The importance of a constructed near-nature-like Danube fish by-pass as a lifecycle fish habitat for spawning, nurseries, growing and feeding: a long-term view with remarks on management
Paul Meulenbroek A C , Silke Drexler A , Christoffer Nagel B , Michael Geistler A and Herwig Waidbacher AA University of Natural Resources and Life Sciences, Vienna, Institute of Hydrobiology and Aquatic Ecosystem Management, Gregor-Mendel-Strasse 33, A-1180 Vienna, Austria.
B Technical University of Munich, Department for Ecology and Ecosystem Management, Chair of Aquatic Systems Biology, Mühlenweg 22, D-85354 Freising, Germany.
C Corresponding author. Email: paul.meulenbroek@boku.ac.at
Marine and Freshwater Research 69(12) 1857-1869 https://doi.org/10.1071/MF18121
Submitted: 26 March 2018 Accepted: 2 August 2018 Published: 17 October 2018
Journal Compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
Major sections of today’s rivers are man made and do not provide the essential requirements for riverine fish. A nature-like fish by-pass system in Vienna-Freudenau was assessed for its function as a fish habitat. The study was conducted continuously over 3 years; 15 years after construction of the by-pass. The chosen nature-like construction of the by-pass system functions like natural tributaries. More than 17 000 fish and 43 species, including several protected and endangered species, in all life stages, including eggs, larvae, juveniles and adults, were captured. Furthermore, the indicator species of the free-flowing Danube, nase (Chondrostoma nasus) and barbel (Barbus barbus), migrated into the fish by-pass and successfully spawned before returning. Therefore, our results suggest that by-pass systems can function as an important habitat for the conservation of native fish fauna. The heterogenic habitat configuration provides conditions for all ecological guilds and, consequently, increases biodiversity. Finally, approved management tools are discussed. We suggest that fish by-pass channels may be suitable at other sites in the Danube catchment.
Additional keywords: Barbus barbus, by-pass management, Chondrostoma nasus, cyprinids, large river.
Introduction
Hydromorphological alterations for navigation, flood protection, hydroelectric power generation, as well as the disconnection of tributaries, have resulted in riverine habitat degradation and fragmentation, especially in large rivers such as the Danube (Schiemer 2000; Morley and Karr 2002; Dudgeon et al. 2006). These habitat modifications affect the integrity and diversity of freshwater biota (Karr et al. 1985; Allan and Flecker 1993; Richter et al. 1996). Lack of functional spawning grounds, nursery habitats and reduced connectivity are now considered to be limiting factors for riverine fish populations (Keckeis and Schiemer 2002; Jungwirth et al. 2003; Pander and Geist 2010; Jungwirth et al. 2014).
According to the key objective of the European Water Framework Directive (WFD), all waterbodies in the EU need to achieve good ecological status. This is defined in Annex V of the Water Framework Proposal as a slight departure from the biological community (fish, benthic invertebrates and aquatic flora) that would be expected in conditions of minimal anthropogenic impact (European Parliament and the Council of the European Union 2000). For WFD implementation, among others, the Austrian legal framework (NGP, Gewässerbewirtschaftungsplan 2009) focuses on the provision of a longitudinal migration for aquatic organisms by installing fish by-passes. Investigations showed that a free passage alone does not improve the ecological status of a river satisfactorily in many cases (Schmutz 2012; Reyjol et al. 2014; Harreiter et al. 2015). To improve connectivity, near-natural fish by-passes provide passage for a wider range of species, age classes and sizes and are, therefore, preferred over hard technical fish by-passes (Jungwirth et al. 1998; Calles and Greenberg 2007; Tummers et al. 2016).
There are many river restoration projects (e.g. the creation of gravel banks, riparian bays and channel systems or lateral connections of waterbodies) that attempt to create and restore important key habitats for the different life stages of endangered species such as spawning grounds, and larval and juvenile habitats to strengthen fish populations (Schiemer and Waidbacher 1992; Barlaup et al. 2008; Pulg et al. 2013; Geist and Hawkins 2016; Pander and Geist 2016; Zauner et al. 2016; Meulenbroek et al. 2018; Waidbacher et al. 2018). Near-natural by-pass solutions can provide both, namely, possibility of migration as well as the provision of the abovementioned key habitats. There are limited studies, mostly focusing on salmonids at by-passes in smaller rivers, showing this multifunctional role for different species (Eberstaller et al. 1998; Parasiewicz et al. 1998; Calles and Greenberg 2007; Gustafsson et al. 2013; Pander et al. 2013; Tamario et al. 2018). However, to the best of our knowledge, the present study is the first on a large river such as the Danube (mean annual discharge 1910 m3 s–1, Niederösterreich 2018) that considers all life stages of fish deriving from a broad range of species. Besides improved connectivity, shown by Eberstaller et al. (2001) in 2000, there have been first indications of possible additional benefits, such as spawning activities during this time.
The objective of the present study is to assess the near-natural by-pass system in Freudenau-Vienna as a habitat. We hypothesised that the fish by-pass would provide habitat for spawning, nurseries, growth and feeding. Furthermore, following the principles of ecological guilds (Balon 1990; Schiemer and Waidbacher 1992), the heterogenic configuration should provide conditions for different species compositions. Therefore, we sampled fish larvae, juveniles, and adult fish and analysed species occurrences and spatial and temporal differences of assemblage structure.
Materials and methods
Study sites
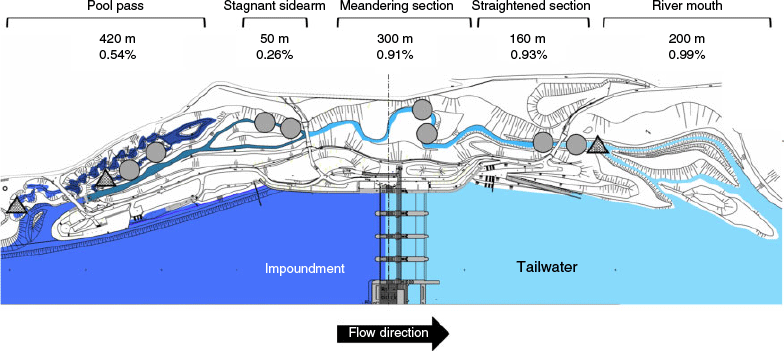
The Hydropower plant (HPP) Vienna-Freudenau is the newest HPP in the Danube (mean discharge 1910 m3 s–1) and was built in 1998. A fish migration by-pass system was incorporated with two major components, namely, a near-natural by-pass channel and a near-natural pool pass (Fig. 1). The fish by-pass starts 500 m downstream of the HPP, with a delta system in the tailwater that has calm, shallow waters over some 200 m with two permanent wetted channels. The subsequent semi-natural by-pass channel has an average slope of 0.7% and is situated in a 7-m-wide riverbed with and an average current speed of ~0.6 m s–1. The first 160-m length is straight (hereafter called the straightened section), followed by a 300-m-long meandering section (hereafter meandering section) and a 140-m-long branched section.
|
One of the branches is blocked by a beaver dam and has calm to stagnant water for ~50 m (hereafter stagnant sidearm). The remaining section of 170 m up to the weir is straight again. The total length of this free-flowing section is ~1000 m. The channel bottom was constructed with a 1-m-thick layer of gravel and sand; subjacent is a 0.4-m-thick silt layer that seals the fish by-pass. Some rifle-pool sequences are developed and very dense riparian vegetation has been well established, consisting mainly of willows and alders.
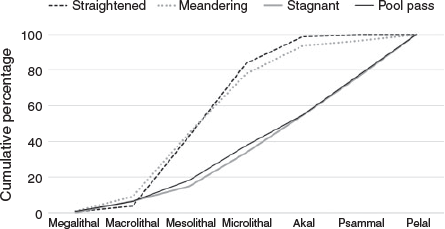
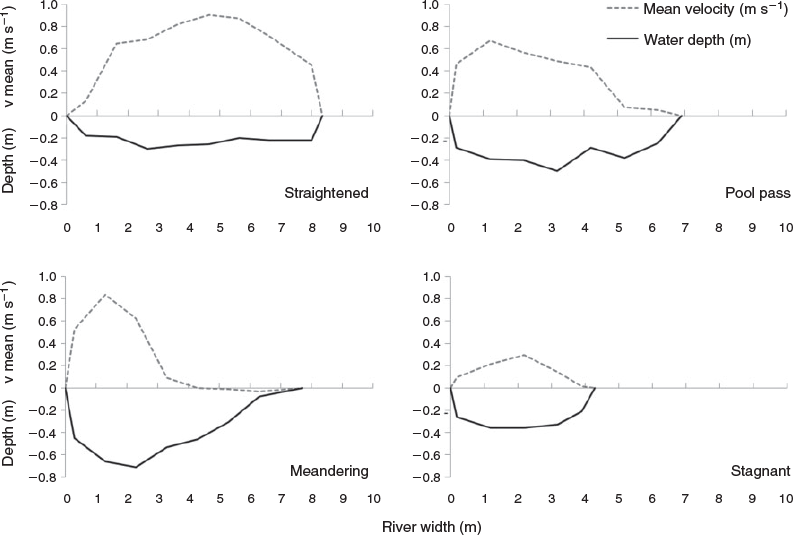
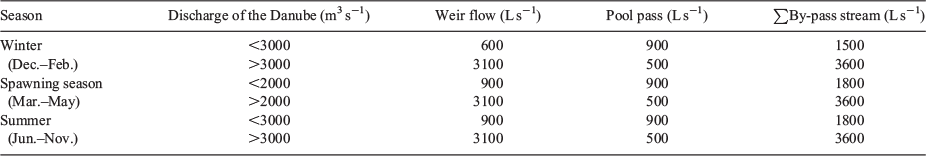
The uppermost part of the system is a pool pass of 19 pools (20–40 m in length and 3–16 m in width), with a minimum of 70 m2 per pool, a water-level difference of 11 cm from pool to pool, and a total length of 420 m (hereafter pool pass). It is characterised by a pool depth of 1.5 m, different flow conditions (from reverse flow to velocities up to 1 m s–1), high abundances of reeds and macrophytes. There are big boulders (30–50 cm) at the ramps between the pools and different substrate patterns, ranging from gravel to very fine sediments with high quantities of xylal. Sediments ranged from megalithal to pelal (Önorm-6232 1995; Fig. 2). The straightened and meandering sections were rather similar, showing a high percentage (~80%) of lithal (mega, macro, meso, microlithal) fractions. In contrast, the pool pass and the stagnant sidearm exhibited higher percentages (~65%) of finer fractions (Akal, Psammal and Pelal). At the fishing points, water depth, river width and flow velocities were measured (Fig. 3). Mean flow velocity was calculated following Kreps (1975). For a detailed description of the by-pass, see Eberstaller et al. (1998). The discharge of the by-pass is not constant but changes depending on the discharge of the Danube and the season, ranging from 1.5 m3 s–1 in winter to a maximum of 3.6 m3 s–1 during higher discharges in the main river (Table 1).
|
|
|
Field sampling, identification and data analyses
The main sampling campaign comprised point abundance sampling by electrofishing (EF) of juvenile and adult fish (Copp and Peňáz 1988) from January 2014 to December 2015. For EF, the backpack-generator ELT60-IIH (Hans Grassl GmbH, Schönau am Königssee, Germany) was used, according to the code of practice and national standard in Austria (Haunschmid et al. 2010). The generator operates with direct current at 1.3 kW and 500 V. The same two points (~20 m2) in each section (straightened section, meandering section, pool pass and stagnant sidearm) were fished approximately every 2 weeks, with a total of 225 sampling events. A constant fishing effort was applied for each sampling event and the catch per unit effort (CPUE) was used for further analyses on the basis of these data. Additional fishing was undertaken at selected habitats in 2013 and 2016 (22 sampling events). Fish larvae were sampled from April to September 2015 with the same drift nets as described by Meulenbroek et al. (2018), in 27 sampling events at the following three different locations: one at the beginning of the pool pass, one at the end of the pool pass and one at the end of the straightened section (triangles in Fig. 1). Fish larvae samples were taken approximately once per week, always during the night with an exposure time of 12 h (CPUE). All juvenile and adult specimens were identified to species level by using morphological characters (Wiesner and Zauner 1999), counted, and their total length was measured. Early life stages of fish were identified to family level and further processed as described in Meulenbroek et al. (2018). A subsample of 560 individuals was analysed with mt-DNA barcoding to species level (Hebert et al. 2003). The selection criteria for the individuals to be barcoded were the abundance within each family found in a sampling site for each calendar week and the potential number of species. The latter represents the proportions of potential species for each family (Cyprinidae: 32 species; Gobiidae and Cottidae: 5 species; Percidae: 9 species; compare with Meulenbroek et al. 2018). The calculated number of individuals for barcoding was then randomly selected by the randomise tool in Excel (2016). The primers FishCo1-F and FishCo1-R (Baldwin et al. 2009) were used, and for some individuals we also used the cytochrome b primers KAI_F and KAI_R (Kotlík et al. 2008).
The affiliation to guilds followed Schiemer and Waidbacher (1992), as well as Zauner and Eberstaller (1999), and was expanded for Neogobius melanostumus, Ponticola kessleri, Babka gymnotrachelus and Lepomis gibbosus (compare with Table 2; Kottelat and Freyhof 2007). Kruskal–Wallis test was performed using SPSS (IBM SPSS Statistics for Windows, ver. 24.0, IBM Corp., Armonk, NY, USA), followed by a Dunn–Bonferroni post hoc test. Significance was accepted at P < 0.05. Frequency-of-use graphs (FUG) were calculated as normalised probability density functions ranging from 0 to 1 (Raleigh et al. 1984; Melcher and Schmutz 2010):

where ∫i is the class frequency and ∫[max] is the maximum class frequency.
|
To visualise spatial differences, 95% confidence intervals (Sachs 2004), histograms, line plots and a Venn diagram were compiled. Non-metric multidimensional scaling (NMS; Kruskal 1964) was applied to the ecological guild data (densities were ln(x + 1) transformed, for juveniles and adults separately). Indicator species analysis (ISA; Dufrêne and Legendre 1997) identified fish species and life stages that serve as indicators for different habitats and seasons. Only species with an indicator value (IV) of >25 and P < 0.05 (Monte Carlo permutation test) were considered. NMS and ISA were performed with PC-ORD (ver. 5.33, MjM Software, Gleneden Beach, OR, USA). Length–frequency diagrams were used to illustrate population structure, showing the relationships between length classes and relative frequency.
Results
During the entire study period, 17 200 fish were caught, comprising 6800 adults, 3900 juveniles and 6500 larvae. In total, 43 species from 12 families were detected with spatial and seasonal variations.
Habitat function
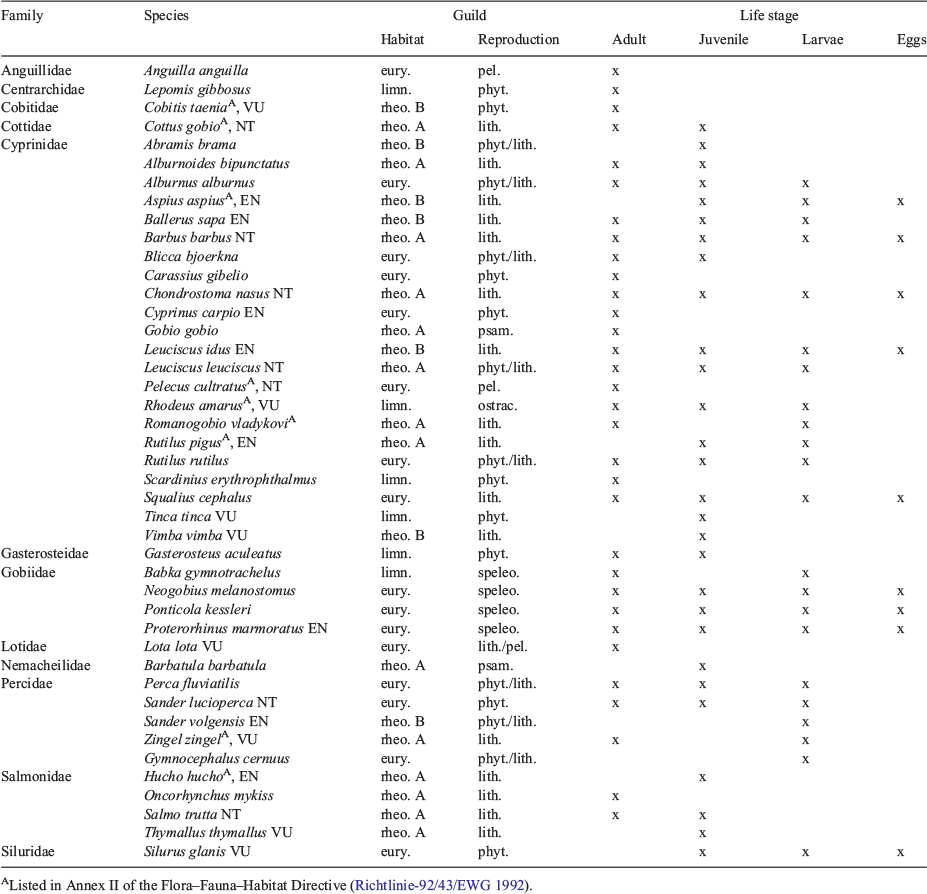
Most of the Danube fish species used the system at all life stages every year (compare with Table 2). There is an apparent increase in numbers of species from downstream (17) to upstream (29; Fig. 4). The straightened section is characterised by a relatively low number of species (17) and has the highest proportion of rheophilic species (58% of juveniles, 69% of adults), such as the nase (Chondrostoma nasus) and the barbel (Barbus barbus). The subsequent meandering section provides habitats for at least 27 species, with an increased proportion of eurytopic specimens (49% of juveniles, 54% of adults). In the meandering section, we caught all species that occurred in the straightened section in similar quantities, plus additionally 10 further species. Stagnophilic species such as European bitterling (Rhodeus amarus), three-spined stickleback (Gasterosteus aculeatus), rudd (Scardinius erythrophthalmus) or tench (Tinca tinca) were almost exclusively caught in the pool pass (5% of juveniles, 8% of adults) and in the stagnant sidearm (22% of juveniles, 35% of adults). The latter exhibits the highest proportion of this guild. Furthermore, hundreds of juvenile cyprinid fish used the dam cavities for overwintering.
|
The pool pass was dominated by eurytopic species (86% of juveniles, 77% of adults). There were highly significant differences in the distribution of adult and juvenile fish for the different flow guilds among sections in the by-pass (Kruskal–Wallis; P < 0.05). The post hoc test showed the following significant differences (Dunn–Bonferroni test, P < 0.05): fish communities differed between the straightened section and stagnant sidearm for all guilds; the straightened section and the pool pass differed for all guilds except for rheophilic B; the meandering section and the stagnant sidearm differed for all guilds except for rheophilic A, whereas only the stagnophilic showed significant differences between the meandering section and the pool pass; when comparing the stagnant sidearm and the pool-pass, only rheophilic B and eurytopic guilds differed.
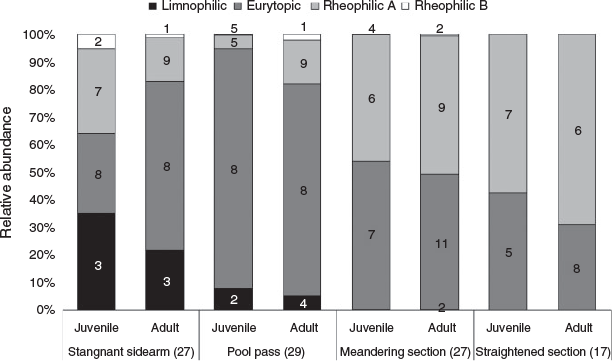
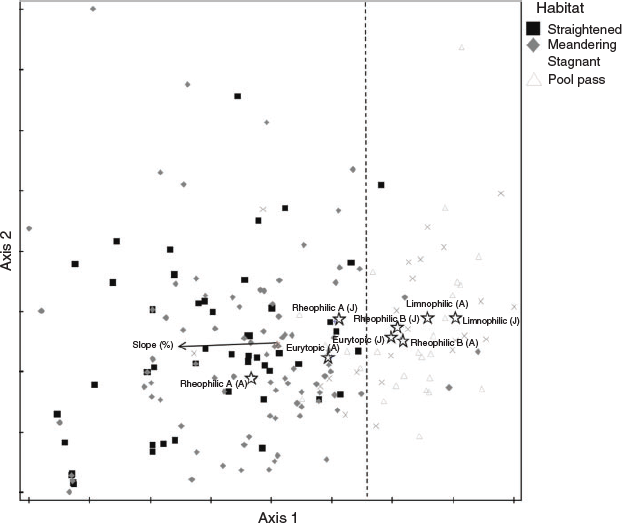
The results of the NMS analysis (Fig. 5), presented as joint plot (cut-off value r2 = 0.3), showed a relationship between riverbed slope and ecological traits of juvenile and adult fish species. On the basis of the NMS scatterplot, samples from sections with a higher slope were noticeably separated from the remaining samples. Samples from the straightened and meandering section were close to each other on the NMS scatterplot, exhibiting a rather similar faunal composition. In contrast, samples from the pool pass and stagnant sidearm were predominantly on the right side of the dashed line. Fifteen species were caught in all sections, whereas four species occurred only in the stagnant sidearm, one only in the straightened section, three only in the Meandering section, and three only in the Pool pass (Fig. 6). Overlaps indicated the number of species caught in more than one section (e.g. 22 species were caught in both the stagnant sidearm and the pool pass).
|
|
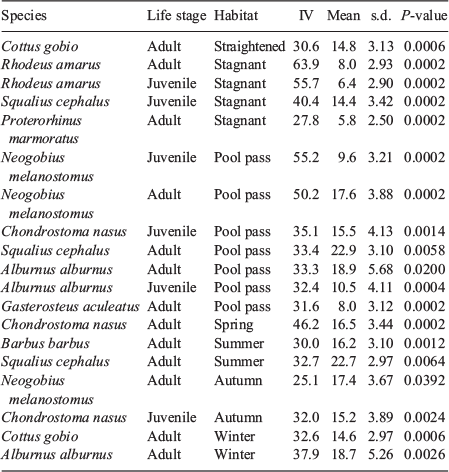
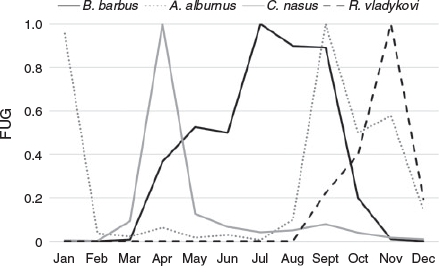
The indicator species analyses (Table 3) showed some fish species that serve as indicators for the different sections of the by-pass. For the pool pass, those were Neogobius melanostomus, Alburnus alburnus, adult individuals of Squalius cephalus and Gasterosteus aculeatus, and juvenile Chondrostoma nasus. The best indication was given by Neogobius melanostomus (IV: juvenile = 55.2; adult = 50.2). For the straightened section, only adult Cottus gobio was listed. For the meandering section, no species met the chosen indicator species criteria (IV > 25, Monte Carlo permutation test: P < 0.05). For the stagnant sidearm, the best indicator species were Rhodeus amarus (IV: juvenile = 55.7; adults = 63.9), followed by juvenile Squalius cephalus and adult Proterorhinus marmoratus. Eleven species were detected throughout the whole year in the system (chub (Squalius cephalus), trout (Salmo trutta), bullhead (Cottus gobio), bleak (Alburnus alburnus), roach (Rutilus rutilus), spirlin (Alburnoides bipunctatus), round goby (Neogobius melanostomus), barbel, bitterling, three-spined stickleback, and nase). Some species, such as grayling (Thymallus thymallus), gudgeon (Gobio gobio), Danube salmon (Hucho hucho), carp (Cyprinus carpio), stone loache (Barbatula barbatula) and sichel (Pelecus cultratus), were detected only once or very rarely in catches. However, there were also seasonal differences in the occurrence of species. In general, the lowest abundances were found for most species during winter (November–February), except for bleak, Danube whitefin gudgeon (Romanogobio vladykovi) and dace (Leuciscus leuciscus), which inhabited the system numerously in this period. This seasonal pattern is clearly illustrated in Fig. 7, which illustrates the frequency of species occurring in the by-pass for adult individuals of Barbus barbus (most abundant from May to September), Alburnus alburnus (most abundant from September to January), Chondrostoma nasus (maximum in April) and Romanogobio vladykovi (most abundant from October to December). On the basis of these data, the only significant (P ≤ 0.05) indicator species identified as colonising the by-pass system in spring was the adult stages of Chondrostoma nasus. In summer, the adult stages of Barbus barbus and Squalius cephalus were identified as indicator species. Adult Neogobius melanostomus and juvenile Chondrostoma nasus were indicator species in autumn, and adult Cottus gobio and Alburnus alburnus were indicator species in winter (Table 3).
|
|
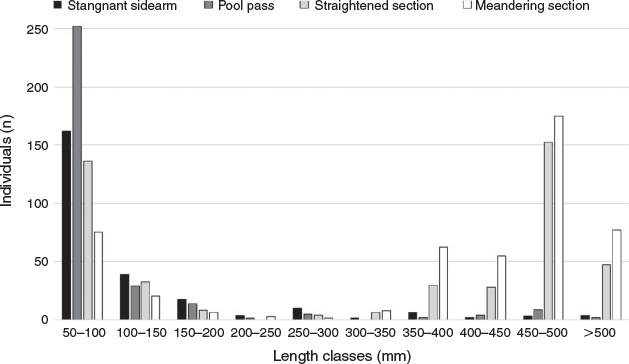
There were noticeable differences for all caught nase for the four investigated sections (Fig. 8), specifically the following:
-
length classes from 200 to 350 mm were highly underrepresented;
-
juveniles were found in all four sections, with higher abundance in the stagnant sidearm and especially the pool pass; and
-
larger individuals (>350 mm) were almost exclusively found in the meandering and straightened sections.
|
Spawning and fish larval drift
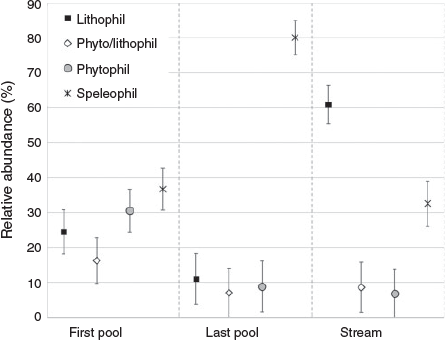
Nase showed a distinct seasonal pattern. In both years, the adult individuals migrated in high numbers into the fish by-pass at the beginning of April and remained there for ~4 weeks. The nase, together with chub, which were most frequent in May, and barbel in July, were the most frequently found species. Spawning activities were observed multiple times, especially in riffle sections, for these species (Fig. 3). A single pool and one riffle section were fished carefully quantitatively during a single spawning event, showing massive spawning runs of nase. In the pool, which had a surface area of 30 m2, 44 adult individuals were caught with a mean weight of 1.5 kg. A further 10 individuals were caught in the adjacent 20-m2 riffle section. Estimates of fish biomass calculated from these data equated to 22 t of fish per hectare in the pool and 7 t of fish per hectare in the riffle section. We collected a total of 6557 fish larvae, representing 22 species, from three sampling points. The following results presented a clear picture of the spatial distribution and spawning-guild composition:
-
in the first pool, a mixed set of fish larvae drifted into the system (n = 2465);
-
the sampling point downstream at the end of the pool pass (n = 2734) was dominated by speleophilic (75–85%) and equal shares of lithophilic and phytophilic (1–16%) species; and
-
in contrast, in the stream section (n = 1358), most of the larvae caught consisted of lithophilic species (55–66%), followed by speleophilic species (26–39%; Fig. 9).
|
These pronounced differences were also reflected in family and species compositions at the three sampling sites (Table 4). In the uppermost pool of the pool pass, the majority of the caught fish larvae were from European catfish (Silurus glanis: 37%), followed by invasive Gobiidae, namely the round goby (Neogobius melanostomus: 20%) and bighead goby (Ponticola kessleri: 11%). In addition, barbel formed a relatively high percentage (14%) of the fish larvae caught. The remaining 12 species were found in lower frequencies. The species information from the last pool and the stream presented a contrasting picture: whereas the stream was dominated by cyprinids (68%), the pool pass clearly showed high proportions of Gobiidae (84%). Of the 22 species of fish larvae collected, nine were also detected by mt-DNA analysis of drifting eggs (Table 4).
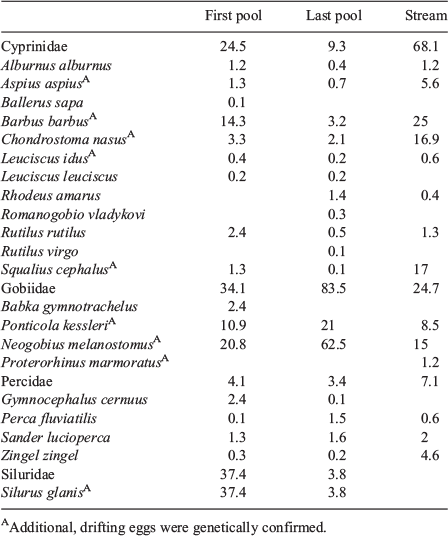
|
Discussion
Our observations and the occurrence of the majority of the Danube fish species from different life stages validates our hypothesis that the fish by-pass provides habitat for spawning, nurseries, growing and feeding for a wide range of species. In total, 43 species deriving from 12 families were detected. These included eight species classified as endangered, eight species classified as vulnerable and a further seven species classified as near threatened for Austria (Wolfram and Mikschi 2007). On a European scale, nine species are listed in Annex II of the Flora–Fauna–Habitat Directive (Richtlinie-92/43/EWG 1992) and, consequently, locations where these species occur must be managed in accordance with the ecological needs of the species. Furthermore, the occurrence of various life stages of protected species such as Aspius aspius, Barbus barbus, Chondrostoma nasus, Leuciscus idus, Proterorhinus marmoratus, Rhodeus amarus, Ballerus sapa or Rutilus pigus highlighted the importance of such fish by-passes for their conservation. In total, the 43 verified species represent three-quarters of all species that were sampled in all habitat assemblages of the Viennese Danube waterbodies in 2013–2016 (Waidbacher et al. 2016). Most of the missing species are rare in the area, such as Acipenser ruthenus, Misgurnus fossilis, Romanogobio kesslerii or Ballerus ballerus. It remains unclear why other species, such as Gymnocephalus schraetser, Esox lucius or Zingel streber do not inhabit the fish pass, and why the abundance of top predators seems to be low.
Whether the sampled juvenile and adult fish originate from downstream or also from upstream sections remains uncertain because no traps were used. In a monitoring study conducted by Eberstaller et al. (2001) in 2000, the downstream migration was evaluated as negligible, mainly consisting of a few juvenile individuals of Alburnus alburnus and Blicca bjoerkna. These authors also found that mainly ‘indifferent’ fish species, especially the bleak, white bream, European roach, vimba and zobel, traverse the entire by-pass system into the impoundment. Only a few individuals of stagnophilic species were detected; however, they are also rare in the tailwater of the power plant. During the spawning season in spring, nase and barbel migrate into the bypass channel in high abundance. Whereas barbel frequently ascends into the impoundment via the pool pass, comparatively few nase traverse the entire system. These authors concluded that the Freudenau bypass channel can be classified as broadly functional (Eberstaller et al. 2001).
Habitat function for fish species, young-of-the-year classes and adults
The taxonomic composition and distribution of the fish fauna varied among the different sections and seasons, and it is likely that this was related to the high variability of the habitat conditions (such as, for example, water depth, flow velocities and substrate). This is in line with one of the key elements of ecology, namely, that habitat heterogeneity increases biodiversity (Ricklefs and Schluter 1993). Additionally, large organic debris is often added from the well-stocked riparian zone, which also has a positive effect on the richness of biota (Crook and Robertson 1999; Dossi et al. 2018). Noteworthy is the stagnant sidearm, which is clogged by a beaver dam, with its calm-water conditions that rarely exist in by-passes. This section supplements the range of available habitats and this is reflected in the proven fish community, which shows a high proportion of stagnophilic species. The increase in species number from the straightened section to the meandering section can be explained by the complex hydrodynamics of convex and concave riverbanks in short succession that produce the sequence of shallow, calm-flowing habitats and deep fast-running sections. This fast-changing sequence produces a variety of essential habitat types in immediately adjacent spots. (Gorman and Karr 1978; Garcia et al. 2012).
The pool pass differs substantially in its habitat specifications, by providing deeper areas with low flow velocity, large boulders at the ramps between the pools, and a well-established riparian vegetation with high proportions of reed and different substrate patterns, ranging from gravel to very fine sediments with a high component of xylal. It shows an abundance of the invasive round goby (Neogobius melanostomus), which prefers the above-mentioned large boulders or riprap structures (Ahnelt et al. 1998; Borcherding et al. 2013; Brandner et al. 2015; Meulenbroek et al. 2018). The reed belt provides shelters and habitats for small species and masses of young-of-the-year fish from all guilds.
It remains unclear whether the apparently under-represented length classes (200–350 mm) of Chondrostoma nasus were caused by either
-
the low attractiveness of the by-pass system for these length classes, or
-
the limited abundance of these length classes in the tailwater of the main river channel.
Evidence in support of the second point comes from the monitoring of the by-pass entrance conducted by Eberstaller et al. (2001) who reported that nase in the 200–350-mm length classes were migrating into the system in the Year 2000, and from our survey of the Danube from 2013 to 2015, in which these length classes were under-represented in the main channel (Waidbacher et al. 2016).
Little is known about a self-sustaining Salmo trutta population within the river Danube, but this species is considered rare (Schiemer and Spindler 1989). It is worth mentioning that some of the caught individuals are most likely to derive from stocking activities, indicated by body pigmentation and deformations of gills and fins (Arndt et al. 2001; Aparicio et al. 2005), and some from autochthonous populations. In total, we caught 48 Salmo trutta individuals throughout the years and seasons, ranging from 100 mm to 350 mm in total length. Most of the adults were caught in late autumn, which corresponds to their spawning season, whereas the presence of some smaller individuals in summer indicated that reproduction had occurred in the system.
The peaks of Chondrostoma nasus and Barbus barbus are linked to their spawning seasons, whereas those of Romanogobio vladykovi and Alburnus alburnus indicate their use of the system as a winter habitat. The latter was also confirmed in the indicator-species analyses. The provision and accessibility of winter habitats are essential for fish communities, especially in highly degraded river systems (e.g. Schlosser 1995; Cunjak 1996).
Spawning function and fish larvae drift
The observed migration of the indicator species of the free-flowing Danube, nase and barbel, and their multiple spawning acts within the fish by-pass are comparable to those described in natural streams and tributaries of the Danube (Keckeis 2001; Ovidio and Philippart 2008; Melcher and Schmutz 2010) and highlight the quality of the fish by-pass system as a functional spawning habitat.
In total, the 22 genetically verified species of fish larvae represent nearly half of all species sampled in the by-pass. This does not necessarily mean that the others do not reproduce there, because they might either spawn at other sites or avoid drifting (Reichard and Jurajda 2007). Artificially built systems often provide functional spawning grounds (Pander and Geist 2016; Meulenbroek et al. 2018), which can be assessed by the occurrence of early life stages of fish (Pavlov 1994). The differences in composition and abundance of larval species among the three sampling points are particularly pronounced and indicate a locally separated reproduction of different fish species.
Most of the catfish larvae (Silurus glanis) in the first pool were caught on a single day together with catfish eggs. This indicates that spawning took place in the area upstream of the first net. Other species found in the first net drifted in a balanced distribution and were derived from somewhere in the Danube upstream. Not all drifting larvae were collected in the first net; some of the species by-passed the first net and were found in the second net in the last pool of the pool pass. The large differences between the two sampling points demonstrated the contribution of the stretch between as a reproduction area. As mentioned above, the high proportion of speleophilic species (in particular Gobiidae) at the second sampling point originates from the rock habitats found in ramps and ripraps within the pool pass. The repeated capture of Rhodeus amarus larvae indicated the occurrence of mussels, which are a prerequisite for the reproduction of this ostracophilic species (Mills and Reynolds 2003).
The third and most downstream larval sampling point in the stream section was clearly dominated by lithophilic cyprinids, primarily by nase, barbel and chub. This showed that the observed spawning acts resulted in successful reproduction. In comparison to a larval-drift investigation undertaken several kilometres upstream (Meulenbroek et al. 2018), additional species of drifting larvae (Silurus glanis, Proterorhinus marmoratus, Ballerus sapa and Romanogobio vladykovi) were detected only in the described fish by-pass. Further investigations are needed to provide a clear explanation for this. However, the distribution of the caught larvae in the stream section of the by-pass is comparable to that of gravel bars in the remaining free-flowing Danube and its tributaries. The distribution of larvae caught in the pool pass is more similar to that of riprap sections in the main channel (Lechner et al. 2010, 2014; Melcher and Schmutz 2010; Ramler et al. 2016; Meulenbroek et al. 2018).
Management aspects
Even though close-to-nature types of fish passes are easier to maintain (Food and Agriculture Organization of the United Nations and the Deutscher Verband für Wasserwirtschaft und Kulturbau e.V. 2002), these artificial systems need continuous management to function sustainably. Besides maintenance of all technical facilities, ecological maintenance needs to be implemented. Currently, the plant operator needs to ensure a free passage of fish by an official notification. This comprises mainly the yearly removal of beaver dams and log or driftwood jams (Renner 2012).
In general, higher discharge provides better passage, but it needs to be in accordance with the morphology of the fish pass (Food and Agriculture Organization of the United Nations and the Deutscher Verband für Wasserwirtschaft und Kulturbau e.V. 2002). The critical swimming capacity (Plaut 2001) of different species and life stages needs to be taken into account to facilitate a complete passage through the by-pass. Furthermore, the stability of the spawning habitats must be guaranteed for nearly 4 weeks for successful spawning to occur (Hauer et al. 2007). In 2016, a high discharge occurred for a long time shortly after migrating nase arrived, resulting in zero catches and the loss of a whole generation of young-of-the-year fish (P. Meulenbroek and H. Waidbacher, unpubl. data). Hydrological disturbances or dynamic floods are still recommended as they can ‘clean up’ the interstitial of the sediments, which enhances the habitat not only for fish and egg development but also for other organisms such as macroinvertebrates (Dudgeon et al. 2006; Dole-Olivier 2011) or biofilms (Boulton 2007). Implementing this ‘cleaning up’ of the sediments before the migration season, which is not linked to a particular date but more to water temperature, discharge and other parameters (Northcote 1984), is recommended.
Another fact that should be considered is the deepening of the by-pass riverbed. After 17 years of operation, the whole system deepened by an average of 24 cm, resulting in a loss of more than 3000 m3 of gravel (Hagel and Westermayr 2016). Compensation is required to ensure system stability and to fill up the developed washouts, which can impede some species from swimming upstream and we recommend implementing this at 10-yearly intervals. Gravel addition could also create or improve suitable spawning grounds (Pulg et al. 2013). This demonstrates the absolute need for continuous management actions to secure the positive ecological values for fish and other riverine faunal elements.
Conclusions
Most species from the Austrian Danube were observed using a man-made by-pass and some have accepted the surroundings as habitats for different life stages. The diversity of species and sizes of the colonised fish, as well as the evident reproduction of some, correspond to a situation in a natural sidearm or tributary (Haunschmid et al. 2006). Therefore, the fish by-pass may serve as a key habitat for the conservation of a variety of endangered species. Furthermore, we have shown that the heterogenic configuration provides conditions for different ecological guilds and, consequently, increases biodiversity. The spatial extent of by-passes is limited in comparison to the degradation and disconnection of former habitats, and the habitat quality of these artificial systems may be lower than that of natural habitats. Nevertheless, near-nature fish by-passes have high potential as a remediating or mitigating measure (Quigley and Harper 2006; Tamario et al. 2018) and this was clearly visible in the present study. Future studies should focus on the influence of near-nature fish by-passes on the population size, population dynamics, and the production of offspring of the protected and endangered fish species in the context of the lost habitats and fish populations in the river system. However, such artificial systems need to be managed continuously to function sustainably.
Almost 60% of all Austrian waterbodies are affected by interruption of river continuity, whereby, in larger rivers (>100 km2 catchment), 26% derives from hydropower plants. More than 70% of these facilities are not passable at present (Gewässerbewirtschaftungsplan 2015). Until now, the focus for the implementation of fish by-passes has mostly been to enable migration corridors. Accepting the Danube as an originally braided river with highly diverse habitats (Hohensinner et al. 2013), a systematic approach to the creation and connection of habitats is necessary to improve the ecological situation of such a system under pressure and to achieve the requirements formulated in the EU–WFD. Especially in highly modified waterbodies, the provision of functioning spawning and juvenile habitats are two of the most essential tasks to strengthen the remaining fish stocks (Keckeis and Schiemer 2002; Pander and Geist 2010; Jungwirth et al. 2014; Waidbacher et al. 2018). In planning river modifications, interruptions, or by-passes, the ecological functioning of these key habitats must be incorporated.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors thank C. Dorninger for technical assistance and P. Rauch, D. Stadler, W. Westermayr and T. Hagl who assisted with field work. H. Meulenbroek improved the English. S. Krumböck and V. Waidbacher are thanked for laboratory assistance. The study was partly financed and enabled by Verbund Hydro Power GmbH; special thanks go to Roswitha Renner and Mr Ringel.
References
Ahnelt, H., Banarescu, P., Spolwind, R., Harka, A., and Waidbacher, H. (1998). Occurrence and distribution of three gobiid species (Pisces, Gobiidae) in the middle and upper Danube region: examples of different dispersal patterns? Biologia-Bratislava 53, 665–678.Allan, J. D., and Flecker, A. S. (1993). Biodiversity conservation in running waters. Bioscience 43, 32–43.
| Biodiversity conservation in running waters.Crossref | GoogleScholarGoogle Scholar |
Aparicio, E., García‐Berthou, E., Araguas, R., Martínez, P., and García‐Marín, J. (2005). Body pigmentation pattern to assess introgression by hatchery stocks in native Salmo trutta from Mediterranean streams. Journal of Fish Biology 67, 931–949.
| Body pigmentation pattern to assess introgression by hatchery stocks in native Salmo trutta from Mediterranean streams.Crossref | GoogleScholarGoogle Scholar |
Arndt, R. E., Routledge, M. D., Wagner, E. J., and Mellenthin, R. F. (2001). Influence of raceway substrate and design on fin erosion and hatchery performance of rainbow trout. North American Journal of Aquaculture 63, 312–320.
| Influence of raceway substrate and design on fin erosion and hatchery performance of rainbow trout.Crossref | GoogleScholarGoogle Scholar |
Baldwin, C. C., Mounts, J. H., Smith, D. G., and Weigt, L. A. (2009). Genetic identification and colour descriptions of early life-history stages of Belizean Phaeoptyx and Astrapogon (Teleostei: Apogonidae) with comments on identification of adult Phaeoptyx. Zootaxa 2008, 1–22.
Balon, E. K. (1990). Epigenesis of an epigeneticist: the development of some alternative concepts on the early ontogeny and evolution of fishes. Guelph Ichthyology Reviews 1, 1–22.
Barlaup, B. T., Gabrielsen, S. E., Skoglund, H., and Wiers, T. (2008). Addition of spawning gravel: a means to restore spawning habitat of Atlantic salmon (Salmo salar L.), and anadromous and resident brown trout (Salmo trutta L.) in regulated rivers. River Research and Applications 24, 543–550.
| Addition of spawning gravel: a means to restore spawning habitat of Atlantic salmon (Salmo salar L.), and anadromous and resident brown trout (Salmo trutta L.) in regulated rivers.Crossref | GoogleScholarGoogle Scholar |
Borcherding, J., Dolina, M., Heermann, L., Knutzen, P., Krüger, S., Matern, S., van Treeck, R., and Gertzen, S. (2013). Feeding and niche differentiation in three invasive gobies in the Lower Rhine, Germany. Limnologica-Ecology and Management of Inland Waters 43, 49–58.
| Feeding and niche differentiation in three invasive gobies in the Lower Rhine, Germany.Crossref | GoogleScholarGoogle Scholar |
Boulton, A. J. (2007). Hyporheic rehabilitation in rivers: restoring vertical connectivity. Freshwater Biology 52, 632–650.
| Hyporheic rehabilitation in rivers: restoring vertical connectivity.Crossref | GoogleScholarGoogle Scholar |
Brandner, J., Auerswald, K., Schäufele, R., Cerwenka, A. F., and Geist, J. (2015). Isotope evidence for preferential dispersal of fast-spreading invasive gobies along man-made river bank structures. Isotopes in Environmental and Health Studies 51, 80–92.
| Isotope evidence for preferential dispersal of fast-spreading invasive gobies along man-made river bank structures.Crossref | GoogleScholarGoogle Scholar |
Calles, E., and Greenberg, L. (2007). The use of two nature‐like fishways by some fish species in the Swedish River Emån. Ecology Freshwater Fish 16, 183–190.
Copp, G. H., and Peňáz, M. (1988). Ecology of fish spawning and nursery zones in the flood plain, using a new sampling approach. Hydrobiologia 169, 209–224.
| Ecology of fish spawning and nursery zones in the flood plain, using a new sampling approach.Crossref | GoogleScholarGoogle Scholar |
Crook, D., and Robertson, A. (1999). Relationships between riverine fish and woody debris: implications for lowland rivers. Marine and Freshwater Research 50, 941–953.
| Relationships between riverine fish and woody debris: implications for lowland rivers.Crossref | GoogleScholarGoogle Scholar |
Cunjak, R. A. (1996). Winter habitat of selected stream fishes and potential impacts from land-use activity. Canadian Journal of Fisheries and Aquatic Sciences 53, 267–282.
| Winter habitat of selected stream fishes and potential impacts from land-use activity.Crossref | GoogleScholarGoogle Scholar |
Dole-Olivier, M.-J. (2011). The hyporheic refuge hypothesis reconsidered: a review of hydrological aspects. Marine and Freshwater Research 62, 1281–1302.
| The hyporheic refuge hypothesis reconsidered: a review of hydrological aspects.Crossref | GoogleScholarGoogle Scholar |
Dossi, F., Leitner, P., Pauls, S., and Graf, W. (2018). In the mood for wood-habitat specific colonization patterns of benthic invertebrate communities along the longitudinal gradient of an Austrian river. Hydrobiologia 805, 245–258.
| In the mood for wood-habitat specific colonization patterns of benthic invertebrate communities along the longitudinal gradient of an Austrian river.Crossref | GoogleScholarGoogle Scholar |
Dudgeon, D., Arthington, A. H., Gessner, M. O., Kawabata, Z.-I., Knowler, D. J., Lévêque, C., Naiman, R. J., Prieur-Richard, A.-H., Soto, D., and Stiassny, M. L. (2006). Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews of the Cambridge Philosophical Society 81, 163–182.
| Freshwater biodiversity: importance, threats, status and conservation challenges.Crossref | GoogleScholarGoogle Scholar |
Dufrêne, M., and Legendre, P. (1997). Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs 67, 345–366.
Eberstaller, J., Hinterhofer, M., and Parasiewicz, P. (1998). The effectiveness of two nature-like bypass channels in an upland Austrian river. In ‘Fish Migration and Fish Bypasses’. (Eds M. Jungwirth, S. Schmutz, and S. Weiss.) pp. 363–383. (Fishing News Books: Oxford, UK.)
Eberstaller, J., Pinka, P., and Honsowitz, H. (2001). Fischaufstiegshilfe Donaukraftwerk Freudenau Forschung im Verbund, Schriftenreihe 72, 177–196.
European Parliament and the Council of the European Union (2000). Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy as amended by Decision 2455/2001/EC and Directives 2008/32/EC, 2008/105/EC and 2009/31/EC. Available at https://eur-lex.europa.eu/eli/dir/2000/60/2014-01-01 [Verified 4 September 2018].
Food and Agriculture Organization of the United Nations and the Deutscher Verband für Wasserwirtschaft und Kulturbau e.V. (2002) Fish passes – design, dimensions, and monitoring. (FAO and DVWK: Rome, Italy.)
Garcia, X.-F., Schnauder, I., and Pusch, M. (2012). Complex hydromorphology of meanders can support benthic invertebrate diversity in rivers. Hydrobiologia 685, 49–68.
| Complex hydromorphology of meanders can support benthic invertebrate diversity in rivers.Crossref | GoogleScholarGoogle Scholar |
Geist, J., and Hawkins, S. J. (2016). Habitat recovery and restoration in aquatic ecosystems: current progress and future challenges. Aquatic Conservation 26, 942–962.
| Habitat recovery and restoration in aquatic ecosystems: current progress and future challenges.Crossref | GoogleScholarGoogle Scholar |
Gewässerbewirtschaftungsplan, N. (2009). NGP 2009. Available at https://www.bmnt.gv.at/wasser/wasser-oesterreich/plan_gewaesser_ngp/nationaler_gewaesserbewirtschaftungsplan-ngp/ngp2009.html [Verified 4 September 2018].
Gewässerbewirtschaftungsplan, N. (2015). NGP 2015. Available at https://www.bmnt.gv.at/wasser/wisa/fachinformation/ngp/ngp-2015/text/textdokument_ngp2015.html [Verified 4 September 2018].
Gorman, O. T., and Karr, J. R. (1978). Habitat structure and stream fish communities. Ecology 59, 507–515.
| Habitat structure and stream fish communities.Crossref | GoogleScholarGoogle Scholar |
Gustafsson, S., Österling, M., Skurdal, J., Schneider, L. D., and Calles, O. (2013). Macroinvertebrate colonization of a nature-like fishway: the effects of adding habitat heterogeneity. Ecological Engineering 61, 345–353.
| Macroinvertebrate colonization of a nature-like fishway: the effects of adding habitat heterogeneity.Crossref | GoogleScholarGoogle Scholar |
Hagel, T., and Westermayr, W. (2016). Räumliche Veränderung der FAH Freudenau 15 Jahre nach Einstau. M.Sc. Thesis, Institut für Hydrobiologie, Gewässermanagement (IHG), BOKU-Universität für Bodenkultur, Vienna, Austria.
Harreiter, H., Frik, G., Schmalfuß, R., and Reckendorfer, W. (2015). Implementation of the EU Water Framework Directive at the Danube River in Austria: experiences and perspectives. Wasserwirtschaft 105, 14–19.
| Implementation of the EU Water Framework Directive at the Danube River in Austria: experiences and perspectives.Crossref | GoogleScholarGoogle Scholar |
Hauer, C., Unfer, G., Schmutz, S., and Habersack, H. (2007). The importance of morphodynamic processes at riffles used as spawning grounds during the incubation time of nase (Chondrostoma nasus). Hydrobiologia 579, 15–27.
| The importance of morphodynamic processes at riffles used as spawning grounds during the incubation time of nase (Chondrostoma nasus).Crossref | GoogleScholarGoogle Scholar |
Haunschmid, R., Wolfram, G., Spindler, T., Honsig-Erlenburg, W., Wimmer, R., Jagsch, A., Kainz, E., Hehenwarter, K., Wagner, B., and Konecny, R. (2006). ‘Erstellung einer fischbasierten Typologie österreichischer Fließgewässer sowie einer Bewertungsmethode des fischökologischen Zustandes gemäß EU – Wasserrahmenrichtlinie’ (Bundesamt für Wasserwirtschaft: Vienna, Austria.)
Haunschmid, R., Schotzko, N., Petz-Glechner, R., Honsig-Erlenburg, W., Schmutz, S., Spindler, T., Unfer, G., Graf, W., Bammer, V., and Hundritsch, L. (2010). ‘Leitfaden zur Erhebung der Biologischen Qualitätselemente Teil A1: Fische.’ (Bundesministerium für Land- und Forstwirtschaft, Umwelt und Wasserwirtschaft: Vienna, Austria.)
Hebert, P. D., Cywinska, A., and Ball, S. L. (2003). Biological identifications through DNA barcodes. Proceedings of the Royal Society of London – B. Biological Sciences 270, 313–321.
| Biological identifications through DNA barcodes.Crossref | GoogleScholarGoogle Scholar |
Hohensinner, S., Sonnlechner, C., Schmid, M., and Winiwarter, V. (2013). Two steps back, one step forward: reconstructing the dynamic Danube riverscape under human influence in Vienna. Water History 5, 121–143.
| Two steps back, one step forward: reconstructing the dynamic Danube riverscape under human influence in Vienna.Crossref | GoogleScholarGoogle Scholar |
Jungwirth, M., Schmutz, S., and Weiss, S. (1998). ‘Fish Migration and Fish Bypasses.’ (Fishing News Books: Oxford, UK.)
Jungwirth, M., Haidvogel, G., Moog, O., Muhar, S., and Schmutz, S. (2003). ‘Angewandte Fischökologie an Fließgewässern.’ (Facultas Universitätsverlag: Vienna, Austria.)
Jungwirth, M., Haidvogel, G., Hohensinner, S., Waidbacher, H., and Zauner, G. (2014). ‘Österreichs Donau.’ (Landschaft–Fisch–Geschichte. Institut für Hydrobiologie und Gewässermanagement, BOKU: Vienna, Austria.)
Karr, J. R., Toth, L. A., and Dudley, D. R. (1985). Fish communities of midwestern rivers: a history of degradation. Bioscience 35, 90–95.
| Fish communities of midwestern rivers: a history of degradation.Crossref | GoogleScholarGoogle Scholar |
Keckeis, H. (2001). Influence of river morphology and current velocity conditions on spawning site selection of Chondrostoma nasus (L.). Archiv für Hydrobiologie – Large Rivers 12, 341–356.
Keckeis, H., and Schiemer, F. (2002). ‘Understanding Conservation Issues of the Danube River. Fishery Science: the Unique Contribution of Early Life Stages.’ (Blackwell Publishing: Oxford. UK.)
Kotlík, P., Markova, S., Choleva, L., Bogutskaya, N. G., Ekmekcl, F. G., and Ivanova, P. P. (2008). Divergence with gene flow between Ponto‐Caspian refugia in an anadromous cyprinid Rutilus frisii revealed by multiple gene phylogeography. Molecular Ecology 17, 1076–1088.
| Divergence with gene flow between Ponto‐Caspian refugia in an anadromous cyprinid Rutilus frisii revealed by multiple gene phylogeography.Crossref | GoogleScholarGoogle Scholar |
Kottelat, M., and Freyhof, J. R. (2007). ‘Handbook of European Freshwater Fishes.’ (Publications Kottelat: Delémont, Switzerland.)
Kreps, H. (1975). ‘Praktische Arbeit in der Hydrographie.’ (Bundesministerium für Land- und Forstwirtschaft: Vienna, Austria.)
Kruskal, J. B. (1964). Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 29, 1–27.
| Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis.Crossref | GoogleScholarGoogle Scholar |
Lechner, A., Schuldermann, E., Keckeis, H., Humphries, P., and Tritthart, M. (2010). Jungfischdrift in der österreichischen Donau: taxonomische Zusammensetzung, Entwicklungsstadien und Driftdichte. Oesterreichs Fischerei 63, 96–100.
Lechner, A., Keckeis, H., Schludermann, E., Loisl, F., Humphries, P., Glas, M., Tritthart, M., and Habersack, H. (2014). Shoreline configurations affect dispersal patterns of fish larvae in a large river. ICES Journal of Marine Science 71, 930–942.
| Shoreline configurations affect dispersal patterns of fish larvae in a large river.Crossref | GoogleScholarGoogle Scholar |
Melcher, A. H., and Schmutz, S. (2010). The importance of structural features for spawning habitat of nase Chondrostoma nasus (L.) and barbel Barbus barbus (L.) in a pre-Alpine river. River Systems 19, 33–42.
| The importance of structural features for spawning habitat of nase Chondrostoma nasus (L.) and barbel Barbus barbus (L.) in a pre-Alpine river.Crossref | GoogleScholarGoogle Scholar |
Meulenbroek, P., Drexler, S., Rauch, P., Stauffer, C., Huemer, D., Gruber, S., Zwettler, M., Zirgoi, S., Krumboeck, S., Waidbacher, V., and Waidbacher, H. (2018). Species-specific fish larvae drift in anthropogenically constructed riparian zones on the Vienna impoundment of the River Danube, Austria: species occurrence, frequencies and seasonal patterns based on DNA Barcoding. River Research and Applications 34, 854–862.
| Species-specific fish larvae drift in anthropogenically constructed riparian zones on the Vienna impoundment of the River Danube, Austria: species occurrence, frequencies and seasonal patterns based on DNA Barcoding.Crossref | GoogleScholarGoogle Scholar |
Mills, S. C., and Reynolds, J. D. (2003). Operational sex ratio and alternative reproductive behaviours in the European bitterling, Rhodeus sericeus. Behavioral Ecology and Sociobiology 54, 98–104.
Morley, S. A., and Karr, J. R. (2002). Assessing and restoring the health of urban streams in the Puget Sound basin. Conservation Biology 16, 1498–1509.
| Assessing and restoring the health of urban streams in the Puget Sound basin.Crossref | GoogleScholarGoogle Scholar |
Niederösterreich, L. (2018). Wasserstandnachrichten Korneuburg. 2018. (Österreichische Wasserstraßen-Gesellschaft mbH. und der Verbund Hydro Power AG.) Available at http://www.noel.gv.at/wasserstand/static/stations/207241P/station.html [Verified 4 September 2018].
Northcote, T. (1984). Mechanisms of fish migration in rivers. In ‘Mechanisms of Migration in Fishes’. (Eds J. D. McCleave, J. J. Dodson, and W. H. Neill.) pp. 317–355. (Plenum: New York, NY, USA.)
Önorm-6232 (1995) ‘6232 (Austrian Standards): Guidelines for the Ecological Study and Assessment of Rivers.’ pp. 1–38. (Österreichisches Normungsinstitut: Vienna, Austria.)
Ovidio, M., and Philippart, J.-C. (2008). Movement patterns and spawning activity of individual nase Chondrostoma nasus (L.) in flow‐regulated and weir‐fragmented rivers. Journal of Applied Ichthyology 24, 256–262.
| Movement patterns and spawning activity of individual nase Chondrostoma nasus (L.) in flow‐regulated and weir‐fragmented rivers.Crossref | GoogleScholarGoogle Scholar |
Pander, J., and Geist, J. (2010). Seasonal and spatial bank habitat use by fish in highly altered rivers: a comparison of four different restoration measures. Ecology Freshwater Fish 19, 127–138.
| Seasonal and spatial bank habitat use by fish in highly altered rivers: a comparison of four different restoration measures.Crossref | GoogleScholarGoogle Scholar |
Pander, J., and Geist, J. (2016). Can fish habitat restoration for rheophilic species in highly modified rivers be sustainable in the long run? Ecological Engineering 88, 28–38.
| Can fish habitat restoration for rheophilic species in highly modified rivers be sustainable in the long run?Crossref | GoogleScholarGoogle Scholar |
Pander, J., Mueller, M., and Geist, J. (2013). Ecological functions of fish bypass channels in streams: migration corridor and habitat for rheophilic species. River Research and Applications 29, 441–450.
| Ecological functions of fish bypass channels in streams: migration corridor and habitat for rheophilic species.Crossref | GoogleScholarGoogle Scholar |
Parasiewicz, P., Eberstaller, J., Weiss, S., and Schmutz, S. (1998). Fish migration and fish bypasses. In ‘Conceptual Guidelines for Nature-like Bypass Channels’. (Eds M. Jungwirth, S. Schmutz, and S. Weiss.) pp. 348–362. (Fishing News Books: Oxford, UK.)
Pavlov, S. (1994). The downstream migration of young fishes in rivers: mechanisms and distribution. Folia Zoologica-UZPI 43, 193–208.
| The downstream migration of young fishes in rivers: mechanisms and distribution.Crossref | GoogleScholarGoogle Scholar |
Plaut, I. (2001). Critical swimming speed: its ecological relevance. Comparative Biochemistry and Physiology – A. Molecular & Integrative Physiology 131, 41–50.
| Critical swimming speed: its ecological relevance.Crossref | GoogleScholarGoogle Scholar |
Pulg, U., Barlaup, B. T., Sternecker, K., Trepl, L., and Unfer, G. (2013). Restoration of spawning habitats of brown trout (Salmo trutta) in a regulated chalk stream. River Research and Applications 29, 172–182.
| Restoration of spawning habitats of brown trout (Salmo trutta) in a regulated chalk stream.Crossref | GoogleScholarGoogle Scholar |
Quigley, J. T., and Harper, D. J. (2006). Effectiveness of fish habitat compensation in Canada in achieving no net loss. Environmental Management 37, 351–366.
| Effectiveness of fish habitat compensation in Canada in achieving no net loss.Crossref | GoogleScholarGoogle Scholar |
Raleigh, R. F., Zuckerman, L. D., and Nelson, P. C. (1984). ‘Habitat Suitability Index Models and Instream Flow Suitability Curves: Brown Trout.’ (US Fish and Wildlife Service: Washington, DC, USA.)
Ramler, D., Ahnelt, H., Nemeschkal, H., and Keckeis, H. (2016). The drift of early life stages of Percidae and Gobiidae (Pisces: Teleostei) in a free-flowing section of the Austrian Danube. Hydrobiologia 781, 199–216.
| The drift of early life stages of Percidae and Gobiidae (Pisces: Teleostei) in a free-flowing section of the Austrian Danube.Crossref | GoogleScholarGoogle Scholar |
Reichard, M., and Jurajda, P. (2007). Seasonal dynamics and age structure of drifting cyprinid fishes: an interspecific comparison. Ecology Freshwater Fish 16, 482–492.
| Seasonal dynamics and age structure of drifting cyprinid fishes: an interspecific comparison.Crossref | GoogleScholarGoogle Scholar |
Renner, R. (2012). Donaukraftwerk Freudenau – Umgehungsgerinne mit Fischaufstieg – Vorläufige Betriebsordnung. Verbund AG, Vienna, Austria.
Reyjol, Y., Argillier, C., Bonne, W., Borja, A., Buijse, A. D., Cardoso, A. C., Daufresne, M., Kernan, M., Ferreira, M. T., and Poikane, S. (2014). Assessing the ecological status in the context of the European Water Framework Directive: where do we go now? The Science of the Total Environment 497–498, 332–344.
| Assessing the ecological status in the context of the European Water Framework Directive: where do we go now?Crossref | GoogleScholarGoogle Scholar |
Richter, B. D., Baumgartner, J. V., Powell, J., and Braun, D. P. (1996). A method for assessing hydrologic alteration within ecosystems. Conservation Biology 10, 1163–1174.
| A method for assessing hydrologic alteration within ecosystems.Crossref | GoogleScholarGoogle Scholar |
Richtlinie-92/43/EWG (1992). Richtlinie 92/43/EWG des rates vom 21. Mai 1992 zur Erhaltung der natürlichen Lebensräume sowie der wildlebenden Tiere und Pflanzen. Amtsblatt der Europäischen Gemeinschaften, Reihe L. 206, 7–50.
Ricklefs, R. E., and Schluter, D. (1993). Species diversity: regional and historical influences. Species Diversity in Ecological Communities 3, 50–363.
Sachs, L. (2004). Weitere Prüfverfahren. In ‘Angewandte Statistik’. (Ed. L. Sachs.) pp. 404–489. (Springer: Berlin, Germany.)
Schiemer, F. (2000). Fish as indicators for the assessment of the ecological integrity of large rivers. Hydrobiologia 422, 271–278. https://doi.org/10.1023/A:1017086703551
Schiemer, F., and Spindler, T. (1989). Endangered fish species of the Danube River in Austria. Regulated Rivers: Research and Management 4, 397–407.
| Endangered fish species of the Danube River in Austria.Crossref | GoogleScholarGoogle Scholar |
Schiemer, F., and Waidbacher, H. (1992). Strategies for conservation of a Danubian fish fauna. River Conservation and Management 26, 363–382.
Schlosser, I. J. (1995). Critical landscape attributes that influence fish population dynamics in headwater streams. Hydrobiologia 303, 71–81.
| Critical landscape attributes that influence fish population dynamics in headwater streams.Crossref | GoogleScholarGoogle Scholar |
Schmutz, S. (2012). ‘Was Bringt die Durchgängigkeit für den Guten Zustand? ÖWAV – Tagung Fischaufstiegshilfen: Neue Anforderungen und Erfahrungen aus der Praxis. 5.10.2012.’ (ÖWAV: Vienna, Austria.)
Tamario, C., Degerman, E., Donadi, S., Spjut, D., and Sandin, L. (2018). Nature‐like fishways as compensatory lotic habitats. River Research and Applications 34, 253–261.
| Nature‐like fishways as compensatory lotic habitats.Crossref | GoogleScholarGoogle Scholar |
Tummers, J. S., Hudson, S., and Lucas, M. C. (2016). Evaluating the effectiveness of restoring longitudinal connectivity for stream fish communities: towards a more holistic approach. The Science of the Total Environment 569–570, 850–860.
| Evaluating the effectiveness of restoring longitudinal connectivity for stream fish communities: towards a more holistic approach.Crossref | GoogleScholarGoogle Scholar |
Waidbacher, H., Drexler, S., and Meulenbroek, P. (2016). ‘Donau–Stauraum Freudenau; Ökosystem-Response 15 Jahre nach Einstau, Fachbereiche Fischökologie, Limnologie sowie Ausgewählte Begleitdisziplinen.’ (Universität für Bodenkultur: Vienna, Austria.)
Waidbacher, H., Drexler, S.-S., and Meulenbroek, P. (2018). Danube under pressure: hydropower rules the fish. In ‘Riverine Ecosystem Management’. (Eds S. Schmutz and J. Sendzimir.) pp. 473–489. (Springer: Cham, Switzerland.)
Wiesner, C., and Zauner, G. (1999). ‘Bestimmungsschlüssel für heimische Fisch-und Neunaugenarten.’ (Abt. f. Hydrobiologie, Fischereiwirtschaft und Aquakultur, Universität für Bodenkultur: Vienna, Austria.)
Wolfram, G., and Mikschi, E. (2007). Rote liste der fische (Pisces) Österreichs, In ‘Rote Listen gefährdeter Tiere Österreichs: Checklisten, Gefährdungsanalysen, Handlungsbedarf. Teil 2. Grüne Reihe des Lebensministeriums Band 14/2’. (Ed. K. P. Zulka.) pp. 61–198. (Böhlau-Verlag: Vienna, Austria.)
Zauner, G., and Eberstaller, J. (1999). Klassifizierungsschema der österreichischen Flußfischfauna in bezug auf deren Lebensraumansprüche. Oesterreichs Fischerei 52, 198–205.
Zauner, G., Jung, M., Ratschan, C., and Mühlbauer, M. (2016). Ökologische Sanierung von Fließstrecken und Stauhaltungen der österreichischen Donau: auf dem Weg zur Zielerreichung nach Wasserrahmenrichtlinie. Österreichische Wasser-und Abfallwirtschaft 68, 503–518.
| Ökologische Sanierung von Fließstrecken und Stauhaltungen der österreichischen Donau: auf dem Weg zur Zielerreichung nach Wasserrahmenrichtlinie.Crossref | GoogleScholarGoogle Scholar |


