Kills in the Darling: assessing the impact of the 2018–20 mass fish kills on the fish communities of the Lower Darling–Baaka River, a large lowland river of south-eastern Australia
Jerom R. Stocks A E , Iain M. Ellis
A E , Iain M. Ellis  B , Dylan E. van der Meulen
B , Dylan E. van der Meulen  A , Jonathon I. Doyle C and Katherine J. M. Cheshire
A , Jonathon I. Doyle C and Katherine J. M. Cheshire  D
D
A NSW Department of Primary Industries, Batemans Bay Fisheries Centre, PO Box 17, Batemans Bay, NSW 2536, Australia.
B NSW Department of Primary Industries, Buronga, PO Box 363, Buronga, NSW 2739, Australia.
C NSW Department of Primary Industries, Narrandera Fisheries Centre, PO Box 182, Narrandera, NSW 2700, Australia.
D NSW Department of Primary Industries, Port Stephens Fisheries Institute, Locked Bag 1, Nelson Bay, NSW 2316, Australia.
E Corresponding author. Email: jerom.stocks@dpi.nsw.gov.au
Marine and Freshwater Research 73(2) 159-177 https://doi.org/10.1071/MF20340
Submitted: 24 November 2020 Accepted: 23 August 2021 Published: 9 September 2021
Journal Compilation © CSIRO 2022 Open Access CC BY-NC-ND
Abstract
Understanding the impacts of extreme events is essential to effective fisheries management. During the summer of 2018–19 millions of native fish died in Lower Darling–Baaka River adjacent to Menindee, New South Wales, Australia. Hypoxia during a period of protracted low flow, triggered by climatic events, was responsible for the fish kills. From June 2019 to March 2020, further broader-scale fish kills occurred throughout ~600 km of the Darling–Baaka River as disconnected refuge pools contracted and water quality deteriorated. This study examined the status of the remnant fish populations, compared the fish assemblage of the Menindee fish death reach with an unaffected reach and monitored change of the fish community over 18 months after the initial fish kills. Significantly lower abundances of Murray cod (Maccullochella peelii), bony herring (Nematalosa erebi), carp gudgeon (Hypseleotris spp.) and freshwater prawn (Macrobrachium australiense) were captured within the Menindee fish death reach compared with the unaffected reach. Varied responses were observed in species abundances within the affected reach in the 18 months after the initial fish kills, attributed to the various life-history traits and reproductive strategies. The results presented highlight a fish community in continued stress. Continued monitoring will guide and track the effectiveness of recovery management interventions in the region.
Keywords: environmental water management, fish death, freshwater fish, hypoxia, life history guild, river regulation.
Introduction
In the past 100 years, the amount of water withdrawn globally by humans and the land area under irrigation have risen exponentially (Jackson et al. 2001). It is estimated that, globally, 54% of accessible freshwater run-off is appropriated for human use (Postel et al. 1996). Irrigated agriculture accounts for 70% of the total global water withdrawals and meets 45% of the global food demand (Steduto et al. 2018). In the 20th century, the global water demand has been growing more than twice the rate of population growth (Steduto et al. 2018). This demand on the water resources of rivers in dryland areas is often high (Thoms and Sheldon 2000). Dryland rivers exhibit extreme hydrological regimes, where long periods of low flow and drought can be interrupted by extensive flooding (Humphries et al. 1999; Thoms and Sheldon 2000). As a result, many rivers have been regulated through the construction of dams, weirs and other water-diversion structures. This has altered natural flow regimes and associated ecological processes for many inland river systems around the world (Gehrke et al. 1995; Bunn and Arthington 2002; Mallen-Cooper and Zampatti 2018; Ngor et al. 2018). It is estimated that only 37% of rivers longer than 1000 km remain free flowing over their entire length (Grill et al. 2019). For dryland rivers globally, trade-offs occur between consumptive water use and aquatic ecosystem integrity (Vörösmarty et al. 2010; Mallen-Cooper and Zampatti 2020). Consumptive water use coupled with climatic change is affecting the ecological resilience of dryland rivers (Vertessy et al. 2019). It has been widely recognised that extreme events, such as high and low temperature, heavy precipitation and floods and droughts, occur more frequently under the impact of climate change (Du et al. 2014). Climate change has the potential to substantially alter river flow regimes (Arnell and Gosling 2013). By 2050, it is estimated that the effects of climate change may have had a larger impact on flow regimes than what dams and water withdrawals have had until now (Döll and Zhang 2010).
Given the extent of flow regime alteration and climate change predictions, fish kills present an underappreciated risk to riverine fish communities (Small et al. 2014). Although fish kills can be a natural phenomenon, human alteration of aquatic and terrestrial systems is increasing fish kill frequency and scale worldwide (La and Cooke 2011). Approximately 67% of fish kills result from anthropogenic activities, whereas only 10% are caused by natural events and the remaining are ‘cause unknown’ (La and Cooke 2011). Furthermore, human-induced global environmental alterations such as climate change can modulate these natural causes (La and Cooke 2011). For example, extreme changes in temperatures and low dissolved oxygen combined accounted for ~12% of all surveyed fish kills (La and Cooke 2011). The occurrence of hypoxic events is increasing globally, likely accelerated by human activities (Diaz 2001). Fish kills are not isolated events and are relatively common throughout the Australian Murray–Darling Basin (MDB), being frequently associated with hypoxia (Lugg 2000; Koehn 2004; King et al. 2012). Hypoxia is often driven by overbank flooding and the associated blackwater events (King et al. 2012; Whitworth et al. 2012), but may also eventuate as a consequence of extended low–no flows and associated thermal stratification and destratification of the water column (Vertessy et al. 2019).
Since the 1960s, the hydrology of the Barwon–Darling River, an intermittent dryland river system of south-eastern Australia’s MDB, has been substantially modified by water storages and flow regulation (Thoms and Sheldon 2000; Mallen-Cooper and Zampatti 2020). Water storage and diversion have increased the frequency and duration of zero flows, reduced the magnitude of low flows and reduced the magnitude of near-annual flow pulses by over 90% (Mallen-Cooper and Zampatti 2020). Such alterations to the flow regime have had significant ecological consequences in the Darling River, including wide-scale cyanobacterial blooms (Bowling and Baker 1996; Mitrovic et al. 2011) and the death of thousands of native fish in fish kill events, most notably in 2004 (Ellis and Meredith 2004; Koehn 2004) and 2018–20, with these generally associated with low- or zero-flow conditions (Mallen-Cooper and Zampatti 2020).
Healthy and diverse populations of native fish and crustaceans of the Australian MDB provide significant cultural, social, recreational and economic values (McIlgorm and Pepperell 2013; Murray–Darling Basin Authority 2020). Fish have been integral to the cultural heritage of Indigenous people for over 30 000 years and, more recently, to non-Indigenous communities throughout the Barwon–Darling system (Mathews 1903; Balme 1995; Humphries 2007; Koehn 2015; Garvey 2017). A recent Native Title Determination was followed by a request for dual naming of the Lower Darling River, respecting the deep cultural and spiritual connections of the Barkandji people with the Baaka. In this paper we refer to the Lower Darling River as the Darling–Baaka River. The Darling–Baaka River is significant for native fish species in the MDB, including iconic species such as golden perch and Murray cod (nationally listed as vulnerable), threatened species, including silver perch and freshwater catfish Tandanus tandanus, and a suite of important small-bodied native fish species. The region is also important in a basin-wide context for the breeding and recruitment of golden perch (Sharpe 2011; Zampatti et al. 2015; Sharpe and Stuart 2018; Zampatti et al. 2018).
Over three separate events between 15 December 2018 and 28 January 2019, millions of native fish died along a 30- to 40-km reach of the Darling–Baaka River adjacent to the town of Menindee, New South Wales (NSW), in the weir pool between Weir 32 and the Menindee Lakes Main Weir (New South Wales Department of Primary Industries 2019). A series of investigations concurred that a lack of dissolved oxygen, related to weather-driven destratification of thermally stratified water, during a period of protracted low flow caused each of the three fish kill events (Baldwin 2019; Moritz et al. 2019; Vertessy et al. 2019). Severe drought from 2017 and extreme hot and dry conditions during late 2018, extending into 2019, preceded the Menindee fish kill events (Moritz et al. 2019; Vertessy et al. 2019). Although the drought and extreme conditions were contributing factors, the fish kills were not solely attributed to the biophysical processes of protracted drought. Excess upstream diversion of water for irrigation was concluded to have reduced the resilience of the Darling–Baaka riverine ecosystem (Moritz et al. 2019; Vertessy et al. 2019; Jackson and Head 2020).
Visual estimates undertaken by NSW Department of Primary Industries Fisheries officers indicated that affected fish in the Menindee weir pool reach in the summer of 2018–19 were predominantly bony herring (Nematalosa erebi), numbering in the millions, with thousands of golden perch (Macquaria ambigua) and silver perch (Bidyanus bidyanus), as well as hundreds of Murray cod (Maccullochella peelii) and small numbers (tens to hundreds) of common carp (Cyprinus carpio). For each species, a range of size and age classes were affected, although for Murray cod and common carp mostly larger individuals were reported (New South Wales Department of Primary Industries 2020a). Over the 14 months following the Menindee fish kills of the summer of 2018–19, further broad-scale fish kills occurred throughout the Darling–Baaka River downstream of Menindee. The Darling–Baaka River ceased to flow in January 2019 and flows did not resume until March 2020. This protracted duration of no flow was a rare occurrence in historical flow records of the Darling–Baaka River before flow regulation. For example, from 1885 to 1950 before river regulation, only three events of zero flow >6 months were recorded, with the longest being 11 months (Mallen-Cooper and Zampatti 2020). During this unregulated period, the Darling River flowed for more than 90% of the time and was characterised by short spells (generally <1 month) of zero flow (Mallen-Cooper and Zampatti 2020). The 2019–20 fish kills occurred throughout ~600 km of the Darling River downstream of Weir 32 from January 2019 through to March 2020 (just before the arrival of flows), as refuge pools contracted and water quality deteriorated (I. M. Ellis, unpubl. obs., 28 August 2020). Incidents of fish kills were also reported upstream of Menindee during the protracted dry spell in 2019, although, due to remoteness and low human population density, few verified reports were documented (I. M. Ellis, unpubl. obs., 28 August 2020). Emergency drought actions were undertaken by the NSW Department of Primary Industries Fisheries and partner agencies during the protracted cease-to-flow period in 2019 and early 2020 in order to protect native fish (New South Wales Department of Primary Industries 2020a). This included installation of aeration devices in 10 key refuge areas throughout the Darling–Baaka, and the rescue and relocation of over 1600 large native fish from drying pools with the support from sanctioned volunteer groups.
Despite the well-documented occurrence of fish kills, there is limited quantitative evidence of the ecological consequences of low–no flow hypoxia-related fish kills on fish communities in the short to medium term (La and Cooke 2011). This study examined the status of the remnant fish population in the Darling–Baaka River, compared the fish assemblage in the vicinity of the Menindee fish kills 6 months after the initial summer of 2018–19 Menindee fish kills to that of an unaffected downstream reach, monitored further changes to the Darling–Baaka River fish community at 8 and 18 months after the initial Menindee fish kills and examined the effects of the fish kills on fish health and condition. This study improves our understanding of the short-term effects of low–no flow hypoxic events on fish communities, which is critical to the development and implementation of effective management and policy to prevent such events, as well as fish recovery activities following large-scale fish kills.
Materials and methods
Study area and sampling design
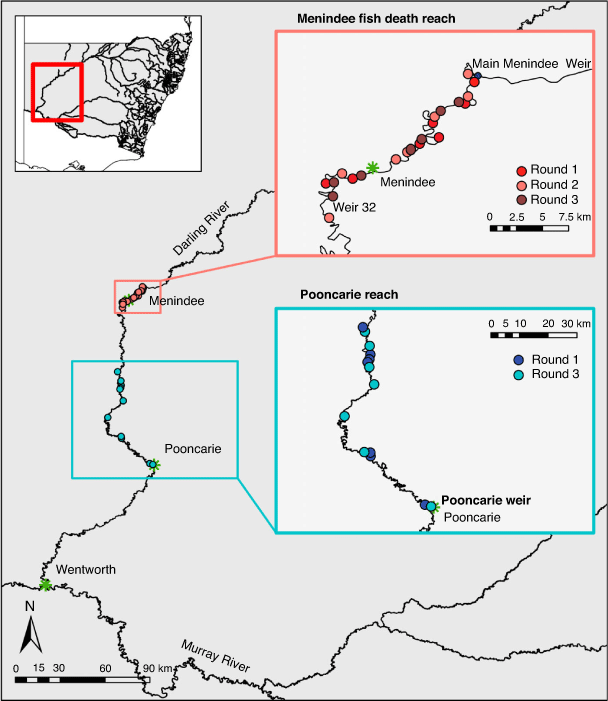
The Barwon–Darling River (hereafter referred to as the Darling River) is an intermittent dryland river (Puckridge et al. 1998). It has a catchment area of 650 000 km2 and runs for 2740 km draining the north-western section of the MDB (Fig. 1). The catchment is characterised by extreme climatic variability, receiving low rainfall. Eight headwater dams and diversion of flows for irrigated agriculture have resulted in a highly modified flow regime (Thoms and Sheldon 2000; Mallen-Cooper and Zampatti 2020). The Lower Darling (which we refer to as the Darling–Baaka in this paper) includes the Menindee Lakes (including the upstream extent of Lake Wetherell) and the river channel downstream of Menindee to Wentworth (Fig. 1). Flows in the Darling–Baaka River are largely regulated by the Menindee Lakes, which are a series of naturally occurring ephemeral lakes that were converted for water storage in the 1960s.
|
The nature of the Darling–Baaka river system during the sampling period in this study presented several complications in terms of study design. First, the identification of control sites or reaches to compare against the reach affected by the catastrophic Menindee Fish Kills in the summer of 2018–19 was problematic. Although natural field-based ecological studies often encounter problems identifying suitable controls, this issue was magnified in the present case. We acknowledge there are inherent differences in hydraulic character between the two study reaches. The Menindee pool is a long, slow-flowing and stratification-prone weir pool, whereas the Pooncarie reach represents habitat that is generally free flowing river. However, no other comparable large isolated weir pools existed in the Darling River downstream of Menindee at the time of this study that would provide a suitable ‘control’ reach. Furthermore, fish may ‘accumulate’ over time in the Menindee Weir pool due to the lack of upstream passage at the Menindee Main Weir, potentially resulting in non-random distribution of fish in the fish death reach.
Fish community sampling was conducted across 35 sites in the Darling–Baaka River over three rounds of sampling: Round 1, June 2019 (6 months after the Menindee fish kills of 2018–19); Round 2, October 2019 (10 months after the Menindee fish kills); and Round 3, June 2020 (18 months after the Menindee fish kills and 2 months after flow returned to the Darling–Baaka). Sampling sites (each approximately an 800-m river segment) were distributed between two reaches, the Menindee fish death reach between Menindee Main Weir and Weir 32 (an ~39-km river reach) and the Pooncarie reach, downstream of Weir 32 between Karoola Station and Pooncarie Weir, as a comparison (an ~180-km river reach; Fig. 1). Seven sites were randomly selected within each reach for each round of monitoring. This delivered a total of 21 observational units within the Menindee fish death reach (three rounds of sampling) and 14 within the Pooncarie reach (only sampled in Rounds 1 and 3; Fig. 1). Within the Menindee fish death reach the minimum, maximum and mean distance by river between sites during Round 1 sampling was 3, 9 and 5 km respectively. For Round 2, these respective distances were 3, 12 and 7 km, and for Round 3 they were 2, 11 and 5 km. Within the Pooncarie reach, the minimum, maximum and mean distance by river between sites during Round 1 was 2, 81 and 27 km, and 10, 67 and 29 km respectively for Round 3.
During Round 1 of sampling, the river reach between Pooncarie Weir and Weir 32 presented the most suitable comparison reach to compare fish assemblages between the affected Menindee fish death reach and an unaffected reach. The fish communities sampled at the comparison sites (Pooncarie reach during Round 1) were subjected to the same low-flow hydrological conditions as the fish in the Menindee fish death reach. The comparison reach was also known to contain the same suite of species as occurred within the Menindee fish death reach (NSW Fisheries, unpubl. data). A key distinction was that the populations in the Menindee Main Weir to Weir 32 reach (Menindee fish death reach) had been affected by the hypoxic events that caused the summer 2018–19 Menindee fish kills. Despite inherent differences in hydraulic character between the two reaches, no other comparable large, isolated weir pools existed downstream of Menindee at the time of this study.
During Round 1 of sampling, the scarcity of accessible water within the Pooncarie reach deep enough for boat electrofishing restricted the selection of comparison sites to large, isolated refuge pools. The Menindee fish death reach remained as one interconnected river segment throughout the entirety of the project’s sampling period. With regard to the statistical independence of sampling sites, the potential of fish movement between sites during a sampling round was considered minimal given the distance between sites (mean distance between sites 7 and 28 km for the Menindee fish death reach and Pooncarie reach respectively), the short temporal period over which sampling was conducted within a reach during a sampling round (5–6 days) and the lack of significant river discharge during the sampling rounds that is known to cue fish movements (Reynolds 1983).
To minimise sampling-related stress to the fish community, Round 1 sampling was conducted between 18 and 27 June 2019, when cooler temperatures supported more favourable water quality conditions (in particular, higher dissolved oxygen concentrations throughout the water profile). Water quality parameters for each sampling round and reach are presented in Table 1. After Round 1, fish relocation efforts were conducted and broad-scale fish kills occurred throughout the Pooncarie reach as refuge pools contracted (New South Wales Department of Primary Industries 2020a). Round 2 of fish community sampling (October 2019; 10 months after the initial Menindee fish kills) was therefore constrained to the Menindee fish death reach to prevent further stress to fish in the Pooncarie reach. With flows returning to both study reaches in March 2020, Round 3 sampling (June 2020; 18 months after the initial Menindee fish kills) was conducted within both the Menindee fish death reach and the Pooncarie reach. The Pooncarie reach no longer comprised isolated refuge pools, but was an interconnected river reach. Thus, given the changing hydrology, widespread deaths of fish and the management interventions during the course of this sampling period, the Pooncarie reach after Round 1 no longer represented a suitable comparison reach. Rather, the Pooncarie reach now presented an opportunity to examine the effects of protracted no-flow and relocation efforts on fish communities in a ‘before–after’-like experimental design.
Fish community sampling followed the Sustainable Rivers Audit standard methods for riverine fish (Davies 2008). Standardised fish sampling was performed using a combination of boat electrofishing and unbaited concertina-style traps with the addition of five baited opera house traps deployed at each site.
Specimens were collected under Animal Research Authority number 98/14 granted by the NSW Department of Primary Industries (Fisheries Animal Care and Ethics Committee) and Scientific Collection Permit P01/0059(A).
Data analyses
No comparable historical electrofishing fish community data were available from the Menindee fish death reach before the summer 2018–19 fish kills. Standardised historical fish community data were available within the Pooncarie reach dating back to 2004. These historical data were used to examine longer-term temporal trends in the fish community composition of the Darling–Baaka River.
Species abundance
The abundance of each species caught was compared between reaches and sampling rounds using analysis of variance (ANOVA). Assumptions of ANOVA, including normality and homogeneity of variance, were investigated. Data were log10 transformed to meet ANOVA assumptions. To examine pairwise comparisons within species, Tukey’s honest significant difference (HSD) tests were performed. Each site sampled was independent from those of earlier sampling rounds.
Historical catch-per-unit-effort fish community data were available for the Pooncarie reach dating back to 2004. The length data of individual fish were used to classify each fish as either young-of-the-year (YOY), juvenile or sexually mature. Each species was also classified as either short lived (~1–2 years), moderate lived (~3–6 years) or long lived (~6 years or greater; D. Gilligan, L. Cameron, and J. R. Stocks, unpubl. data). To reduce interannual variation introduced by the high variability in annual recruitment success, the mean abundance of moderate- and long-lived species captured per site was plotted as a function of year, excluding YOY fish. For short-lived species, the combined abundance of YOY, juveniles and sexually mature fish was plotted.
The capture probability of selected species (juvenile and sexually mature fish) was examined for the Pooncarie reach between 2004 and 2020. Capture probability was studied for species that were absent from >50% of sites sampled in the Pooncarie reach in 2020, excluding silver perch, spangled perch, eastern gambusia and Murray–Darling rainbowfish due to sporadic historical captures of those species. A binary indicator (1 = no catch, 0 = catch > 0) was created to assess the effect of year on the probability of catching no examples of a species by logistic regression. The year 2018 was removed from analysis because only one site was sampled that year. Change in model deviance due to adding the year effect was assessed by Chi-Square test.
Length–frequency distributions were constructed for each species, reach and sampling round. Because subsampling of species occurred when a large number (>50) of a species was caught, length–frequency data were scaled to match abundance data for each sampling event. Kolmogorov–Smirnov (K-S) tests were used for statistical comparison of length–frequency distributions for each species between reaches and sampling rounds. Only length–frequency distributions with n > 15 were used in the analysis.
Multivariate analyses of the fish community
Catches from all electrofishing operations, bait traps and opera house traps for a site were pooled for analysis. Multivariate analyses of the fish community composition were performed in PRIMER (ver. 7.0, Plymouth Marine Laboratory). To compare variation in the fish community composition, species abundances were transformed to the fourth root after shade plot inspection. Similarities between fish communities for each sample were calculated using a Bray–Curtis similarity matrix (Bray and Curtis 1957). Non-metric multidimensional scaling (nMDS; PRIMER, ver. 7) ordination plots were constructed for visual comparisons of dissimilarity between the fish communities. A permutational multivariate analysis of variance (PERMANOVA; PRIMER, ver. 7) was used to test for any significant differences in the fish community composition between: (1) Round 1 data comparing the 2018–19 Menindee fish death reach and Pooncarie reach; (2) Menindee fish death reach between Rounds 1, 2 and 3; and (3) Pooncarie reach between Round 1, Round 3 and historical data (2004–18). Similarity percentage analysis (SIMPER) was used to examine the contribution of each species to the observed dissimilarity between 2018–19 Menindee fish death reach and the Pooncarie reach during Round 1 of sampling.
Food resources
The mean abundance of food resources available to higher-trophic-level predators (large fish) was compared between reaches and sampling rounds using ANOVA. All fish ≤100 mm in length (fork length, FL, for species with a forked tail; total length, TL, for species with a rounded tail) and all large decapods (freshwater prawns, Macrobrachium australiense; shrimp, Atyidae; and yabbies, Cherax destructor) were classified as a food resource (i.e. two food classes). ANOVA compared: (1) the mean abundance of fish and decapod prey items (class) between the Menindee fish death reach and Pooncarie reach during Round 1 of sampling; (2) the mean abundance of fish and decapod prey items (class) in the Menindee fish death reach between Rounds 1, 2 and 3; and (3) the mean abundance of fish and decapod prey items (class) in the Pooncarie reach between Rounds 1 and 3. Assumptions of ANOVA were investigated and data were log10 transformed.
Fish health and condition index
Length and weight data collected for large-bodied species were used in the calculation of a relative body condition (RBC) index. Established length–weight relationships for each species (New South Wales Department of Primary Industries 2020b) were used to estimate the ‘expected weight’ of individuals based on their length. The RBC of each individual fish was calculated as follows:
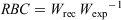
where Wrec is recorded weight and Wexp is expected weight. Values >1 indicate better-than-average condition, whereas values <1 indicate poorer-than-average condition relative to established length–weight relationships in NSW. Fish with good body condition are typically more resistant to negative environmental factors and have greater reproductive potential (Balcombe et al. 2012). Differences in the RBC between reaches and sampling rounds for each large-bodied species where >10 individuals were caught within each reach and sampling round were analysed using ANOVA. Data were examined for normality and homogeneity of variance. For pairwise comparisons, Tukey’s HSD test was performed.
Parasites can affect fish health, growth and survival and can be indicators of ecological stress (Dalu et al. 2012). Using rapid visual assessment for parasites, the number of external Lernaea cyprinacea infecting the left side of all fish was recorded (Davies 2008). The mean percentage of each species affected by Lernaea was compared between reaches and sampling rounds using ANOVA. This was only examined for species with a sufficient sample size for analysis (n > 30 in each round and reach, with the species caught at greater than or equal to six of the seven sample sites for each round and reach). The Lernaea loading of infected fish (i.e. number of Lernaea counted per fish) was compared between reaches during Round 1 for golden perch using the non-parametric Wilcoxon rank-sum test due to violation of ANOVA assumptions. Lernaea loading was only compared in Round 1 of sampling between reaches because the number of fish infected by Lernaea in Rounds 2 and 3 did not provide a sufficient sample size for analysis (n < 3 infected fish per species in each sampling round and reach).
Results
Fish community composition
Round 1: Menindee fish death reach v. Pooncarie reach
In total, 2684 fish and 94 decapods were caught during Round 1 of sampling (June 2019) in the Pooncarie reach, compared with just 532 fish and no decapods within the 2018–19 Menindee fish death reach. The largest differences in total fish abundance were associated with bony herring. Nine fish species and two decapod species were captured in the Pooncarie reach, compared with seven fish species and no decapod species within the 2018–19 Menindee fish death reach. Goldfish (Carassius auratus), freshwater prawn and eastern gambusia (Gambusia holbrooki) were not captured within the Menindee fish death reach, although they were recorded in the Pooncarie reach (Table 2).
An ANOVA comparing the abundance of each species between the Menindee fish death reach and Pooncarie reach indicated a significant interaction between reach and species (F10,132 = 6.151; P < 0.0001). Tukey’s HSD post hoc pairwise comparison within species indicated a significantly greater abundance of bony herring (P < 0.0001), carp gudgeon (Hypseleotris) species complex (P < 0.0001), Murray cod (P < 0.001), freshwater prawn (P < 0.01) and goldfish (P < 0.01) within the Pooncarie reach compared with the Menindee fish death reach. There was no significant difference in the abundance of golden perch, Australian smelt and common carp between the two reaches (P > 0.05; Fig. 2).
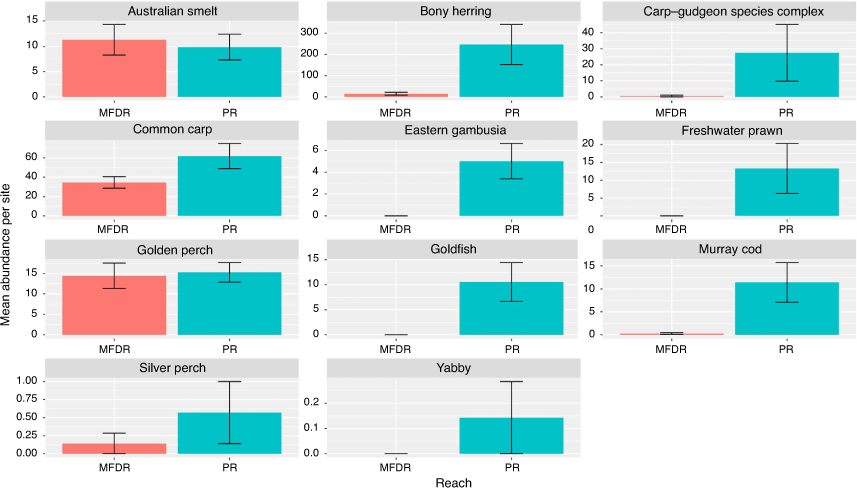
|
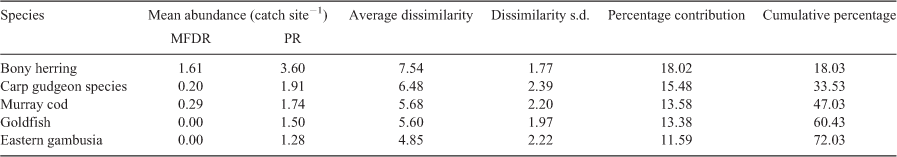
Multivariate analysis of fish community composition indicated a significant difference in the fish community between the Menindee fish death reach and Pooncarie reach for Round 1 of sampling (PERMANOVA, F = 19.12; P = 0.0008), and when including all historical data from the Pooncarie reach (PERMANOVA, F = 7.19; P = 0.0001). For visual comparison, nMDS plots display dissimilarity in the fish community composition between sampling reaches (Fig. 3). SIMPER revealed that bony herring, carp gudgeon and Murray cod were the primary species responsible for the dissimilarity between the Menindee fish death reach and Pooncarie reach during Round 1 (Table 3).
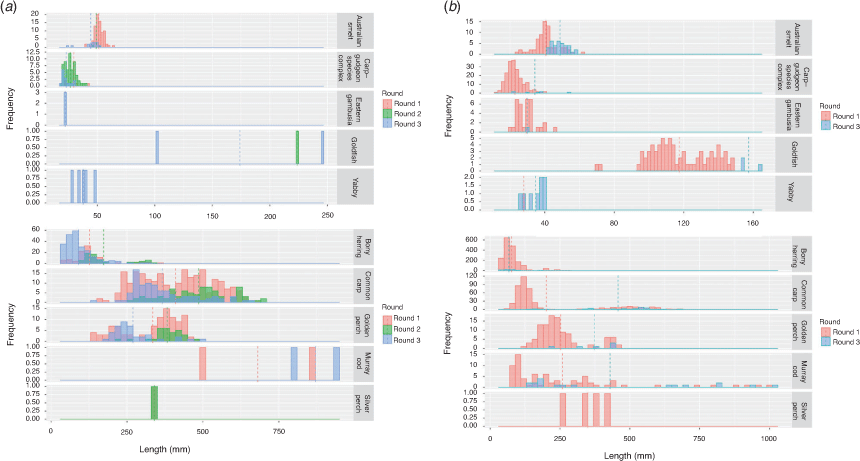
Length–frequency distributions for each species are shown in Fig. 4. K-S tests indicated significant differences in the length–frequency distributions of the Menindee fish death reach and Pooncarie reach for each species examined during Round 1 of sampling (Table 4).
Fish death reach: comparisons of Rounds 1, 2 and 3
An ANOVA comparing the abundance of each species in the Menindee fish death reach between Rounds 1, 2 and 3 indicated a significant interaction between round and species (F20,198 = 6.151; P < 0.0001). Tukey’s HSD post hoc pairwise comparison within species indicated that the abundance of Australian smelt (Retropinna semoni) in Round 1 was significantly greater than the abundance in Rounds 2 and 3. Bony herring abundance increased significantly from Round 2 to Round 3. The abundance of carp gudgeon was significantly greater in Round 2 than in Rounds 1 and 3. The abundance of common carp in Round 2 was significantly less than in Round 1. Freshwater prawn abundance was significantly greater in Round 3 than in Rounds 1 and 2. All other pairwise comparisons for a single species between sampling rounds were non-significant (P > 0.05; Fig. 5a). That is, the abundance of golden perch, Murray cod and silver perch did not substantially change in the 18 months after the fish kills in the Menindee fish death reach. Length–frequency distributions for each species are shown in (Fig. 6a). From these length–frequency distributions, there was no evidence of recruitment for the culturally and recreationally important golden perch, Murray cod and silver perch.
Multivariate analysis of fish community composition indicated a significant effect of sampling round on the fish community of the Menindee fish death reach (PERMANOVA, F = 10.62; P = 0.0001). Further pairwise analysis indicated that the fish community was significantly different between each round of sampling (Round 1 v. Round 2: t = 3.578, P < 0.0007; Round 1 v. Round 3: t = 3.609, P < 0.0006; Round 2 v. Round 3: t = 2.522, P < 0.0002). For visual comparison, nMDS plots display dissimilarity in the fish community composition between sampling rounds in the Menindee fish death reach (Fig. 7a).
Pooncarie reach: Round 1, Round 3 and historical data
An ANOVA comparing the abundance of each species in the Pooncarie reach between Round 1 and Round 3 (before and after most of the widespread fish kills and subsequent resumption of flows within this reach) indicated a significant interaction between round and species (F10,132 = 5.073; P < 0.0001). Tukey’s HSD post hoc pairwise comparisons within species indicated significant reductions in goldfish (P < 0.05), golden perch (P < 0.0001), carp gudgeon (P < 0.002), common carp (P < 0.0002) and bony herring (P < 0.00001). All other pairwise comparison for species in the Pooncarie reach between Rounds 1 and 3 were non-significant (P > 0.05; Fig. 5b).
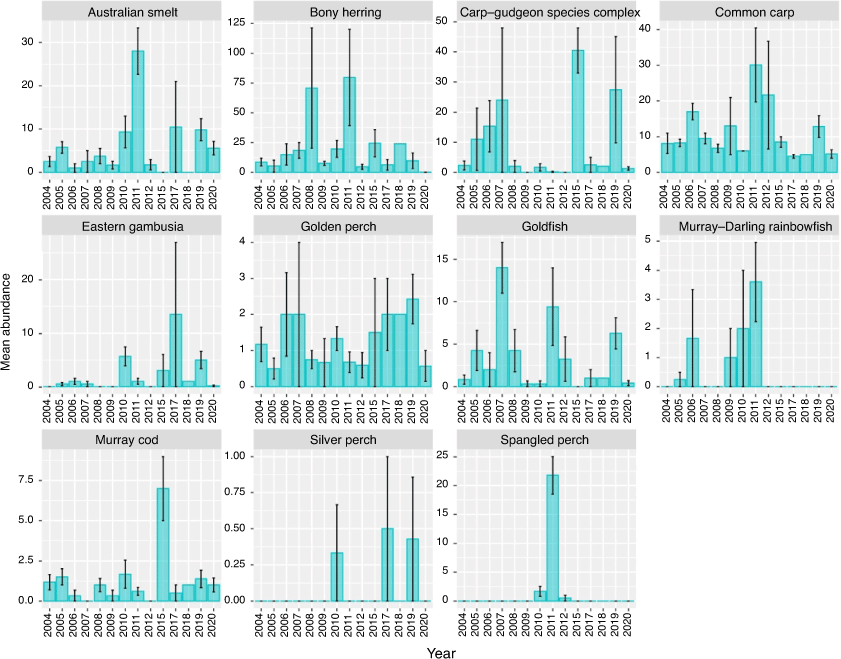
The mean abundance of species captured per site between 2004 and 2020 are shown in Fig. 8. Temporal variability was observed in the abundance of all species, likely attributed to the limited sampling conducted in the region. No Murray–Darling rainbowfish (Melanotaenia fluviatilis) or spangled perch (Leiopotherapon unicolor) have been captured in the Pooncarie reach since 2011 and 2012 respectively. Juvenile and sexually mature bony herring, golden perch and goldfish were absent from >50% (>3/7 sites) of sites sampled in the Pooncarie reach in 2020. For bony herring and goldfish, a significant year effect was observed on non-detection probability (P < 0.05). In 2020, there was an 86% chance (95% confidence interval (CI) 42–98%) of non-detection of bonny herring at sites sampled in the Pooncarie reach. For the 10 years examined before the fish kills within this reach, dating back to 2005, bony herring non-detection probability was 0%, except for 2012 and 2006, when non-detection probability was <33%. In 2020, goldfish non-detection probability was 71% (95% CI 34–93%); however, this estimate was similar to that of most of the 12 preceding years.
Multivariate analysis of fish community composition indicated a significant difference in the fish community of the Pooncarie reach between Round 1, Round 3 and historical data (2004–17; PERMANOVA, F = 5.29; P = 0.0001). Further pairwise analysis indicated that the fish community was significantly different between each round of sampling and the historical data (Round 1 v. Round 3: t = 3.3965, P < 0.0005; Round 1 v. historical data: t = 1.8011, P < 0.05; Round 3 v. historical data: t = 2.4979, P < 0.0005). For visual comparison, nMDS plots display dissimilarity in the fish community composition for the Pooncarie reach between Round 1, Round 3 and historical data (Fig. 7b).
Length–frequency distributions were significantly different between Round 1 and Round 3 within the Pooncarie reach for each species examined, with the exception of bony herring, for which no significant difference was observed in the length–frequency distributions (Table 4; Fig. 6b).
Food resources
An ANOVA comparing the mean abundance of fish and decapod prey items (class) between the Menindee fish death reach and Pooncarie reach during Round 1 of sampling indicated a significantly greater mean abundance of prey items in the Pooncarie reach (F1,22 = 26.854; P < 0.0001) and a significantly greater mean abundance of fish prey items than decapod prey items (F1,22 = 62.504; P < 0.0001); no significant reach × class interaction was observed (F1,22 = 0.549; P > 0.1; mean ± s.e. fish and decapod abundance 20.33 ± 5.45 and 0 ± 0 respectively for the Menindee fish death reach and 283.00 ± 104.89 and 13.43 ± 6.98 respectively for the Pooncarie reach).
An ANOVA comparing the mean abundance of fish and decapod prey items (class) in the Menindee fish death reach between Rounds 1, 2 and 3 indicated a significant interaction between class and round (F2,34 = 12.000; P < 0.001). Tukey’s HSD post hoc pairwise comparisons indicated a significant increase in the abundance of decapod prey items between all three sampling rounds (P < 0.05; mean ± s.e., 0 ± 0, 3.86 ± 1.87 and 17.71 ± 2.76 in Rounds 1, 2 and 3 respectively); no significant difference was observed for fish prey items (P > 0.05).
An ANOVA comparing the mean abundance of fish and decapod prey items (class) in the Pooncarie reach between Round 1 and Round 3 indicated a significant interaction between class and round (F1,24 = 5.668; P < 0.05). Tukey’s HSD post hoc pairwise comparisons indicated a significant decrease in the abundance of fish prey items between Round 1 and Round 3 of sampling (P < 0.001; mean ± s.e., 283.00 ± 104.89 and 14.57 ± 2.07 in Rounds 1 and 3 respectively); no significant difference was observed for decapods (P > 0.05).
Fish health and condition index
An ANOVA comparing the RBC of large-bodied species between the Menindee fish death reach and Pooncarie reach during Round 1 of sampling indicated a significant interaction between reach and species (F2,992 = 12.917; P < 0.001). Tukey’s HSD post hoc pairwise comparisons indicated a significantly greater RBC of common carp within the Menindee fish death reach than the Pooncarie reach (mean ± s.e., 1.00 ± 0.01 v. 0.97 ± 0.01 respectively; P < 0.05E), a significantly greater RBC of golden perch within the Pooncarie reach than Menindee fish death reach (mean ± s.e., 1.09 ± 0.01 v. 1.01 ± 0.01 respectively; P < 0.01), and no significant difference in the RBC of bony herring between the Pooncarie reach and Menindee fish death reach (mean ± s.e., 0.74 ± 0.01 v. 0.78 ± 0.01 respectively; P = 0.538).
An ANOVA comparing the RBC of large-bodied species in the Menindee fish death reach between Rounds 1, 2 and 3 indicated a significant interaction between round and species (F4,768 = 6.394; P < 0.0001). Tukey’s HSD post hoc pairwise comparisons indicated a significantly greater RBC of bony herring in Round 3 than Round 2 (mean ± s.e., 0.943 ± 0.252 v. 0.834 ± 0.002 respectively; P < 0.05), a significantly greater RBC of common carp in Rounds 1 and 2 than in Round 3 (mean ± s.e., 1.004 ± 0.010 and 1.012 ± 0.011 v. 0.879 ± 0.037 respectively; P < 0.00001) and no significant difference in the RBC of golden perch between sampling rounds (P > 0.05).
An ANOVA comparing the RBC of large-bodied species in the Pooncarie reach between Round 1 and Round 3 indicated a significant interaction between round and species (F1,437 = 6.357; P < 0.05). Tukey’s HSD post hoc pairwise comparisons indicated a significantly greater RBC of common carp in Round 1 than in Round 3 (mean ± s.e., 0.966 ± 0.008 v. 0.859 ± 0.026 respectively; P < 0.001) and no significant difference in the RBC of Murray cod between Rounds 1 and 3 (P > 0.05).
An ANOVA comparing the percentage of common carp, golden perch and bony herring infected with Lernaea during Round 1 of sampling between the Menindee fish death reach and Pooncarie reach indicated a significant interaction between reach and species (F2,35 = 6.845; P < 0.005). Further investigation of relevant interactions using Tukey’s HSD post hoc pairwise comparisons indicated a significant difference in the percentage of common carp infected with Lernaea in the Menindee fish death reach compared with Pooncarie reach (mean ± s.e., 0.53 ± 0.53% v. 6.21 ± 2.31% respectively; P < 0.05). No significant differences were observed in the percentages of golden perch and bony herring infected with Lernaea during Round 1 of sampling between the Menindee fish death reach and Pooncarie reach (P > 0.05). Data were log10 transformed to meet normality assumptions.
A Wilcoxon rank test indicated Lernaea loadings (i.e. the number of Lernaea per infected fish) of golden perch were significantly greater in the Menindee fish death reach than the Pooncarie reach (mean ± s.e., 11.58 ± 1.06 v. 3.16 ± 0.60 Lernaea per fish respectively; W = 141, P < 0.0001). Lernaea loadings were only compared in Round 1 of sampling between reaches because the number of fish infected by Lernaea in Rounds 2 and 3 did not provide a sufficient sample size for analysis (n < 3 infected fish per species in each sampling round and reach).
An ANOVA comparing the percentage of common carp, golden perch and bony herring infected with Lernaea in the Menindee fish death reach between Rounds 1, 2 and 3 indicated a significant interaction between round and species (F4,52 = 13.68; P < 0.0001). Further investigation of relevant interactions using Tukey’s HSD post hoc pairwise comparisons indicated that a significantly greater proportion of golden perch was infected by Lernaea in Round 1 of sampling than in Rounds 2 and 3 (mean ± s.e., 51.50 ± 11.54% v. 2.78 ± 2.78% and 1.59 ± 1.59% respectively; P < 0.05). No significant differences were observed for common carp and bony herring (P > 0.05).
An ANOVA comparing the percentage of common carp and bony herring infected with Lernaea in the Pooncarie reach between Round 1 and Round 3 indicated a significant interaction between round and species (F1,24 = 14.25; P < 0.001). Further investigation of relevant interactions using Tukey’s HSD post hoc pairwise comparisons indicated that a significantly greater proportion of common carp was infected by Lernaea in Round 1 than in Round 3 (mean ± s.e., 6.21 ± 2.31% v. 0.00 ± 0.00% respectively; P < 0.05). No significant differences were observed for golden perch and bony herring (P > 0.05).
Discussion
Fish kill impacts and species responses
The dramatic extent of the impact the 2018–20 fish kills had on the Darling–Baaka River fish community is evident in the differences in species abundance, diversity and size composition between and within the two reaches after the fish kill events, and in the year following. During the 18 months after the initial Menindee fish kills, varied responses were observed in species abundances within the affected reaches. This can be attributed to the differences in water quality tolerances, life history requirements and reproductive strategies of the different fish species (Crook et al. 2010). The hydrological conditions experienced after major fish kill events will largely determine population dynamics and species-specific recovery rates (Kennedy et al. 2012; Thiem et al. 2017), primarily by providing recruitment opportunities and hydrological connectivity for recolonisation (Kennedy et al. 2012).
Fish species can be grouped into several guilds that exhibit similar reproductive responses to abiotic conditions such as hydrology, water temperature and seasonality (Winemiller 1989). Some Australian freshwater fish species can recruit independent of flow, others require stable moderate flows and some require increased river discharge to cue spawning (Humphries et al. 1999; Growns 2004; Kerezsy et al. 2011; Baumgartner et al. 2014; Stocks et al. 2021). In the present study, species-specific responses can be related to abiotic drivers of recruitment. For example, carp gudgeon showed an increase in abundance in the Menindee fish death reach 8 months after the initial fish kills, before the recommencement of flows. Existing literature details carp gudgeon prefer stable water levels and can spawn in aquarium tanks independent of flows (Lake 1967; Anderson et al. 1971). A progressive decline was observed in the abundance of Australian smelt in the Menindee fish death reach up to 18 months after the initial fish kill events. Being short lived, poor recruitment of Australian smelt in the Menindee fish death reach during the subsequent breeding season may account for their progressive decline. Similar trends were observed by King et al. (2012) for some small-bodied species after the 2010 Murray River blackwater event. The abundance of bony herring in the Menindee fish death reach showed an increase at 18 months after the initial fish kill events. This increase in abundance, primarily driven by juvenile fish, was observed after flows returned throughout the Darling River in March 2020. Existing literature indicates bony herring spawn independent of flooding, but flow events may play an important role in the magnitude of recruitment success (Llewellyn 1983; Puckridge and Walker 1990; Gehrke et al. 1995; Southwell et al. 2015). No freshwater prawns were captured in the Menindee fish death reach 6 months after the initial Menindee fish kill events. Similarly, McCarthy et al. (2014) identified an 81% reduction in the abundance of the decapod Euastacus armatus after a large-scale hypoxic blackwater event in the Murray River, Australia. At 8 months after the initial Menindee fish kills, before the recommencement of flows, freshwater prawns were captured and continued to increase in abundance at 18 months after the initial fish kills. This is consistent with existing literature that details freshwater prawns breed between December and April independent of hydrological patterns (Richardson et al. 2004).
Various factors influence the recovery time of fish communities from fish kills events, including the duration, spatial extent and severity of the event, proximity to recolonisation sources and the life history traits of the affected species (Olmsted and Cloutman 1974; Gresswell 1999; Weng et al. 2001; Adams and Warren 2005). For example, after severe fish kill in the Munster Blackwater, Northern Ireland, salmonid abundance returned to control levels within 1 year, total salmonid biomass recovered within 2 years and age structure returned to background levels within 3 years (Kennedy et al. 2012). In contrast, algal blooms in the Pecos River, Texas, caused large-scale declines in abundance and population instability that persisted for many years (Rhodes and Hubbs 1992). In the 18 months after the initial Menindee fish kills, no significant change in abundance was observed for Murray cod, golden perch and silver perch within the Menindee fish death reach; nor have YOY recruits been detected for these species. Natural recovery requires either that the structure of the surviving population is sufficient to allow recovery or that unimpeded immigration from outside the affected area can occur relatively quickly (Thiem et al. 2017). It is possible that: (1) the small remaining population of Murray cod and silver perch within the Menindee fish death reach was insufficient to allow recovery under the temporal scale examined; (2) further broad-scale fish kills throughout 600 km of the Darling–Baaka River both upstream and downstream of the Menindee fish death reach limited the potential for Murray cod and silver perch immigration; or (3) hydrological conditions following the Menindee fish kills were not conducive to support recovery. Although no new recruits of golden perch (<125 mm TL) were detected, golden perch larvae were sampled upstream of the study reach (upstream of Menindee Main Weir) in larval drift nets following spawning in the Barwon, Darling, Moonie, Warrego and Culgoa rivers during March 2020 flow events (J. D. Thiem, pers. comm., 18 May 2020). There was limited opportunity during the period reported here for these YOY fish to disperse into the Menindee fish death reach (or further downstream) because most inflows to the Menindee Lakes were either retained upstream of Menindee Main Weir, diverted into Lake Pamamaroo or delivered into the Menindee fish death reach via an undershot regulator on the Lake Wetherell outlet, which is likely to have resulted in the death of a large proportion of young fish passing through it (Baumgartner et al. 2006).
Although the majority of media attention was concentrated on the Menindee fish kill events, further broader-scale fish kills occurred throughout ~600 km of the Darling–Baaka River as disconnected refuge pools contracted and water quality deteriorated from June 2019 to March 2020. Spatial imagery indicated that only 12% of the 272 km of river channel between Weir 32 and Pooncarie Weir was habitable pools for native fish (K. Danaher, pers. comm., 16 June 2021). The Pooncarie reach is representative of the broader Darling River that experienced similar hydrological conditions, poor water quality and widespread fish kills. Within the Pooncarie reach, significant reductions in the abundance of several species were observed after fish kills and fish translocations within this reach. These differences in species abundances can likely be attributed to a combination of: (1) the widespread fish kills and translocations that occurred throughout the Darling–Baaka River as the refuge pools contracted throughout 2019–20; (2) a reduction in catchability associated with electrofishing efficiency (discussed further below); and (3) fish dispersal with the resumption of flows. It is also possible that declines in the abundance of some short-lived species were masked by recruitment in the 3 months between the fish kills within this reach and Round 3 of sampling after the resumption on flows. No significant decline was observed in the abundance of Murray cod after the fish kills throughout the Pooncarie reach, although this can likely be attributed to the sample size and associated statistical power to detect change. Estimates of 2000–8000 large Murray cod perished over 600 km of the Darling River downstream of Weir 32 (I. M. Ellis, unpubl. obs., 28 August 2020) and a further ≥950 Murray cod were translocated and collected for broodstock as part of ‘fish rescue’ efforts (New South Wales Department of Primary Industries 2020a). When historical data were examined for the Pooncarie reach between 2004 and 2020, temporal variability was observed in the abundance of species. However, the limited available annual standardised fish community sampling data throughout the Darling–Baaka between 2004 and 2018 (New South Wales Department of Primary Industries 2020b) created high variance in species abundance between sites within years.
The severity of the effect of fish kill events can vary across taxonomic groups (King et al. 2012). Behavioural or physiological attributes can influence the susceptibility of a species to environmental stressors such as low dissolved oxygen (McNeil and Closs 2007; Crook et al. 2010). During Round 1 of sampling, 6 months after the initial Menindee fish kills, no significant differences in the abundance of golden perch, Australian smelt and common carp were observed between the Menindee fish death reach and Pooncarie reach. Menindee Main Weir acts as a barrier to upstream fish movement; however, local flow velocity below Menindee Main Weir during the Menindee fish kill events appears to have created a short (~8–10 km) zone of mixing, and hence dissolved oxygen refugia. Given that golden perch and Australian smelt can be highly mobile (Reynolds 1983; Lintermans 2007), it is possible that many individuals from these species moved into this zone to seek reprieve from the low dissolved oxygen. Golden perch have been observed to move to areas of normoxic refuge during hypoxia events (Thiem et al. 2020). Although thousands of golden perch died during the summer 2018–19 Menindee fish kills, enough survived to result in an abundance comparable to that of the unaffected reach; this may also be attributed to higher initial abundances in the Menindee fish death reach as a result of aggregation (detailed further below). Australian smelt and common carp are also more adept to using the surface water interface, where there is increased dissolved oxygen, known as ‘aquatic surface respiration’ (Sargent and Galat 2002). Common carp were observed ‘gasping’ at the water surface in the early morning during periods of the lowest dissolved oxygen (J. R. Stocks, pers. comm., 30 January 2019), and high abundances of live Australian smelt were observed occupying this surface layer during the Menindee fish kills (J. McGowan, pers. comm., 10 January 2019). McNeil and Closs (2007) identified that carp gudgeon can maintain aquatic surface respiration in severe hypoxia, but significantly lower abundances were observed in the Menindee fish death reach compared with the Pooncarie reach. This may be attributed to the species being less mobile and thus a reduced ability to move into areas of normoxic refuge.
Large-sale fish kills can affect food web dynamics and nutrient balance (Holmlund and Hammer 1999; Ruuhijärvi et al. 2010; Oh et al. 2019). During Round 1 of sampling RBC of golden perch within the reasonably unaffected Pooncarie reach was significantly greater than that of fish from the Menindee fish death reach. This may be attributed to the significantly greater abundance of both fish and decapod prey items within the unaffected Pooncarie reach. Such association between fish condition and prey abundance illustrates the likely effects that the fish kills had on the food web and trophodynamics of the Darling River. Although this study focused on the impacts on the fish and decapod community, it is also highly likely that other components of the ecosystem were affected by poor water quality during late 2018 and 2019, including lower-trophic-order taxa, such as other macroinvertebrates and zooplankton (King et al. 2012). These lower-trophic-level taxa form the base of the aquatic food web and thus have significant implications for native fish recovery.
Study design
The present study had important design complications that limited our ability to confidently identify the impacts of the summer 2018–19 Menindee fish kill on the fish community of the Menindee Weir pool (Menindee fish death reach). Because no suitable pre-fish kill data were available for the Menindee fish death reach, we were only able to implement a ‘control–impact’-like study design, a common trait of fish kill assessment studies (King et al. 2012). Although the Pooncarie reach at the commencement of this study was the most appropriate comparison reach available, there were clear inherent differences between the two reaches, including hydraulic conditions and fish movement, which, anecdotally, creates aggregations in the Menindee Weir pool (i.e. Menindee fish death reach). As described by Vertessy et al. (2019), high-flow events in 2012 and 2016 combined with the use of Commonwealth environmental water connected the river system to create good conditions for fish spawning, recruitment and dispersal into the Menindee lakes. By the end of 2018, high fish biomass from the recruitment events, water releases from the lakes by NSW and the inability of fish to move upstream or downstream due to the restriction of weirs resulted in the aggregation of fish populations in the Menindee Weir pool upstream of Weir 32. Further, the suitability of the Pooncarie reach as a comparison reach became less appropriate during the course of the present study as a cease to flow extended, resulting in disconnection of the river into isolated pools. Therefore, we were unable to exclude the potential that the observed differences in mean abundance between the two reaches were not the result of inherent site differences.
During periods of low flow, fish species may seek refuge pools; and, as refuge pool volumes recede, fish densities may further increase in the short term due to the decreasing water volume, but this becomes more complex due to the increased predator–prey interactions (Magoulick and Kobza 2003). Ultimately, fish in these circumstances will die if conditions exceed a species tolerance threshold (Crook et al. 2010), as observed in the Pooncarie reach through 2019 and early 2020. This study documented a substantial impact on the fish community in the Pooncarie reach between June 2019 and June 2020. These data for this reach alone demonstrate a ‘before–after’ impact on the fish community in the Pooncarie reach in the 12 months following the cease to flow below Menindee. This result reflects a high number of reports of fish kills in isolated pools during this period (I. M. Ellis, unpubl. obs., 28 August 2020). These deaths were not as well documented because they occurred over multiple months, as opposed to sudden mass deaths in a reach adjacent to a population centre. Thus, our data demonstrate another more innocuous detrimental effect of drought and low-flow conditions.
Electrofishing capture probability can be influenced by environmental conditions (Lyon et al. 2014). Factors that may exacerbate differences in fish abundances between sampling events and reaches include variables that can affect electrofishing efficiency. With returned flows in March 2020, water turbidity, conductivity and depth during Round 3 differed significantly to those in Rounds 1 and 2, likely affecting electrofishing efficiency between the sampling rounds (Miranda and Kratochvíl 2008; Lyon et al. 2014).
This study highlights the problematic nature of dynamic ecosystems in designing research programs, as well as the paucity of representative baseline data in freshwater ecosystems that can be used to measure the effects of catastrophic events. Despite the limitations experienced in this study, we suggest that this study makes a significant contribution to our understanding of the effects of fish kills on fish communities. This study also provides the ability to monitoring changes in the fish communities of the Darling–Baaka in response to restoration activities such as adaptive environmental flows, fish stocking and remediation works.
Management implications and recovery actions
Although signs of short-term recovery are evident so far for some species with the return of moderate flows to the Darling River, the results presented here highlight a fish community in continued stress. It is also expected that the impacts on the fish communities of the Darling–Baaka will be extended throughout the broader MDB. It has become widely accepted in contemporary population models that the Barwon–Darling and Darling–Baaka are critical sources for spawning and recruitment of golden perch, and that the Menindee Lakes system provides a key nursery area supporting basin-wide recruitment (Sharpe 2011; Zampatti et al. 2015; Stuart and Sharpe 2020). Golden perch that originate in the Barwon–Darling and Menindee Lakes can disperse widely and contribute substantially to subpopulations throughout the entire MDB. The impacts of the 2018–20 fish kills on the population of golden perch throughout the MDB have not been fully considered. Furthermore, Koehn (2004) estimated it would take 52 years to re-establish Murray cod populations similar to those lost in the 2004 Darling River fish kills. The 2018–20 Darling River fish kills are therefore another significant blow to an already degraded fish community.
Significant management actions will be needed to protect native fish from further decline and support the recovery and restoration of native fish populations in the Darling–Baaka River (Koehn et al. 2014), including the following:
-
Revision of minimum flow requirements to ensure base flows are released that minimise the threat of perverse water quality outcomes linked to thermal stratification and blue–green algal or cyanobacteria blooms.
-
Refinement of the delivery of operational water (in line with ecological advice) and the delivery of water for the environment to promote the movement, connectivity, spawning and recruitment of native fish. Each of these flow management improvements will clearly need to be linked to refined water management upstream in the northern MDB (Vertessy et al. 2019).
-
Improvements in water quality and algal monitoring capacity to support enhanced predicting capability and better river operations in mitigating poor water quality events.
-
Conservation stocking, particularly in the early stages of recovery, that may complement natural recruitment. This could be supported through the management of water for the environment, but stocking must not be considered a substitute for overall flow reform, from which natural recruitment can vastly exceed restocking outcomes, as evident from the 2010–11 Edward–Wakool blackwater fish kills (Thiem et al. 2017).
-
Strategic investment in on-ground actions, including water off-take screening to prevent fish entrainment, resnagging with complex large woody habitat and riverbank rehabilitation through fencing and native vegetation restoration. Undershot weirs could be removed or replaced with overshot weirs to minimise the death of drifting larvae (Baumgartner et al. 2006). Fish passage should also be provided or improved at all weirs and barriers to facilitate passage throughout the Barwon–Darling, promoting genetic mixing, population resilience, fish immigration and recruitment.
This study details the short-term impacts of major hypoxic events and protracted drying on aquatic biota within a large dryland river. However, these changes were not generic among species, life stages and short-term temporal scales. This study highlights how different behavioural and physiological attributes, life history requirements and reproductive strategies of a species affect the extent of the impact and recovery after major fish kill events. Just prior to the publication of this paper, the Menindee Lakes system is filling as floodwater makes its way through the Darling River system, providing favourable conditions for breeding and recruitment of some native fish species. Continued monitoring will provide an opportunity to both guide and track the effective recovery management interventions and determine the mid- to long-term impacts of such fish kills on the fish communities.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This study was funded by the Murray–Darling Basin Authority.
Acknowledgements
The authors thank New South Wales Fisheries personnel who provided technical advice and assistance, particularly Nicholas O’Brien, Ian Wooden, Tom Butterfield, Patrick Martin, Michael Rodgers, Dean Gilligan, Anthony Townsend and Craig Boys. The authors thank Steve Morris for biometric support and Ivor Stuart for constructive comments on the manuscript. The authors also thank the property owners for allowing access to the study sites.
References
Adams, S. B., and Warren, M. L. (2005). Recolonization by warmwater fishes and crayfishes after severe drought in upper coastal plain hill streams. Transactions of the American Fisheries Society 134, 1173–1192.| Recolonization by warmwater fishes and crayfishes after severe drought in upper coastal plain hill streams.Crossref | GoogleScholarGoogle Scholar |
Anderson, J., Lake, J., and Mackay, N. (1971). Notes on reproductive behaviour and ontogeny in two species of Hypseleotris (= Carassiops) (Gobiidae: Teleostei). Marine and Freshwater Research 22, 139–146.
| Notes on reproductive behaviour and ontogeny in two species of Hypseleotris (= Carassiops) (Gobiidae: Teleostei).Crossref | GoogleScholarGoogle Scholar |
Arnell, N. W., and Gosling, S. N. (2013). The impacts of climate change on river flow regimes at the global scale. Journal of Hydrology 486, 351–364.
| The impacts of climate change on river flow regimes at the global scale.Crossref | GoogleScholarGoogle Scholar |
Balcombe, S. R., Lobegeiger, J. S., Marshall, S. M., Marshall, J. C., Ly, D., and Jones, D. N. (2012). Fish body condition and recruitment success reflect antecedent flows in an Australian dryland river. Fisheries Science 78, 841–847.
| Fish body condition and recruitment success reflect antecedent flows in an Australian dryland river.Crossref | GoogleScholarGoogle Scholar |
Baldwin, D. S. (2019). Stratification, mixing and fish deaths in the lower Darling River. A report prepared for the Murray–Darling Basin Authority. (Rivers and Wetlands.) Available at https://www.mdba.gov.au/sites/default/files/pubs/Independent-report-stratification-mixing-and-fish-deaths-in-the-Lower-Darling-River.pdf
Balme, J. (1995). 30 000 years of fishery in western New South Wales. Archaeology in Oceania 30, 1–21.
| 30 000 years of fishery in western New South Wales.Crossref | GoogleScholarGoogle Scholar |
Baumgartner, L. J., Reynoldson, N., and Gilligan, D. M. (2006). Mortality of larval Murray cod (Maccullochella peelii peelii) and golden perch (Macquaria ambigua) associated with passage through two types of low-head weirs. Marine and Freshwater Research 57, 187–191.
| Mortality of larval Murray cod (Maccullochella peelii peelii) and golden perch (Macquaria ambigua) associated with passage through two types of low-head weirs.Crossref | GoogleScholarGoogle Scholar |
Baumgartner, L. J., Conallin, J., Wooden, I., Campbell, B., Gee, R., Robinson, W. A., and Mallen-Cooper, M. (2014). Using flow guilds of freshwater fish in an adaptive management framework to simplify environmental flow delivery for semi-arid riverine systems. Fish and Fisheries 15, 410–427.
| Using flow guilds of freshwater fish in an adaptive management framework to simplify environmental flow delivery for semi-arid riverine systems.Crossref | GoogleScholarGoogle Scholar |
Bowling, L., and Baker, P. (1996). Major cyanobacterial bloom in the Barwon–Darling River, Australia, in 1991, and underlying limnological conditions. Marine and Freshwater Research 47, 643–657.
| Major cyanobacterial bloom in the Barwon–Darling River, Australia, in 1991, and underlying limnological conditions.Crossref | GoogleScholarGoogle Scholar |
Bray, J. R., and Curtis, J. T. (1957). An ordination of the upland forest communities of southern Wisconsin. Ecological Monographs 27, 325–349.
| An ordination of the upland forest communities of southern Wisconsin.Crossref | GoogleScholarGoogle Scholar |
Bunn, S. E., and Arthington, A. H. (2002). Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environmental Management 30, 492–507.
| Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity.Crossref | GoogleScholarGoogle Scholar | 12481916PubMed |
Crook, D. A., Reich, P., Bond, N. R., McMaster, D., Koehn, J. D., and Lake, P. S. (2010). Using biological information to support proactive strategies for managing freshwater fish during drought. Marine and Freshwater Research 61, 379–387.
| Using biological information to support proactive strategies for managing freshwater fish during drought.Crossref | GoogleScholarGoogle Scholar |
Dalu, T., Nhiwatiwa, T., Clegg, B., and Barson, M. (2012). Impact of Lernaea cyprinacea Linnaeus 1758 (Crustacea: Copepoda) almost a decade after an initial parasitic outbreak in fish of Malilangwe Reservoir, Zimbabwe. Knowledge and Management of Aquatic Ecosystems 406, 03.
| Impact of Lernaea cyprinacea Linnaeus 1758 (Crustacea: Copepoda) almost a decade after an initial parasitic outbreak in fish of Malilangwe Reservoir, Zimbabwe.Crossref | GoogleScholarGoogle Scholar |
Davies, P. E. (2008). Sustainable Rivers Audit: SRA report 1, June 2008: a report on the ecological health of rivers in the Murray–Darling Basin, 2004–2007. Murray–Darling Basin Commission, Canberra, ACT, Australia.
Diaz, R. J. (2001). Overview of hypoxia around the world. Journal of Environmental Quality 30, 275–281.
| Overview of hypoxia around the world.Crossref | GoogleScholarGoogle Scholar | 11285887PubMed |
Döll, P., and Zhang, J. (2010). Impact of climate change on freshwater ecosystems: a global-scale analysis of ecologically relevant river flow alterations. Hydrology and Earth System Sciences Discussions 14, 783–799.
| Impact of climate change on freshwater ecosystems: a global-scale analysis of ecologically relevant river flow alterations.Crossref | GoogleScholarGoogle Scholar |
Du, H., Xia, J., Zeng, S., She, D., and Liu, J. (2014). Variations and statistical probability characteristic analysis of extreme precipitation events under climate change in Haihe River Basin, China. Hydrological Processes 28, 913–925.
| Variations and statistical probability characteristic analysis of extreme precipitation events under climate change in Haihe River Basin, China.Crossref | GoogleScholarGoogle Scholar |
Ellis, I., and Meredith, S. (2004). An independent review of the February 2004 Lower Darling River fish deaths: guidelines for future release effects on Lower Darling River fish populations. A report to the NSW Department of Infrastructure Planning and Natural Resources. Technical Report 7, Murray–Darling Freshwater Research Centre, Wodonga, Vic., Australia.
Garvey, J. (2017). Australian Aboriginal freshwater shell middens from late Quaternary northwest Victoria: prey choice, economic variability and exploitation. Quaternary International 427, 85–102.
| Australian Aboriginal freshwater shell middens from late Quaternary northwest Victoria: prey choice, economic variability and exploitation.Crossref | GoogleScholarGoogle Scholar |
Gehrke, P. C., Brown, P., Schiller, C. B., Moffatt, D. B., and Bruce, A. M. (1995). River regulation and fish communities in the Murray–Darling river system, Australia. Regulated Rivers 11, 363–375.
| River regulation and fish communities in the Murray–Darling river system, Australia.Crossref | GoogleScholarGoogle Scholar |
Gresswell, R. E. (1999). Fire and aquatic ecosystems in forested biomes of North America. Transactions of the American Fisheries Society 128, 193–221.
| Fire and aquatic ecosystems in forested biomes of North America.Crossref | GoogleScholarGoogle Scholar |
Grill, G., Lehner, B., Thieme, M., Geenen, B., Tickner, D., Antonelli, F., Babu, S., Borrelli, P., Cheng, L., and Crochetiere, H. (2019). Mapping the world’s free-flowing rivers. Nature 569, 215–221.
| Mapping the world’s free-flowing rivers.Crossref | GoogleScholarGoogle Scholar | 31068722PubMed |
Growns, I. (2004). A numerical classification of reproductive guilds of the freshwater fishes of south-eastern Australia and their application to river management. Fisheries Management and Ecology 11, 369–377.
| A numerical classification of reproductive guilds of the freshwater fishes of south-eastern Australia and their application to river management.Crossref | GoogleScholarGoogle Scholar |
Holmlund, C. M., and Hammer, M. (1999). Ecosystem services generated by fish populations. Ecological Economics 29, 253–268.
| Ecosystem services generated by fish populations.Crossref | GoogleScholarGoogle Scholar |
Humphries, P. (2007). Historical Indigenous use of aquatic resources in Australia’s Murray–Darling Basin, and its implications for river management. Ecological Management & Restoration 8, 106–113.
| Historical Indigenous use of aquatic resources in Australia’s Murray–Darling Basin, and its implications for river management.Crossref | GoogleScholarGoogle Scholar |
Humphries, P., King, A. J., and Koehn, J. D. (1999). Fish, flows and flood plains: links between freshwater fishes and their environment in the Murray–Darling River system, Australia. Environmental Biology of Fishes 56, 129–151.
| Fish, flows and flood plains: links between freshwater fishes and their environment in the Murray–Darling River system, Australia.Crossref | GoogleScholarGoogle Scholar |
Jackson, S., and Head, L. (2020). Australia’s mass fish kills as a crisis of modern water: understanding hydrosocial change in the Murray–Darling Basin. Geoforum 109, 44–56.
| Australia’s mass fish kills as a crisis of modern water: understanding hydrosocial change in the Murray–Darling Basin.Crossref | GoogleScholarGoogle Scholar |
Jackson, R. B., Carpenter, S. R., Dahm, C. N., McKnight, D. M., Naiman, R. J., Postel, S. L., and Running, S. W. (2001). Water in a changing world. Ecological Applications 11, 1027–1045.
| Water in a changing world.Crossref | GoogleScholarGoogle Scholar |
Kennedy, R. J., Rosell, R., and Hayes, J. (2012). Recovery patterns of salmonid populations following a fish kill event on the River Blackwater, Northern Ireland. Fisheries Management and Ecology 19, 214–223.
| Recovery patterns of salmonid populations following a fish kill event on the River Blackwater, Northern Ireland.Crossref | GoogleScholarGoogle Scholar |
Kerezsy, A., Balcombe, S. R., Arthington, A. H., and Bunn, S. E. (2011). Continuous recruitment underpins fish persistence in the arid rivers of far-western Queensland, Australia. Marine and Freshwater Research 62, 1178–1190.
| Continuous recruitment underpins fish persistence in the arid rivers of far-western Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
King, A. J., Tonkin, Z., and Lieshcke, J. (2012). Short-term effects of a prolonged blackwater event on aquatic fauna in the Murray River, Australia: considerations for future events. Marine and Freshwater Research 63, 576–586.
| Short-term effects of a prolonged blackwater event on aquatic fauna in the Murray River, Australia: considerations for future events.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D. (2004). The loss of valuable Murray cod in fish kills: a science and management perspective. Arthur Rylah Institute for Environmental Research, Melbourne, Vic., Australia.
Koehn, J. (2015). Managing people, water, food and fish in the Murray–Darling Basin, south-eastern Australia. Fisheries Management and Ecology 22, 25–32.
| Managing people, water, food and fish in the Murray–Darling Basin, south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., King, A. J., Beesley, L., Copeland, C., Zampatti, B. P., and Mallen-Cooper, M. (2014). Flows for native fish in the Murray–Darling Basin: lessons and considerations for future management. Ecological Management & Restoration 15, 40–50.
| Flows for native fish in the Murray–Darling Basin: lessons and considerations for future management.Crossref | GoogleScholarGoogle Scholar |
La, V. T., and Cooke, S. J. (2011). Advancing the science and practice of fish kill investigations. Reviews in Fisheries Science 19, 21–33.
| Advancing the science and practice of fish kill investigations.Crossref | GoogleScholarGoogle Scholar |
Lake, J. (1967). Rearing experiments with five species of Australian freshwater fishes. I. Inducement to spawning. Marine and Freshwater Research 18, 137–154.
| Rearing experiments with five species of Australian freshwater fishes. I. Inducement to spawning.Crossref | GoogleScholarGoogle Scholar |
Lintermans, M. (2007). ‘Fishes of the Murray–Darling Basin: an Introductory Guide.’ (Murray–Darling Basin Commission: Canberra, ACT, Australia.)
Llewellyn, L. (1983). The distribution of fish in New South Wales. Special Publication 7, Australian Society for Limnology, Sydney, NSW, Australia.
Lugg, A. (2000). ‘Fish Kills in NSW.’ (NSW Fisheries: Sydney, NSW, Australia.)
Lyon, J. P., Bird, T., Nicol, S., Kearns, J., O’Mahony, J., Todd, C. R., Cowx, I. G., and Bradshaw, C. J. A. (2014). Efficiency of electrofishing in turbid lowland rivers: implications for measuring temporal change in fish populations. Canadian Journal of Fisheries and Aquatic Sciences 71, 878–886.
| Efficiency of electrofishing in turbid lowland rivers: implications for measuring temporal change in fish populations.Crossref | GoogleScholarGoogle Scholar |
Magoulick, D. D., and Kobza, R. M. (2003). The role of refugia for fishes during drought: a review and synthesis. Freshwater Biology 48, 1186–1198.
| The role of refugia for fishes during drought: a review and synthesis.Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M., and Zampatti, B. P. (2018). History, hydrology and hydraulics: rethinking the ecological management of large rivers. Ecohydrology 11, e1965.
| History, hydrology and hydraulics: rethinking the ecological management of large rivers.Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M., and Zampatti, B. P. (2020). Restoring the ecological integrity of a dryland river: why low flows in the Barwon–Darling River must flow. Ecological Management & Restoration 21, 218–228.
| Restoring the ecological integrity of a dryland river: why low flows in the Barwon–Darling River must flow.Crossref | GoogleScholarGoogle Scholar |
Mathews, R. H. (1903). The aboriginal fisheries at Brewarrina. Journal and Proceedings of the Royal Society of New South Wales 37, 146–156.
McCarthy, B., Zukowski, S., Whiterod, N., Vilizzi, L., Beesley, L., and King, A. (2014). Hypoxic blackwater event severely impacts Murray crayfish (Euastacus armatus) populations in the Murray River, Australia. Austral Ecology 39, 491–500.
| Hypoxic blackwater event severely impacts Murray crayfish (Euastacus armatus) populations in the Murray River, Australia.Crossref | GoogleScholarGoogle Scholar |
McIlgorm, A., and Pepperell, J. (2013). Developing a cost effective state-wide expenditure survey method to measure the economic contribution of the recreational fishing sector in NSW in 2012. University of Wollongong, Wollongong, NSW, Australia.
McNeil, D. G., and Closs, G. P. (2007). Behavioural responses of a south-east Australian floodplain fish community to gradual hypoxia. Freshwater Biology 52, 412–420.
| Behavioural responses of a south-east Australian floodplain fish community to gradual hypoxia.Crossref | GoogleScholarGoogle Scholar |
Miranda, L. E., and Kratochvíl, M. (2008). Boat Electrofishing Relative to Anode Arrangement. Transactions of the American Fisheries Society 137, 1358–1362.
| Boat Electrofishing Relative to Anode Arrangement.Crossref | GoogleScholarGoogle Scholar |
Mitrovic, S. M., Hardwick, L., and Dorani, F. (2011). Use of flow management to mitigate cyanobacterial blooms in the Lower Darling River, Australia. Journal of Plankton Research 33, 229–241.
| Use of flow management to mitigate cyanobacterial blooms in the Lower Darling River, Australia.Crossref | GoogleScholarGoogle Scholar |
Moritz, C., Blackall, L., Davis, J., Flannery, T., Godden, L., Head, L., Jackson, S., Kingsford, R., Wheeler, S., and Williams, J. (2019). Investigation of the causes of mass fish kills in the Menindee Region NSW over the summer of 2018–2019. Australian Academy of Science, Canberra, Australia.
Murray–Darling Basin Authority (2020). Native Fish Recovery Strategy. Working together for the future of native fish. MDBA, Canberra, ACT, Australia.
New South Wales Department of Primary Industries (2019). Fish death interim investigation report – Lower Darling River fish death event, Menindee 2018/19. Department of Primary Industries, Taylor’s Beach, NSW, Australia.
New South Wales Department of Primary Industries (2020a). Recovering the Lower Darling 2019/20: Lower Darling fish recovery reach coordinator report. NSW Department of Primary Industries.
New South Wales Department of Primary Industries (2020b). Aquatic Ecosystems Research Database. 20/06/2021 edn. (NSW Department of Primary Industries: Batemans Bay, NSW, Australia.)
Ngor, P. B., Legendre, P., Oberdorff, T., and Lek, S. (2018). Flow alterations by dams shaped fish assemblage dynamics in the complex Mekong-3S river system. Ecological Indicators 88, 103–114.
| Flow alterations by dams shaped fish assemblage dynamics in the complex Mekong-3S river system.Crossref | GoogleScholarGoogle Scholar |
Oh, H.-J., Oda, Y., Ha, J.-Y., Nagata, T., Hanazato, T., Miyabara, Y., Sakamoto, M., and Chang, K.-H. (2019). Responses of daphnids and other zooplankton populations to massive fish kill in Lake Suwa. Ecological Research 34, 856–863.
| Responses of daphnids and other zooplankton populations to massive fish kill in Lake Suwa.Crossref | GoogleScholarGoogle Scholar |
Olmsted, L. L., and Cloutman, D. G. (1974). Repopulation after a fish kill in Mud Creek, Washington County, Arkansas following pesticide pollution. Transactions of the American Fisheries Society 103, 79–87.
| Repopulation after a fish kill in Mud Creek, Washington County, Arkansas following pesticide pollution.Crossref | GoogleScholarGoogle Scholar |
Postel, S. L., Daily, G. C., and Ehrlich, P. R. (1996). Human appropriation of renewable fresh water. Science 271, 785–788.
| Human appropriation of renewable fresh water.Crossref | GoogleScholarGoogle Scholar |
Puckridge, J., and Walker, K. (1990). Reproductive biology and larval development of a gizzard shad, Nematalosa erebi (Gunther) (Dorosomatinae: Teleostei), in the River Murray, South Australia. Marine and Freshwater Research 41, 695–712.
| Reproductive biology and larval development of a gizzard shad, Nematalosa erebi (Gunther) (Dorosomatinae: Teleostei), in the River Murray, South Australia.Crossref | GoogleScholarGoogle Scholar |
Puckridge, J. T., Sheldon, F., Walker, K. F., and Boulton, A. J. (1998). Flow variability and the ecology of large rivers. Marine and Freshwater Research 49, 55–72.
| Flow variability and the ecology of large rivers.Crossref | GoogleScholarGoogle Scholar |
Reynolds, L. F. (1983). Migration patterns of five fish species in the Murray–Darling River system. Marine and Freshwater Research 34, 857–871.
| Migration patterns of five fish species in the Murray–Darling River system.Crossref | GoogleScholarGoogle Scholar |
Rhodes, K., and Hubbs, C. (1992). Recovery of Pecos River fishes from a red tide fish kill. The Southwestern Naturalist 37, 178–187.
| Recovery of Pecos River fishes from a red tide fish kill.Crossref | GoogleScholarGoogle Scholar |
Richardson, A., Growns, J., and Cook, R. (2004). Distribution and life history of caridean shrimps in regulated lowland rivers in southern Australia. Marine and Freshwater Research 55, 295–308.
| Distribution and life history of caridean shrimps in regulated lowland rivers in southern Australia.Crossref | GoogleScholarGoogle Scholar |
Ruuhijärvi, J., Rask, M., Vesala, S., Westermark, A., Olin, M., Keskitalo, J., and Lehtovaara, A. (2010). Recovery of the fish community and changes in the lower trophic levels in a eutrophic lake after a winter kill of fish. Hydrobiologia 646, 145–158.
| Recovery of the fish community and changes in the lower trophic levels in a eutrophic lake after a winter kill of fish.Crossref | GoogleScholarGoogle Scholar |
Sargent, J. C., and Galat, D. L. (2002). Fish mortality and physicochemistry in a managed floodplain wetland. Wetlands Ecology and Management 10, 113–119.
| Fish mortality and physicochemistry in a managed floodplain wetland.Crossref | GoogleScholarGoogle Scholar |
Sharpe, C. P. (2011). Spawning and recruitment ecology of golden perch (Macquaria ambigua Richardson, 1845) in the Murray and Darling Rivers. Ph.D. Thesis, Griffith University.
Sharpe, C., and Stuart, I. (2018). Environmental flows in the Darling River to support native fish populations. Commonwealth Environmental Water Office, Canberra, ACT, Australia.
Small, K., Kopf, R. K., Watts, R. J., and Howitt, J. (2014). Hypoxia, blackwater and fish kills: experimental lethal oxygen thresholds in juvenile predatory lowland river fishes. PLoS One 9, e94524.
| Hypoxia, blackwater and fish kills: experimental lethal oxygen thresholds in juvenile predatory lowland river fishes.Crossref | GoogleScholarGoogle Scholar | 24728094PubMed |
Southwell, M., Wilson, G., Ryder, D., Sparks, P., and Thoms, M. (2015). Monitoring the ecological response of Commonwealth Environmental Water delivered in 2013–14 in the Gwydir River system. A report to the Department of Environment. University of New England, Armidale, NSW, Australia.
Steduto, P., Schultz, B., Unver, O., Ota, S., Vallee, D., Kulkarni, S., and Dagnino-Johns Garcia, M. (2018). Food security by optimal use of water: synthesis of the 6th and 7th world water forums and developments since then. Irrigation and Drainage 67, 327–344.
| Food security by optimal use of water: synthesis of the 6th and 7th world water forums and developments since then.Crossref | GoogleScholarGoogle Scholar |
Stocks, J. R., Davis, S., Anderson, M. J., Asmus, M. W., Cheshire, K. J. M., Meulen, D. E. d., Walsh, C. T., and Gilligan, D. M. (2021). Fish and flows: abiotic drivers influence the recruitment response of a freshwater fish community throughout a regulated lotic system of the Murray–Darling Basin, Australia. Aquatic Conservation , aqc.3636.
| Fish and flows: abiotic drivers influence the recruitment response of a freshwater fish community throughout a regulated lotic system of the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Stuart, I. G., and Sharpe, C. P. (2020). Riverine spawning, long distance larval drift, and floodplain recruitment of a pelagophilic fish: a case study of golden perch (Macquaria ambigua) in the arid Darling River, Australia. Aquatic Conservation 30, 675–690.
| Riverine spawning, long distance larval drift, and floodplain recruitment of a pelagophilic fish: a case study of golden perch (Macquaria ambigua) in the arid Darling River, Australia.Crossref | GoogleScholarGoogle Scholar |
Thiem, J. D., Wooden, I. J., Baumgartner, L. J., Butler, G. L., Forbes, J. P., and Conallin, J. (2017). Recovery from a fish kill in a semi-arid Australian river: can stocking augment natural recruitment processes? Austral Ecology 42, 218–226.
| Recovery from a fish kill in a semi-arid Australian river: can stocking augment natural recruitment processes?Crossref | GoogleScholarGoogle Scholar |
Thiem, J. D., Wooden, I. J., Baumgartner, L. J., Butler, G. L., Taylor, M. D., and Watts, R. J. (2020). Hypoxic conditions interrupt flood-response movements of three lowland river fish species: implications for flow restoration in modified landscapes. Ecohydrology 13, e2197.
| Hypoxic conditions interrupt flood-response movements of three lowland river fish species: implications for flow restoration in modified landscapes.Crossref | GoogleScholarGoogle Scholar |
Thoms, M., and Sheldon, F. (2000). Water resource development and hydrological change in a large dryland river: the Barwon–Darling River, Australia. Journal of Hydrology 228, 10–21.
| Water resource development and hydrological change in a large dryland river: the Barwon–Darling River, Australia.Crossref | GoogleScholarGoogle Scholar |
Vertessy, R., Barma, D., Baumgartner, L., Bond, N., Mitrovic, S., and Sheldon, F. (2019). Independent assessment of the 2018–19 fish deaths in the lower Darling. Independent assessment, Canberra, ACT, Australia.
Vörösmarty, C. J., McIntyre, P. B., Gessner, M. O., Dudgeon, D., Prusevich, A., Green, P., Glidden, S., Bunn, S. E., Sullivan, C. A., and Liermann, C. R. (2010). Global threats to human water security and river biodiversity. Nature 467, 555–561.
| Global threats to human water security and river biodiversity.Crossref | GoogleScholarGoogle Scholar | 20882010PubMed |
Weng, Z., Mookerji, N., and Mazumder, A. (2001). Nutrient-dependent recovery of Atlantic salmon streams from a catastrophic flood. Canadian Journal of Fisheries and Aquatic Sciences 58, 1672–1682.
| Nutrient-dependent recovery of Atlantic salmon streams from a catastrophic flood.Crossref | GoogleScholarGoogle Scholar |
Whitworth, K. L., Baldwin, D. S., and Kerr, J. L. (2012). Drought, floods and water quality: drivers of a severe hypoxic blackwater event in a major river system (the southern Murray–Darling Basin, Australia). Journal of Hydrology 450–451, 190–198.
| Drought, floods and water quality: drivers of a severe hypoxic blackwater event in a major river system (the southern Murray–Darling Basin, Australia).Crossref | GoogleScholarGoogle Scholar |
Winemiller, K. O. (1989). Patterns of variation in life history among South American fishes in seasonal environments. Oecologia 81, 225–241.
| Patterns of variation in life history among South American fishes in seasonal environments.Crossref | GoogleScholarGoogle Scholar | 28312542PubMed |
Zampatti, B., Wilson, P., Baumgartner, L. J., Koster, W., Livore, J., McCasker, N., and Thiem, J. (2015). Reproduction and recruitment of golden perch (Macquaria ambigua ambigua) in the southern Murray–Darling Basin 2013–2014: an exploration of river-scale response, connectivity and population dynamics. South Australian Research and Development Institute, Henley Beach, SA, Australia.
Zampatti, B. P., Strawbridge, A., Thiem, J., Tonkin, Z., Mass, R., Woodhead, J., and Fredberg, J. (2018). Golden perch (Macquaria ambigua) and silver perch (Bidyanus bidyanus) age demographics, natal origin and migration history in the River Murray, Australia. Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.