Estimating sea cucumber abundance and exploitation rates using removal methods
James Prescott A E , Camille Vogel B , Kenneth Pollock B , Samuel Hyson A , Dian Oktaviani C and Anthony Sisco Panggabean DA Australian Fisheries Management Authority, Canberra, ACT 2610, Australia.
B Centre for Fish and Fisheries Research, Murdoch University, Murdoch, WA 6150, Australia.
C Research Center for Fisheries Management and Conservation, Agency for Marine and Fisheries Research, Ministry of Marine Affairs and Fisheries, Jl. Pasir Putih I, Ancol Timur, 14430 Jakarta, Indonesia.
D Research Center for Capture Fisheries, Agency for Marine and Fisheries Research, Ministry of Marine Affairs and Fisheries, Muara Baru Street, Samudera Fisheries Port, North Jakarta, Indonesia.
E Corresponding author. Email: jim.prescott@afma.gov.au
Marine and Freshwater Research 64(7) 599-608 https://doi.org/10.1071/MF12081
Submitted: 21 March 2012 Accepted: 4 March 2013 Published: 8 May 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
Removal methods were used to estimate key fishery parameters, abundance and exploitation rate for five species of tropical sea cucumbers harvested by Indonesian fishers at Scott Reef, north-western Australia. Detailed catch records were kept by the traditional fishers over a period of 58 days as needed for this method, whereas effort was estimated from aerial surveillance. Concurrently, ~1007 artificial sea cucumber surrogates, were distributed and rewards were paid for recovered surrogates. Both datasets were analysed using the Huggins closed-population procedure in program MARK to obtain maximum-likelihood estimates. This procedure allowed inclusion of effort and tide covariates and an initial search phase followed by an exploitation phase. We accounted for extreme over-dispersion which is a common problem in fishery removal data. Our results strongly suggested that some surrogates became unavailable to the fishers. However, results from both datasets demonstrated strong evidence of extreme rates of exploitation on the shallow, drying reef-top habitat. Closed-removal or depletion methods are shown to be a viable method to estimate abundance and exploitation rate for sea cucumbers harvested with intense fishing pressure during a short fishing season.
Additional keywords: invertebrate, management, program MARK, traditional fishery.
Introduction
Worldwide, most tropical sea cucumber fisheries have come under intense fishing pressure that reflects growing demand for the products in China and growing human populations in regions where the species are exploited, often being accompanied by poverty and limited livelihood opportunities. Many fisheries have been over-exploited and many of the most valuable species have been replaced in the catch by comparatively low-value species (Anderson et al. 2011). Contributing to this situation is the fact that sea cucumbers pose particular stock-assessment challenges because of their lack of hard parts for ageing, difficulty in marking them (Uthicke et al. 2004) and plastic size and shape (Perry et al. 1999). Compounding the management dilemma, knowledge of the ecological role of sea cucumbers is poor and makes management targets even less certain in terms of maintaining healthy and fully functional ecosystems.
Some sea cucumber fisheries have persisted, usually undocumented but generally thought to be subject to ‘boom and bust’ periods (Anderson et al. 2011), for centuries. Macassan fishers made voyages to the mainland Australia to harvest sea cucumbers, probably because of overfishing in their home waters (Schwerdtner and Ferse 2010), for about two centuries until Australia curtailed their fishing in 1906 (Russell 2004; Schwerdtner and Ferse 2010). However, other fishers from Indonesia continued to harvest sea cucumbers on some Australian offshore reefs, including Scott Reef (~14.1°S, 121.85°E) Fig. 1., for another 100 years (Fox and Sen 2002), making it the oldest extant commercial fishing activity in Australia. Since 1974, the fishery at Scott Reef has operated under a memorandum of understanding between Australia and Indonesia that requires the fishers to access the area in non-motorised vessels and to employ only non-motorised equipment. Despite these intuitively restrictive measures, hundreds of fishers have seasonally harvested sea cucumbers from Scott Reef during the ‘sailing season’ that has its peak activity in August and September. It is likely that the intensity of fishing activity has increased in recent decades, coinciding with the boom that has been a general global trend (Friedman et al. 2011).
|
Belt-transect surveys revealed a low population abundance for all exploited sea cucumber species (Skewes et al. 1999a; J. Prescott, unpubl. data) relative to other reefs subject to less fishing ~200 km north of Scott Reef, for example in the Ashmore Reef Marine National Reserve and in the Torres Strait. However, the precision of the estimates was poor, which would preclude detection of any trends in abundance. The survey results also did not reflect the known species diversity nor the apparent abundance observed among the catches highlighting the difficulty of acquiring reliable sea cucumber stock-abundance data through visual census methods. Although the fishery is of minor economic importance on the scale of Australian and most Indonesian fisheries, it is a key livelihood activity for the participants who come from some of the poorest regions of Indonesia. Because of the fishers’ economic dependence on the fishery, Indonesia has insisted a sound scientific basis for any management measures which would, inevitably, have near-term impacts on the livelihoods of their traditional fishers. Consequently, an alternative to the approach of a belt-transect survey assessment, that would provide more reliable and relevant information, was sought.
Estimating exploitation rates, which have a direct relation to fishing effort, was considered to be an attractive alternative – if exploitation could be estimated. Exploitation rates for sea cucumbers are not typically reported in the literature because they cannot be estimated by many of the methods ordinarily used for this purpose. However, Dissanayake and Stefansson (2012) recently estimated exploitation rates for sea cucumbers in two Sri Lankan fisheries and Trianni (2002) estimated abundance and exploitation rates for two species harvested in the Northern Marianas Islands by using depletion methods over a 6-month fishing season. At Scott Reef, where it was expected that stocks would be depleted during the relatively short but intensive season, a depletion or removal approach was considered to be feasible. It would also be expected that the effects of fishing would overwhelm the effects of the small levels of natural mortality and recruitment because of the short season. We also knew that the fishers had participated willingly in a pilot catch-recording program in 2008, were able to identify most species reliably and, although access to education had been limited for most, were able to count and do arithmetic reliably. Consequently, our study set out to estimate the exploitation rates and population (stock) sizes of as many of the exploited sea cucumber species as possible by using removal methods. Although fishers caught more species than presented here, the species-specific data analysed accounted for most of the catch.
To provide supplementary information about exploitation rates and gain additional insights into the factors moderating exploitation, artificial (cast concrete) sea cucumber surrogates were distributed on the reef in three plots.
Materials and methods
Study site
Scott Reef is Australia’s largest individual offshore reef and comprises two separate carbonate (predominantly scleractinian coral) reefs (Heap and Harris 2008) at the outer margins of the continental shelf, ~400 km north of Broome, Western Australia. The northern and southern reefs are separated by a narrow 400-m deep channel that effectively isolates sea cucumbers once they have settled from the larval stages and taken up their benthic lives. The semi-circular south reef is ~28 km wide and 18 km long from north to south, with a large lagoon open to the north. The eastern, southern and western sides of the reef are bounded seaward by a well developed reef crest that becomes emergent at spring low tides. Lagoon-ward of the reef crest, there is a shallow reef top that in places is interspersed with enclosed or semi-enclosed shallow lagoons. In total, the shallow reef-top habitat of the southern reef is more than 9000 ha. Although fishing takes place on both northern and southern reefs and fishers move between them during their fishing trips, lasting up to 60 days, our study was confined to southern Scott Reef for logistical reasons.
Scott Reef, like the rest of the region, has diurnal tides with a relatively large tidal range which, at spring tides during the present study, was as much as 3.7 m. Tidal cycles play an important role in fishing operations that are most intensive when the spring tides leave much of the shallow reef exposed at low tide.
Fishery study
Fishing crews, which on average included eight men, were recruited as they were encountered and every crew present at southern Scott Reef during the initial recruitment phase agreed to participate in the study. Each crew was issued with pictorial catch data sheets. Next to an image and the local common name for each species were spaces to record the corresponding number of that species caught during each fishing episode. Fishers were able to readily identify a list of species with which they were familiar. However, taxonomic uncertainty is a problem for fishers and scientists alike with regard to some sea cucumber species. The most problematic species was Holothuria whitmaei, known as koro batu to the traditional fishermen, which as a juvenile was very frequently confused with the congener H. fuscogilva because the small juveniles of H. whitmaei share a colour pattern similar to the colour pattern of the latter species (Uthicke et al. 2010). To resolve this issue, we combined the recorded catches of H. whitmaei with H. fuscogilva and analysed them as the single species H. whitmaei.
Normally, there were two episodes per day corresponding with low tides. Consequently, one episode took place mostly during the day and the other at night. Catch numbers were summed across episodes and vessels, to produce a daily recorded catch.
Because most crews had never been involved in any project like this previously, vessels were revisited as quickly and as frequently as possible, to ensure that they understood the catch-recording instructions and were recording catches correctly. On most visits, we attempted to count the numbers of each species that had been captured during the most recent fishing episode and compared our counts with the fisher’s records. As part of their training to collect accurate catch data, when differences were detected, the fisher’s records were adjusted and the changes explained to them.
Fishers present on the reef originated from three ‘islands’ and had their own approaches to fishing. Two groups, Rotinese and Alorese, generally reef-gleaned at low tide or dived on the shallow reef edges, while the third group, Madurese, dived in deeper waters. Importantly, for the aerial censuses of fishing effort, the latter group used distinctly different vessels that were readily separable from those of the former. Low-altitude passes were made by Australian Coastwatch aircraft on most days of the study and counts of vessels were reported to and later retrieved from Australian Border Protection Command. Because we were interested only in the reef-top fishery, we excluded the catch and effort data of the group that dived in deeper waters. After excluding the catch by the Madurese, the recorded catch of sea cucumbers of the five species analysed was 63 448.
Some vessel-type and reef misclassifications were evident on some days in the aerial surveillance data, which led to vessel numbers being over- or under-reported at a day scale. To reduce the daily variation in the recorded effort that these errors caused, the number of sightings by a vessel type was regressed on the date during four periods picked by eye. The daily vessel numbers derived by this approach were broadly consistent with our observations during the period we spent at the reef. This process smoothed the over and under counts but left the effort time trends intact.
Recorded daily catches were scaled up by the ratio of vessels recording their catch to the total number of vessels belonging to the two groups of reef-top fishers present at the southern Scott Reef each day. A maximum of 23 vessels recorded their catch at the southern Scott Reef on any one day and there were as many as 31 vessels present on the reef. Overall, the catch-weighted mean number of vessels at the reef recording their catches was 64% of the total, but this varied from day to day.
Statistical approach
The sea cucumber-fishery removal (depletion) data were analysed following the approach developed in detail by Gould and Pollock (1997). This used a multinomial model and maximum-likelihood estimation (Seber 1982; Gould and Pollock 1997; Williams et al. 2002) to analyse the fishery removal data. This is in contrast to earlier linear-regression methods (Ricker 1958; Seber 1982). A closed model was used because natural mortality would be low in the relatively short fishing season and because it enabled the inclusion of effort and tidal effects as covariates, by using a logistic link function (Gould and Pollock 1997). We used the MARK software designed to obtain maximum-likelihood estimates for capture–recapture and removal data (White and Burnham 1999; Cooch and White 2010). The Huggins closed-captures procedure, which is specifically designed for situations where there are covariates such as fishing effort and tidal deviations (Pollock 2002; White 2002), was used. The covariates were standardised as advocated by Cooch and White (2010).
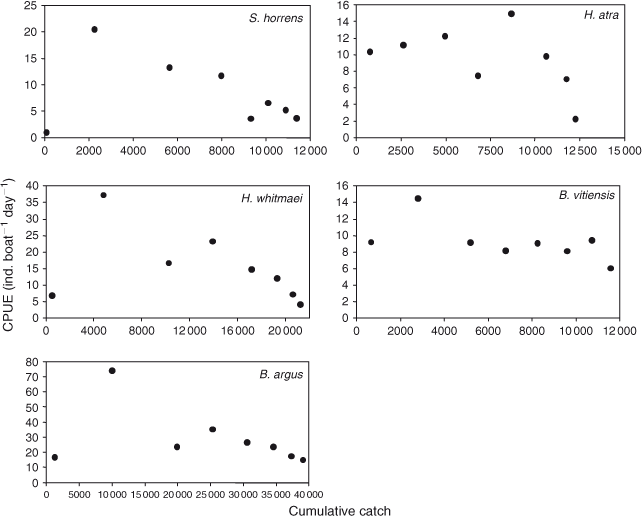
Models were compared and the standard errors were adjusted using the quasi-likelihood adjusted Akaike’s information criterion (QAIC) (Burnham and Anderson 2002). Unlike the Akaike’s information criterion (AIC), QAIC is adjusted for over-dispersion resulting from lack of independence in the removal of the animals. A large amount of over-dispersion was expected in our removal data, as also found by Gould and Pollock (1997) in their examples using commercial fisheries. Over-dispersion is unlikely to cause bias in estimates, but the standard errors need to be adjusted and are scaled up by the square root of the estimated over-dispersion parameter (McCullagh and Nelder 1989; Gould and Pollock 1997, Williams et al. 2002). The over-dispersion parameter was estimated on the basis of a chi-squared goodness-of-fit test and the equation, as follows:
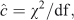
where χ2 is the value of the statistic and df is the degrees of freedom.
Catch and effort data were pooled into eight 7-day periods for analysis to attempt to reduce measurement errors in the catch and increase the precision of the estimates. Models were based on a biological understanding of the fishery. A search phase was expected at the start of the harvest; it translated in modelling a separate first-removal parameter, independent from the later expected depletion pattern. The possibility that this removal parameter was a function of effort and tidal deviations was considered. Depletion after the initial search phase was first considered as a constant rate of removal through time (constant parameter). However, we also considered an alternative that allowed the possibility of a linear increase in catchability over time. That is, the efficiency in fishing increases throughout the study period and therefore we fitted models where removal (after the initial search phase) was composed of a constant base plus a linear increment through time. We also allowed for the possible inclusion of the covariate effort and tide deviations in this component as well. Finally, we estimated the exploitation rate for each species (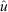 ) by dividing the total number of animals removed by the estimated population size.
) by dividing the total number of animals removed by the estimated population size.
Surrogates study
Three different-sized concrete surrogate sea cucumbers were cast in sand moulds. The sizes produced were small (20 × 40 × 100 mm, ~80 cm3), medium (30 × 60 × 150 mm, ~270 cm3) and large (37 × 75 × 187 mm, ~527 cm3). These corresponded to the size range of many of the living sea cucumbers that were being harvested from the reef top. They were produced as natural concrete colour or black by adding oxide. Each surrogate was numbered using a DYMO punch (Esselte Dymo Australia) label secured with epoxy putty. Most, but not all, numbers were unique because a small number of duplicate numbers were applied and some numbers could be read two ways, e.g. 998 could be 866. However most of these problems were resolved using the size, colour and the presence or absence of mark applied to 5% of the surrogates denoting a higher reward.
Three study areas on the southern Scott Reef were selected on the basis of reef-habitat characterisations (Skewes et al. 1999b). Fifty-six randomly selected positions were allocated to each 14.4-ha area (Fig. 2). The majority of these positions were dry at spring low tides. Time and depth (measured by a digital depth sounder) were recorded at each position and this was later used to calculate the depth (or emergent height) at 10-min intervals throughout the period of the study, on the basis of tide predictions exported from Seafarer Tides (Australian Hydrographic Office, Wollongong). The habitat at each site was also characterised qualitatively in terms of the percentage sand, rubble, boulders, hard substrate (pavement like) or coral present.
Concrete surrogates were distributed at the 166 positions in one event when high tide permitted navigation over the reef. At each site, one of each colour and size was distributed by tossing them haphazardly around the boat, over a radius of about 5 m. The number, size and colour were recorded against the site number as they were distributed. In total, the1007 surrogates were approximately equal in apparent density to two conspicuous shallow-reef species of sea cucumbers (Holothuria edulis and H. atra) observed during a belt-transect survey on Scott Reef in 2008 (J. Prescott, unpubl. data) to try and simulate realistic densities as they were known before the present study.
Fishers were encouraged to return the surrogates to the research party and rewards for their recovery were paid commensurate with the size of the surrogate and average prices paid to the fishers for their catches of similar-sized living sea cucumbers.
Statistical approach
Artificial sea cucumber surrogates in shallow or drying areas of the reef were expected to be removed more rapidly than those in deeper areas and were therefore grouped accordingly (possible because depth of each was known) into ‘shallow’ and ‘overall’, because data were too few to compare shallow and ‘deep’. Daily removal data were pooled into six 4-day periods, similar to the fishery data, to reduce the variance among daily removals. The statistical modelling approach to the surrogate data was identical to that used for the fishery data, using Program MARK and the Huggins closed-captures procedure.
The population estimates (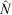 ) obtained for the surrogates using MARK were used to evaluate the percentage negative relative bias arising from the method, because the true population sizes (N) were known in this case. We calculated the percentage negative relative bias (RB) using the equation
) obtained for the surrogates using MARK were used to evaluate the percentage negative relative bias arising from the method, because the true population sizes (N) were known in this case. We calculated the percentage negative relative bias (RB) using the equation
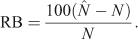
Results
Fishery study
Catch per unit effort (CPUE) data for five species, for which population-size estimates are presented later, are plotted against their respective cumulative catches in Fig. 3. Data were pooled into the same eight 7-day periods as used in the MARK analyses. The CPUE data varied in their steepness and uniformity of the decline among the species. CPUE generally fell following the second 7-day period, except for H. atra, where it did not fall until after the fifth period. A common feature was lower CPUE during the first 7-day period.
|
The best-fitting models with associated QAIC values and model weights are presented in Table 1. Over-dispersion parameter estimates (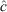 ) (measuring the goodness-of-fit of the model to the data) ranged from 4.08 (Holothuria whitmaei) to 186.5 (Holothuria atra). Estimates of population size (
) (measuring the goodness-of-fit of the model to the data) ranged from 4.08 (Holothuria whitmaei) to 186.5 (Holothuria atra). Estimates of population size (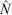 ) were obtained from the MARK software for all species analysed, except Bohadschia koellikeri. This species did not indicate any depletion, even though we estimated that ~74 000 were harvested. Consequently, the method failed and cannot be used to find an estimate of the population size for this species (Seber 1982).
) were obtained from the MARK software for all species analysed, except Bohadschia koellikeri. This species did not indicate any depletion, even though we estimated that ~74 000 were harvested. Consequently, the method failed and cannot be used to find an estimate of the population size for this species (Seber 1982).
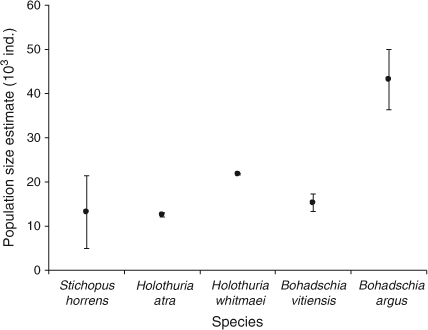
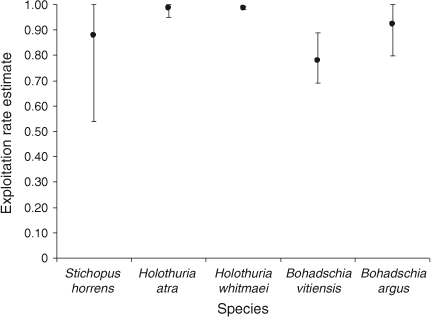
Population-size estimates are presented in Fig. 4. The confidence intervals (95%) around the estimated population numbers varied widely among species. Bohadschia argus was the most abundant species for which we obtained a population estimate, with an estimated size of 43 204 with 95% confidence limits of ±6785 and a high estimate of over-dispersion parameter of 110.89. QAICc remained high for all fits of the best models; QAICc weight varied between 35% (B. vitiensis) and 73% (S. horrens). Tide was an important covariate for all five species, whereas Effort was important for two of the five species. All species fitted a model where the removal probability in the first period behaved differently from that in the remaining seven periods. Removal probabilities increased with an additional linear trend for three species (H. atra, H. whitmaei and B. argus) following the first removal period. Estimates of the exploitation rate are presented in Fig. 5. Densities on the shallow reef, corresponding to the population estimates, for the five species were all low, ranging from ~1.4 to 4.8 individuals ha–1.
|
|
Surrogate study
We found that the surrogates were removed at very high rates. In addition, estimates of the population size obtained from MARK were equal to the number of surrogates recovered for all sizes and colour classes. This means that the model is estimating that all surrogates available for removal (i.e. that were visible) were recovered. We consider the issue of realism of the surrogates as representing live sea cucumbers and the potential for them to become unavailable for detection in the Discussion below.
The population estimates obtained for the surrogates were used to evaluate the systematic underestimates of 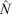 (negative bias of
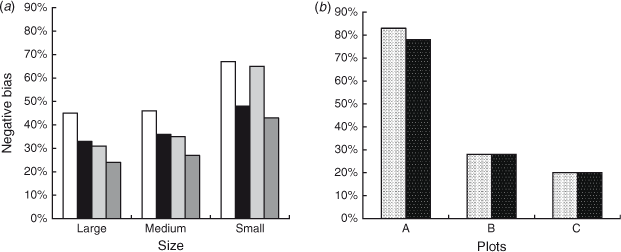
(negative bias of 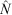 ) arising from the removal method, because the true number of surrogates was known in this case. We found that the removal approach had very high negative biases for all sizes and both colours of surrogates, and that there was also great variation between the different sizes and colours of surrogates (Fig. 6). Black surrogates had a lower percentage relative bias than did the grey ones, and large and medium surrogates had a lower one than did small surrogates. Further the percentage relative bias of surrogates in shallow water (where the reef is emergent at low tide) was lower than that in parts of the reef that remained submerged at low tide (Fig. 6), except for the small ones where the percentage relative bias was similar for shallow and deeper water. Estimates for small surrogates were much more negatively biased (that is much less well recovered) than were those for the other two classes independently of colour. (The average percentage negative relative bias was as follows: large = 33.25%, medium = 36%, small = 55.75%). Reef position also had a very large influence on the negative bias. The reef top (Plot B) and the reef crest (Plot C) grouped together, with values being much less biased than on the lagoon edge (Plot A) (the average values were 28%, 20% and 80.5%, respectively). Negative bias was lesser on the reef crest (20%) than in any of the size classes and colours previously tested. The lagoon edge had the deepest zones on average (mean: 1.57 m; range: –0.76 m (emergent) to 14.72 m).
) arising from the removal method, because the true number of surrogates was known in this case. We found that the removal approach had very high negative biases for all sizes and both colours of surrogates, and that there was also great variation between the different sizes and colours of surrogates (Fig. 6). Black surrogates had a lower percentage relative bias than did the grey ones, and large and medium surrogates had a lower one than did small surrogates. Further the percentage relative bias of surrogates in shallow water (where the reef is emergent at low tide) was lower than that in parts of the reef that remained submerged at low tide (Fig. 6), except for the small ones where the percentage relative bias was similar for shallow and deeper water. Estimates for small surrogates were much more negatively biased (that is much less well recovered) than were those for the other two classes independently of colour. (The average percentage negative relative bias was as follows: large = 33.25%, medium = 36%, small = 55.75%). Reef position also had a very large influence on the negative bias. The reef top (Plot B) and the reef crest (Plot C) grouped together, with values being much less biased than on the lagoon edge (Plot A) (the average values were 28%, 20% and 80.5%, respectively). Negative bias was lesser on the reef crest (20%) than in any of the size classes and colours previously tested. The lagoon edge had the deepest zones on average (mean: 1.57 m; range: –0.76 m (emergent) to 14.72 m).
|
Discussion
Few studies of sea cucumbers have successfully estimated abundance, catchability and exploitation rate using depletion methods, despite the fact that most sea cucumber fisheries are likely to adhere to the assumptions of the methods (Trianni 2002). The fishery at Scott Reef presented a relatively uncommon opportunity to use these methods. Because of close cooperation with a relatively high proportion of the fishers present on the reef, it was possible to collect a large dataset. The reefs are discrete, so the study area is easily defined and understood by the fishers who had to record the reef fished correctly for each fishing episode. Most importantly, the fishery exhibits a very intense period of activity, with as many as several hundred fishers actively harvesting sea cucumbers day and night and, as our study indicates, having a substantial impact on the abundance of many species.
Implicit in the use of removal methods is the assumption that the removal process (fishing) proceeds in a non-systematic fashion, i.e. that the process does not begin in one part of the exploited area and move systematically through the area (that is, all components of the fishery are subject to repeated removals over time). In the present study, fishing vessels were anchored along the protected lagoon edges of the southern reef, from the north-eastern tip to the north-western tip and points in between. Although some areas provided better shelter than others and attracted more fishers, the fishers worked away from the lagoon anchorages in their small dugout ‘sampans’. Vessels regularly repositioned from the western part of the reef to the eastern part and vice versa. On the reef flats, fishers regularly left coral boulders overturned and so left clues that the spot had been previously fished. However, at night, when a majority of the catch was taken, it would not be possible to see these clues easily and, in any case, it is clear that many sea cucumbers are not available at any particular time; thus, revisiting an area can be expected to produce additional catch. Furthermore, we would not have expected to see such high exploitation rates if fishers were able to systematically remove the sea cucumbers. Thus, we are comfortable that this assumption was not violated.
Uthicke et al. (2010) noted that a single, presumed juvenile, specimen of H. whitmaei collected in Palau matched the gene sequences of adults of this species and confirmed that juveniles had a black and white colour pattern that has caused confusion between H. whitmaei and H. fuscogilva. Although we initially analysed data separately for these two species, we concluded that it was necessary to combine both sets of data and analyse them as H. whitmaei. Even though this may have led to the inclusion of a small number of true H. fuscogilva in the analysis, we consider this to have had a negligible effect because the numbers observed in the catches from the reef-top habitat were extremely small. In fact, the population estimate for H. whitmaei increased only by 4%, being a number approximately equal to our initial population estimate of H. fuscogilva.
Estimates of over-dispersion (Table 1) were similar in magnitude to those obtained by Gould and Pollock (1997) for commercial-fishery removal data. Standard errors were scaled up by factors ranging from 1.56 to 10.82. The best model fits to the data were the ones that allowed the removal probability to vary independently during the first 7-day period. The graphs of CPUE (Fig. 3) illustrate clearly why this was the case. We attributed this to the fact that the study started when the boats first arrived at the reef for the season and took some time to refine their search areas. That is, we view the removal process as involving a complex spatial process where there is an initial search phase, followed by a local exploitation phase of dense local areas. Despite the inherent attractiveness of this hypothesis, we cannot rule out other factors we may be unaware of. Estimating this removal probability separately improved the model fits but had the effect of reducing our estimates of stock size and, consequently, increasing the estimates of exploitation rate. Noting that our estimates of exploitation rate are, with one exception, nearly 80% or higher, we consider them to be the upper limits of exploitation rate.
Interestingly, the most numerous species observed and recorded in the catch, Bohadschia koellikeri, was the only species for which we tried but could not estimate population size. We note that this species did not appear on any of the 334 belt transects conducted during two surveys in 1998 and 2008 on the southern Scott Reef or on the 224 belt transects conducted on the northern reef during the same surveys. It is also interesting that during 2011, this species was unseen on 16 belt transects conducted in a 20-ha plot on the shallow reef top of the southern Scott Reef that covered 4.8% of the plot area. Nevertheless, over a period of eight nights immediately following the transect survey, fishers removed a total of 347 individuals of this species from the same 20-ha plot during a closely supervised fish-down experiment (J. Prescott, unpubl. data). The evidence strongly suggests that this species is highly cryptic. As a consequence, we believe removal probabilities may be too low for this species to use removal methods successfully. Several other species were not analysed because they were comparatively uncommon in the catch, although this is unlikely to be due solely to low availability.
The estimated exploitation rates were unquestionably extreme. They exceeded some of the few other exploitation-rate estimates from a tropical fishery (<0.5) in Sri Lanka (Dissanayake and Steffansson 2012), but were similar to rates estimated in the Marianas Islands (0.78–0.90) by Trianni (2002). The extreme rates are strongly supported by the rates of removal we observed with the concrete surrogates. Despite negative biases, which are discussed below, an overall 64% of the surrogates were removed over a period of 24 days. We note that near the reef crest (Plot C), where the substrate was predominantly hard, the removals were more than 80%, despite this part of the reef being most distant from the anchorage used by the traditional fishers and subjected to strong wave action much of the time. We can surmise from the strong negative biases among both colours and sizes, that the surrogates became less available as a result of burial in sand and becoming encrusted with coralline algae (observed on the surrogates recovered from approximately midway through the experiment).
The surrogates provided a unique opportunity to learn about the effects of colour and size on removal probability. In most analyses of such effects, the analyst attempts to estimate the effects on the basis of a population of unknown sizes and colours. In contrast, in our analysis, we knew the number of each type (size and colour) and we readily determined that the removal probability was higher for black than for natural concrete colour and, regardless of colour, removal probability increased between small and medium sizes but was constant between medium and large sizes.
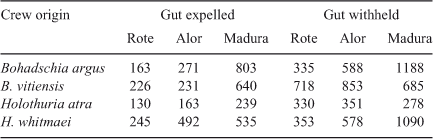
By inference from the lower removal probabilities for the small surrogates, we can draw the conclusion that the removal probabilities, exploitation rates and our population estimates are for sea cucumbers that are vulnerable to the fishing process because of the sizes the individuals have attained and their distribution with respect to the fishing effort. Clearly, some of the sea cucumber population is invulnerable or unavailable to be removed during part or all of the fishing season because of their small sizes, depth distribution and cryptic behaviour. This helps explain the incongruence of the very high exploitation rates we estimated for the shallow reef-top populations and the very persistence of sea cucumber populations on Scott Reef. It is also noteworthy that sea cucumbers harvested in deeper water were heavier on average than were their reef-top counterparts, as shown in Table 2 and, being larger, it is likely that a greater proportion of these were breeding individuals, although we have no maturity data to confirm this.
|
By modifying the surrogates so that they are not so readily encrusted or buried, they could be a very useful way to derive a reference level of exploitation and perhaps an approximation of exploitation rates for sedentary species that do not move, such as e.g. giant clams, or exhibit strongly cryptic behaviour. However, even with the current limitations, the surrogates were useful to identify where and when individuals fished and they neatly separated, spatially, the activities of the fishers who fished on the reef-top habitats and those who fished in deeper water. They can also be used to test other hypotheses, e.g. that sheltering under coral boulders increases human predation, i.e. the target’s removal probability because the search image is amplified by the size of the boulder.
Our study did not cover the whole fishing season; however, the exploitation rates calculated by species would be expected to be representative of the whole season, unless there was some significant change in the effectiveness of the fishing between the period we estimated over-exploitation and the unstudied part of the season. Regardless, our study covered the most intensive part of the fishing season and accounted for 69% of the total fishing effort for the year.
Similar to Gould and Pollock (1997), we found that the degree of over-dispersion in fishery removal data is vastly greater than that in capture–recapture data, which are also modelled with multinomial models. We suspect that most of this over-dispersion is real and a result of a lack of independence between the removal of different animals. Any discovery of a patch of the sea cucumbers would presumably lead to a series of dependent captures, whereas the multinomial model assumes this process to be independent. However, we acknowledge that because we are measuring a general lack of fit using a chi-squared statistic, there could be other assumption violations, such as measurement errors in the effort or catch, that may have caused some bias.
We believe that removal methods should be used more extensively as conditions allow, for the assessment of sea cucumber fisheries. The approach can overcome problems of low availability that compromises the utility of many visual-transect surveys, particularly diurnal surveys of species that are predominately nocturnal. The products of the approach, population size, catch and exploitation rate are directly relevant to management and can be used to advocate more effectively than density estimates alone, for management controls. Furthermore, the removals approach requires engagement with the fishers and it can have a strong educational role which is one of the foundations of effective fishery management. Similarly, the use of surrogates is a powerful way to demonstrate how rapidly anything on the surface of a substrate can be depleted.
Acknowledgements
Our study relied entirely on the participation of traditional fishers to keep detailed records of their catches and to report the concrete surrogates they removed. We could not have hoped or asked for greater cooperation from the participants. The study was made possible by the Australian Border Protection Command making the vessel ASHMORE GUARDIAN available to us and to the Australian Customs and Guardline crews who supported our daily field activities enthusiastically. AFMA officers Nikki Alber and Andrew Browne capably assisted with the collection of the data in the field. Andrew Heap, Geoscience Australia, provided the random positions and maps for deployment of the concrete surrogates.
References
Anderson, S. C., Mills-Flemming, J., Watson, R., and Lotze, H. K. (2011). Serial exploitation of global sea cucumber fisheries. Fish and Fisheries 12, 317–339.| Serial exploitation of global sea cucumber fisheries.Crossref | GoogleScholarGoogle Scholar |
Burnham, K. P., and Anderson, D. R. (2002). ‘Model Selection and Multi-Model Inference: a Practical Information-Theoretic Approach’. 2nd edn. (Springer-Verlag: New York.)
Cooch, E., and White, G. C. (Eds) (2010). ‘Program MARK, a Gentle Introduction.’ 9th edn. (Colorado State University: Fort Collins, CO.)
Dissanayake, D. C. T., and Stefansson, G. (2012). Present status of the commercial sea cucumber fishery off the north-west coast of Sri Lanka. Journal of the Marine Biological Association of the United Kingdom 92, 831–841.
| Present status of the commercial sea cucumber fishery off the north-west coast of Sri Lanka.Crossref | GoogleScholarGoogle Scholar |
Fox, J., and Sen, S. (2002). A study of socio-economic issues facing traditional Indonesian fishers who access the MoU Box. Environment Australia, Canberra.
Friedman, K., Eriksson, H., Tardy, E., and Pakoa, K. (2011). Management of sea cucumber stocks: patterns of vulnerability and recovery of sea cucumber stocks impacted by fishing. Fish and Fisheries 12, 75–93.
| Management of sea cucumber stocks: patterns of vulnerability and recovery of sea cucumber stocks impacted by fishing.Crossref | GoogleScholarGoogle Scholar |
Gould, W. R., and Pollock, K. H. (1997). Catch-effort maximum likelihood estimation of important population parameters. Canadian Journal of Fisheries and Aquatic Sciences 54, 890–897.
| Catch-effort maximum likelihood estimation of important population parameters.Crossref | GoogleScholarGoogle Scholar |
Heap, A., and Harris, P. T. (2008). Geomorphology of the Australian margin and adjacent sea floor. Australian Journal of Earth Sciences 55, 555–585.
| Geomorphology of the Australian margin and adjacent sea floor.Crossref | GoogleScholarGoogle Scholar |
McCullagh, P., and Nelder, J. A. (1989). ‘Generalized Linear Models.’ (Chapman and Hall: New York.)
Perry, R. I., Walters, C. J., and Boutillier, J. A. (1999). A framework for providing scientific advice for the management of new and developing invertebrate fisheries. Reviews in Fish Biology and Fisheries 9, 125–150.
| A framework for providing scientific advice for the management of new and developing invertebrate fisheries.Crossref | GoogleScholarGoogle Scholar |
Pollock, K. H. (2002). The use of auxilliary variables in capture-recapture modelling: an overview. Journal of Applied Statistics 29, 85–102.
| The use of auxilliary variables in capture-recapture modelling: an overview.Crossref | GoogleScholarGoogle Scholar |
Ricker, W. E. (1958). ‘Handbook of Computation for biological statistics of Fish populations.’ Bulletin 119. (Fisheries Research Board of Canada: Ottawa.)
Russell, D. (2004). Aboriginal–Makassan interactions. Australian Aboriginal Studies 1, 3–17.
Schwerdtner, M. K., and Ferse, S. C. A. (2010). The history of Makassan trepang fishing and trade. PLoS ONE 5, e11346.
| The history of Makassan trepang fishing and trade.Crossref | GoogleScholarGoogle Scholar |
Seber, G. A. F. (1982). ‘The Estimation of Animal Abundance and Related Parameters.’ 2nd edn. (The Blackburn Press: Caldwell, NJ.)
Skewes, T. D., Dennis, D. M., Jacobs, D. R., Gordon, S. R., Taranto, T. J., Haywood, M., Pitcher, C. R., Smith, G. P., Milton, D., and Poiner, I. R. (1999a). Survey and stock size estimates of the shallow reef (0–15 m deep) and shoal area (15–50 m deep) marine resources and habitat mapping within the Timor Sea MOU74 box. Vol. 1: stock estimates and stock status. CSIRO Marine Research, Brisbane.
Skewes, T. D., Gordon, S. R., McLeod, I. R., Taranto, T. J., Dennis, D. M., Jacobs, D. R., Pitcher, C. R., Haywood, M., Smith, G. P., Poiner, I. R., Milton, D. A., Griffin, D., and Hunter, C. (1999b). Survey and stock size estimates of the shallow reef (0–15 m deep) and shoal area (15 – 50 m deep) marine resources and habitat mapping within the MOU74 box. Vol. 2: habitat mapping and coral dieback. CSIRO Marine Research, Brisbane.
Trianni, M. S. (2002). Evaluation of the resource following the sea cucumber fishery of Saipan, Northern Marianas Islands. In ‘Proceedings of the 9th International Coral Reef Symposium, Bali, Indonesia 23–27 October 2000. Vol. 2’. (Eds M. K. Moosa, S. Soemodihardjo, A. Soegiarto, K. Romimohtarto, A. Nontji, Soekarno and Suharsono.) pp. 829–834. Ministry of Environment, the Indonesian Institute of Sciences and the International Society for Reef Studies. Jakarta.
Uthicke, S., Welch, D., and Benzie, J. A. H. (2004). Slow growth and lack of recovery in overfished holothurians on the Great Barrier Reef: evidence from DNA fingerprints and repeated large-scale surveys. Conservation Biology 18, 1395–1404.
| Slow growth and lack of recovery in overfished holothurians on the Great Barrier Reef: evidence from DNA fingerprints and repeated large-scale surveys.Crossref | GoogleScholarGoogle Scholar |
Uthicke, S., Byrne, M., and Conand, C. (2010). Genetic barcoding of commercial Bêche-de-mer species (Echinodermata: Holothuroidea). Molecular Ecology Resources 10, 634–646.
| Genetic barcoding of commercial Bêche-de-mer species (Echinodermata: Holothuroidea).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpsFSltrg%3D&md5=351668449fab186549746984aa13cc5fCAS | 21565068PubMed |
White, G. C. (2002). Discussion comments on: the use of auxilliary variables in capture–recapture modelling. An overview. Journal of Applied Statistics 29, 103–106.
| Discussion comments on: the use of auxilliary variables in capture–recapture modelling. An overview.Crossref | GoogleScholarGoogle Scholar |
White, G. C., and Burnham, K. P. (1999). Program MARK: survival estimation from populations of marked animals. Bird Study 46, S120–S139.
| Program MARK: survival estimation from populations of marked animals.Crossref | GoogleScholarGoogle Scholar |
Williams, B. K., Nichols, J. D., and Conroy, M. J. (2002). ‘Analysis and Management of Animal Populations.’ (Academic Press: San Diego, CA.)




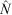 , ±95% confidence intervals, CI) returned by MARK (CIs are corrected for over-dispersion) for five species, namely Stichopus horrens, Holothuria atra, H. whitmaei, Bohadschia vitiensis and B. argus, found over 9000 ha of shallow reef flat on southern Scott Reef.
, ±95% confidence intervals, CI) returned by MARK (CIs are corrected for over-dispersion) for five species, namely Stichopus horrens, Holothuria atra, H. whitmaei, Bohadschia vitiensis and B. argus, found over 9000 ha of shallow reef flat on southern Scott Reef.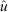 ) estimated for the following five species: Stichopus horrens, Holothuria atra, H. whitmaei, Bohadschia vitiensis and B. argus. Vertical bars indicate the upper and lower limits to exploitation, based on the estimated population size and catch.
) estimated for the following five species: Stichopus horrens, Holothuria atra, H. whitmaei, Bohadschia vitiensis and B. argus. Vertical bars indicate the upper and lower limits to exploitation, based on the estimated population size and catch.