Spatial variation and drivers of vegetation structure and composition in coastal freshwater wetlands of subtropical Australia
Rebekah Grieger A B D , Samantha J. Capon A B , Wade L. Hadwen A B C and Brendan Mackey A C
A B D , Samantha J. Capon A B , Wade L. Hadwen A B C and Brendan Mackey A C
A Griffith University, School of Environment and Science, Nathan, Qld 4111, Australia.
B Griffith University, Australian Rivers Institute, Nathan, Qld 4111, Australia.
C Griffith University, Climate Action Beacon, Southport, Qld 4222, Australia.
D Corresponding author. Email: rebekah.grieger@griffithuni.edu.au
Marine and Freshwater Research 72(12) 1746-1759 https://doi.org/10.1071/MF21023
Submitted: 21 January 2021 Accepted: 11 August 2021 Published: 31 August 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC
Abstract
Coastal freshwater wetlands (CFWs) are among the most understudied wetlands globally and are highly vulnerable to projected climate changes. To address CFW knowledge gaps in south-east Queensland, Australia, we surveyed the floristic composition and structure of wooded CFWs and explored variation in vegetation patterns in relation to selected environmental drivers. Understorey and shrub assemblages were surveyed using a cover-class scale and stem counts for tree species abundance. Vegetation structure attributes (stem density, basal area) were calculated from survey data. Redundancy analysis was used to investigate drivers of vegetation structure and the species composition of each stratum. Vegetation structure patterns were associated with gradients of rainfall, soil moisture, salinity and pH. Understorey species composition was associated with wallum wetland species, native perennial grass and herb species, and vegetation patterns of the canopy. Common CFW species, namely Melaleuca quinquenervia and Eucalyptus tereticornis, dominated tree assemblage variation. Overall, CFW vegetation exhibited strong associations with gradients of salinity, rainfall, groundwater dependence and disturbance. Alterations to key drivers of vegetation pattern with future climate changes are likely to markedly influence the composition, structure and function of CFW vegetation communities. Action is therefore required to maintain CFW vegetation communities and ecological function in these diverse and unique wetland systems.
Keywords: climate change, floodplain wetlands, salinity, sea level rise, tidal freshwater wetlands.
Introduction
Coastal wetlands sit at a crossroads. Supporting many critical ecosystem services (Mitsch and Gosselink 2015a; An and Verhoeven 2019), wetlands are among the most vulnerable coastal environments, threatened by both intensive development (Torio and Chmura 2013) and climate change (Nicholls 2004; Schuerch et al. 2018). Research concerning coastal wetlands and their vulnerability to climate change has mainly focused on saline systems (i.e. saltmarsh and mangroves), with non-saline coastal wetlands (e.g. coastal floodplain wetlands, freshwater swamps) largely overlooked (Williams et al. 2019). Climate change is expected to lead to sea level rise (SLR), salinisation of groundwaters, shoreline retreat, altered rainfall, warming and increased severity of extreme weather events (floods, fires, cyclones), all of which may significantly alter the composition, structure, and function of coastal freshwater wetlands (CFWs; IPCC 2014; Grieger et al. 2020), exacerbating other pressures. There is an urgent need to understand the value and environmental determinants of these poorly described ecosystems, as well as the likely effects of climate change, to guide potential adaptation strategies.
Responses of saline coastal wetlands to climate-related impacts have been observed globally over recent decades, often involving altered ecosystem functioning including sediment accretion, nutrient dynamics and productivity (Tobias and Neubauer 2019; Grieger et al. 2020). Also apparent is a landward migration of communities, especially that of mangroves into saltmarsh and saltmarsh into upland or freshwater wetlands (Morris et al. 2002; Enwright et al. 2016; Schuerch et al. 2018). Non-saline coastal wetlands, hereafter referred to as CFWs, are similarly expected to be able to migrate landward in response to overland and groundwater salinisation (Boon 2012), but this has not been observed. In many situations, there is often limited space for CFWs to migrate landward due to hydrological constraints coupled with high levels of urban and agricultural development (Grieger et al. 2020).
CFWs are variously referred to as tidal freshwater forested wetlands (TFFW) and tidal freshwater marshes in the US, tidal várzeas in South America and coastal floodplain wetlands or Melaleuca swamps in Australia, where their vegetation tends to differ mainly in relation to local hydrology, geology, and the degree of tidal or saline influence (Boon 2012). Here, we define CFWs as freshwater wetlands in coastal lowlands with the potential to be directly affected by coastal drivers such as increasing salinity and tidal influence.
Salinity and hydrology are important drivers of vegetation patterns in coastal wetlands, with clear zonation often apparent (e.g. saltmarsh–forest ecotones; Mitsch and Gosselink 2015b). Within CFWs, vegetation also responds strongly to these drivers, particularly through differences in composition and structure. For example, bald cypress Taxodium distichum swamps, common in TFFWs of the south-east US, exhibit greater basal areas and growth rates in areas of lower salinity and shorter flood periods (Krauss et al. 2009). Composition also differed from marsh to eucalypt forest across a saline–fresh tidal gradient in south-east Queensland, Australia (Grieger et al. 2019). Significant changes in salinity or hydrology due to disturbance events (e.g. cyclones, storm surges) can lead to marked transitions in vegetation composition and structure (Hoeppner et al. 2008; Tate and Battaglia 2013; Middleton 2016b). Saline intrusion in coastal floodplain wetlands of northern Australia have led to widespread Melaleuca forest dieback (Bowman et al. 2010; Sloane et al. 2019) and expansion of saltpans and mangrove forests (Bell et al. 2001). Groundwater salinisation is a key concern in many non-tidal CFWs that rely on rainfall and fresh groundwater to maintain their ecological function (Hatton and Evans 1998). Fire also shapes Australian Melaleuca swamps (Franklin et al. 2007) and ‘wallum’ wetlands, where many species require fire to stimulate regeneration (Turner et al. 1997; Griffith et al. 2004).
The effects of climate change on CFWs remain largely unknown globally (Grieger et al. 2020), despite the risk of permanent marine inundation in most cases because rates of sediment accumulation are generally outstripped by rates of local SLR (Grieger et al. 2020). Further, CFW vegetation communities tend to be ill-adapted to saline and more frequently inundated conditions with large-scale tree diebacks (Kirwan and Gedan 2019) and transitions to communities dominated by halophytic species observed in many North American CFWs (Langston et al. 2017). Similar diebacks have been observed in salt-affected CFWs of northern Australia (Bowman et al. 2010; Sloane et al. 2019), but assessments of salinity risk in other regions of Australia are rare.
In Australia, assessments of climate risks to these ecosystems are hampered by a paucity of basic descriptions of CFWs, especially in subtropical regions. Here, we seek to address this knowledge gap by describing floristic diversity and structure of CFW vegetation communities within south-east Queensland (SEQ). Spatial patterns in vegetation structure and the composition of both tree and understorey plant assemblages are explored and potential environmental drivers of these, especially in relation to projected climate change (rainfall, salinity, sea level), are investigated. Overall, we sought to improve understanding of vegetation patterns to assess the vulnerability of CFWs and their floristic diversity to the effects of climate change in the region.
Materials and methods
Study area
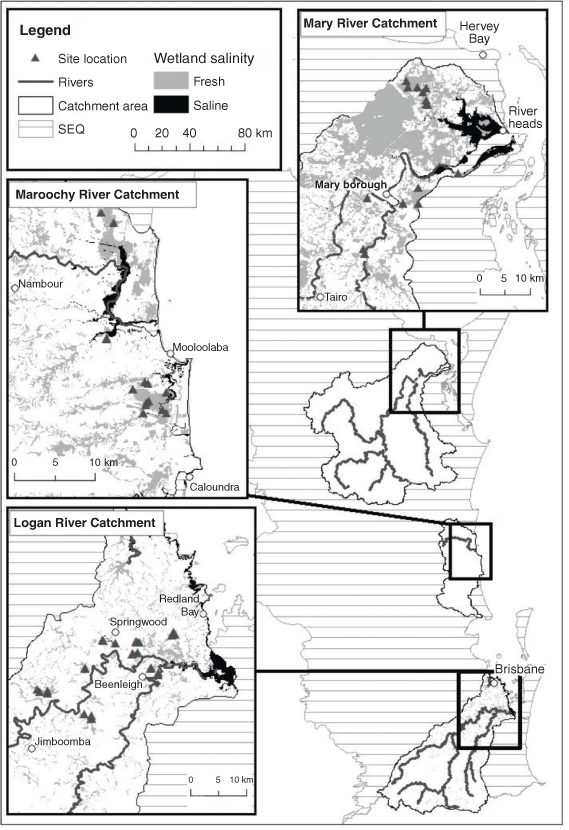
SEQ, Australia, has a humid subtropical climate with mild winters (12.7–22.8°C, rainfall 450 mm) and warm, wet summers (19.7–28.8°C, rainfall 688 mm; Bureau of Meteorology 2019). The SEQ bioregion extends from the New South Wales border north to Gladstone and west along the Great Dividing Range, and is home to more than 70% of Queensland’s population (Queensland Government Statistician’s Office 2019), although urban development only occupies 3.5% of its area. Livestock grazing is the primary land use (82.5%), with crops and plantations occupying ~3.7% and conservation reserves a further 9% (Department of Environment and Science 2014). Wetlands occupy 5.5% of the region, with CFWs comprising ~2% (WetlandInfo 2020a). This study focused on wooded CFW areas that, under the Queensland wetland classifications, are delineated as coastal and subcoastal tree swamps (dominated by Melaleuca and eucalypt species) and coastal and subcoastal wet heath swamps.
CFW areas within three SEQ river catchments (Logan–Albert River, Mary River, Maroochy–Mooloolah River) were identified using the state wetland mapping (Environmental Protection Agency 2005), all with large extents of wetlands and in conservation areas. Catchments were selected to represent: (1) an urban–rural gradient; (2) a latitudinal gradient reflecting climatic variation; and (3) areas of coastal lowlands predicted to be significantly affected by SLR. Sixty sites (Fig. 1) were selected across these catchments in wetland areas managed by local government (i.e. conservation areas, environmental reserves, with only three in national parks). Selected sites were all located in wooded CFWs (i.e. a dominant tree or shrub layer, not grassy wetlands without canopy), in accessible areas, representative of the surrounding vegetation, in relatively undisturbed conditions (i.e. not artificial wetlands or areas with lots of rubbish) and within the mapped extent of projected SLR (3-km buffer added; see Table S1 of the Supplementary material). Sites within the same continuous wetland patch (i.e. a single mapped wetland area) were separated by at least 100 m.
|
Data collection
Field surveys were conducted during summer (November 2018–April 2019) in a 10-m × 10-m area representative of the surrounding vegetation for each site. A summary data table was compiled for each site recording location, water inundation, evidence of fire and disturbance and canopy cover (Table 1).
At each site, vegetation was surveyed in three strata: tree layer (>3 m), shrub layer (1–3 m) and understorey (<1 m). All trees within the plot were recorded, along with species, height, diameter at breast height (DBH; 1.3 m from the tree base, measured using a diameter tape) and the number of stems at 1.3 m. The abundance of woody tree seedlings was also recorded for each species at each site.
The presence and foliage projective cover of species present in the understorey and shrub layers were recorded using a modified Braun–Blanquet cover scale as follows: 1, few individuals (<10); 2, plentiful but small cover (<5%); 3, 5–25% cover; 4, 25–50% cover; 5, 50–75% cover; and 6, >75% cover sensu lato (Braun-Blanquet 1932; Mueller-Dombois and Ellenberg 1974). Other types of groundcover (i.e. bare ground, leaf litter, woody debris, logs, rocks, water) were also recorded using the same cover categories. Where possible, species were identified in the field, with clippings of unknown species taken and identified following the nomenclature of Leiper et al. (2019).
Approximately 4000 cm3 of soil was collected from each 10- × 10-m site by aggregating 10 random subsamples of 400 cm3 of the top 10 cm of soil collected with a hand shovel. Leaf litter and vegetation were removed from the soil surface before collection. Samples were transported in heavy plastic bags and stored in the dark in a glasshouse at Griffith University (Brisbane, Qld, Australia).
Soil pH and salinity were analysed using pHM250 and ECM250 MeterLab probes (Copenhagen, Denmark) and soil was diluted to a ratio of 1:5 in deionised water to investigate the effects of pH and salinity on the structuring of vegetation communities of CFWs, because these parameters are important drivers of assembly in other coastal wetland communities and soil chemistry is likely to be affected by climate change (Neubauer et al. 2013; McKee et al. 2016). Prior to analysis, samples were thoroughly homogenised in a bucket, from which ~5 g soil from each site was used for analysis.
Potential environmental drivers were delineated into regional and local variables to investigate CFW vegetation responses at differing spatial scales. Regional drivers included annual rainfall, hydrological information (highest astronomical tide, groundwater-dependent ecosystem areas) and the predicted extent of SLR from spatial mapping (Table 1). Local drivers comprised elevation, land use (categories in Table 1), soil chemical properties (pH and salinity) and observed disturbances (fire and other). Tree structural attributes (i.e. stem density, canopy height) and the log-transformed abundance of the five most common tree species were included as potential local drivers for the understorey community.
Data analysis
All data analyses were conducted in RStudio using R (ver. 4.0.1, R Foundation for Statistical Computing, Vienna, Austria), using functions from the packages tidyverse (ver. 1.3.0, see https://CRAN.R-project.org/package=tidyverse; Wickham et al. 2019), vegan (ver. 2.5-6, J. Oksanen, F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs, and H. Wagner, see https://cran.r-project.org/web/packages/vegan/index.html), Zetadiv (ver. 1.1.1, G. Latombe, M. A. McGeoch, D. A. Nipperess, and C. Hui, see https://CRAN.R-project.org/package=zetadiv), and ggplot2 (ver. 3.3.3, see https://CRAN.R-project.org/package=ggplot2; Wickham 2016), which are referred to with the notation package::function.
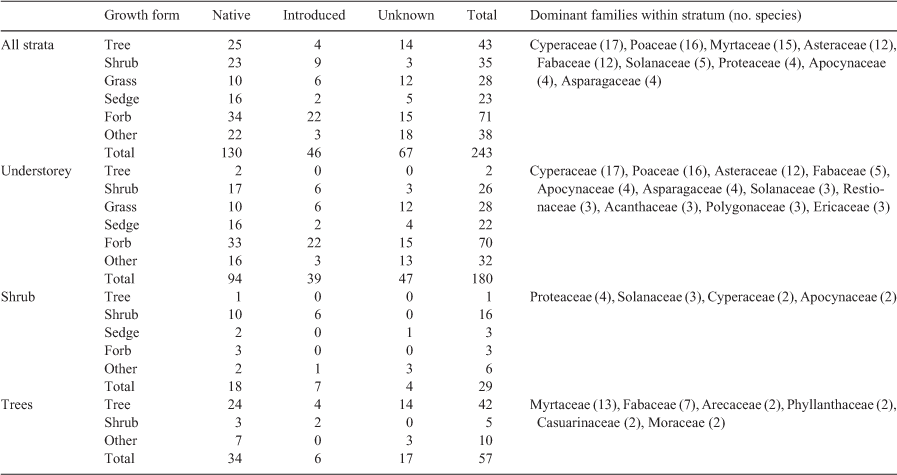
To describe the floristic composition of CFWs, observed species were summarised by family, growth form, life span, endemicity and wetland indicator species status (Table 2). These data were then summarised for the region and each stratum (understorey, shrubs, trees).

|
The similarity of stratum assemblages between sites was explored through zeta (ζ) diversity (zetadiv::Zeta.decline.mc), expressed as the mean number of species shared between i sites (ζi; Hui and McGeoch 2014), where ζ1 is the mean number of species at any one site (analogous to species richness), ζ2 is the mean species shared between any two sites and ζn is the mean shared between all sites. The use of zeta diversity in this way explores the rarity and commonality of the species pool; for example, a community with little species variation between sites would have both high low-order zetas (i.e. ζ1 – ζ4) and high late-order zetas (i.e. ζi – ζnumber of sites), whereas a community with high spatial variability but some dominant species would have small low-order zetas (capturing the rare species at each site) and higher late-order zetas (capturing the dominant species in the study area; McGeoch et al. 2019). Data were presence or absence transformed, with rare species (occurring in fewer than three sites) removed before analysis, which did not alter the results notably.
To explore patterns in woody vegetation structure, a range of structural attributes was calculated for each site: stem density, total basal area (BA), shrubiness, canopy height and canopy cover (Table 1). In addition, the total cover of each growth form category in the groundcover stratum (Table 2) and the total cover of each other ground cover (leaf litter, woody debris, logs, rocks) were calculated.
Redundancy analysis (RDA; vegan::rda) was used to analyse patterns in vegetation structure, composition and distribution in canopy and understorey (groundcover and shrub) vegetation strata in relation to environmental variables. RDA combines regression and ordination techniques to explore the constrained relationship between a response and explanatory data matrix (Borcard et al. 2018). Understorey cover category and tree species abundance data were Hellinger transformed, whereas vegetation structure data were zero mean and unit variance standardised before analysis. Species occurring in three sites or fewer were removed to reduce the noise of rare species. To identify environmental variables that explain significant proportions of variation in vegetation structure and species composition, stepwise forward selection (vegan::ordi2step) of zero mean- and unit variance-standardised (vegan::decostand, method = ‘standardize’) numerical environmental variables (factor data not standardised) was conducted, based on a 5% significance level and adjusted r2 of the global model. Environmental variables included for vegetation structure and tree assemblage models are listed in Table 1. Additional variables (tree structural attributes and transformed tree species abundance) were included for the understorey model to investigate the relationship between canopy structure and composition and understorey composition, as well as the influence of environmental variables. Collinearity between forward-selected variables was explored with the variance inflation factor (VIF) where variables were removed if the VIF was >10. Selected variables were then used to explore their contribution to variance in species composition through RDA, the significance of which was tested with 1000 permutations. The results of RDA were extracted and plotted with ggplot2, where sites were plotted as points, numerical environmental variables were plotted as vectors and factorial environmental variables were plotted as points. The length and direction of environmental vectors reflect their importance to the ordination axes and each other (i.e. vectors in opposite directions are negatively correlated; Borcard et al. 2018).
Results
Floristic composition of CFWs in SEQ
During the surveys, 243 vascular plant species were identified (Table S1). Of these species, 130 are native to SEQ, 46 are considered exotic but naturalised in the region (i.e. introduced but established and widespread) and 9 are listed as invasive species in the Biosecurity Act 2014 (Qld). The remaining 67 species could not be identified to a sufficient taxonomic resolution to determine their endemicity (Table S1). Forbs were the most prevalent growth form, followed by tree, grass and sedges (Table 3). Cyperaceae, Poaceae, Myrtaceae, Asteraceae and Fabaceae were the most speciose families (Table 3). Approximately 40% of all species were only observed in one site, whereas only 8% occurred in more than 10 sites. Two species of significant conservation value were recorded: Melaleuca irbyana (endangered) and Samadera bidwillii (vulnerable; Nature Conservation Act 1992 (Qld)).
|
Understorey vegetation was present at all sites encompassing 178 species, 20% of which were wetland indicator taxa. Perennial herbs were the most common life form, with Lomandra longifolia, Ageratum houstonianum and Commelina sp. observed in most sites. Perennial sedges and grasses were also common, with Cyperus polystachyos and Paspalum distichum the most widely distributed species. Commonly represented families in the understorey included Cyperaceae, Poaceae and Asteraceae (Table 3). In total, 52% of understorey species were native, with 21.6% considered naturalised and seven species of these were invasive. The mean (±s.d.) species richness of the understorey was 10.6 ± 4.1 species per site, with low similarity between sites and a mean of one species shared between any two sites (mean ± s.d., ζ2 1.1 ± 1.3).
Shrubs occurred in 35 sites, comprising 29 species, of which 10 were native perennials and 6 were wetland indicator taxa. Nineteen families were represented in the shrub stratum, the most common being Proteaceae, Solanaceae, Cyperaceae and Apocynaceae (Table 3). Shrub species cover varied between sites, with some rarer species having high local densities (e.g. Alpinia caerulea, Banksia oblongifolia) whereas widespread species occurred at lower densities (e.g. Lantana camara). The mean species richness for sites with shrubs was low (mean ± s.d., 1.2 ± 1.0 species per site), with similarity between sites also low (i.e. <1 species shared between any two sites; mean ± s.d., ζ2 0.16 ± 0.42).
Trees occurred in all but 2 sites and comprised 56 species and 18 families. Where trees were not observed, sites were still considered woody due to dense shrub layers of Banksia robur. The typical wetland species Melaleuca quinquenervia and Lophostemon suaveolens were the most abundant and widespread trees. Other typical wetland species, such as Casuarina glauca and Melaleuca linariifolia, had constrained distributions but were relatively abundant locally. Eucalyptus tereticornis and Alphitonia excelsa were also widespread. Two invasive tree species, namely Celtis sinensis and Schinus terebinthifolius, occurred at isolated sites. Mean tree species richness per site (mean ± s.d., 3.3 ± 1.8) was lower than understorey richness but greater than shrub richness. Approximately one tree species was shared by any two sites (mean ± s.d., ζ2 0.99 ± 0.94).
Vegetation structure
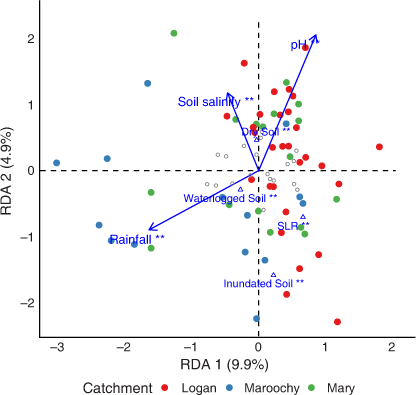
A significant relationship between vegetation structure and environmental variables (F = 2.65, P = 0.001) explained ~26% of variation in the dataset. The first axis explained ~10% of variation (F = 6.99, P = 0.001) in vegetation structure between sites and was correlated with an annual rainfall gradient (Fig. 2; Table 4). Axis 2 explained 5% of the variation and was correlated with a soil salinity and soil pH gradient (Fig. 2; Table 4). Annual rainfall explained ~4% of variation in vegetation structure and captured an additional moisture gradient in Maroochy and Mary sites. Soil pH showed a strong negative correlation to annual rainfall and explained ~16.3% of variation, largely associated with variation in Logan sites.
|
Vegetation composition
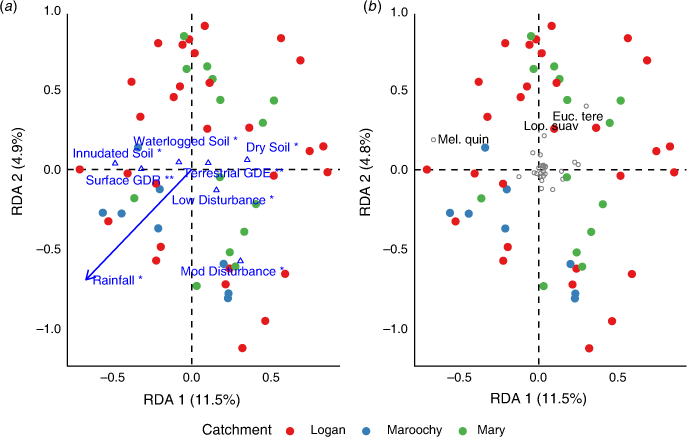
There was a significant relationship between understorey (shrub and groundcover) vegetation composition and selected environmental variables (F = 1.834, P = 0.001), which was represented by the first four significant axes (P ≤ 0.05). Approximately 32% of the variation in understorey vegetation was explained by forward-selected environmental variables. Axis 1 explained 6.6% of variation (F = 4.56, P = 0.001), which represented a gradient of soil moisture (Fig. 3b; Table 4). The occurrence of M. quinquenervia in the canopy was also negatively correlated with Axis 1 (Fig. 3a). Axis 2 explained 5.3% of the constrained variation (F = 3.65, P = 0.001) and was correlated with a stem density gradient where typical ‘wallum’ wetland sites occurred on the negative second axis (Fig. 3; Table 4). Canopy species L. suaveolens and M. linariifolia were positively correlated with Axis 2. Axis 3 explained 3.6% of variation and represented an east–west spatial gradient and captured variation associated with dry soils and the occurrence of M. linariifolia. Axis 4 explained 2.9% of variation with which inundated soils and L. suaveolens were correlated (Table 4).
For tree composition, a significant relationship between species composition and environmental variables (F = 2.13, P = 0.001) explained ~28% of variation largely explained in the first axis. Axis 1 explained 11.5% of variation (F = 7.96, P = 0.001), whereas Axis 2 explained 4.9% of variation (F = 3.43, P = 0.098). Environmental variables were largely correlated with Axis 1, representing a soil moisture gradient (Fig. 4a; Table 4). Most tree species were centred in the ordination plot, but M. quinquenervia occurred in areas with wetter soils (inundated and waterlogged) and where groundwater was expressed at the surface. E. tereticornis tended to occur in areas where annual rainfall was low.
Discussion
Diversity of CFW vegetation communities in SEQ
CFWs in SEQ support diverse vegetation communities, with over 200 species observed, mostly within the understorey. Over two-thirds of species are native to SEQ, but the naturalised species A. houstonianum (i.e. non-native but widespread with minimal ecological or economic threat) and invasive species L. camara (i.e. non-native and threatening to environment, industry and health) were also common (Batianoff and Butler 2002). Distinct species assemblages were apparent in each stratum, with only a handful of species observed in multiple strata, but rarely within the same site. Species richness varied throughout the region with little similarity between sites (low zeta diversity). Zoete (2001) noted similar variability in species composition and vegetation structure, with distinct vegetation strata, in M. quinquenervia wetlands of southern SEQ.
Local distinctiveness of species assemblages, overall high diversity and the presence of two species of ecological significance (i.e. M. irbyana and S. bidwillii; Nature Conservation Act 1992 (Qld)) highlight the conservation significance of CFWs in SEQ. Many of the surveyed CFWs occurred in small patches along waterway corridors or depressions and in close proximity to urban and agricultural land uses, which are known to influence vegetation composition through changes in water quality and soil chemistry (Akasaka et al. 2010; Fierro et al. 2017). We did not detect a strong influence of surrounding land use on vegetation composition and structure of CFWs, but strong influences of hydrology (annual rainfall, soil moisture, groundwater dependence) and soil salinity (Table S2) suggest potentially high sensitivity to projected changes in climate and associated SLR.
Variation and drivers of CFW vegetation structure and composition
Vegetation structure varied in relation to annual rainfall, soil moisture, soil pH and soil salinity, the latter being a potential indicator of natural salinity, saline intrusion or tidal influence. Larger diameters and taller trees occurred in sites within projected SLR inundation areas, whereas grasses were dominant and tree stands were more dense in high-salinity soils. Many studies have described gradients in vegetation structure and composition along tidal gradients, as well as observations in the variability of stem densities of trees, dominant understorey growth form and the abundance of seedlings in TFFWs in the eastern US (Baldwin 2007; Anderson et al. 2013; Johnson and Simenstad 2015; Liu et al. 2017). In these wetlands, dense stands of smaller trees tend to occur in tidal areas (Anderson et al. 2013). Keith and Scott (2005) also note denser stands of melaleucas and eucalypts in disturbed or regrowth areas of coastal floodplain wetlands in New South Wales, Australia. This concurs with our findings, suggesting that increasing disturbance and salinity with rising sea levels could threaten the large, old trees and lead to a period of woody thickening in CFWs.
The composition of understorey vegetation was highly variable, but assemblages differed broadly among sites dominated by P. distichum and Persicaria decipiens and those dominated by L. longifolia (Fig. 3). Importantly, soil moisture, groundwater-dependent areas and soil salinity emerged as key drivers of understorey vegetation distribution. Drier soils were associated with higher densities of L. longifolia, Commelina sp. and Ipomoea cairica. The abundance of dominant tree species may also influence understorey composition, with a high abundance of M. quinquenervia associated with high understorey cover of wetland species P. decipiens and Juncus sp. Tree stem density was also associated with understorey variation, particularly in ‘wallum’ sites in which trees were rare and, instead, supported a dense shrub stratum, commonly comprising B. robur and Leptospermum liversidgei.
Tree species composition was less variable than that of shrub and understorey assemblages, with patterns largely associated with annual rainfall and disturbance. Communities dominated by E. tereticornis and L. suaveolens tended to occur in areas of low annual rainfall, whereas M. quinquenervia was associated with more inundated soils. Disturbance-affected sites tended to be similar in tree composition but showed no indicative species.
Clear clustering of CFW vegetation communities was not apparent in SEQ and a large proportion of variation remains unexplained. However, a hydrological–salinity gradient explains some patterns in vegetation structure and composition. TFFWs in the southern US are similarly structured along a tidal gradient of differing salinity and flooding (Mitsch and Gosselink 2015c), reflected by variation in vegetation structure, species richness and diversity and tree regeneration (Baldwin 2007; Krauss et al. 2009; Anderson et al. 2013; Liu et al. 2017). Within subtropical Australia, dominant CFW canopy species shift along landward elevational gradients, reflecting changes in salinity and flooding (Grieger et al. 2019). For example, the structure of Melaleuca swamp forests in northern Australia differs with the duration of seasonal flooding, with denser canopies generally occurring in areas that are inundated for up to 8 months (Finlayson et al. 1989). Further, C. glauca typically occurs in saline areas, whereas M. quinquenervia occurs in freshwater areas but can tolerate some salinity (Keith and Scott 2005).
Projected climate change impacts
Climate change will significantly alter the composition and distribution of CFWs globally, both directly through changes to temperature and rainfall and indirectly through SLR, salinisation of groundwaters and associated shoreline retreat (Grieger et al. 2020). In SEQ, SLR is ~2.4 mm year–1 (Lovelock et al. 2011), with sea levels expected to rise up to 0.87 m by 2090 (Dowdy et al. 2015). This change in hydrology will likely result in the migration of salt-tolerant understorey species landward, into areas of CFW, particularly species that can regenerate from stem fragments (i.e. Phragmites australis, Sporobolus virginicus) and persist within the soil seed bank (Kottler and Gedan 2020). The greater disturbance and extreme events likely with climate change will further favour these species. In TFFWs of the US, transitions of freshwater wetland to saltmarsh have been widely observed in response to saline intrusion and local SLR (Williams et al. 1999; Desantis et al. 2007; Doyle et al. 2007; Krauss et al. 2009; Langston et al. 2017; Kirwan and Gedan 2019). This transition typically influences groundcover vegetation more rapidly than the canopy because trees tend to exhibit greater tolerance of flooding and salinity but have reduced capacity to regenerate under saline conditions (Krauss et al. 2009; Anderson et al. 2013). As a result, successive disturbance events (e.g. storms, further saline intrusion) can trigger the death of the canopy, resulting in a ghost forest of standing dead trees and a salt marsh understorey (Grieger et al. 2019; Kirwan and Gedan 2019). Similar responses have been observed in coastal Melaleuca forests of northern Australia, where Traditional Owners and scientists attribute the forest dieback to increased salinity from rising sea levels and the effects of feral ungulates (Bowman et al. 2010; Sloane et al. 2019).
Communities most likely to be influenced by SLR in SEQ are those with a dominant M. quinquenervia canopy, because these are associated with groundwater-dependent areas and lie within the extent of projected SLR (Fig. 4). Palaeoecological findings from northern Australia suggest that freshwater wetlands were replaced by mangrove forests during the early Holocene SLR (Woodroffe et al. 1985). Current CFW environments then developed in areas previously occupied by mangrove forest as sea levels declined and stabilised (Clark and Guppy 1988; Rowe 2007; Mackenzie et al. 2020). Tolerance of mature CFW trees can also be inferred from the distribution of CFWs along the east coast of Australia, where casuarina wetlands typically occur in more saline areas, closer to the coast, whereas melaleuca wetlands are generally associated with ‘fresher’ areas (Keith and Scott 2005). Although seedlings of M. quinquenervia can tolerate periods of increased salinity or flooding, mortality can occur when such conditions are prolonged (Grieger et al. 2019). Although the mature tree community may tolerate some degree of SLR, regeneration and recruitment are very likely to be constrained, adding to the vulnerability of these communities and progression into ghost forests (Kirwan and Gedan 2019).
Access to groundwater is important for many wetlands, particularly those in coastal areas, providing a key freshwater resource for those not receiving regular rainfall or overland flow inputs (Winter 1999), although very little is known about groundwater hydrology in many CFWs. Salinisation of groundwater and incursion of marine groundwater into fresh coastal aquifers can occur with reduced freshwater inputs (e.g. during drought or with increased freshwater extraction), which has been observed as a precursor to overland saline inundation. Groundwater salinisation will continue to occur with climate change-induced drought and SLR, with the potential to affect more wetlands than direct inundation by SLR alone (Costall et al. 2020a, 2020b). The effects of groundwater salinisation on CFW vegetation communities are similar to those of SLR, which negatively affects vegetation through reduced growth rates, productivity, regeneration and tolerance to flooding (Williams et al. 1999; Grieger et al. 2020). Trees, which are generally more tolerant than understorey species to surface water salinisation by retrieving water from fresh groundwater, can act as early warning systems of groundwater salinisation and SLR. This has been observed as mass tree dieback in many regions of the US (Langston et al. 2017; Kirwan and Gedan 2019). However, some CFW tree species (e.g. Melaleuca and bald cypress) can tolerate short-term groundwater changes by altering their root function to draw freshwater from the surface and can draw water from deeper when the water table recedes (Mensforth and Walker 1996; Hsueh et al. 2016), although this adaptive function would be impeded with the sustained groundwater salinisation and reduced freshwater surface flows that are projected with climate change.
Annual rainfall patterns will be strongly affected by warming in subtropical regions under climate change, leading to longer droughts, heavier precipitation events and stronger but less frequent cyclones (Hoegh-Guldberg et al. 2018). Drought has an overall negative effect on TFFW vegetation, manifested through reduced recruitment, tree mortality, increased litter production and lower primary productivity (Williams et al. 2003; Hoeppner et al. 2008). Reduced freshwater inputs during drought can lead to periods of saline intrusion in ground and surface waters, causing further reductions in productivity and recruitment, and greater mortality as vegetation becomes salt and water stressed (Williams et al. 2003; Hoeppner et al. 2008). Zoete (2001) also noted reduced height and stem density of M. quinquenervia in areas of sandy soil that undergo periodic water stress. As freshwater scarcity worsens, it is possible that species that are drought and salinity tolerant may come to dominate CFW communities. However, it is likely that SLR poses a more immediate threat to CFWs as salinity-tolerant communities migrate into newly inundated freshwater areas.
Fire has been noted as a key driver of vegetation structure and composition in CFWs, particularly in northern Australia and the south-east US (Turner et al. 1997; Franklin et al. 2007). Although fire was not significant in describing vegetation patterns in the present study, annual rainfall was important, where declines in rainfall patterns, projected with climate change, are likely to increase the threat of fire. Zoete (2001) suggests that greater fire frequencies in SEQ Melaleuca forests will favour species able to regenerate and produce seeds quickly (e.g. annual grass species and trees like M. quinquenervia; Turner et al. 1997). However, frequent fire can be detrimental to the regeneration of tolerant species, particularly when return fires occur before seedlings mature, leading to mortality (Myers et al. 2001). Further, more frequent fires can limit the release and recruitment of seeds and epicormic regeneration of some species (e.g. Banksia ericifolia and Sprengelia sprengelioides), altering vegetation structure and composition (Griffith et al. 2003; Myerscough and Clarke 2007). Invasive species may benefit from the increased disturbance associated with fire, particularly in areas where native vegetation regeneration is affected by SLR. Conversely, fire has been used as an effective management technique for controlling invasive para grass (Urochloa mutica) in tropical floodplain wetlands of northern Queensland (Grice and Nicholas 2011). Projected climate changes (increased temperatures, reduced rainfall) will produce more frequent fire conditions that can further alter vegetation patterns, but this interaction requires further investigation.
Under projected climate changes, it is unlikely that CFWs would face each of these stressors in isolation, and combined effects will be more stressful and damaging. The effects of multiple climate stressors on CFWs have received little research attention (see Grieger et al. 2020), but should be addressed to better understand how climate change will affects these wetlands and how best to manage them.
Adaptation options
CFWs are increasingly recognised for their provision of many highly valuable ecosystem services, including nutrient cycling (Ensign et al. 2008; Hopfensperger et al. 2009; Von Korff et al. 2014), coastal storm buffering (Middleton 2016a), carbon sequestration (Loomis and Craft 2010; Tran and Dargusch 2016; Krauss et al. 2018; Adame et al. 2020) and the provision of habitat for threatened species (Mitsch and Gosselink 2015c; Taillie et al. 2019). Climate change poses a significant threat to these ecosystems and the services they provide, particularly through shifting key drivers shown here to be important determinants of vegetation structure and composition (i.e. salinity, hydrology, drought). It can be anticipated that CFWs will not persist in their current state and distribution as rising sea levels push saline coastal wetlands inland (Boon 2012; Kirwan and Gedan 2019). Landward migration of saline wetlands has been observed globally for many years (Morris et al. 2002; Enwright et al. 2016; Borchert et al. 2018), but there is little information to suggest that CFWs will be able to similarly migrate at a rate that is sufficient to keep pace with SLR (Grieger et al. 2020). Further, there is generally little available area with the specific hydrological and geological conditions that favour CFWs to migrate into, because they are often the most landward of coastal wetland ecosystems (Torio and Chmura 2013).
CFWs, particularly in densely populated coastal regions, are commonly cleared for urban development because they are seen as ‘weedy swamps’ and do not experience tidal fluctuations (Greenway 1998; Novoa et al. 2020). Salt marshes in eastern Australia have species diversities comparable to CFWs (Boon et al. 2015), but in the US salt marshes have much lower diversity (Weilhoefer et al. 2013; Janousek and Folger 2014). Australian mangrove forests, although nationally diverse (at least 41 species), are regionally dominated by a few species that commonly form monotypic stands and exhibit distinct species zonation (Snedaker 1982). The present study has shown that CFWs in SEQ have a diverse species composition, largely composed of native species, and are relatively unique from wetland to wetland, suggesting that the loss of even small patches of CFWs could constitute a significant loss of local biodiversity.
To sustain biodiversity, function and ecosystem service provision of CFWs under a changed climate, significant management interventions are likely to be required. One option is to provide space for wetland migration through the expansion of protected areas that currently contain CFWs (Shoo et al. 2014) or the reversion of agricultural lands that were historically CFWs. The reversion of agricultural lands has been successful for tidal freshwater wetland restoration in the US (for examples, see Baldwin et al. 2019) and is currently underway in areas of Queensland (Waltham et al. 2019). Monitoring of restored areas is important to understand trajectories of change and inform ongoing management actions.
To understand the effects of projected climate change on CFW vegetation, particularly outside the US, further research is required into the tolerances and migration potential of CFW vegetation, at both a species and community level, and to a range of relevant scenarios (e.g. saline flooding, storm surge events, warming). The effects of climate warming and associated influences of drought and disturbances have received little research attention (Grieger et al. 2020), but are identified here as key drivers of vegetation composition. One possible management action could be the provision of environmental flows during drought periods to offset water and salinity stress. The management of a single aspect of climate change may be suitable in the short term (e.g. to deal with immediate effects of SLR), but the synergistic nature of climate drivers requires an adaptive and holistic approach. Grieger et al. (2020) highlight the importance of long-term, field-based observational and manipulative research for understanding the ‘real-life’ effects of climate change to CFWs, but they suggest that experimentation and modelling methods can better capture the multiplicative effects of multiple climate drivers, where particular focus should be given to CFWs outside the US.
Here, we have provided one of the first descriptions of CFW vegetation for the SEQ bioregion and have highlighted the importance of rainfall, salinity and groundwater as drivers of vegetation composition and structure. This study can be used as a baseline from which future research, adaptation and management decisions can be drawn, expanding the knowledge around CFWs and the impacts of climate change.
Data availability statement
Data and the R code used to generate the results in this paper are available upon reasonable request to the corresponding author.
Conflicts of interest
Samantha Capon is an Associate Editor for Marine and Freshwater Research but did not at any stage have access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this Journal. Marine and Freshwater Research encourages its editors to publish in the Journal and they are kept totally separate from the decision-making processes for their manuscripts. The authors have no further conflicts of interest to declare.
Declaration of funding
This project was funded under an Australian Government Research Training Program Scholarship and the Griffith University Higher Degree Research student support funding as part of the Ph.D. candidature of R. Grieger.
Acknowledgements
We acknowledge traditional owners of the land where this work was conducted and pay our respects to their elders, past, present and emerging. The authors thank M. Millington, E. Henderson, P. Zivec, E. Grieger and many volunteers for their assistance with fieldwork, C. Pratt for assistance with soil analysis and S. Price for assistance with mapping.
References
Adame, M. F., Reef, R., Wong, V. N. L., Balcombe, S. R., Turschwell, M. P., Kavehei, E., Rodríguez, D. C., Kelleway, J. J., Masque, P., and Ronan, M. (2020). Carbon and nitrogen sequestration of melaleuca floodplain wetlands in tropical Australia. Ecosystems 23, 454–466.| Carbon and nitrogen sequestration of melaleuca floodplain wetlands in tropical Australia.Crossref | GoogleScholarGoogle Scholar |
Akasaka, M., Takamura, N., Mitsuhashi, H., and Kadono, Y. (2010). Effects of land use on aquatic macrophyte diversity and water quality of ponds. Freshwater Biology 55, 909–922.
| Effects of land use on aquatic macrophyte diversity and water quality of ponds.Crossref | GoogleScholarGoogle Scholar |
An, S., and Verhoeven, J. T. A. (2019). Wetland functions and ecosystem services: Implications for wetland restoration and wise use. In ‘Wetlands: Ecosystem Services, Restoration and Wise Use’. (Eds S. An and J. T. A. Verhoeven.) pp. 1–10. (Springer International Publishing: Cham, Switzerland.)
Anderson, C. J., Lockaby, B. G., and Click, N. (2013). Changes in wetland forest structure, basal growth, and composition across a tidal gradient. American Midland Naturalist 170, 1–13.
| Changes in wetland forest structure, basal growth, and composition across a tidal gradient.Crossref | GoogleScholarGoogle Scholar |
Baldwin, A. H. (2007). Vegetation and seed banks studies of salt-pulsed swamps of the Nanticoke River, Chesapeake Bay. In ‘Ecology of Tidal Freshwater Forested Wetlands of the Southeastern United States’. (Eds W. H. Conner, T. W. Doyle, and K. W. Krauss.) pp. 139–160. (Springer: Dordrecht, Netherlands.)
Baldwin, A. H., Hammerschlag, R. S., and Cahoon, D. R. (2019). Evaluating restored tidal freshwater wetlands. In ‘Coastal Wetlands – An Integrated Ecosystem Approach’, 2nd edn. (Eds G. M. E. Perillo, E. Wolanski, D. R. Cahoon, and C. S. Hopkinson.) pp. 889–915. (Elsevier: Amsterdam, Netherlands.)
Batianoff, G. N., and Butler, D. W. (2002). Assessment of invasive naturalised plants in south-east Queensland. Plant Protection Quarterly 17, 27–34.
Bell, D., Menges, C. H., and Bartolo, R. E. (2001). Assessing the extent of saltwater intrusion in a tropical coastal environment using radar and optical remote sensing. Geocarto International 16, 45–52.
| Assessing the extent of saltwater intrusion in a tropical coastal environment using radar and optical remote sensing.Crossref | GoogleScholarGoogle Scholar |
Boon, P. (2012). Coastal wetlands of temperate eastern Australia: will Cinderella ever go to the ball? Marine and Freshwater Research 63, 845–855.
| Coastal wetlands of temperate eastern Australia: will Cinderella ever go to the ball?Crossref | GoogleScholarGoogle Scholar |
Boon, P. I., Allen, T., Carr, G., Frood, D., Harty, C., Mcmahon, A., Mathews, S., Rosengren, N., Sinclair, S., White, M., and Yugovic, J. (2015). Coastal wetlands of Victoria, south-eastern Australia: providing the inventory and condition information needed for their effective management and conservation. Aquatic Conservation 25, 454–479.
| Coastal wetlands of Victoria, south-eastern Australia: providing the inventory and condition information needed for their effective management and conservation.Crossref | GoogleScholarGoogle Scholar |
Borcard, D., Gillet, F., and Legendre, P. (2018). ‘Numerical Ecology with R’, 2nd edn. (Springer: Cham, Switzerland.)
Borchert, S. M., Osland, M. J., Enwright, N. M., and Griffith, K. T. (2018). Coastal wetland adaptation to sea level rise: quantifying potential for landward migration and coastal squeeze. Journal of Applied Ecology 55, 2876–2887.
| Coastal wetland adaptation to sea level rise: quantifying potential for landward migration and coastal squeeze.Crossref | GoogleScholarGoogle Scholar |
Bowman, D. M. J. S., Prior, L. D., and De Little, S. C. (2010). Retreating Melaleuca swamp forests in Kakadu National Park: evidence of synergistic effects of climate change and past feral buffalo impacts. Austral Ecology 35, 898–905.
| Retreating Melaleuca swamp forests in Kakadu National Park: evidence of synergistic effects of climate change and past feral buffalo impacts.Crossref | GoogleScholarGoogle Scholar |
Braun-Blanquet, J. (1932). ‘Plant Sociology.’ (McGraw-Hill: New York, USA.)
Brown, G. K., and Bostock, P. D. (2019). Census of the Queensland flora 2019. (Queensland Department of Environment and Science: Brisbane, Qld, Australia.) Available at https://www.data.qld.gov.au/dataset/census-of-the-queensland-flora-2019
Bureau of Meteorology (2019). Greater Brisbane in 2019: a very warm and dry year. Available at http://www.bom.gov.au/climate/current/annual/qld/brisbane.shtml [Verified 10 February 2020].
Clark, R. L., and Guppy, J. C. (1988). A transition from mangrove forest to freshwater wetland in the monsoon tropics of Australia. Journal of Biogeography 15, 665–684.
| A transition from mangrove forest to freshwater wetland in the monsoon tropics of Australia.Crossref | GoogleScholarGoogle Scholar |
Costall, A. R., Harris, B. D., Teo, B., Schaa, R., Wagner, F. M., and Pigois, J. P. (2020a). Groundwater throughflow and seawater intrusion in high quality coastal aquifers. Scientific Reports 10, 9866.
| Groundwater throughflow and seawater intrusion in high quality coastal aquifers.Crossref | GoogleScholarGoogle Scholar | 32555499PubMed |
Costall, A. R., Harris, B. D., Teo, B., Schaa, R., Wagner, F. M., and Pigois, J. P. (2020b). Publisher Correction: Groundwater throughflow and seawater intrusion in high quality coastal aquifers. Scientific Reports 10, 19088.
| Publisher Correction: Groundwater throughflow and seawater intrusion in high quality coastal aquifers.Crossref | GoogleScholarGoogle Scholar | 33127942PubMed |
Crisp, M. D., and Cummings, D. J. (1977). How to use field note books. Available at https://www.anbg.gov.au/cpbr/herbarium/collecting/field-note-book.html [Verified 10 March 2020].
Department of Environment and Science (2014). Land use mapping – 1999 to 2013 – South East Queensland NRM. (Queensland Department of Environment and Science.)
Desantis, L. R. G., Bhotika, S., Williams, K., and Putz, F. E. (2007). Sea-level rise and drought interactions accelerate forest decline on the Gulf Coast of Florida, USA. Global Change Biology 13, 2349–2360.
| Sea-level rise and drought interactions accelerate forest decline on the Gulf Coast of Florida, USA.Crossref | GoogleScholarGoogle Scholar |
Dowdy, A., Abbs, D., Bhend, J. F. C., Church, J., Ekström, M., Kirono, D., Lenton, A., Lucas, C., McInnes, K., Moise, A., Monselesan, D., Mpelasoka, F., Webb, L., and Whetton, P. (2015). Climate change in Australia, projections for Australia’s natural resource management regions: cluster reports. CSIRO and Bureau of Meteorology, Australia.
Doyle, T. W., O’Neil, C. P., Melder, M. P., From, A. S., and Palta, M. M. (2007). Tidal freshwater swamps of the southeastern United States: effects of land use, hurricanes, sea-level rise, and climate change. In ‘Ecology of Tidal Freshwater Forested Wetlands of the Southeastern United States’. pp. 1–28. (Springer.)
| Crossref |
Ensign, S. H., Piehler, M. F., and Doyle, M. W. (2008). Riparian zone denitrification affects nitrogen flux through a tidal freshwater river. Biogeochemistry 91, 133–150.
| Riparian zone denitrification affects nitrogen flux through a tidal freshwater river.Crossref | GoogleScholarGoogle Scholar |
Environmental Protection Agency (2005). ‘Wetland Mapping and Classification Methodology – Overall Framework – A Method to Provide Baseline Mapping and Classification for Wetlands in Queensland. Version 1.2.’ (Queensland Government: Brisbane, Qld, Australia.)
Enwright, N. M., Griffith, K. T., and Osland, M. J. (2016). Barriers to and opportunities for landward migration of coastal wetlands with sea-level rise. Frontiers in Ecology and the Environment 14, 307–316.
| Barriers to and opportunities for landward migration of coastal wetlands with sea-level rise.Crossref | GoogleScholarGoogle Scholar |
Fierro, P., Bertrán, C., Tapia, J., Hauenstein, E., Peña-Cortés, F., Vergara, C., Cerna, C., and Vargas-Chacoff, L. (2017). Effects of local land-use on riparian vegetation, water quality, and the functional organization of macroinvertebrate assemblages. The Science of the Total Environment 609, 724–734.
| Effects of local land-use on riparian vegetation, water quality, and the functional organization of macroinvertebrate assemblages.Crossref | GoogleScholarGoogle Scholar | 28763669PubMed |
Finlayson, C. M., Bailey, B. J., and Cowie, I. D. (1989). Macrophytic vegetation of the Magela flood plain, Alligator Rivers Region, Northern Territory. Supervising Scientist for the Alligator Rivers Region, Research Report 5. (Australian Government Publishing Service: Canberra, ACT, Australia.) Available at https://www.environment.gov.au/system/files/resources/3ce1901c-7b9b-47ae-a024-8f07c08d0a04/files/rr5.pdf
Franklin, D. C., Brocklehurst, P. S., Lynch, D., and Bowman, D. (2007). Niche differentiation and regeneration in the seasonally flooded Melaleuca forests of northern Australia. Journal of Tropical Ecology 23, 457–467.
| Niche differentiation and regeneration in the seasonally flooded Melaleuca forests of northern Australia.Crossref | GoogleScholarGoogle Scholar |
Greenway, M. (1998). Melaleuca wetlands – wastelands or wonderlands: their benefits, threats and management. In ‘Wetlands in a Dry Land: Understanding for Management’. (Ed. W. D. Williams.) pp. 163–170. (Environment Australia: Canberra, ACT, Australia.)
Grice, T., and Nicholas, M. (2011). Using fire to restore Australian wetlands from invasive grasses. RIRDC Publication number 11/028, Rural Industries Research and Development Corporation, Canberra, ACT, Australia.
Grieger, R., Capon, S., and Hadwen, W. (2019). Resilience of coastal freshwater wetland vegetation of subtropical Australia to rising sea levels and altered hydrology. Regional Environmental Change 19, 279–292.
| Resilience of coastal freshwater wetland vegetation of subtropical Australia to rising sea levels and altered hydrology.Crossref | GoogleScholarGoogle Scholar |
Grieger, R., Capon, S. J., Hadwen, W. L., and Mackey, B. (2020). Between a bog and a hard place: a global review of climate change effects on coastal freshwater wetlands. Climatic Change 163, 161–179.
| Between a bog and a hard place: a global review of climate change effects on coastal freshwater wetlands.Crossref | GoogleScholarGoogle Scholar |
Griffith, S. J., Bale, C., Adam, P., and Wilson, R. (2003). Wallum and related vegetation on the NSW North Coast: description and phytosociological analysis. Cunninghamia 2, 202–252.
Griffith, S. J., Bale, C., and Adam, P. (2004). The influence of fire and rainfall upon seedling recruitment in sand-mass (wallum) heathland of north-eastern New South Wales. Australian Journal of Botany 52, 93–118.
| The influence of fire and rainfall upon seedling recruitment in sand-mass (wallum) heathland of north-eastern New South Wales.Crossref | GoogleScholarGoogle Scholar |
Hatton, T., and Evans, R. (1998). Dependence of ecosystems on groundwater and its significance to Australia. Land and Water Resources Research and Development Corporation, Canberra, ACT, Australia.
Hoegh-Guldberg, O., Jacob, D., Taylor, M., Bindi, M., Brown, S., Camilloni, I., Diedhiou, A., Djalante, R., Ebi, K. L., Engelbrecht, F., Guiot, J., Hijioka, Y., Mehrotra, S., Payne, A., Seneviratne, S. I., Thomas, A., Warren, R., and Zhou, G. (2018). Impacts of 1.5°C global warming on natural and human systems. In ‘Global Warming of 1.5°C. An IPCC Special Report on the Impacts of Global Warming of 1.5°C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty’. (Eds V. Masson-Delmotte, P. Zhai, H.-O. Pörtner, D. Roberts, J. Skea, P. R. Shukla, A. Pirani, W. Moufouma-Okia, C. Péan, R. Pidcock, S. Connors, J. B. R. Matthews, Y. Chen, X. Zhou, M. I. Gomis, E. Lonnoy, T. Maycock, M. Tignor, and T. Waterfield.) pp. 175–311. (IPCC.) Available at https://www.ipcc.ch/site/assets/uploads/sites/2/2019/06/SR15_Chapter3_Low_Res.pdf
Hoeppner, S. S., Shaffer, G. P., and Perkins, T. E. (2008). Through droughts and hurricanes: tree mortality, forest structure, and biomass production in a coastal swamp targeted for restoration in the Mississippi River Deltaic Plain. Forest Ecology and Management 256, 937–948.
| Through droughts and hurricanes: tree mortality, forest structure, and biomass production in a coastal swamp targeted for restoration in the Mississippi River Deltaic Plain.Crossref | GoogleScholarGoogle Scholar |
Hopfensperger, K. N., Kaushal, S. S., Findlay, S. E. G., and Cornwell, J. C. (2009). Influence of plant communities on denitrification in a tidal freshwater marsh of the Potomac River, United States. Journal of Environmental Quality 38, 618–626.
| Influence of plant communities on denitrification in a tidal freshwater marsh of the Potomac River, United States.Crossref | GoogleScholarGoogle Scholar | 19202032PubMed |
Hsueh, Y. H., Chambers, J. L., Krauss, K. W., Allen, S. T., and Keim, R. F. (2016). Hydrologic exchanges and baldcypress water use on deltaic hummocks, Louisiana, USA. Ecohydrology 9, 1452–1463.
| Hydrologic exchanges and baldcypress water use on deltaic hummocks, Louisiana, USA.Crossref | GoogleScholarGoogle Scholar |
Hui, C., and McGeoch, M. A. (2014). Zeta Diversity as a concept and metric that unifies incidence-based biodiversity patterns. American Naturalist 184, 684–694.
| Zeta Diversity as a concept and metric that unifies incidence-based biodiversity patterns.Crossref | GoogleScholarGoogle Scholar |
IPCC (2014). ‘Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change.’ (Eds R. K. Pachauri and L. A. Meyer.) Number 9291691437. (IPCC, Geneva, Switzerland.)
Janousek, C. N., and Folger, C. L. (2014). Variation in tidal wetland plant diversity and composition within and among coastal estuaries: assessing the relative importance of environmental gradients. Journal of Vegetation Science 25, 534–545.
| Variation in tidal wetland plant diversity and composition within and among coastal estuaries: assessing the relative importance of environmental gradients.Crossref | GoogleScholarGoogle Scholar |
Johnson, L. K., and Simenstad, C. A. (2015). Variation in the flora and fauna of tidal freshwater forest ecosystems along the Columbia River Estuary gradient: controlling factors in the context of river flow regulation. Estuaries and Coasts 38, 679–698.
| Variation in the flora and fauna of tidal freshwater forest ecosystems along the Columbia River Estuary gradient: controlling factors in the context of river flow regulation.Crossref | GoogleScholarGoogle Scholar |
Keith, D. A., and Scott, J. (2005). Native vegetation of coastal floodplains – a diagnosis of the major plant communities in New South Wales. Pacific Conservation Biology 11, 81–104.
| Native vegetation of coastal floodplains – a diagnosis of the major plant communities in New South Wales.Crossref | GoogleScholarGoogle Scholar |
Kirwan, M. L., and Gedan, K. B. (2019). Sea-level driven land conversion and the formation of ghost forests. Nature Climate Change 9, 450–457.
| Sea-level driven land conversion and the formation of ghost forests.Crossref | GoogleScholarGoogle Scholar |
Kottler, E. J., and Gedan, K. (2020). Seeds of change: characterizing the soil seed bank of a migrating salt marsh. Annals of Botany 125, 335–344.
| Seeds of change: characterizing the soil seed bank of a migrating salt marsh.Crossref | GoogleScholarGoogle Scholar | 31408516PubMed |
Krauss, K. W., Duberstein, J. A., Doyle, T. W., Conner, W. H., Day, R. H., Inabinette, L. W., and Whitbeck, J. L. (2009). Site condition, structure, and growth of Baldcypress along tidal/non-tidal salinity gradients. Wetlands 29, 505–519.
| Site condition, structure, and growth of Baldcypress along tidal/non-tidal salinity gradients.Crossref | GoogleScholarGoogle Scholar |
Krauss, K. W., Noe, G. B., Duberstein, J. A., Conner, W. H., Stagg, C. L., Cormier, N., Jones, M. C., Bernhardt, C. E., Lockaby, B. G., From, A. S., Doyle, T. W., Day, R. H., Ensign, S. H., Pierfelice, K. N., Hupp, C. R., Chow, A. T., and Whitbeck, J. L. (2018). The role of the upper tidal estuary in wetland blue carbon storage and flux. Global Biogeochemical Cycles 32, 817–839.
| The role of the upper tidal estuary in wetland blue carbon storage and flux.Crossref | GoogleScholarGoogle Scholar |
Langston, A. K., Kaplan, D. A., and Putz, F. E. (2017). A casualty of climate change? Loss of freshwater forest islands on Florida’s Gulf Coast. Global Change Biology 23, 5383–5397.
| A casualty of climate change? Loss of freshwater forest islands on Florida’s Gulf Coast.Crossref | GoogleScholarGoogle Scholar | 28675588PubMed |
Leiper, G., Glazebrook, J., Cox, D., and Rathie, K. (2019). ‘Mangroves to Mountains: a Field Guide to the Native Plants of South-East Queensland’, revised edn. (Society for Growing Australian Plants (Queensland Region), Logan River Branch: Logan, Qld, Australia.)
Liu, X. J., Conner, W. H., Song, B., and Jayakaran, A. D. (2017). Forest composition and growth in a freshwater forested wetland community across a salinity gradient in South Carolina, USA. Forest Ecology and Management 389, 211–219.
| Forest composition and growth in a freshwater forested wetland community across a salinity gradient in South Carolina, USA.Crossref | GoogleScholarGoogle Scholar |
Loomis, M. J., and Craft, C. B. (2010). Carbon sequestration and nutrient (nitrogen, phosphorus) accumulation in river-dominated tidal marshes, Georgia, USA. Soil Science Society of America Journal 74, 1028–1036.
| Carbon sequestration and nutrient (nitrogen, phosphorus) accumulation in river-dominated tidal marshes, Georgia, USA.Crossref | GoogleScholarGoogle Scholar |
Lovelock, C. E., Bennion, V., Grinham, A., and Cahoon, D. R. (2011). The role of surface and subsurface processes in keeping pace with sea level rise in intertidal wetlands of Moreton Bay, Queensland, Australia. Ecosystems 14, 745–757.
| The role of surface and subsurface processes in keeping pace with sea level rise in intertidal wetlands of Moreton Bay, Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Mackenzie, L., Moss, P., and Ulm, S. (2020). A late-Holocene record of coastal wetland development and fire regimes in tropical northern Australia. The Holocene 30, 1379–1390.
| A late-Holocene record of coastal wetland development and fire regimes in tropical northern Australia.Crossref | GoogleScholarGoogle Scholar |
McGeoch, M. A., Latombe, G., Andrew, N. R., Nakagawa, S., Nipperess, D. A., Roigé, M., Marzinelli, E. M., Campbell, A. H., Vergés, A., Thomas, T., Steinberg, P. D., Selwood, K. E., Henriksen, M. V., and Hui, C. (2019). Measuring continuous compositional change using decline and decay in zeta diversity. Ecology 100, e02832.
| Measuring continuous compositional change using decline and decay in zeta diversity.Crossref | GoogleScholarGoogle Scholar | 31323117PubMed |
McKee, M., White, J. R., and Putnam-Duhon, L. A. (2016). Simulated storm surge effects on freshwater coastal wetland soil porewater salinity and extractable ammonium levels: Implications for marsh recovery after storm surge. Estuarine, Coastal and Shelf Science 181, 338–344.
| Simulated storm surge effects on freshwater coastal wetland soil porewater salinity and extractable ammonium levels: Implications for marsh recovery after storm surge.Crossref | GoogleScholarGoogle Scholar |
Mensforth, L. J., and Walker, G. R. (1996). Root dynamics of Melaleuca halmaturorum in response to fluctuating saline groundwater. Plant and Soil 184, 75–84.
| Root dynamics of Melaleuca halmaturorum in response to fluctuating saline groundwater.Crossref | GoogleScholarGoogle Scholar |
Middleton, B. A. (2016a). Differences in impacts of Hurricane Sandy on freshwater swamps on the Delmarva Peninsula, Mid-Atlantic Coast, USA. Ecological Engineering 87, 62–70.
| Differences in impacts of Hurricane Sandy on freshwater swamps on the Delmarva Peninsula, Mid-Atlantic Coast, USA.Crossref | GoogleScholarGoogle Scholar |
Middleton, B. A. (2016b). Effects of salinity and flooding on post-hurricane regeneration potential in coastal wetland vegetation. American Journal of Botany 103, 1420–1435.
| Effects of salinity and flooding on post-hurricane regeneration potential in coastal wetland vegetation.Crossref | GoogleScholarGoogle Scholar | 27539261PubMed |
Mitsch, W. J., and Gosselink, J. G. (2015a). Wetland ecosystem services. In ‘Wetlands’, 5th edn. pp. 527–562. (Wiley: Hoboken, NJ, USA.)
Mitsch, W., and Gosselink, J. G. (2015b). Wetland hydrology. In ‘Wetlands’, 5th edn. pp. 111–161. (Wiley: Hoboken, NJ, USA.)
Mitsch, W., and Gosselink, J. G. (2015c). Tidal marshes: tidal freshwater wetlands. In ‘Wetlands’, 5th edn. pp. 285–309. (Wiley: Hoboken, NJ, USA.)
Morris, J. T., Sundareshwar, P. V., Nietch, C. T., Kjerfve, B., and Cahoon, D. R. (2002). Responses of coastal wetlands to rising sea level. Ecology 83, 2869–2877.
| Responses of coastal wetlands to rising sea level.Crossref | GoogleScholarGoogle Scholar |
Mueller-Dombois, D., and Ellenberg, H. (1974). ‘Aims and Methods of Vegetation Ecology.’ (Wiley: Hoboken, NJ, USA.)
Myers, R. L., Belles, H. A., and Snyder, J. R. (2001). Prescribed fire in the management of Melaleuca quinquenervia in subtropical Florida. In ‘Proceedings of Invasive Species Workshop: Role of Fire in the Control and Spread of Invasive Species; Fire Conference 2000: 1st National Congress on Fire Ecology, Prevention, and Management’, 27 November–1 December 2000, San Diego, CA, USA. (Eds K. E. M. Galley, T. P. Wilson, and P. Tyrone.) pp. 132–140. (Tall Timbers Research Station: Tallahassee, FL, USA.)
Myerscough, P. J., and Clarke, P. J. (2007). Burnt to blazes: landscape fires, resilience and habitat interaction in frequency burnt coastal heath. Australian Journal of Botany 55, 91–102.
| Burnt to blazes: landscape fires, resilience and habitat interaction in frequency burnt coastal heath.Crossref | GoogleScholarGoogle Scholar |
Neubauer, S. C., Franklin, R. B., and Berrier, D. J. (2013). Saltwater intrusion into tidal freshwater marshes alters the biogeochemical processing of organic carbon. Biogeosciences 10, 8171–8183.
| Saltwater intrusion into tidal freshwater marshes alters the biogeochemical processing of organic carbon.Crossref | GoogleScholarGoogle Scholar |
Nicholls, R. J. (2004). Coastal flooding and wetland loss in the 21st century: changes under the SRES climate and socio-sconomic scenarios. Global Environmental Change 14, 69–86.
| Coastal flooding and wetland loss in the 21st century: changes under the SRES climate and socio-sconomic scenarios.Crossref | GoogleScholarGoogle Scholar |
Novoa, V., Rojas, O., Ahumada-Rudolph, R., Saez, K., Fierro, P., and Rojas, C. (2020). Coastal wetlands: ecosystems affected by urbanization? Water 12, 698.
| Coastal wetlands: ecosystems affected by urbanization?Crossref | GoogleScholarGoogle Scholar |
Queensland Department of Environment and Heritage Protection (2013). ‘Coastal Hazard Technical Guide: Determining Coastal Hazard Areas.’ (Queensland Government: Brisbane, Qld, Australia.)
Queensland Department of Science (2015). Queensland groundwater dependent ecosystem mapping method: a method for providing baseline mapping of groundwater dependent ecosystems in Queensland. Queensland Department of Science, Information Technology and Innovation, Brisbane, Qld, Australia.
Queensland Government Statistician’s Office (2019). Queensland regional profiles: resident profile for SEQ region. Available at https://statistics.qgso.qld.gov.au/qld-regional-profiles?report-type=RES®ion-type=LGA-2016®ion-ids=19711,19713,19730,19731,19732,19734,19737,19743,19750,19755,19762,19765,19766,19769&custom-name=SEQ®ion-type-comp=S [Verified 15 May 2020].
Rowe, C. (2007). Vegetation change following mid-Holocene marine transgression of the Torres Strait shelf: a record from the island of Mua, northern Australia. Holocene 17, 927–937.
| Vegetation change following mid-Holocene marine transgression of the Torres Strait shelf: a record from the island of Mua, northern Australia.Crossref | GoogleScholarGoogle Scholar |
Schuerch, M., Spencer, T., Temmerman, S., Kirwan, M. L., Wolff, C., Lincke, D., McOwen, C. J., Pickering, M. D., Reef, R., Vafeidis, A. T., Hinkel, J., Nicholls, R. J., and Brown, S. (2018). Future response of global coastal wetlands to sea-level rise. Nature 561, 231.
| Future response of global coastal wetlands to sea-level rise.Crossref | GoogleScholarGoogle Scholar | 30209368PubMed |
Shoo, L. P., O’Mara, J., Perhans, K., Rhodes, J. R., Runting, R. K., Schmidt, S., Traill, L. W., Weber, L. C., Wilson, K. A., and Lovelock, C. E. (2014). Moving beyond the conceptual: specificity in regional climate change adaptation actions for biodiversity in South East Queensland, Australia. Regional Environmental Change 14, 435–447.
| Moving beyond the conceptual: specificity in regional climate change adaptation actions for biodiversity in South East Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Sloane, D. R., Ens, E., Wunungmurra, J., Falk, A., Marika, G., Maymuru, M., Towler, G., Preece, D., the Yirralka Rangers (2019). Western and Indigenous knowledge converge to explain Melaleuca forest dieback on Aboriginal land in northern Australia. Marine and Freshwater Research 70, 125–139.
| Western and Indigenous knowledge converge to explain Melaleuca forest dieback on Aboriginal land in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Snedaker, S. C. (1982). Mangrove species zonation: why? In ‘Contributions to the Ecology of Halophytes’. pp. 111–125. (Springer.)
Taillie, P. J., Moorman, C. E., Smart, L. S., and Pacifici, K. (2019). Bird community shifts associated with saltwater exposure in coastal forests at the leading edge of rising sea level. PLoS One 14, e0216540.
| Bird community shifts associated with saltwater exposure in coastal forests at the leading edge of rising sea level.Crossref | GoogleScholarGoogle Scholar | 31071148PubMed |
Tate, A. S., and Battaglia, L. L. (2013). Community disassembly and reassembly following experimental storm surge and wrack application. Journal of Vegetation Science 24, 46–57.
| Community disassembly and reassembly following experimental storm surge and wrack application.Crossref | GoogleScholarGoogle Scholar |
Tobias, C., and Neubauer, S. C. (2019). Salt marsh biogeochemistry – an overview. In ‘Coastal Wetlands – an Integrated Ecosystem Approach’, 2nd edn. (Eds G. M. E. Perillo, E. Wolanski, D. R. Cahoon, and C. S. Hopkinson.) pp. 539–597. (Elseiver: Amsterdam, Netherlands.)
Torio, D. D., and Chmura, G. L. (2013). Assessing coastal squeeze of tidal wetlands. Journal of Coastal Research 290, 1049–1061.
| Assessing coastal squeeze of tidal wetlands.Crossref | GoogleScholarGoogle Scholar |
Tran, D. B., and Dargusch, P. (2016). Melaleuca forests in Australia have globally significant carbon stocks. Forest Ecology and Management 375, 230–237.
| Melaleuca forests in Australia have globally significant carbon stocks.Crossref | GoogleScholarGoogle Scholar |
Turner, C. E., Center, T. D., Burrows, D. W., and Buckingham, G. R. (1997). Ecology and management of Melaleuca quinquenervia, an invader of wetlands in Florida, USA. Wetlands Ecology and Management 5, 165–178.
| Ecology and management of Melaleuca quinquenervia, an invader of wetlands in Florida, USA.Crossref | GoogleScholarGoogle Scholar |
Von Korff, B. H., Piehler, M. F., and Ensign, S. H. (2014). Comparison of denitrification between river channels and their adjoining tidal freshwater wetlands. Wetlands 34, 1047–1060.
| Comparison of denitrification between river channels and their adjoining tidal freshwater wetlands.Crossref | GoogleScholarGoogle Scholar |
Waltham, N. J., Burrows, D., Wegscheidl, C., Buelow, C., Ronan, M., Connolly, N., Groves, P., Marie-Audas, D., Creighton, C., and Sheaves, M. (2019). Lost floodplain wetland environments and efforts to restore connectivity, habitat, and water quality settings on the Great Barrier Reef. Frontiers in Marine Science 6, 71.
| Lost floodplain wetland environments and efforts to restore connectivity, habitat, and water quality settings on the Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Weilhoefer, C. L., Nelson, W. G., Clinton, P., and Beugli, D. M. (2013). Environmental determinants of emergent macrophyte vegetation in Pacific Northwest estuarine tidal wetlands. Estuaries and Coasts 36, 377–389.
| Environmental determinants of emergent macrophyte vegetation in Pacific Northwest estuarine tidal wetlands.Crossref | GoogleScholarGoogle Scholar |
WetlandInfo (2020a). Southeast Queensland (SEQ) bioregion – facts and maps. Available at https://wetlandinfo.des.qld.gov.au/wetlands/facts-maps/bioregion-southeast-queensland-seq/ [Verified 7 May 2020].
WetlandInfo (2020b). Flora Wetland Indicator Species List. Available at https://wetlandinfo.des.qld.gov.au/wetlands/ecology/components/flora/flora-indicator-species-list.html [Verified 7 May 2020].
Wickham, H. (2016). ‘ggplot2: Elegant Graphics for Data Analysis.’ (Springer-Verlag New York: New York, USA)
Wickham, H., Averick, M., Bryan, J., Chang, W., McGowan, L. D., François, R., Grolemund, G., Hayes, A., Henry, L., Hester, J., Kuhn, M., Pedersen, T. L., Miller, E., Bache, S. M., Müller, K., Ooms, J., Robinson, D., Seidel, D. P., Spinu, V., Takahashi, K., Vaughan, D., Wilke, C., Woo, K., and Yutani, H. (2019). Welcome to the tidyverse. Journal of Open Source Software 4, 1686.
| Welcome to the tidyverse.Crossref | GoogleScholarGoogle Scholar |
Williams, K., Ewel, K. C., Stumpf, R. P., Putz, F. E., and Workman, T. W. (1999). Sea-level rise and coastal forest retreat on the west coast of Florida, USA. Ecology 80, 2045–2063.
| Sea-level rise and coastal forest retreat on the west coast of Florida, USA.Crossref | GoogleScholarGoogle Scholar |
Williams, K., MacDonald, M., and Sternberg, L. D. L. (2003). Interactions of storm, drought, and sea-level rise on coastal forest: A case study. Journal of Coastal Research 19, 1116–1121.
Williams, T., Amatya, D., Conner, W., Panda, S., Xu, G., Dong, J., Trettin, C., Dong, C., Gao, X., Shi, H., Yu, K., and Wang, H. (2019). Tidal forested wetlands: mechanisms, threats, and management tools. In ‘Wetlands: Ecosystem Services, Restoration and Wise Use’. (Eds S. An and J. T. A. Verhoeven.) pp. 129–158. (Springer International Publishing: Cham, Switzerland.)
Winter, T. C. (1999). Relation of streams, lakes, and wetlands to groundwater flow systems. Hydrogeology Journal 7, 28–45.
| Relation of streams, lakes, and wetlands to groundwater flow systems.Crossref | GoogleScholarGoogle Scholar |
Woodroffe, C. D., Thom, B. G., and Chappell, J. (1985). Development of widespread mangrove swamps in mid-Holocene times in northern Australia. Nature 317, 711–713.
| Development of widespread mangrove swamps in mid-Holocene times in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Zoete, T. (2001). Variation in the vegetation of Melaleuca quinquenervia dominated forested wetlands of the Moreton region. Plant Ecology 152, 29–57.
| Variation in the vegetation of Melaleuca quinquenervia dominated forested wetlands of the Moreton region.Crossref | GoogleScholarGoogle Scholar |