A systematic review of methods used to study fish in saltmarsh flats
Violet Harrison-Day A B , Vishnu Prahalad A , Jamie B. Kirkpatrick A and Melinda McHenry A
A B , Vishnu Prahalad A , Jamie B. Kirkpatrick A and Melinda McHenry A
A Discipline of Geography and Spatial Sciences, University of Tasmania, Private Bag 78, Hobart, Tas. 7001, Australia.
B Corresponding author. Email: violet.harrisonday@utas.edu.au
Marine and Freshwater Research 72(2) 149-162 https://doi.org/10.1071/MF20069
Submitted: 4 March 2020 Accepted: 5 May 2020 Published: 13 June 2020
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
There is a growing body of research highlighting the importance of saltmarshes as habitats for fish for feeding, refuge from predation and reproduction. However, more work is needed on fish on vegetated marsh flats (or surfaces). We reviewed 60 studies that used 21 methods to sample fish assemblages on saltmarsh flats. Drop samplers, fyke nets and pop nets were most frequently employed, with considerably more studies being conducted in graminoid than succulent marsh. Reporting of sampling temporal and tidal details, environmental variables and fish attributes was inconsistent. Most of the papers focussed on one or more of conservation management, comparisons among habitat types, and the use of saltmarsh (including fish activity type or residency status). Important potential areas of research include the relationships between the fish assemblages of saltmarsh flats and coastal fisheries, the effects of invasive plant species and marsh restoration efforts in areas outside the United States, and the potential effects of sea-level rise on vegetated flats as fish habitat. Sampling methods that provide density measures are likely to be most useful for most of this research. Thus, drop samplers and pop nets are an appropriate choice, the former in graminoid saltmarshes and the latter in succulent saltmarshes.
Additional keywords: estuary, fish community, research design, Sarcocornia, Spartina, tidal marsh.
Introduction
Saltmarshes form in low-energy coastal environments such as estuaries and embayments. They are defined by the presence of salt-tolerant plants, occurring in areas that are flooded regularly to occasionally. These habitats provide foraging, refuge and nursery sites for resident and transient fish (e.g. Hettler 1989; Minello and Zimmerman 1992; Peterson and Turner 1994; West and Zedler 2000; Minello et al. 2003; Crinall and Hindell 2004; Platell and Freewater 2009). In addition to providing fish habitat, saltmarshes contribute to broader coastal seascape productivity through outwelling and the export of nutrients, organic matter and live organisms (Odum 2002; Kneib 2003; Creighton et al. 2019). Through these and several other ecological functions, saltmarshes provide a broad range of highly regarded provisioning, supporting and cultural ecosystem services (Barbier et al. 2011; Rogers et al. 2016).
Fish use of saltmarsh habitat is complex, with the effects of seasonality, tidal patterns, water depth, diel period, temperature and salinity influencing fish assemblages (e.g. Connolly et al. 1997; Crinall and Hindell 2004; Mazumder et al. 2005b; Prahalad et al. 2019). Fish either (1) remain on the marsh at all times (finding refuge in pools and depressions during low tide), (2) are present on the marsh at high tide but return to subtidal habitat at low tide, (3) venture only onto the marsh edge at high tide and return to subtidal creeks at low tide, or (4) remain in subtidal creeks without venturing onto the marsh itself (Peterson and Turner 1994).
The ‘saltmarsh habitat’ is necessarily a complex of subhabitats including vegetated marsh flats (or vegetated marsh surfaces), intertidal creeks, subtidal creeks and marsh ponds and pools (Minello et al. 2003; Fig. 1a). These subhabitats, in the context of fish use, can be delineated by frequency of flooding and water availability (Rountree and Able 2007). Of these subhabitats, fish use of saltmarsh creeks has received much attention, particularly in the United States (e.g. Rountree and Able 1992; Able et al. 2001; Hampel et al. 2003; Paterson and Whitfield 2003; Green et al. 2009), as have marsh edges (e.g. Baltz et al. 1993; Kaneko et al. 2019). In comparison, vegetated saltmarsh flats, disproportionate to the area they occupy within saltmarshes (e.g. see Fig. 1a), have received much lesser attention. The results of sampling conducted in creeks cannot be used to demonstrate fish use of adjacent vegetated flats (Peterson and Turner 1994; Connolly 1999).

|
Vegetated marsh flats can be largely dominated by graminoids, such as Spartina spp., Phragmites spp. and Elymus spp. in parts of the United States and Europe (Fig. 1b), or succulents, such as Sarcocornia spp. and Samolus spp. in Australia and New Zealand (Fig. 1c; e.g. Hettler 1989; Connolly et al. 1997; Kneib 2003; Prahalad et al. 2019).
Several previous reviews have described fish use of saltmarshes and survey methods, but none has focussed solely on flats. Existing reviews of fish use of saltmarsh have demonstrated several complexities associated with sampling and surveying flats, perhaps suggesting why consistent sampling strategies and global comparisons of fish activity in this subhabitat have not been undertaken. For instance, Connolly (1999) addressed difficulties in sampling design and methods of sampling nekton in saltmarshes as a whole. He recommended that sampling methods should prioritise transportability (to facilitate replication) and that reporting of flooding regime should be standardised. Minello et al. (2003) discussed the nursery role of saltmarsh as a whole, specifically the patterns of density, growth and survival of nekton. They constrained their review parameters to studies that utilised methods that provide densities of animals per ‘area of bottom’ (i.e. habitat area). Rountree and Able (2007) reviewed sampling design and equipment selection for estimating densities of nekton in saltmarsh and other shallow estuarine habitats. Rozas and Minello (1997) discussed sampling design and methods used in shallow estuarine habitats, including saltmarsh flats, and recommended use of enclosure samplers because of their capacity to measure fish density. As more than 20 years of research has been conducted since the publication of these reviews, and a wide range of methods is still used to survey fish, an updated and more targeted investigation of methodological approaches is needed to support researchers in designing and undertaking future studies in saltmarsh flats.
The primary aim of the present study was to review the methods used to study fish assemblages on saltmarsh flats, including the relationships of field equipment and sampling design to vegetation type and research topic. We identify research gaps and offer a guide for sampling methods in relation to environment (i.e. vegetation type) and other practical considerations (e.g. portability, ease of deployment, catch efficiency). Our focus is on the methods appropriate to fill gaps in our knowledge of the fish ecology of saltmarsh flats. For a review of substantive findings relating to the nursery role of saltmarsh, see Minello et al. (2003), and for marsh function and patterns of fish use, see Rountree and Able (2007).
Materials and methods
We followed established methods (Moher et al. 2009; Pickering and Byrne 2014) to conduct a systematic search of the peer-reviewed literature on fish assemblages of vegetated saltmarsh flats. The electronic databases Google Scholar, Web of Science and Scopus were used to source original research papers published in English language in peer reviewed academic journals, with searches conducted between January and March 2019. The search terms used to identify papers were ‘saltmarsh’ or ‘salt marsh’, ‘fish assemblages’ and ‘sampling’. The keyword ‘fish assemblages’ was used to avoid papers that focussed solely on individual fish species. The keyword ‘sampling’ was used to select for papers that involved field sampling methods. Wildcards were used to ensure we did not omit papers that used other variant endings of the keywords. We did not specify a start or end limit for publication date.
The process of identification, screening and assessment for eligibility and inclusion of papers is represented in Fig. 2 (adapted from the Preferred Reporting Items for Systematic Review Recommendations; Moher et al. 2009). Only papers that describe the results of original research were included. Book chapters were excluded. Papers on single taxa (e.g. Geary et al. 2001; Able et al. 2012), rather than assemblages, were excluded. We also excluded papers that did not include field research. Papers that focussed solely on creeks (e.g. Hampel et al. 2003; Paterson and Whitfield 2003), pools (e.g. Davis et al. 2014) or artificial ponds, ditches and impoundments (e.g. Stevens 2006; Carswell et al. 2015) were excluded. Papers were also excluded in cases where they appeared to address the relevant topic but lacked important details (such as sampling location and design). Searches were concluded when either all results had been assessed for initial suitability (in the cases of Web of Science and Scopus) or when no relevant results were found within six consecutive pages with 10 results per page (in the case of Google Scholar). The reference lists of all selected papers were assessed for any further relevant peer-reviewed papers that met the criteria.

|
From the final selection of papers, we extracted the following information: (1) authorship, year of publication, country and hemisphere where the research was conducted, paper and journal titles; (2) fish-sampling methods; (3) dominant vegetation type; (4) other taxa surveyed in addition to fish; (5) sampling details including tidal magnitude and stage, diel phase, sampling month and number of months sampled; (6) variables measured or described including species identification, fish length, temperature, salinity, depth, dissolved oxygen and pH, and fish biomass; and (7) the research subject, including assemblage composition, food chains, webs or diet, fish use type (fish residency status and also activity type, e.g. feeding), restoration, marsh modification and degradation, fisheries, sampling techniques, temporal variations, and habitat comparisons. Some studies also included other taxa and habitats outside the scope of the present review. In such cases, information on sampling methods used only for the other taxa and habitats was excluded.
The database was then analysed to detect patterns and to identify gaps in the research. For the most frequently used methods, Pearson’s chi-square tests were used to test association between methods and vegetation type, diel period and the more popular subjects of research (temporal variation and marsh restoration). Vegetation type was grouped as either graminoid or succulent. If vegetation had a mixture of graminoids and succulents as dominant species, it was classed as succulent. The statistical software R (R Core Team 2019) and R Studio (RStudio Team 2016) were used to analyse data. Results of publication date, methods used, sampling diel period, sampling month, variables measured and reported, and other taxa surveyed were visualised using the function ggplot (Wickham 2016).
Results
Publication time, journal and study location
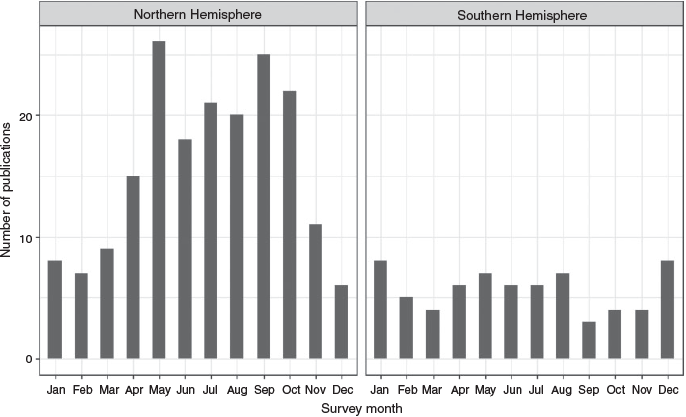
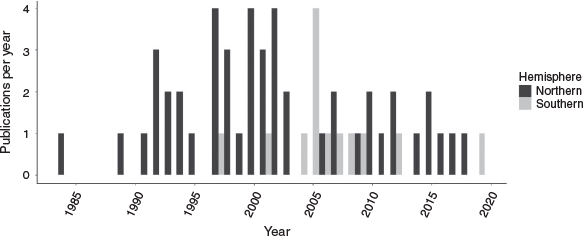
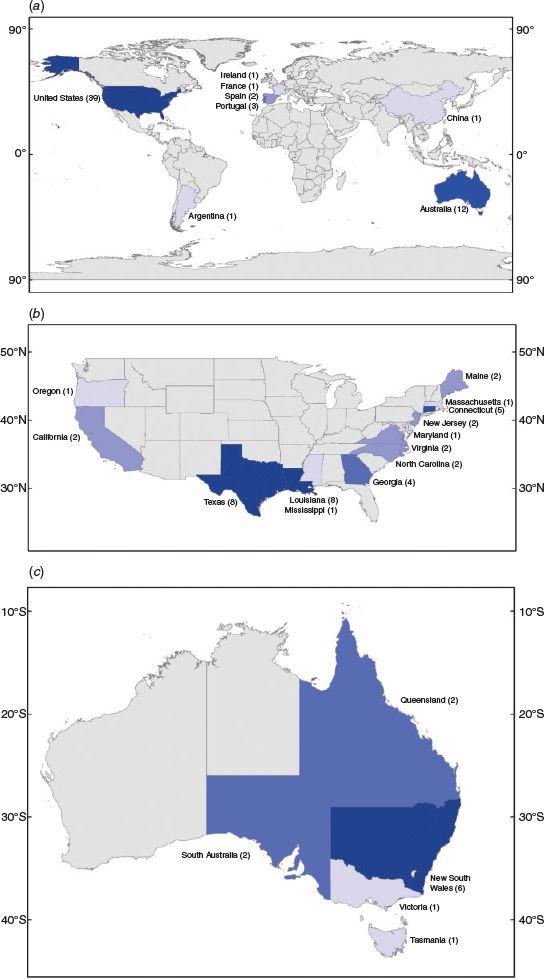
Sixty journal articles were selected, with publication dates ranging from 1984 to 2019 (Fig. 3). Papers were published in 21 journals. Those journals in which most papers were published were Estuaries and Coasts (previously Estuaries) and the Marine Ecology Progress Series, which featured 25% (n = 15) and 22% (n = 13) of publications respectively. Northern hemisphere studies accounted for 78% (n = 47) of papers, whereas southern hemisphere studies accounted for 22% (n = 13; Fig. 4a). Of the northern hemisphere studies, 83% (n = 39) were conducted in the United States. Of the southern hemisphere studies, 92% (n = 12) were conducted in Australia. Within these two countries, there was a strong regional bias, with the states of Louisiana and Texas in the Gulf Coast of the USA and the state of New South Wales in eastern Australia being disproportionately represented (Fig. 4b, c). Of the total number of studies, the USA contributed more than any other country, accounting for 65% (n = 39).
|
|
Methods used to sample fish
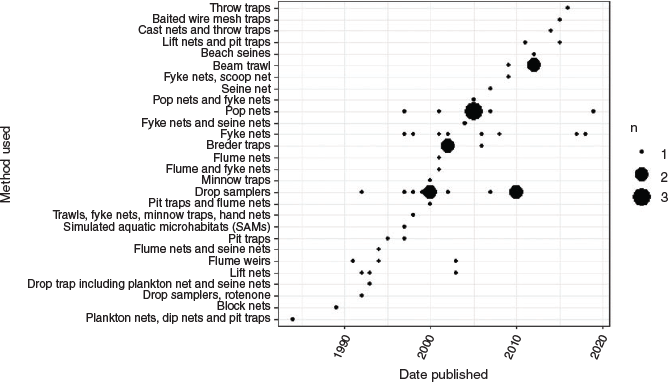
Twenty-one methods were used to sample fish assemblages in vegetated intertidal saltmarsh flats (Table 1). Many studies (22%, n = 13) used a combination of these methods. The most frequently used were drop samplers (18%, n = 11), followed by fyke nets (22%, n = 13) and pop nets (13%, n = 8).
Methods used to sample fish were either active (requiring field personnel to be on site to release nets or traps) or passive (requiring only initial set up or placement before being left to passively collect fish as they move into the trap; Kneib 1991). Active sampling methods include the use of drop samplers, pop nets, lift nets, seine nets, throw traps, trawls, cast nets, drop traps, flume weirs, plankton nets and hand nets. Several of these active methods, including pop nets, lift nets and drop traps, use remote-release systems to trap fish, thus allowing personnel to be stationed further from nets and traps. Passive sampling methods include fyke nets, Breder traps, pit traps, baited wire mesh traps, block nets, flume nets, minnow traps and simulated aquatic microhabitats. Only one study employed chemical methods, with the poison rotenone being used in conjunction with drop samplers (Rakocinski et al. 1992).
Many methods were used only once. Those that were used more frequently (pop nets, fyke nets and drop samplers) were used for more years than were the other methods (Fig. 5).
|
Tide, diel period, sampling month and season
Of the 60 studies, 72% (n = 43) provided details about the tidal conditions that occurred during sampling. Fifty-seven per cent (n = 34) noted that sampling had been conducted at high tide, whereas one study was conducted at low tide (Yozzo and Smith 1997). A further study sampled during both high and low tides (Jovanovic et al. 2007). Two other studies sampled during incoming tides (Lechêne et al. 2018) and incoming, slack high and ebb tides (Kneib and Wagner 1994). Twenty-three studies (38%) reported whether sampling occurred during spring or neap tides. Twenty of these sampled during spring tides, and three sampled during both spring and neap (and other lower-amplitude) tides.
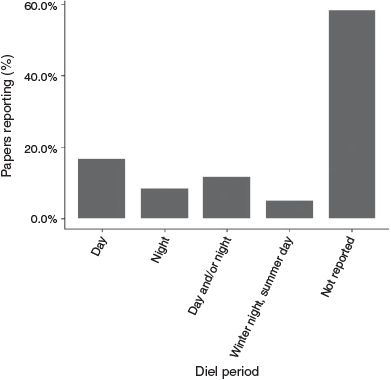
Only 42% of studies (n = 25) reported diel phase (Fig. 6), with 8% conducting sampling at night, 17% during the day, 12% during both day and night, and 5% changing diel phase according to season (winter afternoon or night sampling and summer morning or daytime sampling).
|
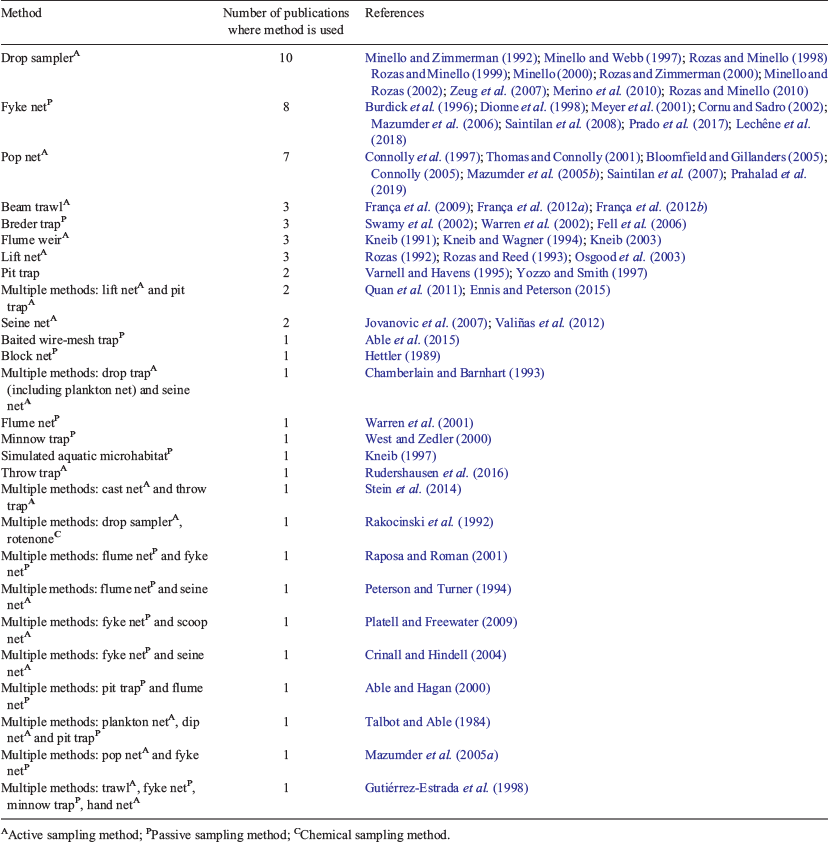
Ninety per cent of studies (n = 54) reported the months when sampling occurred, whereas 7% reported the sampling season without listing sampling months. Two studies (3%) did not report either the sampling month or the season. In the northern hemisphere, sampling peaked in the warmer months (April to October). In the southern hemisphere, sampling was performed during all times of year (Fig. 7).
Environmental variables and fish attributes
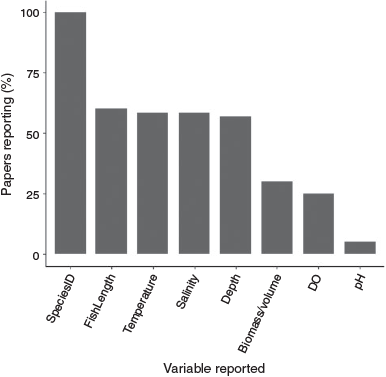
Most studies identified fish to species level. Commonly measured fish attributes included fish length, which was recorded in 60% of studies (n = 36), and fish biomass (either dry or wet weight per fish, per area sampled, or per sampling effort, in milligrams, grams or kilograms), or volume (millilitres per fish), as recorded in 30% of studies (n = 18). Of the environmental variables measured, water temperature was recorded in 58% of studies (n = 35), water salinity in 58% of studies (n = 35), water depth in 57% of studies (n = 34), dissolved oxygen in 25% of studies (n = 15), and pH in 5% of studies (n = 3) (Fig. 8).
|
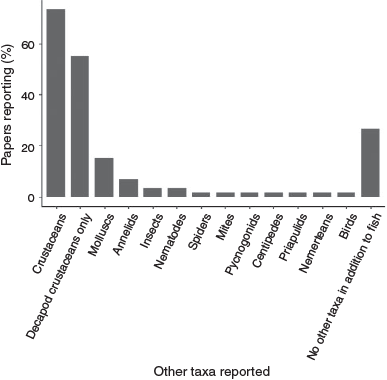
Seventy-three per cent (n = 44) of papers surveyed other taxa as well as fish. These other taxa were mainly invertebrates, including molluscs (gastropods, bivalves and cephalopods), annelids (oligochaetes and polychaetes), spiders, mites, pycnogonids, centipedes, insects (coleopterans, dipterans, hemipterans, orthopterans and collembolans), crustaceans (decapods, amphipods, isopods, mysids, tanaids, ostracods, copepods), priapulids, nematodes and nemerteans. Only one paper (Warren et al. 2002) reported on another vertebrate taxon (birds) in addition to fish. Decapods (crabs, prawns and shrimp) were the most common taxa studied in addition to fish (surveyed in 70% of studies, n = 42). Of these, 55% of studies (n = 33%) surveyed only decapods in addition to fish (Fig. 9).
|
Vegetation
Most papers (87%, n = 52) provided a description of the dominant vegetation type found at the sampling location. The most common marsh vegetation, reported in 58% of papers (n = 35), was graminoid marsh. Mixed graminoid and succulent vegetation was reported in 17% of papers (n = 10), and succulent vegetation was reported as dominant in 12% of papers (n = 7).
Topics investigated
All papers reported assemblage composition (a criterion for inclusion in the present review). Forty-seven per cent of papers (n = 28) discussed types of fish use of saltmarsh flats (including fish use of the flats for foraging, as a refuge, or as a nursery, and habitat occupancy, whether flats are used as a permanent habitat for resident fish or temporary habitat for transient fish).
Seventeen per cent of papers (n = 10) discussed the contribution of saltmarsh flats to fish diet, food chains and in terms of production more broadly. Many studies (87%, n = 52) included comparisons among habitats, whether within saltmarsh (e.g. vegetated flats, ponds, edges or creeks, and flats of different elevations), between saltmarsh and other habitats (e.g. mangrove, seagrass, non-vegetated flats and freshwater wetlands) or among saltmarsh modifications (e.g. hydrologically restricted and unrestricted saltmarshes, introduced and native vegetation types, and restored or created saltmarshes and unaltered saltmarshes). Sixty-three per cent of studies (n = 38) included analysis of temporal variation (whether tidal, diel or seasonal) in the fish assemblages using saltmarsh flats.
Thirty-five per cent of papers (n = 21) investigated marsh modification or degradation (hydrological modification, coastal development, pollution, and invasive plant species). Of papers investigating marsh modification and degradation, 18% (n = 11) addressed hydrological modification of saltmarshes (and implications for fish assemblages), 12% (n = 7) discussed invasive plant species, 5% (n = 3) discussed the effects of coastal development on saltmarsh fish assemblages and 3% (n = 2) looked at the effects of pollution (including nutrient input and oil spills). Invasive fish species (and the implications of their presence for native fish assemblages) were discussed only in 5 per cent of papers (n = 3). Thirty per cent of papers (n = 18) discussed saltmarsh restoration or artificial creation. Twenty-two per cent of papers (n = 13) discussed the contribution of saltmarsh fish to fisheries.
In addition to those papers investigating topics related to fish habitat use, ecology and management considerations, 13% of papers (n = 8) focussed on sampling techniques, either comparing sampling gear types or describing newly developed techniques.
Relationships between research context and method selection
Marsh restoration studies did not differ from the rest in terms of the methods used (χ2 = 3.186, d.f. = 2, P = 0.203), with there being no clear preference in the method used for this research focus. Studies that compared saltmarsh flats with other fish habitat also showed no clear preference for a method (χ2 = 3.214, d.f. = 2, P = 0.201). Pop nets and drop samplers were more frequently used in studies that investigated temporal variation in fish assemblages, whereas fyke nets were used in studies that did not investigate temporal variation (χ2 = 6.227, d.f. = 2, P = 0.045). Pop nets were used only in succulent-dominated vegetation and drop samplers were mostly used in graminoid vegetation (χ2 = 11.54, d.f. = 2, P = 0.003). Fyke nets were used in both vegetation types. Drop samplers were used only during daytime sampling and pop and fyke nets were used in sampling that included night and day and/or night sampling (χ2 = 11.66, d.f. = 2, P = 0.003).
Discussion
Geographic distribution
Our data showed a broader geographic range in studies of the fish assemblages of saltmarsh flats than has previously been observed for saltmarsh in general. We found the percentage of papers published on the USA saltmarshes to be lower than in previous reviews (65%, n = 39), with a greater variation in study location. This may partly relate to the different foci of the reviews, as well as increases in studies outside the USA. New studies represent areas including Australia, Argentina, China, Ireland, France, Portugal and Spain. Yet, there are still many regions poorly represented in the literature (Fig. 4a). For example, fish use of southern African marsh creeks has been studied (e.g. Paterson and Whitfield 2003), but the use of the surrounding flats has not yet been studied. Similarly, research has been published recently in Europe and Asia that provides information on creek fish assemblages, but not on fish assemblages of the flats (e.g. Hampel et al. 2003; Kaneko et al. 2019).
Sampling methods
The need to overcome difficulties associated with sampling in often densely vegetated shallow-water environments may be related to the development of the wide range of sampling methods observed in the present study and earlier reviews (Rozas and Minello 1997; Connolly 1999). In addition to vegetation type, other important considerations when sampling fish in vegetated flats include tidal and landscape patterns, issues with access, fish-movement patterns and data requirements such as density and size class.
Connolly (1999) recommended use of sampling equipment that is easily transportable, allowing for repeated random sampling. Rozas and Minello (1997) focussed on the importance of gear types meeting the requirements of the data, and specific study objectives, and the necessity of having high and temporally stable catch efficiency (defined by Rozas and Minello (1997) as the proportion of target animals collected from the sample unit area). In addition, if nekton densities are to be compared over time or among locations, quantitative sampling methods that allow the sample-unit area to be known, and methods that sample the whole water column are necessary (Rozas and Minello 1997). Meta-analyses, such as that by Minello et al. (2003), are enabled by studies that provide measures of density. To these previously recognised requirements of portability, catch efficiency and density measurement, we add suitability for different saltmarsh vegetation types (tall graminoid compared with succulent vegetation) and suitability for use in different diel periods. Because diel patterns in fish diversity and abundance on saltmarsh flats have been reported (Prahalad et al. 2019), it is important that method choice takes suitability for use at night as well as day into account.
Of the 21 methods employed, drop samplers were used most frequently. Drop samplers are often chosen to provide a precise measure of density and to efficiently capture organisms within a defined area, usually enclosing between 1 and 2.6 m2 (Minello 2000; Merino et al. 2010). Drop samplers have been commonly used in the dense, tall graminoid vegetation that is dominant in many saltmarshes in the USA (e.g. Minello 2000; Rozas and Zimmerman 2000; Zeug et al. 2007). They are often preferentially used in vegetation dominated by graminoids because they can be successfully deployed from a boat over this vegetation where use of other methods may be hindered. A major disadvantage with drop samplers is that, because they require access by boat for deployment, their use is limited by water depth. This restricts drop samplers from being used in marsh flats with shallow depths (Peterson and Turner 1994). We also noted that drop samplers were used only during the day, possibly because boat access may be more difficult at night.
Fyke nets were the second-most frequently employed method. Fyke nets passively sample fish retreating from the marsh and are composed of a long funnel-shaped net with wings extending from the net mouth, channelling fish in as the tide recedes. Fyke nets are frequently chosen owing to their portability and ease of deployment, low cost (Dionne et al. 1998) and because, as a passive capture method, there is less disturbance caused by the presence of researchers during deployment. In some cases, fyke nets have also been found to catch more species than do other methods (Mazumder et al. 2005a). Fyke nets have been used in both tall graminoid marshes and succulent marshes; so, they may be useful if comparisons between marsh vegetation types are needed. They have also been used in both day and night sampling. Fyke nets can be put in place at low tide while the marsh is not flooded, which may make sampling during the night less difficult. The most notable disadvantage of fyke nets is that, unlike the other two most commonly used methods, they do not provide an immediate measure of fish density, although some studies have calculated the area of marsh drained and sampled by the net (e.g. Dionne et al. 1998). An additional disadvantage of fyke nets (and also block nets and flume nets) is that, because they are generally located at drainage points on the marsh, often near a marsh edge, they cannot provide as detailed a picture of marsh subhabitat utilisation as do other gear types. They catch fish from the wider area drained, including species that do not venture further than the edge of the flats (Peterson and Turner 1994). Because fyke nets capture fish as they retreat during the falling tide, they are also less useful for sampling during different tidal stages.
Pop nets were the third-most frequently employed method. Pop nets are installed at low tide and held down by weights until release at high tide. Fish are captured when the buoyant-topped net walls are remotely released by removing the weights. Like drop samplers, pop nets also provide a measure of density with good catch efficiency, but for larger areas than do drop samplers (net area commonly 25 m2). We found that, along with drop samplers (which also provide a measure of density), pop nets were more frequently used than were other methods in investigations of temporal variation in fish assemblages. This may be due to their providing a standardised, readily replicable measure that can be applied year-round. Although pop nets have previously not been recommended for use in tidal marsh (Rozas and Minello 1997), they have since been frequently successfully employed in Australia. Indeed, all studies using pop nets were conducted in Australia (e.g. Bloomfield and Gillanders 2005; Prahalad et al. 2019), in locations where the vegetation is commonly composed of lower, succulent-dominated vegetation. It is possible that the recommendation against this method by Rozas and Minello (1997) was made considering only tall graminoid marshes. Like fyke nets, pop nets are highly portable, facilitating replication, and do not require a boat for deployment. They have been used in both day and night sampling and, unlike fyke nets, can be used during both flood and ebb tides.
Other methods were less frequently used. Among the rarely used older methods, poisoning may have been abandoned because of its unnecessary lethality for target and non-target species. Many studies that do not require fish to be retained after capture (e.g. for biomass measures) release fish once identified and measured. Other infrequently used methods employed recently, such as cast nets (Stein et al. 2014), have been used in other habitats, such as estuarine wetland pools (Sheaves and Johnston 2008). Cast nets were chosen because of their covering a large area per sampling effort, and their suitability for catching large fast-moving nekton (Stein et al. 2014). Other methods used recently, such as seines and beam trawls, have also been used elsewhere in different shallow habitats such as seagrass because they are easy to deploy over larger areas (Guest et al. 2003).
Additional, less frequently used, gear types (including pit traps and simulated aquatic microhabitats) are better suited for catching small resident species that remain in marsh subhabitats such as pools and ditches during low tide (e.g. Kneib 1997; Able and Hagan 2000), than species that return to other habitats when the tide ebbs (Peterson and Turner 1994). Smaller highly portable traps, such as Breder traps, also capture resident marsh fish but may exclude larger fish (Fell et al. 2006). These physically small traps may have less overall catch efficiency but they are highly portable, facilitating replication. The opposite is true for the flume weir (Kneib 1991), a large semipermanent structure that cannot be easily moved among locations but catches a high proportion of species present over an extensive area (100 m2). This type of method has, therefore, provided valuable information on seasonal and tidal variation in fish use (Kneib 1991; Kneib and Wagner 1994), but with limited spatial replication.
There are methods that have been used in other shallow habitats that are yet to be used on vegetated saltmarsh flats. These include underwater digital video cameras (Meynecke et al. 2008) and high-resolution imaging sonar (Rieucau et al. 2015). Within saltmarsh, they have been used in an intertidal creek and saltmarsh pool respectively. The benefits of these recording techniques include their low impact on organisms compared with trap or net gear types, their independence from human presence and ability to generate a large volume of data relative to sampling effort. Their limitations include inability to provide a positive species identification where the visuals are unclear, and not being able to reliably indicate either diversity or density.
Studies that focus on comparing sampling methods are highly valuable in providing information on relative catch efficiency and ease of deployment and replication. Where new methods are being employed, descriptions of these methods can be particularly useful when they are trialled with other previously established methods, providing direct in situ comparisons (e.g. Stein et al. 2014). This approach would be appropriate in future studies that use novel techniques or equipment not previously employed in saltmarsh flats.
Fish attributes and environmental factors
Some of the tidal and temporal variables assessed were found to be more consistently reported than were others. Tidal conditions during sampling, for example, were frequently reported (in 72% of reviewed papers). Sampling most commonly took place during high tides, with papers often specifying spring high tides, which in many locations is the only time when the marsh flats are fully inundated and accessible to fish (e.g. Bloomfield and Gillanders 2005; Quan et al. 2011). Several sampling techniques, such as, for example, fyke nets, block nets and flume nets, depend on the ebb tide to passively catch fish leaving the marsh surface (Hettler 1989; Dionne et al. 1998; Warren et al. 2001). Because diel phase can influence fish species richness and numbers (Prahalad et al. 2019), the frequent absence of information relating to diel phase during sampling is notable. Although assessing fish-assemblage responses to the patterns of environmental conditions of diel, tidal and seasonal scales can be challenging practically (Rountree and Able 2007), reporting the details of when sampling took place (and the associated environmental conditions present) is important for any study of fish on vegetated flats, because tidal variation, diel period and seasonal variation have all been found to influence saltmarsh-flat fish assemblages (e.g. Kneib and Wagner 1994; Thomas and Connolly 2001; Crinall and Hindell 2004). Previous reviews have noted the diversity of sampling methods and lack of standardisation in terms of reporting in studies of fish use of saltmarsh (Rozas and Minello 1997; Connolly 1999). Reporting of tides and flooding regimes has been an important recommendation (Connolly 1999). The variation in environmental variables, fish attributes and taxa recorded reflects the range of research subjects being investigated, but also limits the potential for comparing among studies.
Ecological relationships and management considerations
The most frequent subject of research has been comparisons between vegetated saltmarsh flats and other habitats, including other saltmarsh subhabitats and neighbouring habitats outside saltmarsh. Differences in the habitats being compared and the methods employed limit the potential for meta-analyses. Minello et al. (2003) found that only 32 studies met their research subject and density measurement criteria, and, of these studies, only six included vegetated inner marsh. For studies to be used in meta-analyses, methods that provide a measure of the number of fish per marsh area (as used by Minello et al. 2003) need to be employed, and environmental details such as landscape structure and tidal conditions and patterns need to be reported (Connolly 1999).
The influence of marsh modification and degradation on fish assemblages, including hydrological modification and the effects of invasive species, has been frequently investigated. The influence of less direct impacts on marsh hydrology on fish assemblages have also been investigated. These less direct impacts include changed watershed imperviousness associated with coastal development (Rudershausen et al. 2016) and marsh submergence associated with changes in sediment supply (Rozas and Reed 1993).
The effects of invasive plant species were also frequently investigated. With the exception of one study conducted in China, which compared invasive S. alterniflora marshes to native Scirpus mariqueter marshes (Quan et al. 2011), studies on the effects of invasive plant species were confined to the United States, where the invasive reed Phragmites australis has become increasingly prevalent along north-eastern coasts. There is, therefore, an opportunity for future research in places such as Australia where invasive plant species (such as Spartina anglica) are present (Prahalad 2014) and the implications for fish habitat are not yet known.
The potential effects of sea-level rise on saltmarsh was mentioned briefly in some studies (e.g. Ennis and Peterson 2015; Lechêne et al. 2018) but this was not specifically studied in terms of implications for fish habitat. Coastal saltmarsh is vulnerable to climate change and rising sea levels. Changes in saltmarsh plant communities associated with climate change have already been documented (Prahalad et al. 2011). Changes to saltmarsh area and vegetation type as a result of rising sea levels (particularly if inland retreat is not possible), as well as changes in climate, may affect fish habitat availability and requires research. ‘Within-range’ expansion of mangrove into saltmarsh flats, related to climate change and rising sea levels, also has implications for fish habitat and requires research (Kelleway et al. 2017).
Many regions have faced widespread pressures on formerly large areas of marsh, including subsidence as a result of large-scale inland land-use changes, impoundments, coastal development and invasive plant species, with some extensive restoration projects taking place as a result (e.g. Warren et al. 2002). Consequently, several studies have focussed on the effects of these restoration efforts on fish assemblages. In parts of the USA, particularly the Atlantic and Gulf Coasts, marsh restoration research has been ongoing for nearly 40 years (Warren et al. 2002). There is a dearth of information on restoration works and implications for fish assemblages in other parts of the world (Creighton et al. 2019).
The relationships between fisheries and saltmarsh flats was addressed less frequently in the literature we reviewed. The limited number of studies that investigate this subject may be related to the challenge of distinguishing the contribution of saltmarsh flats compared with other potential saltmarsh habitats, and the influence of other confounding variables such as changes in fishing effort (Connolly 1999). Because many estuarine species rely on several habitats during their life histories, determining the direct contribution of saltmarsh to particular fisheries can be challenging (Kneib 2003). Several studies have reported fisheries species caught on saltmarsh flats (e.g. Connolly et al. 1997; Prahalad et al. 2019), and the dietary contribution of saltmarsh flats for fish is a reasonably frequently studied subject of research (e.g. Warren et al. 2002; Crinall and Hindell 2004; Platell and Freewater 2009), but demonstrating links to commercial and recreational fisheries species can be more difficult. Connolly (1999) suggested that large-scale, long-term adaptive-management studies are needed to demonstrate any link between saltmarsh loss and reduced fisheries, or, alternatively, small-scale studies on calorific requirements or isotopic studies to portray food webs. A different approach was taken by Saintilan et al. (2008), who studied changes in saltmarsh fish assemblages following the closure of commercial fishing, rather than investigating the effects of changes to saltmarsh habitat itself on fisheries. Future studies using techniques such as isotopic analysis will be beneficial in understanding links between saltmarsh-flat habitat and fisheries.
Conclusions, research gaps and recommendations
Although the variety of methods noted in previous reviews is still apparent, the present review found that three methods were used more frequently than others. Drop samplers, pop nets and fyke nets all have positive attributes that make them suitable choices in relation to environment, research questions and practical considerations. On the basis of suitability for vegetation type, capacity to directly measure density, portability, and suitability for use during both day and night, recommendations for methods can be made. Drop samplers are more suitable for tall graminoid marsh, where access for deployment of other nets and traps is more difficult, whereas pop nets are more suitable for succulent marsh. Fyke nets can be used in both vegetation types. Drop samplers and pop nets both provide measures of density, which is valuable not only for comparisons being made within a single study, but also allows data to be used in meta-analyses of multiple studies and to compare different habitats or changes in habitat condition. Fyke nets have been used in both succulent and graminoid vegetation types, but they do not allow fish density to be directly measured. All three are portable, facilitating replication in sampling design, although drop samplers rely on boat access for deployment. Both pop and fyke nets do not require a boat for deployment and can be used for both day and night sampling. An additional important environmental consideration is tide stage. Unlike drop samplers and pop nets, fyke nets may be less suitable for studies investigating variation in fish use by tidal stage.
There are many regions where research on fish use of vegetated saltmarsh flats is still absent or limited. Given the global variation in vegetated-flat habitat, including differences in marsh plant community composition and inundation patterns, this represents a distinct gap in the literature. Research in locations currently understudied will be valuable, particularly given the ongoing loss and degradation of saltmarsh. The relationships between the fish assemblages of vegetated saltmarsh flats and coastal fisheries, the influence of invasive plant species on fish assemblages in Australia and areas where this has not yet been investigated, and the potential effects of climate change, sea-level rise and mangrove incursion are also worthy of more research.
Our review will enable investigations that build on the important previous work and reviews to expand knowledge of how fish use saltmarsh flats. In particular, our inventory of methods and design will help inform sampling approaches to under-researched questions.
Conflict of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Research was supported by an Australian Government Research Training Program Scholarship and a Holsworth Wildlife Research Endowment Scholarship.
References
Able, K. W., and Hagan, S. M. (2000). Effects of common reed (Phragmites australis) invasion on marsh surface macrofauna: response of fishes and decapod crustaceans. Estuaries 23, 633–646.| Effects of common reed (Phragmites australis) invasion on marsh surface macrofauna: response of fishes and decapod crustaceans.Crossref | GoogleScholarGoogle Scholar |
Able, K. W., Nemerson, D. M., Bush, R., and Light, P. (2001). Spatial variation in Delaware Bay (USA) marsh creek fish assemblages. Estuaries 24, 441–452.
| Spatial variation in Delaware Bay (USA) marsh creek fish assemblages.Crossref | GoogleScholarGoogle Scholar |
Able, K. W., Vivian, D. N., Petruzzelli, G., and Hagan, S. M. (2012). Connectivity among salt marsh subhabitats: residency and movements of the mummichog (Fundulus heteroclitus). Estuaries and Coasts 35, 743–753.
| Connectivity among salt marsh subhabitats: residency and movements of the mummichog (Fundulus heteroclitus).Crossref | GoogleScholarGoogle Scholar |
Able, K. W., López-Duarte, P. C., Fodrie, F. J., Jensen, O. P., Martin, C. W., Roberts, B. J., Valenti, J., O’Connor, K., and Halbert, S. C. (2015). Fish assemblages in Louisiana salt marshes: effects of the Macondo oil spill. Estuaries and Coasts 38, 1385–1398.
| Fish assemblages in Louisiana salt marshes: effects of the Macondo oil spill.Crossref | GoogleScholarGoogle Scholar |
Baltz, D. M., Rakocinski, C., and Fleeger, J. W. (1993). Microhabitat use by marsh-edge fishes in a Louisiana estuary. Environmental Biology of Fishes 36, 109–126.
| Microhabitat use by marsh-edge fishes in a Louisiana estuary.Crossref | GoogleScholarGoogle Scholar |
Barbier, E. B., Hacker, S. D., Kennedy, C., Koch, E. W., Stier, A. C., and Silliman, B. R. (2011). The value of estuarine and coastal ecosystem services. Ecological Monographs 81, 169–193.
| The value of estuarine and coastal ecosystem services.Crossref | GoogleScholarGoogle Scholar |
Bloomfield, A. L., and Gillanders, B. M. (2005). Fish and invertebrate assemblages in seagrass, mangrove, saltmarsh, and nonvegetated habitats. Estuaries 28, 63–77.
| Fish and invertebrate assemblages in seagrass, mangrove, saltmarsh, and nonvegetated habitats.Crossref | GoogleScholarGoogle Scholar |
Burdick, D. M., Dionne, M., Boumans, R. M., and Short, F. T. (1996). Ecological responses to tidal restorations of two northern New England salt marshes. Wetlands Ecology and Management 4, 129–144.
| Ecological responses to tidal restorations of two northern New England salt marshes.Crossref | GoogleScholarGoogle Scholar |
Carswell, B. L., Peterson, J. T., and Jennings, C. A. (2015). Tidal management affects sub-adult fish assemblages in impounded South Carolina marshes. Wetlands Ecology and Management 23, 1015–1031.
| Tidal management affects sub-adult fish assemblages in impounded South Carolina marshes.Crossref | GoogleScholarGoogle Scholar |
Chamberlain, R. H., and Barnhart, R. A. (1993). Early use by fish of a Mitigation Salt Marsh, Humboldt Bay, California. Estuaries 16, 769–783.
| Early use by fish of a Mitigation Salt Marsh, Humboldt Bay, California.Crossref | GoogleScholarGoogle Scholar |
Connolly, R. M. (1999). Saltmarsh as habitat for fish and nektonic crustaceans: challenges in sampling designs and methods. Austral Ecology 24, 422–430.
| Saltmarsh as habitat for fish and nektonic crustaceans: challenges in sampling designs and methods.Crossref | GoogleScholarGoogle Scholar |
Connolly, R. M. (2005). Modification of saltmarsh for mosquito control in Australia alters habitat use by nekton. Wetlands Ecology and Management 13, 149–161.
Connolly, R. M., Dalton, A., and Bass, D. A. (1997). Fish use of an inundated saltmarsh flat in a temperate Australian estuary. Australian Journal of Ecology 22, 222–226.
| Fish use of an inundated saltmarsh flat in a temperate Australian estuary.Crossref | GoogleScholarGoogle Scholar |
Cornu, C. E., and Sadro, S. (2002). Physical and functional responses to experimental marsh surface elevation manipulation in Coos Bay’s south slough. Restoration Ecology 10, 474–486.
| Physical and functional responses to experimental marsh surface elevation manipulation in Coos Bay’s south slough.Crossref | GoogleScholarGoogle Scholar |
Creighton, C., Prahalad, V. N., McLeod, I., Sheaves, M., Taylor, M. D., and Walshe, T. (2019). Prospects for seascape repair: three case studies from eastern Australia. Ecological Management & Restoration 20, 182–191.
| Prospects for seascape repair: three case studies from eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Crinall, S. M., and Hindell, J. S. (2004). Assessing the use of saltmarsh flats by fish in a temperate Australian embayment. Estuaries 27, 728–739.
| Assessing the use of saltmarsh flats by fish in a temperate Australian embayment.Crossref | GoogleScholarGoogle Scholar |
Davis, B., Baker, R., and Sheaves, M. (2014). Seascape and metacommunity processes regulate fish assemblage structure in coastal wetlands. Marine Ecology Progress Series 500, 187–202.
| Seascape and metacommunity processes regulate fish assemblage structure in coastal wetlands.Crossref | GoogleScholarGoogle Scholar |
Dionne, M., Short, F. T., and Burdick, D. M. (1998). Fish utilization of restored, created, and reference salt-marsh habitat in the Gulf of Maine. American Fisheries Society Symposium 22, 384–404.
Ennis, B., and Peterson, M. S. (2015). Nekton and macro-crustacean habitat use of Mississippi micro-tidal salt marsh landscapes. Estuaries and Coasts 38, 1399–1413.
| Nekton and macro-crustacean habitat use of Mississippi micro-tidal salt marsh landscapes.Crossref | GoogleScholarGoogle Scholar |
Fell, P. E., Warren, R. S., Curtis, A. E., and Steiner, E. M. (2006). Short-term effects on macroinvertebrates and fishes of herbiciding and mowing Phragmites australis-dominated tidal marsh. Northeastern Naturalist 13, 191–212.
| Short-term effects on macroinvertebrates and fishes of herbiciding and mowing Phragmites australis-dominated tidal marsh.Crossref | GoogleScholarGoogle Scholar |
França, S., Costa, M. J., and Cabral, H. N. (2009). Assessing habitat specific fish assemblages in estuaries along the Portuguese coast. Estuarine, Coastal and Shelf Science 83, 1–12.
| Assessing habitat specific fish assemblages in estuaries along the Portuguese coast.Crossref | GoogleScholarGoogle Scholar |
França, S., Vasconcelos, R. P., Fonseca, V. F., Tanner, S. E., Reis-Santos, P., Costa, M. J., and Cabral, H. N. (2012a). Predicting fish community properties within estuaries: Influence of habitat type and other environmental features. Estuarine, Coastal and Shelf Science 107, 22–31.
| Predicting fish community properties within estuaries: Influence of habitat type and other environmental features.Crossref | GoogleScholarGoogle Scholar |
França, S., Vasconcelos, R. P., Reis-Santos, P., Fonseca, V. F., Costa, M. J., and Cabral, H. N. (2012b). Vulnerability of Portuguese estuarine habitats to human impacts and relationship with structural and functional properties of the fish community. Ecological Indicators 18, 11–19.
| Vulnerability of Portuguese estuarine habitats to human impacts and relationship with structural and functional properties of the fish community.Crossref | GoogleScholarGoogle Scholar |
Geary, B. W., Rooker, J. R., and Webb, J. W. (2001). Utilization of saltmarsh shorelines by newly settled sciaenids in a Texas estuary. Gulf and Caribbean Research 13, 29–41.
| Utilization of saltmarsh shorelines by newly settled sciaenids in a Texas estuary.Crossref | GoogleScholarGoogle Scholar |
Green, B. C., Smith, D. J., Earley, S. E., Hepburn, L. J., and Underwood, G. J. C. (2009). Seasonal changes in community composition and trophic structure of fish populations of five salt marshes along the Essex coastline, United Kingdom. Estuarine, Coastal & Shelf Science 85, 247–256.
| Seasonal changes in community composition and trophic structure of fish populations of five salt marshes along the Essex coastline, United Kingdom.Crossref | GoogleScholarGoogle Scholar |
Guest, M. A., Connolly, R. M., and Loneragan, N. R. (2003). Seine nets and beam trawls compared by day and night for sampling fish and crustaceans in shallow seagrass habitat. Fisheries Research 64, 185–196.
| Seine nets and beam trawls compared by day and night for sampling fish and crustaceans in shallow seagrass habitat.Crossref | GoogleScholarGoogle Scholar |
Gutiérrez-Estrada, J. C., Prenda, J., Oliva-Paterna, F. J., and Fernandez-Delgado, C. (1998). Distribution and habitat preferences of the introduced mummichog Fundulus heteroclitus (Linneaus) in south-western Spain. Estuarine, Coastal and Shelf Science 46, 827–835.
| Distribution and habitat preferences of the introduced mummichog Fundulus heteroclitus (Linneaus) in south-western Spain.Crossref | GoogleScholarGoogle Scholar |
Hampel, H., Cattrijsse, A., and Vincx, M. (2003). Tidal, diel and semi-lunar changes in the faunal assemblage of an intertidal salt marsh creek. Estuarine, Coastal and Shelf Science 56, 795–805.
| Tidal, diel and semi-lunar changes in the faunal assemblage of an intertidal salt marsh creek.Crossref | GoogleScholarGoogle Scholar |
Hettler, W. (1989). Nekton use of regularly-flooded salt-marsh cordgrass habitat in North Carolina, USA. Marine Ecology Progress Series 56, 111–118.
| Nekton use of regularly-flooded salt-marsh cordgrass habitat in North Carolina, USA.Crossref | GoogleScholarGoogle Scholar |
Jovanovic, B., Longmore, C., O’Leary, Á., and Mariani, S. (2007). Fish community structure and distribution in a macro-tidal inshore habitat in the Irish Sea. Estuarine, Coastal and Shelf Science 75, 135–142.
| Fish community structure and distribution in a macro-tidal inshore habitat in the Irish Sea.Crossref | GoogleScholarGoogle Scholar |
Kaneko, S., Kanou, K., and Sano, M. (2019). Comparison of fish assemblage structures among microhabitats in a salt marsh in Lake Hinuma, eastern Japan. Fisheries Science 85, 113–125.
| Comparison of fish assemblage structures among microhabitats in a salt marsh in Lake Hinuma, eastern Japan.Crossref | GoogleScholarGoogle Scholar |
Kelleway, J. J., Cavanaugh, K., Rogers, K., Feller, I. C., Ens, E., Doughty, C., and Saintilan, N. (2017). Review of the ecosystem service implications of mangrove encroachment into salt marshes. Global Change Biology 23, 3967–3983.
| Review of the ecosystem service implications of mangrove encroachment into salt marshes.Crossref | GoogleScholarGoogle Scholar | 28544444PubMed |
Kneib, R. (1991). Flume weir for quantitative collection of nekton from vegetated intertidal habitats. Marine Ecology Progress Series 75, 29–38.
| Flume weir for quantitative collection of nekton from vegetated intertidal habitats.Crossref | GoogleScholarGoogle Scholar |
Kneib, R. T. (1997). Early life stages of resident nekton in intertidal marshes. Estuaries 20, 214–230.
| Early life stages of resident nekton in intertidal marshes.Crossref | GoogleScholarGoogle Scholar |
Kneib, R. (2003). Bioenergetic and landscape considerations for scaling expectations of nekton production from intertidal marshes. Marine Ecology Progress Series 264, 279–296.
| Bioenergetic and landscape considerations for scaling expectations of nekton production from intertidal marshes.Crossref | GoogleScholarGoogle Scholar |
Kneib, R. T., and Wagner, S. L. (1994). Nekton use of vegetated marsh habitats at different stages of tidal inundation. Marine Ecology Progress Series 106, 227–238.
| Nekton use of vegetated marsh habitats at different stages of tidal inundation.Crossref | GoogleScholarGoogle Scholar |
Lechêne, A., Boët, P., Laffaille, P., and Lobry, J. (2018). Nekton communities of tidally restored marshes: a whole-estuary approach. Estuarine, Coastal and Shelf Science 207, 368–382.
| Nekton communities of tidally restored marshes: a whole-estuary approach.Crossref | GoogleScholarGoogle Scholar |
Mazumder, D., Saintilan, N., and Williams, R. (2005a). Comparisons of fish catches using fyke nets and buoyant pop nets in a vegetated shallow water saltmarsh flat at Towra Point, NSW. Wetlands Australia 23, 37–46.
Mazumder, D., Saintilan, N., and Williams, R. J. (2005b). Temporal variations in fish catch using pop nets in mangrove and saltmarsh flats at Towra Point, NSW, Australia. Wetlands Ecology and Management 13, 457–467.
| Temporal variations in fish catch using pop nets in mangrove and saltmarsh flats at Towra Point, NSW, Australia.Crossref | GoogleScholarGoogle Scholar |
Mazumder, D., Saintilan, N., and Williams, R. J. (2006). Fish assemblages in three tidal saltmarsh and mangrove flats in temperate NSW, Australia: a comparison based on species diversity and abundance. Wetlands Ecology and Management 14, 201–209.
| Fish assemblages in three tidal saltmarsh and mangrove flats in temperate NSW, Australia: a comparison based on species diversity and abundance.Crossref | GoogleScholarGoogle Scholar |
Merino, J. H., Rozas, L. P., Minello, T. J., and Sheridan, P. F. (2010). Effects of marsh terracing on nekton abundance at two locations in Galveston Bay, Texas. Wetlands 30, 693–704.
| Effects of marsh terracing on nekton abundance at two locations in Galveston Bay, Texas.Crossref | GoogleScholarGoogle Scholar |
Meyer, D., Johnson, J., and Gill, J. (2001). Comparison of nekton use of Phragmites australis and Spartina alterniflora marshes in the Chesapeake Bay, USA. Marine Ecology Progress Series 209, 71–83.
| Comparison of nekton use of Phragmites australis and Spartina alterniflora marshes in the Chesapeake Bay, USA.Crossref | GoogleScholarGoogle Scholar |
Meynecke, J.-O., Poole, G. C., Werry, J., and Lee, S. Y. (2008). Use of PIT tag and underwater video recording in assessing estuarine fish movement in a high intertidal mangrove and salt marsh creek. Estuarine, Coastal and Shelf Science 79, 168–178.
| Use of PIT tag and underwater video recording in assessing estuarine fish movement in a high intertidal mangrove and salt marsh creek.Crossref | GoogleScholarGoogle Scholar |
Minello, T. J. (2000). Temporal development of salt marsh value for nekton and epifauna: utilization of dredged material marshes in Galveston Bay, Texas, USA. Wetlands Ecology and Management 8, 327–342.
| Temporal development of salt marsh value for nekton and epifauna: utilization of dredged material marshes in Galveston Bay, Texas, USA.Crossref | GoogleScholarGoogle Scholar |
Minello, T. J., and Rozas, L. P. (2002). Nekton in gulf coast wetlands: fine-scale distributions, landscape patterns, and restoration implications. Ecological Applications 12, 441–455.
| Nekton in gulf coast wetlands: fine-scale distributions, landscape patterns, and restoration implications.Crossref | GoogleScholarGoogle Scholar |
Minello, T., and Webb, J. (1997). Use of natural and created Spartina alterniflora salt marshes by fishery species and other aquatic fauna in Galveston Bay, Texas, USA. Marine Ecology Progress Series 151, 165–179.
| Use of natural and created Spartina alterniflora salt marshes by fishery species and other aquatic fauna in Galveston Bay, Texas, USA.Crossref | GoogleScholarGoogle Scholar |
Minello, T., and Zimmerman, R. (1992). Utilization of natural and transplanted Texas salt marshes by fish and decapod crustaceans. Marine Ecology Progress Series 90, 273–285.
| Utilization of natural and transplanted Texas salt marshes by fish and decapod crustaceans.Crossref | GoogleScholarGoogle Scholar |
Minello, T., Able, K., Weinstein, M., and Hays, C. (2003). Salt marshes as nurseries for nekton: testing hypotheses on density, growth and survival through meta-analysis. Marine Ecology Progress Series 246, 39–59.
| Salt marshes as nurseries for nekton: testing hypotheses on density, growth and survival through meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and The, P. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 6, e1000097.
| Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.Crossref | GoogleScholarGoogle Scholar | 19753108PubMed |
Odum, E. P. (2002). Tidal marshes as outwelling/pulsing systems. In ‘Concepts and Controversies in Tidal Marsh Ecology’. (Eds M. P. Weinstein and D. A. Kreeger.) pp. 3–7. (Kluwer Academic Publishers: New York, NY, USA.) https://doi.org/
Osgood, D. T., Yozzo, D. J., Chambers, R. M., Jacobson, D., Hoffman, T., and Wnek, J. (2003). Tidal Hydrology and habitat utilization by resident nekton in phragmites and non-phragmites marshes. Estuaries 26, 522–533.
| Tidal Hydrology and habitat utilization by resident nekton in phragmites and non-phragmites marshes.Crossref | GoogleScholarGoogle Scholar |
Paterson, A. W., and Whitfield, A. K. (2003). The fishes associated with three intertidal salt marsh creeks in a temperate southern African estuary. Wetlands Ecology and Management 11, 305–315.
| The fishes associated with three intertidal salt marsh creeks in a temperate southern African estuary.Crossref | GoogleScholarGoogle Scholar |
Peterson, G. W., and Turner, R. E. (1994). The value of salt marsh edge vs interior as a habitat for fish and decapod crustaceans in a Louisiana tidal marsh. Estuaries 17, 235–262.
| The value of salt marsh edge vs interior as a habitat for fish and decapod crustaceans in a Louisiana tidal marsh.Crossref | GoogleScholarGoogle Scholar |
Pickering, C., and Byrne, J. (2014). The benefits of publishing systematic quantitative literature reviews for PhD candidates and other early-career researchers. Higher Education Research & Development 33, 534–548.
| The benefits of publishing systematic quantitative literature reviews for PhD candidates and other early-career researchers.Crossref | GoogleScholarGoogle Scholar |
Platell, M. E., and Freewater, P. (2009). Importance of saltmarsh to fish species of a large south-eastern Australian estuary during a spring tide cycle. Marine and Freshwater Research 60, 936–941.
| Importance of saltmarsh to fish species of a large south-eastern Australian estuary during a spring tide cycle.Crossref | GoogleScholarGoogle Scholar |
Prado, P., Alcaraz, C., Jornet, L., Caiola, N., and Ibanez, C. (2017). Effects of enhanced hydrological connectivity on Mediterranean salt marsh fish assemblages with emphasis on the endangered Spanish toothcarp (Aphanius iberus). PeerJ 5, e3009.
| Effects of enhanced hydrological connectivity on Mediterranean salt marsh fish assemblages with emphasis on the endangered Spanish toothcarp (Aphanius iberus).Crossref | GoogleScholarGoogle Scholar | 28265500PubMed |
Prahalad, V. N. (2014). Human impacts and saltmarsh loss in the Circular Head coast, north-west Tasmania, 1952–2006: implications for management. Pacific Conservation Biology 20, 272–285.
| Human impacts and saltmarsh loss in the Circular Head coast, north-west Tasmania, 1952–2006: implications for management.Crossref | GoogleScholarGoogle Scholar |
Prahalad, V. N., Kirkpatrick, J. B., and Mount, R. E. (2011). Tasmanian coastal saltmarsh community transitions associated with climate change and relative sea level rise 1975–2009. Australian Journal of Botany 59, 741–748.
| Tasmanian coastal saltmarsh community transitions associated with climate change and relative sea level rise 1975–2009.Crossref | GoogleScholarGoogle Scholar |
Prahalad, V., Harrison-Day, V., McQuillan, P., and Creighton, C. (2019). Expanding fish productivity in Tasmanian saltmarsh wetlands through tidal reconnection and habitat repair. Marine and Freshwater Research 70, 140–151.
| Expanding fish productivity in Tasmanian saltmarsh wetlands through tidal reconnection and habitat repair.Crossref | GoogleScholarGoogle Scholar |
Quan, W., Shi, L., and Chen, Y. (2011). Comparison of nekton se for cordgrass Spartina alterniflora and bulrush Scirpus mariqueter marshes in the Yangtze River estuary, China. Estuaries and Coasts 34, 405–416.
| Comparison of nekton se for cordgrass Spartina alterniflora and bulrush Scirpus mariqueter marshes in the Yangtze River estuary, China.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2019). A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at https://www.R-project.org/ [verified 15 May 2020].
Rakocinski, C. F., Baltz, D. M., and Fleeger, J. W. (1992). Correspondence between environmental gradients and the community structure of marsh-edge fishes in a Louisiana estuary. Marine Ecology Progress Series 80, 135–148.
| Correspondence between environmental gradients and the community structure of marsh-edge fishes in a Louisiana estuary.Crossref | GoogleScholarGoogle Scholar |
Raposa, K. B., and Roman, C. T. (2001). Seasonal habitat-use patterns of nekton in a tide-restricted and unrestricted New England salt marsh. Wetlands 21, 451–461.
| Seasonal habitat-use patterns of nekton in a tide-restricted and unrestricted New England salt marsh.Crossref | GoogleScholarGoogle Scholar |
Rieucau, G., Boswell, K. M., Kimball, M. E., Diaz, G., and Allen, D. M. (2015). Tidal and diel variations in abundance and schooling behavior of estuarine fish within an intertidal salt marsh pool. Hydrobiologia 753, 149–162.
| Tidal and diel variations in abundance and schooling behavior of estuarine fish within an intertidal salt marsh pool.Crossref | GoogleScholarGoogle Scholar |
Rogers, K., Boon, P. I., Branigan, S., Duke, N. C., Field, C. D., Fitzsimons, J. A., Kirkman, H., Mackenzie, J. R., and Saintilan, N. (2016). The state of legislation and policy protecting Australia’s mangrove and salt marsh and their ecosystem services. Marine Policy 72, 139–155.
| The state of legislation and policy protecting Australia’s mangrove and salt marsh and their ecosystem services.Crossref | GoogleScholarGoogle Scholar |
Rountree, R. A., and Able, K. W. (1992). Fauna of polyhaline subtidal marsh creeks in southern New Jersey: composition. Abundance and Biomass 15, 171–185.
Rountree, R. A., and Able, K. W. (2007). Spatial and temporal habitat use patterns for salt marsh nekton: implications for ecological functions. Aquatic Ecology 41, 25–45.
| Spatial and temporal habitat use patterns for salt marsh nekton: implications for ecological functions.Crossref | GoogleScholarGoogle Scholar |
Rozas, L. (1992). Bottomless lift net for quantitatively sampling nekton on intertidal marshes. Marine Ecology Progress Series 89, 287–292.
| Bottomless lift net for quantitatively sampling nekton on intertidal marshes.Crossref | GoogleScholarGoogle Scholar |
Rozas, L. P., and Minello, T. J. (1997). Estimating densities of small fishes and decapod crustaceans in shallow estuarine habitats: a review of sampling design with focus on gear selection. Estuaries 20, 199–213.
| Estimating densities of small fishes and decapod crustaceans in shallow estuarine habitats: a review of sampling design with focus on gear selection.Crossref | GoogleScholarGoogle Scholar |
Rozas, L. P., and Minello, T. J. (1998). Nekton use of salt marsh, seagrass, and nonvegetated habitats in a south Texas (USA) estuary. Bulletin of Marine Science 63, 481–501.
Rozas, L. P., and Minello, T. J. (1999). Effects of structural marsh management on fishery species and other nekton before and during a spring drawdown. Wetlands Ecology and Management 7, 121–139.
| Effects of structural marsh management on fishery species and other nekton before and during a spring drawdown.Crossref | GoogleScholarGoogle Scholar |
Rozas, L. P., and Minello, T. J. (2010). Nekton density patterns in tidal ponds and adjacent wetlands related to pond size and salinity. Estuaries and Coasts 33, 652–667.
| Nekton density patterns in tidal ponds and adjacent wetlands related to pond size and salinity.Crossref | GoogleScholarGoogle Scholar |
Rozas, L., and Reed, D. (1993). Nekton use of marsh-surface habitats in Louisiana (USA) deltaic salt marshes undergoing submergence. Marine Ecology Progress Series 96, 147–157.
| Nekton use of marsh-surface habitats in Louisiana (USA) deltaic salt marshes undergoing submergence.Crossref | GoogleScholarGoogle Scholar |
Rozas, L., and Zimmerman, R. (2000). Small-scale patterns of nekton use among marsh and adjacent shallow nonvegetated areas of the Galveston Bay Estuary, Texas (USA). Marine Ecology Progress Series 193, 217–239.
| Small-scale patterns of nekton use among marsh and adjacent shallow nonvegetated areas of the Galveston Bay Estuary, Texas (USA).Crossref | GoogleScholarGoogle Scholar |
RStudio Team (2016). RStudio: integrated development for R. RStudio, Inc., Boston, MA, USA. Available at http://www.rstudio.com/ [verified 21 May 2020].
Rudershausen, P. J., Buckel, J. A., Dueker, M. A., Poland, S. J., and Hain, E. (2016). Comparison of fish and invertebrate assemblages among variably altered tidal creeks in a coastal landscape. Marine Ecology Progress Series 544, 15–35.
| Comparison of fish and invertebrate assemblages among variably altered tidal creeks in a coastal landscape.Crossref | GoogleScholarGoogle Scholar |
Saintilan, N., Hossain, K., and Mazumder, D. (2007). Linkages between seagrass, mangrove and saltmarsh as fish habitat in the Botany Bay estuary, New South Wales. Wetlands Ecology and Management 15, 277–286.
| Linkages between seagrass, mangrove and saltmarsh as fish habitat in the Botany Bay estuary, New South Wales.Crossref | GoogleScholarGoogle Scholar |
Saintilan, N., Mazumder, D., and Cranney, K. (2008). Changes to fish assemblages visiting estuarine wetlands following the closure of commercial fishing in Botany Bay, Australia. Aquatic Ecosystem Health & Management 11, 441–449.
| Changes to fish assemblages visiting estuarine wetlands following the closure of commercial fishing in Botany Bay, Australia.Crossref | GoogleScholarGoogle Scholar |
Sheaves, M., and Johnston, R. (2008). Influence of marine and freshwater connectivity on the dynamics of subtropical estuarine wetland fish metapopulations. Marine Ecology Progress Series 357, 225–243.
| Influence of marine and freshwater connectivity on the dynamics of subtropical estuarine wetland fish metapopulations.Crossref | GoogleScholarGoogle Scholar |
Stein, W., Smith, P. W., and Smith, G. (2014). The cast net: an overlooked sampling gear. Marine and Coastal Fisheries 6, 12–19.
| The cast net: an overlooked sampling gear.Crossref | GoogleScholarGoogle Scholar |
Stevens, P. W. (2006). Sampling fish communities in saltmarsh impoundments in the northern Indian River Lagoon, Florida: cast net and culvert trap gear testing. Florida Scientist 69, 135–147.
Swamy, V., Fell, P. E., Body, M., Keaney, M. B., Nyaku, M. K., McIlvain, E. C., and Keen, A. L. (2002). Macroinvertebrate and fish populations in a restored impounded salt marsh 21 years after the reestablishment of tidal flooding. Environmental Management 29, 516–530.
| Macroinvertebrate and fish populations in a restored impounded salt marsh 21 years after the reestablishment of tidal flooding.Crossref | GoogleScholarGoogle Scholar | 12071502PubMed |
Talbot, C. W., and Able, K. W. (1984). Composition and distribution of larval fishes in New Jersey high marshes. Estuaries 7, 434–443.
| Composition and distribution of larval fishes in New Jersey high marshes.Crossref | GoogleScholarGoogle Scholar |
Thomas, B. E., and Connolly, R. M. (2001). Fish use of subtropical saltmarshes in Queensland, Australia: relationships with vegetation, water depth and distance onto the marsh. Marine Ecology Progress Series 209, 275–288.
| Fish use of subtropical saltmarshes in Queensland, Australia: relationships with vegetation, water depth and distance onto the marsh.Crossref | GoogleScholarGoogle Scholar |
Valiñas, M. S., Molina, L. M., Addino, M., Montemayor, D. I., Acha, E. M., and Iribarne, O. O. (2012). Biotic and environmental factors affect Southwest Atlantic saltmarsh use by juvenile fishes. Journal of Sea Research 68, 49–56.
| Biotic and environmental factors affect Southwest Atlantic saltmarsh use by juvenile fishes.Crossref | GoogleScholarGoogle Scholar |
Varnell, L. M., and Havens, K. J. (1995). A comparison of dimension-adjusted catch data methods for assessment of fish and crab abundance in intertidal salt marshes. Estuaries 18, 319–325.
| A comparison of dimension-adjusted catch data methods for assessment of fish and crab abundance in intertidal salt marshes.Crossref | GoogleScholarGoogle Scholar |
Warren, R. S., Fell, P. E., Grimsby, J. L., Buck, E. L., Rilling, G. C., and Fertik, R. A. (2001). Rates, patterns, and impacts of Phragmites australis expansion and effects of experimental phragmites control on vegetation, macroinvertebrates, and fish within tidelands of the lower Connecticut River. Estuaries 24, 90–107.
| Rates, patterns, and impacts of Phragmites australis expansion and effects of experimental phragmites control on vegetation, macroinvertebrates, and fish within tidelands of the lower Connecticut River.Crossref | GoogleScholarGoogle Scholar |
Warren, R. S., Fell, P. E., Rozsa, R., Brawley, A. H., Orsted, A. C., Olson, E. T., Swamy, V., and Niering, W. A. (2002). Salt marsh restoration in Connecticut: 20 years of science and management. Restoration Ecology 10, 497–513.
| Salt marsh restoration in Connecticut: 20 years of science and management.Crossref | GoogleScholarGoogle Scholar |
West, J. M., and Zedler, J. B. (2000). Marsh-creek connectivity: fish use of a tidal salt marsh in southern California. Estuaries 23, 699–710.
| Marsh-creek connectivity: fish use of a tidal salt marsh in southern California.Crossref | GoogleScholarGoogle Scholar |
Wickham, H. (2016). ‘ggplot2: Elegant Graphics for Data Analysis.’ (Springer-Verlag: New York, NY, USA.)
Yozzo, D. J., and Smith, D. E. (1997). Composition and abundance of resident marsh-surface nekton: comparison between tidal freshwater and salt marshes in Virginia, USA. Hydrobiologia 362, 9–19.
| Composition and abundance of resident marsh-surface nekton: comparison between tidal freshwater and salt marshes in Virginia, USA.Crossref | GoogleScholarGoogle Scholar |
Zeug, S. C., Shervette, V. R., Hoeinghaus, D. J., and Davis, S. E. (2007). Nekton assemblage structure in natural and created marsh-edge habitats of the Guadalupe Estuary, Texas, USA. Estuarine, Coastal and Shelf Science 71, 457–466.
| Nekton assemblage structure in natural and created marsh-edge habitats of the Guadalupe Estuary, Texas, USA.Crossref | GoogleScholarGoogle Scholar |