Variability of the carbonate chemistry in a shallow, seagrass-dominated ecosystem: implications for ocean acidification experiments
Roberta C. Challener A D , Lisa L. Robbins B and James B. McClintock CA Department of Biology, Bellarmine University, Louisville, KY 40205, USA.
B Saint Petersburg Coastal and Marine Science Center, US Geological Survey, St Petersburg, FL 33701, USA.
C Department of Biology, University of Alabama at Birmingham, Birmingham, AL 35294, USA.
D Corresponding author. Email rchallener@bellarmine.edu
Marine and Freshwater Research 67(2) 163-172 https://doi.org/10.1071/MF14219
Submitted: 30 July 2014 Accepted: 3 February 2015 Published: 25 May 2015
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Open ocean observations have shown that increasing levels of anthropogenically derived atmospheric CO2 are causing acidification of the world’s oceans. Yet little is known about coastal acidification and studies are just beginning to characterise the carbonate chemistry of shallow, nearshore zones where many ecologically and economically important organisms occur. We characterised the carbonate chemistry of seawater within an area dominated by seagrass beds (Saint Joseph Bay, Florida) to determine the extent of variation in pH and pCO2 over monthly and daily timescales. Distinct diel and seasonal fluctuations were observed at daily and monthly timescales respectively, indicating the influence of photosynthetic and respiratory processes on the local carbonate chemistry. Over the course of a year, the range in monthly values of pH (7.36–8.28), aragonite saturation state (0.65–5.63), and calculated pCO2 (195–2537 μatm) were significant. When sampled on a daily basis the range in pH (7.70–8.06), aragonite saturation state (1.86–3.85), and calculated pCO2 (379–1019 μatm) also exhibited significant range and indicated variation between timescales. The results of this study have significant implications for the design of ocean acidification experiments where nearshore species are utilised and indicate that coastal species are experiencing far greater fluctuations in carbonate chemistry than previously thought.
Additional keywords: benthic zone, climate change, echinoderms
Introduction
The field of ocean acidification (OA) research has grown immensely over the past decade in an effort to predict the potential consequences of increasing carbon dioxide (CO2) concentrations on marine ecosystems (e.g. Orr et al. 2005; Fabry et al. 2008; Doney et al. 2009; Hofmann et al. 2010). There has been a rapid increase in laboratory experiments investigating the effect of altered seawater carbonate chemistry associated with increases in atmospheric CO2 (e.g. increased partial pressure of CO2 (pCO2) and dissolved inorganic carbon (DIC), reduced pH and calcium carbonate polymorph saturation states) on marine organisms (for example, see Dupont et al. 2010; Hendriks et al. 2010; Kroeker et al. 2010; Byrne 2011 for reviews and data meta-analyses). These experiments are largely designed around the use of experimental pCO2 and pH values projected by the Intergovernmental Panel on Climate Change emission scenarios (IPCC 2007) rather than values that more closely model environments from which organisms are collected (see McElhany and Busch 2013; Waldbusser and Salisbury 2014, for a discussion). This is partially due to the lack of information on nearshore carbonate chemistry and the focus on coastal species with important ecological and economic roles. Therefore, there is a need for studies that investigate the local variability in the seawater carbonate chemistry of ecosystems where targeted model study organisms occur (Wahl et al., in press). Such data will allow researchers to establish relevant target experimental pH and pCO2 values and put into context the results that have been generated on previous OA studies in terms of the organism’s current physiological tolerance for pH and pCO2 variability.
Significant fluctuations in seawater carbonate chemistry have been documented for many marine ecosystems (e.g. DeGrandpre et al. 1995; Ohde and van Woesik 1999; Hales et al. 2005; Wootton et al. 2008; Hofmann et al. 2011). In coastal areas there are a variety of processes that can influence the carbonate chemistry of a body of water over various timescales, most notably community production and respiration (see Waldbusser and Salisbury 2014, for a discussion). On short timescales (24 h) in shallow environments with dense beds of macroalgae or seagrasses, photosynthesis increases pH of the surrounding water while decreasing DIC (mainly through uptake of CO2 and HCO3–) (e.g. Invers et al. 1997; Beer et al. 2006; Middelboe and Hansen 2007; Semesi et al. 2009). Conversely, respiration by marine organisms decreases pH but increases DIC due to the release of CO2. Furthermore, shallow-water coastal environments may be subject to large annual and daily temperature and salinity fluctuations that in turn control the carbonate chemistry at a given site. Diurnal fluctuations >1 pH unit have been observed in seagrass meadows (Semesi et al. 2009) and increases in pH of up to 0.38 units have been associated with the presence of seagrass meadows (Unsworth et al. 2012). In Florida Bay where seagrass beds are common, extensive variation in pH (7.85–8.1) and pCO2 (325–725 μatm) were observed in samples taken every 2 months (Millero et al. 2001). Additionally, Florida Bay and Tampa Bay, FL, exhibited average diurnal differences (over a 3-day period) of 0.22 pH units and 218 μatm pCO2 (Yates et al. 2007). These two studies demonstrate the need to understand the variability of the carbonate chemistry of coastal areas, particularly where seagrass beds dominate.
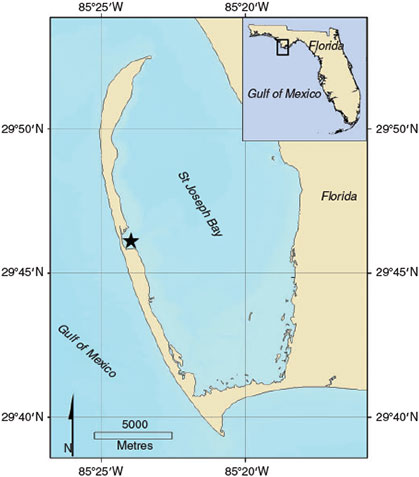
Saint Joseph Bay is a shallow, subtropical lagoon located on the north-west coast of Florida (29.8°N, 85.3°W) that is semienclosed by the Saint Joseph Peninsula (Fig. 1). Other than freshwater runoff, the bay does not have any significant sources of freshwater such as those from rivers, and has been characterised as having pristine waters with no elevated concentrations of nutrients or chlorophyll (FDEP 2012). Approximately 15 miles (~24 km) long and 6 miles (~9.7 km) wide, the average water depth in the bay is 6.4 m, with the deepest area (9–18 m) located in the north-west region of the bay. The bay contains ~30 km2 of dense, stable populations of continuous and discontinuous seagrass beds (FERI 2006; FDEP 2012) primarily comprising Thalassia testudium with scattered patches of well sorted, siliceous sand (Valentine and Heck 1993; Beddingfield and McClintock 2000). Several invertebrate and vertebrate species have been observed to occur in high densities associated with the seagrass beds within the bay including amphipods, mussels, crabs, scallops, gastropods, sea urchins, sand dollars, sea turtles and a variety of juvenile fish (Valentine and Heck 1993; Heck et al. 2000; Heck et al. 2003).
|
The complexity of coastal seawater chemistry can be attributed to many factors, including the influence of nearby riverine inputs and pollution. Therefore, lack of large freshwater sources and its pristine state make Saint Joseph Bay an excellent site to obtain information on the variability in carbonate chemistry of nearshore, subtropical seagrass ecosystems. Moreover, in shallow seagrass zones, the degree of fluctuation in carbonate chemical parameters may be influenced by wave action, tidal flushing, and water exchange with the open ocean, factors that are minimal in the low-energy, microtidal (less than 2 m) regime of Saint Joseph Bay lagoon (Heck et al. 2000). Characterising the natural variation in the carbonate system of Saint Joseph Bay will identify conditions that marine organisms are experiencing in the field and therefore contribute to understanding the potential response of these organisms to near-future ocean acidification conditions in nearshore seagrass ecosystems.
The objective of the current study was to characterise the carbonate chemistry of a nearshore seagrass ecosystem on multiple timescales (monthly and daily) to ascertain the extent of variation in pH and pCO2 that marine organisms are experiencing.
Methods
Sample collection
To obtain carbonate chemistry parameters, we chose to take discrete water samples from within Eagle Harbor, an area that is located within the Saint Joseph Peninsula State Park (Fig. 1). Three sampling sites within 1 km of each other that had visibly high densities of seagrass were sampled in triplicate at each sampling period (morning and afternoon). One-way analysis of equal variances (ANOVA) using Minitab 17 (Minitab Inc., State College, PA, USA) indicated that the three sites were statistically the same for salinity and temperature (monthly dataset: F2,212 = 0.2, P = 0.971 and F2,212 = 0.19, P = 0.830 respectively). In addition, monthly pH was also tested between sites and was not significantly different (ANOVA, F2,212 = 0.37, P = 0.693). Based on how close the sites were and these statistical results, samples were combined (n = 9 per time sampling). As many of the marine organisms in Saint Joseph Bay have been observed associated with the seagrass beds (e.g. Valentine and Heck 1993; Heck et al. 2000, 2003), we chose to collect benthic water samples. Water samples for carbonate chemistry were collected monthly throughout 2012 and daily during the week of 28 July–4 August 2012. At the time of each sampling at all sites, depth (0.3–3 m) was recorded and temperature and dissolved oxygen (DO) were measured using a YSI-85 meter (Cole-Parmer Instrument Company, LLC North America, Vernon Hills, IL, USA). To collect benthic water samples without allowing gas exchange with the surface (and therefore avoid potential perturbation of seawater chemistry), we modified a dissolved oxygen sampling device used for shallow tidal pool samples (see Daniel and Boyden 1975, fig. 2) designed to minimise gas exchange between the water sample and atmosphere. This device provided consistency in sampling and did not disrupt sediments in the extremely shallow waters (<1 m) that occur at low tide in Eagle Harbor. The device consisted of a 300-mL borosilicate glass bottle with a rubber stopper plumbed with silicone tubing (Fisher Scientific, Thermo Fisher Scientific, Inc., Waltham, MA, USA). The rubber stopper did not allow the overlying water column to mix with water sampled at or near the seafloor as the bottle was brought to the surface. The tubing used to allow air from the bottle to escape was significantly longer in length (2 m) in order to extend from the bottle up to the water’s surface. Therefore water coming in near the seafloor replaced air that was in the bottle slowly, such that bubbles were not created. The air travelled up the tubing to the surface. The stoppered bottle was attached to a 2-m length of PVC pipe (Home Depot, Birmingham, AL, USA) in order to allow the device to be quickly moved to and from the seafloor with minimal gas exchange.
The device was placed on the seafloor and the tubing at the air–water interface was unplugged. Air in the bottle was allowed to escape to the surface, ensuring that water that entered the bottle was from the vicinity of the bottom of the seafloor and no mixing occurred with ambient air. Once water began travelling up the tubing towards the surface (indicating the sampling bottle was full of bottom water) the tubing was again plugged and the bottle was quickly brought to the surface. Triplicate samples were taken from each of three locations over seagrass beds in Eagle Harbor within 1 h of dawn and again later in the day once per month for 12 months and each day for 6 days at the end of July 2012. Upon collection, samples were immediately fixed with 100 μL of mercuric chloride (Fisher Scientific) and stored according to the methods of Dickson et al. (2007) until analysis at the University of Alabama at Birmingham. Analysis was completed within 1–5 months after sampling.
Carbonate chemistry analysis
In the laboratory, water samples were immediately analysed at 25°C for pH (total scale) using a Honeywell DL421 sensor module equipped with a DuraFET III pH electrode (Seelaus Instrument Co., Miamisburg, OH) calibrated with Tris buffers (Dickson, University of California, San Diego, Scripps Institute of Oceanography). The DuraFET III electrode was chosen over spectrophotometric measurement to ensure consistency, as we expected some pH values to be outside the range detected using m-cresol purple. Samples were then analysed for total alkalinity following the methods of Dickson et al. (2007) using a Mettler-Toledo T50 open cell titrator with pH probe (Model DGi115-SC, Columbus, OH, USA) and HCl (Dr A. Dickson laboratory, Scripps Institute of Oceanography, University of California, San Diego, CA, USA) of known concentration and density. Certified reference materials (CRM, Dickson laboratory) for total alkalinity (TA) and the titration method were found to have an average error of 6.80 μmol kg–1 ± 1.19 s.e.m. (n = 29) and a calculated average error for pCO2 of ±2.37 µatm. Internal testing on the precision of the pH probe was performed periodically on samples of Tris buffer (average error 0.0095 ± 0.011 s.e.m., n = 21). Salinity was measured using an Orion 3 Star conductivity meter with an Orion 013005MD conductivity probe (Thermo Fisher Scientific). Carbonate saturation states, dissolved inorganic carbon (DIC), and pCO2 were calculated using pH, temperature, total alkalinity, and salinity using CO2SYS 14 (Lewis and Wallace 1998; Pelletier et al. 2007) and CO2calc (Robbins et al. 2010) with the K1 and K2 constants of Millero et al. (2006) and the KHSO4 constant of Dickson (1990), and total boron constant of Lee et al. (2010). DIC and total alkalinity were normalised for open Gulf of Mexico salinity 35 and are designated as nDIC and nTA respectively.
Correlation coefficients between parameters were obtained using SigmaPlot 11.0.
Results
Monthly seawater chemistry
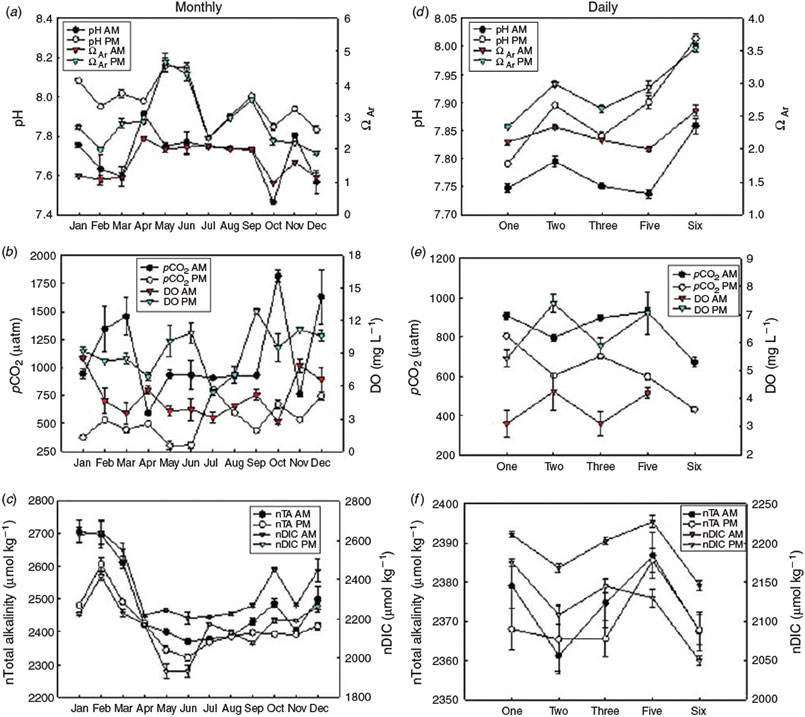
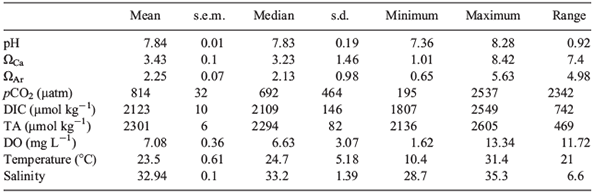
A summary of several descriptive statistics including the range (maximum value – minimum value in the complete dataset for the year) for all parameters for the entire year can be found in Table 1. The annual average pH was 7.84 ± 0.01. Carbonate saturation states did reach values below saturation (minimum of 0.65 ΩAr and 1.01 ΩCa) but mean values were well above saturation (2.25 ΩAr and 3.43 ΩCa). Average pCO2 was 814 ± 32 μatm.
|
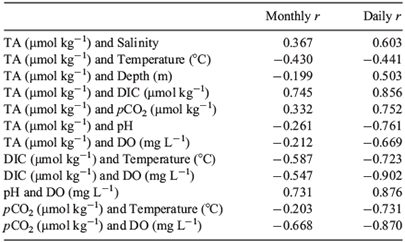
Relationships between parameters measured and calculated on a monthly basis (all P ≤ 0.003: Table 2) indicated that TA showed weak positive correlation with salinity and weak negative correlations with temperature and depth. Importantly, DO was negatively correlated with DIC and pCO2 and was positively correlated with pH and ΩAr.
|
Samples taken on a monthly basis exhibited clear trends for carbonate parameters. Values of pH and carbonate saturation states (ΩCa and ΩAr) were lower in the morning relative to afternoon values with the highest afternoon values observed in May and June 2012 (Fig. 2A). Conversely, pCO2, nDIC (DIC normalised for salinity at 35), and nTA (TA normalised for salinity at 35) values were higher in the morning relative to afternoon values with the lowest afternoon values of pCO2 and nDIC observed in May and June (Fig. 2B, 2C). DO was also consistently lower in the mornings than the afternoons, with highest afternoon values observed in September (Fig. 2B).
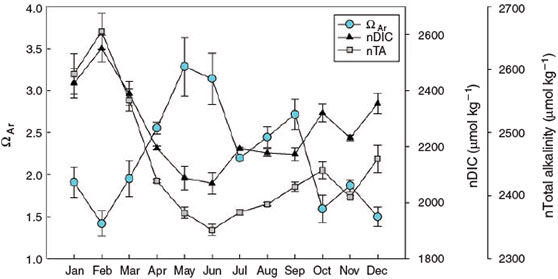
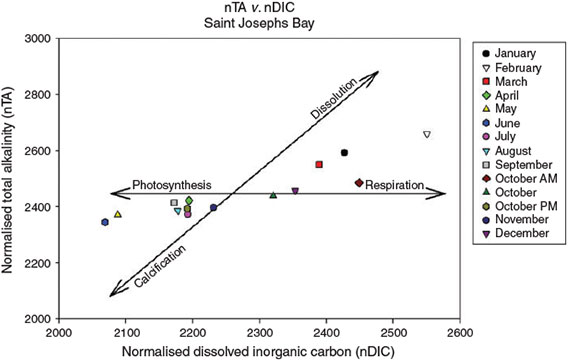
Throughout the year, aragonite saturation state was inversely related to nDIC and, to a lesser extent, nTA, with highest values of ΩAr being observed during April–September, and lowest values during autumn and winter months (Fig. 3). Correlations observed between TA, DIC, pCO2 and pH are expected because of the interdependency of these variables in the carbonate system. The lowest mean nTA and nDIC values occurred primarily during April–September, spring to summer months, with increasing temperatures. Highest mean nTA and nDIC values were observed in winter. Following the methods of Suzuki and Kawahata (2003), we established a nTA v. nDIC plot (Fig. 4). Respiration and dissolution processes appear to dominate during winter (December–March) whereas photosynthesis and calcification dominate April–November, with the exception of October (Fig. 4). In October, respiration and dissolution dominated in the morning whereas photosynthesis and calcification processes dominated in the afternoon.
|
|
Daily seawater chemistry
Summary statistics including the range (maximum value – minimum value in the complete dataset for the week) for all parameters for the entire week can be found in Table 3. Average ranges in pH, ΩAr, and pCO2 were extensive: 0.35 units, 1.99 units and 639 μatm respectively.
Distinct trends in the carbonate parameters were observed each day during the week of 28 July–2 August 2012. Similar to the monthly pattern, pH and ΩAr were lower in the morning relative to afternoon values on a daily timescale (Fig. 2D) and pCO2, nDIC, and nTA were higher in the morning relative to afternoon values (Fig. 2E, 2F). DO was consistently lower in the morning each day relative to afternoon values (Fig. 2E).
Various parameters on daily samples exhibited stronger correlations than that of monthly samples (all P ≤ 0.003: Table 2). Most notably, TA was negatively correlated with DO level. A strong positive correlation was observed between pH and DO and negative correlations between DIC and DO, and pCO2 and DO.
Discussion
Factors influencing carbonate chemistry
Seawater parameters measured at Saint Joseph Bay, Florida, a subtropical estuary adjacent to the Gulf of Mexico, provides insight into the carbonate chemistry and range of variability that a coastal embayment demonstrates over the course of days, months and year. The biogeochemistry of seagrass meadows is highly variable (Marbà et al. 2006). Temperature, salinity, magnitude and timing of tidal cycle controlling residence time of bay water, and biological influences all likely contribute to the observed variability. Biogeochemical processes such as photosynthesis, respiration, carbonate precipitation and dissolution demonstrate predictable relationships in TA and DIC (Gattuso et al. 1999). As photosynthesis occurs, CO2 is removed from the water column and pCO2 and DIC decrease, whereas respiration results in the opposite trends. Calcification (precipitation of calcium carbonate) results in a decrease in DIC and TA, whereas dissolution of calcium carbonate results in increases in these parameters (for a discussion, see Wolf-Gladrow et al. 2007). Therefore, a significant proportion of the variability of the seawater parameters may be attributed to biological influences. For example, seasonal changes in seagrass biomass (e.g. leaf abscission) over an annual cycle (primarily due to changes in light and temperature) likely contribute significantly to the seasonal variability observed in the current study.
The mean annual pH observed in this study (7.84) is significantly lower than the global open ocean average of 8.1. Annual average pH values for Saint Joseph Bay from 1983 to 2008 varied from 7.18 to 8.49 (FDEP 2012). The average pCO2 of the bay was remarkably high (mean 813 μatm) and the reason(s) for this are not clear. Highest monthly pCO2 values were recorded in morning samples taken in February, October, and December and correspond with high nDIC values, suggesting that dissolution and respiration were dominant at the sample sites (Fig. 4). Highest mean pCO2 values were found in March, October, and December when pH showed some of the lowest values (Fig. 2). These months also exhibited low morning values for DO, suggesting that respiration dominated in the morning and photosynthesis dominated in the afternoon (e.g. October DO ranged from 2.7 mg L–1 in the morning v. 9.5 mg L–1 in the afternoon: Fig. 2b). Other bays in Florida, such as Tampa Bay, have been examined for diurnal trends in carbonate parameters (e.g. Yates et al. 2007) and exhibit similar trends in seawater chemistry to those observed in the current study, but considerably lower pCO2 values (maximum 580 μatm for Tampa Bay and 497 μatm for Florida Bay). High pCO2 values may indicate that microbial respiration of organic matter is particularly high in Saint Joseph Bay during the autumn and winter months. Although seagrass beds, especially those dominated by Thalassia sp., are some of the most productive ecosystems in the world (Van Tussenbroek et al. 2006, and references within), they support a significant amount of respiring biomass and therefore may not exhibit high photosynthesis to respiration ratios, or may even become heterotrophic (Marbà et al. 2006, and references within). Seagrasses in temperate latitudes have a distinct annual cycle, with highest leaf abscission rates (and therefore increased sediment organic matter) occurring in late summer–early autumn (Mateo and Romero 1997). An increase in detritus and sediment organic matter has been observed to increase microbial substrates (Gacia and Duarte 2001) and therefore it is possible that microbial respiration rates may be highest during the autumn months in a subtropical bay such as Saint Joseph Bay when fresh leaf litter (and attached epiphytes) has entered the detrital pathway. Large piles of decomposing Thalassia testudium leaves were observed on the beach of the Harbor repeatedly throughout the late summer and autumn (July–October). The lack of significant above-ground seagrass biomass during winter (relative to spring and summer) is also a likely contributing factor to the observed high pCO2 values during winter.
Potential sources of error include the fact that samples in the current study were not filtered before chemical analysis. Although no particulates were observed in the samples tested for total alkalinity, it is possible that calcium carbonate particulates may have contributed to the total alkalinity titration resulting in higher total alkalinity values (and therefore higher calculated pCO2 values under low pH conditions). We recommend the use of a new method for filtration of samples that does not affect total DIC or pH analysis (Bockmon and Dickson 2014). An additional source of error may have been the sampling method used. Although it was possible that gas exchange during sample collection may have occurred, this seems unlikely as pCO2 values would expected to be lower, not higher, and therefore the observed values may be underestimates.
TA and salinity were not strongly correlated at any time scale. Although seawater nutrient data were not available for the current study, Saint Joseph Bay is classified as oligotrophic, with nutrient and chlorophyll-a concentrations within the bay consistently low and stable from 2001 to 2011 (long-term geometric means of total nitrogen = 246.6 μg L–1, total phosphorus = 13.8 μg L–1 and chlorophyll-a = 2.6 μg L–1: FDEP 2012). In some locations, tidal fluctuation can also be an important factor in carbonate chemistry (e.g. Borges and Frankignoulle 1999). In the current study, because of weather, samples could not be taken at the same time during tidal cycles and therefore a portion of the variability observed may be due to the microtidal exchange of Saint Joseph Bay. As the sampling design in the current study involved discrete measurements at only two time points during the day, the results may mask even greater diurnal changes in carbonate parameters occurring in Eagle Harbor. Further studies that involve hourly samples over 48–72 h and at consistent points during tidal cycles between months may better determine the community respiration and photosynthetic dynamics, the influence of tides, as well as the extent and duration of extreme values within the bay.
Calcification and photosynthesis in Saint Joseph Bay
The enclosed embayment of Saint Joseph Bay has a low energy regime and restricted opening to the ocean. Therefore in comparison to non-restricted bays, Saint Joseph Bay has a relatively low volume exchange and high residence time (Heck et al. 2000). The bay also has an abundance of calcifying epiphytes and marine invertebrates (FDEP 2012). The strong positive correlation between pH and DO and negative correlation between pCO2 and DO support the significance of the biological influence on the seawater carbonate chemistry of Saint Joseph Bay (Table 2) particularly in the autumn and winter. As photosynthesis occurs pCO2 and DIC decrease and pH, DO and carbonate saturation states increase. Respiration results in the opposite trends. In addition, TA was strongly, positively correlated with DIC on all time scales (Table 2) and indicates the degree of biological calcification (CaCO3) occurring in the bay at any particular time. Generally, decreases in DIC and TA reflect precipitation, whereas dissolution of CaCO3 results in increases in these parameters (for a discussion, see Wolf-Gladrow et al. 2007). For example, in this study, the morning average values of DIC, TA, and ΩAr over the course of the year indicate that precipitation is more likely to occur April–September (when ΩAr is higher: Fig. 3).
Monthly nTA and nDIC values (normalised for salinity at 35) of Saint Joseph Bay reflect whether net calcification or dissolution, or photosynthesis or respiration are the dominant metabolic processes occurring in a community (Gattuso et al. 1999) (Fig. 4). The lowest mean nTA and nDIC values occurred primarily during April–September, spring to summer, with increasing temperatures. Highest mean nTA and nDIC values occur in the winter. These data reflect the predominance of respiration and dissolution in winter, whereas photosynthesis and calcification dominate in summer and early autumn (Fig. 4). Importantly, daily data indicate that both calcification and photosynthesis may occur daily within Eagle Harbor. As an example, October data indicate that respiration and dissolution are occurring in the morning, whereas photosynthesis and calcification are occurring in the afternoon (Fig. 4).
Implications for ocean acidification experiments
The results of the current study support the recommendations of McElhany and Busch (2013) to take care in choosing appropriate experimental pCO2 levels and thus have important implications for the design of ocean acidification experiments that employ nearshore benthic organisms, both in terms of target carbonate chemistry values and fluctuations of those values. In addition to observing changes in pH, it is important to document changes in nearshore carbonate saturation states as that parameter indicates whether conditions are favourable for calcification. Historically, OA researchers were encouraged to use target experimental values predicted for near-future conditions that reflect atmospheric concentrations of CO2 following IPPC model scenarios (e.g. pCO2 values of 750 μatm, pH 7.7: Riebesell et al. 2010). The mean values reported in the present study (pH 7.84, pCO2 813.83 μatm) suggest that the control values typically employed in OA experiments (reflecting ambient atmospheric CO2 concentrations, or pH 8.1, pCO2 400 μatm) with nearshore benthic organisms do not represent natural conditions for seagrass environments in coastal waters such as Saint Joseph Bay. Future experiments employing organisms that reside in seagrass ecosystems should take into account not only the daily fluctuations, but also seasonal variability that is characteristic of the particular seagrass species that is dominant in the area from which organisms are collected.
These observations have important implications for interpreting OA results. For example, in Saint Joseph Bay, high gonadal indices (gonad wet weight per whole urchin wet weight) have been reported during late July–early August for the sea urchin Lytechinus variegatus (Beddingfield and McClintock 2000), indicating gonad maturation and that spawning and fertilisation events likely take place during that time of year. The current study found daily benthic pH values in the range of 7.70–8.06, pCO2 values of 379.31–1018.53 μatm (with a mean of 7.83 pH units and 733.30 μatm) and aragonite saturation state values of 1.86–3.85 (average 2.56) during late July–early August, suggesting that the larvae of L. variegatus that live in the bay experience reduced pH and high pCO2 conditions. Laboratory studies on the early development of L. variegatus indicate that larval echinoplutei exhibit delayed development and altered larval shape if exposed to low pH and high pCO2 values for an extended period (Challener et al. 2013). Yet the population of L. variegatus at Eagle Harbor has been stable for over 25 years (Stephen Watts, pers. obs.). Therefore, the low pH and high pCO2 conditions of OA experiments may be more representative of the current natural conditions and thus more indicative of the natural developmental rates of echinoids in the field.
Extensive diel fluctuations observed in the current study are also important aspects to consider in the design of OA experiments. Maintaining static pH and pCO2 values over the course of an experiment does not reflect environmental conditions. These fluctuations may serve as critical periods of organismal recovery from hypercapnic conditions. For example, accretion rates of coral reef sites have been observed to be higher at sites that exhibit longer periods of high pH values (Price et al. 2012). Populations of marine organisms within Saint Joseph Bay have been characterised as highly productive and healthy (with the exception of sporadic red tide events: FDEP 2012, and references within), suggesting that these organisms have either acclimatised or adapted successfully to extensive variation in pH and pCO2 values. The physiological mechanisms underlying the ability to cope with such dramatic fluctuations in both physical and chemical parameters are not well understood and the biological consequences of increasing ocean acidification superimposed on these natural fluctuations are unknown. If periods of high pH and low pCO2 conditions are essential for organismal function, then the negative consequences of a high pCO2 future (i.e. where the ‘high pH’ is no greater than 7.8) may be significant (see Shaw et al. 2013 for a discussion). To date, the ability of echinoids to acclimate to hypercapnic conditions within 16 months has been observed in one species (Dupont et al. 2012), highlighting the need for additional studies on the capacity for acclimation and potential acclimatisation in the field. In addition, experimental design should compare the exterior surface pH of study organisms (e.g. the surface layer of the organism) to the bulk carbonate chemistry measurements made in the field to ascertain the actual values that organisms are experiencing (e.g. Flynn et al. 2012). Long-term OA studies that incorporate the natural diurnal and seasonal variation into both control and experimental treatments are needed to determine nearshore organismal resilience to near-future ocean acidification conditions.
Acknowledgements
This research was a part of R. C. Challener’s dissertation at the University of Alabama at Birmingham. Many thanks to John, Travis, and Forrest Thompson for their assistance in the field; to Andrew Dickson, Emily Bockman, and Michael O’Donnell for their helpful input on chemical analysis methods and equipment use. Laura Enzor and Kirk Sato designed the PVC modification to the sampling device. We are grateful to John Lisle for providing comments on earlier drafts. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the USA Government. JBM acknowledges support provided by NSF grant ANT-1041022. L. L. Robbins acknowledges support provided by the USGS Coastal and Marine Geology Program.
References
Beddingfield, S. D., and McClintock, J. B. (2000). Demographic characteristics of Lytechinus variegatus (Echinoidea: Echinodermata) from three habitats in a North Florida bay, Gulf of Mexico. Marine Ecology (Berlin) 21, 17–40.| Demographic characteristics of Lytechinus variegatus (Echinoidea: Echinodermata) from three habitats in a North Florida bay, Gulf of Mexico.Crossref | GoogleScholarGoogle Scholar |
Beer, S., Mtolera, M., Lyimo, T., and Björk, M. (2006). The photosynthetic performance of the tropical seagrass Halophila ovalis in the upper intertidal. Aquatic Botany 84, 367–371.
| The photosynthetic performance of the tropical seagrass Halophila ovalis in the upper intertidal.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XivVGkurY%3D&md5=0e4c95d2969521e8cefc8209713ac09fCAS |
Bockmon, E. E., and Dickson, A. G. (2014). Seawater filtration method suitable for total dissolved inorganic carbon and pH analyses. Limnology and Oceanography, Methods 12, 191–195.
Borges, A. V., and Frankignoulle, M. (1999). Daily and seasonal variations of the partial pressure of CO2 in surface seawater along Belgian and southern Dutch coastal areas. Journal of Marine Systems 19, 251–266.
| Daily and seasonal variations of the partial pressure of CO2 in surface seawater along Belgian and southern Dutch coastal areas.Crossref | GoogleScholarGoogle Scholar |
Byrne, M. (2011). Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Oceanography and Marine Biology - an Annual Review 49, 1–42.
Challener, R. C., McClintock, J. B., and Makowsky, R. (2013). Effects of reduced carbonate saturation state on early development in the common edible sea urchin Lytechinus variegatus: implications for land-based aquaculture. Journal of Applied Aquaculture 25, 154–175.
| Effects of reduced carbonate saturation state on early development in the common edible sea urchin Lytechinus variegatus: implications for land-based aquaculture.Crossref | GoogleScholarGoogle Scholar |
Daniel, M. J., and Boyden, C. R. (1975). Diurnal variations in physico-chemical conditions within intertidal rockpools. Field Studies 4, 161–176.
DeGrandpre, M. D., Hammar, T. R., Smith, S. P., and Sayles, F. L. (1995). In situ measurements of seawater pCO2. Limnology and Oceanography 40, 969–975.
| In situ measurements of seawater pCO2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXosFent7o%3D&md5=9d599d09757e2b704ecfe5663face39dCAS |
Dickson, A. G. (1990). Thermodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 318.15K. Deep-Sea Research. Part A, Oceanographic Research Papers 37, 755–766.
| Thermodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 318.15K.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXivFKmug%3D%3D&md5=9496c8d1342e8b4b73b844cb9e9cafacCAS |
Dickson, A. G., Sabine, C. L., and Christian, J. R. (Eds) (2007). ‘Guide to Best Practices for Ocean CO2 Measurements.’ PICES Special Publication number 3. Sidney, BC, Canada.
Doney, S. C., Fabry, V. J., Feely, R. A., and Kleypas, J. A. (2009). Ocean acidification: the other CO2 problem. Annual Review of Marine Science 1, 169–192.
| Ocean acidification: the other CO2 problem.Crossref | GoogleScholarGoogle Scholar | 21141034PubMed |
Dupont, S., Ortega-Martínez, O., and Thorndyke, M. (2010). Impact of near-future ocean acidification on echinoderms. Ecotoxicology (London, England) 19, 449–462.
| Impact of near-future ocean acidification on echinoderms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjtVGjsbk%3D&md5=095a115b95dd627912ff7ce95a80916eCAS |
Dupont, S., Dorey, N., Stumpp, M., Melzner, F., and Thorndyke, M. (2012). Long-term and trans-life-cycle effects of exposure to ocean acidification in the green sea urchin Strongylocentrotus droebachiensis. Marine Biology , .
| Long-term and trans-life-cycle effects of exposure to ocean acidification in the green sea urchin Strongylocentrotus droebachiensis.Crossref | GoogleScholarGoogle Scholar | 24391285PubMed |
Fabry, V. J., Seibel, B. A., Feely, R. A., and Orr, J. C. (2008). Impacts of ocean acidification on marine fauna and ecosystem processes. ICES Journal of Marine Science 65, 414–432.
| Impacts of ocean acidification on marine fauna and ecosystem processes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntFegtL4%3D&md5=51e793d49d48944e0ade5a432c1e3adeCAS |
FDEP (2012). Site-specific information in support of establishing numeric nutrient criteria for St Joseph Bay. Division of Environmental Assessment and Restoration, Standards and Assessment Section, Florida Department of Environmental Protection. Tallahassee, FL. Available at http://www.dep.state.fl.us/water/wqssp/nutrients/docs/estuaries/st_joe_bay_nutrie nt-tsd.pdf [Verified 25 November 2014].
Florida Environmental Research Institute (FERI) (2006). St Joseph Bay Aquatic Preserve – hyperspectral imaging. Tampa, FL. Available at https://feri.s3.amazonaws.com/pubs_ppts/Final+Report_StJoe_w_Appendices.pdf [Verified 25 November 2014].
Flynn, K. J., Blackford, J. C., Baird, M. E., Raven, J. A., Clark, D. R., Beardall, J., Brownlee, C., Fabian, H., and Wheeler, G. L. (2012). Changes in pH at the exterior surface of plankton with ocean acidification. Nature Climate Change 2, 510–513.
| Changes in pH at the exterior surface of plankton with ocean acidification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XptVGiu7w%3D&md5=1124ab66598a6d9222e16c3382d21cdfCAS |
Gacia, E., and Duarte, C. M. (2001). Sediment retention by a Mediterranean Posidonia oceanica meadow: the balance between deposition and resuspension. Estuarine, Coastal and Shelf Science 52, 505–514.
| Sediment retention by a Mediterranean Posidonia oceanica meadow: the balance between deposition and resuspension.Crossref | GoogleScholarGoogle Scholar |
Gattuso, J.-P., Frankignoulle, M., and Smith, S. V. (1999). Measurement of community metabolism and significance in the coral reef CO2 source–sink debate. Proceedings of the National Academy of Sciences of the United States of America 96, 13017–13022.
| Measurement of community metabolism and significance in the coral reef CO2 source–sink debate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXns1Gmsro%3D&md5=b26a858c6a096292ed3bd284cd9700dfCAS | 10557265PubMed |
Hales, B., Takahashi, T., and Bandstra, L. (2005). Atmospheric CO2 uptake by a coastal upwelling system. Global Biogeochemical Cycles 19, .
| Atmospheric CO2 uptake by a coastal upwelling system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXktlaht7o%3D&md5=f8ce7b2b558d74e6184727aa325e077aCAS |
Heck, K. L., Pennock, J. R., Valentine, J. F., Coen, L. D., and Sklenar, S. A. (2000). Effects of nutrient enrichment and small predator density on seagrass ecosystems: an experimental assessment. Limnology and Oceanography 45, 1041–1057.
| Effects of nutrient enrichment and small predator density on seagrass ecosystems: an experimental assessment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXlvFCrtro%3D&md5=99bfc041ecb22aa4a3f781023b025a93CAS |
Heck, K. L., Hays, G., and Orth, R. J. (2003). Critical evaluation of the nursey role hypothesis for seagrass meadows. Marine Ecology Progress Series 253, 123–136.
| Critical evaluation of the nursey role hypothesis for seagrass meadows.Crossref | GoogleScholarGoogle Scholar |
Hendriks, I. E., Duarte, C. E., and Álvarez, M. (2010). Vulnerability of marine biodiversity to ocean acidification: a meta-analysis. Estuarine, Coastal and Shelf Science 86, 157–164.
| Vulnerability of marine biodiversity to ocean acidification: a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhs1aqtbvM&md5=46fde8894a127e160f4b10f053c4c2e8CAS |
Hofmann, G. E., Barry, J. P., Edmunds, P. J., Gates, R. D., Hutchins, D. A., Klinger, T., and Sewell, M. A. (2010). The effect of ocean acidification on calcifying organisms in marine ecosystems: an organism-to-ecosystem perspective. Annual Review of Ecology Evolution and Systematics 41, 127–147.
| The effect of ocean acidification on calcifying organisms in marine ecosystems: an organism-to-ecosystem perspective.Crossref | GoogleScholarGoogle Scholar |
Hofmann, G.E., Smith, J.E., Johnson, K.S., Send, U., Levin, L.A., Micheli, F., Paytan, A., Price, N.N., Peterson, B., Takeshita, Y., Matson, P.G., Crook, E.D., Kroeker, K.J., Gambi, M.C., Rivest, E.B., Frieder, C.A., Yu, P.C., and Martz, T. R. (2011). High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS ONE 6, .
| High-frequency dynamics of ocean pH: a multi-ecosystem comparison.Crossref | GoogleScholarGoogle Scholar | 22205986PubMed |
Invers, O., Romero, J., and Pérez, M. (1997). Effects of pH on seagrass photosynthesis: a laboratory and field assessment. Aquatic Botany 59, 185–194.
| Effects of pH on seagrass photosynthesis: a laboratory and field assessment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXhsFCgsg%3D%3D&md5=381465d4effb12ece596b5c60c1a8827CAS |
IPCC (2007). ‘Intergovernmental Panel on Climate Change: Climate Change 2007: The Physical Science Basis – Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change.’ (Cambridge University Press: Cambridge, UK).
Kroeker, K. J., Kordas, R. L., Crim, R. N., and Singh, G. G. (2010). Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecology Letters 13, 1419–1434.
| Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms.Crossref | GoogleScholarGoogle Scholar | 20958904PubMed |
Lee, K., Kim, T.-W., Byrne, R. H., Millero, F. J., Feely, R. a., and Liu, Y.-M. (2010). The universal ratio of boron to chlorinity for the North Pacific and North Atlantic oceans. Geochimica et Cosmochimica Acta 74, 1801–1811.
| The universal ratio of boron to chlorinity for the North Pacific and North Atlantic oceans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhvFaltbc%3D&md5=71b8c684ba76ce39276e0520b68cd67aCAS |
Lewis, E., and Wallace, D. W. R. (1998). Program developed for CO2 system calculations. ORNL/CDIAC-105. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, TN.
Marbà, N., Holmer, M., Gacia, E., and Barrón, C. (2006). Seagrass beds and coastal biogeochemistry. In ‘Seagrasses: Biology, Ecology and Conservation’. (Eds W. D. Larkum, R. J. Orth, and C. M. Duarte.) pp. 135–157. (Springer: Dordrecht, the Netherlands).
Mateo, M. A., and Romero, J. (1997). Detritus dynamics in the seagrass Posidonia oceanica: elements for an ecosystem carbon and nutrient budget. Marine Ecology Progress Series 151, 43–53.
| Detritus dynamics in the seagrass Posidonia oceanica: elements for an ecosystem carbon and nutrient budget.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXmtF2qtr8%3D&md5=1bdb2633b59404878e2d7b169beccf82CAS |
McElhany, P., and Busch, S. D. (2013). Appropriate pCO2 treatments in ocean acidification experiments. Marine Biology 160, 1807–1812.
| Appropriate pCO2 treatments in ocean acidification experiments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht1GgtbzP&md5=3874241c5ac15635a82379c0f7f93ec3CAS |
Middelboe, A. L., and Hansen, P. J. (2007). High pH in shallow-water macroalgal habitats. Marine Ecology Progress Series 338, 107–117.
| High pH in shallow-water macroalgal habitats.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXot1Sgt70%3D&md5=bd6ca86ed3258deef8e506c8023da84bCAS |
Millero, F. J., Hiscock, W. T., Huang, F., Roche, M., and Zhang, J. Z. (2001). Seasonal variation of the carbonate system in Florida Bay. Bulletin of Marine Science 68, 101–123.
Millero, F. J., Graham, T. B., Huang, F., Bustos-Serrano, H., and Pierrot, D. (2006). Dissociation constants of carbonic acid in seawater as a function of salinity and temperature. Marine Chemistry 100, 80–94.
| Dissociation constants of carbonic acid in seawater as a function of salinity and temperature.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjsFegsLk%3D&md5=253ac4b32aec31f7f795640aeefc4482CAS |
Ohde, S., and van Woesik, R. (1999). Carbon dioxide flux and metabolic processes of a coral reef, Okinawa. Bulletin of Marine Science 65, 559–576.
Orr, J. C., Fabry, V. J., Aumont, O., Bopp, L., Doney, S. C., Feely, R. A., Gnanadesikan, A., Gruber, N., Ishida, A., Joos, F., Key, R. M., Lindsay, K., Maier-Reimer, E., Matear, R., Monfray, P., Mouchet, A., Najjar, R. G., Plattner, G.-K., Rodgers, K. B., Sabine, C. L., Sarmiento, J. L., Schlitzer, R., Slater, R. D., Totterdell, I. J., Weirig, M. F., Yamanaka, Y., and Yool, A. (2005). Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686.
| Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVCjsL%2FE&md5=8dac831b674316b69dde821d4e9bf4a8CAS | 16193043PubMed |
Pelletier, G., Lewis, E., and Wallace, D. W. R. (2007). CO2SYS.XLS. A calculator for the CO2 system in seawater for Microsoft Excel/MBA. Washington State Department of Ecology, Olympia, Washington, and Brookhaven National Laboratory, Upton, NY.
Price, N. N., Martz, T. R., Brainard, R. E., and Smith, J. E. (2012). Diel variability in seawater pH relates to calcification and benthic community structure on coral reefs. PLoS ONE 7, e43843.
| Diel variability in seawater pH relates to calcification and benthic community structure on coral reefs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1yktLbJ&md5=61443f6b24a8ebac58b2930be1003935CAS | 22952785PubMed |
Riebesell, U., Fabry, V. J., Hansson, L., and Gattuso, J.-P. (Eds) (2010). ‘Guide to Best Practices for Ocean Acidification Research and Data Reporting.’ (Publications Office of the European Union: Luxembourg.)
Robbins, L. L., Hansen, M. E., Kleypas, J. A., and Meylan, S. C. (2010). CO2calc – a user-friendly seawater carbon calculator for Windows, Max OSX, and iOS (iPhone). US Geological Survey Open-File Report 2010–1280 (version 1.2.9).
Semesi, I. S., Beer, S., and Björk, M. (2009). Seagrass photosynthesis controls rates of calcification and photosynthesis of calcareous macroalgae in a tropical seagrass meadow. Marine Ecology Progress Series 382, 41–47.
| Seagrass photosynthesis controls rates of calcification and photosynthesis of calcareous macroalgae in a tropical seagrass meadow.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnvFaisrg%3D&md5=8a153f85213e41f4eeefb168e96f0dd5CAS |
Shaw, E. C., Munday, P. L., and McNeil, B. I. (2013). The role of CO2 variability and exposure time for biological impacts of ocean acidification. Geophysical Research Letters 40, 4685–4688.
| The role of CO2 variability and exposure time for biological impacts of ocean acidification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsFynsb3J&md5=e5c3ec4914afee8053bdd6621e4b2a2fCAS |
Suzuki, A., and Kawahata, H. (2003). Carbon budget of coral reef systems: an overview of observations in fringing reefs, barrier reefs and atolls in the Indo-Pacific regions. Tellus. Series B, Chemical and Physical Meteorology 55, 428–444.
| Carbon budget of coral reef systems: an overview of observations in fringing reefs, barrier reefs and atolls in the Indo-Pacific regions.Crossref | GoogleScholarGoogle Scholar |
Unsworth, R. K. F., Collier, C. J., Henderson, G. M., and McKenzie, L. J. (2012). Tropical seagrass meadows modify seawater carbon chemistry: implications for coral reefs impacted by ocean acidification. Environmental Research Letters 7, 024026.
| Tropical seagrass meadows modify seawater carbon chemistry: implications for coral reefs impacted by ocean acidification.Crossref | GoogleScholarGoogle Scholar |
Valentine, J. F., and Heck, K. L. (1993). Mussels in seagrass meadows: their influence on macroinvertebrate abundance and secondary production in the northern Gulf of Mexico. Marine Ecology Progress Series 96, 63–74.
| Mussels in seagrass meadows: their influence on macroinvertebrate abundance and secondary production in the northern Gulf of Mexico.Crossref | GoogleScholarGoogle Scholar |
Van Tussenbroek, B. I., Vonk, J. A., Stapel, J., Erftemeijer, P. L. A., Middelburg, J. J., and Zieman, J. C. (2006). The biology of Thalassia: paradigms and recent advances in research. In ‘Seagrasses: Biology, Ecology and Conservation’. (Eds W. D. Larkum, R. J. Orth, and C. M. Duarte.) pp. 409–439. (Springer: Dordrecht, the Netherlands.)
Wahl, M., Sawall, Y., and Saderne, V. (in press). How good are we at assessing the impact of ocean acidification in coastal systems? Limitations, omissions and strengths of commonly used experimental approaches with a special emphasis on the neglected role of fluctuations. Marine and Freshwater Research , .
| How good are we at assessing the impact of ocean acidification in coastal systems? Limitations, omissions and strengths of commonly used experimental approaches with a special emphasis on the neglected role of fluctuations.Crossref | GoogleScholarGoogle Scholar |
Waldbusser, G. G., and Salisbury, J. E. (2014). Ocean acidification in the coastal zone from an organism’s perspective: multiple system parameters, frequency domains, and habitats. Annual Review of Marine Science 6, 221–247.
| Ocean acidification in the coastal zone from an organism’s perspective: multiple system parameters, frequency domains, and habitats.Crossref | GoogleScholarGoogle Scholar | 23987912PubMed |
Wolf-Gladrow, D. A., Zeebe, R. E., Klaas, C., Körtzinger, A., and Dickson, A. G. (2007). Total alkalinity: the explicit conservative expression and its application to biogeochemical processes. Marine Chemistry 106, 287–300.
| Total alkalinity: the explicit conservative expression and its application to biogeochemical processes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXovFSmurc%3D&md5=592830d17db1a6af02db24da5cba0969CAS |
Wootton, J. T., Pfister, C. A., and Forester, J. D. (2008). Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset. Proceedings of the National Academy of Sciences of the United States of America 105, 18848–18853.
| Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsV2rtL3M&md5=2f8311fdad878c6620bfbb2942f47fc5CAS | 19033205PubMed |
Yates, K. K., Dufore, C., Smiley, N., Jackson, C., and Halley, R. B. (2007). Diurnal variation of oxygen and carbonate system parameters in Tampa Bay and Florida Bay. Marine Chemistry 104, 110–124.
| Diurnal variation of oxygen and carbonate system parameters in Tampa Bay and Florida Bay.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXitFSmtrc%3D&md5=312b1efe3113d472bffde3fe5e61a5e3CAS |