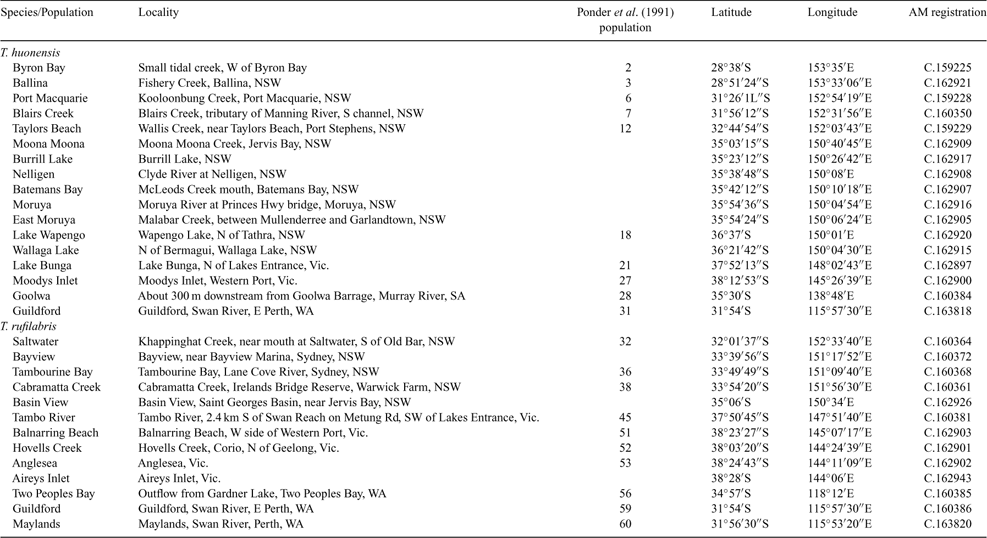
The mitochondrial DNA haplotypes of snails of the estuarine hydrobiid genus Tatea cross species and biogeographic boundaries
D. J. Colgan A B and P. da Costa AA Research Branch, The Australian Museum, 6 College Street, Sydney, NSW 2010, Australia.
B Corresponding author. Email: don.colgan@austmus.gov.au
Marine and Freshwater Research 60(8) 861-872 https://doi.org/10.1071/MF08200
Submitted: 30 June 2008 Accepted: 8 February 2009 Published: 27 August 2009
Abstract
Investigations of estuarine taxa can provide a perspective on phylogeography that complements studies of marine littoral organisms. For example, reductions in gene flow between populations and increased genetic structuring would be expected in estuarine species. The substantial amount of information about marine species and the habitat diversity along long latitudinal spans makes south-eastern Australia an excellent potential location for comparing marine and estuarine taxa. To investigate this potential, we studied the phylogeography of the two species in the estuarine gastropod genus Tatea. These have extensive and broadly overlapping distributions that encompass known marine phylogeographic boundaries. Against expectation, both Tatea species showed a remarkable lack of geographic and inter-specific variability in mitochondrial 12S rRNA (107 specimens) and cytochrome c oxidase subunit I (39) DNA sequences. No major phylogeographic discontinuities were revealed in either species and there was minimal haplotype divergence between them for either 12S rRNA or COI. The patterns of mitochondrial DNA variation discovered in Tatea may be due to a recent selective sweep or range expansion from a population in which there was little variability. Both possibilities are complicated by having to explain the similarity of the patterns in the two species.
Additional keywords: cytochrome c oxidase, Gastropoda, phylogeography, 12S rRNA, south-eastern Australia.
Acknowledgements
We thank Winston Ponder for his continuing support and encouragement and for reading a draft of this manuscript. We thank two anonymous referees for detailed and helpful comments on a previous version of the manuscript. The Australian Museum provided financial support for this work. We also thank the collectors of the material including those who collected for the Ponder et al. (1991) survey and Winston Ponder, Stephanie Clark, Des Beechey and Roger de Keyzer who collected the additional material used here.
Ayre, D. J. , Read, J. , and Wishart, J. (1991). Genetic subdivision within the eastern Australian population of the sea anemone Actinia tenebrosa. Marine Biology 109, 379–390.
| Crossref | GoogleScholarGoogle Scholar |
Clement, M. , Posada, D. , and Crandall, K. (2000). TCS: a computer program to estimate gene genealogies. Molecular Ecology 9, 1657–1659.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed |
Dawson, M. N. (2001). Phylogeography in coastal marine animals: a solution from California? Journal of Biogeography 28, 723–736.
| Crossref | GoogleScholarGoogle Scholar |
Farrington, L. W. , Austin, C. M. , and Coutin, P. C. (2000). Allozyme variation and stock structure in the black bream, Acanthopagrus butcheri (Munro) (Sparidae) in southern Australia: implications for fisheries management, aquaculture and taxonomic relationship with Acanthopagrus australis (Gunther). Fisheries Management and Ecology 7, 265–279.
| Crossref | GoogleScholarGoogle Scholar |
Mills, C. E. , Hadwen, W. L. , and Hughes, J. M. (2008). Looking through glassfish: marine genetic structure in an estuarine species. Marine and Freshwater Research 59, 627–637.
| Crossref | GoogleScholarGoogle Scholar | CAS |
Tamura, K. , Dudley, J. , Nei, M. , and Kumar, S. (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4. 0. Molecular Biology and Evolution 24, 1596–1599.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed |