’ (though Agelenidae is no longer included: we now know that Neoramia Forster & Wilton, 1973 is not related to true agelenids, e.g. Tegenaria Latreille, 1804, Coelotes Blackwall, 1841, Agelenopsis Giebel, 1869). Here, we do not use the paracribellar character in the matrix (we have only one cribellate spider from this group, Stiphidion facetum), but the name is still useful. Stiphidiidae do not cluster with the remaining taxa of the ‘Fused Paracribellar clade’ in our analyses.
Dionycha
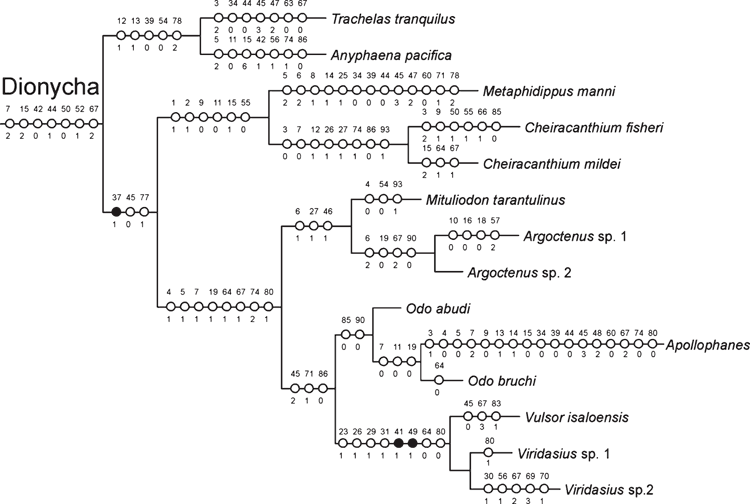
Dionycha appears as sister-group of the ‘Oval Calamistrum clade’ (Fig. 1). Classical Dionycha taxa, including Anyphaena Sundevall, 1833 (Anyphaenidae), Trachelas L. Koch, 1872 (Trachelidae), Metaphidippus F. O. P.-Cambridge, 1901 (Salticidae), Apollophanes O. P.-Cambridge, 1898 (Philodromidae), Cheiracanthium C. L. Koch, 1839 (Eutichuridae) and Mituliodon Raven & Stumkat, 2003 (Miturgidae), cluster together in a well supported clade (Figs 1, 5). Cheiracanthium and Mituliodon were both formerly Miturgidae, but Ramírez (2014) recently moved Cheiracanthium into Eutichuridae, a result corroborated by our total-evidence analysis. Odo Keyserling, 1887, Argoctenus L. Koch, 1878 and the remaining Miturgidae do not form a monophyletic clade but cluster with the remaining Dionycha represented in our analysis (Figs 1, 5). The Viridasiinae clade, formed by two species of Viridasius Simon, 1889 and Vulsor Simon, 1889, cluster with Apollophanes (Philodromidae) and two species of Odo (Miturgidae), indicating that viridasiines arose independently of the remaining Ctenidae (Figs 1, 5). Bayer and Schönhofer (2013) used one species of Viridasius in their phylogenetic analysis and recover the same relation within the Dionycha. Viridasiinae were proposed by Lehtinen (1967) and comprise, to date, two genera, Vulsor and Viridasius. There are several new species and probably some new genera that belong to this clade, all from Madagascar (D. Silva Dávila, pers. comm.). Dionycha have separately been subject to an intensive, taxon-dense analysis (Ramírez 2014), so our conclusions must be considered provisional, but we believe that the monophyly and placement of the viridasiines are secure, and we suggest raising this taxon to family level (see Systematics).
‘Oval Calamistrum clade’
The ‘Oval Calamistrum clade’ (OC clade) (Figs 1, 6) was proposed by Griswold (1993) to include spiders with an oval to rectangular arrangement of calamistral setae, which grouped Lycosoidea (from which only Peschridae and Ctenidae have cribellate members) with zoropsids, zorocratids and tengellids. This clade was recovered in Griswold et al. (1999) and Griswold et al. (2005). In the parsimony analysis of concavity k = 6 and Bayesian analysis (Figs 1, 3, 6) the ‘OC clade’ is sister-group of Dionycha and in the parsimony analysis with equal weights it is sister-group of Oxyopidae (Fig. 2). The ‘OC clade’ appears in almost all partitioned analyses of Bayesian inference and implied weights of concavity k = 6 (Fig. 8).
Udubids
Ububa Simon, 1880, Raecius Simon, 1892, Zorodictyna Strand, 1907 and three other undescribed species from Madagascar form a well supported clade (Figs 1, 6), which appears in all the analyses with different methods or weight schemes. These genera are currently placed in Zorocratidae, but do not appear closely related to the type species of the family, Zorocrates fuscus Simon, 1888. Udubids are recovered in all the total-evidence analyses (Figs 1–3, 8) and the clade is supported by several homoplastic synapomorphies (Fig. 6). Zorocrates Simon, 1888 lacks these udubid synapomorphies. Udubids also appear in almost all partitioned analyses of Bayesian inference and implied weights of concavity k = 6 (Fig. 8). We suggest raising this taxon to family level (see Systematics).
Lycosoidea
Lycosoidea was defined by Homann (1971) by the presence of a grate-shaped tapetum in the indirect eyes. Griswold (1993) presented a phylogeny with the families having a grate-shaped tapetum (including the stiphidiid Stiphidion Simon and miturgid Mituliodon) plus Tengella Dahl (Tengellidae), the latter of which share an oval calamistrum with the grate-shaped tapetum taxa. Analyses beginning with that of Griswold et al. (1999) suggested that Stiphidiidae were more closely related to the Agelenidae, Amphinectidae, Desidae and Neolanidae, and that the grate-shaped tapetum appears as convergent in Stiphidiidae and remaining Lycosoidea. Our results relimit the superfamily Lycosoidea, which now comprises species placed in Lycosidae, Pisauridae (including species placed in Halidae), Oxyopidae, Psechridae, Thomisidae and Ctenidae. In our analyses Pisauridae and Ctenidae are not monophyletic, and viridasiine ctenids are placed far from Lycosoidea. Trechaleidae, not included in our analysis, remains in Lycosoidea because of the similarities with the remaining lycosoids.
‘GST clade’
The ‘grate-shaped tapetum clade’ (or ‘GST clade’) was proposed by Silva Dávila (2003) to include the lycosoids, tengelloids and ctenoids and was supported by the presence of the grate-shaped tapetum (with four reversals to canoe-shaped) and several other characters. In the parsimony analysis of concavity k = 6 and Bayesian inference (Figs 1, 3) we recover a similar group (with the exception of the udubids, miturgids and zorids), also supported by the presence of the grate-shaped tapetum. But our analyses suggest that evolutionary history of the grate-shaped tapetum is more complex than recovered by previous analyses (Griswold 1993; Silva Dávila 2003; Raven and Stumkat 2005). Not only does the grate-shaped tapetum reverse to a canoe shape within the ‘GST clade’, but the GST also appears at least two more times independently (Fig. 9).
Zoropsids
Under all total-evidence analyses, Zorocrates, Tengella, Titiotus Simon, 1897, and Senoculus Taczanowski, 1872 group with Zoropsis Simon, 1878, suggesting that these genera comprise a monophyletic group (Figs 1–3, 6, 8). Raven and Stumkat (2005) recently proposed expansion of Zoropsidae to include the taxa previously placed in Zorocratidae, but our analysis excludes from the zoropsids Raecius, Uduba and Zorodictyna, which comprise the core of our new Udubidae. Also associated with Zoropsis are Austrotengella Raven, 2012, the Griswoldiinae (Griswoldia Dippenaar-Schoeman & Jocqué and Phanotea Simon) and the Uliodoninae (Uliodon L. Koch and Kilyana Raven & Stumkat). We consider that there is enough evidence to support the synonymy of Zorocratidae and Tengellidae with Zoropsidae (the oldest family group name and the valid senior synonym). Morphological evidence to support our newly relimited Zoropsidae is weak, comprising only a few homoplastic synapomorphies: characters 23, 25 and 26, in total-evidence analysis of concavity k = 6 (Fig. 6) and only character 23 in total-evidence with equal weights (Fig. 2). Whereas it is difficult to recover this clade using just morphological data, the total-evidence analyses are consistent in grouping Zorocrates, Tengella, Titiotus with Zoropsis.
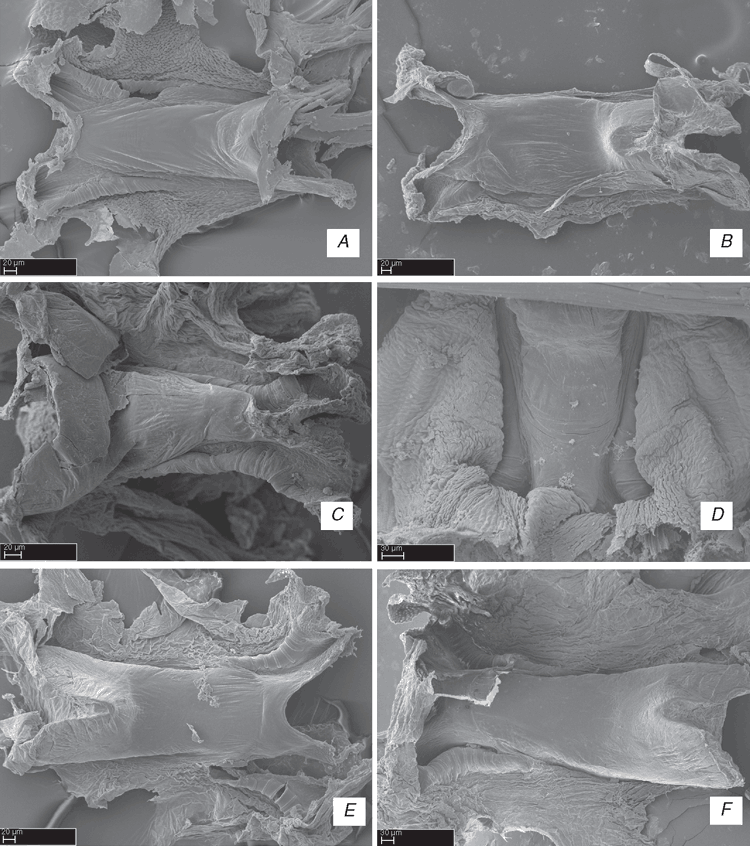
The synonymy of Zoropsidae, a relimited Zorocratidae (without Raecius, Uduba and Zorodictyna), and Tengellidae would require a substantial expansion of Zoropsidae (which has priority over the other family names), to include ~25 genera, most of them not included in this analysis. Nevertheless, we see strong evidence grouping the unrepresented genera with our exemplars. From among the taxa assigned to Zoropsidae (World Spider Catalog 2015), Akamasia Bosselaers, 2002 and Takeoa Lehtinen, 1967 are similar to Zoropsis (Griswold 1993; Bosselaers 2002). Cauquenia Piacentini, Ramírez & Silva, 2013 (Piacentini et al. 2013), Devendra Lehtinen, 1967 (Griswold 1993) and Itatiaya Mello-Leitão, 1915 (Polotow and Brescovit 2010) have been confidently placed within the Griswoldiinae, represented in our matrix by Griswoldia and Phanotea. Birrana Raven & Stumkat, 2005, Krukt Raven & Stumkat, 2005, and Megateg Raven & Stumkat, 2005 have been placed near Kilyana (Raven and Stumkat 2005). Huntia Gray & Thompson, 2001, Pseudoctenus Caporiacco, 1949 and Uliodon have also been associated with the Zoropsinae (Raven and Stumkat 2005). From the taxa assigned to the Tengellidae (World Spider Catalog 2015) we have Austrotengella, Tengella and Titiotus. Anachemmis Chamberlin, 1919, Liocranoides Keyserling, 1881 and Socalchemmis Platnick & Ubick, 2001 are closely related to our exemplar Titiotus (Platnick and Ubick 2008). Calamistrula Dahl is a synonym of Uduba (Udubidae) (Griswold, pers. obs.). Lauricius Simon, 1888 and Wiltonia Koçak & Kemal, 2008 (Tengellidae) remain mysteries, but there is no better place for them than to accompany other tengellids into our newly circumscribed Zoropsidae. Previous analyses already suggest that the limits of Zoropsidae, Zorocratidae and Tengellidae are not well defined (Silva Dávila 2003; Raven and Stumkat 2005). Although our analyses fail to reveal many diagnostic characters to support the synonymy of these four families, they also fail to support the monophyly of, and provide diagnosis for, each family separately. Most problematic may be the position of Senoculidae within the Zoropsidae. This is unexpected, but supported both by Parsimony and Bayesian analyses (Figs 1–3). Some partitioned data, like the protein-coding gene fragments, show Senoculidae as a separate family within the Lycosoidea, a more conservative relationship for the family. Senoculus is on a long morphology branch (with 21 transformations), indicating that it might cluster with different clades when analysing only morphological characters. Despite our results, the synonymy of Senoculidae with Zoropsidae will be postponed, because this family is a classical Lycosoidea and this relation should be further explored.
Ctenids (tropical wolf spiders)
Ctenidae, as currently delimited, are polyphyletic in our results. Some previous phylogenetic analyses based only on morphology cluster Viridasiinae and Cupiennius Simon, 1891 with the remaining Ctenidae (Silva Dávila 2003; Polotow and Brescovit 2014) by several convergent traits, especially in the eye position and genital characters, though in other analyses (Ramírez 2014) viridasiines arise separately from other Ctenidae. In our results, all the total-evidence and partitioned analyses cluster Viridasiinae with Dionycha (Figs 1–3). The only exception was the morphological partition under equal-weights analysis of parsimony, which did not cluster Viridasiinae with the remaining Ctenidae or Dionycha. Cupiennius appears as sister-group of Lycosidae and part of Pisauridae (Figs 1–3, 7). The position of Cupiennius in the partitioned analyses is more complex, clustering with different clades in different partitions, but none of the analyses recover a monophyletic Ctenidae including Cupiennius. The remaining Ctenidae in our analysis cluster in a clade with low support in the parsimony analysis; this clade is not always present in the partitions (Fig. 8). Ctenidae (with a derived Psechridae as sister-group of Acanthoctenus Keyserling, 1877) are supported by six homoplastic synapomorphies in the parsimony analysis under implied weight k = 6 (Fig. 7): presence of four retromarginal teeth (character 15), cup-shaped median apophysis (character 35), presence of adhesive setae on tarsus (character 52), four or more pairs of spines on the ventral metatarsus of leg I and II (character 67), five pairs of spines on the ventral tibia of leg I and II (character 69) and overlapping spines on ventral tibia (character 70) (Fig. 7). The Bayesian analysis shows a Ctenidae clade (except Viridasiinae and Cupiennius) with posterior probability greater than 95% (Fig. 1).
Psechrids (giant funnel web and ‘pseudo-orb’ weavers)
The monophyly of Psechridae, comprising Psechrus Thorell, 1878 and Fecenia Simon, 1887, has been well established (Griswold 1993; Bayer and Schönhofer 2013; Agnarsson et al. 2013b). The grate-shaped tapetum of psechrids mandated inclusion in Lycosoidea (Homann 1971). In our analysis Psechridae (represented by Psechrus) cluster among the Lycosoidea, as has been the case in all previous quantitative analyses (Griswold 1993; Griswold et al. 1999; Silva Dávila 2003; Raven and Stumkat 2005; Griswold et al. 2005; Bayer and Schönhofer 2013; Agnarsson et al. 2013a; Agnarsson et al. 2013b). In our analyses psechrids appear as sister group of the clade formed by Oxyopidae, Thomisidae, Pisauridae, Lycosidae, Cupiennius and Nilus O. P.-Cambridge, 1876 in the Bayesian analysis (Fig. 1), and as a derived ctenid, sister group of Acanthoctenus, in the parsimony analyses with equal weights and implied weights concavity k = 6 (Figs 2, 3). Like Oxyopidae (Rovner 1980; Griswold 1983; Mora 1986), psechrids hang beneath sheet webs. Even the so-called ‘pseudo-orb’ of Fecenia adults has been shown to develop from a juvenile conical sheet web (Robinson and Lubin 1979). Recently, Bayer and Schönhofer (2013) investigated the phylogenetic relationships of Psechridae, and their results also show Psechridae within Lycosoidea. The relation of Psechridae and Ctenidae was not tested in their analysis, because the only ctenid species that they used was a Viridasius Simon, 1889 (here placed in Dionycha) (Bayer and Schönhofer 2013). As cited above, Viridasiinae do not cluster with Ctenidae in any of our analyses; therefore is not possible to compare the two analyses in this regard.
Psechridae species present some morphological characters that could relate them to derived Ctenidae, as the parsimony analysis suggests, such as the third claw and claw tufts (as several species of Ctenidae), whereas the retention of cribellum and calamistrum (as Acanthoctenus) and separate lorum I and II of the pedicel (fused in most Lycosoidea) distinguish psechrids from most other Lycosoidea. The molecular partition under parsimony also clusters Psechridae with Ctenidae (Fig. 8), but no other partition recovered this relation. Our Bayesian analyses never clustered Psechridae with Ctenidae: psechrids appear at the base of Lycosoidea as sister group of Lycosidae, Pisauridae, Cupiennius, Nilus, Oxyopidae and Thomisidae (Fig. 1).
Pisaurids
Pisauridae is represented in this analysis by four terminals. Only parsimony analysis under equal weights shows Nilus clustered with the remaining Pisauridae, in a monophyletic clade (Fig. 2). Thaumasia Perty, 1833, Hala Jocqué, 1994 and Architis Simon, 1898, although not a highly supported clade, appears in all analyses and all partitions as sister-group of Lycosidae (Figs 1, 3, 7). The Madagascar-endemic family Halidae was proposed by Jocqué (1994) and comprises two genera (Hala and Tolma Jocqué, 1994) from Madagascar. Jocqué and Dippenaar-Schoeman (2006) suggested that Halidae is a junior synonym of Pisauridae, but this idea has not previously been tested phylogenetically. Our results under all total-evidence analyses and most of the partitions (Figs 1–3, 7–8) place Hala in the Pisauridae as sister-group of Architis and Thaumasia, thus confirming the family synonymy.
Cupiennius – a model spider
Cupiennius is one of the most intensively studied genera of spiders and can be considered a model organism for understanding the physiology, behaviour and morphology of spiders (Barth 2002, and references therein). The genus comprises large spiders from the Neotropics, which can be easily bred in laboratory conditions (Barth 2002). Cupiennius was described in Ctenidae and kept as part of this family since then (Simon 1891). Our results do not corroborate the position of Cupiennius within Ctenidae and consistently show this genus as sister-group of Lycosidae and Pisauridae (Figs 1–3).
Lynx spiders (Oxyopidae) and crab spiders (Thomisidae)
Oxyopidae and Thomisidae frequently form a clade in our analyses, but their position varies greatly according to the different analysis parameters. In the parsimony analysis with equal weights (and also with concavity values of k = 3, 20, 50 and 99) Thomisidae appear as sister-group of Oxyopidae plus the ‘GST clade’ (Fig. 2). In the parsimony analysis with concavity k = 1, both families form a clade, sister-group of the ‘GST clade’. Thomisidae and Oxyopidae form a clade and appear in very different positions in the analyses using Bayesian and parsimony with concavity of k = 6 and 9, within the Lycosoidea and sister-group of the Lycosidae, Pisauridae, Cupiennius and Nilus (Figs 1, 3). None of the positions have strong support or are recovered by most partitions (Fig. 8). Bayer and Schönhofer (2013) and Agnarsson et al. (2013b) also recovered Thomisidae and Oxyopidae within Lycosoidea.
Systematics
In the following sections, we discuss morphological character support for certain groupings, suggesting how those groups might be diagnosed and identified. Also we present the taxonomic changes proposed on the basis of the results of this paper. The diagnoses are based on the results obtained by the parsimony analysis with concavity k = 6 (Figs 3–7).
Dionycha
The clade Dionycha (Fig. 5), represented in our analysis by exemplars from Anyphaenidae, Trachelidae, Eutichuridae, Mitugidae, Philodromidae, Salticidae, and the new family Viridasiidae, is supported by seven homoplastic synapomorphies, which distinguish them from other ‘RTA clade’ taxa: tapetum perforated with pores (character 7), two retromarginal teeth on chelicerae (character 15), absence of an additional tegular process on male palp (character 42), embolus with flexible base (character 44), third claw absent (character 50), presence of adhesive setae on tarsus (character 52) and two pairs of ventral spines on metatarsus I (character 67).
‘Oval Calamistrum clade’
The ‘Oval Calamistrum clade’ (Fig. 6), comprising Lycosoidea, our enlarged Zoropsidae and the new family Udubidae, is consistently recovered in our analyses and supported by seven homoplastic synapomorphies: subapical serrula (character 19), presence of locking lobes on the male palp (characters 29 and 31), tarsal trichobothrium proximal plate with transversal ridges (character 64), four pairs of ventral spines on tibia I (character 69), a unique oval calamistrum (character 73), trochanters of leg I with asymmetric and shallowly notched border (character 74) and minor ampullate gland spigots close together (character 89).
‘Grate-shaped Tapetum clade’ (GST clade)
This clade comprises seven family-level taxa (Fig. 6) – Zoropsidae (including Zorocratidae syn. nov. and Tengellidae syn. nov.), Lycosidae, Pisauridae, Oxyopidae, Thomisidae, Ctenidae and Trechaleidae (the latter not represented in our analysis) – and at least two genera that did not cluster with the former families – Nilus and Cupiennius. Our concept of the ‘GST clade’ differs from that of Silva-Dávila (2003) by including the Thomisidae but excluding some former zorocratids here placed in Udubidae, e.g. Raecius, Uduba, the viridasiines (formerly placed in Ctenidae, here proposed as the separate family Viridasiidae), and some miturgids and Odo. This clade is supported by three homoplastic synapomorphies (Fig. 6), which can be used to distinguish them from other ‘RTA clade’ taxa: posterior eye row recurved (character 5), ALE larger than AME (character 6) and presence of grate-shaped tapetum in the indirect eyes (character 7), reversed to canoe-shaped in some members.
Lycosoidea
Comprising seven families – Ctenidae, Oxyopidae, Thomisidae, Psechridae, Lycosidae, Pisauridae, Trechaleidae (not represented as a terminal in our analysis) – and at least two genera not clustered with the former families – Nilus and Cupiennius – our superfamily Lycosoidea differs from former concepts (Griswold 1993, Silva-Dávila 2003, Raven and Stumkat 2005) by including the Thomisidae but excluding Zoropsidae. The Lycosoidea are supported by one unique and two homoplastic synapomorphies (Fig. 7): egg sac carried by the chelicerae (later transformed to carried by the spinnerets) (character 96), anterior eye row (anterior view) recurved (character 4) and ALE smaller than AME (character 6).
A large clade of Lycosoidea excluding the Ctenidae (Pisauridae, including junior synonym Halidae, Lycosidae, Thomisidae, and Oxyopidae) is supported by several characters, four of them from the male pedipalp (Fig. 7): absence of the subtegulum lobe (character 29), absence of the lobe at the base of the embolus (character 31), absence of an additional tegular process (character 42), embolus with flexible base (character 44) and embolus with basal origin (character 45). The clade is also supported by six homoplastic somatic synapomorphies: sternum extending between coxae IV (character 13), presence of teeth on third claw (character 51), smooth proximal plate of the tarsal trichobothrium (character 64), presence of a distal spine on ventral metatarsus (character 66), border of trochanter on leg I deeply notched (character 74) and fused lorum I and II of the pedicel (character 75).
PISAURIDAE Simon, 1890
Types
Notes, synonymy
Our analysis includes three traditional pisaurids (Nilus, Thaumasia and Architis) plus Hala, which was described in its own family (Jocqué 1994) and later placed as a junior synonym of Pisauridae (Jocqué and Dippenaar-Schoeman 2006). The clade (Fig. 7) containing Nilus, Thaumasia and Architis is paraphyletic with respect to Lycosidae (Draposa and Alopecosa) and Cupiennius (currently placed in Ctenidae: World Spider Catalog 2015). Our analysis contains no member of Pisaura, and the circumscription and composition of Pisauridae is beyond the scope of this contribution. At least Hala groups with the pisaurids Thaumasia and Architis, corroborating the synonymy of Halidae and Pisauridae.
Distribution
Worldwide.
UDUBIDAE Griswold & Polotow, fam. nov.
Types
Diagnosis
The Udubidae (Figs 3, 6) are supported by several homoplastic synapomorphies, which can be used to distinguished them from other Oval Calamistrum clade families: absence of a serrula on the movable chela of the chelicerae (character 16), tibia of male palp with ventral apophysis (character 22), oval cross section of the embolus (character 48), tarsal organ with a keyhole-shaped opening (character 57), tarsal trichobothria of equal length (character 61), male leg tibiae with crack (character 68), uniquely textured spermathecae (character 88) and elongated posterior lateral spinnerets (character 92).
Composition
Compostichomma Karsch, 1891, Raecius Simon, 1892, Uduba Simon, 1880 and Zorodictyna Strand, 1907.
Distribution
Tropical and subtropical Africa, Madagascar, and Sri Lanka.
Note
Campostichomma was not included as a terminal in our analysis, but it presents the morphological diagnostic characters of Udubidae. The Udubidae fauna from Madagascar is diverse although only five species are currently recognised, i.e. Calamistrula evanescens Dahl, Uduba dahli Simon, 1903 and U. madagascariensis (Vinson, 1863), and Zorodictyna inhonesta (Simon, 1906) and Z. oswaldi (Lenz, 1891), there are nearly 100 new species to be described. Calamistrula Dahl 1901 is a junior synonym of Uduba (Griswold, pers. obs.), which genus is under revision (Griswold, Ledford and Ubick, in prep.).
VIRIDASIIDAE Lehtinen, 1967, rank.nov.
Types
Notes
Although traditionally placed in Ctenidae, the peculiar nature of these spiders has been long recognised. Lehtinen considered Viridasiinae ‘a close relative of an extinct cribellate branch of Lycosoidea’ (Lehtinen 1967: 378). Viridasiinae were placed as a basal clade of Ctenidae by Silva Dávila (2003). In our analysis our three viridasiine exemplars, Vulsor isaloensis (Ono, 1993) and two species of Viridasius, cluster far from the other Ctenidae and nearer to Philodromidae and Odo, suggesting that viridasiines be excluded from Ctenidae (Figs 1–3).
Diagnosis
Several homoplasies and two unique synapomorphies support Viridasiidae. The unique synapomorphies are a tegular process at the conductor base (character 41) and the presence of a pars pendula on the embolus (character 49). Homoplasious synapomorphies include a type 2 ventral tibial process on the male palp (character 23), a cymbial retrolateral process (character 26), a subtegulum locking lobe (character 29), a locking lobe at the base of the embolus (character 31), tarsal trichobothrium with proximal plate smooth, more than three longitudinal rows of dorsal trichobothria on the metatarsus (character 65) and the epigynum lateral sector with a tooth (character 80).
Composition
Viridasius and Vulsor. There are many new species and some new genera that belong to this clade, all from Madagascar (D. Silva Dávila, pers. comm.).
Distribution
Madagascar.
ZOROPSIDAE Bertkau, 1882
Types
Synonymy
We radically relimit this family and propose that Zorocratidae Dahl, 1913 (syn. nov.) and Tengellidae Dahl, 1908 (syn. nov.) be synonymised.
Diagnosis
This family circumscription is consistently supported by molecular data, as well as by three homoplastic morphological synapomorphies on the male palp (Fig. 6): presence of a type 2 ventral tibial process on the tibia (character 23), a rounded to oval cymbium (character 25) and a cymbial retrolateral process (character 26).
Composition
Included genera may be distributed among four subfamilies. Griswoldiinae Raven & Stumkat 2005, which are from south Asia (Sri Lanka), Australia, southern Africa and southern South America, comprise Austrotengella, Cauquenia, Devendra, Griswoldia, Itatiaya and Phanotea. Synapomorphies for Griswoldiinae comprise the absence of a cymbial retrolateral process (character 26), a cup-shaped median apophysis (character 35), no additional tegular process (character 42), a tooth on the epigynum lateral sector (character 80), and elongated gland tubes on the copulatory ducts (character 83). Uliodoninae Lehtinen, 1967, which are from Australia and New Zealand, comprise Huntia and Uliodon. Synapomorphies for Uliodoninae comprise a flexible embolic base (character 44), no inferior tarsal (third) claw (ITC) on leg I (character 50) and no gland tubes on the head of the spermathecae (character 86). Kilyana groups with Uliodoninae in this analysis, though in the more genera-rich analysis of Raven and Stumkat (2005), Kilyana groups with Zoropsinae. Also, following Raven and Stumkat (2005) and our analysis, we suggest that Zoropsinae Bertkau, 1882, which is a worldwide taxon, may comprise Akamasia, Birrana, Kilyana, Krukt, Megateg, Takeoa and Zoropsis. The ‘tengellids’ Anachemmis, Liocranoides, Socalchemmis and Titiotus may also be zoropsines. Synapomorphies for Zoropsinae comprise subtegulum with a shallow, cup-shaped excavation as a locking lobe (character 30), a hooked, bifid median apophysis apex (character 36) (Fig. 15A), embolus shape in cross-section laminar (character 48), highly spinose legs with four pairs of ventral spines on metatarsus I (character 67), six or more pairs of ventral spines on tibia I (character 69) and tibia I with ventral spines overlapping (character 70), with the head of the spermathecae lacking gland tubes (character 86) and the minor ampullate gland spigots separated by their diameter (character 89). Tengellinae Dahl comprise only the cribellate genera Tengella and Zorocrates. Synapomorphies for Tengellinae comprise some characters that may be considered reversals, e.g. posterior eye row (dorsal view) straight (Fig. 10D) (character 5), tapetum canoe-shaped (character 7), no tenant plates bearing claw tufts (character 54), and tarsal trichobothria lengths all equal (character 61); other synapomorphies include endite serrula position apical (character 19) and copulatory ducts with elongated gland tubes (character 83) (Fig. 20F). The single three-nucleotide gap in COI for the terminals Tengella radiata and Tengella sp. can be considered a synapomorphy for the genus Tengella. Pseudoctenus, Lauricius and Wiltonia are incertae sedis in our relimited Zoropsidae.

|
|
|

|
Distribution
Worldwide.
Conclusions
Results are consistent across several analytical parameters and methods for some of the groups found in the analyses. We recover the same clades and the same relations among clades in different analyses. These results imply that many morphological characters show substantial convergence. On the basis of the findings of this study, we provide additional evidence corroborating the non-monophyly of the grate-shaped tapetum. The reflective grate-shaped tapetum is related to a nocturnal and predatory behaviour and it appears to have evolved multiple times, through elongation of the canoe-shaped tapetum, which remains constrained by the diameter of the eye, which in turn leads to compression of the elongated tapetum as a series of loops or grids.
Based on the above analyses and discussion, our taxonomic conclusions can be summarised as follows:
-
The monophyly of the Oval Calamistrum clade appears to be unequivocal, with high support.
-
The grate-shaped tapetum appears independently at least three times and has a complex evolutionary history, with several reversions.
-
The superfamily Lycosoidea should be restricted to seven families: Lycosidae, Pisauridae (including the junior synonym Halidae), Ctenidae, Psechridae, Thomisidae and Oxyopidae. Trechaleidae (not included in our dataset) is also a member of Lycosoidea.
-
As currently circumscribed (World Spider Catalog 2015), the following families are not monophyletic: Ctenidae, Miturgidae, Pisauridae, Tengellidae, Zorocratidae and Zoropsidae.
-
Senoculidae (Senoculus) do not cluster with the remaining Lycosoidea but instead as part of Zoropsidae.
-
The parsimony analysis clusters Psechridae (Psechrus) within Ctenidae as sister-group of Acanthoctenus; this result is not supported by the Bayesian analysis. Placement of Psechridae within the Lycosoidea is unequivocal, but the sister group to Psechridae remains elusive.
-
Udubidae is proposed as a new family including the clade formed by former zorocratid genera Uduba, Raecius and Zorodictyna, plus the south Asian genus Campostichomma. These do not cluster with the zorocratid type species Zorocrates fuscus.
-
Viridasiidae is proposed as a new family, comprising some former Ctenidae endemic to Madagascar.
-
Zoropsidae is enlarged to include the genera of Zorocratidae and Tengellidae.
Acknowledgements
Financial support was provided by the Exline-Frizzell Fund for Arachnological Research and Lindsay Expedition Fund (both California Academy of Sciences, CAS) and by The Schlinger Foundation; DP is grateful for CNPq, Conselho Nacional de Desenvolvimento Científico e Tecnológico (PDE program) and a Bill and Maria Peck Fellowship (CAS); AC and CG thank the Hagey Research Investment Fund (CAS). The Lakeside Fund, CAS, supported the ‘Lycosoidea Summit’: including the authors and Lina Almeida, Estevam Cruz da Silva, Luis Piacintini, Diana Silva Dávila, Petra Sierwald and Darrell Ubick. Some of the ideas considered herein were spawned by the fruitful encounter in September 2011. Aspects of this research were supported by NSF grants: DEB-0072713 – ‘Terrestrial Arthropod Inventory of Madagascar’ (to CG and Brian Fisher), DEB 9296271 – ‘Systematics and Biogeography of Afromontane Spiders‘ (to CG), DEB-0613775 ‘PBI: Collaborative Research – The Megadiverse, Microdistributed Spider Family Oonopidae’ (to CG) and EAR-0228699 – ‘Assembling the Tree of Life: Phylogeny of Spiders’ (Ward Wheeler, PI). The program TNT was made available by the Willi Hennig Society. Specimens were provided by Martín Ramírez, Adalberto Santos and Darci Battesti. Professor Dr Wolfgang Nentwig from University of Bern kindly sent us the specimen of Cupiennius salei. We are thankful to Mark Harvey, Martin Ramírez and Cor Vink for the helpful comments and corrections in this manuscript.
References
Agnarsson, I., Gregorič, M., Blackledge, T. A., and Kuntner, M. (2013a). The phylogenetic placement of Psechridae within Entelegynae and the convergent origin of orblike spider webs. Journal of Zoological Systematics and Evolutionary Research 51, 100–106.| The phylogenetic placement of Psechridae within Entelegynae and the convergent origin of orblike spider webs.Crossref | GoogleScholarGoogle Scholar |
Agnarsson, I., Coddington, J., and Kuntner, M. (2013b). Systematics – progress in the study of spider diversity and evolution. In ‘Spider Research in the 21st Century: Trends and Perspectives’. (Ed. D. Penney.) pp. 58–111. (Siri Scientific Press: Rochdale, UK.)
Altschul, A. F., Madden, T. L., Schäffer, A. A., Zhang, J., Zhang, Z., Miller, W., and Lipman, D. J. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research 25, 3389–3402.
| 1:CAS:528:DyaK2sXlvFyhu7w%3D&md5=f96006b92451a7cea9470cbc39c47b39CAS |
Álvarez-Padilla, F., and Hormiga, G. (2007). A protocol for digesting internal soft tissues and mounting spiders for scanning electron microscopy. The Journal of Arachnology 35, 538–542.
| A protocol for digesting internal soft tissues and mounting spiders for scanning electron microscopy.Crossref | GoogleScholarGoogle Scholar |
Arnedo, M., Oromi, P., and Ribera, C. (2001). Radiation of the spider genus Dysdera (Araneae, Dysderidae) in the Canary Islands: cladistic assessment based on multiple data sets. Cladistics 17, 313–353.
| Radiation of the spider genus Dysdera (Araneae, Dysderidae) in the Canary Islands: cladistic assessment based on multiple data sets.Crossref | GoogleScholarGoogle Scholar |
Baccetti, B., and Bedini, C. (1964). Research on the structure and physiology of the eyes of a lycosid spider. I. Microscopic and ultramicroscopic structure. Archives Italiennes de Biologie 102, 97–122.
Barth, F. G. (2002). ‘A Spider‘s World. Senses and Behavior.’ (Springer-Verlag: Heidelberg.)
Bayer, S., and Schönhofer, A. L. (2013). Phylogenetic relationships of the spider family Psechridae inferred from molecular data, with comments on the Lycosoidea (Arachnida: Araneae). Invertebrate Systematics 27, 53–80.
| Phylogenetic relationships of the spider family Psechridae inferred from molecular data, with comments on the Lycosoidea (Arachnida: Araneae).Crossref | GoogleScholarGoogle Scholar |
Bennett, R. G. (1992). The spermathecal pores of spiders with special reference to dictynoids and amaurobioids (Araneae, Araneomorphae, Araneoclada). Proceedings of the Entomological Society of Ontario 123, 1–21.
Berger, M. P., and Munson, P. J. (1991). A novel randomized iterative strategy for aligning multiple protein sequences. Computer Applications in the Biosciences 7, 479–484.
| 1:CAS:528:DyaK38Xht1Chu7s%3D&md5=f940e2987590a71181f0bca6f073f776CAS | 1747779PubMed |
Bertkau, P. (1882). Über das Cribellum und Calamistrum. Ein Beitrag zur Histologie, Biologie und Systematik der Spinnen. Archiv für Naturgeschichte 48, 316–362.
Blackledge, T. A., Scharff, N., Coddington, J. A., Szüts, T., Wenzel, J. W., Hayashi, C. Y., and Agnarsson, I. (2009). Reconstructing web evolution and spider diversification in the molecular era. Proceedings of the National Academy of Sciences of the United States of America 106, 5229–5234.
| Reconstructing web evolution and spider diversification in the molecular era.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXksVertL8%3D&md5=dd0de03b49447364b5bfefb0cadffbd0CAS | 19289848PubMed |
Bremer, K. (1994). Branch support and tree stability. Cladistics 10, 295–304.
Bosselaers, J. (2002). A cladistic analysis of Zoropsidae (Araneae), with the description of a new genus. Belgian Journal of Zoology 132, 141–154.
Clerck, C. (1757). ‘Svenska spindlar, uti sina hufvud-slågter indelte samt under några och sextio särskildte arter beskrefne och med illuminerade figurer uplyste.’ (Stockholmiae.)
Coddington, J. A., and Levi, H. W. (1991). Systematics and evolution of spiders (Araneae). Annual Review of Ecology and Systematics 22, 565–592.
| Systematics and evolution of spiders (Araneae).Crossref | GoogleScholarGoogle Scholar |
Coddington, J., and Scharff, N. (1994). Problems with zero-length branches. Cladistics 10, 415–423.
| Problems with zero-length branches.Crossref | GoogleScholarGoogle Scholar |
Colgan, D. J., McLauchlan, A., Wilson, G. D. F., Livingston, S. P., Edgecombe, G. D., Macaranas, J., Cassis, G., and Gray, M. R. (1998). Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution. Australian Journal of Zoology 46, 419–437.
| Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution.Crossref | GoogleScholarGoogle Scholar |
Dahl, F. (1901). Nachtrag zur Uebersicht der Zoropsiden. Sitzungs-Berichte der Gesellschaft Naturforschender Freunde, Berlin 1901, 244–255.
Dahl, F. (1908). Die Lycosiden oder Wolfsspinnen Deutschlands und ihre Stellung im Haushalt der Natur. Nach statistichen Untersuchungen dargestellt. Nova Acta Academiae Caesareae Leopoldino-Carolinae Germanicae Naturae Curiosorum 88, 175–678.
Dahl, F. (1913). ‘Vergleichende Physiologie und Morphologie der Spinnentiere unter besonderer Berucksichtigung der Lebensweise. 1. Die Beziehungen des Körperbaues und der Farben zur Umgebung.’ (Jena.) pp. 1–113.
Dufour, L. (1820). Descriptions de cinq arachnides nouvelles. Annales générales des Sciences physiques 5, 198–209.
Farris, J. S. (1969). A successive approximations approach to character weighting. Systematic Zoology 18, 374–385.
Felsenstein, J. (1985). Phylogenies and the comparative method. American Naturalist 125, 1–15.
Fenk, L. M., and Schmid, A. (2010). The orientation-dependent visual special cut-off frequency in a spider. The Journal of Experimental Biology 213, 3111–3117.
| The orientation-dependent visual special cut-off frequency in a spider.Crossref | GoogleScholarGoogle Scholar | 20802111PubMed |
Fitch, W. M. (1971). Toward defining the course of evolution: minimal change for a specific tree topology. Systematic Zoology 20, 406–416.
| Toward defining the course of evolution: minimal change for a specific tree topology.Crossref | GoogleScholarGoogle Scholar |
Foelix, R. F. (2011). ‘Biology of Spiders.’ 3rd edn. (Oxford University Press: New York.)
Folmer, O., Black, M., Hoeh, W., Lutz, R., and Vrijenhoek, R. (1994). DNA primers for the amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3, 294–299.
| 1:CAS:528:DyaK2MXjt12gtLs%3D&md5=c84f72a2d796a7fee91c4e2e01175599CAS | 7881515PubMed |
Forster, R. R. (1970). The spiders of New Zealand. Part III. Otago Museum Bulletin 3, 1–184.
Forster, R. R., and Wilton, C. L. (1973). The spiders of New Zealand. Part IV. Otago Museum Bulletin 4, 1–309.
Giribet, G., and Wheeler, W. C. (1999). On gaps. Molecular Phylogenetics and Evolution 13, 132–143.
| 1:CAS:528:DyaK1MXmt1Omsbs%3D&md5=14682f4f5ca7266a03e6ccfa32dd314bCAS | 10508546PubMed |
Giribet, G., and Edgecomb, G. (2006). Conflict between datasets and phylogeny of centipedes: an analysis based on seven genes and morphology. Proceedings. Biological Sciences 273, 531–538.
| Conflict between datasets and phylogeny of centipedes: an analysis based on seven genes and morphology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XltFeqtLo%3D&md5=52040c0713d3079a05dc0300c97a872dCAS |
Giribet, G., Vogt, L., Pérez González, A., Sharma, P., and Kury, A. B. (2010). A multilocus approach to harvestmen (Arachnida: Opiliones) phylogeny with emphasis on biogeography and the systematics of Laniatores. Cladistics 26, 408–437.
Goloboff, P. A. (1993). Estimating character weights during tree search. Cladistics 9, 83–91.
Goloboff, P. A., Farris, J. S., Källersjö, M., Oxelman, B., Ramírez, M. J., and Szumik, C. A. (2003). Improvements to resampling measures of group support. Cladistics 19, 324–332.
| Improvements to resampling measures of group support.Crossref | GoogleScholarGoogle Scholar |
Goloboff, P. A., Farris, J. S., and Nixon, K. (2008a). TNT, a free program for phylogenetic analysis. Cladistics 24, 774–786.
Goloboff, P. A., Carpenter, J. M., Arias, J. S., and Miranda Esquivel, D. R. (2008b). Weighting against homoplasy improves phylogenetic analysis of morphological data sets. Cladistics 24, 758–773.
| Weighting against homoplasy improves phylogenetic analysis of morphological data sets.Crossref | GoogleScholarGoogle Scholar |
Golubchik, T., Wise, M. J., Easteal, S., and Jermiin, L. S. (2007). Mind the gaps: evidence of bias in estimates of multiple sequence alignments. Molecular Biology and Evolution 24, 2433–2442.
| Mind the gaps: evidence of bias in estimates of multiple sequence alignments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlGmsbfM&md5=2b59eb723a037216c617eabfd1053748CAS | 17709332PubMed |
Gotoh, O. (1993). Optimal alignment between groups of sequences and its application to multiple sequence alignment. Computer Applications in the Biosciences 9, 361–370.
| 1:CAS:528:DyaK2cXnvVer&md5=0be355dedc00e165e9a7663ae748b0eaCAS | 8324637PubMed |
Griswold, C. E. (1983). Tapinillus longipes Taczanowski, a web-building lynx spider from the American tropics (Araneae: Oxyopidae). Journal of Natural History 17, 979–985.
| Tapinillus longipes Taczanowski, a web-building lynx spider from the American tropics (Araneae: Oxyopidae).Crossref | GoogleScholarGoogle Scholar |
Griswold, C. E. (1993). Investigations into the phylogeny of the lycosoid spiders and their kin (Arachnida: Araneae: Lycosoidea). Smithsonian Contributions to Zoology 539, 1–39.
| Investigations into the phylogeny of the lycosoid spiders and their kin (Arachnida: Araneae: Lycosoidea).Crossref | GoogleScholarGoogle Scholar |
Griswold, C. E., Coddington, J. A., Platnick, N. I., and Forster, R. R. (1999). Towards a phylogeny of Entelegyne spiders (Araneae, Araneomorphae, Entelegynae). The Journal of Arachnology 27, 53–63.
Griswold, C. E., Ramírez, M. J., Coddington, J. A., and Platnick, N. I. (2005). Atlas of phylogenetic data for entelegyne spiders (Araneae: Araneomorphae: Entelegynae) with comments on their phylogeny. Proceedings of the California Academy of Sciences 56, 1–324.
Hedin, M. C., and Maddison, W. P. (2001). A combined molecular approach to phylogeny of the jumping spider subfamily Dendryphantinae (Araneae: Salticidae). Molecular Phylogenetics and Evolution 18, 386–403.
| A combined molecular approach to phylogeny of the jumping spider subfamily Dendryphantinae (Araneae: Salticidae).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXit1ansbg%3D&md5=269ae178510d5ccbf65ffed910e1b58fCAS | 11277632PubMed |
Homann, H. (1928). Beiträge zur Physiologie der Spinnenaugen. I. Untesuchungsmethoden. II. Das Sehvermögen der Salticiden. Zeitschrift fur Vergleichende Physiologie 7, 201–268.
| Beiträge zur Physiologie der Spinnenaugen. I. Untesuchungsmethoden. II. Das Sehvermögen der Salticiden.Crossref | GoogleScholarGoogle Scholar |
Homann, H. (1971). Die Augen der Araneae: Anatomie, Ontogenie und Bedeutung für die Systematik (Chelicerata, Arachnida). Zeitschrift für Morphologie der Tiere 69, 201–272.
| Die Augen der Araneae: Anatomie, Ontogenie und Bedeutung für die Systematik (Chelicerata, Arachnida).Crossref | GoogleScholarGoogle Scholar |
Hormiga, G., Arnedo, M., and Gillespie, R. (2003). Speciation on a conveyor belt: sequential colonization of the Hawaiian Islands by Orsonwelles spiders (Araneae, Linyphiidae). Systematic Biology 52, 70–88.
| Speciation on a conveyor belt: sequential colonization of the Hawaiian Islands by Orsonwelles spiders (Araneae, Linyphiidae).Crossref | GoogleScholarGoogle Scholar | 12554442PubMed |
Huelsenbeck, J. P., and Ronquist, F. (2001). MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755.
| MRBAYES: Bayesian inference of phylogenetic trees.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MvotV2isw%3D%3D&md5=cd4de43d633ceeff10f03392cbc4da97CAS | 11524383PubMed |
Jocqué, R. (1994). Halidae, a new spider family from Madagascar (Araneae). Bulletin of the British Arachnological Society 9, 281–289.
Jocqué, R., and Dippenaar-Schoeman, A. S. (2006). ‘Spider Families of the World.’ (Musée Royal de l‘Afrique Central: Tervuren.)
Katoh, K., Misawa, K., Kuma, K., and Miyata, T. (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30, 3059–3066.
| 1:CAS:528:DC%2BD38XlslOqu7s%3D&md5=062ddb93ea5f1aa3de915bbe559e94aaCAS | 12136088PubMed |
Katoh, K.,, Kuma, K., Toh, H., and Miyata, T. (2005). MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Research 33, 511–518.
| 1:CAS:528:DC%2BD2MXhtV2qsbc%3D&md5=cc62409e78bbc10a2c4ef2a613b5707dCAS |
Katoh, K., and Toh, H. (2008). Recent developments in the MAFFT multiple sequence alignment program. Briefings in Bioinformatics 9, 286–298.
| 1:CAS:528:DC%2BD1cXpt1artrs%3D&md5=236425aba2026540cc17978d4fca1cdaCAS | 18372315PubMed |
Land, M. F. (1972). The physics and biology of animal reflectors. Progress in Biophysics and Molecular Biology 24, 75–106.
| The physics and biology of animal reflectors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2cXltVajtr0%3D&md5=b1b273731f73283d461936190fcba2afCAS | 4581858PubMed |
Land, M. F. (1985). The morphology and optics of spider eyes. In ‘Neurobiology of Arachnids’. (Ed. F. G. Barth.) pp. 53–76. (Spring-Verlag: Berlin, Heidelberg.)
Latiolais, J. M., Taylor, M. S., Kaustuv, R., and Hellberg, M. E. (2006). A molecular phylogenetic analysis of strombid gastropod morphological diversity. Molecular Phylogenetics and Evolution 41, 436–444.
| 1:CAS:528:DC%2BD28XhtVagtbzL&md5=8be3fc700825a2f8e817a9af03b81bccCAS | 16839783PubMed |
Lehtinen, P. T. (1967). ‘Classification of the Cribellate Spiders and some Allied Families: with Notes on the Evolution of the Suborder Araneomorpha.’ (Societas Zoologica Botanica Fennica Vanamo: Helsinki.)
Lenz, H. (1886). Beiträge zur Kenntniss der Spinnenfauna Madagascars. Zoologische Jahrbucher. Abteilung fur Systematik, Ökologie und Geographie der Tiere 1, 379–408.
Lenz, H. (1891). Spinnen von Madagascar und Nossi-Bé. Jahrbuch der Hamburgischen Wissenschaftlichen Anstalten 9, 153–181.
Maddison, W. P., and Maddison, D. R. (2011). ‘Mesquite: a Modular System for Evolutionary Analysis.’ Version 2.75. Available at http://mesquiteproject.org
Miller, J. A., Carmichael, A., Ramírez, M. J., Spagna, J. C., Haddad, C. R., Rezac, M., Johannesen, J., Kral, J., Wang, X., and Griswold, C. E. (2010). Phylogeny of entelegyne spiders: affinities of the family Penestomidae (new rank), generic phylogeny of Eresidae, and asymmetric rates of change in spinning organ evolution (Araneae, Araneoidea, Entelegynae). Molecular Phylogenetics and Evolution 55, 786–804.
| Phylogeny of entelegyne spiders: affinities of the family Penestomidae (new rank), generic phylogeny of Eresidae, and asymmetric rates of change in spinning organ evolution (Araneae, Araneoidea, Entelegynae).Crossref | GoogleScholarGoogle Scholar | 20206276PubMed |
Mora, G. (1986). Use of web by Tapinillus longipes (Araneae: Oxyopidae). In ‘Proceedings of the Ninth International Congress of Arachnology, Panama 1983’. (Eds W. G. Eberhard, Y. D. Lubin and B. C. Robinson.) pp. 173–175. (Smithsonian Institution Press: Washington, DC.)
Needleman, S. B., and Wunsch, C. D. (1970). A general method applicable to the search for similarities in the amino acid sequence of two proteins. Journal of Molecular Biology 48, 443–453.
| 1:CAS:528:DyaE3cXktVShu74%3D&md5=396d52dabee57a39fc51795591f10fccCAS | 5420325PubMed |
Notredame, C., Higgins, D. G., and Heringa, J. (2000). T-Coffee: a novel method for fast and accurate multiple sequence alignment. Journal of Molecular Biology 302, 205–217.
| 1:CAS:528:DC%2BD3cXmtVGntr8%3D&md5=eae33ad4c7c01053fb8ba50d65fb37d2CAS | 10964570PubMed |
Nylander, J. A. A. (2008). MrModeltest Version 2.3. Evolutionary Biology Centre, Uppsala University. Available from: http://www.abc.se/~nylander/.
Ono, H. (1993). Spiders (Araneae) from Madagascar. Acta Arachnologica Tokyo 42, 55–67.
Piacentini, L. N., Ramírez, M. J., and Silva, D. (2013). Systematics of Cauquenia (Araneae: Zoropsidae), with comments on the patterns of evolution of cribellum and male tibial crack on Lycosoidea. Invertebrate Systematics 27, 567–577.
| Systematics of Cauquenia (Araneae: Zoropsidae), with comments on the patterns of evolution of cribellum and male tibial crack on Lycosoidea.Crossref | GoogleScholarGoogle Scholar |
Platnick, N. I. (1977). The hypochiloid spiders: a cladistic analysis, with notes on the Atypoidea (Arachnida, Araneae). American Museum Novitates 2627, 1–23.
Platnick, N. I., and Gertsch, W. J. (1976). The suborders of spiders: a cladistic analysis. American Museum Novitates 2607, 1–15.
Platnick, N. I., and Ubick, D. (2008). A revision of the endemic Californian spider genus Titiotus Simon (Araneae, Tengellidae). American Museum Novitates 3608, 1–33.
| A revision of the endemic Californian spider genus Titiotus Simon (Araneae, Tengellidae).Crossref | GoogleScholarGoogle Scholar |
Platt, A. R., Woodhall, R. W., and George, A. L. J. (2007). Improved DNA sequencing quality and efficiency using an optimized fast cycle sequencing protocol. BioTechniques 43, 58–62.
| 1:CAS:528:DC%2BD2sXotVyku7s%3D&md5=d8045465a5d053ef8553adf4dfdf139fCAS | 17695253PubMed |
Polotow, D., and Brescovit, A. D. (2010). Phylogenetic relationships of the Neotropical spider genus Itatiaya (Araneae). Zoologica Scripta 40, 187–193.
Polotow, D., and Brescovit, A. D. (2014). Phylogenetic analysis of the tropical wolf spider subfamily Cteninae (Arachnida, Araneae, Ctenidae). Zoological Journal of the Linnean Society 170, 333–361.
Prendini, L., Crowe, T., and Wheeler, W. (2003). Systematics and biogeography of the family Scorpionidae (Chelicerata: Scorpiones), with a discussion on phylogenetic methods. Invertebrate Systematics 17, 185–259.
| Systematics and biogeography of the family Scorpionidae (Chelicerata: Scorpiones), with a discussion on phylogenetic methods.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmslals7o%3D&md5=15986575bc4531117392470a7b3a894aCAS |
Rambaut, A., and Drummond, A. J. (2007). Tracer: MCMC Trace Analysis Tool. Version 1.4. Available at http://beast.bio.ed.ac.uk/Tracer
Ramírez, M. J. (2003). The spider subfamily Amaurobioidinae (Araneae, Anyphaenidae): a phylogenetic revision at the generic level. Bulletin of the American Museum of Natural History 277, 1–262.
| The spider subfamily Amaurobioidinae (Araneae, Anyphaenidae): a phylogenetic revision at the generic level.Crossref | GoogleScholarGoogle Scholar |
Ramírez, M. J. (2014). The morphology and phylogeny of dionychan spiders (Araneae: Araneomorphae). Bulletin of the American Museum of Natural History 390, 1–374.
| The morphology and phylogeny of dionychan spiders (Araneae: Araneomorphae).Crossref | GoogleScholarGoogle Scholar |
Raven, R. J., and Stumkat, K. S. (2005). Revisions of Australian ground-hunting spiders: II. Zoropsidae (Lycosoidea: Araneae). Memoirs of the Queensland Museum 50, 347–423.
Robinson, M. H., and Lubin, Y. D. (1979). Specialists and generalists: the ecology and behavior of some web-building spiders from Papua New Guinea. II. Psechrus argentatus and Fecenia sp. (Araneae: Psechridae). Pacific Insects 21, 133–164.
Ronquist, F., and Huelsenbeck, J. P. (2003). MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574.
| 1:CAS:528:DC%2BD3sXntlKms7k%3D&md5=6c22440a111ffd7c39b671a54462b45bCAS | 12912839PubMed |
Rovner, J. S. (1980). Adaptations for prey capture in oxyopid spiders: phylogenetic implications. In ‘8th International Congress of Arachnology, Vienna’. (Ed. J. Gruber.) pp. 233–237.
Sierwald, P. (1989). Morphology and ontogeny of female copulatory organs in American Pisauridae, with special reference to homologous features (Arachnida: Araneae). Smithsonian Contributions to Zoology 484, 1–24.
| Morphology and ontogeny of female copulatory organs in American Pisauridae, with special reference to homologous features (Arachnida: Araneae).Crossref | GoogleScholarGoogle Scholar |
Sierwald, P. (1990). Morphology and homologous features in the male palp organ in Pisauridae and other spider families, with notes on the taxonomy of Pisauridae (Arachnidae: Araneae). Nemouria 35, 1–59.
Silva Dávila, D. (2003). Higher-level relationships of the spider family Ctenidae (Araneae: Ctenoidea). Bulletin of the American Museum of Natural History 274, 1–86.
| Higher-level relationships of the spider family Ctenidae (Araneae: Ctenoidea).Crossref | GoogleScholarGoogle Scholar |
Simon, E. (1880). Révision de la famille des Sparassidae (Arachnides). Actes de la Société linnéenne de Bordeaux 34, 223–351.
Simon, E. (1891). Descriptions de quelques arachnides du Costa Rica communiqués pa M. A. Getaz (de Genève). Bulletin de la Société Zoologique de France 16, 109–112.
Simon, E. (1906). Etude sur les araignées de la section des cribellates. Annales de la Société Entomologique de Belgique 50, 284–308.
Simon, C., Frati, F., Beckenbach, A., Crespi, B., Liu, H., and Flook, P. (1994). Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Annals of the Entomological Society of America 87, 651–701.
| Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXis1Wiu7g%3D&md5=2841a8ddd2ecccad310722bcd729b341CAS |
Spagna, J. C., and Gillespie, R. G. (2008). More data, fewer shifts: molecular insights into the evolution of the spinning apparatus in non-orb-weaving spiders. Molecular Phylogenetics and Evolution 46, 347–368.
| More data, fewer shifts: molecular insights into the evolution of the spinning apparatus in non-orb-weaving spiders.Crossref | GoogleScholarGoogle Scholar | 17928240PubMed |
Strand, E. (1907). Diagnosen neuer Spinnen aus Madagaskar und Sansibar. Zoologischer Anzeiger Leipzig 31, 725–748.
Swofford, D. L., and Maddison, W. P. (1987). Reconstructing ancestral character states under Wagner parsimony. Mathematical Biosciences 87, 199–229.
| Reconstructing ancestral character states under Wagner parsimony.Crossref | GoogleScholarGoogle Scholar |
Swofford, D. L., and Maddison, W. P. (1992). Parsimony, character-state reconstructions, and evolutionary inferences. In ‘Systematics, Historical Ecology, and North American Freshwater Fishes’. (Ed. R. L. Mayden.) pp. 187–223. (Stanford University Press: Stanford.)
Vinson, A. (1863). Aranéides des îles de la Réunion. Maurice et Madagascar. Paris i-cxx, 1–337.
Whiting, M. F., Carpenter, J. M., Wheeler, Q. D., and Wheeler, W. C. (1997). The Strepsiptera problem: phylogeny of the holometabolous insect orders inferred from 18S and 28S ribosomal DNA sequences and morphology. Systematic Biology 46, 1–68.
| 1:STN:280:DC%2BD383js1yqtQ%3D%3D&md5=d44f1c97028888e070e932607539b111CAS | 11975347PubMed |
Wolff, J. O., and Gorb, S. N. (2012). Comparative morphology of pretarsal scopulae in eleven spider families. Arthropod Structure & Development 41, 419–433.
Wolff, J. O., Nentwig, W., and Gorb, S. N. (2013). The great silk alternative: multiple co-evolution of web loss and sticky hairs in spiders. PLoS One 8, e62682.
| The great silk alternative: multiple co-evolution of web loss and sticky hairs in spiders.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnsFWqs7k%3D&md5=94a1283fd68379f89ba272fcdc45ee44CAS | 23650526PubMed |
World Spider Catalog (2015). World Spider Catalog. Natural History Museum Bern. Online at http://wsc.nmbe.ch, [version 16, accessed January 2015]

|

|