Description of the mechanoreceptive lateral line and electroreceptive ampullary systems in the freshwater whipray, Himantura dalyensis
Teagan A. Marzullo A D , Barbara E. Wueringer A , Lyle Squire Jnr B and Shaun P. Collin A CA Sensory Neurobiology Group, School of Biomedical Sciences, The University of Queensland, Brisbane, Qld 4072, Australia.
B Cairns Marine, Stratford, Qld 4870, Australia.
C School of Animal Biology and the UWA Oceans Institute, The University of Western Australia, Crawley, WA 6009, Australia.
D Corresponding author. Email: teagan.marzullo@uqconnect.edu.au
Marine and Freshwater Research 62(6) 771-779 https://doi.org/10.1071/MF10156
Submitted: 18 June 2010 Accepted: 17 January 2011 Published: 24 June 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
Mechanoreceptive and electroreceptive anatomical specialisations in freshwater elasmobranch fishes are largely unknown. The freshwater whipray, Himantura dalyensis, is one of a few Australian elasmobranch species that occur in low salinity (oligohaline) environments. The distribution and morphology of the mechanoreceptive lateral line and the electroreceptive ampullae of Lorenzini were investigated by dissection and compared with previous studies on related species. The distribution of the pit organs resembles that of a marine ray, Dasyatis sabina, although their orientation differs. The lateral line canals of H. dalyensis are distributed similarly compared with two marine relatives, H. gerrardi and D. sabina. However, convolutions of the ventral canals and proliferations of the infraorbital canal are more extensive in H. dalyensis than H. gerrardi. The intricate nature of the ventral, non-pored canals suggests a mechanotactile function, as previously demonstrated in D. sabina. The ampullary system of H. dalyensis is not typical of an obligate freshwater elasmobranch (i.e. H. signifer), and its morphology and pore distribution resembles those of marine dasyatids. These results suggest that H. dalyensis is euryhaline, with sensory systems adapted similarly to those described in marine and estuarine species.
Additional keywords: ampullae of Lorenzini, Himantura polylepis, mechanosensory lateral line, pit organs.
Introduction
The mechanoreceptive lateral line and electroreceptive ampullae of Lorenzini are two well developed sensory systems in elasmobranch fishes. Despite these unique senses being extensively studied over recent years (i.e. Maruska and Tricas 1998; Kajiura 2003; Wueringer and Tibbetts 2008), there is still a paucity of research focussing on these senses in freshwater elasmobranchs. In addition, there is a severe lack of information on the recently described Himantura dalyensis, supposedly one of Australia’s few oligohaline elasmobranchs (Thorburn et al. 2004; Last et al. 2008).
The lateral line organ in fishes is used to detect near-field hydrodynamic movement across the body surface (Dijkgraaf 1963). Neuromasts, which form the sensory epithelium of the lateral line system, are composed of bundles of sensory hair cells and associated supportive cells enclosed in a gelatinous cupula (Maruska 2001). There are three types of mechanosensory lateral line organs: (1) pit organs, also called free neuromasts, which are located across the skin surface and exposed directly to the external environment; (2) canal neuromasts, which form a sub-epidermal network of pored or non-pored canals; and (3) vesicles of Savi, found in sub-epidermal pockets of some elasmobranchs (Nickel and Fuchs 1974; Coombs 1994; Maruska 2001).
In rays, pit organs are associated with grooves in the skin surface, whereas the pit organs of sharks are found between modified denticles (Maruska 2001; Peach 2003). Pit organs encode water velocity and in Heterodontus portusjacksoni they are used to orientate the body in water currents (Peach 2001). Pit organs are distributed asymmetrically across the body, and to date, there has been no indication of any left or right-handedness in pit organ counts (Peach 2003). Contrary to teleosts, elasmobranch pit organ counts remain constant throughout their life stages (Tester and Nelson 1967). Peach (2003) observed significant levels of intraspecific variation in several shark and ray species, with some individuals lacking entire groups of pit organs.
Canals of the lateral line form a subcutaneous network and are either non-pored or connected to the external environment by pores (Maruska 2001). They contain a nearly continuous sensory epithelium (Maruska 2001). Pored canals are used by elasmobranchs to detect the acceleration of external hydrodynamic flow near the skin, whereas non-pored canals are used to determine hydrodynamic velocity (Maruska and Tricas 2004). Non-pored canals are also highly responsive to direct skin displacement, and are thought to have a mechanotactile function on the ventral surface in some benthic rays (Maruska and Tricas 2004).
The ampullary system of elasmobranchs is used for passive electroreception (Kalmijn 1971). Ampullae of Lorenzini are located subcutaneously and are linked to the external environment via a canal that connects to a somatic pore. Ampullae are clustered and are often surrounded by capsules of connective tissue, thereby eliminating interference produced by the animal’s own electric field (Murray 1974; Wueringer and Tibbetts 2008). Three types of ampullary systems were described by Andres and von Düring (1988): (1) macro-ampullae, which possess elongated canals that terminate in ampullary bulbs, are the most common type of ampullae of Lorenzini, common in marine elasmobranchs, and can be clustered together; (2) micro-ampullae, which are highly reduced ampullae of Lorenzini (Raschi et al. 1997), found primarily in hexanchid sharks and holocephali; and (3) mini-ampullae, which are microscopic ampullae of Lorenzini of freshwater rays in the genus Potamotrygon (Szabo et al. 1972), are not located in clusters, most likely due to their minute size.
Differences in medium conductance, skin resistance and thus electrical loading between freshwater and marine elasmobranchs contribute to variations in the morphology of the ampullary system (Raschi et al. 1997). For example, the skin resistance in marine species is lower than in freshwater species, making them more prone to being invaded by large, external electrical fields (Szabo et al. 1972; Raschi et al. 1997; McGowan and Kajiura 2009). This results in a small voltage differential across the skin. Consequently, long canals aid in increasing sensitivity, thus enabling the sensory cells to adequately measure the potential difference between the pore and the internal ampullary ending (Raschi et al. 1997; McGowan and Kajiura 2009). In contrast, the comparatively high skin resistance of freshwater species results in weak voltage potentials invading the body tissue (Raschi et al. 1997). Given that the potential of the internal environment is nearly equal to that of the external environment, the drop across the skin is a large enough stimulus to be detected. This also eliminates the need for the suppression of self-generated background noise, minimising the need for ampullary aggregations (Raschi et al. 1997).
This study describes the morphology of the mechanosensory lateral line and electrosensory ampullae of Lorenzini in the freshwater whipray, Himantura dalyensis. H. dalyensis is one of five Australian elasmobranch species found in low salinity (oligohaline) environments (Thorburn et al. 2004). It has been mistaken for other species such as Dasyatis fluviorum (Merrick and Schmida 1984; Herbert and Peeters 1995), as well as the South-east Asian H. polylepis (Bleeker 1852), but was recently redescribed by Last and Manjaji-Matsumoto (2008).
The following hypotheses are tested: (1) the ventral lateral line canal system is highly complex, as demonstrated in marine dasyatids; (2) the pit organs of H. dalyensis do not differ greatly in distribution or abundance compared with marine dasyatids; (3) the electroreceptive ampullae of H. dalyensis are mini-ampullae, as found in obligate freshwater species, compared with the macro-ampullae of marine dasyatids; and (4) electroreceptive pore counts and pore distributions are comparable to other benthic dasyatids.
Materials and methods
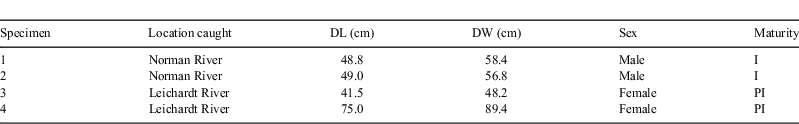
Four specimens of Himantura dalyensis were caught on line and hook by Cairns Marine (Queensland, Australia) in the tidal waters of the Norman and Leichardt Rivers in North Queensland. The animals died of unknown causes and were donated to us frozen. The following measurements were recorded (±1 mm): disk length, disk width, and sex (Table 1). Each specimen was thawed in 10% neutrally buffered formalin. After 24 h, specimens were placed into a second change of 10% neutrally buffered formalin. One week before dissections, specimens were transferred into 70% ethanol.
Sensory system dissections
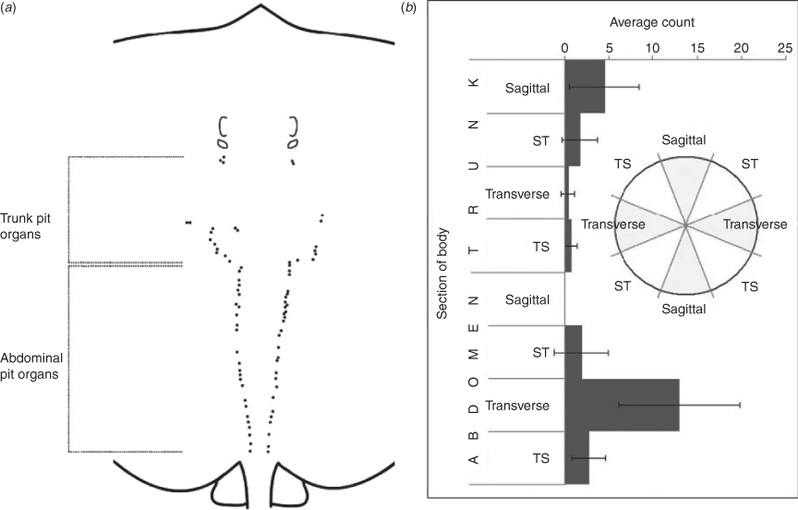
The general distribution of the lateral line and ampullary systems was assessed via dissections. Methylene blue (0.3%) was utilised as a stain following the method of Wueringer and Tibbetts (2008). Specimens were viewed under a Wild Heerbrugg M650 dissecting microscope (Heerbrugg, Switzerland). Structures associated with the lateral line sense (i.e. end pore, canal and tubules) were tracked and further identified via canal dissection. Injection of methylene blue was used to confirm the presence of pores along the lateral line canals. The angle of each pit organ was measured between the midline of the body and the slit in the papillum of a pit organ. The following pit organ orientations and their associated planes were defined (Fig. 1b inset): sagittal (0–22.4° and 157.5–180°); sagittal-transverse (ST; 22.50–67.4°); transverse (67.5–112.4°); and transverse-sagittal (TS; 112.50–157.49°). A map of the lateral line sensory structures was created. After applying Janus green (0.1%) to a dissected canal, the epithelium lining the bottom of the canal was dissected out and viewed under a Leitz Laborlux S fluorescent microscope (Wetzlar, Germany). Nerves perforating a lateral line canal indicated the presence of a neuromast.
Ampullary pores were counted and their general distribution assessed. To determine if pores belonged to the ampullary or lateral line system, the canal of each pore was stained with methylene blue and exposed via dissection. To alleviate double counting of pores in areas of high pore density (i.e. around the mouth), a grid system composed of nylon thread was superimposed over the skin segments when necessary. Once identified as an ampullary pore, the location of each pore was marked on a life-size schematic diagram. Electroreceptor canals were traced to their ampullae, which were found to be either free or in a cluster. The locations of free ampullae and clusters were recorded, as well as canal lengths for two specimens. Pore counts were recorded for all four specimens. Gross ampullae morphology was observed under a Leitz Laborlux S fluorescent microscope and images were taken using an Olympus DP70 camera (Sydney, Australia).
Terminology of the ampullary and lateral line structures follows Chu and Wen (1979) and Maruska and Tricas (1998). Pit organs were identified and compared with Peach (2003) and Maruska and Tricas (1998). Ampullary cluster innervation was identified and traced to the central nervous system. Terminology of the nervous system follows Ashley and Chiasson (1988).
Data analysis
Diagrams were drawn using Adobe Illustrator CS4 and Adobe Photoshop CS4 (www.adobe.com). The sensory epithelium was measured using ImageJ (http://rsb.mto.nih.gov/ij/). All measurements are presented as mean ± standard deviation. Data analyses were carried out using the statistical software ‘R’ (http://r-project.org). A one-way ANOVA was used to determine if there was a difference in pit organ number between specimens. A paired t-test was used to determine if there was any significant difference in pit organ counts between the left and right sides of the body. A three-factor ANOVA was used to test for differences in number of pit organs, orientation and location (on the body) across the four specimens. To investigate a potential relationship between ampullary canal length and specimen size, a regression was performed. A paired t-test was used to determine a potential difference between the total number of pores on the dorsal and ventral surface. To investigate if pore number was related to specimen size, a regression analysis was performed.
Results
The lateral line system
Himantura dalyensis has an extensive bilaterally symmetrical network of lateral line canals on both the dorsal and ventral surfaces. Canals are either pored or non-pored. Pored canals are connected to the skin surface via tubules, which can be branched. Canals that are situated within the fibrous layer may penetrate between muscles and connect with canals on the opposite surface. Both pored and non-pored canals contain a nearly continuous sensory epithelium, but neuromasts are absent in tubules.
On the dorsal surface, the hyomandibular canal extends posteriorly along the base of the pectoral fins (Fig. 2). The canal possesses lateral branches but not medial ones. Each branch ends in a set of highly ramified tubules, which terminate in multiple pores close to the margin of the pectoral fins. The most posterior part of the hyomandibular canal connects to the scapular canal. The anterior end of the hyomandibular canal extends lateral to the eye, where it connects to the infraorbital canal before dissociating from the dermis and continuing on the other surface. The anterior part of the supraorbital canal extends to the margin of the rostral apex. Posteriorly, this canal extends medial to the eye in the posterior direction. Here, this canal forms a junction with the infraorbital and postorbital canals. The part of the supraorbital canal that extends between the orbits has several highly ramified tubules extending from this section. From the junction of the infraorbital and postorbital canal, the infraorbital canal extends between the eye and spiracle, where it loses its association with the fibrous layer of the dermis. From here, the infraorbital canal continues anteriorly towards the rostral apex, where it dissociates from the dermis and breaks through to the ventral surface. Along its length on the dorsal surface, the infraorbital canal forms straight branches. From the junction of the supraorbital, infraorbital and postorbital canals, the postorbital canal extends posteriorly past the endolymphatic pores. There, the supratemporal canal traverses bilaterally over the neurocranium behind the endolymphatic pores. At the connection between those two canals, the posterior canal extends posteriorly. The posterior canal continues along the length of the midline and possesses highly ramified tubules. Above the pectoral girdle, the scapular canals branch off the posterior canal and extend laterally. The posterior canal continues further down the length of the tail, possessing multiple branched tubules. As the canal continues caudally along the abdomen, its tubules gradually become shorter and more branched.
As predicted, the ventral lateral line canal system is highly complex (Fig. 2). On the ventral surface, the anterior part of the hyomandibular canal closely follows the outer margin of the pectoral fin, while the posterior part of the canal is located more medially. The hyomandibular canal is slightly convoluted and the anterior third of these convolutions contain non-branched tubules that extend laterally to the pectoral fin margin, where each then terminates in a single pore. The posterior part of the hyomandibular canal almost reaches the pelvis, from where it continues anteriorly and lateral to the gill slits until it connects to the nasal canal, lateral to the nasal flap. The presence of tubules at the base of this canal varies between specimens. While the tubules of the hyomandibular canal are associated with pores, no pores are present along the main canal. On the ventral surface, the infraorbital canal extends from its connection with the dorsal infraorbital canal posteriorly and forms several non-pored infraorbital canal loops. Lateral of the junction between the hyomandibular and nasal canals, the infraorbital canal connects to the supraorbital canal. The exact location of this junction varies between specimens. From there, the supraorbital canal draws anteriorly, lateral to the nasal flap. Mid-rostrum, the supraorbital canal turns posteriorly, and continues along the nasal flap, from where it turns and continues anteriorly along the rostrum. The nasal canal extends along the lower margin of the nasal cavity and passes deeply through the inter-nasal cartilage, connecting to the pre-nasal canal on the nasal flap. There, it penetrates deep into the musculature, before continuing anteriorly to the exterior margin of the rostrum’s apex. The mandibular canal is located on the mandible, where it transverses across the midline. It is not connected to any other lateral line canal and lacks both pores and tubules.
Himantura dalyensis possesses numerous pit organs restricted to the dorsal surface. Each pit organ appears as a swollen open groove in the skin, with dark pigmentation lining the area of the raised papillum around the slit. There is no difference in pit organ number between specimens (n = 4, one-way ANOVA, F3,60 = 1.29, P = 0.29, data were log-transformed to fit the assumptions of an ANOVA) or between the left (25.3 ± 9.4) and right (24.8 ± 9.2) dorsal surface (n = 4, paired t-test, t3 = 0.24, P = 0.83). Pit organs are located lateral of the vertebrae of H. dalyensis (Fig. 1a) and have a large degree of asymmetry. Along the abdomen and tail pit, organs form a nearly continuous line. The orientation of the pit organs appears to be correlated with the section of body on which they are situated (n = 4, three-way ANOVA, F3,56 = 21.92, P < 0.001) (Fig. 1b). Along the trunk, the pit organs are generally orientated along the sagittal axis. Pit organs situated across the abdomen and tail are generally orientated transversely.
The ampullary system
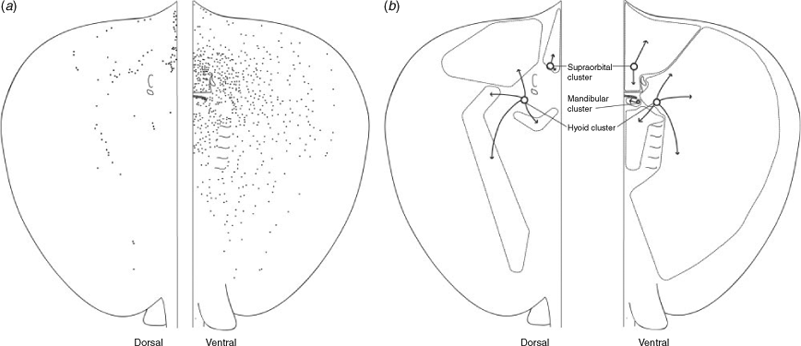
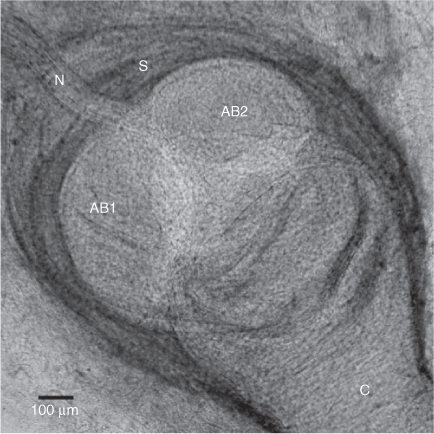
Himantura dalyensis has both free ampullae and clustered ampullae. Free ampullae differ from those found within clusters, as each one is surrounded by a collagen sheath (Fig. 3). Free ampullae are confined to the anterior half of the ventral surface of the pectoral fins where an average of 53 ± 13.37 free ampullae are located. Clustered ampullae are ampullae grouped together in three bilaterally symmetrical clusters situated below the skin or embedded in muscle tissue (Fig. 4b). These clusters have three distinct locations: hyoid, supraorbital and mandibular. The hyoid ampullary cluster is located lateral to the mandible, where it is embedded between the muscles. This is the largest cluster and its canals draw to pores on both surfaces. It is innervated by the hyomandibular branch of the anterior lateral line nerve. The mandibular cluster is located ventrally, between the mandible and dermis, and is innervated by the hyomandibular branch of the anterior lateral line nerve. This cluster is the smallest and only contains ampullae associated with the ventral surface. The supraorbital cluster is located anterior to the nares and orbits. It is innervated by the superficial ophthalmic branch of the anterior lateral line nerve. All ampullae are macroscopic macro-ampullae of marine elasmobranchs. The number of ampullary bulbs ranges from two to four.
|
Ventrally, the pores of the ampullae of Lorenzini are grouped in three pore fields (Fig. 4b) and scattered over the entire body surface, with the exception of the posterior portion of the pectoral fins, and caudal to the cloacal opening. The largest pore field belongs to the hyoid cluster, which possesses canals drawing in all directions. Canals of this cluster that extend towards the midline overlap across the midline in some specimens. The ventral mandibular cluster has the smallest pore field and its canals radiate in all directions. The second largest pore field is associated with the supraorbital cluster. Its canals radiate anteriorly, extending to the apex of the rostrum, and posteriorly across the nasal flap.
Dorsally, the pores of the ampullae of Lorenzini are not as scattered as on the ventral surface (Fig. 4a). Pores are situated towards the inner margin of the pectoral fins down to the base of the tail and originate from two clusters. Two pore fields were distinguished: supraorbital and hyoid pore fields. Hyoid canals draw in four main directions: the anterior canals stretch from the base of the rostral apex to the ampullary cluster; the outer canals extend laterally across the trunk, terminating in pores along the pectoral fin; the posterior canals stretch caudally from the cluster, with the most posterior canal terminating into pores located above the base of the pectoral fins; and the inner canals extend from the cluster towards the midline, located anterior to the endolymphatic pores to caudal to the endolymphatic pores. The majority of the supraorbital canals extend anteriorly to the apex of the rostrum; however, some canals from this cluster terminate in pores located in the skin posterior to the supraorbital cluster.
Ventrally, the pore densities of the ampullae of Lorenzini appear to decrease from the mouth towards the margin of the body (Fig. 4a). As predicted, the dorsal surface contains fewer ampullary pores than the ventral surface (dorsal: 136.0 ± 55.4, ventral: 1120.8 ± 279.4, Table 2; n = 4, paired t-test t3 = –8.02, P < 0.001). There is no correlation between specimen size and total pore count (linear regression analysis, r2 = 0.51, P = 0.29).
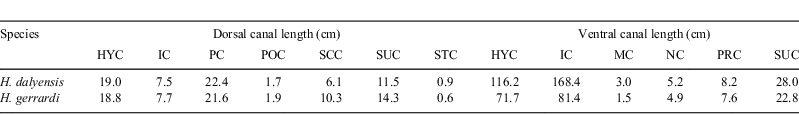
The canals of H. dalyensis can be up to tens of centimetres in length and, like in other elasmobranchs, are filled with gel. Only 13% of the variance of the length of the ampullary canals was explained by the size of the specimen (r2 = 0.13). Therefore, body size does not significantly depend on specimen size, with larger specimens having longer canals than smaller specimens (Table 2, linear regression analysis, P < 0.0012, data were log-transformed). In both specimens, the canals of the hyoid cluster are the longest (Table 2).
Discussion
The lateral line system
The morphology and distribution of the mechanosensory lateral line canals are comparable among closely related taxa, but variations within the system determine the properties of biologically important stimuli detected by an organism (Maruska 2001). This is confirmed in the present study, as the lateral line canal distribution of Himantura dalyensis is similar to Dasyatis sabina and Himantura gerrardi, but minor morphological differences exist.
The pored dorsal lateral line canal system of H. dalyensis is similar to other dasyatids (Chu and Wen 1979). The complex organisation of the highly ramified tubules may increase the sampling area on the skin, thereby increasing its sensitivity to hydrodynamic flow across this plane (Wueringer and Tibbetts 2008). This may enhance the abilities of H. dalyensis to detect prey and conspecifics and also to navigate in turbid river systems. Ramification of tubules appears unrelated to maximum species size, as H. gerrardi is a considerably smaller species than H. dalyensis and exhibits the same degree of ramification.
The results support the hypothesis of a highly complex ventral lateral line that is more distinct than in other dasyatid species. The extensive convolutions of the ventral non-pored infraorbital canal appear to be a unique phylogenetic trait of the genus Himantura (i.e. H. uarnak and H. gerrardi; Chu and Wen 1979). As they are not present in any Dasyatis species studied to date (Maruska and Tricas 1998), the convolutions may be related to the ecological niche and feeding biology of H. dalyensis. The extensive system of convolutions along the canal greatly increases the canal length and consequently allows for greater prey localisation capacity. Furthermore, the function of non-pored canals can be explained 2-fold: first, they cannot be disrupted by sand particles that may potentially block the pores (Maruska and Tricas 2004); and second, the mechanotactile hypothesis suggests that the non-pored canals along the ventral surface act as tactile receptors that facilitate the localisation of benthic prey (Maruska and Tricas 1998; Maruska and Tricas 2004). In D. sabina, the sensitivity of non-pored canals to direct skin depression velocity is higher than to hydrodynamic stimulation near the skin surface (Maruska and Tricas 2004).
The lateral line sensory epithelium is nearly continuous in elasmobranchs (Maruska 2001), which is confirmed in H. dalyensis. No neuromasts were recorded in tubules. The ventral infraorbital lateral line canal is lined by neuromasts, which has great implications for its mechanotactile function. In a hypothetical situation where H. gerrardi and H. dalyensis are the same size, the ventral infraorbital and hyomandibular canals in H. dalyensis are much longer, and cover a greater surface area (Table 3), therefore providing H. dalyensis with more efficient localisation during benthic prey capture.
Pit organs in H. dalyensis are distributed in a pattern similar to those of D. sabina, but their orientation differs. In D. sabina, the axis of the trunk and tail neuromasts ranges between 90 and 135 degrees, while pit organs around the spiracle are found along a slightly larger axis (100–160 degrees) (Maruska and Tricas 1998). The pit organs of H. dalyensis on the trunk are mainly orientated on the sagittal axis, while the transverse axis dominates along the tail. Sagittally oriented pit organs, such as on the trunk in H. dalyensis, are mostly stimulated by water flow along the same axis that corresponds to a forward swimming motion. Slits orientated along a transverse axis, enhance water flow parallel to the slit, particularly when the ray is inactive (Maruska 2001). Simultaneously, this orientation may reduce stimulation of the neuromast receptors during forward movement (Maruska 2001). Both species are regularly found in tropical brackish and fresh waters, and therefore differences in pit organ orientation may be related to behavioural differences rather than ecological ones. However, as almost nothing is known about the behaviour of H. dalyensis, no definite conclusion can be drawn for now. As in other elasmobranch species, the distribution of the pit organs on the body is asymmetrical (Peach 2003), but there is no difference in the number of pit organs between the left and right dorsal surface.
The ampullary system
The electrosense is an ancient sensory modality, with its importance being emphasised by its independent evolution in several taxa. The significance of the electrosensory system is demonstrated by the increased ventral pore count, which is eight times greater than the number of pores located on the dorsal surface of Himantura dalyensis. This provides further evidence for the importance of close-range senses for a benthic diet, which predominantly consists of benthic crustaceans and benthic teleost fishes (Last and Stevens 2009). Similar to the findings of Raschi (1986) on Raja species, the highest density of ampullary pores in H. dalyensis is located around the mouth, providing the necessary resolution to direct a strike after prey localisation (Raschi 1986). Urobatis halleri, Pteroplatytrygon violacea and Myliobatis californica all exhibit a similar pattern, despite the varying ventral pore counts and different ecological niches (Table 4; Jordan 2008), suggesting that this high pore density is not restricted to benthic species. The ventral ampullary counts of H. dalyensis are similar to U. halleri (Urolophidae; Jordan 2008). Pore counts of H. dalyensis are much greater than those of P. violacea, as well as the rhinobatids Glaucostegus typus and Aptychotrema rostrata (Jordan 2008; Wueringer and Tibbetts 2008). The difference in total ventral pore counts between H. dalyensis and P. violacea may reflect their dissimilar ecological niches. Pteroplatytrygon violacea is a pelagic predator (Jordan 2008) and may rely more on other senses, such as vision and olfaction (Raschi 1986). Dorsal counts of H. dalyensis and P. violacea are highly similar, which may reflect phylogenetic conservation of this characteristic. Pore counts have no correlation with size and are fixed throughout development. The differences in the size of all species studied (Table 4) should be considered in these comparisons, as almost all specimens sampled were immature, and although no ontogenetic shifts have been demonstrated, they may still be an influencing factor on the traits listed.

|
Contrary to our hypothesis, H. dalyensis, unlike its freshwater relative H. signifer or any other obligate freshwater ray, has macro-ampullae, a feature consistent with marine and euryhaline elasmobranchs (Raschi et al. 1997; Tam et al. 2003; e.g. Carcharhinus leucas Whitehead 2002). In a comparison of the ampullary morphology between two freshwater rays, D. garouaensis and H. signifer, Raschi et al. (1997) suggested that electrical problems caused by canals that are not fully adapted to the freshwater environment (i.e. longer with less insulated canal walls) may be counteracted by increased luminal gel production. At present, the results suggest that the ampullae of H. dalyensis most likely function best in higher salinities, unlike the environment where H. dalyensis has been reported (salinity 0.5–5 g kg–1; Thorburn et al. 2004). Therefore, further investigation is needed identify how this species copes with a freshwater medium.
The presence of free ampullae also appears to be another unique feature of H. dalyensis. Free ampullae have not been described in any other dasyatid species, and may represent a gradual adaptation to low salinity. The production of ampullary gel may be energy consuming, and may cause a reduction in the canal length when long ampullary canals are not needed. The reduction of canal length in free ampullae may move the ampullary bulb out of an ampullary capsule, which may increase background noise (Kalmijn 1974). The decrease in sensitivity may not matter because the electrical signals detected in fresh water are much stronger than those detected in the marine environment (Bodznick and Montgomery 2005).
Himantura dalyensis, like D. sabina (Puzdrowski and Leonard 1993), D. zugei, H. gerrardi and H. uarnak (Chu and Wen 1979), possesses three groups of ampullary clusters. Innervations of the clusters are similar to D. sabina (Puzdrowski and Leonard 1993). Externally, the ampullary bulb morphology corresponds to the ampulla morphology of H. gerrardi and D. zugei by having a single canal that opens into three to four alveoli, which surround a central lumen. The ampullary canal distributions across both surfaces are similar in H. dalyensis and H. gerrardi (Chu and Wen 1979). Like H. dalyensis, most other stingray species have only three clusters, all of which are typically located around the nasal, hyoid and mandibular regions (Chu and Wen 1979; Puzdrowski and Leonard 1993). The number of clusters and locations, however, does vary between families (e.g. rajids have four clusters (Raschi 1986), whereas myliobatids can have between one and four clusters (Chu and Wen 1979)).
Here, we show that Himantura dalyensis has very well developed hydrodynamic and electroreceptive sensory systems. The development of the ventral non-pored lateral line canal system is most likely related to its feeding ecology and has a mechanotactile function. The proliferation of the ventral infraorbital canal loops appears to be restricted to the Himantura genus, but this needs to be confirmed by further research on other dasyatids, both Himantura species and Dasyatis species. While the pit organs of H. dalyensis are distributed similarly to other dasyatids, studies regarding the orientation of these components in other species are lacking. The high ventral pore density of the ampullary system provides evidence of its significance as a close-range sense for a benthic diet. Unexpectedly, the species possesses macro-ampullae, a feature common to marine and euryhaline elasmobranchs, which leads us to hypothesise that H. dalyensis could be euryhaline, as opposed to oligohaline. How these macro-ampullae function efficiently in a freshwater medium requires further investigation. Behavioural and physiological experiments, as well as information on movement patterns and the life history of H. dalyensis, would clarify its exact relationship with low salinity environments.
Acknowledgements
The authors thank Cairns Marine for the generous donation of these rare specimens. Thanks to Geoff Oke for sharing insights into species biology. Specimen donation and dissections were done according to the following permits: UQ Animal Ethics Committee VTHRC/835/07, VTHRC/717/06, VTHRC/974/08/(NF), DPI&F 87591. This work was funded by an ARC Linkage Grant to SPC LP0989676. We would like to thank the anonymous referees for their valuable comments.
References
Andres, K. H., and von Düring, M. (1988). Comparative anatomy of vertebrate electroreceptors. Progress in Brain Research 74, 113–131.| Comparative anatomy of vertebrate electroreceptors.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1M%2Fkt1ylsQ%3D%3D&md5=14e5484c396378b150d48c5ba8db647eCAS | 3055044PubMed |
Ashley, L. M., and Chiasson, R. B. (1988). ‘Laboratory Anatomy of the Shark.’ (Brown and Benchmark: New York, NY.)
Bleeker, P. (1852). Bijdrage tot de kennis der Plagiostomen van den Indischen Archipel. Bataviaasch Genootschap der Kunsten en Wetenschappen 24, 1–92.
Bodznick, D., and Montgomery, J. C. (2005). The physiology of low frequency electrosensory systems. In ‘Electroreception’. (Eds T. H. Bullock, C. D. Hopkins, A. N. Popper and R. R. Fay.) pp. 132–153. (Springer: New York, NY.)
Chu, Y. T., and Wen, M. C. (1979). ‘A Study of the Lateral – Line Canal System and that of the Lorenzini Ampullae and Tubules of Elasmobranchiate Fishes of China: Monograph of Fishes of China.’ (Academic Press: Shanghai.)
Coombs, S. (1994). Nearfield detection of dipole sources by the goldfish (Carassius auratus) and the mottled sculpin (Cottus bairdi). The Journal of Experimental Biology 190, 109–129.
| 1:STN:280:DyaK2M%2FltlKgsw%3D%3D&md5=3eb06481455588f189f3262ddbad7de3CAS | 7964388PubMed |
Dijkgraaf, S. (1963). Functioning and significance of lateral-line organs. Biological Reviews of the Cambridge Philosophical Society 38, 51–105.
| Functioning and significance of lateral-line organs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaF383hvFynuw%3D%3D&md5=749fa1dc4e7d6b2a084c3b66fd34f96eCAS | 14027866PubMed |
Herbert, B., and Peeters, J. (1995). ‘Freshwater Fishes of Far North Queensland.’ (Department of Primary Industries: Brisbane.)
Jordan, L. K. (2008). Comparative morphology of stingray lateral line canal and electrosensory systems. Journal of Morphology 269, 1325–1339.
| Comparative morphology of stingray lateral line canal and electrosensory systems.Crossref | GoogleScholarGoogle Scholar | 18655157PubMed |
Kajiura, S. M. (2003). Electroreception in neonatal bonnethead sharks, Sphyrna tiburo. Marine Biology 143, 603–611.
| Electroreception in neonatal bonnethead sharks, Sphyrna tiburo.Crossref | GoogleScholarGoogle Scholar |
Kalmijn, A. J. (1971). Electric sense of sharks and rays. The Journal of Experimental Biology 55, 371–383.
| 1:STN:280:DyaE38%2FislGrug%3D%3D&md5=0500a54cee429a86ffada2d24e974d61CAS | 5114029PubMed |
Kalmijn, A. J. (1974). The detection of electric fields from inanimate and animate sources other than electric organs. In ‘Electroreceptors and Other Specialized Receptors in Lower Vertebrates’. (Eds T. H. Bullock, A. Fessard and R. H. Hartline.) pp. 147–200. (Springer-Verlag: Berlin.)
Last, P. R., and Manjaji-Matsumoto, B. M. (2008). Himantura dalyensis sp. nov., a new estuarine whipray (Myliobatoidei: Dasyatidae) from northern Australia. In ‘Descriptions of New Australian Chondrichthyans’. (Eds P. R. Last, W. T. White and J. J. Pogonoski.) pp. 283–291. (CSIRO Marine and Atmospheric Research: Canberra.)
Last, P. R., and Stevens, J. D. (2009). ‘Sharks and Rays of Australia.’ 2nd edn. (CSIRO Publishing: Melbourne.)
Last, P. R., White, W. T., and Pogonoski, J. J. (2008). ‘Descriptions of New Australia Chondrichthyans.’ (CSIRO Marine and Atmospheric Research: Hobart.)
Martin, L. K., and Cailliet, G. M. (1988). Aspects of the reproduction of the bat ray, Myliobatis californica, in Central California. Copeia 3, 754–762.
| Aspects of the reproduction of the bat ray, Myliobatis californica, in Central California.Crossref | GoogleScholarGoogle Scholar |
Maruska, K. P. (2001). Morphology of the mechanosensory lateral line system in elasmobranch fishes: ecological and behavioral considerations. Environmental Biology of Fishes 60, 47–75.
| Morphology of the mechanosensory lateral line system in elasmobranch fishes: ecological and behavioral considerations.Crossref | GoogleScholarGoogle Scholar |
Maruska, K. P., and Tricas, T. C. (1998). Morphology of the mechanosensory lateral line system in the Atlantic stingray, Dasyatis sabina: the mechanotactile hypothesis. Journal of Morphology 238, 1–22.
| Morphology of the mechanosensory lateral line system in the Atlantic stingray, Dasyatis sabina: the mechanotactile hypothesis.Crossref | GoogleScholarGoogle Scholar |
Maruska, K. R., and Tricas, T. C. (2004). Test of the mechanotactile hypothesis: neuromast morphology and response dynamics of mechanosensory lateral line primary afferents in the stingray. The Journal of Experimental Biology 207, 3463–3476.
| Test of the mechanotactile hypothesis: neuromast morphology and response dynamics of mechanosensory lateral line primary afferents in the stingray.Crossref | GoogleScholarGoogle Scholar | 15339942PubMed |
McGowan, D. W., and Kajiura, S. M. (2009). Electroreception in the euryhaline stingray, Dasyatis sabina. The Journal of Experimental Biology 212, 1544–1552.
| Electroreception in the euryhaline stingray, Dasyatis sabina.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MzhvVahtw%3D%3D&md5=23dbb435a93562112e622108f70d17ecCAS | 19411548PubMed |
Merrick, J. R., and Schmida, G. E. (1984). ‘Australian Freshwater Fishes: Biology and Management.’ (J. R. Merrick: Sydney.)
Murray, R. W. (1974). The ampullae of Lorenzini. In ‘Electroreceptors and Other Specialized Receptors in Lower Vertebrates’. (Eds T. H. Bullock, A. Fessard and R. H. Hartline.) pp. 125–146. (Springer-Verlag: Berlin.)
Nickel, E., and Fuchs, S. (1974). Organization and ultrastructure of mechanoreceptors (Savi vesicles) in the elasmobranch Torpedo. Journal of Neurocytology 3, 161–177.
| Organization and ultrastructure of mechanoreceptors (Savi vesicles) in the elasmobranch Torpedo.Crossref | GoogleScholarGoogle Scholar |
Peach, M. B. (2001). The dorso-lateral pit organs of the Port Jackson shark contribute sensory information for rheotaxis. Journal of Fish Biology 59, 696–704.
| The dorso-lateral pit organs of the Port Jackson shark contribute sensory information for rheotaxis.Crossref | GoogleScholarGoogle Scholar |
Peach, M. B. (2003). Inter- and intraspecific variation in the distribution and number of pit organs (free neuromasts) of sharks and rays. Journal of Morphology 256, 89–102.
| Inter- and intraspecific variation in the distribution and number of pit organs (free neuromasts) of sharks and rays.Crossref | GoogleScholarGoogle Scholar | 12616576PubMed |
Puzdrowski, R. L., and Leonard, R. B. (1993). The octavolateral systems in the stingray, Dasyatis sabina. 1. Primary projections of the octaval and lateral line nerves. The Journal of Comparative Neurology 332, 21–37.
| The octavolateral systems in the stingray, Dasyatis sabina. 1. Primary projections of the octaval and lateral line nerves.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3s3psVSltg%3D%3D&md5=745d3977b795fb47073b05f3c45fb37aCAS | 8514920PubMed |
Raschi, W. (1986). A morphological analysis of the ampullae of Lorenzini in selected skates (Pisces, Rajoidei). Journal of Morphology 189, 225–247.
| A morphological analysis of the ampullae of Lorenzini in selected skates (Pisces, Rajoidei).Crossref | GoogleScholarGoogle Scholar |
Raschi, W., Keithan, E. D., and Rhee, W. C. H. (1997). Anatomy of the ampullary electroreceptor in the freshwater stingray, Himantura signifer. Copeia 1, 101–107.
| Anatomy of the ampullary electroreceptor in the freshwater stingray, Himantura signifer.Crossref | GoogleScholarGoogle Scholar |
Szabo, T., Enger, P. S., Kalmijn, A. J., and Bullock, T. H. (1972). Microampullary organs and a submandibular sense organ in a fresh water ray, Potamotrygon. Journal of Comparative Physiology 79, 15–27.
| Microampullary organs and a submandibular sense organ in a fresh water ray, Potamotrygon.Crossref | GoogleScholarGoogle Scholar |
Tam, W. L., Wong, W. P., Loong, A. M., Hiong, K. C., Chew, S. F., et al. (2003). The osmotic response of the Asian freshwater stingray (Himantura signifer) to increased salinity: a comparison with marine (Taeniura lymma) and Amazonian freshwater (Potamotrygon motoro) stingrays. The Journal of Experimental Biology 206, 2931–2940.
| The osmotic response of the Asian freshwater stingray (Himantura signifer) to increased salinity: a comparison with marine (Taeniura lymma) and Amazonian freshwater (Potamotrygon motoro) stingrays.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXnslKnsbc%3D&md5=3e156207c55f5b292e2d0771424399c5CAS | 12878662PubMed |
Tester, A. L., and Nelson, G. J. (1967). Free neuromasts (pit organs) in sharks. In ‘Sharks, Skates and Rays’. (Eds P. W. Gilbert, R. F. Matthewson and D. P. Rall.) pp. 503–531. (Johns Hopkins Press: Baltimore, MD.)
Thorburn, D. C., Morgan, D. L., Rowland, A. J., and Gill, H. (2004). Elasmobranchs of the Fitzroy River, Western Australia. Report to the Natural Heritage Trust, Department of Agriculture, Fisheries and Forestry, Canberra.
Whitehead, D. L. (2002). Ampullary organs and electroreception in freshwater Carcharhinus leucas. Journal of Physiology, Paris 96, 391–395.
| Ampullary organs and electroreception in freshwater Carcharhinus leucas.Crossref | GoogleScholarGoogle Scholar | 14692487PubMed |
Wueringer, B. E., and Tibbetts, I. R. (2008). Comparison of the lateral line and ampullary systems of two species of shovelnose ray. Reviews in Fish Biology and Fisheries 18, 47–64.
| Comparison of the lateral line and ampullary systems of two species of shovelnose ray.Crossref | GoogleScholarGoogle Scholar |