Systematic revision of the Ogyris idmo (Hewitson, 1862) species group (Lepidoptera: Lycaenidae): implications for the conservation management of Australia’s most threatened butterflies
Ethan P. Beaver A B * , Michael F. Braby
A B * , Michael F. Braby  A B , Richard V. Glatz
A B , Richard V. Glatz  C D E and D. Andy Young
C D E and D. Andy Young  E
E
A
B
C
D
E
Invertebrate Systematics 37(10) 677-701 https://doi.org/10.1071/IS23032
Submitted: 17 June 2023 Accepted: 14 September 2023 Published: 11 October 2023
Abstract
Lycaenid butterflies of the Ogyris idmo species group are endemic to Australia and obligatorily associated with Camponotus ants. Several species are threatened with extinction, but there are considerable uncertainties with the present classification. Here, the taxonomy of the species group is revised based on molecular and morphological data. Mitochondrial sequence data were obtained from GenBank for Ogyris Angas, 1847, from cytochrome oxidase I (COI) and cytochrome b (cytb) (total of 1203 bp), and a phylogeny of the genus was reconstructed using Maximum Likelihood methods. Based on these molecular data, adult morphology and other evidence, the following eight taxa are recognised in this species group: Ogyris otanes (C. & R. Felder, 1865), Ogyris arcana M.R. Williams & Hay, 2001 stat. rev., Ogyris arcana arcana M.R. Williams & Hay, 2001 comb. nov., Ogyris arcana sublustris M.R. Williams & Hay, 2001 comb. nov., Ogyris halmaturia (Tepper, 1890), Ogyris halmaturia halmaturia (Tepper, 1890), Ogyris halmaturia waterhouseri (Bethune-Baker, 1905) stat. rev., Ogyris idmo (Hewitson, 1862), Ogyris subterrestris Field, 1999 and Ogyris petrina Field, 1999 stat. rev. The female of Ogyris halmaturia halmaturia is described for the first time. Phylogenetic relationships among the six species are as follows: (O. otanes + O. arcana) + (O. halmaturia + (O. idmo + (O. subterrestris + O. petrina))). The life history switch from phytophagy (O. otanes and O. arcana) to entomophagy (suspected myrmecophagy) within this species group has led to diversification of four species, a most unusual evolutionary pattern within the Lycaenidae globally. The taxonomic changes proposed herein affect some of the most threatened Australian butterflies and their conservation status is discussed.
ZooBank: urn:lsid:zoobank.org:pub:B9A6F558-DD47-47DF-AC9C-A71270B6EE09
Keywords: Camponotus, conservation status, critically endangered, endangered, entomophagy, Loranthaceae, myrmecophagy, Santalaceae, systematics, Theclinae, threatened species, vulnerable.
Introduction
The genus Ogyris Angas, 1847 is a diverse thecline genus in the tribe Ogyrini endemic to Australia and New Guinea, with several distinct species groups defined on the basis of ecological or structural relationships (Braby and Müller 2022). The Ogyris idmo (Hewitson, 1862) species group is currently understood to include four morphologically similar species, all endemic to southern and south-western Australia. Although members of all other Ogyris species groups are phytophagous, with larvae specialising on aerial-stem mistletoes in the family Loranthaceae (Braby 2000), most members of the O. idmo species group are entomophagous, with suspected predatory associations with Camponotus terebrans (Lowne, 1865) (Formicinae) ants (Pierce 1995; Field 1999; Braby and Douglas 2008; Grund 2010; Schmidt et al. 2014; Williams et al. 2020). The exception to this is Ogyris otanes (C. & R. Felder, 1865), which is ecologically unique in being the only species in which the larvae are associated with the foliage of root hemiparasites in the family Santalaceae (Burns and Angel 1952; Williams et al. 1992; Hart and Powell 1997; Williams and Hay 2001). Santalaceae is closely related to Loranthaceae and both families are classified in the order Santalales (Nickrent et al. 2010).
The taxonomy and nomenclature of this group of butterflies have been contentious and highly dynamic for the duration of the investigation period. This uncertainty has been compounded by the fact that the species in this complex are generally rarely encountered without targeted efforts and that the species can be highly localised. As such, previous workers often had few (usually poor quality) specimens available for study and this has hindered species delimitation in this group of morphologically similar species.
At least five taxa in this species group are of conservation significance. At the state level, Ogyris otanes otanes and O. halmaturia (Tepper, 1890) are listed as Critically Endangered (CR), and O. subterrestris subterrestris Field, 1999 is listed as Endangered (EN) in Victoria under the Flora and Fauna Guarantee Act 1988 (Taylor et al. 2018). Additionally, O. subterrestris petrina is listed as CR in Western Australia under the Biodiversity Conservation Act 2016 and is also listed as CR under the federal Environmental Protection and Biodiversity Conservation Act (1999), and O. halmaturia is listed internationally as EN under the IUCN Red List Categories (Young et al. 2021). Ogyris otanes sublustris M.R. Williams & Hay, 2001 is recognised as a taxon of national concern (Geyle et al. 2021) but is currently not listed under any legislation. Although notably less threatened, concerns for the protection of O. idmo populations have also been raised, with the species extirpated from almost a third of all known sites (Williams et al. 2020).
The purpose of this study is to re-examine the morphology of the Ogyris idmo species group and re-analyse the mtDNA sequence data presented by Schmidt et al. (2014) in this context. Taxonomic resolution of the O. idmo species group is imperative because all taxa are of conservation concern and many are threatened with extinction.
Materials and methods
Taxon sampling and molecular data
The mitochondrial DNA regions cytochrome oxidase I (COI) and cytochrome b (cytb) were obtained from NCBI GenBank (Supplementary Table S1) and included in the phylogenetic analysis. This dataset comprised 83 sequences covering seven in-group taxa and 12 outgroup taxa. The data were assembled, trimmed and aligned using Geneious (ver. 11.1.5, Biomatters, New Zealand, see https://www.geneious.com). Information on taxon sampling, PCR and sequencing is discussed by Schmidt et al. (2014) and Beaver et al. (2023) who obtained these sequences prior to our study.
Phylogenetic analysis
Phylogenetic relationships of Ogyris species were inferred by Maximum Likelihood methods. The COI and cytb alignments were concatenated in Geneious and the final alignment was a contig of 83 sequences with a total length of 1203 nucleotides (COI 626 bp, cytb 577 bp). Maximum Likelihood analysis was performed using IQ-TREE (ver. 2.2.0, see https://www.iqtree.org/; Minh et al. 2020). The best model was GTR + F + I + G4 that was chosen according to Bayesian Information Criterion using ModelFinder (see https://www.iqtree.org/ModelFinder/; Kalyaanamoorthy et al. 2017). Branch and clade support were assessed by ultrafast bootstrapping approximation with 1000 replicates (Hoang et al. 2018) and the Shimodaira–Hasegawa-like approximate likelihood-ratio test (SH-like aLRT) with 1000 replicates. Node values were considered robust if these were recovered with ultrafast bootstrap values of or above 95, and Shimodaira–Hasegawa-like approximate likelihood-ratio test values of or above 80. The resulting best tree was rooted with Acrodipsas myrmecophila as the outgroup and visualised using FigTree (ver. 1.4.4, see https://tree.bio.ed.ac.uk/ software/figtree/) and Adobe Photoshop (ver. ver. 2022, 24.0, Adobe Inc., San Jose, CA, USA).
Morphological data
Internal morphology of the male genitalia was examined through dissection. The abdomen was removed and soaked in 10% KOH for 8–15 min at 85–90°C to dissolve internal musculature and viscera. Cleared sclerites were stained with Chlorazol black diluted with 30% ethanol. Completed dissections were fixed in 95% ethanol and 100% isopropanol. Dissections were stored within glycerol and assigned a unique identifier that was also attached to the pinned specimen. Stored dissections were either pinned next to the specimen or affixed to the same pin on which the set specimen was mounted. Dissections were imaged and measured using a Leica M205A microscope and stacked using Helicon Focus (ver. 5.3, see https://helicon-focus.software.informer.com/5.3/) with the dissection floating in gel as described by Su (2016).
The genitalia of male Ogyris spp. were studied and compared by dissecting specimens of each taxon as follows. Ogyris otanes otanes: 1♂ Genitalia No. EPB-169 – Kingscote, KI, SA, 21 JAN. 1952, F.E. Parsons, ANIC Database no. 31 020437 (ANIC); 1♂ Genitalia No. EPB-183 – Big Desert, V. 27 m. N. Yanac, 6 DEC. 1973. B. D.F. Crosby, ANIC Database no. 31-020454 (ANIC); Ogyris otanes arcana: 1♂ Genitalia No. EPB-170 – Stirling Ranges, WA. 2 NOV 1988, R.W. Hay, ANIC Database no. 31 019933 (ANIC); Ogyris otanes sublustris: 1♂ Genitalia No. EPB-171 – 2 km S of Leeman, WA. 16 OCT 1985, R.W. Hay, ANIC Database no. 31 019976 (ANIC); Ogyris subterrestris subterrestris: 1♂ Genitalia No. EPB-185 – 13.5 km ENE of Waikerie, SA, 19 OCT. 2021, E.P. Beaver & E.F.M. Fisher (ANIC); Ogyris subterrestris petrina: 1♂ Genitalia No. EPB-184 – Lake Douglas, Kalgoolie, WA, 28 SEP. 1988, J.W.C. d’Apice, ANIC database no. 31 034646 (ANIC); 1♀ Genitalia slide M1127 – Lake Douglas, 12 km SW of Kalgoorlie, WA, 1 FEB. 82, A.J. Graham, PARATYPE, ANIC Database no. 31 019585 (ANIC); 1♂ Genitalia slide M1132 – Lake Douglas, 12 km SW of Kalgoorlie, WA, 22 OCT 1985, A.J. Graham, PARATYPE, ANIC Database no. 31 019589 (ANIC); 1♂ Genitalia tube 208 – Lake Douglas, 12 km SW of Kalgoorlie, WA, 1 FEB. 82, A.J. Graham, PARATYPE, ANIC Database no. 31 019584 (ANIC); Ogyris idmo: 1♂ Genitalia No. MFB-029 – Port Denison, WA, 29 SEP. 2002, M.F. Braby (ANIC); 1♂ Genitalia No. MFB-157 – South Perth, WA, 10 DEC. 1901, H.M. Giles (ANIC); 1♂ Genitalia No. MFB-161 – Mundaring, WA, 26 NOV. 1985, R. Hay (ANIC). Ogyris halmaturia: 1♂ Genitalia No. MFB-158 – Flinders Chase NP [exact locational data withheld], Kangaroo Island, SA, NOV. 2014, R.V. Glatz & D.A. Young (DESS); 1♂ Genitalia No. MFB-159 – Flinders Chase NP [exact locational data withheld], Kangaroo Island, SA, NOV. 2014, R.V. Glatz & D.A. Young (DESS); 1♂ Genitalia No. MFB-162 – Flinders Chase NP [exact locational data withheld], Kangaroo Island, SA, NOV. 2015, R.V. Glatz & D.A. Young (DESS); 1♂ Genitalia No. MFB-028 – Ngarkat CP, 28 km N Keith, SA, 10 NOV. 2005, M. Moore (ANIC); 1♂ Genitalia No. MFB-160 – Ngarkat CP, 28 km N Keith, SA, 30 OCT. 2007, M.F. Braby & M. Moore (ANIC).
The following abbreviations refer to repositories at which material has been examined:
AMS, Australian Museum, Sydney.
ANIC, Australian National Insect Collection, Canberra.
DESS, D’Estrees Entomology and Science Services, Kangaroo Island, SA.
NHMUK, Natural History Museum, London (formerly BMNH).
NMV, Museums Victoria, Melbourne.
SAMA, South Australian Museum, Adelaide.
WAM, Western Australian Museum, Perth.
Results
Molecular phylogeny
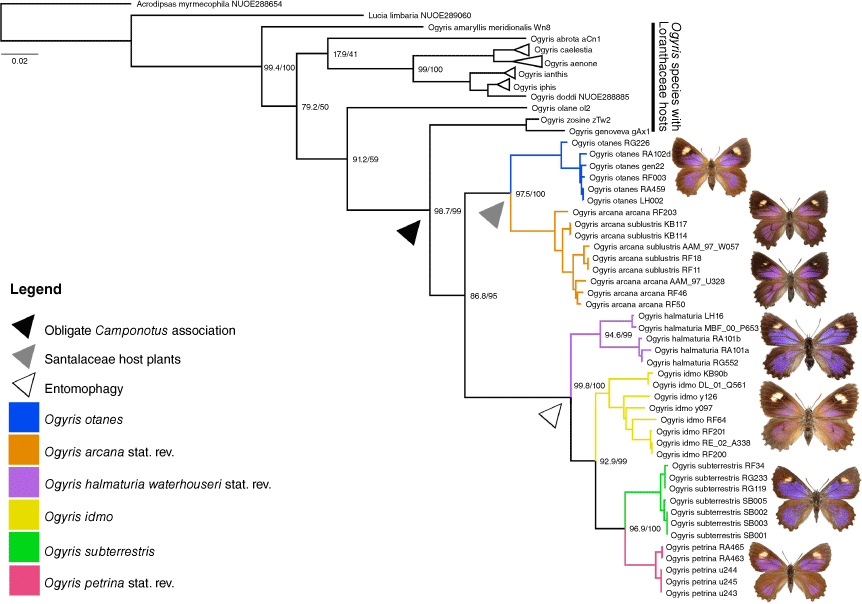
The Ogyris idmo species group was resolved as a monophyletic lineage with strong support (BS = 95, SH aLRT = 86.8, BI = 0.99) (Fig. 1). A larger clade containing all descendants with a Camponotus-associated life history was also strongly supported (BS = 99, SH aLRT = 98.7, BI = 1.0) (Fig. 1). Based on the well-supported phylogeny, relationships of the six species we recognised in the O. idmo group were as follows: (O. otanes + O. arcana stat. rev.) + (O. halmaturia + (O. idmo + (O. subterrestris + O. petrina stat. rev.))). Topology and support values for the O. idmo species group were largely congruent with those in Schmidt et al. (2014). The notable differences were that in our study that included additional Ogyris taxa, the clade containing O. idmo + (O. subterrestris + O. petrina stat. rev.) had a bootstrap support of 99 compared with 95 in Schmidt et al. (2014) and the clade containing O. otanes + O. arcana stat. rev. was resolved as a sister lineage to the remainder of the O. idmo species group with higher support (BS = 95) compared with only weak support (BS = 57) in Schmidt et al. (2014).
Maximum Likelihood phylogeny of Ogyris species inferred from mitochondrial COI and cytb, showing our revised taxonomy. Focal clades for six species comprising the Ogyris idmo group are highlighted in colour. The obligate host-ant Camponotus association is designated by a black arrowhead and ant entomophagy is designated with a white arrowhead. Node labels represent SH-aLRT values (left) and bootstrap support values (right). Twelve outgroup taxa are included and the tree is rooted with Acrodipsas myrmecophila. Scale bar represents substitutions per site.
Uncorrected p-distances between the species of interest are summarised in Table 1 as median values. Variation of within-group sequence similarity was wide, ranging from 0–0.5% for outgroup O. ianthis to 0.319–4.31% for O. halmaturia.
| O. otanes | O. arcana | O. halmaturia | O. idmo | O. subterrestris | O. petrina | ||
|---|---|---|---|---|---|---|---|
| O. otanes | |||||||
| O. arcana | 5.07 | ||||||
| O. halmaturia | 9.07 | 8.71 | |||||
| O. idmo | 9.11 | 9.14 | 5.68 | ||||
| O. subterrestris | 9.39 | 9.23 | 6.55 | 5.10 | |||
| O. petrina | 10.09 | 9.56 | 6.87 | 5.19 | 3.62 |
Subspecies of O. arcana are not displayed here as these were non-monophyletic (see Taxonomy section) and sequences of O. halmaturia halmaturia were not available for inclusion.
Taxonomy
The genus Ogyris was named twice with the same name based on different type species, first by Angas (1847) and later by Westwood in Doubleday and Westwood (1851). This confusing nomenclatural problem was clarified by Edwards (1996). The legacy of this synonym is apparent in the nomenclatural accounts for each taxon in this work, whereby most authors post-1996 refer to Ogyris Angas, 1847, but earlier authors were unaware of Angas (1847), and referred to the genus Ogyris Westwood, 1851 instead.
The Ogyris idmo species group (Fig. 1–8) as referred to by Schmidt et al. (2014) is informally defined by the combination of: (1) the presence of a cream forewing postmedian spot or patch in females; (2) ecological association with Camponotus ants; (3) behaviour of adults resting on the ground; (4) the hindwing termen relatively rounded with the tornus not produced or sometimes weakly produced in the male; and (5) all species have subterranean life histories. The larvae emerge from the ground to feed nocturnally in two species. The species pairs O. genoveva (Hewitson, 1853) and O. zosine (Hewitson, 1853) are closely related and sister to the O. idmo group but do not meet criteria 3 and 4. These species are canopy butterflies instead and the hindwing tornus is strongly produced.
On the basis of molecular and morphological evidence, we recognise eight taxa in the strictest sense (six species and two subspecies) in the O. idmo group as follows: Ogyris otanes (C. & R. Felder, 1865), Ogyris arcana M.R. Williams & Hay, 2001 stat. rev., Ogyris arcana arcana M.R. Williams & Hay, 2001 comb. nov., Ogyris arcana sublustris M.R. Williams & Hay, 2001 comb. nov., Ogyris halmaturia (Tepper, 1890), Ogyris halmaturia halmaturia (Tepper, 1890), Ogyris halmaturia waterhouseri (Bethune-Baker, 1905) stat. rev., Ogyris idmo (Hewitson, 1862), Ogyris subterrestris Field, 1999 and Ogyris petrina Field, 1999 stat. rev.
The O. idmo species group contains taxa that are morphologically very similar to each other. Careful corroboration of locality and biological information will assist in confirming identification. Females may be distinguished from males by the presence of a yellowish-cream postmedian spot or patch on the dorsal surface of the forewing.
| 1 | Dorsal forewing with narrow dark brown margin, 1.0–1.8 mm at apex...O. idmo, Fig. 5a Dorsal forewing with broad dark brown margin, greater than 2 mm at apex...2 |
| 2 | Hindwing without prominent tornal projection...3 Hindwing with prominent tornal projection...6 |
| 3 | Ventral forewing with blue scales below discal cell, postmedian band angled sharply towards tornus and not displaced medially...O. subterrestris, Fig. 5f Ventral forewing without blue scales below discal cell, postmedian band displaced medially...4 |
| 4 | Ventral colouration predominately cinnamon–brown, medial area of hindwing without distinct black marking...O. petrina, Fig. 5j Ventral colouration predominately silver–grey, medial area of hindwing with distinctive black patch of scales...5 |
| 5 | Dorsal wing margins broader, 4 mm, ventral forewing apex with double line of charcoal–black tending to brown scales against light grey background...O. halmaturia halmaturia, Fig. 4a, b Dorsal wing margins narrower, 2.0–2.5 mm, ventral forewing apex unmarked...O. halmaturia waterhouseri, Fig. 4i, j |
| 6 | Ventral surface light yellowish to cinnamon–brown, hindwing tornal projections shorter, narrower...O. otanes, Fig. 3b Ventral surface dark brown to grey–brown, with distinctive hindwing markings, tornal projections longer, broader...7 |
| 7 | Ventral hindwing generally plain dark brown ...O. arcana arcana, Fig. 3f Ventral hindwing generally suffused with light grey scales, particularly in background area around markings...O. arcana sublustris, Fig. 3j |
| 1 | Ventral forewing with blue scales below discal cell, postmedian band angled in straight line towards termen, not curved or displaced along any part...O. subterrestris, Fig. 5h Ventral forewing with blue scales in discal cell, postmedian band not straight...2 |
| 2 | Ventral ground colour predominately light brown or light cinnamon brown...3 Ventral ground colour predominately silver–grey or dark brown, with only highlights of lighter brown at most...4 |
| 3 | Dorsal forewing with double black line at end of discal cell, set on lighter reddish-brown scales...O. petrina, Fig. 5k Dorsal forewing with only black scales at end of discal cell...O. otanes, Fig. 3c |
| 4 | Ventral hindwing without distinctive central patch of black scales, tornal lobes prominent...5 Ventral hindwing with distinctive central patch of black scales, tornal lobes reduced...6 |
| 5 | Ventral wings dark brown, with markings similar colouration to background, cream dorsal forewing postmedian spot larger...O. arcana arcana, Fig. 3g, h Ventral wings covered with light grey suffusion, predominately over background but occasionally on hindwing markings, cream dorsal forewing postmedian spot smaller...O. arcana sublustris, Fig. 3k, l |
| 6 | Dorsal forewing with violet or blue scales not reaching cream postmedian spot, overall wing margins broader, cinnamon-coloured scales extensive in basal areas...O. idmo, Fig. 5c Dorsal forewing with purple or blue scales meeting with cream postmedian spot, margins narrower, with or without very few cinnamon-coloured scales in basal wing area...7 |
| 7 | Cream forewing postmedian spot reduced, rarely extending beyond vein M3 as a scattering of cream scales, ventral forewing apex with double line of brown markings over light grey background...O. halmaturia halmaturia, Fig. 4c–f Cream forewing postmedian spot large, usually extending beyond vein M3 as a distinct clean patch of scales, ventral forewing apex plain or with few broken brown markings...O. halmaturia waterhouseri, Fig. 4g, h, k, l |
Ogyris otanes (C. & R. Felder, 1865)
Ogyris otanes C. & R. Felder, 1865, p. 217, pl. XXVIII, fig. 1–3; Kirby (1871), p. 425; Miskin (1890), pp. 23–24; Lower (1893), p. 9; Tepper (1893), pp. 284–285; Anderson and Spry (1893), p. 101; Seitz (1926), p. 940, fig. 161g; Waterhouse (1903b), p. 249; Waterhouse (1903a), p. 29; Bethune-Baker (1905), pp. 275–277; Waterhouse and Lyell (1914), p. 121, pl. 18, fig. 404, 408, pl. 19, fig. 426, 427; Bethune-Baker (1916), p. 390; Tindale (1923), pp. 347–348, pl. XXIX, fig. 16–19; Waterhouse (1932), p. 184, pl. XXV, fig. 10; Waterhouse (1937), p. 120; Common (1964), p. 96, fig. 370–371; Burns and Rotherham (1969), p. 98; D’Abrera (1971), p. 320; McCubbin (1971), p. 84; Common and Waterhouse (1972), p. 342, pl. 34, fig. 10, 10A; Crosby (1972), pp. 6–7; Quick (1973), p. 15; Crosby (1974b), p. 58; Quick (1974), pp. 60–61; Crosby (1976), pp. 15–16; Fisher (1978), pp. 194–196, pl. 11, fig. 7–9; Common and Waterhouse (1981), pp. 480–481, pl. 41, fig. 10, 10A; Fisher (1985), p. 7; Hill and Michaelis (1988), pp. 14, 36; Fisher (1993), pp. 11–12; F. Douglas (unpubl. data, 1995), pp. 18–23, fig. 3; Grund (1996), p. 97; Grund (1999), pp. 47–48; Grund (2003), p. 71. [Genus Ogyris Westwood, 1851].
Ogyris atanes C. & R. Felder, 1865 – Burns and Angel (1952), pp. 183–186, pl. VII. [Misspelling of O. otanes].
Ogyris otanes (C. & R. Felder, 1865). – Edwards (1996), p. 250; Field (1997), p. 391; Hunt et al. (1998), p. 116; Eastwood and Fraser (1999), pp. 511; Field (1999), pp. 252; Edwards et al. (2001), pp. 258–259; Sands and New (2002), pp. 288–290; Pierce (2002), pp. 747; Young (2004), pp. 64–65, fig. 11; Orr and Kitching (2010), pp. 244–245; Braby et al. (2011), pp. 29–36. [Genus Ogyris Angas, 1847].
Ogyris otanes C. & R. Felder, 1865 ‘local form 1’ – Dunn and Dunn (1991), pp. 367, 458.
Ogyris otanes (C. & R. Felder, 1865) ‘eastern form’ – Braby (2000), pp. 396, 710–712, pl. 50, fig. 3.
Ogyris otanes otanes (C. & R. Felder, 1865). – Williams and Hay (2001), p. 55–56, 62; Braby (2004), pp. 242–243, 316; Braby and Douglas (2008), pp. 315–318, 326; Grund (2010), pp. 114–120; Braby (2010), pp. 14, 16, 35, 70; New (2011), pp. 101–103, 142, 148, 154, 163; Schmidt et al. (2014), pp. 473–484; Braby (2016), pp. 266–267, 350; Taylor et al. (2018), table S3; Sankowsky (2020), p. 315; Geyle et al. (2021), pp. 101, 103.
Ogyris idmo Hewitson, 1862 – Tepper (1893), pp. 284–285. [Incorrectly considered to be a senior synonym of O. otanes].
Ogyris halmaturia Tepper, 1890 – Waterhouse (1903b), p. 249; Waterhouse (1903a), p. 29; Waterhouse and Lyell (1914), p. 121; Bethune-Baker (1916), p. 390; Tindale (1923), pp. 347–348. [Incorrectly considered to be a junior synonym of O. otanes in whole or in part].
Ogyris halmaturia (Tepper, 1890). – Braby and Douglas (2008), pp. 315–319, fig. 7–9. [Incorrectly considered to be a junior synonym of O. otanes].
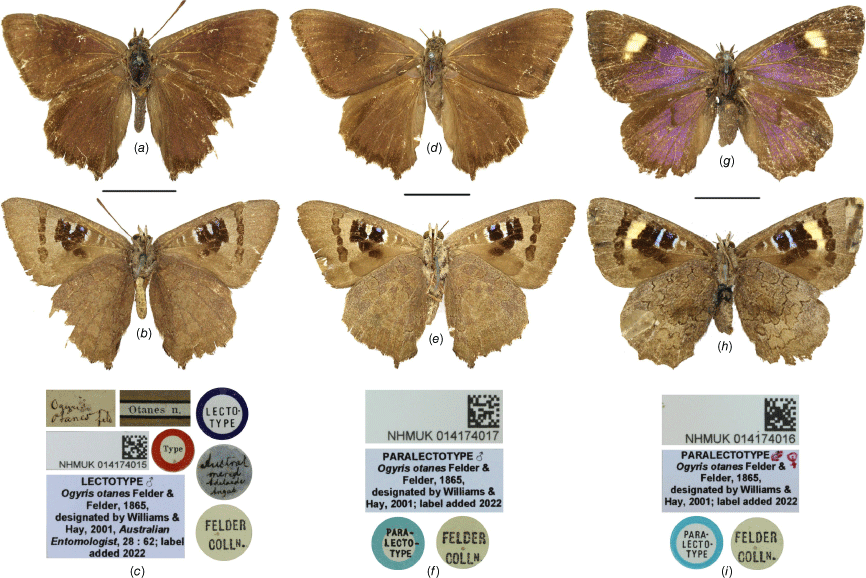
Lectotype. Australia: South Australia: ♂ ‘Austral. Merid. Adelaide Angas’, ‘Ogyris otanes Feld.’, ‘FELDER COLLN.’, ‘Otanes n.’, ‘Type’ [on red label], ‘LECTO-TYPE’ [on blue label], ‘LECTOTYPE ♂ Ogyris otanes Felder & Felder, 1865 designated by Williams & Hay, 2001, Australian Entomologist, 28: 62; label added 2022’, ‘NHMUK 014174015’ (NHMUK) (Fig. 2a–c).
Paralectotypes. Australia: South Australia: 1♂ ‘PARA-LECTO-TYPE’ [on blue label], ‘FELDER COLLN.’, ‘PARALECTOTYPE ♂ Ogyris otanes Felder & Felder, 1865, designated by Williams & Hay, 2001; label added 2022’, ‘NHMUK 014174015’, ‘NHMUK 014174017’ (NHMUK) (Fig. 2d–f). 1♀ ‘PARA-LECTO-TYPE’ [on blue label], ‘FELDER COLLN.’, ‘PARALECTOTYPE ♀ Ogyris otanes Felder & Felder, 1865, designated by Williams & Hay, 2001; label added 2022’, ‘NHMUK 014174015’, ‘NHMUK 014174016’ (NHMUK) (Fig. 2g–h).
85 ♂, 70 ♀ in the ANIC, variously from across the known South Australian and Victorian distribution.
Felder and Felder (1865) originally described and illustrated both sexes of Ogyris otanes C. & R. Felder, 1865 based on three syntypes (2♂, 1♀, now in NHMUK) but no types were referenced (Edwards et al. 2001). Bethune-Baker (1905) examined and referred to the syntypes of Felder and Felder (1865) but did not refer to an individual specimen. Tindale (1923) referred to types, stating that, ‘The type of O. otanes came from Adelaide…’ (p. 347). Edwards et al. (2001) interpreted Tindale’s (1923) reference to a type as qualifying as a lectotype designation but this is questionable because Tindale (1923) did not indicate which of the three syntypes were being referred to. Subsequently, Williams and Hay (2001, p. 62) made an intentional, unambiguous and explicit lectotype designation by providing the label data of one of the syntype males in NHMUK. We consider the action of Williams and Hay (2001) to constitute a valid lectotypification under Article 74 of the ‘International Code of Zoological Nomenclature’ (International Commission on Zoological Nomenclature 1999), thereby fixing the name otanes to this species. The three types were not illustrated; the lectotype male (Fig. 2a–c) is illustrated for comparison with the paralectotype male (Fig. 2d–f) and paralectotype female (Fig. 2g–i).
Type specimens of Ogyris otanes C. & R. Felder, 1865 in the NHMUK showing dorsal and ventral views, and labels: (a–c) lectotype male; (d–f) paralectotype male; (g–i) paralectotype female. Scale bar: 10 mm. Images by J. Tennant.
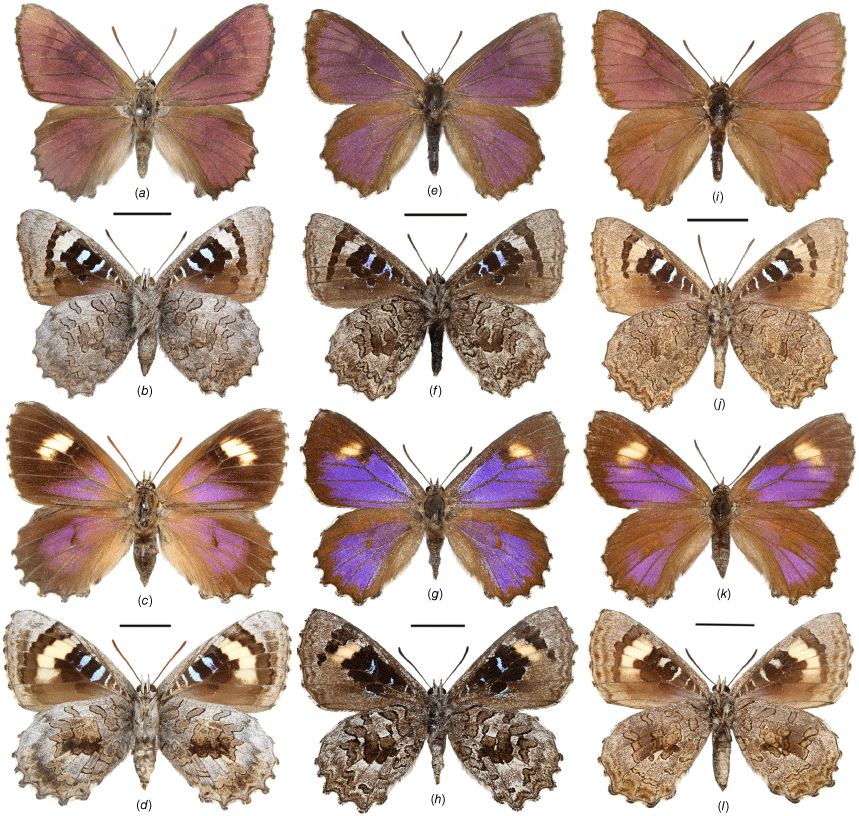
Adults of Ogyris otanes and O. arcana stat. rev. showing dorsal and ventral views: (a–d) O. otanes; (a, b) male from Big Desert, VIC; (c, d) female from Kingscote, SA; (e–l) O. arcana stat. rev.; (e–h) O. arcana arcana comb. nov. from Stirling Range, WA; (e, f) male; (g, h) female; (i–l) O. arcana sublustris comb. nov. from Leeman, WA; (i, j) male; (k, l) female. Scale bars: 10 mm.
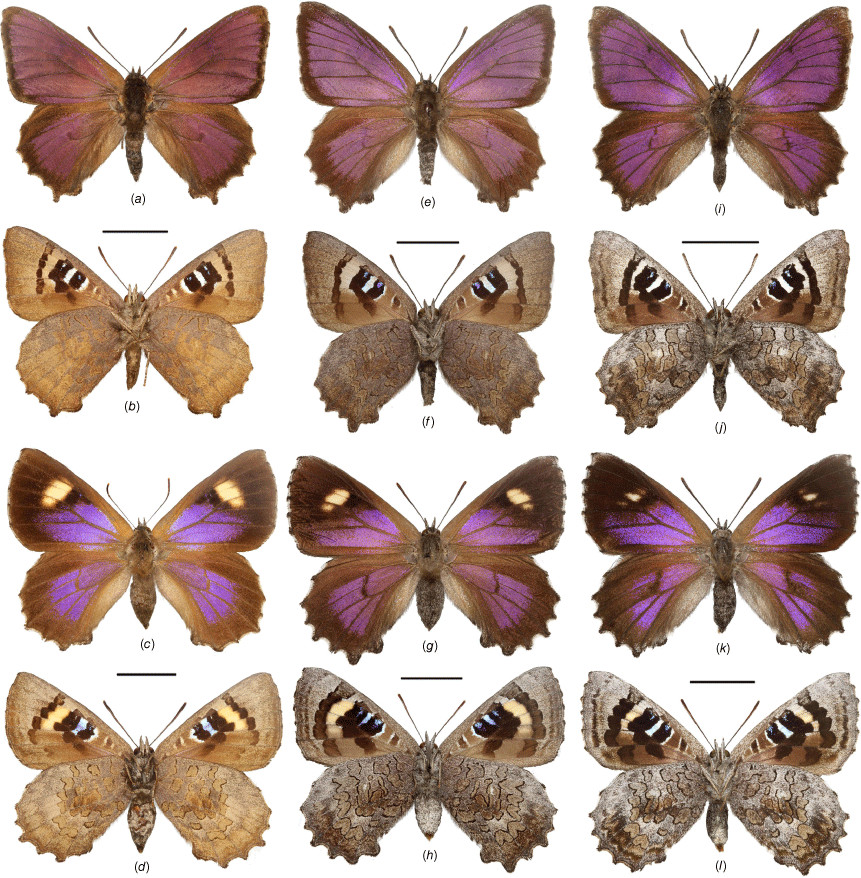
Adults of Ogyris halmaturia showing dorsal and ventral views: (a–f) O. halmaturia halmaturia from Kangaroo Island, SA; (a, b) male; (c, d) female; (e, f) female showing variation with reduced forewing spot; (g–l) O. halmaturia waterhouseri stat. rev. from Ngarkat, SA; (g, h) O. halmaturia waterhouseri stat. rev. female showing variation with reduced forewing spot; (i, j) male; (k, l) female. Scale bars: 10 mm. Images g, h by F. Douglas.
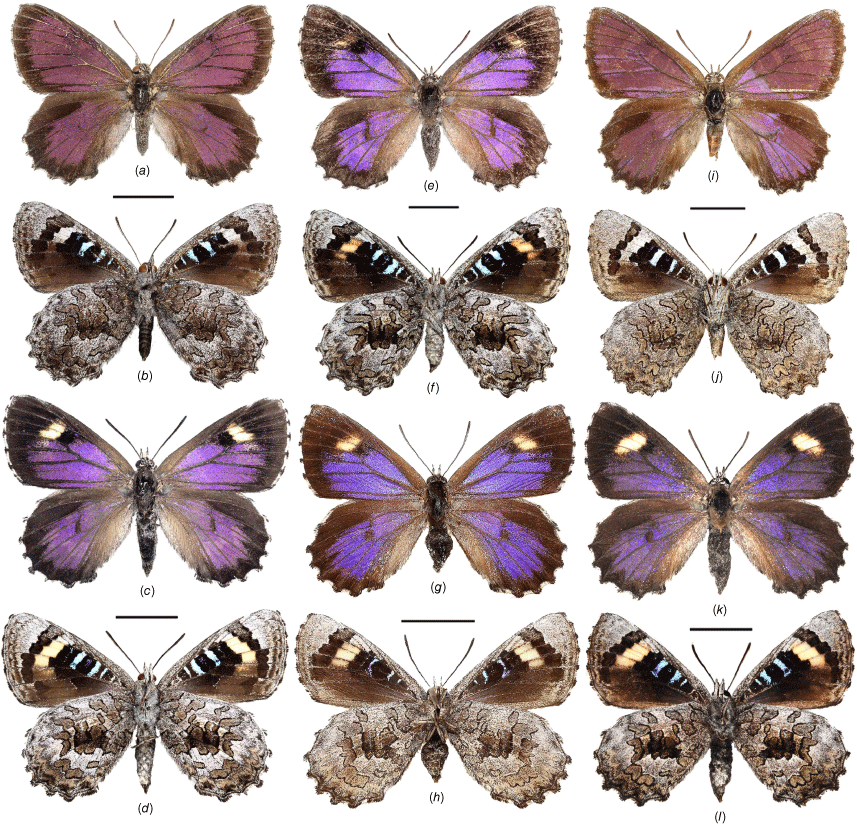
Adults of Ogyris idmo, O. subterrestris and O. petrina stat. rev. showing dorsal and ventral views: (a–d) O. idmo from Mundaring, WA; (a, b) male; (c, d) female; (e–h) O. subterrestris; (e, f) male from 6 km south of Colignan, VIC; (g, h) female from Pooginook, SA; (i–l) O. petrina stat. rev. from 12 km SW of Kalgoorlie, WA; (i, j) male; (k, l) female. Scale bars: 10 mm.
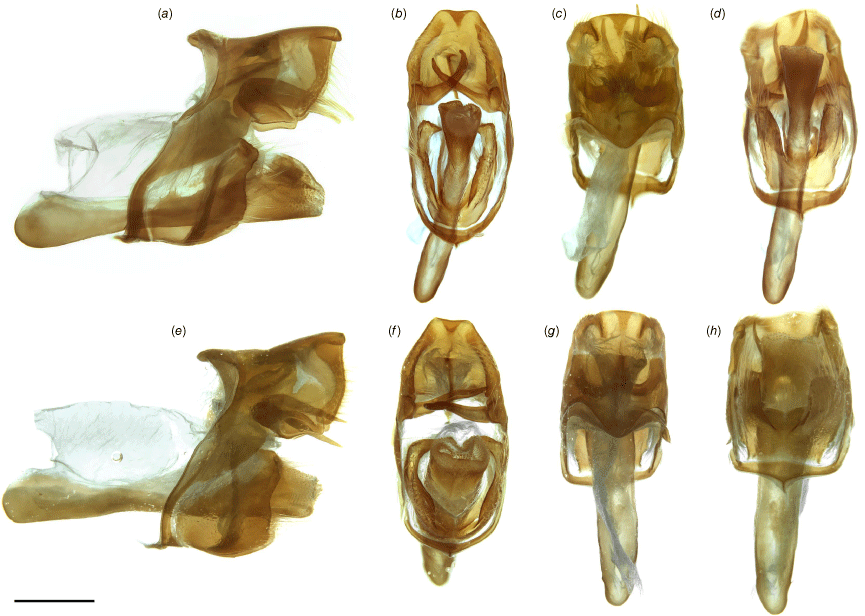
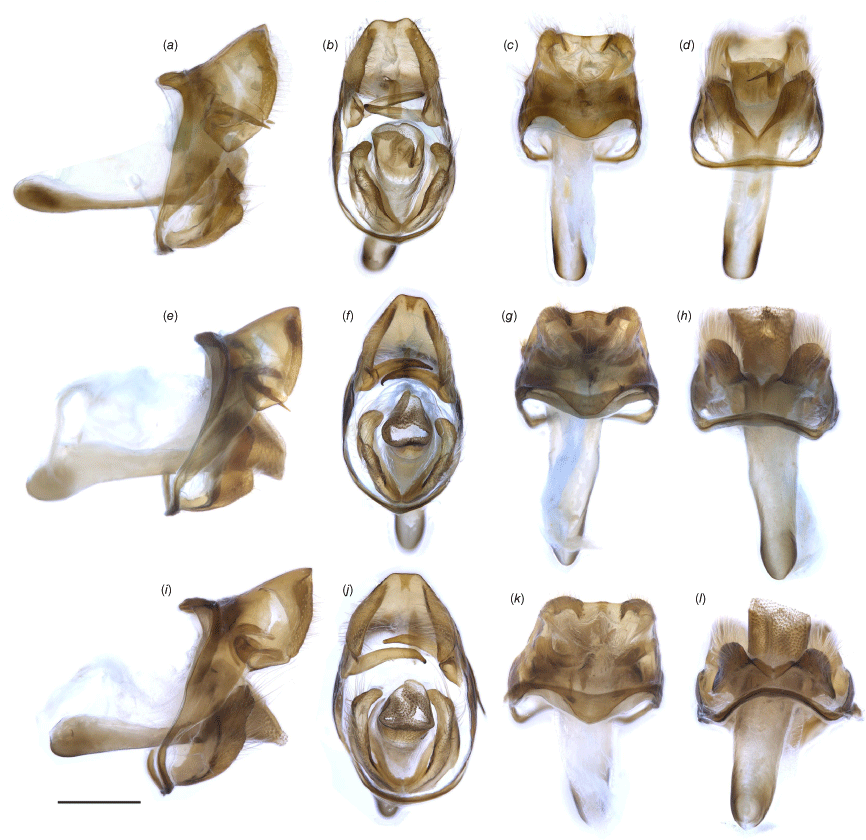
Male genitalia of Ogyris spp.: (a–d) O. otanes, genitalia number EPB-183 (ANIC); (e–h) O. arcana arcana comb. nov., genitalia number EPB-170 (ANIC). (i–l) O. arcana sublustris comb. nov., genitalia number EPB-171 (ANIC). Shown are lateral (a, e, i), posterior (b, f, j), dorsal (c, g, k) and ventral (d, h, l) views. Scale bar: 500 μm.
This species occurs patchily in the Gulf Country and Lower South-East South Australia and north-western Victoria (Braby 2000, 2016) and formerly occurred in western New South Wales where the species is currently considered locally extinct.
Ogyris arcana M.R. Williams & Hay, 2001, stat. rev.
Two subspecies are recognised here, the nominate subspecies O. arcana arcana M.R. Williams & Hay, 2001 comb. nov. and O. arcana sublustris M.R. Williams & Hay, 2001 comb. nov.
Ogyris arcana arcana M.R. Williams & Hay, 2001, comb. nov.
Ogyris otanes C. & R. Felder, 1865 – Waterhouse and Lyell (1914), p. 121; Waterhouse (1932), p. 184; Common (1964), p. 96; Burns and Rotherham (1969), p. 98; McCubbin (1971), p. 84; Common and Waterhouse (1972), p. 342; Crosby (1972), p. 7; Fisher (1978), p. 196; Williams et al. (1992), pp. 55–60; Fisher (1993), p. 11; Hay et al. (1994), pp. 31, 31, pl. 4, fig. 7–9. [Genus Ogyris Westwood, 1851]. [Misidentifications in literature].
Ogyris sp. – Field (1987), p. 113.
Ogyris otames C. & R. Felder, 1865 – Hay (1989), pp. 42, 43. [Misspelling of O. otanes].
Ogyris otanes C. & R. Felder, 1865 ‘local forms 2 & 3’ – Dunn and Dunn (1991), p. 367. [Genus Ogyris Westwood, 1851].
Ogyris otanes (C. & R. Felder, 1865) – Williams et al. (2012), pp. 50–51. [Misidentification in literature]. [Genus Ogyris Angas, 1847].
Ogyris otanes (C. & R. Felder, 1865) ‘south-western form’ – Braby (2000), pp. 710–712, pl. 50, fig. 3b. [Genus Ogyris Angas, 1847].
Ogyris otanes arcana M.R. Williams & Hay, 2001, pp. 59–61, fig. 5–8; Sands and New (2002), p. 288; Braby (2010), p. 35; Schmidt et al. (2014), pp. 473–484; Braby (2016), pp. 266–267, 350; Sankowsky (2020), p. 315.
Holotype. Australia: Western Australia: ♂ ‘Stirling Ra, 7 Dec. 1990, M.R. Williams’ (WAM). [Illustrated in Williams and Hay (2001), p. 57, fig. 5].
20 ♂, 17 ♀ in the ANIC, from the Stirling Range, Wylie Scarp, and Cape Arid, WA.
Ogyris arcana can be distinguished from O. otanes by the following 10 character states that are more easily discerned in freshly emerged or reared specimens: (1) The forewing of O. arcana males is more elongate, with a straighter termen and more pointed apex than that of O. otanes males. This character appears to hold true for females but is most evident in males. (2) The hindwing of O. arcana males is more elongate or slightly narrower than that of O. otanes males. (3) The tornal projection at the end of vein CuA2 on the hindwing is more pronounced in both sexes of O. arcana than that in O. otanes. (4) In females, the yellow postmedian spot on the upperside of the forewing is smaller in O. arcana than that in O. otanes, especially in the subspecies O. arcana sublustris. In O. otanes, the spot is not only generally broader but almost always extends beyond vein M3, whereas in O. arcana the spot extends beyond this vein in only about half of the specimens examined (n = 38); when present the spot is usually very faintly developed. (5) In O. arcana females, the upperside of the hindwing has a distinct black transverse bar along the discocellular vein, whereas in O. otanes females this bar is usually absent or if present, very faintly developed as a narrow black line. (6, 7) In both sexes of O. arcana, the apex on the upperside of the forewing and tornus (tornal projection and tornal lobe) on the upper side of the hindwing have a dusting or suffusion of grey scales, a pattern element that is absent in O. otanes. (8, 9) The underside (i.e. subapical region of forewing and entire hindwing) ground colour is dark brown to grey in both sexes of O. arcana, being particularly dark in O. arcana arcana males, but light yellowish to cinnamon–brown in both sexes of O. otanes. Moreover, the underside markings of the hindwing in O. arcana are more pronounced and well developed (reminiscent of Ogyris oroetes), with a darker hue or tone towards the termen, especially in the subspecies O. arcana sublustrus, whereas in O. otanes the markings are usually more obscure and the colour pattern more uniform, particularly in males. One exception to this is that female specimens particularly from Kangaroo Island are occasionally more distinctly patterned in this respect. (10) The cilia (scale fringe) are white and conspicuous in both wings of O. arcana, whereas these are grey and less conspicuous in O. otanes.
Williams and Hay (2001) noted that males of O. arcana are darker than those of O. otanes; however, the upperside ground colour varies in males of both species. Williams and Hay (2001) also noted that the anal or tornal lobe of the hindwing is more accentuated in O. arcana but this feature is not applicable due to also being well developed in O. otanes. In females, the iridescent purple areas on the upperside vary in extent and colour in both species and are therefore not diagnostic.
The two species further differ in the male genitalia according to the following five character states: (1) the space at which the vinculum meets with the uncus is more concave in O. arcana, less so in O. otanes; (2) the valvae are elongate in O. arcana with a subtle medial concavity, whereas in O. otanes this concavity is deeper with the dorsal and ventral lobes more prominent, and ventral lobe more rounded; (3) the phallus is medially broader in O. arcana, whereas this is very narrow in O. otanes; (4) the sacculus of O. arcana is rugose with a basal triangular projection, whereas this is smooth in O. otanes; (5) the brachia are thicker and more heavily sclerotised in O. arcana but much finer and less sclerotised at the apex in O. otanes.
A new description is not required (see Williams and Hay 2001).
Waterhouse and Lyell (1914, p. 121) first drew attention to the western population from the Stirling Range, WA, provisionally placing the material under O. otanes (C. & R. Felder, 1865) but noted that these ‘possibly represent a distinct race’. Waterhouse and Lyell’s (1914) comment was based on three worn specimens (1♂, 2♀) collected in 1911 preserved in AMS (Williams et al. 1992). The population was subsequently rediscovered in 1988 by P.S. Valentine, H.H. Bollam and R.W. Hay (Hay 1989; Williams et al. 1992). Hay (1989, p. 43) remarked that, ‘Classification of the species is being researched and all indications point to a distinct new W.A. species.’ Williams et al. (1992) and Hay et al. (1994), however, later regarded this to most likely be an undescribed subspecies of O. otanes. Braby (2000), following Williams et al. (1992) and Dunn and Dunn (1991), provisionally treated the population as a distinct form of O. otanes and referred to this as the ‘south-western form’. Subsequently, Williams and Hay (2001) formally described and illustrated the taxon as a subspecies of O. otanes, listing 81 paratypes (38♂, 43♀) in CALM (now in WAM) and various private collections. Braby (2000) and Williams and Hay (2001) provided a diagnosis and collectively listed five character states by which Ogyris arcana differs from O. otanes. Examination of additional material preserved in the ANIC since that publication have confirmed these differences but also revealed an additional five characters. Therefore we treat O. arcana as a species distinct from O. otanes based on the large number of morphological differences including differences in the male genitalia (total of 15 characters), together with the molecular phylogenetic evidence presented by Schmidt et al. (2014) (see also Fig. 1) and the large p-distance, which is >5% (Table 1).
Ogyris arcana arcana occurs in the south-west of Western Australia, from the Stirling Range to as far east as Wylie Scarp (Williams and Hay 2001; Braby 2016).
Dark bronze azure. Williams et al. (1992) proposed the name ‘western dark azure’ for this species, a name that we suggest modifying to include the group name ‘bronze azure’.
Ogyris arcana sublustris M.R. Williams & Hay, 2001, comb. nov.
Ogyris sp. – Field (1987), pp. 112–113.
Ogyris otanes C. & R. Felder, 1865 – Field (1990), pp. 77, 78; Williams et al. (1995), p. 95; Hart and Powell (1997), pp. 185–190. [Genus Ogyris Westwood, 1851]. [Misidentifications in literature].
Ogyris otanes C. & R. Felder, 1865 ‘local form 2’ – Dunn and Dunn (1991), p. 367. [Genus Ogyris Westwood, 1851].
Ogyris sp. aff. otanes – Grund (1998), p. 66.
Ogyris otanes (C. & R. Felder, 1865) ‘western coastal form’ – Braby (2000), pp. 710–712, pl. 50, fig. 3c. [Genus Ogyris Angas, 1847].
Ogyris otanes sublustris M.R. Williams & Hay, 2001, pp. 56–59, fig. 1–4; Sands and New (2002), p. 288; Braby (2010), p. 35; Schmidt et al. (2014), pp. 473–484; Braby (2016), pp. 266–267, 350; Sankowsky (2020), p. 315.
Holotype. Australia: Western Australia: ♂ ‘Port Denison, 13 Nov. 1993, M.R. Williams’ (WAM). [Illustrated in Williams and Hay (2001), p. 57, fig. 1].
Williams and Hay (2001) provided a comprehensive diagnosis on how Ogyris arcana sublustris is distinguished from the nominate subspecies. Several character state differences were listed, including: (1) smaller body size; (2) paler (grey) underside ground colour in both sexes; (3) reduced pale yellow postmedian spot on the forewing in females; and (4) a more pronounced subterminal band on the underside of forewing in males. The male genitalia were not included in the original diagnosis; however, we have discovered that the two subspecies differ slightly in shape of the male valvae, with O. arcana sublustris (Fig. 6i–l) having smoother, less concave valvae than nominate O. arcana (Fig. 6e–h).
Field (1987) first drew attention to the distinctiveness of this taxon from Western Australia, classifying this as a species of Ogyris allied to O. otanes (C. & R. Felder, 1865). Dunn and Dunn (1991) commented on the unusual wing phenotype of the adults and referred to the population(s) as a local form (‘No. 2’) of O. otanes. Braby (2000) illustrated a female, diagnosed the population and treated this as the ‘western coastal form’ of O. otanes. Subsequently, Williams and Hay (2001) formally described and illustrated the taxon as a subspecies of O. otanes and provided a detailed diagnosis on how O. otanes sublustris M.R. Williams & Hay, 2001 is distinguished from O. otanes arcana M.R. Williams & Hay, 2001. These authors listed 124 paratypes (39♂, 85♀) in CALM (now in WAM) and various private collections. Braby (2010) again highlighted the distinctive wing pattern elements of O. otanes sublustris and called for further evidence to establish whether the observed morphological differences were correlated with molecular, ecological and behavioural characteristics. Following the molecular phylogeny based on mtDNA (COI and cytb) published by Schmidt et al. (2014) (see also Fig. 1), sublustris clearly belongs with O. arcana and not with O. otanes.
Ogyris arcana sublustris occurs on the western coast of Western Australia, from near Lancelin northwards to Port Denison (Hart and Powell 1997; Williams and Hay 2001; Braby 2016).
Ogyris halmaturia (Tepper, 1890)
Two subspecies are recognised here, the nominate subspecies O. halmaturia halmaturia (Tepper, 1890) and O. halmaturia waterhouseri (Bethune-Baker, 1905) stat. rev.
Ogyris halmaturia halmaturia (Tepper, 1890)
Ogyris halmaturia Tepper, 1890, p. 12; Tepper (1893), p. 285; Hill and Michaelis (1988), pp. 14, 36. [Genus Ogyris Westwood, 1851].
Ogyris halmaturea Tepper. – Seitz (1926), p. 940. [Misspelling of O. halmaturia].
Ogyris halmaturia (Tepper). – Grund (2010), pp. 114–120; Braby et al. (2011), pp. 29–36; New (2011), pp. 21, 99, 103–104, 148; Schmidt et al. (2014), pp. 473–484; Braby (2016), pp. 268–269, 350. [Genus Ogyris Angas, 1847].
Ogyris halmaturia halmaturia Tepper. – Tindale (1923), p. 348, pl. XXIX, fig. 20. [Genus Ogyris Westwood, 1851].
Ogyris idmo Hewitson, 1862. – Lower (1893), p. 9; Waterhouse (1903a), p. 29. [Incorrectly considered to be a senior synonym of O. halmaturia].
Ogyris idmo (Hewitson, 1862). – Edwards (1996), p. 250. [Incorrectly considered to be a senior synonym of O. halmaturia].
Ogyris idmo halmaturia Tepper. – Waterhouse (1932), pp. 179–180; Common (1964), p. 96; Burns and Rotherham (1969), p. 98; D’Abrera (1971), p. 320; McCubbin (1971), p. 84; Common and Waterhouse (1972), pp. 341–342; Fisher (1978), pp. 193–194; Common and Waterhouse (1981), p. 479; Fisher (1985), p. 7; Dunn and Dunn (1991), pp. 366, 457. [Genus Ogyris Westwood, 1851].
Ogyris idmo halmaturia (Tepper). – Edwards (1996), p. 250; Moore (1999), p. 12; Field (1999), pp. 252–253; Braby (2000), pp. 396, 712–714; Edwards et al. (2001), pp. 254–255; Sands and New (2002), pp. 282–284. [Genus Ogyris Angas, 1847].
Ogyris waterhouseri (Bethune-Baker, 1905). – Braby and Douglas (2008), pp. 315–329, fig. 4–6.
Lectotype. Australia: South Australia: ♂ ‘Queenscliffe, 1 mile N.W. very shy, ♀, 20.11.86. Tepper’ [in Tepper’s original handwriting]; ‘Ogyris halmaturia Tepper, Type female = ♂, Kangaroo Island | n348, vide, TRSSA. 1923’; ‘SAMA Database No. 31-001699’ (SAMA). [♂ O. halmaturia, illustrated in Tindale (1923), pl. XXIX, fig. 20; Braby and Douglas (2008), p. 319, fig. 4–6].
Paralectotypes. Australia: South Australia: 1♂ ‘Queenscliffe, ♂, 1 m. N.W. very shy, 21.11.86. Tepper’ [in Tepper’s original handwriting]; ‘Ogyris halmaturia Tepper, Type male, =not type, Kangaroo Island | vide TRSSA 1923, p. 389’, ‘Ogyris otanes ♂ not halmaturia’; ‘SAM Database No. 31-001700’ (SAMA) [=♂ O. otanes, illustrated in Tindale (1923), pl. XXIX, fig. 16]. 1♂ ‘Queenscliffe, 1 m. N.W. very shy, 20.11.86. Tepper’ [in Tepper’s original handwriting]; ‘Ogyris halmaturia Tepper, Cotype male, Kangaroo Island | vide TRSSA 1923, p. 389’; ‘Ogyris otanes ♂ not halmaturia’; ‘SAM Database No. 31-001701’ (SAMA) [=♂ O. otanes]. 1♂ ‘Queenscliffe, 1 mile N.W., in scrub. ♂, 20.11.86. Tepper’ [in Tepper’s original handwriting]; ‘Ogyris halmaturia, Queenscliffe, Kang. Island, Nov. 1886., legit J.G.O. Tepper’; ‘Bethune-Baker Coll. B.M. 1927-471.’ (NHMUK) [=♂ O. otanes, illustrated in Braby and Douglas (2008), p. 319, fig. 7–9].
An additional 40 specimens (23♂, 17♀) were examined in the ANIC and DESS, from Kangaroo Island, SA.
Dorsally and ventrally covered in dense light grey scales. Antennae 5–7 mm long, broadest distally, dorsally densely packed with scales, ventral surface without scales; dark brown, slight reddish hue in middle, lateral two-thirds from head with white scales on distal aspect of each antennomere; 45–47 antennomeres, each with scattering of short black spines on lateral and ventral surfaces, particularly towards distal end; scape in deep socket, ovoid, appressed mandarin-like. Labial palp elongate, with three palpomeres, light grey to white, middle longest, apex acuminate, tipped with darker scales.
Patagium dark grey; tegula cinnamon–brown; meso- and metathorax dark grey interspersed with cinnamon-coloured scales. Ventral surface predominately light grey, interspersed with dark grey scales. Legs light grey, midleg with antenna brush organ vestigial, almost entirely absent, tibia and tarsi of all legs ventrally and laterally covered with many short black spines; tarsi with paired prominent claws and paired associated setae; aerolium triangular, larger on mid- and hindlegs.
Forewing length 15–23 mm (x̄ = 20.6 mm ± 2.54 s.d., n = 12); near triangular, with costa slightly convex, termen convex medially, tornus rounded, dorsum straight; upperside dark iridescent rich violet over brown; costal, marginal and apical areas black, light cinnamon–brown in basal area of costa; veins brown; black spot at end of discal cell; small cream–yellowish to cream postmedian spot between vein M3 and M1, rarely larger between M3 and R5; cilia white, brown at ends of veins; underside ground colour, dark charcoal to brown basally, light silvery-grey at apex, grey at tornus, with complex patterns; discal cell black with 4–5 irregular longitudinal sky-blue markings, interspersed with dark blue; yellowish-cream to cream postmedian band from M3 to CuA1, variable in extent and suffused with mid-brown in basal median area, followed by charcoal-coloured postmedian band from costa to CuA2, sometimes merging with dark background, slightly displaced distally at M3; two dark brown parallel subterminal bands, variable in intensity but always present; cilia white, brown at ends of veins. Hindwing sub-ovoid in shape, with costa convex, apex indistinct from termen, termen crenulate with short, rounded projections at ends of veins, tornal lobe reduced, dorsum with weakly defined anal lobe, otherwise slightly convex medially; upperside colour pattern similar to forewing except without cream spot; inner margin light cinnamon–grey; prominent dark brown band at end of discal cell; underside ground colour, broadly light silvery-grey interspersed with highly variable complex markings from basal to marginal area, markings ovoid, irregularly squarish in basal area, becoming heart-shaped towards marginal area, each marking bordered by black, interiorly interspersed with cinnamon–brown and grey; variable black central patch between irregularly shaped markings; submarginal area light brown, with broken black subterminal line; cilia light grey, black at ends of veins.
Tergites covered with dark grey scales; sternites with light grey to white scales. Female genitalia not dissected – this feature is diagnostically uninformative in Ogyris spp. among closely related species (Beaver et al. 2023).
Variation was observed in the size and shape of the dorsal and ventral postmedian forewing spot, the ventral discal forewing cell patterns and the ventral hindwing patterns.
Braby and Douglas (2008) and Braby et al. (2011) discussed the taxonomic status and complex nomenclatural history of this species. For over 75 years (from 1932 to 2008) Ogyris halmaturia (Tepper, 1890) was considered to be a junior synonym or subspecies of O. idmo (Hewitson, 1862) but both Braby and Douglas (2008) and Grund (2010) treated this as a distinct species according to fundamental differences in juvenile and adult morphology. Tindale (1923) had previously considered O. halmaturia to be a distinct species but this was not accepted by Waterhouse (1932). Confusion in nomenclature of this species has arisen because Tepper (1890) had a mixed series representing two different species, one of Tepper’s (1890) syntypes was incorrectly sexed (the male was thought to be the female), and historically most previous authors (Lower 1893; Tepper 1893; Waterhouse 1903a, 1903b; Waterhouse and Lyell 1914; Bethune-Baker 1916; Tindale 1923) had failed to render a valid and unambiguous lectotype designation. The introduction of a second name, that is, Ogyris waterhouseri (Bethune-Baker, 1905), added further complexity. Braby et al. (2011) concluded that the name O. halmaturia had priority over O. waterhouseri based on the action of Tindale (1923) who was the first to make a valid lectotype designation, even though Tindale’s (1923) type designation was confusing in that Tepper’s (1893) concept of O. halmaturia was partly synonymised under O. otanes.
The type locality of O. halmaturia is north-west of Kingscote, SA and we restrict the concept of the nominate subspecies to Kangaroo Island. The South Australian Gulf Country and Kangaroo Island region have a complex geological and climactic history, with many potential biogeographic filters present that may have influenced subspeciation in the recent past. Indeed, the Kangaroo Island population is genetically distinct (D. J. Lohman, pers. comm.) from the mainland populations. The subspecies was last recorded on the island at ‘Rocky River’, in 1934, leading Braby and Douglas (2008) to conclude that it was possibly extinct. However, the subspecies was rediscovered on Kangaroo Island in 2014 after a lapse of 80 years. Prior to the rediscovery, only two females were historically known from Kangaroo Island, both in AMS (G.A. Waterhouse Collection): a specimen that was collected in December 1926 by F. W. Jones and another is without a locality label. While both historical localities for this species are valid historical Kangaroo Island place names, the actual collection locations referred to may likely not necessarily coincide with the areas currently recognised by these terms in the present day. Since Tepper (1890) only described the male of O. halmaturia (that was mistaken for the female), the female of the nominate subspecies has not been described. Hence, we provide a description and illustrations of female O. halmaturia halmaturia to distinguish the taxon and for comparison with females of the mainland subspecies.
Eastern bronze azure. Braby and Douglas (2008) proposed the common name ‘eastern bronze azure’ for this species, a name that we recommend be adopted for O. halmaturia.
Ogyris halmaturia waterhouseri (Bethune-Baker, 1905), stat. rev.
Ogyris idmo Hewitson, 1862. – Miskin (1890), p. 24; Anderson and Spry (1893), pp. 101, 104; Lower (1893), p. 9; Waterhouse (1903a), p. 29. [Misidentifications in literature or incorrectly considered to be a senior synonym of O. halmaturia].
Ogyris waterhouseri Bethune-Baker, 1905, pp. 273–274; Waterhouse and Lyell (1908), pp. 162, 165–166; Kershaw (1908), pp. 163–164; Seitz (1926), p. 940; Peters (1971), p. 26. [Genus Ogyris Westwood, 1851].
Ogyris waterhouseri (Bethune-Baker, 1905). – Braby and Douglas (2008), pp. 315–329, fig. 1–3, 14–17. [Genus Ogyris Angas, 1847].
Ogyris idmo waterhouseri Bethune-Baker. – Waterhouse and Lyell (1914), p. 122, pl. 18; Burns (1931), pp. 131–132; Burns and Angel (1952), p. 183; Common (1964), p. 96; Burns and Rotherham (1969), p. 98; D’Abrera (1971), p. 320; McCubbin (1971), p. 84; Quick (1972), pp. 9–10.
Ogyris idmo waterhousei Bethune-Baker. – Common (1964), p. 96. [Misspelling of O. waterhouseri].
Ogyris halmaturia waterhouseri Bethune-Baker. – Tindale (1923), p. 348.
Ogyris idmo halmaturia Tepper. – Waterhouse (1932), pp. 179–180; Common and Waterhouse (1972), pp. 341–342; Crosby (1974a), p. 65; Fisher (1978), pp. 193–194, pl. 10, fig. 11–13; Common and Waterhouse (1981), p. 479; Dunn and Dunn (1991), pp. 366, 457; F. Douglas (unpubl. data, 1995), pp. 6–10, fig. 1; Grund (1999), pp. 47–48; Grund (2003), p. 71. [Genus Ogyris Westwood, 1851]. [Considered to be a senior synonym of O. waterhouseri].
Ogyris idmo halmaturia (Tepper). – Edwards (1996), p. 250; Hunt et al. (1998), pp. 113–116; Moore (1999), p. 12; Field (1999), pp. 252–254, 259, fig. 13–16; Braby (2000), pp. 396, 712–714, pl. 50, fig. 5b; Edwards et al. (2001), pp. 254–255; Sands and New (2002), pp. 282–284; Braby (2004), pp. 242–243, 316; New et al. (2007), p. 245. [Genus Ogyris Angas, 1847]. [Considered to be a senior synonym of O. waterhouseri].
Ogyris halmaturia Tepper. – Hill and Michaelis (1988), pp. 14, 36. [Genus Ogyris Westwood, 1851].
Ogyris halmaturia (Tepper). – Grund (2010), pp. 114–120; Braby et al. (2011), pp. 29–36; New (2011), pp. 21, 99, 103–104, 148; Schmidt et al. (2014), pp. 473–484; Braby (2016), pp. 268–269, 350. [Genus Ogyris Angas, 1847].
Lectotype. Australia: Victoria: ♂ [Grampians]; ‘Victoria’; ‘Type’; L128’; ‘L128, Ogyris waterhouseri B-B’; ‘G.A. Waterhouse Collection’; ‘FIG. 400 Underside IN ‘THE BUTTERFLIES OF AUSTRALIA’, by WATERHOUSE & LYELL, was taken from this specimen | KL20205’ (AMS). [Illustrated in Waterhouse and Lyell (1914), pl. 18, fig. 400; Braby and Douglas (2008) p. 319, fig. 1–3].
Paralectotype. Australia: Victoria: ♀ [Grampians]; ‘Ogyris waterhouseri B-B’; ‘Ex wll Miskin’; ‘G.A. Waterhouse Collection’; ‘FIG. 402 Underside IN ‘THE BUTTERFLIES OF AUSTRALIA’, by WATERHOUSE & LYELL, was taken from this specimen | KL20208’ (AMS) [Illustrated in Waterhouse and Lyell (1914), pl. 18, fig. 402].
Braby and Douglas (2008) listed 54 specimens (29 ♂, 25 ♀) within the ANIC, AMS, NMV and SAM, variously from mainland South Australia and Western Victoria that we have re-examined.
The following four character states distinguish O. halmaturia waterhouseri from O. halmaturia halmaturia: (1) males have the black dorsal forewing margin narrower than that of the nominate subspecies; (2) females usually have the cream dorsal forewing postmedian spot larger, whereas in O. halmaturia halmaturia the spot is smaller, never extending cleanly beyond vein M3; indeed in some specimens the spot is almost absent (Fig. 4e). Sometimes, O. halmaturia halmaturia specimens have a few cream scales beyond M3 (Fig. 4c); however, these do not coalesce to form a discrete patch as seen in typical O. halmaturia waterhouseri. The extent of this pattern is somewhat variable in O. halmaturia waterhouseri; typically, specimens (Fig. 4k) exhibit a large clean spot but in some atypical specimens (Fig. 4g) this may be reduced. (3) Both sexes of O. halmaturia waterhouseri have the apical area of the ventral forewing unmarked, particularly between the costa and M1, whereas in O. halmaturia halmaturia this area has a double broken charcoal–black tending to dark brown line of crescent-shaped markings; (4) females have the dorsal basal area blue, whereas in the nominate subspecies this is violet purple, with a richer basal cinnamon suffusion.
There are also several diagnostic differences in the male genitalia of the two subspecies: (1) the valvae of O. halmaturia waterhouseri (Fig. 7e–h) are uniformly broader, with the apices rounded, whereas in O. halmaturia halmaturia (Fig. 7a–d) the apices are more truncate and with a more concave posterior margin; (2) the saccus of O. halmaturia waterhouseri is narrower than in the nominate subspecies, particularly in lateral view; and (3) the brachia of O. halmaturia waterhouseri are slightly shorter and broader than in O. halmaturia halmaturia.
Miskin (1890) provided a description of the male of this taxon based on material from Victoria and South Australia under the name Ogyris idmo Hewitson, 1862, not realising this was an undescribed species. Anderson and Spry (1893) described and illustrated both sexes from the Grampians in western Victoria (see Waterhouse and Lyell 1908), also under the name O. idmo and similarly failed to realise this was an unnamed species. Miskin’s (1890) revision of Ogyris was published (in February 1890) before Tepper (1890) introduced the name O. halmaturia, 15 years before Bethune-Baker (1905) described Ogyris waterhouseri. Edwards et al. (2001) and Braby and Douglas (2008) reviewed the type material, type locality and nomenclature of Ogyris waterhouseri (Bethune-Baker, 1905). Bethune-Baker (1905) described the species from Victoria based on material dispatched by G. A. Waterhouse (see Waterhouse and Lyell 1908) but did not indicate the number of specimens available. Braby and Douglas (2008) concluded that Bethune-Baker had at most three syntypes (1♂, 2♀) and that the type locality was most likely the Grampians based on the plate index for the lectotype male illustrated by Waterhouse and Lyell (1914, pl. 18, fig. 400) and referred to by Peters (1971) as the holotype. However, re-examination of O. waterhouseri material in AMS and G. A. Waterhouse’s original register indicated that only two specimens (1♂, 1♀) were sent to England and subsequently returned to Australia. These type specimens have the label ‘Ogyris waterhouseri B-B’ in Bethune-Baker’s original handwriting. Edwards et al. (2001) considered Peters' (1971) incorrect reference to a ‘holotype’ as a lectotype designation. We agree with the conclusion of Edwards et al. (2001) and consider the second (female) specimen, referred to above, in AMS and illustrated by Waterhouse and Lyell (1914, pl. 18, fig. 402), a paralectotype.
Ogyris halmaturia waterhouseri was at one time broadly distributed across mainland Gulf Country South Australia and western Victoria; however, the species is locally extinct in most localities and currently known only from the central-southern section of the Eyre Peninsula and Ngarkat Conservation Park, South Australia (Braby and Douglas 2008), in which the presence has not been confirmed for a number of years.
Ogyris idmo (Hewitson, 1862)
Ogyris idmo Hewitson, 1862, p. 2, pl. I, fig. 3–4; Hewitson (1863), p. 2; Kirby (1871), p. 425; Miskin (1891), p. 72; Waterhouse (1903b), pp. 248–249; Waterhouse (1903a), p. 29; Bethune-Baker (1905), pp. 269, 271, 273–275, 290, fig. 10, 10a; Seitz (1926), p. 940, fig. 161g; Waterhouse (1937), p. 120; Hay et al. (1994), p 31, fig. 10–13; Grund (1998), p. 66. [Genus Ogyris Westwood, 1851].
Ogyris idmo (Hewitson). – Edwards (1996), p. 250; Eastwood and Fraser (1999), pp. 510; Pierce (2002), pp. 747, 750; Braby and Douglas (2008), pp. 315–326, fig. 10–12; Williams (2009), pp. 37–43; Grund (2010), pp. 114–120; Orr and Kitching (2010), pp. 244–245; Braby (2010), pp. 16, 71; New (2011), pp. 101, 103; Williams et al. (2012), pp. 52–53; Schmidt et al. (2014), pp. 473–484; King and Williams (2014), pp. 199–201, fig. 1; Braby (2016), pp. 268–269, 350; Williams et al. (2020), pp. 221–247, fig. 4–6; Sankowsky (2020), p. 316. [Genus Ogyris Angas, 1847].
Ogyris idmo idmo Hewitson. – Waterhouse and Lyell (1914), pp. 121–122, pl. 18, fig. 405–406; Waterhouse (1932), p. 179, pl. XXV, fig. 3; Common (1964), p. 96, fig. 368; Burns and Rotherham (1969), pp. 98–99, fig. 73; McCubbin (1971), p. 84; D’Abrera (1971), p. 320; Common and Waterhouse (1972), p. 341, pl. 34, fig. 3, 3A; Atkins (1978), pp. 25–27; Common and Waterhouse (1981), pp. 478–479, pl. 41, fig. 3, 3A; D’Abrera (1984), p. 127; Hill and Michaelis (1988), p. 36; Field (1990), pp. 76–77; Dunn and Dunn (1991), p. 366; Field (1992), pp. 12–16; Williams et al. (1995), p. 94. [Genus Ogyris Westwood, 1851].
Ogyris idmo idmo (Hewitson). – Field (1997), pp. 390–391; Moore (1999), p. 12; Field (1999), pp. 251–259, fig. 9–12, 17–20; Braby (2000), pp. 712–714, pl. 50, fig. 5; Edwards et al. (2001), p. 255; Sands and New (2002), p. 285; Grund (2003), p. 71. [Genus Ogyris Angas, 1847].
Ogyris sp. aff. idmo – Hay et al. (1994), p 31, fig. 14–15. [Cape Arid population].
Ogyris orontas Hewitson, 1862, p. 2, pl. I, fig. 8–9; Hewitson (1863), p. 2; Kirby (1871), p. 425; Miskin (1890), p. 24; Anderson and Spry (1893), p. 101; Waterhouse (1903b), pp. 248–249; Waterhouse (1903a), p. 29; Bethune-Baker (1905), pp. 269, 274–275; Seitz (1926), p. 940; Edwards (1996), p. 250; Field (1999), pp. 252. [Junior objective synonym of O. idmo].
Ogyris otrontas Hewitson. – Tepper (1893), p. 285. [Misspelling of orontas].
Lectotype. Ogyris idmo. Australia: Western Australia: ♀ ‘Swan River., 43.14. | Ogyris idmo Type Hewitson’; ‘Type’; ‘LECTO-TYPE’; ‘NHMUK 014175794’; ‘LECTOTYPE ♀ Ogyris idmo Hewitson, 1862’ (NHMUK). [Illustrated in Hewitson (1862), p. 2, pl. I, fig. 3–4; Braby and Douglas (2008), p. 319, fig. 10–12].
Paralectotype. Ogyris idmo. Australia: Western Australia: ♀ ‘Swan River | 43 14’; ‘Swan River.’; ‘PARA-LECTO-TYPE’; ‘NHMUK 014175795’; ‘PARALECTOTYPE Ogyris idmo Hewitson, 1862’ (NHMUK).
Holotype. Ogyris orontas. Australia: Western Australia: ♂ ‘Orontas Hewitson’; ‘Type’; ‘Holo-type’; ‘Type’; ‘Bethune-Baker Coll. B.M. 1927-471’; ‘NHMUK 014175793’; ‘HOLOTYPE ♂ Ogyris orontas Hewitson, 1862’ (NHMUK) [Illustrated in Hewitson (1862), p. 2, pl. I, fig. 8–9].
Hewitson (1862) described and illustrated Ogyris idmo Hewitson, 1862 based on two females and Ogyris orontas Hewitson, 1862 based on a single male, all in the NHMUK. The following year, Hewitson (1863) realised the sexes of the two species were conspecific and synonymised O. orontas under O. idmo (Hewitson 1863), an action subsequently followed by Kirby (1871), Miskin (1890), Waterhouse (1903a, 1903b) and Bethune-Baker (1905) amongst others. The type locality of O. orontas was simply given as ‘Australia’ but no further detail was provided about from which part of Western Australia the specimen was collected. Braby and Douglas (2008) examined Hewitson’s (1863) syntypes of O. idmo from Western Australia and designated one of the females from the Swan Coastal Plain near Perth as the lectotype.
Recent collections of this species indicate a species complex in south-western Western Australia (Williams et al. 2020) in which there are four distinct populations that we are currently investigating: (1) a bivoltine population restricted to banksia woodland or jarrah–banksia–wandoo woodland in the Swan Coastal Plain and from sites 60–80 km east of Perth (east of the Darling Escarpment) in which adults fly in late spring (November–December) and again in early autumn (February–April); both sexes fly during the morning and afternoon, and males perch to locate females; and the dorsal iridescent basal areas of females are purple. (2) A more widely distributed population that occurs in a variety of habitats in the mesic, higher rainfall areas of the south-western corner (generally >500 mm mean annual rainfall) that is strictly univoltine (mainly in October and November but in some locations adults appear as early as September or as late as December); diurnal activity of the sexes is temporally partitioned, with females flying during the morning (10:00–12:00 hours) and males in the afternoon (>13:00 hours), and where males patrol to locate females; and the iridescent areas of females are purple, with the colour varying from bright purple to dull dark purple. (3) A semi-arid population restricted to mallee heath or mallee shrubland in the south-eastern subcoastal area, from Scaddan to Cape Arid, in which the mean annual rainfall varies from ~400–500 mm; adults are univoltine, flying in spring (September–November); females fly predominately in the morning and males in the afternoon; and the iridescent areas of females are blue. (4) A semi-arid population that occurs in shrubland in the transition zone between the South-western and Eyrean Provinces of low rainfall (~300–400 mm mean annual rainfall); adults are univoltine, emerging mainly in spring (September–November) and males and females appear to fly together during the day, from mid-morning to early afternoon; and the iridescent areas of females are violet. Further work is being undertaken by the authors to clarify the ecological and taxonomic boundaries of these four populations.
Ogyris idmo occurs in south-western Western Australia; see Williams et al. (2020) and the above remarks concerning distribution within this region.
Ogyris subterrestris Field, 1999
Ogyris idmo halmaturia Tepper, 1890. – Common and Waterhouse (1981), p. 479; Dunn and Dunn (1991), p. 366. [Genus Ogyris Westwood, 1851]. [Misidentifications in literature].
Ogyris sp. aff. idmo – F. Douglas (unpubl. data, 1995), pp. 11–17, fig. 2; Field (1997), pp. 389–392; Grund (1997), pp. 8–9; Moore (1999), p. 12–17; Eastwood and Fraser (1999), pp. 510.
Ogyris subterrestris subterrestris Field, 1999, pp. 251–259, fig. 1–4; Braby (2000), pp. 714–715, pl. 50, fig. 4b; Edwards et al. (2001), p. 259; Pierce (2002), pp. 747, 750; Douglas and Allen (unpub. data, 2002, pp. 1–16); Sands and New (2002), pp. 293–295; Grund (2003), p. 71; Braby (2004), pp. 242–243, 316; Braby and Douglas (2008), pp. 315, 326, 327; Williams and Williams (2008), p. 16; Grund (2010), pp. 114–120; Braby (2010), pp. 19, 71; New (2011), pp. 101–105; Douglas (2012), pp. 1–37; Field (2013), pp. 248–249; Schmidt et al. (2014), pp. 473–484; Braby (2016), pp. 268–269, 350; Taylor et al. (2018), table S3; Geyle et al. (2021), pp. 101, 103; Orr and Kitching (2010), pp. 244–245; Sankowsky (2020), p. 316; Murdoch (2021), pp. 7–8; Murdoch (2022), pp. 22–24; Murdoch and Murdoch (2023), p. 21; Ley (2023), pp. 86–87.
Holotype. Australia: Victoria: ♀ ‘Pink Lakes, Murray-Sunset NP, 15 km N Lima, Victoria, 35°03.45′S, 142°43.I3′E, 20.x.1996, R.P. Field’; ‘NMV T-17264’ (NMV). [Illustrated in Field (1999), p. 254, fig. 1–2].
Paratypes. Australia: Victoria: 1♀, 1 ♂: ‘Mildura. Vic. 16 October. 1972, B.H. Vardy’; D.F. Crosby collection donated ANIC 1987’; ‘M f 14’ [male with label ‘M m 2’]; (ANIC). Field (1999) listed a further 19 paratypes (14 ♂, 5 ♀) in NMV and the private collection of B.H. Vardy.
Although O. subterrestris was named and described relatively recently, historical specimens of this species were collected 51–111 years ago: 1♂ from Broken Hill, NSW (1912), 1♀ from Lake Hattah (given as Lake Waltah) (1918) and 2♂, 4♀ from Mildura, Vic (1972) (Field 1999). The specimens from Broken Hill and Mildura were referred to by Common and Waterhouse (1981) and Dunn and Dunn (1991) under the name Ogyris idmo halmaturia before the realisation that these specimens belonged to a distinct species. Field (1999) and Braby (2000) also placed two females from Koonibba Mission near Ceduna, SA (in SAMA) under this species but this is erroneous because these specimens are actually O. halmaturia waterhouseri (see Braby and Douglas 2008).
Ogyris subterrestris occurs in the South Australian Riverland area and in far north-western Victoria (Field 1999; Braby 2000, 2016; Braby and Douglas 2008). The species is considered to be locally extinct in south-western New South Wales.
Mallee bronze azure. The name ‘mallee bronze azure’ is suggested for this species (Braby et al. 1997). Ogyris subterrestris has previously been known as arid bronze azure, as proposed by Field (1999) but the reasons for suggesting this change are twofold. Field (1999) reasoned that ‘arid bronze azure’ was a more appropriate name as the species was known ‘from mallee and non-mallee areas’. Contrary to this, the localities referred to, Broken Hill, NSW, Mildura, Vic and near Ceduna, SA, are all localities at which mallee is or was historically present or where O. subterrestris does not actually occur. A further impetus for this common name reappraisal is that the name arid bronze azure has been utilised extensively for conservation planning for O. petrina stat. rev., a species that also occurs within a drier environment than O. subterrestris and is plausibly more suitable.
Ogyris petrina Field, 1999, stat. rev.
Ogyris sp. – Field (1987), p. 113; Field (1990), pp. 77, 80; Field (1992), pp. 13–16; Field (1997), pp. 389–391.
Ogyris idmo idmo Hewitson, 1862 – Dunn and Dunn (1991), p. 366. [Genus Ogyris Westwood, 1851]. [Misidentification in literature].
Ogyris sp. aff. idmo – Eastwood and Fraser (1999), pp. 510–511.
Ogyris subterrestris petrina Field, 1999, pp. 255–257, fig. 5–8; Braby (2000), pp. 714–715, pl. 50, fig. 4a; Edwards et al. (2001), p. 259; F. Douglas and G. G. Allen (unpubl. data, 2002), p. 1; Sands and New (2002), pp. 291–292; Braby (2004), pp. 242–243, 316; Williams and Williams (2006), pp. 20–23; Braby and Douglas (2008), p. 326; Williams et al. (2008), pp. 1–13; Williams and Williams (2008), pp. 1–17, fig. 1–2; Braby (2010), p. 71; Gamblin et al. (2010), pp. 55–58; New (2011), p. 105; Douglas (2012), pp. v, 1, 17; Braby et al. (2014), p. 108; King and Williams (2014), p. 199–201; Schmidt et al. (2014), pp. 475–481; Braby (2016), pp. 268–269, 350; Taylor et al. (2018), table S3; Williams et al. (2020), pp. 223, 239, 244; Sankowsky (2020), p. 316; Geyle et al. (2021), pp. 101, 103; Australian Geographic (2022), p. 97; Western Australian Biodiversity Science Institute (2022), pp. 1–20, fig. 1a.
Ogyris subterrestris Field, 1999 – Orr and Kitching (2010), p. 244; Williams et al. (2012), pp. 54–55. [Misidentification in literature].
Holotype. Australia: Western Australia: ♀ ‘Lake Douglas, 12 km SW of Kalgoorlie. 12.xi.1989, R.P. Field’; ‘NMVT-17268’ (NMV). [Illustrated in Field (1999), p. 254, fig. 5–6].
Paratypes. Australia: Western Australia: 10 ♂, 3 ♀: 1 ♂ labelled as for the holotype except with date ‘1.2.82’; ‘genitalia tube 208’; ‘K m 20’; ‘ANIC Database No. 31 019584’. 1 ♂ labelled similarly except with date ‘22 Oct 1985, ‘K m 19’; ‘Genitalia slide M1132’; ‘Legs on slide’. ‘ANIC Database No. 31 019589’. A further 7 ♂ and 2 ♀ with date ‘16 Dec 1986’, one female with ‘K f 16’; ‘ANIC Database No. 31 019587’, the other with ‘K f 19’. 1 ♀ labelled similarly except with date ‘17 Dec 1986’; ‘K f 17’; ‘ANIC Database No. 31 019586’. 1 ♂ ‘Lake Douglas WA, 22 Nov 1991, L.R. Ring’ (ANIC). Field (1999) listed a further 58 paratypes (32 ♂, 26 ♀) in NMV and WAM.
Ogyris petrina can be distinguished from O. subterrestris by the following 14 character states: (1) In female O. petrina, the pale yellow dorsal forewing postmedian spot is irregular but larger and distinctly divided by vein M3, forming a small faint cream patch posteriorly between M3 and CuA1, whereas this spot is smaller in O. subterrestris. (2) In female O. petrina, the dorsal iridescent purple central area on the hindwing does not extend anteriorly to vein M1 and does not extend proximally to the base, whereas the blue area is far more extensive in O. subterrestris. (3) In both sexes, the dorsal forewing of O. petrina is predominately brownish-purple without a strong bronze sheen to brown marginal areas, whereas in O. subterrestris this is bluer with a cinnamon–bronze sheen. (4) In both sexes of O. petrina, the brown markings on the ventral hindwing are poorly defined, compared to the highly complex and distinct grey markings in O. subterrestris; furthermore, there is a prominent dark grey to charcoal central marking in O. subterrestris that is absent in O. petrina. (5) The ventral hindwing of O. petrina lacks the contrasting grey postmedian patches between veins M3 and CuA2 that are prominent in O. subterrestris. (6) In female O. petrina, the dorsal forewing is deep iridescent purple, whereas in O. subterrestris this is brighter bluish-purple or blue. (7) In female O. petrina, the dorsal forewing has a double line of black scales at the end of the discal cell, divided medially by cinnamon-coloured scales, whereas in O. subterrestris this marking consists of a single broad black bar. (8) In female O. petrina, the dorsal hindwing discal bar is very obscure and surrounded by brown scales but is distinct in O. subterrestris. (9) The ventral ground colour of both sexes of O. petrina is a distinctive light cinnamon–brown but is generally dark grey in O. subterrestris, with highlights of light silvery-grey. (10) In both sexes of O. petrina, the ventral forewing has the area below the cubital vein a cinnamon-coloured gradient, from dark brown basally to light brown towards the dorsum and termen, whereas in O. subterrestris this feature is extensively and uniformly charcoal–grey towards the dorsum and termen without a gradient. (11) The ventral double subterminal bands in both wings of both sexes of O. petrina are more widely spaced, whereas this feature is narrower and less extensive in O. subterrestris, especially in the forewing. (12) In both sexes of O. petrina, the posterior section of the dark brown ventral forewing postmedian band is parallel to the termen, whereas this band is inclined sharply towards the termen or tornus in O. subterrestris. (13) In both sexes of O. subterrestris, the ventral forewing has two small patches of black edged with blue below the discal cell, whereas in O. petrina these patches are absent or, if present, dark brown without the blue markings. (14) The ventral forewing of both sexes of O. petrina has the patterns in the discal cell cream and usually transverse, whereas these are usually circular and blue in O. subterrestris.
The following diagnostic male genitalia characters have been identified between O. petrina (Fig. 8i–l) and O. subterrestris (Fig. 8e–h): (1) the vinculum of O. petrina has a strong medio-lateral expansion, whereas in O. subterrestris this is expressed as a small point; (2) the vinculum apex and saccus in O. petrina appear straight in lateral view, whereas in O. subterrestris the vinculum is displaced dorsally so as to appear recurved. In ventral view (Fig. 8h) this is apparent by way of a more triangular saccus in O. subterrestris and not straight as in O. petrina (Fig. 8l). (3) In O. petrina, the uncus is narrower and more triangular, with the dorsal projection enlarged compared with that of O. subterrestris; and (4) the valvae of O. petrina have a clearly defined ventral lobe or projection, with the apex broad and blunt, whereas in O. subterrestris the ventral lobe is poorly defined, weakly triangular and the apex is distinctly acuminate.
The adult stage has previously been illustrated in colour by Field (1999), Braby (2000) and Williams and Williams (2006).
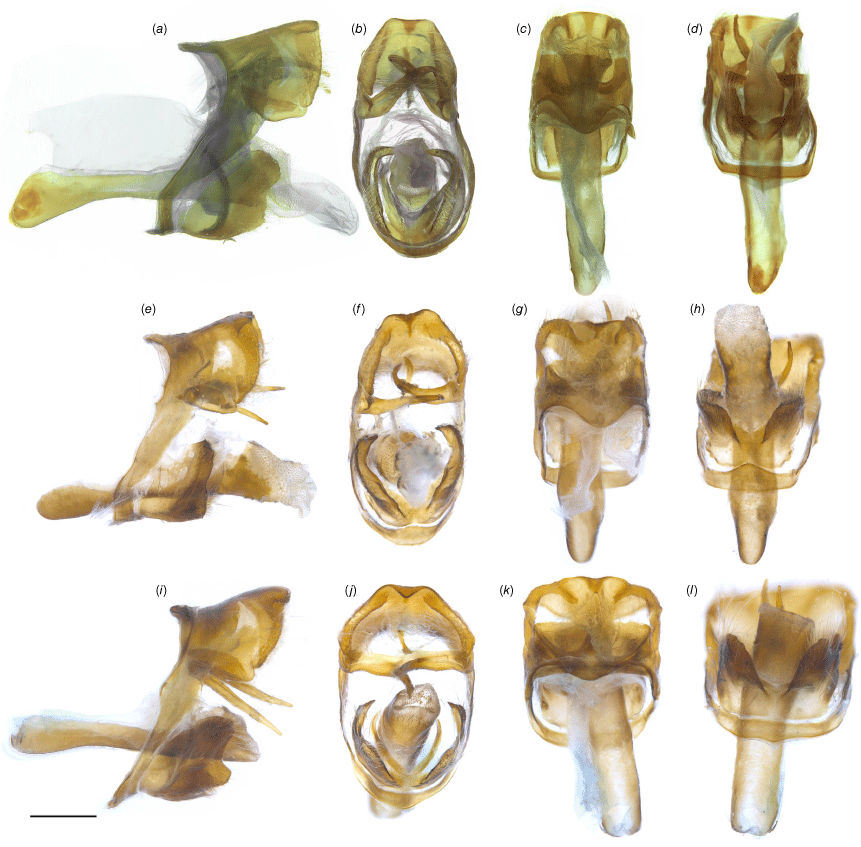
Male genitalia of Ogyris spp.: (a–d) O. idmo, genitalia number MFB-161 (ANIC); (e–h) O. subterrestris, genitalia number EPB-185 (ANIC). (i–l) O. petrina stat. rev., genitalia number EPB-184 (ANIC). Shown are lateral (a, e, i), posterior (b, f, j), dorsal (c, g, k) and ventral (d, h, l) views. Scale bar: 500 μm.
This species was originally discovered from Lake Douglas near Kalgoorlie, WA, by the late Alan Graham in 1982 (Braby 2000; Williams and Williams 2006; Gamblin et al. 2010), although investigations of material in the NHMUK revealed that a historic specimen (a single female) had been collected from the Kalgoorlie district in 1911 but the true identity had remained unrecognised (Field 1999; Williams and Williams 2006). Over many years, the taxonomy of the species was subsequently investigated by Field (1987, 1990, 1992, 1997), who considered this to be an undescribed taxon closely related to Ogyris idmo (Hewitson, 1862). Field (1999) eventually described and treated this as a subspecies of Ogyris subterrestris Field, 1999 although no justification was provided for this taxonomic arrangement. Field (1999) gave a diagnosis for the species O. subterrestris but not O. petrina. The only diagnostic information given for the latter taxon is provided in the Key on page 252, where two character states for females and three character states for males were included by which this taxon can be differentiated from O. subterrestris. The male genitalia were never examined in Field’s (1999) treatment of this taxon; only those of O. subterrestris were examined (Field 1999, fig. 22). Examination of a larger series of specimens in the ANIC and comparative study of the male genitalia have revealed additional characters by which the two taxa are distinguished. Schmidt et al. (2014) demonstrated that, based on molecular phylogenetic data, O. petrina was a monophyletic lineage more closely related to O. subterrestris from eastern Australia than to O. idmo. We treat O. petrina as a species distinct from O. subterrestris based on the large number of morphological differences including differences in the male genitalia (total of 18 characters), together with the molecular phylogenetic evidence presented by Schmidt et al. (2014) (see also Fig. 1) and the large p-distance, that is >3% (Table 1).
Ogyris petrina is known only from a few scattered localities near Kalgoorlie and Mukinbudin in the Goldfields and north-eastern Wheatbelt regions of Western Australia (Field 1999; Williams and Williams 2006; Gamblin et al. 2010; Western Australian Biodiveristy Science Institute 2022). The population near Kalgoorlie was extirpated in 1993 (Williams et al. 2012).
Discussion
We currently consider the O. idmo species group to contain six species and two subspecies (i.e. 8 taxa) based on an integrative taxonomic approach that includes a detailed appraisal of adult morphology, including wing colour and pattern elements, and morphology of male genitalia, together with a reanalysis of the molecular phylogenetic data presented in Schmidt et al. (2014). This taxonomic revision brings the total number of species recognised in Ogyris to 18 and in the tribe Ogyrini to 20.
Recognition of both Ogyris arcana and O. petrina as distinct species is based on the divergent morphology correlated with the reciprocal monophyly compared with that of the previously proposed sister subspecies status. Moreover, the uncorrected p-distances between these taxa (Table 1) are larger than those of sister species in the O. aenone complex (Beaver et al. 2023). Furthermore, the total number of adult morphological character state differences between these two species pairs (15 for O. arcana and O. otanes, and 18 for O. petrina and O. subterrestris) is similar to that recorded for the sister species O. aenone (Waterhouse, 1902) and O. caelestia Beaver & Braby, 2023, in which there are 20 character state differences (Beaver et al. 2023). This number does not include other known character differences, such as divergent life histories or differences in the morphology of the immature stages. These differences, coupled with the apparently deeper molecular divergence, may suggest that the O. idmo species group is morphologically conservative compared with other Ogyris species groups. Although molecular data from this group have been publicly accessible since 2014 (Schmidt et al. 2014), no additional morphological investigation has been undertaken until this study. While often useful for species delimitation, mitochondrial sequence data alone were notably not useful for resolving subspecies concepts within Ogyris in two other studies (Schmidt and Hughes 2007; Schmidt et al. 2014).
Two species, O. arcana and O. halmaturia, contain allopatric subspecies. In Western Australia, the two taxa O. arcana arcana and O. arcana sublustris are not reciprocally monophyletic according to neutral divergence markers (mtDNA) (Schmidt et al. 2014) (Fig. 1) but are otherwise allopatric, morphologically distinct according to wing colour pattern and male genitalia (total of five character state differences), and do not share mtDNA haplotypes. The lack of clear phylogenetic structure between O. arcana arcana and O. arcana sublustris is possibly due to a relatively recent divergence, incomplete lineage sorting or historical gene flow. Schmidt et al. (2014) argued that these taxa may be at an ‘intermediate’ state of genetic divergence in that the speciation process is not yet completed. Therefore, under the general lineage species concept and criteria for delineating species and subspecies in butterflies (Braby et al. 2012) we recognise two species in the O. otanes complex and two subspecies within O. arcana. Similarly, in this work we treat O. halmaturia waterhouseri as a subspecies of O. halmaturia given that all mainland populations of this species from Victoria and South Australia are sufficiently and consistently distinct from material of O. halmaturia halmaturia from Kangaroo Island, SA, a fact first pointed out 100 years ago by Tindale (1923). There are fewer morphological character state differences (a total of seven) between these two allopatric populations than between known sister species within the O. idmo species group. Additionally, there are currently no known biological differences between the mainland and Kangaroo Island populations; therefore, subspecific rank for waterhouseri is more appropriate than species-level designation.
The Ogyris idmo species group has undergone two significant host shifts away from aerial Loranthaceae, onto root-parasitic Santalaceae and a presumed further shift to entomophagy. The exact nature of this entomophagy, whether ant brood predation (myrmecophagy), brood parasitism (trophallaxis), or predation or parasitism of other ant nest residents, is unconfirmed due to the concealed nature of the subterranean larvae. The obligate association with the largely terrestrial-nesting Camponotus ants may have encouraged the foliage-dwelling ancestors of this clade to live in galleries or shelter at the entrances of ant nests, as is still seen in O. otanes and O. arcana, and in the nearest relatives of the O. idmo species group, O. zosine and O. genoveva. This shelter is particularly important for O. otanes and O. arcana, as no loose bark or cracks are usually present on the Santalaceae shrub hosts. A concealed, underground lifestyle is likely to add further protection within the fire-prone environments of southern and south-western Australia. The close cohabitation of lycaenid larvae within the ant nests may have been a driver for the suspected entomophagous behaviour exhibited by some species (Fig. 1). Species diversification in the Theclinae-Polyommatinae assemblage is known to be correlated with ant entomophagy (Pierce et al. 2002; Pierce and Dankowicz 2022a). Among the non-monophyletic Theclinae, the Ogyris idmo species group is one of only two lineages to have undergone significant diversification that is correlated with entomophagy (Fig. 1), the other lineage being the genus Acrodipsas Sands, 1980 that is also endemic to Australia. Generally, other thecline genera are phytophagous, with only a single or very few species that are predatory, with species richness decreasing as obligate interactions increase (Pierce and Dankowicz 2022b). The diversity of species adopting an entophagous larval feeding strategy in this small section of the Australian Theclinae resembles that of the host strategies adopted by the Miletinae elsewhere. The dynamics of the asymmetrical relationship between ant and butterfly in the O. idmo species group require further study, although the larvae are likely to be entomophagous (sensu Pierce et al. 2002). Lycaenids that engage in entomophagy are known to be morphologically conservative (Heath 1997), a condition apparent in the O. idmo group. Curiously, the four suspected entomophagous Ogyris species associate with only a single ant species, Camponotus terebrans, although C. terebrans very likely represents a complex of similar species (R. G. Eastwood, pers. comm.). Additionally, the evolution of the O. idmo species group was influenced by biogeographic factors (Schmidt et al. 2014), however this may not be truly independent of ant association if these factors have also affected the host ants.
Entomophagous relationships within Lycaenidae are always unidirectional and the highly specialised lifestyle has ultimately left these taxa more vulnerable to extinction. Indeed, butterflies in the Ogyris idmo species group are among the most threatened Australian Lepidoptera – our revised taxonomic classification of the group, together with the conservation status, is summarised in Table 2. Six (75%) of the eight recognised taxa are considered to be threatened, either nationally or regionally (state level). However, only one species (Ogyris petrina) is listed nationally under federal legislation (EPBC Act) and only one (Ogyris halmaturia) is listed internationally under the IUCN Red List categories, highlighting the disparity between conservation need and current listing. Clearly, there is an urgent need for re-assessment of conservation status at the national and international levels and nomination for protective legislation for all taxa. Like other lycaenids with obligate ant interactions, these butterflies require a very high density of the associated host species within the environment. This is a biological strategy that can fail if the environment is altered by extensive habitat loss or fragmentation so as to be unfavourable for supporting high densities of host ant colonies. This risk is increased due to the species generally being highly localised. Accordingly, the species in this group are primarily threatened by widespread habitat loss and fragmentation by way of land clearing for agriculture and mining activities (Sands and New 2002).
| Taxon | Occurrence | International (IUCN) | National (EPBC) | State (FFG, BC) | Comments | |
|---|---|---|---|---|---|---|
| Ogyris otanes | NSW, VIC, SA | CR (VIC: FFG) | Considered to be EX in NSW, EN in VIC and possibly VU in SA (Sands and New 2002) | |||
| Ogyris arcana stat. rev. | WA | Western coastal population considered to be Thr nationally | ||||
| Ogyris arcana arcana comb. nov. | WA | No conservation significance | ||||
| Ogyris arcana sublustris comb. nov. | WA | Considered to be Thr nationally (Geyle et al. 2021) | ||||
| Ogyris halmaturia | VIC, SA | EN | Considered to be EN internationally (Young et al. 2021) | |||
| Ogyris halmaturia halmaturia | SA | Previously considered to be EX (Field 1997; Braby and Douglas 2008) but now most likely CR since rediscovery in 2014 | ||||
| Ogyris halmaturia waterhouseri stat. rev. | VIC, SA | CR (VIC: FFG) | Considered to be EX in VIC and EN in SA (Sands and New 2002; Braby and Douglas 2008) | |||
| Ogyris idmo | WA | No conservation significance, but current taxonomy is uncertain and range is highly fragmented (Williams et al. 2020) | ||||
| Ogyris subterrestris | NSW, VIC, SA | EN (VIC: FFG) | Considered to be EX in NSW, EN in VIC and VU in SA (Field 1997; Sands and New 2002; Douglas 2012) | |||
| Ogyris petrina stat. rev. | WA | CR | CR (WA: BC) | Considered to be CR nationally (Geyle et al. 2021) |
EX, Extinct in the wild; CR, Critically Endangered; EN, Endangered; VU, Vulnerable; Thr, Threatened; IUCN, International Union for Conservation of Nature Red List Categories; EPBC, Environmental Protection and Biodiversity Act 1999; FFG, Flora and Fauna Guarantee Act 1988; BC, Biodiversity Conservation Act 2016; NSW, New South Wales; VIC, Victoria, SA, South Australia; WA, Western Australia.
Supplementary material
GenBank Accession Numbers and general locality information for the molecular component of this study are included in Supplementary Table S1. Supplementary material is available online.
Data availability
Molecular data for this project can be found on GenBank, see Supplementary Table S1 for GenBank accession numbers. Author ZooBank numbers are as follows: Ethan Beaver, urn:lsid:zoobank.org:author:E85BA5D9-54F9-4920-9D2A-DC7262F44BBC; Michael Braby, urn:lsid:zoobank.org:author:4D3A7605-EBD0-40F6-A5F2-7F67F59E3D60; Richard V. Glatz, urn:lsid:zoobank.org:author:EB9E57BA-0B47-4302-8D4E-848789AA3B27.
Acknowledgements
We thank the various staff members of museum collections who facilitated examination of specimens or photography of specimens, including Russell Cox, Derek Smith and Natalie Tees (AMS), Simon Hinkley (NMV), You Ning Su, Andreas Zwick (ANIC), Ben Parslow (SAMA) and John Tennent (NHMUK). Additionally, we thank Fabian Douglas and Graham Wurtz for sharing images and experience. We thank Fred Jaya (ANU) and Yi-Kai Tea (AM) for assistance with molecular analysis.
References
Atkins AF (1978) A collecting trip to Western Australia: a list of butterflies captured and some biological notes. Victorian Entomologist 8, 25-29.
| Google Scholar |
Beaver EP, Braby MF, Mikheyev AS (2023) Systematics of the Ogyris aenone (Waterhouse, 1902) complex (Lepidoptera: Lycaenidae): threatened Australian butterflies of national conservation significance. Invertebrate Systematics 37(7), 457-497.
| Crossref | Google Scholar |
Bethune-Baker GT (1905) A monograph of the genus Ogyris. Transactions of the Entomological Society of London 1905, 269-292, pl XV.
| Google Scholar |
Bethune-Baker GT (1916) XLIV.—Notes on the synonymy of the genus Ogyris. Annals and Magazine of Natural History 17, 386-390.
| Crossref | Google Scholar |
Braby MF (2010) The merging of taxonomy and conservation biology: a synthesis of Australian butterfly systematics (Lepidoptera: Hesperioidea and Papilionoidea) for the 21st century. Zootaxa 2707, 1-76.
| Crossref | Google Scholar |
Braby MF, Douglas F (2008) The nomenclature, taxonomy and conservation status of Ogyris waterhouseri (Bethune-Baker, 1905) stat. nov. (Lepidoptera: Lycaenidae), a threatened butterfly from southern Australia. Australian Journal of Entomology 47, 315-329.
| Crossref | Google Scholar |
Braby MF, Müller CJ (2022) Pseudogyris gen. nov. (Lepidoptera: Lycaenidae), a new genus for two rare thecline butterflies from New Guinea, including the description of a new species. Tropical Lepidoptera Research 32, 1-15.
| Google Scholar |
Braby MF, Atkins AF, Dunn KL, Woodger TA, Quick WNB (1997) A provisional list of common names for Australian butterflies. Australian Journal of Entomology 36(3), 197-212.
| Crossref | Google Scholar |
Braby MF, Douglas F, Willan RC (2011) The nomenclature of Ogyris halmaturia (Tepper, 1890) (Lepidoptera: Lycaenidae). The Australian Entomologist 38, 29-36.
| Google Scholar |
Braby MF, Eastwood R, Murray N (2012) The subspecies concept in butterflies: has its application in taxonomy and conservation biology outlived its usefulness? Biological Journal of the Linnean Society 106, 699-716.
| Crossref | Google Scholar |
Braby MF, Douglas F, Peterson M (2014) New and interesting records of Ogyris zosine (Hewitson, [1853]) (Lepidoptera: Lycaenidae) from inland Western Australia. The Australian Entomologist 41, 107-114.
| Google Scholar |
Burns AN (1931) Habits and life histories of some Victorian lycaenid butterflies. The Victorian Naturalist 48, 129-136.
| Google Scholar |
Burns AN, Angel F (1952) The small brown azure (Ogyris atanes [sic] Feld) (description and notes on the life history). The Victorian Naturalist 68, 183-186.
| Google Scholar |
Crosby DF (1972) Some butterflies of the Victorian Big Desert. Victorian Entomologist 2, 5-7.
| Google Scholar |
Crosby DF (1974a) Insects of the Victorian National Parks. Part 1 – butterflies. Victorian Entomologist 4, 62-65.
| Google Scholar |
Crosby DF (1974b) Notes on the insects of the Big Desert (Victoria). Part Two. Victorian Entomologist 4, 58-59.
| Google Scholar |
Crosby DF (1976) Further notes on the butterflies of the Big Desert. Victorian Entomologist 6, 15-16.
| Google Scholar |
Eastwood R, Fraser AM (1999) Associations between lycaenid butterflies and ants in Australia. Australian Journal of Ecology 24, 503-537.
| Crossref | Google Scholar |
Felder C, Felder R (1865) ‘Lepidoptera. Heft I–III. Rophalocera. Reise der Österreichischen Fregatte Novara um die Erde in den Jahren 1857, 1858, 1859 unter den Befehlen des Commodore B. von Wüllerstorf-Ubair’. Zoologischer Theil, Band 2, Abtheilung 2(1–3). (Kaiserlich-Königliche Hof- und Staatsdruckerei: Vienna, Austrian Empire) [In German]
Field RP (1987) Notes on butterflies collected in south-west Western Australia, September–October, 1987. Victorian Entomologist 17, 111-114.
| Google Scholar |
Field RP (1990) Range extensions and the biology of some Western Australian butterflies. Victorian Entomologist 20, 76-82.
| Google Scholar |
Field RP (1992) Research grant report: life history studies and species determination of the Ogyris idmo Hewitson (Lepidoptera: Lycaenidae) complex in Western Australia. Myrmecia 28, 12-17.
| Google Scholar |
Field RP (1997) The Ogyris idmo Hewitson complex (Lepidoptera: Lycaenidae) as flagship species for conservation in southern Australia. Memoirs of the Museum of Victoria 56, 389-392.
| Crossref | Google Scholar |
Field R (1999) A new species of Ogyris Angas (Lepidoptera: Lycaenidae) from southern arid Australia. Memoirs of Museum Victoria 57, 251-259.
| Crossref | Google Scholar |
Fisher RH (1985) Butterflies (Lepidoptera: Hesperiidae, Papilionoidea) of Kangaroo Island, South Australia. Australian Entomological Magazine 12, 1-8.
| Google Scholar |
Fisher RH (1993) Report on the conservation status of the small brown azure butterfly (Ogryis otanes C. & R. Felder) in South Australia. Xanthopus. Nature Conservation Society of South Australia Inc., Newsletter 149, 11-12.
| Google Scholar |
Gamblin T, Williams MR, Williams AAE (2010) The ant, the butterfly, the leafhopper and the bulldozer. Landscope 25, 54-58.
| Google Scholar |
Geyle HM, Braby MF, Andren M, et al. (2021) Butterflies on the brink: identifying the Australian butterflies (Lepidoptera) most at risk of extinction. Austral Entomology 60, 98-110.
| Crossref | Google Scholar |
Grund R (1996) Range extensions, new foodplant recordings and biology for some South Australian butterflies. Victorian Entomologist 26, 93-100.
| Google Scholar |
Grund R (1997) Additional range extensions, new foodplant recordings and biology for some rarer South Australian butterflies including the life history for Candalides cyprotus cyprotus (Oliff). Victorian Entomologist 27, 7-14.
| Google Scholar |
Grund R (1998) New foodplant recordings and biological observations for some Western Australian butterflies. Victorian Entomologist 28, 65-68.
| Google Scholar |
Grund R (1999) Additional range extensions, new foodplant recordings and biology for some rarer butterflies from north-east Eyre Peninsula, South Australia. Victorian Entomologist 29, 45-50.
| Google Scholar |
Grund R (2003) A note on the first instar larva of Ogyris idmo halmaturia Tepper (Lepidoptera: Lycaenidae). Victorian Entomologist 33, 71.
| Google Scholar |
Grund R (2010) The taxonomy of Ogyris halmaturia (Tepper, 1890) stat. nov. (Lepidoptera: Lycaenidae). Australian Journal of Entomology 49, 114-120.
| Crossref | Google Scholar |
Hart R, Powell M (1997) Status of the northern population of the butterfly, the western dark azure (Ogyris otanes) in Western Australia. Western Australian Naturalist 21, 185-190.
| Google Scholar |
Hay RW (1989) In search of a butterfly. Landscope 4, 42-43.
| Google Scholar |
Heath A (1997) A review of African genera of the tribe Aphnaeini (Lepidoptera: Lycaenidae). Metamorphosis 2, 1-60.
| Google Scholar |
Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS (2018) UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35, 518-522.
| Crossref | Google Scholar | PubMed |
Hunt L, Moore M, Moore D (1998) Rediscovery of Ogyris idmo halmaturia (Tepper 1890). Victorian Entomologist 28, 113-116.
| Google Scholar |
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods 14, 587-589.
| Crossref | Google Scholar | PubMed |
Kershaw JA (1908) No title. In reports, general business, papers, natural history notes and exhibits of the Field Naturalists’ Club of Victoria. The Victorian Naturalist 24, 161-164.
| Google Scholar |
King D, Williams AAE (2014) An inland range extension for Ogyris idmo (Hewitson) (Lepidoptera: Lycaenidae). The Australian Entomologist 41, 199-201.
| Google Scholar |
Ley G (2023) Engaging the community. Victorian Entomologist 53(4), 86-87.
| Google Scholar |
Lower OB (1893) List of South Australian Rhopalocera. Transactions and Proceedings and Report of the Royal Society of South Australia 17, 1-12.
| Google Scholar |
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R (2020) IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37, 1530-1534.
| Crossref | Google Scholar | PubMed |
Miskin WH (1890) A revision of the Australian genus Ogyris, with description of a new species. Proceedings of the Linnean Society of New South Wales 5, 23-28.
| Crossref | Google Scholar |
Miskin WH (1891) A synonymical catalogue of the Lepidoptera Rhopalocera (butterflies) of Australia with full bibliographical reference; including descriptions of some new species. Annals of the Queensland Museum 1, 1-93.
| Google Scholar |
Moore MD (1999) Some field notes on the as yet unnamed Ogyris species (formerly included in the species Ogyris idmo halmaturia) from Waikerie. Victorian Entomologist 29, 12-17.
| Google Scholar |
Murdoch F (2021) Mildura Ogyris butterfly. Victorian Entomologist 51, 7-8.
| Google Scholar |
Murdoch F (2022) Notes from a talk by Fiona Murdoch from Mallee Conservation. Victorian Entomologist 52, 22-26.
| Google Scholar |
Murdoch F, Murdoch P (2023) Mallee conservation. Restoring private land habitat. Victorian Entomologist 53, 21.
| Google Scholar |
New TR, Field RP, Sands DPA (2007) Victoria’s butterflies in a national conservation context. The Victorian Naturalist 124, 243-249.
| Google Scholar |
Nickrent DL, Malécot V, Vidal-Russell R, Der JP (2010) A revised classification of Santalales. Taxon 59, 538-558.
| Crossref | Google Scholar |
Pierce NE (1995) Predatory and parasitic Lepidoptera: carnivores living on plants. Journal of the Lepidopterists’ Society 49, 412-453.
| Google Scholar |
Pierce NE, Dankowicz E (2022a) The natural history of caterpillar–ant associations. In ‘Caterpillars in the Middle. Fascinating Life Sciences’. (Eds RJ Marquis, S Koptur) pp. 319–391. (Springer: Cham, Switzerland) 10.1007/978-3-030-86688-4_11
Pierce NE, Dankowicz E (2022b) Behavioral, ecological and evolutionary mechanisms underlying caterpillar-ant symbioses. Current Opinion in Insect Science 52, 100898.
| Crossref | Google Scholar |
Pierce NE, Braby MF, Heath A, et al. (2002) The ecology and evolution of ant association in the Lycaenidae (Lepidoptera). Annual Review of Entomology 47, 733-771.
| Crossref | Google Scholar | PubMed |
Quick WNB (1972) Collected Ogyris idmo waterhouseri. Victorian Entomologist 2, 9-10.
| Google Scholar |
Quick WNB (1973) The Big Desert – September 15–17, 1973. Victorian Entomologist 3, 15-18.
| Google Scholar |
Quick WNB (1974) The Big Desert – September, 1974. Victorian Entomologist 4, 60-61.
| Google Scholar |
Schmidt DJ, Hughes JM (2007) Genetic affinities among subspecies of a widespread Australian lycaenid butterfly, Ogyris amaryllis (Hewitson). Australian Journal of Zoology 54, 429-446.
| Crossref | Google Scholar |
Schmidt DJ, Grund R, Williams MR, Hughes JM (2014) Australian parasitic Ogyris butterflies: east-west divergence of highly specialized relicts. Biological Journal of the Linnean Society 111, 473-484.
| Crossref | Google Scholar |
Su YN (2016) A simple and quick method of displaying liquid-preserved morphological structures for microphotography. Zootaxa 4208, 592-593.
| Crossref | Google Scholar | PubMed |
Taylor GS, Braby MF, Moir ML, et al. (2018) Strategic national approach for improving the conservation management of insects and allied invertebrates in Australia. Austral Entomology 57, 124-149.
| Crossref | Google Scholar |
Tepper JGO (1893) Notes and remarks on South Australian Rhopalocera. Transactions, Proceedings and Reports of the Royal Society of South Australia 17, 281-286.
| Google Scholar |
Tindale NB (1923) On Australian Rhopalocera. Transactions and Proceedings of the Royal Society of South Australia 47, 342-354.
| Google Scholar |
Waterhouse GA (1903a) A catalogue of the Rhopalocera of Australia. Memoirs of the New South Wales Naturalists’ Club 1, 1-49.
| Google Scholar |
Waterhouse GA (1903b) Notes on Australian Rhopalocera: Lycaenidae. Part III Revisional. Proceedings of the Linnean Society of New South Wales 28, 132-275.
| Google Scholar |
Waterhouse GA (1937) Presidential address: The biology and taxonomy of the Australian butterflies. Report of the Australian and New Zealand Association for the Advancement of Science, Auckland Meeting, January 1937 23, 101-133.
| Google Scholar |
Waterhouse GA, Lyell G (1908) Some Dimboola butterflies. The Victorian Naturalist 24, 165-166.
| Google Scholar |
Williams MR (2009) Butterflies and day-flying moths in a fragmented urban landscape, south-west Western Australia: patterns of species richness. Pacific Conservation Biology 15, 32-46.
| Crossref | Google Scholar |
Williams MR, Hay RW (2001) Two new subspecies of Ogyris otanes C.&R. Felder (Lepidoptera: Lycaenidae) from Western Australia. The Australian Entomologist 28, 55-63.
| Google Scholar |
Williams AAE, Williams MR (2006) Endangered or extinct? Kalgoorlie’s arid bronze azure. Landscope 21, 19-23.
| Google Scholar |
Williams MR, Atkins AF, Hay RW, Bollam HH (1992) The life history of Ogyris otanes C. & R. Felder in the Stirling Range, Western Australia (Lepidoptera: Lycaenidae). The Australian Entomologist 19, 55-60.
| Google Scholar |
Williams MR, Williams AAE, Lundstrom TD, Hay RW, Bollam HH, Graham AJ (1995) Range extensions and natural history notes for some Western Australian butterflies. Victorian Entomologist 25, 94-96.
| Google Scholar |
Williams AAE, Williams MR, Heterick BE (2020) Notes on the distribution, habitat, behaviour and flight times of the large bronze azure, Ogyris idmo (Hewitson, 1862) (Lepidoptera: Lycaenidae), a rare myrmecophilous butterfly from south-western Western Australia. The Australian Entomologist 47, 221-247.
| Google Scholar |
Young DA (2004) Observations on the Lepidoptera of Vivonne Bay, Kangaroo Island, South Australia: spring 2003–autumn 2004. Victorian Entomologist 34, 63-67.
| Google Scholar |
Young DA, Glatz RV, Field RP, Braby MF (2021) Eastern bronze azure Ogyris halmaturia. In ‘The IUCN Red List of Threatened Species 2021’. e.T189545680A195996978. (International Union for Conservation of Nature and Natural Resources) Available at https://www.iucnredlist.org/species/189545680/195996978