Uterine fluid composition of the dwarf ornate wobbegong shark (Orectolobus ornatus) during gestation
Megan T. Ellis A B and Nicholas M. Otway AA Industry and Investment NSW, Port Stephens Fisheries Institute, Taylors Beach, NSW 2316, Australia.
B Corresponding author. Email: sharkymegs@hotmail.com
Marine and Freshwater Research 62(6) 576-582 https://doi.org/10.1071/MF10138
Submitted: 15 June 2010 Accepted: 8 January 2011 Published: 24 June 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
Low fecundity in chondrichthyans makes them extremely susceptible to fishing, so understanding the various reproductive strategies in this group is vital for management. Knowledge of the uterine fluid (UF) composition throughout gestation is fundamental to this understanding, yet is restricted to a few species. This study focussed on the UF composition of the wobbegong (Orectolobus ornatus), which inhabits coastal waters off eastern Australia. The UF was quantified throughout pregnancy. Fluids surrounding uterine eggs had a complex composition, with mean urea (98.48 mmol L–1), sodium (560.25 mmol L–1) and potassium (13.93 mmol L–1) concentrations significantly greater than those in seawater. A change in composition, from complex to simple, occurred after 3–4 months gestation. Major electrolyte concentrations then resembled seawater for the remainder of gestation, suggesting the flushing of the uteri with seawater and evidenced by fluctuating low levels of urea. The gestation period reflected the time for metabolism of yolk stores, osmotic and ionic adjustment, development of functioning immunological systems and prevention of external yolk sac damage. Our study is the first documentation of UF composition for a wobbegong shark and increases understanding of its reproductive biology.
Additional keywords: biochemistry, elasmobranch, reproduction.
Introduction
Chondrichthyan embryos can develop external to the mother’s body (oviparous) or within the mother’s body (viviparous) (Wourms et al. 1988; Hamlett and Koob 1999; Hamlett et al. 2005b). The source of nutrition for the developing embryos can also vary, and ranges from strict lecithotrophy (reliant on yolk) to matrotrophy (reliant on maternal secretions). All chondrichthyan embryos initially rely on yolk sequestered in an external yolk sac for nutrition (Hamlett and Koob 1999) and oviparous species and strictly lecithotrophic viviparous species remain solely reliant on yolk throughout gestation (Hamlett and Koob 1999; Hamlett et al. 2005b). Once yolk stores are depleted for matrotrophic species, developing embryos rely on nutrients from maternal sources to augment yolk stores, for at least a portion of embryonic development. Maternal sources include uterine secretions (histotrophy), ova (ovatrophy), siblings (intrauterine cannibalism or adelphotrophy) or placental transfer (placentatrophy) (Gilmore et al. 2005; Hamlett et al. 2005a, 2005b). During gestation, maternal and foetal tissues are modified to assist with the various processes associated with embryonic nutrition and development (Hamlett et al. 1993; Hamlett and Hysell 1998; Hamlett and Koob 1999).
The uterine fluid (UF) of chondrichthyans is secreted by the epithelium of the uterine wall during gestation and provides a hydrostatic environment for developing embryos. UF facilitates respiration, waste removal, restricted movement and, in some species, provides nutrition (Lombardi et al. 1993; Hamlett and Koob 1999). The UF of a limited number of species has been analysed biochemically and these analyses have focussed on quantifying the levels of major electrolytes, free amino acids, proteins, lipids and carbohydrates. The electrolyte UF composition has been quantified for the piked dogfish (Squalus acanthias) (Price and Daiber 1967; Evans et al. 1982; Kormanik 1988, 1992, 1993), the dusky smooth-hound (Mustelus canis) (Price and Daiber 1967) and the bull shark (Carcharhinus leucas) (Thorson and Gerst 1972). Free amino acids, total protein, total lipid, glucose and osmolarity were measured in the UF of S. acanthias, M. canis, the sandbar shark (Carcharhinus plumbeus) and the Atlantic sharpnose shark (Rhizoprionodon terraenovae) (Jones et al. 1990; Files and Lombardi 1993; Lombardi et al. 1993), while total protein and total carbohydrate were measured in the UF of the common sawshark (Pristiophorus cirratus) but not during the latter part of gestation (Stevens 2001). Although these informative papers include species that are strict lecithotrophic, placentatrophic and limited histotrophic, they are limited to six species of shark and, therefore, any studies that can expand the taxonomic diversity on this topic will be important contributions.
Wobbegong sharks (family Orectolobidae) are bottom-dwelling sharks found in temperate to tropical waters off Australia, Indonesia, the north-western and western Pacific and eastern Indian Oceans (Compagno 2002; Last and Stevens 2009). The dwarf ornate wobbegong (Orectolobus ornatus), the spotted wobbegong (O. maculatus) and the ornate wobbegong (O. halei) are found in NSW coastal waters and are caught in commercial fisheries (Huveneers 2006; Huveneers et al. 2007a; Scandol et al. 2008) and are listed as Vulnerable in NSW waters under the World Conservation Union (IUCN) Red List Assessment (Cavanagh et al. 2003). Detailed information on their ecology, reproduction, and physiology has only just begun to emerge, with recent studies describing patterns of abundance, localised movements, diet and reproduction (Carraro and Gladstone 2006; Huveneers 2007; Corrigan et al. 2008). Despite this, no data have been published on UF composition in relation to reproduction for these three wobbegong species. This paper is the first to document the UF composition of O. ornatus throughout gestation and tests the hypothesis that the composition remains consistent throughout gestation. In doing so, the study expands the knowledge of elasmobranch uterine environments and provides information on the reproductive mode and maternal roles of this shark species, which may assist in their fisheries management, husbandry and long-term conservation.
Materials and methods
Field sampling
Over 13 months (February 2007 to February 2008), O. ornatus individuals were collected by a commercial fisher operating off Nambucca Heads, northern NSW (30°38.731′S 153°01.149′E). Wobbegongs were caught on rocky reefs in 10 to 20 m of water using wire-mesh lobster traps (650 × 450 mm with a circular opening 20 cm in diameter). Traps were baited with fresh fish (various species), set after sunrise and retrieved within 24 h, and were capable of catching a maximum of two O. ornatus individuals. When traps were retrieved, the wobbegongs were placed in plastic tubs containing aerated seawater. Each shark was then examined externally to determine sex via the presence of claspers in males, sexual maturity (for males, total length (TL) and clasper calcification; for females, TL and the presence/absence of a cloacal hymen), and any external injuries associated with capture or the animal’s prior existence were noted. Females that had laterally expanded abdominal regions were palpated between the pectoral and pelvic fins to assess potential pregnancy. Females that were possibly pregnant were separated from the remaining catch. During each field sampling, three replicate seawater samples were taken from the site of capture. These samples were then transferred to 1.5-mL cryovials, transported in a portable freezer to the laboratory and stored at –20°C until analysed.
Necropsies
Females suspected of being pregnant were necropsied first. Each shark was euthanased by severing the spinal cord with a sharp knife and then subjected to a standardised necropsy during which total weight (TW), liver weight, gonad weight, reproductive condition and various morphometric measurements were recorded. In females, sexual maturity was confirmed by a combination of ovarian, oviducal gland and uterine development. The diameters of the three largest ovarian follicles were measured to the nearest millimetre and then each follicle was weighed to the nearest gram. The maximum width of the oviducal glands and uteri were measured to the nearest millimetre. With each pregnant female, separate samples of UF were carefully removed from the left and right uteri (avoiding any contamination with blood and/or contact with eggs or embryos) using a sterile 18-gauge needle attached to a 10-mL syringe. The UF samples were then transferred to 1.5-mL cryovials and transported in a portable freezer to the laboratory where they were stored at –20°C until analysed. The uterine eggs and or embryos were then removed from the uterus for further examination. For uterine eggs, TW and maximum diameter were recorded. For embryos with an external yolk sac (EYS), TW was recorded and then the EYS (including yolk stalk) was removed by cutting the stalk as close to the embryo as possible. Each embryo and its associated EYS were then weighed separately and these weights were recorded together with the diameter of the EYS, measured perpendicular to the yolk stalk. The TL of each embryo and any notable external developmental features were recorded. Following dissection, any internal developmental features were noted and if present/possible, the weight and diameter and/or length of the internal yolk sac (IYS) were recorded.
Biochemical analyses
Biochemical analysis of UF and seawater samples was done with an Olympus AU400 clinical biochemistry analyser (Olympic Diagnostics, Sydney). An ion-selective electrode was used to quantify sodium, potassium and chloride, while automated bichromatic spectrophotometry was used for calcium, magnesium, urea nitrogen, bicarbonate, phosphate, glucose, total protein, cholesterol and triglycerides. Total osmolarity (mmol kg–1) of UF and seawater was determined using freezing point depression by means of a micro-osmometer (Advanced Osmometer, Model 330, Advanced Instruments Inc., Norwood, MA).
Statistical analyses
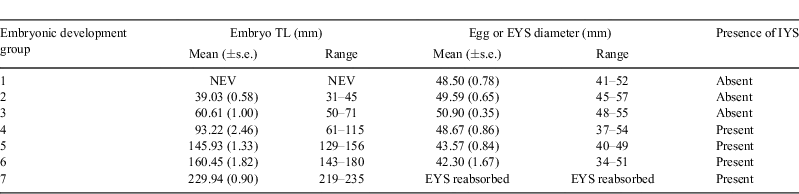
The composition of the catch of O. ornatus was analysed using contingency tables to test the null hypothesis that the proportion of immature and mature male and female wobbegongs did not differ. Relationships between number of embryos and maternal TL were examined using linear regressions, with measurements taken from a subsample (n = 19) of pregnant wobbegongs. Paired t-tests were used to compare the number of male and female embryos in the uteri of pregnant O. ornatus following Kolmogorov–Smirnov tests for normality (Sokal and Rohlf 1969). Embryos were assigned to groups according to EYS diameter, embryo TL, presence/absence of an IYS and uterine fluid composition (Table 1). The UF sampling yielded a maximum possible sample size of n = 38 of which a subsample of 28 was used in the analyses. Concentrations of major constituents (sodium, potassium, calcium, magnesium, chloride and urea), minor constituents (bicarbonate, phosphate, glucose, total protein, cholesterol and triglycerides) and osmolarity of the UF of these groups (7 groups, n = 4 replicates) and seawater (1 group, n = 4 replicates) were each analysed using a single-factor analysis of variance following a Cochran’s test for homoscedasticity (Snecedor and Cochran 1967) to test the null hypothesis of no difference in concentrations among developmental groups and seawater. Significant differences among means, when present, were identified using post-hoc Student–Newman–Keuls (SNK) tests.
Results
Wobbegong population and embryonic development
Over the 12 months of sampling, 113 O. ornatus individuals comprising 38 males (626–972-mm TL, 1.49–7.50-kg TW) and 75 females (570–1000-mm TL, 1.22–7.35-kg TW) were caught. The catch comprised significantly more mature males than immature males (χ12 = 20.50, P < 0.001) and similar numbers of immature and mature females. Of the 38 males caught, 4 were immature (626–858-mm TL) and 34 were mature (649–972-mm TL). For females, 41 individuals were immature (570–958-mm TL) and 34 individuals were mature (812–1000-mm TL). Twenty-seven females were pregnant and covered all stages of gestation. In the subsample (n = 19), two females had 21 in utero eggs, with a mean (±s.e.) of 10.50 (0.50) eggs per female. The remaining 17 females had 165 embryos, with a mean (±s.e.) of 9.71 (0.45) embryos per female. The number of embryos was linearly related to the maternal female TL (r = 0.54, n = 19, P < 0.05) but not to TW (r = 0.29, n = 19, P > 0.10). Seven embryonic development groups were identified based on presence of eggs or embryos, EYS diameter, embryo TL and presence/absence of an IYS (Table 1). Litters comprising uterine eggs (Group 1) were observed in November, embryos of increasing TL with EYS were observed from late January to July (Groups 2–6) and embryos with an IYS and no EYS were observed from August to September. No pregnant animals were captured during May, June or July. Sex of the embryos could only be determined in embryos from Group 3, with a minimum TL of 50 mm.
UF and plasma biochemistry
Major constituents in UF
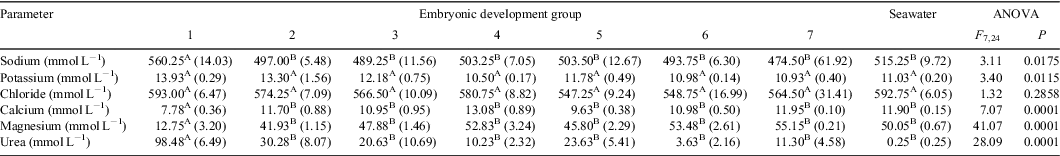
Results comparing the concentrations of the major constituents in seawater and the uterine fluid from pregnant O. ornatus females at different stages of development are summarised in Table 2. Females from Group 1 had UF with mean sodium and urea concentrations that were significantly greater than in seawater and in the UF of pregnant females from Groups 2 to 7. The mean sodium and urea concentrations in the UF bathing the developing embryos from Groups 2 to 7 did not differ from each other or from that in seawater. However, the urea concentrations fluctuated among sharks at different stages of development. The UF of females from Group 1 had mean calcium and magnesium concentrations that were significantly lower than those in seawater and in the UF of pregnant females from Groups 2 to 7, but the concentrations for Groups 2 to 7 did not differ from each other or from seawater. The mean concentration of potassium in the UF of pregnant O. ornatus females differed significantly during gestation; however, the SNK test could not unequivocally identify the differences among means. Inspection of the raw data and the mean values for each group suggested that the UF of pregnant females from Group 1 had a potassium concentration greater than that in seawater and in the UF of pregnant females from Groups 2 to 7, which did not differ from each other or from seawater. The mean chloride concentrations in the UF of pregnant O. ornatus females from Groups 1 to 7 did not differ significantly from each other or from that in seawater.
Minor constituents in UF and osmolarity
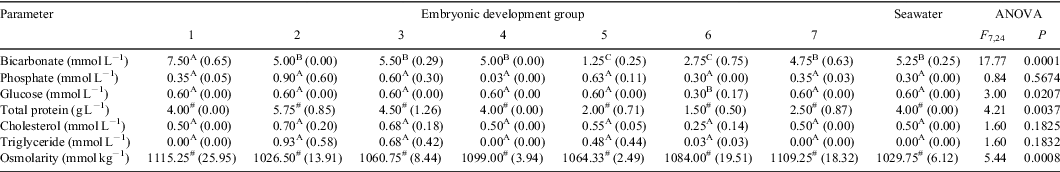
Results comparing the concentrations of the minor constituents in seawater and the uterine fluid from pregnant O. ornatus females at different stages of development are summarised in Table 3. Females from Group 1 had UF with a mean bicarbonate concentration that was significantly greater than that in seawater and in the UF of pregnant females from Groups 2 to 7. The mean bicarbonate concentrations of UF bathing embryos from Group 5 and 6 were significantly lower than in seawater and in the UF of pregnant females from the remaining groups. The mean glucose concentration in UF of pregnant females from Group 6 was significantly lower than that in seawater and in the UF bathing embryos from the remaining groups. The mean concentration of total protein and the osmolarity in the UF of pregnant females differed significantly during gestation. The differences observed and rank order of the means suggested natural fluctuations throughout gestation. The mean phosphate, cholesterol, and triglyceride concentrations in the UF of pregnant females from Groups 1 to 7 did not differ significantly from each other nor from seawater.
Discussion
Comparisons among species
Orectolobus ornatus embryos in the early stages of development had complex UF that was hyperosmotic to seawater owing to significantly elevated concentrations of urea, sodium and potassium. After 4 months of gestation, the UF resembled seawater in composition and the concentrations of all electrolytes examined did not differ significantly from those of seawater. The transition of the UF in pregnant O. ornatus females from complex to resembling seawater is similar to the situation previously documented for S. acanthias. As for O. ornatus, S. acanthias embryos are encased in a tertiary egg covering during the initial stages of development (Chatzispyrou and Megalofonou 2005; Hamlett et al. 2005b). The concentrations of the major constituents in the UF surrounding the early-term embryos (extracapsular fluid) of S. acanthias and O. ornatus differed from seawater (Evans et al. 1982; Kormanik 1993). After 4 and 6 months gestation in O. ornatus (Group 4, Table 1) and S. acanthias, respectively, all embryos had broken out of the tertiary egg coverings and the UF fluid surrounding these embryos (extracapsular fluid) resembled seawater in the concentrations of many constituents (Price and Daiber 1967; Evans et al. 1982; Kormanik 1992). For some species of Squatinidae, a seawater embryonic environment has also been suggested because of the exposure of the uterine environment to the exterior and the presence of leeches on developing embryos; however, the biochemical composition of the UF from pregnant angel sharks has never been examined (Sunye and Vooren 1997).
A uterine seawater environment for developing embryos of viviparous chondrichthyans, such as wobbegongs, is similar to the development of oviparous species where embryos surrounded by egg jelly and enclosed in a hard egg case are laid by the mother and attached to the substrate (Evans 1981; Koob and Straus 1998; Hamlett and Koob 1999). The concentrations of sodium, chloride and potassium in the intra-capsular fluid are less than in seawater while the urea concentrations are greater (Evans 1981). After approximately one-third of the incubation period, the egg jelly liquefies and, as a consequence, any areas in the egg case previously plugged with egg jelly open, permitting the influx of environmental seawater. The embryos then develop in seawater for the remainder of the incubation period (Evans 1981; Koob and Straus 1998; Hamlett and Koob 1999).
Uterine flushing
Encapsulated embryos of S. acanthias and the skate (Raja binoculata) have enzymes associated with the ornithine–urea cycle and are able to maintain urea and trimethylamine oxide at concentrations similar to adults (Needham and Needham 1930; Read 1968a, 1968b). In O. ornatus, there were fluctuations in the UF concentrations of urea, albeit small, at various stages throughout pregnancy, supporting the conclusion that urea is excreted in the urine of the developing embryos (represented by elevated urea levels) and that uterine flushing occurs (represented by decreased urea levels). The increased urea concentrations (23.63 mmol L–1) followed by low urea concentrations (3.63 mmol L–1) from Groups 3 to 7 suggest that uterine flushing occurs intermittently and not continuously. While previous authors, Burger and Loo (1959) experimentally observed ‘spontaneous intermittent emptying and filling of the uterus overnight’, there were no methods outlining these experiments and no results presented. During the course of the current study, several O. ornatus females, whose pregnancy was confirmed by ultrasound, were kept in captivity and potential uterine flushing was observed using underwater remote video cameras. Uterine flushing is an ideal behaviour to maintain a uterine seawater environment and hence one would expect that it occurs in species with this reproductive strategy to assist with respiratory exchanges and waste removal. It is unknown how uterine flushing is initiated and for S. acanthias, there is currently no mechanistic explanation. However, observations recorded during the current study may offer an explanation in O. ornatus (M. T. Ellis and N. M. Otway, unpubl. data).
Osmoregulatory implications
This raises many questions regarding the iono- and osmoregulatory capabilities of embryos. There have been no physiological studies on O. ornatus embryos, but the ability to survive in a seawater environment for the majority of gestation suggests that O. ornatus embryos can regulate their ion concentrations and osmolarity. S. acanthias embryos of 25–30-mm TL are capable of iono- and osmoregulation (Evans et al. 1982). A rectal gland precursor is present in S. acanthias embryos of 30-mm TL and by 100-mm TL, the embryonic rectal gland resembles the structure of the adult rectal gland in several ways (Chan and Phillips 1966). In histological studies of O. ornatus embryos (M. T. Ellis and N. M. Otway, unpubl. data), the rectal gland is present in embryos of 50-mm TL. There is also evidence that the kidneys of a 30-mm-TL S. acanthias embryo may be functional (Witschi 1956 in Price and Daiber 1967; Price and Daiber 1967) and that mitochondria-rich cells are present in the gill arches of embryos 17–20-mm TL (Kormanik et al. 1991).
For S. acanthias, the development of the organs involved in ion homeostasis suggest that embryonic osmoregulatory requirements are met early in gestation (Witschi 1956 in Price and Daiber 1967; Chan and Phillips 1966; Price and Daiber 1967; Kormanik et al. 1991) and that embryos could possibly survive in a natural seawater environment. However, early embryos are still very delicate and both species have an EYS until at least 200-mm TL (9 and 15 months gestation in O. ornatus and S. acanthias respectively). In O. ornatus and S. acanthias, the IYS first appears at a similar time as the transition to uterine seawater. It increases in size throughout gestation and remains as a source of nutrition following birth for up to 1 and 2 months in O. ornatus and S. acanthias, respectively (Jones and Geen 1977; Jones and Ugland 2001). The IYS is created from yolk sequestered in the EYS and, as a result, is connected to the EYS through the body wall and to the intestine so that nutrients can be absorbed (Hamlett and Koob 1999). In O. ornatus, once the EYS is fully depleted, a small scar remains for a short, as yet unknown, period of time. Rupture of the EYS in embryos of S. acanthias (Von Bonde 1945; Gilbert 1958; Jones and Price 1967) and O. ornatus (M. T. Ellis and N. M. Otway, unpubl. data) has resulted in death and in O. ornatus this occurred even when the EYS was reduced to a few millimetres in diameter or completely absent with only the small scar remaining. Hence, retention of developing embryos of O. ornatus provides protection for the EYS and IYS.
General implications
While protection of the yolk sacs is crucial to the survival of the embryos, the embryos of O. ornatus remain within the uterus for a further 1 to 2 months following the complete resorption of the external yolk sac and healing of the yolk sac scar. We have observed the death of O. ornatus embryos, with resorbed yolk sacs and no yolk scar, 2 weeks before birth. We believe this emphasises the importance of a minimum developmental period within the uterus and additional roles undertaken by the uterus, and hence the mother, during gestation. We have already discussed the role of physical protection and preliminary histological results for the uterus of O. ornatus (M. T. Ellis and N. M. Otway, unpubl. data) and previous histological research on the uterus of S. acanthias shows a reduction in the maternal–fetal blood barrier and increased surface area and vascularisation of the uterine wall, all of which are modifications to facilitate respiration (Jollie and Jollie 1967). However, we believe that the uterus may also play another important role in bacterial protection and providing immune responses, if required. While the structural ontogeny of the elasmobranch immune system has been studied (Hart et al. 1986; Lloyd-Evans 1993; Rumfelt et al. 2002; Luer et al. 2004), detailed information on the functional development is lacking (Rumfelt et al. 2002; Luer et al. 2004). These studies suggest that while the organs and cells required for an immune response develop early, these may not function until much later in development when a ‘mature immune-responsive environment’ is present (Rumfelt et al. 2002). As a result, the embryos would be unable to resist bacterial infection until such an environment develops. Currently, it is unknown when this occurs.
The reproductive strategy utilised by S. acanthias and O. ornatus appears to be the next evolutionary step from oviparous species, with the uterus replacing the egg case for accommodation and protection (Wourms 1977; Wourms and Lombardi 1992; Kormanik 1993). While this strategy has only been documented in these two species, it is likely that the embryos of other species within the same families will also spend the latter part of gestation in a seawater environment. The results of ongoing research with the spotted wobbegong (O. maculatus) show that this species also switches to a uterine seawater environment for the developing embryos (M. T. Ellis and N. M. Otway, unpubl. data). It would be of interest to examine the UF of several members of related families to determine if and in how many species this strategy occurs.
The O. ornatus individuals sampled in this study exhibited almost identical biological traits to those described in recent studies on this species (Huveneers 2007; Huveneers et al. 2007a, 2007b). The size composition and patterns of abundance of O. ornatus were similar to those documented by Huveneers (2007) and Huveneers et al. (2007a). Moreover, the timing of the reproductive cycle and embryonic development was entirely consistent with the results of Huveneers et al. (2007b). Therefore, we are confident that the biochemical analyses of the UF are representative of the reproductive cycle of the O. ornatus population found along the east coast of Australia. The results presented may assist in their conservation either in the wild, through appropriate management strategies, or in captivity, by providing better husbandry for pregnant animals and their offspring.
Acknowledgements
This research was done under NSW DPI research permit number P01/0059(A) and animal care and ethics approvals ACEC 99/14 and ACEC 03/04. Funding for this research was provided by Industry and Investment NSW. Our colleagues, particularly B. Louden, J. Gilligan, G. Housefield, M. Field, R. Carraro, C. Venables, A. Smissen, P. Meagher, S. Fenton, K. Bown and A. Ellis, are thanked for their various inputs. We are indebted to R. Brislane for catching the wobbegongs and to the staff at National Marine Science Centre for providing facilities to undertake this work. We are also grateful to the staff of IDEXX Laboratories, particularly J. Roberts and N. MacFarlane. Comments from R. Creese, M. Lowry, the editor, guest editor and anonymous referees greatly improved the manuscript.
References
Burger, J. W., and Loo, T. L. (1959). Bromination of phenol red by the dogfish, Squalus acanthias. Science 129, 778–779.| Bromination of phenol red by the dogfish, Squalus acanthias.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG1MXmvVOhtg%3D%3D&md5=3d21de883c9c843866ef0e9e278f9871CAS | 13635012PubMed |
Carraro, R., and Gladstone, W. (2006). Habitat preferences and site fidelity of the ornate wobbegong shark (Orectolobus ornatus) on rocky reefs of New South Wales. Pacific Science 60, 207–223.
| Habitat preferences and site fidelity of the ornate wobbegong shark (Orectolobus ornatus) on rocky reefs of New South Wales.Crossref | GoogleScholarGoogle Scholar |
Cavanagh, R., Kyne, P., Fowler, S. L., Musick, J. A., and Bennett, M. B. (2003). ‘The Conservation Status of Australasian Chondrichthyans. Report of the IUCN Shark Specialist Group Australia and Oceania regional Red List Workshop.’ (University of Queensland: Brisbane.)
Chan, D. K. O., and Phillips, J. G. (1966). The embryology of the rectal gland of the spiny dogfish Squalus acanthias L. Journal of Anatomy 100, 899–903.
| 1:STN:280:DyaF2s7ns1Wqsg%3D%3D&md5=5d0c0d41d5c179706351f7841700fef1CAS | 5969984PubMed |
Chatzispyrou, A., and Megalofonou, P. (2005). Sexual maturity, fecundity and embryonic development of the spiny dogfish, Squalus acanthias, in the eastern Mediterranean Sea. Journal of the Marine Biological Association of the United Kingdom 85, 1155–1161.
| Sexual maturity, fecundity and embryonic development of the spiny dogfish, Squalus acanthias, in the eastern Mediterranean Sea.Crossref | GoogleScholarGoogle Scholar |
Compagno, L. J. V. (2002). ‘FAO Species Catalogue. Sharks of the World. An Annotated and Illustrated Catalogue of Shark Species Known to Date. Volume 2. Bullhead, Mackerel and Carpet Sharks (Heterodontiformes, Lamniformes and Orectolobiformes).’ (FAO: Rome.)
Corrigan, S., Huveneers, C., Schwartz, T. S., Harcourt, R. G., and Beheregaray, L. B. (2008). Genetic and reproductive evidence for two species of ornate wobbegong shark Orectolobus spp. on the Australian east coast. Journal of Fish Biology 73, 1662–1675.
| Genetic and reproductive evidence for two species of ornate wobbegong shark Orectolobus spp. on the Australian east coast.Crossref | GoogleScholarGoogle Scholar |
Evans, D. H. (1981). The egg case of the oviparous elasmobranch, Raja erinacea, does osmoregulate. The Journal of Experimental Biology 92, 337–340.
Evans, D. H., Oikari, A., Kormanik, G. A., and Mansberger, L. (1982). Osmoregulation by the prenatal spiny dogfish, Squalus acanthias. The Journal of Experimental Biology 101, 295–305.
| 1:CAS:528:DyaL3sXhsFyitrg%3D&md5=0a21670ce0f0c4b4113b11227236753bCAS |
Files, T., and Lombardi, J. (1993). Free amino acids in the uterine fluids of four species of viviparous sharks (Squalus acanthias, Carcharhinus plumbeus, Mustelus canis, and Rhizoprionodon terraenovae). Comparative Biochemistry and Physiology 104B, 583–588.
| 1:CAS:528:DyaK3sXitlSnu7w%3D&md5=cb94f74c0a66d93d62b42ec217a5d107CAS |
Gilbert, P. W. (1958). The ability of yolk-sac stage dogfish pups to survive outside the uterus. Bulletin of the Mount Desert Island Biological Laboratory 1958, 68..
Gilmore, R. G., Jr, Putz, O., and Dodrill, J. W. (2005). Oophagy, intrauterine cannibalism and reproductive strategy in lamnoid sharks. In ‘Reproductive Biology and Phylogeny of Chondrichthyes: Sharks, Batoids and Chimaeras’. (Ed. W. C. Hamlett.) pp. 435–462. (Science Publishers: Enfield, NH.)
Hamlett, W. C., and Hysell, M. K. (1998). Uterine specializations in elasmobranchs. The Journal of Experimental Zoology 282, 438–459.
| Uterine specializations in elasmobranchs.Crossref | GoogleScholarGoogle Scholar |
Hamlett, W. C., and Koob, T. J. (1999) Female reproductive system. In ‘Sharks, Skates and Rays. The Biology of Elasmobranch Fishes’. (Ed. W. C. Hamlett.) pp. 398–443. (The Johns Hopkins University Press: Baltimore, MD.)
Hamlett, W. C., Eulitt, A. M., Jarrell, R. L., and Kelly, M. A. (1993). Uterogestation and placentation in elasmobranchs. The Journal of Experimental Zoology 266, 347–367.
| Uterogestation and placentation in elasmobranchs.Crossref | GoogleScholarGoogle Scholar |
Hamlett, W. C., Jones, C. J. P., and Paulesa, L. R. (2005a). Placentatrophy in sharks. In ‘Reproductive Biology and Phylogeny of Chondrichthyes: Sharks, Batoids and Chimaeras’. (Ed. W. C. Hamlett.) pp. 463–502. (Science Publishers: Enfield, NH.)
Hamlett, W. C., Kormanik, G. A., Storrie, M. T., Stevens, B., and Walker, T. I. (2005b). Chondrichthyan parity, lecithotrophy and matrotrophy. In ‘Reproductive Biology and Phylogeny of Chondrichthyes: Sharks, Batoids and Chimaeras’. (Ed. W. C. Hamlett.) pp. 395–434. (Science Publishers: Enfield, NH.)
Hart, S., Wrathmell, A. B., and Harris, J. E. (1986). Ontogeny of gut-associated lymphoid tissue (GALT) in the dogfish Scyliorhinus canicula L. Veterinary Immunology and Immunopathology 12, 107–116.
| Ontogeny of gut-associated lymphoid tissue (GALT) in the dogfish Scyliorhinus canicula L.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2s%2FitVKhsw%3D%3D&md5=dee4f983c9a820329ef1e2d5938e03f1CAS | 3765334PubMed |
Huveneers, C. (2006). Redescription of two species of wobbegongs (Chondrichthyes: Orectolobidae) with elevation of Orectolobus halei Whitley 1940 to species level. Zootaxa 2006, 29–51.
Huveneers, C. (2007). The ecology and biology of wobbegong sharks (Genus Orectolobus) in relation to the commercial fishery in New South Wales, Australia. PhD Thesis, Macquarie University, Sydney.
Huveneers, C., Otway, N. M., and Harcourt, R. G. (2007). Morphometric relationships and catch composition of wobbegong sharks (Chondrichthyes: Orectolobus) commercially fished in New South Wales, Australia. Proceedings of the Linnean Society of New South Wales 128, 243–249.
Huveneers, C., Walker, T. I., Otway, N. M., and Harcourt, R. G. (2007). Reproductive synchrony of three sympatric species of wobbegong shark (genus Orectolobus) in New South Wales, Australia: reproductive parameter estimates necessary for population modelling. Marine and Freshwater Research 58, 765–777.
| Reproductive synchrony of three sympatric species of wobbegong shark (genus Orectolobus) in New South Wales, Australia: reproductive parameter estimates necessary for population modelling.Crossref | GoogleScholarGoogle Scholar |
Jollie, W. P., and Jollie, L. G. (1967). Electron microscopic observations on accommodations to pregnancy in the uterus of the spiny dogfish, Squalus acanthias. Journal of Ultrastructure Research 20, 161–178.
| Electron microscopic observations on accommodations to pregnancy in the uterus of the spiny dogfish, Squalus acanthias.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaF1c7itlyhuw%3D%3D&md5=d5da857cef052345de5c803249071b9dCAS | 5625089PubMed |
Jones, B. C., and Geen, G. H. (1977). Reproduction and embryonic development of spiny dogfish (Squalus acanthias) in the strait of Georgia, British Columbia. Journal of the Fisheries Research Board of Canada 34, 1286–1292.
Jones, R. T., and Price, K. S. (1967). A method for maintaining spiny dogfish, Squalus acanthias, pups artificially. Copeia 1967, 471–472.
| A method for maintaining spiny dogfish, Squalus acanthias, pups artificially.Crossref | GoogleScholarGoogle Scholar |
Jones, T. S., and Ugland, K. I. (2001). Reproduction of female spiny dogfish, Squalus acanthias, in the Oslofjord. Fish Bulletin 99, 685–690.
Jones, K. B., Wine, R. N., Garrity, C. A., and Lombardi, J. (1990). Composition of uterine fluid in four species of viviparous sharks. American Zoologist 30, 83a..
Koob, T. J., and Straus, J. W. (1998). On the role of egg jelly in Raja erinacea egg capsule. Bulletin of the Mount Desert Island Biological Laboratory 37, 117–119.
Kormanik, G. A. (1988). Time course establishment of the uterine seawater conditions in late-term pregnant spiny dogfish (Squalus acanthias). The Journal of Experimental Biology 137, 443–456.
| 1:STN:280:DyaL1M%2FpsVajug%3D%3D&md5=e406a8c4b7aa7b880f4266e9a65a586dCAS | 3145320PubMed |
Kormanik, G. A. (1992). Ion and osmoregulation in prenatal elasmobranchs: evolutionary implications. American Zoologist 32, 294–302.
Kormanik, G. A. (1993). Ionic and osmotic environment of developing elasmobranch embryos. Environmental Biology of Fishes 38, 233–240.
| Ionic and osmotic environment of developing elasmobranch embryos.Crossref | GoogleScholarGoogle Scholar |
Kormanik, G. A., Lofton, A. J., and Vibbard, D. E. (1991). The ontogeny of mitochondria-rich cells in embryos of the spiny dogfish¸ Squalus acanthias. Bulletin of the Mount Desert Island Biological Laboratory 30, 4–7.
Last, P. R., and Stevens, J. D. (2009). ‘Sharks and Rays of Australia.’ (CSIRO Publishing: Melbourne.)
Lloyd-Evans, P. (1993). Development of the lymphomyeloid system in the dogfish, Scyliorhinus canicula. Developmental and Comparative Immunology 17, 501–514.
| Development of the lymphomyeloid system in the dogfish, Scyliorhinus canicula.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2c7jtFWnug%3D%3D&md5=8ff29c6ad5dc3c581e58252475307904CAS | 8299849PubMed |
Lombardi, J., Jones, K. B., Garrity, C. A., and Files, T. (1993). Chemical composition of uterine fluid in four species of viviparous sharks (Squalus acanthias, Carcharhinus plumbeus, Mustelus canis and Rhizoprionodon terraenovae). Comparative Biochemistry and Physiology 105A, 91–102.
| 1:CAS:528:DyaK3sXktVGhs78%3D&md5=c44eeeda828c78fac1c8515623f4685eCAS |
Luer, C. A., Walsh, C. J., and Bodine, A. B. (2004). The immune system of sharks, skates and rays. In ‘Biology of Sharks and Their Relatives’. (Eds J. C. Carrier, J. A. Musick and M. R. Heithaus.) pp. 369–395. (CRC Press: London.)
Needham, J., and Needham, D. M. (1930). Nitrogen excretion in selachian ontogeny. The Journal of Experimental Biology 7, 7–18.
| 1:CAS:528:DyaA3cXitVensA%3D%3D&md5=15bea361b31d44b696395c26ae065bc9CAS |
Price, K. S., and Daiber, F. C. (1967). Osmotic environments during fetal development of dogfish, Mustelus canis (Mitchill) and Squalus acanthias Linnaeus, and some comparisons with skates and rays. Physiological Zoology 40, 248–260.
| 1:CAS:528:DyaF1cXlslKruw%3D%3D&md5=a3ea296f1cb3ce98f8972ebf69007530CAS |
Read, L. J. (1968). Ornithine–urea cycle enzymes in early embryos of the dogfish Squalus suckleyi and the skate Raja binoculata. Comparative Biochemistry and Physiology 24, 669–674.
| Ornithine–urea cycle enzymes in early embryos of the dogfish Squalus suckleyi and the skate Raja binoculata.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1cXlslOisw%3D%3D&md5=ecb81db3bf4e048974209ab254ffb9dcCAS | 5651305PubMed |
Read, L. J. (1968). Urea and trimethylamine oxide levels in elasmobranch embryos. The Biological Bulletin 135, 537–547.
| Urea and trimethylamine oxide levels in elasmobranch embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1MXjsFCmsA%3D%3D&md5=1f11a9b040e2de584b1e1266d087a210CAS |
Rumfelt, L. L., McKinney, E. C., Taylor, E., and Flajnik, M. F. (2002). The development of primary and secondary lymphoid tissues in the nurse shark Ginglymostoma cirratum: B-cell zones precede dendritic immigration and T-cell zone formation during ontogeny of the spleen. Scandinavian Journal of Immunology 56, 130–148.
| The development of primary and secondary lymphoid tissues in the nurse shark Ginglymostoma cirratum: B-cell zones precede dendritic immigration and T-cell zone formation during ontogeny of the spleen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmvVarsrs%3D&md5=ffeab051698195b600e19d5e260b2641CAS | 12121433PubMed |
Scandol, J., Rowling, K., and Graham, K. (Eds) (2008). Status of fisheries resources in NSW 2006/07. NSW Department of Primary Industries, Sydney.
Snecedor, G. W., and Cochran, G. W. (1967). ‘Statistical Methods.’ (Iowa State University Press: Ames, IA.)
Sokal, R. R., and Rohlf, F. J. (1969). ‘Biometry.’ (WH Freeman: San Francisco, CA.)
Stevens, B. (2001). Uterine and oviducal mechanisms for gestation in the common saw shark, Pristiophorus cirratus. BSc (Hons) Thesis, University of Melbourne, Melbourne.
Sunye, P. S., and Vooren, C. M. (1997). On cloacal gestation in angel sharks from southern Brazil. Journal of Fish Biology 50, 86–94.
| On cloacal gestation in angel sharks from southern Brazil.Crossref | GoogleScholarGoogle Scholar |
Thorson, T. B., and Gerst, J. W. (1972). Comparison of some parameters of serum and uterine fluid of pregnant, viviparous sharks (Carcharhinus leucas) and serum of their near-term young. Comparative Biochemistry and Physiology 42A, 33–40.
Von Bonde, C. (1945). Stages in the development of picked or spiny dogfish, Squalus acanthias Linn. The Biological Bulletin 88, 220–232.
| Stages in the development of picked or spiny dogfish, Squalus acanthias Linn.Crossref | GoogleScholarGoogle Scholar |
Witschi, E. (1956). ‘Development of Vertebrates.’ (W. B. Saunders: Philadelphia, PA.)
Wourms, J. P. (1977). Reproduction and development in chondrichthyan fishes. American Zoologist 17, 379–410.
Wourms, J. P., and Lombardi, J. (1992). Reflections on the evolution of piscine viviparity. American Zoologist 32, 276–293.
Wourms, J. P., Groves, B. D., and Lombardi, J. (1988). The maternal–embryonic relationship in viviparous fishes. In ‘Fish Physiology. Volume 11. Part B, Viviparity and Posthatching Juveniles’. (Eds W. S. Hoar and D. J. Randall.) pp. 1–134. (Academic Press: New York, NY.)