Linkages between reach-scale physical habitat and invertebrate assemblages in upland streams
Victoria S. Milner A D , Nigel J. Willby B , David J. Gilvear C and Charles Perfect BA Institute of Science and the Environment, University of Worcester, Worcester, UK.
B Biological and Environmental Science, University of Stirling, Stirling, UK.
C School of Geography, Earth and Environmental Sciences, Faculty of Science and Technology, Plymouth University, Plymouth, UK.
D Corresponding author. Email: v.milner@worc.ac.uk
Marine and Freshwater Research 66(5) 438-448 https://doi.org/10.1071/MF14008
Submitted: 11 January 2014 Accepted: 22 July 2014 Published: 8 January 2015
Journal Compilation © CSIRO Publishing 2015 Open Access CC BY-NC-ND
Abstract
Determining the influence of physical habitat on biological structure in minimally disturbed settings is important if the effects of alterations to physical habitat are to be understood. This study tested whether reach-scale differences in physical habitat influence macroinvertebrate community composition at 24 sites in the Cairngorm Mountains, Scotland. Stream reaches were classified into channel types based on a geomorphic typology (i.e. step-pool, bedrock, plane-bed and pool-riffle). PERMANOVA indicated an overall significant relationship between the geomorphic typology and macroinvertebrate species-level composition, and among all combinations of channel types (such as step-pool and pool-riffle, step-pool and bedrock). Most channel types were dominated by high abundances of Baetis rhodani, Rhithrogena semicolorata and Leuctra inermis, which are ubiquitous in unpolluted gravel-bedded Scottish streams. However, reflecting significant differences in abundance of commoner taxa between types, indicator value (IndVal) analysis revealed that pool-riffle reaches were characterised by elmids (Limnius sp. and Oulimnius sp.) and Caenis rivulorum, and step-pool reaches by Alainites muticus, B. rhodani, L. inermis and Brachyptera risi. Geomorphic typing of rivers provides a useful basis for the initial assessment of ecological status whereas abundance-based biological data processed at the appropriate taxonomic resolution should be sensitive to physical-habitat modifications.
Additional keywords: channel type, geomorphic typology, macroinvertebrate, physical habitat heterogeneity.
Introduction
Natural river systems show high heterogeneity in physical habitat and associated biotic communities across multiple spatio-temporal scales (Heino et al. 2004). This heterogeneity in physical and biological structures and processes is hierarchically organised within river ecosystems, ranging from catchments, stream segments, reaches, pool-riffle sequences to microhabitats (Frissell et al. 1986). At each spatial scale, differences in environmental conditions contribute to heterogeneity in stream communities (Hildrew and Giller 1994; Poff 1997). At a catchment scale, land use, vegetation, discharge, conductivity, alkalinity (a surrogate for geology) and altitude (broadly indicative of temperature regime) influence macroinvertebrate distributions (Moss et al. 1987; Marchant et al. 1997; Wright et al. 1998; Newson and Newson 2000). At finer spatial scales (e.g. reaches, pool-riffle sequences and microhabitats), stream biota respond to differences in physical-habitat heterogeneity, such as substratum composition (Thomson et al. 2004; Dolédec et al. 2007), hydrological regime including magnitude, duration and timing of flows (Gibbins et al. 2001; Dunbar et al. 2010), depth (Mérigoux and Dolédec 2004), and differences in water chemistry that reflect the underlying geology (Gibbins et al. 2001; Pedersen and Friberg 2009).
Various studies have addressed patterns in macroinvertebrate communities across multiple hierarchical spatial scales (e.g. Li et al. 2001; Townsend et al. 2004; Robson et al. 2005; Campbell and McIntosh 2013; Heino et al. 2013) and variation in environmental conditions (i.e. valley confinement, channel planform, geomorphic units and bed materials) by using nested geomorphic typologies with distinct channel types or classes (e.g. Montgomery and Buffington 1997; Brierley and Fryirs 2005). In hierarchical typologies, habitat features at a specific spatio-temporal scale or within channel types are nested within a larger-scale framework, whereby factors such as valley confinement and discharge constrain their behaviour (Frissell et al. 1986; Hawkins et al. 1993). Ideally, channel types within geomorphic typologies should harbour reasonably distinct physical characteristics and dynamics, which would engender biotic differences. Whereas this goal may be elusive across all geomorphic features and spatial scales (from catchments to microhabitats), hierarchical frameworks potentially offer a useful tool to identify those spatial scales that are ecologically and physically most relevant to biological structure and to link physical processes at multiple scales (Thomson et al. 2004). Different geomorphic units (e.g. pools, riffles, runs, cascades) with discrete physical-habitat characteristics support distinct biological communities, especially for macroinvertebrates (e.g. Brown and Brussock 1991; Braaten and Berry 1997). Because channel types contain different combinations of these geomorphic units, it is logical to expect that the biota will also differ between channel types, at least within the same bioclimatic zone (Thomson et al. 2001). Biological sampling strategies encompassing a range of patches nested within each channel type should capture this physical-habitat heterogeneity more effectively than standard sampling of riffles or pools.
The Scottish Environment Protection Agency (SEPA) uses the Morphological Impact Assessment System tool (MiMAS; SNIFFER 2006) to identify the geomorphic sensitivity of channel morphology to engineering activities (Milner et al. 2013). A modified version of a hierarchical geomorphic typology developed by Montgomery and Buffington (1997, 1998) underpins the MiMAS tool, the principle being that channel types are governed by varying natural geomorphological controls (Milner et al. 2013). However, the ecological relevance of geomorphic typologies or the physical-habitat distinctiveness of channel types has rarely been tested at the reach scale, which is the scale mostly commonly used in ecological status assessments. In the Bega River basin in New South Wales, Australia, Chessman et al. (2006) found that River Styles (i.e. channel types) strongly affected macrophyte and macroinvertebrate assemblages (at the taxonomic family level), due to differences in physical habitat, but did not affect diatoms and fish. Despite finding an overall alignment between the geomorphic typology and macroinvertebrate and macrophyte community composition, the study did not specify which River Styles differed from one another. Thomson et al. (2004), also working in New South Wales, compared macroinvertebrate assemblages (at family level) and the habitat characteristics of pool and run geomorphic units for three different River Styles: a gorge, a bedrock-controlled channel with discontinuous floodplain and a meandering gravel bed. The study found significant differences for pools (although not for those associated with runs) among the three River Styles, but the influence of geographic dependence of reaches was not addressed. Both studies showed that hierarchical geomorphic typologies can partly explain spatial variation in macroinvertebrate assemblages, although most of the variance at the network scale is potentially due to other factors such as altitude and water temperature, and to biological processes such as colonisation and extinction. Determining the influence of physical habitat on biological structure in largely undisturbed settings is important to refine our understanding of geomorphic–biological interactions and to improve detection of the effects of physical alterations on aquatic biota. Geomorphic typing of rivers may therefore be a useful tool for researchers and managers who require a provisional assessment of ecological status or wish to predict the effects of changes in physical habitat.
This paper investigates the links between physical habitat and macroinvertebrate fauna at the reach scale, using several channel types in a geomorphic typology. Our primary question is ‘are channel types characterised by a distinct macroinvertebrate fauna because of intrinsic differences in physical-habitat characteristics?’ By focussing on sites with minimal geomorphological disturbance located within a small geographical area, we avoid the complication of multiple stressors or the confounding effects of different levels of disturbance and bioclimatic variables on biological differences among stream types.
Materials and methods
Study area
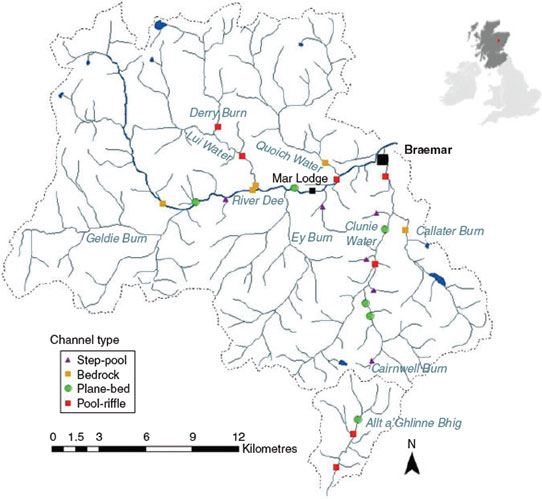
The study was conducted in the upper River Dee catchment (21 reaches), and the adjacent Allt a’Ghlinne Bhig (3 reaches) catchment, in the Cairngorm Mountains, north-eastern Scotland (Fig. 1). The upper River Dee catchment covers an area of ~289 km2, with the nearby Allt a’Ghlinne Bhig possessing a smaller catchment (27.5 km2). Mean flow was 12.3 m3 s–1 during 1982–2010, measured at the Mar Lodge gauging station on the River Dee, which is situated at the centre of the study site (Centre for Ecology and Hydrology 2012; Fig. 1). Both catchments are upland in character, the study reaches being situated between 328 and 615 m above sea level. The mean channel width was 11.2 m (range 2.9–13.0 m). The underlying geology of the catchment is predominantly granite and quartzose–mica–schist, with minor outcrops of limestone, graphitic schist and slate. Most of the study area comprises heather moorland (>92%), which is dominated by Calluna vulgaris on the upper and middle slopes, becoming montane arctic–alpine in character at high altitudes. Coniferous and deciduous managed forests, plus pockets of semi-natural Caledonian pine woodland occupy the lower slopes. Management activities include heather burning for ~10 days each year across a small area of the catchment (<0.1 km2) and the rearing of red deer (typically 1800 animals within the River Dee catchment; Christopher Murphy, pers. comm.). The study reaches were chosen by a random stratified sampling procedure, in which a 2 × 2-km grid was overlain on the upper River Dee and Allt a’Ghlinne catchments, and random coordinates were plotted within the grid (Milner and Gilvear 2012). If a site was positioned within 2 km of a site already selected, a random replacement was chosen. Sampling was not possible in all subcatchments within the upper River Dee because of access restrictions.
|
Geomorphic classification and physical-habitat mapping
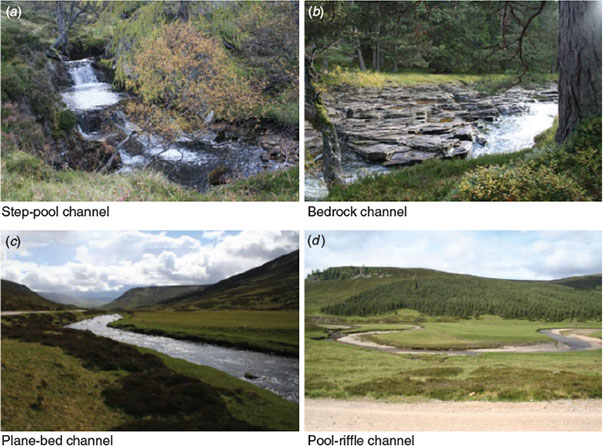
Fieldwork was undertaken during May–September 2007 and April 2008 at low discharges (between 4.2 m3 s–1 (Q80) and 3.0 m3 s–1 (Q90)). Stream reaches were classified into channel types using the Montgomery and Buffington (1997, 1998) process-based typology, which was developed for mountain streams in the north-western USA. Reaches were classified as step-pool (6 reaches), bedrock (5), plane-bed (6) or pool-riffle (7) channel types (Fig. 2). A study reach was defined as 20 channel widths, which was deemed a useful scale to link stream morphology to channel processes (Montgomery and Buffington 1997). The reach scale was chosen owing to its usefulness for describing medium- and long-term effects of human activities on physical habitat (Frissell et al. 1986) and for possessing a predictable combination of geomorphic units. In all study reaches, morphological condition was considered to be good because the longitudinal and lateral connectivity was intact, a range of geomorphic units were present, and the hydrological and sedimentological regime was unmodified (Milner and Gilvear 2012).
|
To characterise the physical habitat of the study reaches, channel cross-sectional geometry, channel bed slope, water depth, substrate and velocity were measured. Channel geometry was measured at a riffle, a glide and a pool, or at representative geomorphic units within each study reach. Channel bed slope was surveyed with an electronic distance meter. Water depth, grain size and mean column velocity were measured at 100 equidistant points across a reach by using a ‘zigzag’ sampling pattern, as proposed by Biggin and Stewardson (2004). Velocity was measured with a propeller current meter (Marsh McBirney Flo-Mate model 2000, Frederick, MD, USA) for 20 s at 0.6 water depth. A pebble plate incorporating the substrate categories of the Wentworth scale was used to measure grain size (Wentworth 1922). Because the upper range of bed material sizes exceeded the Wentworth scale, three additional classes were added (256–512 mm, 512–1024 mm and >1024 mm). Bedrock was assigned to the largest grain-size category. Variations in physical habitat are related to channel type, with a trend for increasing water depth and velocity from step-pool reaches, through plane-bed, pool-riffle and bedrock reaches (Table 1).

|
Macroinvertebrate sampling
A macroinvertebrate and water sample was collected in September 2007 and April 2008 from each of the 24 study reaches. Macroinvertebrates were collected using sweep and kick techniques. Samples were taken in all geomorphic units present at a site to capture physical-habitat heterogeneity, but the duration of kick sampling in each unit was proportional to their spatial coverage within the study reach. Thus each 3-min kick sample was spatially representative of the geomorphic units constituting the reach. Samples were sieved (500-µm mesh), and all invertebrates extracted, counted and identified in the laboratory to the lowest feasible taxonomic level (83% of individuals were identified to genus level or better). Identifications were made using the keys and guides of Hynes (1977), Croft (1986), Edington and Hildrew (1995) and Elliott and Humpesch (2010). Water samples were filtered and analysed for pH, alkalinity and major ions. The pH of a sample was measured by a calibrated electrode. Calcium, sodium, magnesium and potassium were measured using atomic absorption spectrometry.
Statistical analyses
Principal coordinates analysis (PCoA; Gower 1966) was used to visualise spatial patterns in (1) biological assemblage and (2) physical-habitat conditions in unconstrained ordination space. Prior to the analyses, macroinvertebrate abundance data were averaged from spring and autumn samples and square-root transformed, whereas physical-habitat and water-quality data were normalised and a Euclidean distance measure was used. Differences in macroinvertebrate abundances and physical-habitat characteristics among channel types were tested for significance using a one-way PERMANOVA. Owing to the low sample sizes, Monte Carlo P-values were used to detect significant variations among groups (i.e. channel types; Hladyz et al. 2012). When significant differences were present, post hoc comparisons were carried out to identify differences among channel types. The biotic distinctiveness of channel types was tested using both species (i.e. mixed-taxon level, including species-level identifications) and family-level invertebrate data to assess the importance of taxonomic resolution. We also performed PERMDISP (i.e. tests of homogeneity of dispersion; Anderson 2006) to identify dispersion effects in community structural variation among channel types. The test used the ANOVA F-statistic to contrast among-channel type differences in the distance of sites from their type centroid (Heino et al. 2013). The null hypothesis was that biological dispersion was equal across types.
To quantify within and between-type similarity using different levels of taxonomic resolution, the Bray–Curtis index was used as a dissimilarity measure (
A distance-based linear model (DistLM) was carried out to identify which physical-habitat and water-quality variables best explained the spatial variation in macroinvertebrate assemblage. A Bray–Curtis similarity matrix of macroinvertebrate abundances and a matrix of the normalised physical-habitat variables were used in the test. A stepwise selection of the physical-habitat variables (e.g. median and maximum grain size, water depth, velocity, channel slope, relative roughness, pH, alkalinity and sulfate) using the adjusted R2 selection criterion and a permutation test of significance was used (Legendre and Anderson 1999). A distance-based redundancy analysis (dbRDA) was constructed to highlight the influence of physical habitat on macroinvertebrate assemblage structure.
Sites belonging to the same channel type tended to be geographically interspersed with those of other types (Fig. 1), but the spatial proximity of sites could potentially have influenced the similarity of macroinvertebrate assemblages. To disentangle the role of geographical distance as a confounding factor, the stream-network geographic distance between all possible pairs of study reaches was determined in ArcMap 10 (ESRI, Redlands, CA, USA). A matrix of the geographic distances between all possible pairs of study reaches was then constructed, and supplied as a model matrix. Subsequently, the RELATE routine was used to identify whether the dissimilarity in species composition between pairs of samples was systematically related to their geographical position. The low rank-correlation coefficient (ρ = 0.01) indicated that the biotic assemblage was independent of the geographical distance matrix and that distance between sites was therefore not a contributing factor in the biological similarity between channel types.
Finally, species accumulation curves for macroinvertebrate taxa were generated for each channel type by using EcoSim Professional (version 1; Entsminger 2012) based on 1000 iterations per type of the recorded data. A composite curve (representing a channel based on all types) was also created by averaging the abundances of each taxon across each channel type, and subsequently adding the four totals together to generate the curve. The rationale for creating species accumulation curves was to compare richness between types after standardisation for sampling effort (i.e. numbers of individuals recorded), and to identify whether a river network containing high channel-type heterogeneity would possess higher diversity for the same sampling effort as a network comprising a single or small number of channel types. PCoA, PERMANOVA, PERMDISP, DistLM and RELATE were run in PERMANOVA+ for PRIMER (PRIMER-E, Plymouth, UK; Anderson et al. 2008) and the indicator value method was performed in IndVal (Dufrêne and Legendre 1997).
Results
Differences in invertebrate fauna among channel types
The macroinvertebrate assemblage was dominated by Ephemeroptera (proportion of individuals recorded = 46%), Plecoptera (20%) and Diptera (17%). Coleoptera (8%) and Trichoptera (9%) contributed smaller proportions. In total, 65 taxa were recorded in the study, of which 30 were confined to a single sample or occurred at an average abundance of <1 individual per sample (Table 2). The mayflies Rhithrogena semicolorata (Heptageniidae) and Baetis rhodani (Baetidae) were the two most abundant taxa, followed by Simuliidae, Leuctra inermis (Leuctridae), and Limnius (Elmidae). These five taxa together contributed almost two-thirds of the individuals recorded.
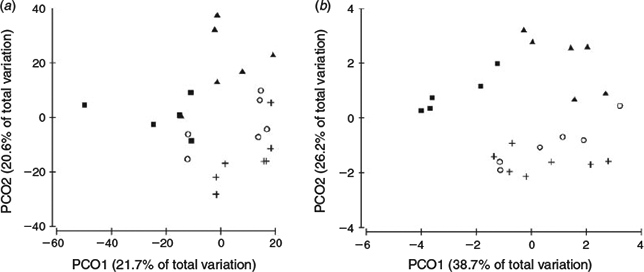
One-way PERMANOVA showed that macroinvertebrate composition differed significantly across the geomorphic types (F-ratio = 3.6, P = 0.001), and between all pairwise comparisons of types. The largest difference was between pool-riffle versus step-pool (P = 0.001) and bedrock samples (P < 0.01). Assemblages in bedrock reaches also differed strongly from step-pool (P < 0.02) and plane-bed reaches (P < 0.02), and those in plane-bed reaches differed from step-pool (P < 0.02) and pool-riffle reaches (P < 0.02). When one-way PERMANOVA was repeated with family-level data, weaker relationships were observed and the assemblages in pool-riffle and plane-bed reaches could no longer be distinguished, indicating a substantially poorer capacity to discriminate among channel types using data identified only to family-level. The PERMANOVA analysis based on mixed genus–species resolution data was visually supported by a PCoA ordination (Fig. 3a). Samples from all channel types tended to group together in the ordination and to separate from other samples, particularly those from pool-riffle and plane-bed reaches. PERMDISP revealed that biological dispersion did not differ significantly across channel types (F = 1.18, P = 0.34) or for any pairwise comparisons (P > 0.05). Community structural variation, as measured by the mean distance from the group centroid, was similar in step-pool (25.6), plane-bed (25.8) and pool-riffle (26.2) channels. Bedrock channels (33.5) possessed higher variability, as indicated in the PCoA ordination (Fig. 3a), but did not differ significantly from the other types. The findings indicated a location effect (e.g. the PERMANOVA output) on macroinvertebrate community assemblage, but not a dispersion effect.
|
The majority (83%) of the 35 commoner taxa occurred in at least three of the four channel types (Table 2). However, there were significant variations in abundance of some of these taxa among channel types that contributed to successful identification of indicator taxa using IndVal. Step-pool and pool-riffle types contained the greatest numbers of indicator taxa. Alainites muticus (Baetidae) and B. rhodani, plus L. inermis and Brachyptera risi (Taeniopterygidae) were strong positive indicators of step-pool reaches, whereas the elmids Limnius and Oulimnius, Caenis rivulorum (Caenidae) and Leuctra hippopus (Leuctridae) were strong indicators of pool-riffle reaches. Plane-bed reaches were clearly distinguishable only by higher abundances of two less common taxa, Siphonoperla torrentium (Chloroperlidae) and Baetis fuscatus (Baetidae). Bedrock reaches had lower abundances of almost all shared taxa, especially the commoner mayflies, presumably as a result of lower habitat suitability, and had only a single weak positive indicator, Protonemura meyeri. The presence of congeneric indicators for contrasting channel types illustrated the importance of high resolution identification where possible.
Individual-based taxon-accumulation curves revealed step-pool reaches to have the lowest overall richness, with pool-riffle types the greatest and plane-bed sites intermediate (Fig. 4). Although bedrock sites contained the lowest numbers of individuals, their pattern of species accumulation was similar to that for the pool-riffle type, indicating a steep rise in diversity with abundance. The position of the curve for the composite sample confirmed that all types contributed to the global species pool and indicated that a river comprising multiple channel types will, on average, for the same sampling effort, contain more species than a river dominated by a single channel type.

|
Effect of physical-habitat characteristics on macroinvertebrate composition
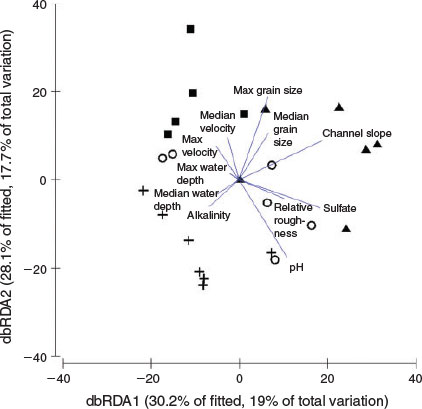
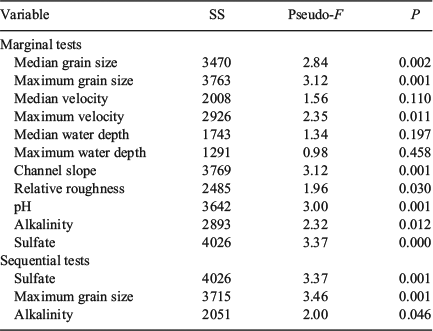
In the PCoA ordination, the first two axes explained 64.9% of the variation in the physical-habitat data (Fig. 3b). PERMANOVA revealed differences in physical habitat among channel types (P = 0.001), and all pairwise comparisons excluding plane-bed and pool-riffle channels (P = 0.14). Physical-habitat distinctions were largest between step-pool and pool-riffle (P = 0.001), bedrock (P ≤ 0.01), and plane-bed samples (P ≤ 0.01). Physical-habitat character in bedrock reaches was also distinct from pool-riffle (P ≤ 0.01) and plane-bed reaches (P ≤ 0.01). Marginal tests within the DistLM procedure revealed that most individual physical-habitat and water-quality variables, including median and maximum grain size, maximum velocity, channel slope, relative roughness, pH, alkalinity and sulfate, had a significant relationship with the macroinvertebrate assemblage structure when considered alone (Table 3). There was no correlation between median (P = 0.2) and maximum water depth (P = 0.46) or maximum velocity (P = 0.11) with macroinvertebrate abundances. In the marginal tests, sulfate, median and maximum grain size, channel slope and pH individually explained a similar proportion of variation in macroinvertebrate community structure (i.e. between 11.4–13.3%). Although, the dbRDA showed that the highest correlation (R2 = 0.63) with the macroinvertebrate assemblage structure was using all 11 physical-habitat and water-quality variables, the F-values revealed that sulfate, maximum grain size and channel slope were the most effective at splitting channel types based on their biota (Table 3). The dbRDA ordination showed that channel slope (R2 = 0.57), sulfate (R2 = 0.55) and also relative roughness (R2 = 0.31) were positively correlated with dbRDA axis 1, whereas maximum grain size (R2 = 0.56) and pH (R2 = –0.53) were correlated with dbRDA axis 2 (Fig. 5). For the stepwise selection based on the R2 criterion, the sequential tests revealed a combination of three variables, namely sulfate, maximum grain size and alkalinity, significantly explained the variation (32.3%) in macroinvertebrate composition (Table 3). The sequential tests highlighted that after fitting these three variables, the remaining environmental variables did not add significantly to the explanation of the residual biological variation (P = 0.45). Therefore, sulfate, maximum grain size and alkalinity are the best variables to account for variation in macroinvertebrate community structure in this system.
|
|
Discussion
Linkages between channel type and invertebrate composition
Upland streams in Britain are generally characterised by a coarse bed and turbulent, well-oxygenated flow and tend to support a species-poor invertebrate fauna (Wright et al. 1998). Despite this, our study revealed a significant association between channel types and macroinvertebrate composition. Although all channel types contributed to the global species pool, the primary distinction among channel types was based on differences in abundance of some of the most ubiquitous species, such as B. rhodani, L. inermis, A. muticus (step-pool), and Limnius sp. (pool-riffle), rather than the presence of specialist taxa. This may partly explain the superior performance of analyses based on genus, as opposed to family-level identification. Other less abundant but still widespread taxa such as Oulimnius, C. rivulorum and Leuctra hippopus had a strong significant association with pool-riffle reaches, consistent with reported habitat preferences (Giller and Malmqvist 2003; Merritt et al. 2008).
Besides identifying the ecological relevance of a geomorphic typology and individual channel types, our study used individual-based species accumulation curves to examine the diversity of macroinvertebrates within and among channel types (Fig. 4). The findings indicated pronounced differences in diversity between some channel types, such as, between pool-riffle (higher) and step-pool (lower) reaches, when standardised for the numbers of individuals recorded. Therefore, a river network with high geomorphic channel-type heterogeneity (i.e. containing multiple channel types) will support greater macroinvertebrate diversity. Physical modifications by anthropogenic activities that result in a homogenisation of the channel structure within a river network may thus be reflected in a loss of biodiversity.
A combination of processes acting at different spatial scales, such as large-scale geographical factors, local-scale physical-habitat characteristics and water chemistry, influences macroinvertebrate community composition across river networks (Li et al. 2012). The marginal tests in the DistLM procedure confirmed that physical habitat (expressed as median and maximum grain size, velocity, channel slope, relative roughness) and water chemistry (expressed as pH, sulfate and alkalinity) were strongly associated with macroinvertebrate composition in the upper Dee catchment when considered independently. The sequential tests in the DistLM analysis also revealed sulfate, maximum grain size and alkalinity to be the best subset of explanatory variables to account for differences in macroinvertebrate community structure. These findings implied that macroinvertebrates respond at the reach scale and to physical-habitat variation in a manner consistent with many other studies (e.g. Robson and Barmuta 1998; Robson and Chester 1999; Thomson et al. 2004), and confirmed the importance of reaches containing a diversity of contrasting microhabitats or longitudinally heterogeneous stream systems in supporting fluvial biodiversity. The findings also highlighted a significant persistent influence of stream chemistry on community structure (e.g. Clenaghan et al. 1998; Gibbins et al. 2001) in unpolluted systems where physical factors may appear most discriminatory. Alkalinity and sulfate may be acting here as a surrogate for some other, more proximate, factor, such as flow regime, biofilm or leaf-litter quality. Physical-habitat variables are also directly relevant to other biota, such as fish, that migrate over larger spatial areas, and have more specific habitat requirements (Thomson et al. 2004). Different geomorphic units may therefore support fish assemblages of differing richness (Cianfrani et al. 2009) or fulfil different life-history functions. For example, runs may act as a feeding source, backwaters and pools as resting or nursery areas and gravel bars as spawning sites (Thomson et al. 2004). Hence, a reach-scale classification of geomorphic units may also be transferable to fish assemblages.
Applications and implications for river management
The relationships between macroinvertebrates or other biota and reach-scale geomorphic typologies are important for river management and assessment purposes. However, much debate exists concerning the appropriateness of geomorphic typologies as a tool to classify stream biota. Hawkins and Vinson (2000) proposed that the poor performance of geomorphic typologies in predicting macroinvertebrate communities is often because the physical-habitat heterogeneity present within sites is omitted within the broad partitions of the classification and the sampling design. Clustering and classification typically focus on average physical-habitat values from a site, and not the variability of these values. In the present study, spatial heterogeneity was incorporated into the design through sampling macroinvertebrates in all dominant habitats (i.e. riffles, pools and glides) within a channel type. By including habitat heterogeneity at this scale, we enhanced our ability to detect differences in biological assemblages among channel types because the relative abundance of commonly occurring geomorphic units varied predictably among channel types. Many studies focussing on multiscale patterns of spatial variation in macroinvertebrate communities have found the highest variability at small spatial scales (Downes et al. 1993; Boyero and Bailey 2001; Li et al. 2001; Robson et al. 2005). For example, Robson and Chester (1999) investigated patterns of macroinvertebrate species richness between two types of riffles (bedrock versus cobble) and their constituent microhabitats in Mountain River, Tasmania. The variation in species density among microhabitats was greater than among riffles of the same type (Robson and Chester 1999), reinforcing the need to consider microhabitat in sampling designs.
SEPA’s use of the geomorphic typology forms an integral part of the MiMAS tool (see Introduction), which quantifies geomorphic sensitivity to engineering pressures (Milner et al. 2013). The present study indicated that there are significant differences in macroinvertebrate fauna and physical habitat across the four key channel types commonly found in upland rivers. The relationship was apparent at a within-catchment scale where biogeographic factors are well controlled, but requires examination at a larger scale, which would also permit a greater variety of types to be compared. Additionally, this study was undertaken on reaches in good morphological and ecological condition. By demonstrating biological differences among minimally affected examples of geomorphic types when biogeographic factors are controlled, these results indicate that macroinvertebrates should be sensitive to local geomorphological alteration within types, which is useful for understanding geomorphic–biotic interactions for management purposes. However, further work is needed to explore the macroinvertebrate communities of similar channel types in moderate and poor morphological condition, as defined under the EU Water Framework Directive (WFD; 2000/60/EC), to determine whether, for example, degradation tends to cause biotic homogenisation, or whether there are type-specific trajectories in response to a given type of degradation.
Conclusions
This study has shown a significant alignment of a geomorphic typology and macroinvertebrate community composition at the reach scale. In stream systems with similar water quality, differences in channel type at the reach scale are likely to reflect biological diversity. The results suggest that fluvial geomorphology, through the influence of physical habitat and water chemistry, affect macroinvertebrate distributions at the reach scale. Geomorphic typologies offer a useful tool for researchers and managers, especially if they can be remotely derived, and may explain some coarser-scale variability in aquatic communities within stream systems, provided that sampling adequately reflects habitat heterogeneity. Although no geomorphic typology or classification will have universal application (Thomson et al. 2004), such typologies offer a useful basis for grouping functionally similar sites for habitat assessments, and ecological and conservation applications.
Acknowledgements
This study was funded by a UK Natural Environment Research Council studentship (NER/S/A/2006/14226) and the Scottish Environment Protection Agency (SEPA). We thank Graham and Lucy Milner, Dr Adel El Metwalli, Dr Jennifer Brown and Claire Elliott for assistance in the field, Robbie Austrums for cartographic support, and Mar Lodge Estate and Invercauld Estate for granting access to their land. We are also grateful for the comments from Prof. Belinda Robson, Dr Ian Maddock, Prof. Bob Clarke and two anonymous reviewers whose encouraging advice greatly improved the paper.
References
Anderson, M. J. (2006). Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62, 245–253.| Distance-based tests for homogeneity of multivariate dispersions.Crossref | GoogleScholarGoogle Scholar | 16542252PubMed |
Anderson, M. J., Gorley, R. N., and Clarke, K. R. (2008). ‘PRIMER+ for PERMANOVA: Guide to Software and Statistical Methods. 214.’ (Primer-E: Plymouth, UK.)
Biggin, M. E., and Stewardson, M. J. (2004). Quantifying hydraulic habitat heterogeneity: the development of a flow type heterogeneity index. In ‘Proceedings of the 4th Australian Stream Management Conference’, 19–22 October 2004, Launceston, Tas.. (Eds I. Rutherford, I. Wiszniewski, M. Asky-Doran, and R. Glazik.) pp. 78–83 (Department of Primary Industries Water and Environment: Launceston, Tas.)
Boyero, L., and Bailey, R. C. (2001). Organization of macroinvertebrate communities at a hierarchy of spatial scales in a tropical stream. Hydrobiologia 464, 219–225.
| Organization of macroinvertebrate communities at a hierarchy of spatial scales in a tropical stream.Crossref | GoogleScholarGoogle Scholar |
Braaten, P. J., and Berry, C. R. (1997). Fish associations with four habitat types in a South Dakota prairie stream. Journal of Freshwater Ecology 12, 477–489.
| Fish associations with four habitat types in a South Dakota prairie stream.Crossref | GoogleScholarGoogle Scholar |
Brierley, G. J., and Fryirs, K. A. (2005). ‘Geomorphology and River Management. Applications of the River Styles Framework.’ (Blackwell Publications: Oxford, UK.)
Brown, A. V., and Brussock, P. P. (1991). Comparisons of benthic invertebrates between riffles and pool. Hydrobiologia 220, 99–108.
| Comparisons of benthic invertebrates between riffles and pool.Crossref | GoogleScholarGoogle Scholar |
Campbell, R. E., and McIntosh, A. R. (2013). Do isolation and local habitat jointly limit the structure of stream invertebrate assemblages? Freshwater Biology 58, 128–141.
| Do isolation and local habitat jointly limit the structure of stream invertebrate assemblages?Crossref | GoogleScholarGoogle Scholar |
Centre for Ecology and Hydrology (2012). National River Flow Archive. Available at http://www.ceh.ac.uk/data/nrfa/data/search.html [Verified 1 April 2013].
Chessman, B. C., Fryirs, K. A., and Brierley, G. J. (2006). Linking geomorphic character, behaviour and condition to fluvial biodiversity: implications for river management. Aquatic Conservation: Marine and Freshwater Ecosystems 16, 267–288.
| Linking geomorphic character, behaviour and condition to fluvial biodiversity: implications for river management.Crossref | GoogleScholarGoogle Scholar |
Cianfrani, C. M., Sullivan, S. M. P., Hession, W. C., and Watzin, M. C. (2009). Mixed stream channel morphologies: implications for fish community diversity. Aquatic Conservation: Marine and Freshwater Ecosystems 19, 147–156.
| Mixed stream channel morphologies: implications for fish community diversity.Crossref | GoogleScholarGoogle Scholar |
Clenaghan, C., Giller, P. S., O’Halloran, J., and Hernan, R. (1998). Stream macroinvertebrate communities in a conifer-afforested catchment in Ireland: relationships to physico-chemical and biotic factors. Freshwater Biology 40, 175–193.
| Stream macroinvertebrate communities in a conifer-afforested catchment in Ireland: relationships to physico-chemical and biotic factors.Crossref | GoogleScholarGoogle Scholar |
Croft, P. S. (1986). A key to the major groups of British freshwater invertebrates. Field Studies 6, 531–579.
Dolédec, S., Lamouroux, N., Fuchs, U., and Mérigoux, S. (2007). Modelling the hydraulic preferences of benthic macroinvertebrates in small European streams. Freshwater Biology 52, 145–164.
| Modelling the hydraulic preferences of benthic macroinvertebrates in small European streams.Crossref | GoogleScholarGoogle Scholar |
Downes, B. J., Lake, P. S., and Schreiber, E. S. G. (1993). Spatial variation in the distribution of stream invertebrates: implications of patchiness for models of community organization. Freshwater Biology 30, 119–132.
| Spatial variation in the distribution of stream invertebrates: implications of patchiness for models of community organization.Crossref | GoogleScholarGoogle Scholar |
Dufrêne, M., and Legendre, P. (1997). Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs 67, 345–366.
Dunbar, M., Pedersen, M. L., Cadman, D., Extence, C., Waddingham, J., Chadd, R., and Larsen, S. E. (2010). River discharge and local-scale physical habitat influence macroinvertebrate LIFE scores. Freshwater Biology 55, 226–242.
| River discharge and local-scale physical habitat influence macroinvertebrate LIFE scores.Crossref | GoogleScholarGoogle Scholar |
Edington, J. M., and Hildrew, A. G. (1995). ‘A revised key to the caseless caddis larvae of the British Isles.’ (FBA Scientific Publications: Ambleside, UK.)
Elliott, J. M., and Humpesch, U. H. (2010). ‘Mayfly larvae (Ephemeroptera) of Britain and Ireland: keys and a review of their biology.’ (FBA Scientific Publications: Ambleside, UK.)
Entsminger, G. L. (2012). ‘EcoSim Professional: Null Modeling Software for Ecologists. Version 1.’ (Acquired Intelligence Inc., Kesey-Bear, & Pinyon Publishing: Montrose, CO.)
Frissell, C. A., Liss, W. J., Warren, C. E., and Hurley, M. D. (1986). A hierarchical framework for stream habitat classification: viewing streams in a watershed context. Environmental Management 10, 199–214.
| A hierarchical framework for stream habitat classification: viewing streams in a watershed context.Crossref | GoogleScholarGoogle Scholar |
Gibbins, C. N., Dilks, C. F., Malcolm, R., Soulsby, C., and Juggins, S. (2001). Invertebrate communities and hydrological variation in Cairngorm mountain streams. Hydrobiologia 462, 205–219.
| Invertebrate communities and hydrological variation in Cairngorm mountain streams.Crossref | GoogleScholarGoogle Scholar |
Giller, P. S., and Malmqvist, B. (2003). ‘The Biology of Streams and Rivers.’ (Oxford University Press: New York.)
Gower, J. C. (1966). Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika 53, 325–338.
| Some distance properties of latent root and vector methods used in multivariate analysis.Crossref | GoogleScholarGoogle Scholar |
Hawkins, C. P., and Vinson, M. R. (2000). Weak correspondence between landscape classifications and stream invertebrate assemblages: implications for bioassessment. Journal of the North American Benthological Society 19, 501–517.
| Weak correspondence between landscape classifications and stream invertebrate assemblages: implications for bioassessment.Crossref | GoogleScholarGoogle Scholar |
Hawkins, C. P., Kershner, J. L., Bisson, P. A., Bryant, M. D., Decker, L. M., Gregory, S. V., McCullough, D. A., Overton, C. K., Reeves, G. H., Steedman, R. J., and Young, M. K. (1993). A hierarchical approach to classifying stream habitat features. Fisheries 18, 3–12.
| A hierarchical approach to classifying stream habitat features.Crossref | GoogleScholarGoogle Scholar |
Heino, J., Louhi, P., and Muotka, T. (2004). Identifying the scales of variability in stream macroinvertebrate abundance, functional composition and assemblage structure. Freshwater Biology 49, 1230–1239.
| Identifying the scales of variability in stream macroinvertebrate abundance, functional composition and assemblage structure.Crossref | GoogleScholarGoogle Scholar |
Heino, J., Grönroos, M., Ilmonen, J., Karhu, T., Niva, M., and Paasivirta, L. (2013). Environmental heterogeneity and β diversity of stream macroinvertebrate communities at intermediate scales. Freshwater Science 32, 142–154.
| Environmental heterogeneity and β diversity of stream macroinvertebrate communities at intermediate scales.Crossref | GoogleScholarGoogle Scholar |
Hildrew, A. G., and Giller, P. S. (1994). Patchiness, species interactions and disturbance in the stream benthos. In ‘Aquatic Ecology: Scale, Pattern and Process’. (Eds P. S. Giller, A. G. Hildrew and D. G. Raffaelli.) pp. 21–62. (Blackwell Science: Oxford, UK.)
Hladyz, S., Nielson, D. L., Suter, P. J., and Krull, E. S. (2012). Temporal variations in organic carbon utilization by consumers in a lowland river. River Research and Applications 28, 513–528.
| Temporal variations in organic carbon utilization by consumers in a lowland river.Crossref | GoogleScholarGoogle Scholar |
Hynes, H. B. N. (1977). A key to the adults and nymphs of the British stoneflies (Plecoptera) with notes on their ecology and distribution, 3rd edition. Scientific Publications of the Freshwater Biological Association 17, 1–92.
| A key to the adults and nymphs of the British stoneflies (Plecoptera) with notes on their ecology and distribution, 3rd edition.Crossref | GoogleScholarGoogle Scholar |
Legendre, P., and Anderson, M. J. (1999). Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological requirements. Ecological Monographs 69, 1–24.
| Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological requirements.Crossref | GoogleScholarGoogle Scholar |
Li, J., Herlihy, A., Gerth, W., Kaufmann, P., Gregory, S., Urquhart, S., and Larsen, D. P. (2001). Variability in stream macroinvertebrates at multiple spatial scales. Freshwater Biology 46, 87–97.
| Variability in stream macroinvertebrates at multiple spatial scales.Crossref | GoogleScholarGoogle Scholar |
Li, F., Chung, N., Mi-Jung, B., Kwon, Y. S., and Park, Y. S. (2012). Relationships between stream macroinvertebrates and environmental variables at multiple spatial scales. Freshwater Biology 57, 2107–2124.
| Relationships between stream macroinvertebrates and environmental variables at multiple spatial scales.Crossref | GoogleScholarGoogle Scholar |
Marchant, R., Hirst, A., Norris, R. H., Butcher, R., Metzeling, L., and Tiller, D. (1997). Classification and prediction of macroinvertebrate assemblages from running waters in Victoria. Journal of the North American Benthological Society 16, 664–681.
| Classification and prediction of macroinvertebrate assemblages from running waters in Victoria.Crossref | GoogleScholarGoogle Scholar |
Mérigoux, S., and Dolédec, S. (2004). Hydraulic requirements of stream communities: a case study on invertebrates. Freshwater Biology 49, 600–613.
| Hydraulic requirements of stream communities: a case study on invertebrates.Crossref | GoogleScholarGoogle Scholar |
Merritt, R. W., Cummins, K. W., and Berg, M. B. (2008). ‘An Introduction to the Aquatic Insects of North America’, 4th edn. (Kendall Hunt Publishing Company: Dubuque, IA).
Milner, V. S., and Gilvear, D. J. (2012). Charaterization of hydraulic habitat and retention across different channel types; introducing a new field-based technique. Hydrobiologia 694, 219–233.
| Charaterization of hydraulic habitat and retention across different channel types; introducing a new field-based technique.Crossref | GoogleScholarGoogle Scholar |
Milner, V. S., Gilvear, D. J., and Willby, N. J. (2013). An assessment of variants in the professional judgement of geomorphologically based channel types. River Research and Applications 29, 236–249.
| An assessment of variants in the professional judgement of geomorphologically based channel types.Crossref | GoogleScholarGoogle Scholar |
Montgomery, D. R., and Buffington, J. M. (1997). Channel-reach morphology in mountain drainage basins. Geological Society of America Bulletin 109, 596–611.
| Channel-reach morphology in mountain drainage basins.Crossref | GoogleScholarGoogle Scholar |
Montgomery, D. R., and Buffington, J. M. (1998). Channel processes, classification and response. In ‘River Ecology and Management: Lessons from the Pacific Coastal Ecoregion’. (Eds R. Naiman and R. Bilby.) pp. 13–42. (Springer-Verlag: New York.)
Moss, D., Furse, M. T., Wright, J. F., and Armitage, P. D. (1987). The prediction of the macro-invertebrate fauna of unpolluted running water sites in Great Britain using environmental data. Freshwater Biology 17, 41–52.
| The prediction of the macro-invertebrate fauna of unpolluted running water sites in Great Britain using environmental data.Crossref | GoogleScholarGoogle Scholar |
Newson, M. D., and Newson, C. L. (2000). Geomorphology, ecology and river channel habitat: mesoscale approaches to basin-scale challenges. Progress in Physical Geography 24, 195–217.
| Geomorphology, ecology and river channel habitat: mesoscale approaches to basin-scale challenges.Crossref | GoogleScholarGoogle Scholar |
Pedersen, M. L., and Friberg, N. (2009). Influence of disturbance on habitats and biological communities in lowland streams. Fundamental and Applied Limnology 174, 27–41.
| Influence of disturbance on habitats and biological communities in lowland streams.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXks1Orurw%3D&md5=80190caaddfb0445d32f95f271fbd542CAS |
Poff, N. L. (1997). Landscape filters and species traits: towards mechanistic understanding and prediction in stream ecology. Journal of the North American Benthological Society 16, 391–409.
| Landscape filters and species traits: towards mechanistic understanding and prediction in stream ecology.Crossref | GoogleScholarGoogle Scholar |
Robson, B. J., and Barmuta, L. A. (1998). The effect of two scales of habitat architecture on benthic grazing in a river. Freshwater Biology 39, 207–220.
| The effect of two scales of habitat architecture on benthic grazing in a river.Crossref | GoogleScholarGoogle Scholar |
Robson, B. J., and Chester, E. T. (1999). Spatial patterns of invertebrate species richness in a river: the relationship between riffles and microhabitats. Australian Journal of Ecology 24, 599–607.
| Spatial patterns of invertebrate species richness in a river: the relationship between riffles and microhabitats.Crossref | GoogleScholarGoogle Scholar |
Robson, B. J., Hogan, M., and Forrester, T. (2005). Hierarchical patterns of invertebrate assemblage structure in stony upland streams change with time and flow permanence. Freshwater Biology 50, 944–953.
| Hierarchical patterns of invertebrate assemblage structure in stony upland streams change with time and flow permanence.Crossref | GoogleScholarGoogle Scholar |
SNIFFER (2006). A new impact assessment tool to support river engineering regulatory decision. Technical Report. Project number WFD49, Scottish and Northern Ireland Forum for Environmental Research, Edinburgh, UK.
Thomson, J. R., Taylor, M. P., Fryirs, K. A., and Brierley, G. J. (2001). A geomorphological framework for river characterization and habitat assessment. Aquatic Conservation: Marine and Freshwater Ecosystems 11, 373–389.
| A geomorphological framework for river characterization and habitat assessment.Crossref | GoogleScholarGoogle Scholar |
Thomson, J. R., Taylor, M. P., and Brierley, G. J. (2004). Are river styles ecologically meaningful? A test of the ecological significance of a geomorphic river characterization scheme. Aquatic Conservation: Marine and Freshwater Ecosystems 14, 25–48.
| Are river styles ecologically meaningful? A test of the ecological significance of a geomorphic river characterization scheme.Crossref | GoogleScholarGoogle Scholar |
Townsend, C. R., Downes, B. J., Peacock, K., and Arbuckle, C. J. (2004). Scale and the detection of land-use effects on morphology, vegetation and macroinvertebrate communities of grassland streams. Freshwater Biology 49, 448–462.
| Scale and the detection of land-use effects on morphology, vegetation and macroinvertebrate communities of grassland streams.Crossref | GoogleScholarGoogle Scholar |
Wentworth, C. K. (1922). A scale of grade and class terms for clastic sediments. The Journal of Geology 30, 377–392.
| A scale of grade and class terms for clastic sediments.Crossref | GoogleScholarGoogle Scholar |
Wright, J. F., Furse, M. T., and Moss, D. (1998). River classification using invertebrates: RIVPACS applications. Aquatic Conservation: Marine and Freshwater Ecosystems 8, 617–631.
| River classification using invertebrates: RIVPACS applications.Crossref | GoogleScholarGoogle Scholar |



