Population demographics of golden perch (Macquaria ambigua) in the Darling River prior to a major fish kill: a guide for rehabilitation
Brenton P. Zampatti A B N , Benjamin G. Fanson
A B N , Benjamin G. Fanson  C , Lee J. Baumgartner
C , Lee J. Baumgartner  D , Gavin L. Butler
D , Gavin L. Butler  E , Steven G. Brooks F , David A. Crook
E , Steven G. Brooks F , David A. Crook  G , Katherine Doyle
G , Katherine Doyle  D , Alison J. King
D , Alison J. King  G , Wayne M. Koster
G , Wayne M. Koster  C , Roland Maas
C , Roland Maas  H , Aleksey Sadekov M , Peter Scott
H , Aleksey Sadekov M , Peter Scott  I , Arron Strawbridge B , Jason D. Thiem
I , Arron Strawbridge B , Jason D. Thiem  D J , Zeb Tonkin
D J , Zeb Tonkin  C , Phillipa J. Wilson
C , Phillipa J. Wilson  B K , Jon Woodhead
B K , Jon Woodhead  H and Ryan Woods L
H and Ryan Woods L
A CSIRO Land and Water, Locked Bag 2, Glen Osmond, SA 5064, Australia.
B Inland Waters and Catchment Ecology Program, South Australian Research and Development Institute (SARDI) – Aquatic Sciences, PO Box 120, Henley Beach, SA 5022, Australia.
C Arthur Rylah Institute for Environmental Research, Department of Environment, Land, Water and Planning, Heidelberg, Melbourne, Vic. 3084, Australia.
D Institute for Land, Water and Society, Charles Sturt University, PO Box 789, Albury, NSW 2640, Australia.
E Department of Primary Industries, Grafton Fisheries Centre, Grafton, NSW 2460, Australia.
F Electrofishing Services, Wamuran, Qld 4512, Australia.
G Centre for Freshwater Ecosystems, La Trobe University, Wodonga, Vic. 3689, Australia.
H School of Geography, Earth and Atmospheric Sciences, The University of Melbourne, Vic. 3010, Australia.
I Centre for Microscopy, Characterisation and Analysis, The University of Western Australia, Perth, WA 6009, Australia.
J Department of Primary Industries, Narrandera Fisheries Centre, Narrandera, NSW 2700, Australia.
K Australian Institute of Marine Science, Indian Ocean Marine Research Centre, The University of Western Australia (M096), Perth, WA 6009, Australia.
L Department of Environment and Science, Ecosciences Precinct, GPO Box 5078, Brisbane, Qld 4001, Australia.
M Ocean Graduate School, The University of Western Australia, Perth, WA 6009, Australia.
N Corresponding author. Email: brenton.zampatti@csiro.au
Marine and Freshwater Research 73(2) 223-236 https://doi.org/10.1071/MF21033
Submitted: 31 January 2021 Accepted: 16 July 2021 Published: 2 September 2021
Journal Compilation © CSIRO 2022 Open Access CC BY-NC-ND
Abstract
An understanding of population demographics and life history processes is integral to the rehabilitation of fish populations. In Australia’s highly modified Murray–Darling Basin, native fish are imperilled and fish deaths in the Darling River in 2018–19 highlighted their vulnerability. Golden perch (Macquaria ambigua) is a long-lived percichthyid that was conspicuous in the fish kills. To guide population rehabilitation in the Darling River, pre-fish kill age structure, provenance and movement of golden perch were explored using otolith microstructure and chemistry (87Sr/86Sr). Across the Lower and Mid-Darling River, recruitment was episodic, with dominant cohorts associated with years characterised by elevated discharge. There was substantial variability in age structure, recruitment source and movement patterns between the Lower and Mid-Darling River. In the Mid-Darling River, tributaries were an important recruitment source, whereas in the Lower Darling fish predominantly originated in the Darling River itself. Downstream movement of juveniles, upstream migration of adults and return movements to natal locations were important drivers of population structure. Restoring resilient golden perch populations in the Darling River will be reliant on mitigating barriers to movement, promoting a connected mosaic of recruitment sources and reinstating the hydrological and hydraulic factors associated with spawning, recruitment and dispersal. Globally, increasing water resource development and climate change will necessitate such integrated approaches to the management of long-lived migratory riverine fishes.
Keywords: dryland river, migration, Murray–Darling Basin, otolith chemistry, river regulation, strontium isotopes.
Introduction
The ecological integrity of many of the world’s rivers is compromised by water resource development for hydropower and consumptive use (Best 2019). Dams and other regulating structures fragment habitats and alter hydrological regimes, leading to species decline and biodiversity loss (Poff et al. 2007; Grill et al. 2015). This is exacerbated by climate change and a range of other human disturbances (Kingsford 2011; Arnell and Gosling 2013). In light of these effects, hydromorphological restoration to rehabilitate the health of riverine ecosystems is becoming more prevalent (Murray–Darling Basin Authority 2014; Kail et al. 2015; Schmutz et al. 2016).
The effective management and rehabilitation of rivers is reliant on knowledge of ecosystem function and form (Ward et al. 2001; Lake 2005). As the increasing prevalence of extreme climatic events causes further degradation, including fish kills, an understanding of biological mechanisms, as well as patterns, is essential to guide the adaptive management of rivers (Tonkin et al. 2019). Knowledge of demographic structure before catastrophic events can provide a template for species rehabilitation and be used to develop management objectives and targets (Kondolf 1995). To this end, natural baselines can be unrealistic (Stanford et al. 1996; Dufour and Piégay 2009), and contemporary or Anthropocene baselines may provide a more representative surrogate (Humphries and Winemiller 2009; Kopf et al. 2015). Simultaneously, there is a need for robust understanding of life histories and the processes that influence population and community dynamics, and their relationships with hydrology (Arthington et al. 2006; Humphries et al. 2020). This will also help identify potential lags in recovery due to variability among taxa and species driven by longevity and life history traits (Thompson et al. 2018).
For riverine fish populations, successful management and rehabilitation require detailed knowledge of the spatial scale of life history processes, including how recruitment source, connectivity and movement influence population demographics (Fausch et al. 2002; Shenton et al. 2012; Cooke et al. 2016). Management interventions can directly affect these drivers of population structure. For example, barriers to migration that interrupt connectivity may be mitigated with fishways (Lucas and Baras 2001). Globally, however, there remains a paucity of data on the population structures and life history processes of many riverine fishes (Reynolds et al. 2005; Cooke et al. 2012).
Australia’s Murray–Darling Basin (MDB) is typical of many river systems worldwide where river regulation, in the form of altered flow regimes and dams and weirs, has caused substantial declines in native fish populations (Barrett 2004). Although the effects of river regulation are widespread across the MDB, they came to the fore in the Darling River in 2018–19 when drought and overextraction of water caused hypoxic conditions that resulted in the deaths of millions of fish (Vertessy et al. 2019), garnering national and worldwide attention (Normile 2019). Fish deaths occurred primarily in a 40-km reach of the Darling River between two regulating structures, but upstream and downstream reaches also received little to no flow over the preceding 18 months (Mallen-Cooper and Zampatti 2020). A range of fish species was conspicuous in the 2018–19 fish kills, including golden perch (Macquaria ambigua) (Thiem et al. 2021).
Golden perch are distributed broadly across the lowland rivers of the MDB (Lintermans 2007). In the Darling River, the species has provided a food source for Aboriginal people for tens of thousands of years (Balme 1995), once supported a commercial fishery (Reid et al. 1997), and remains a popular angling species (Forbes et al. 2015b). Golden perch are a long-lived (~27 years; Forbes et al. 2015a) potamodromous fish that exhibit a periodic life history strategy (sensu Winemiller and Rose 1992). Consequently, like other wide-ranging riverine fishes that exhibit episodic recruitment, golden perch are susceptible to flow regulation and barriers to movement, which ultimately reduce demographic resilience (Winemiller 2005; Olden et al. 2006; Zampatti 2019). In the MDB, altered flow regimes and fragmentation are considered primary causes of declines in golden perch populations and reductions in their range (Cadwallader 1978; Baumgartner et al. 2006; Shams et al. 2020). To arrest this decline and rehabilitate populations, enhancing reproduction, recruitment and movement of golden perch are key objectives for flow management and environmental water allocations throughout the MDB (Koehn et al. 2014; King et al. 2016; Koster et al. 2017).
For rehabilitation programs to be successful, knowledge of population demographics and drivers of population change is essential (Cooke et al. 2012). In the MDB, rehabilitation of native fish populations, including golden perch, constitutes a focus of contemporary and emerging fish management initiatives, particularly following the 2018–19 fish kills in the Darling River (MDBA 2020). Consequently, an understanding of pre-fish kill population demographics of golden perch in the Darling River, and the spatiotemporal characteristics of population processes (recruitment, movement) that influence these, is urgently needed to guide rehabilitation and subsequently assess effectiveness.
In this study, otoliths from golden perch collected in the Darling River in years before the 2018–19 fish kill were used to determine population age structure and the effect of recruitment source and riverine connectivity on population demographics. To reconcile spatial aspects of key life history processes, otolith chemistry was used as a natural tag. Specifically, the delineation in dissolved strontium isotope ratios (87Sr/86Sr) in water samples from across rivers of the MDB (Douglas et al. 1995; Zampatti 2019) was related to otolith 87Sr/86Sr to reconstruct natal origin and lifetime movements. The specific objectives of this study were to: (1) characterise the age structure of golden perch in the Lower and Mid-Darling River; (2) examine 87Sr/86Sr in otolith cores in relation to water 87Sr/86Sr across rivers of the MDB to determine fish provenance; (3) measure 87Sr/86Sr along transects from otolith core to edge to reconstruct the movement history of fish; and (4) compare age structures, natal source and movement histories between fish from the Lower and Mid-Darling River. By integrating knowledge on demographic patterns and underlying population processes, we provide an insight into the factors needed to rehabilitate golden perch populations in the Darling River. The approach used is also applicable to the management of other imperilled long-lived and wide-ranging riverine fishes, particularly in the face of continuing water resource development and climate change.
Methods
Study region
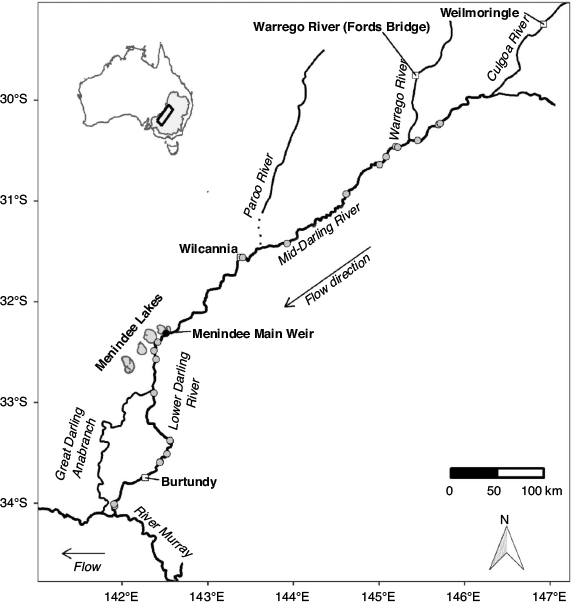
The MDB drains 1 073 000 km2 (17% of Australia’s landmass; Fig. 1). The combined length of the two major rivers, the Murray and the Darling, is ~5500 km, and both flow through predominantly semi-arid and arid landscapes. In their natural states, the Murray and Darling rivers were characterised by highly variable hydrological regimes (Walker et al. 1995; Puckridge et al. 1998), yet they also exhibited distinct but predictable hydrological and hydraulic characteristics, such as seasonal flow pulses and largely perennial lotic habitats (Mallen-Cooper and Zampatti 2018, 2020).
|
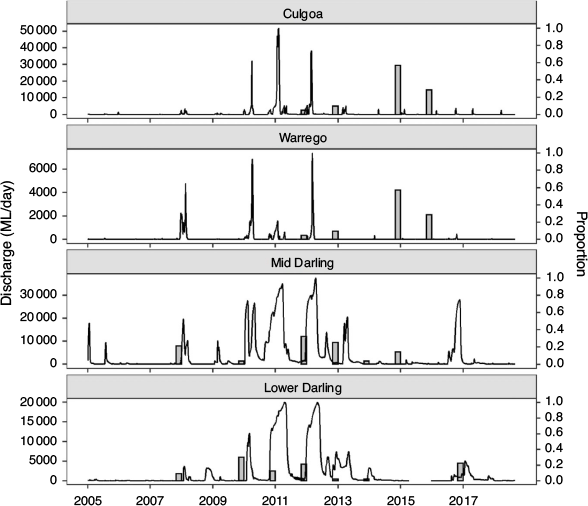
This study focused on golden perch populations in the mid- and lower reaches of the Barwon–Darling river system, which drains the northern river catchments of the MDB (~650 000 km2) and converges with the River Murray ~825 km upstream from the river mouth. The mid-reach of the Barwon–Darling (hereafter the Mid-Darling River) extends south-west for ~900 km from the confluence of the Culgoa River to the Menindee Lakes (Fig. 1); an extensive series of off-channel lakes (457 km2; capacity 1731 GL) used to store and re-regulate water for consumptive use. The Mid-Darling River incorporates the Warrego and Paroo rivers to the west (Fig. 1), although, on average, these catchments only contribute 5% of annual flow to the Darling River, principally from the Warrego River (Thoms et al. 2004). The Lower Darling River extends for ~500 km in a generally southerly direction from the Menindee Lakes to its confluence with the River Murray (Fig. 1).
The hydrological, hydraulic and geomorphological characteristics of the Darling River have been profoundly altered by river regulation in the form of headwater dams, weirs, direct extraction and floodplain harvesting (Thoms et al. 2004; Pearson et al. 2020). Flows in the Mid-Darling River are reduced by ~70%, and much of the river is fragmented and hydraulically modified by in-stream weirs (Thoms and Sheldon 2000; Mallen-Cooper and Zampatti 2020). Along the Mid- and Lower Darling River, there are at least nine substantial instream weirs, ranging from ~2 to 4.5 m high (New South Wales Department of Primary Industries 2006, 2012). Fish passage infrastructure is incorporated into some weirs, particularly those in the Lower Darling River (Baumgartner et al. 2014), but, for those without fishways, upstream fish passage is not feasible until the weirs are drowned-out at discharges ranging from ~2500 to 10 000 ML day–1 (New South Wales Department of Primary Industries 2006, 2012). In addition, the Menindee Lakes Storage Scheme (MLSS), which delineates the Lower and Mid-Darling River, comprises several weirs, regulators, levees and channels, and includes the Menindee Main Weir (Fig. 1), which has a head differential of ~12 m and does not incorporate fish passage infrastructure.
Hydrology
To relate river discharge to recruitment across the age range of golden perch collected in this study, daily mean flow (ML day–1) for the period from 2005 to mid-2018 was obtained for the Lower Darling River at Burtundy (gauge number 425007), Mid-Darling River at Wilcannia (gauge number 425008), Warrego River at Ford’s Bridge (gauge number 423001) and Culgoa River at Weilmoringle (gauge number 422017; Fig. 1). For all gauges, data were sourced from WaterNSW (https://realtimedata.waternsw.com.au/).
Sampling golden perch
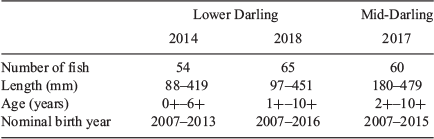
Adult and juvenile golden perch were collected in the Lower Darling River in April 2014 and May 2018 and in the Mid-Darling River in December 2017 as components of broader investigations of golden perch population dynamics in the MDB (see Fig. 1 and Table S1 in the Supplementary material). Fish were sampled by boat electrofishing using a 5- or 7.5-kW Smith Root (Model GPP 5 or 7.5) electrofishing unit during daylight hours, and all littoral and channel habitats were fished. All fish (n = 201) were measured to the nearest millimetre (total length, TL) and subsamples of individuals (n = 54–65), proportionally representing the length frequency of golden perch collected, were retained for ageing and otolith chemistry analysis.
Ageing
Ageing methods previously validated for golden perch (Anderson et al. 1992; Mallen-Cooper and Stuart 2003; Zampatti and Leigh 2013) were used to establish the annual age of each individual and birth year. Briefly, golden perch retained for ageing were euthanased in the field by AQUI-S overdose (AQUI-S, Lower Hutt, New Zealand) and sagittal otoliths were removed. Whole otoliths were embedded in clear casting resin and a single transverse 400- to 600-μm section that incorporated the core was prepared from each otolith. Sections were examined using a dissecting microscope (25×) under transmitted light. Estimates of age were determined independently by three readers by counting the number of discernible opaque zones (annuli) from the primordium to the otolith edge and integrating this information with otolith edge type and capture date. A nominal birth date of 1 October was assigned to each individual. Young-of-year (YOY; <1 year old) fish were defined as individuals lacking clearly discernible annuli.
Otolith chemistry analysis
Otolith strontium isotope ratios (87Sr/86Sr) were used as geochemical signatures to reconstruct the natal origin and lifetime movements of golden perch. This approach has previously been used to discern the provenance and movements of golden perch in the MDB (Zampatti 2019).
Otolith 87Sr/86Sr was determined in a subsample of individuals of known age (n = 43–50), proportionally representing the age frequency of golden perch collected in each region and time period. Using the second otolith of aged fish, an additional transverse 400- to 600-μm section, incorporating the otolith core, was prepared from each otolith. Individual transverse sections were mounted collectively on ‘master’ slides using a thin layer of casting resin, and each section was polished using wetted lapping film (9 µm). Master slides were rinsed in Milli-Q water (Millipore) and air dried overnight in a Class 100 laminar flow cabinet at room temperature.
Laser ablation inductively coupled plasma mass spectrometry
Laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) was used to measure 87Sr/86Sr in the otoliths of golden perch. Otoliths collected in the two different time periods (2014 and 2017–18) were analysed in different facilities using the same methodology, generally following Woodhead et al. (2005).
2014 Lower Darling fish
Analysis was undertaken by The University of Melbourne Isotope Geochemistry Laboratory. The experimental system consisted of a Nu Plasma multicollector LA-ICP-MS (Nu Instruments, Wrexham, UK) coupled to an Australian Scientific Instruments (now Applied Spectra) RESOlution laser ablation system constructed around a COMPex 110 excimer laser (Lambda Physik, Göttingen, Germany) operating at 193 nm.
Otolith mounts were placed in the sample cell and the primordium of each otolith was located visually with a 400× objective and a video imaging system. The intended ablation path on each sample was then digitally plotted using GeoStar software (ver. 6.14). Each otolith was ablated along a transect from the primordium to the dorsal margin at the widest radius using a 6- × 100-μm rectangular laser slit. The laser system was operated with a fluence of 2–3 J cm–2 measured at the sampling site, with a repetition rate of 10 Hz and scan speed of 5 or 10 μm s–1 (depending on the size of the otolith) across the sample.
Corrections for interferences and instrumental mass bias were performed within the iolite software environment (Paton et al. 2011), following protocols outlined in Woodhead et al. (2005). A marine carbonate standard with the isotope composition of modern seawater was run repeatedly throughout the analytical session and, using a spline fit passing through these standard analyses, all unknowns were corrected to the accepted value for modern seawater (~0.709160; e.g. Farrell et al. 1995). Raw values for the marine carbonate were typically within ±50 ppm from the accepted value, and thus the maximum corrections made to the 87Sr/86Sr ratio of the unknowns were of this order.
2017–18 Lower and Mid-Darling fish
Analyses were undertaken by the Advanced Geochemical Facility for Indian Ocean Research at the University of Western Australia. The experimental system consisted of a Thermo Scientific NEPTUNE Plus multicollector ICP-MS coupled with a Teledyne Analyte G2 excimer laser ablation system. Otolith mounts were placed in the sample cell and the primordium of each otolith was located visually with a 400× objective and a video imaging system. The intended ablation path on each sample was then digitally plotted. Otoliths were ablated along a transect from the primordium to the dorsal margin using a 25- × 100-μm rectangular laser slit. The laser was pulsed at 10 Hz and scanned at 10 μm s–1 across the sample. Standardisation and Rb correction were done following Woodhead et al. (2005), and external reproducibility was assessed using three in-house carbonate standards with mean ± 2 s.d. 87Sr/86Sr ratios of 0.709176 ± 0.000025 (n = 20), 0.70592 ± 0.00012 (n = 21) and 0.713017 ± 0.000028 (n = 20). The accuracy for the standards measurements was –22, 27 and 2 ppm and was estimated as an offset between measured values and true values obtained using thermo-ionisation mass spectroscopy.
Natal origin assignment and movement history
As part of a previous study, a Bayesian mixing isotope model (BMIM) was developed to map water 87Sr/86Sr in rivers across the MDB (Zampatti et al. 2019). The BMIM used a similar moving average structure to the spatial stream network model used by Brennan et al. (2016) by incorporating the branching structure of the rivers. The BMIM was based on 726 water samples that were collected between 2011 and 2018 across the MDB and analysed for 87Sr/86Sr ratios and concentrations. The BMIM is temporally static and incorporates temporal variation into the residual error structure (Zampatti et al. 2019).
An assignment algorithm was also developed, based on a modified version of the Bayesian assignment approach used by Brennan and Schindler (2017), for estimating the probability that non-transitory sections of otolith 87Sr/86Sr profiles are from each river section (Zampatti et al. 2019). Briefly, this assignment algorithm first divides otolith 87Sr/86Sr profiles into unique sections using a regression tree approach. Working backwards from the capture location, the algorithm assigns a probability of each stationary section of the 87Sr/86Sr profile based on the 87Sr/86Sr for that section, the estimated mean 87Sr/86Sr (and variance) for each river and the distance of the river section from the current location. The final stationary section, closest to the otolith core, represents the fish’s spatial (natal) origin (for more details, see Zampatti et al. 2019).
Because artificially propagated golden perch are stocked into the Lower and Mid-Darling River, the algorithm includes an additional option for a potential hatchery origin. Water 87Sr/86Sr was obtained for each hatchery that potentially stocks fish into the Darling River (see Zampatti et al. 2019). If a fish had a distinct movement in 87Sr/86Sr between 100 and 800 µm from the otolith core, representing the time period over which a hatchery fish may be released into the wild, then the algorithm checked whether 87Sr/86Sr during this period matched that of a hatchery. If this occurred, then fish were classified as stocked.
Results
Population structure
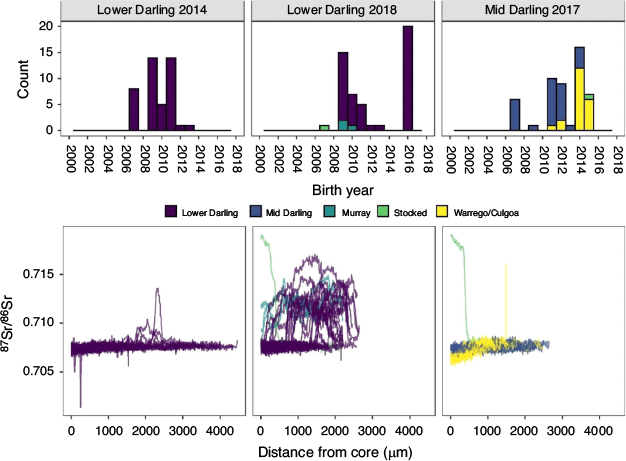
In 2014, golden perch collected from the Lower Darling River ranged in length from 88 to 419 mm and from age-0+ to age-6+, corresponding to birth years from 2007 to 2013 (Table 1; Fig. 2). The most prominent cohorts were 2009 and 2011 (65% of the sampled population; Fig. 2). In 2018, golden perch collected in the Lower Darling River ranged in length from 97 to 451 mm and from age-1+ to age-10+, corresponding to birth years from 2007 to 2016 (Table 1; Fig. 2). The 2009 cohort remained dominant in the 2018 sample, but the 2011 cohort had diminished (Fig. 2). In addition, in 2018, a cohort of age-1+ fish, representing a 2016 birth year, accounted for 40% of the sampled population (Fig. 2).
|
|
Golden perch collected in the Mid-Darling River in 2017 ranged in length from 180 to 479 mm and from age-2+ to age-10+, corresponding to birth years from 2007 to 2015 (Table 1; Fig. 2). The sampled population was characterised by five distinct cohorts: 2007, 2011, 2012, 2014 and 2015, of which the 2014 cohort was the most prominent (32% of fish collected; Fig. 2). Across both regions and in all years, no golden perch older than age-10+ were collected.
Natal origin
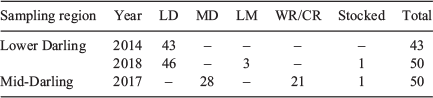
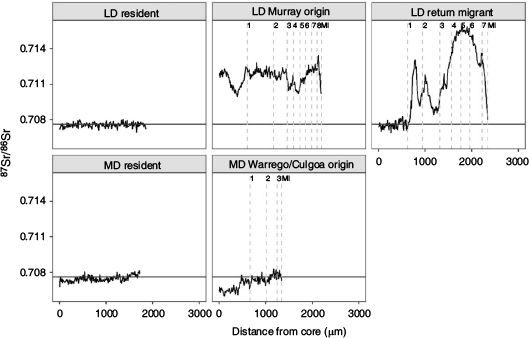
Individual 87Sr/86Sr profiles revealed the Darling River was the predominant natal origin of golden perch collected from the Lower Darling River in 2014 (100%) and 2018 (92%), with the remainder of fish collected in 2018 exhibiting either a Lower River Murray (6%) or hatchery (2%) origin (Table 2; Fig. 2). For golden perch collected from the Mid-Darling River in 2017, 56% had a Darling River natal origin and 42% exhibited distinct natal 87Sr/86Sr signatures representative of the Warrego or Culgoa rivers (Table 2; Fig. 2). In particular, the prominent 2014 and 2015 cohorts in the Mid-Darling River primarily exhibited Warrego or Culgoa river origins (Fig. 2). The natal 87Sr/86Sr signature for these fish ranged from 0.7063 to 0.7068, which is lower than fish assigned to the Mid-Darling River (Fig. 2). The Warrego and Culgoa rivers have distinctly lower water 87Sr/86Sr (mean ± s.e.m. 0.7065 ± 0.0002 and 0.7062 ± 0.0004 respectively) than the Mid-Darling River (0.7077 ± 0.0002), but similarities in water 87Sr/86Sr between the Warrego and Culgoa rivers preclude differentiation between these rivers as a recruitment source. In the Mid-Darling River in 2017, golden perch of hatchery origin accounted for 2% of fish collected (Table 2; Fig. 2).
|
Integrating age and natal origin enabled association of hydrological characteristics at the time and place of birth. Where golden perch originated in the Lower or Mid-Darling River, these cohorts were generally associated with overbank or within-channel flow pulses in the year of birth (Fig. 3), noting that spawning may occur throughout spring–autumn and therefore may straddle calendar years. In the Mid-Darling River, the 2014 and 2015 cohorts that predominantly exhibited a Warrego–Culgoa river natal origin (Fig. 2) were associated with either relatively minor flow events in the Warrego River that were interspersed in otherwise long periods (months–years) of cease-to-flow or, in the Culgoa River, with regular annual within-channel flow pulses (Fig. 3).
|
Movement
Golden perch collected in the Lower Darling River in 2014 and 2018 exhibited three lifetime movement patterns: (1) lifelong residency in the Darling River; (2) a Lower River Murray natal origin and immigration into the Lower Darling River; and (3) a Darling River natal origin, emigration to the Lower River Murray early in life (age-0+ or age-1+) and a return migration to the Lower Darling later in life (age-6+–8+; Fig. 4). In 2014, 93% of golden perch collected from the Lower Darling were lifelong residents and 7% were return migrants (see Fig. S1 in the Supplementary material). In 2018, excluding one fish of hatchery origin, 50% of fish were lifelong residents, 42% were return migrants born in the Darling River and 6% had migrated into the Darling after being born in the Lower Murray (see Fig. S2 in the Supplementary material).
|
In 2017, golden perch collected from the Mid-Darling River exhibited two distinct life time movement patterns: (1) lifelong residency in the Mid-Darling River; and (2) a Warrego–Culgoa river natal origin and movement into the Mid-Darling River early in life (age-0+; Fig. 4). Excluding one fish of hatchery origin, 56% of fish were lifelong residents and 42% were immigrants from tributaries (see Fig. S3 in the Supplementary material).
Discussion
In rivers affected by anthropogenic alteration and climate change, knowledge of demographic patterns and underlying population processes is essential to guide the effective management of fish populations (Ward et al. 2001). In the years preceding the 2018–19 fish kills in the Darling River, there was substantial variability in age structure, recruitment source and movement patterns between samples of golden perch collected from the Lower and Mid-Darling River. In the Mid-Darling River, tributaries were an important recruitment source, whereas in the Lower Darling River fish predominantly originated in the Darling River. Furthermore, variation in lifetime movement patterns between the regions potentially demonstrates the effects of barriers to migration on population structure. These findings suggest that restoring a diversity of recruitment sources, and hydrological and physical connectivity throughout the Darling River, will be critical to promoting golden perch population growth and resilience.
Age structure
Golden perch populations in the Darling River in 2014 and 2017–18 (before the fish kills) were generally characterised by episodic recruitment and variation in age structure between the Lower and Mid-Darling River. The dominance of specific age classes was most marked in the Lower Darling River where, in 2014, the sampled population comprised two distinct cohorts: 2009 and 2011. By contrast, the sampled population of golden perch in the Mid-Darling River was characterised by greater age structure diversity. Across both regions, however, fish >10 years old were absent. For a long-lived fish, the absence of golden perch aged >10 years was unexpected, particularly when individuals 10–25 years old are relatively common in the adjoining River Murray (Zampatti et al. 2018).
Episodic recruitment is common in long-lived freshwater fishes with periodic life histories, where strong cohorts may punctuate periods of poor recruitment (Winemiller 2005). Populations of long-lived fish, like golden perch, can generally withstand these periods of recruitment failure, often caused by adverse climatic conditions (e.g. drought), because of the reproductive potential of strong cohorts (Secor 2007). However, the apparent absence of fish older than age-10+ in the Lower and Mid-Darling River and, in the case of the Lower Darling River, a lack of age structure diversity potentially render these populations susceptible to environmental perturbations (Berkeley et al. 2004).
Various factors could account for the truncated golden perch age structure, including a lack of recruitment, mortality and movement (Wright et al. 2020). Golden perch recruitment is generally associated with periods of increased flow through spring and summer (Mallen-Cooper and Stuart 2003; Zampatti and Leigh 2013). A lack of recruitment in the Darling River before 2007 could be the result of the pervasive impacts of the Millennium Drought across the MDB, leading to long periods of low or no flow (van Dijk et al. 2013; Mallen-Cooper and Zampatti 2020). Cease-to-flow periods in the Darling River may also have increased mortality by concentrating fish in isolated water holes, leading to predation, competition for resources or death from hypoxic or drying events (Balcombe et al. 2006; Close et al. 2014). In addition, when flow resumed, resulting in increased connectivity, fish may have migrated upstream or downstream to alternative reaches, thus restructuring populations.
This study provides a useful comparison for the demographic representativeness of golden perch collected during the 2018–19 fish kills, where an absence of older fish was also apparent (Thiem et al. 2021). In both studies, the 2009 cohort dominated, demonstrating the long-term persistence of this cohort in the Darling River, which has also contributed widely to the golden perch population in the River Murray (Zampatti et al. 2018). Nevertheless, the fish kill sample contained few fish from the 2016 cohort, which dominated in the Lower Darling River in early 2018. Either this cohort had diminished by the summer of 2018–19, when the fish kills occurred, or these juvenile fish were less susceptible to hypoxia, being smaller and having lower oxygen demands (Glencross and Felsing 2006). If these smaller, younger fish survived the hypoxic events and remain present across the ~500 km of the Lower Darling River, they may convey some resilience to golden perch populations in this region.
Natal origin
Although age structures provide insight into population demographics, there is also a need to develop a mechanistic understanding of the effects of population processes (spawning, recruitment and movement) on observed patterns (Gowan and Fausch 1996; Wohl et al. 2005). This includes knowledge of recruitment sources and fish movement, and the effects of flow and connectivity. The Darling River was the predominant (92–100%) natal source of golden perch collected in the Lower Darling River. By contrast, in the Mid-Darling River, a large proportion (42%) of fish, particularly from the 2014 and 2015 cohorts, which were absent from the Lower Darling River, exhibited natal 87Sr/86Sr signatures representative of the Warrego or Culgoa rivers, tributaries of the Darling River. The remaining fish collected in the Mid-Darling River were predominantly of Mid-Darling River origin. Despite stocking of hatchery reared golden perch into the Darling River, pre-fish kill populations of golden perch showed little influence of stocking on population structure. The effectiveness of golden perch stocking is variable among rivers of the MDB (Crook et al. 2016; Thiem et al. 2017). As such, in rivers like the Darling, which demonstrate strong evidence of natural recruitment, a more effective approach to restoration than stocking may be to provide hydrological conditions that support in situ recruitment and connectivity to a diversity of spatially distinct recruitment sources.
Worldwide, there is growing recognition of the potential importance of tributaries to the population dynamics of riverine fish in flow-regulated rivers (Rice et al. 2008; Pracheil et al. 2013; Koster et al. 2021). Tributaries that preserve the hydrological and hydraulic conditions that promote spawning and recruitment may subsequently provide a source of recruits to flow-affected main channel habitats (Pollux et al. 2006; Webber et al. 2013). As such, preserving or reinstating the hydrological, hydraulic and habitat characteristics of tributary recruitment sources is crucial, as is enabling connectivity between tributary and main-stem rivers (Koster et al. 2014). In the Darling River system, a host of tributaries, particularly those with relatively intact flow regimes, may serve as recruitment sources for golden perch (Rolls et al. 2013; Stuart and Sharpe 2020). Furthermore, Mallen-Cooper and Zampatti (2020) highlight the opportunity to increase the diversity of potential fish recruitment sources by rehabilitating the hydrology and hydraulics of flow-affected tributaries in the Barwon–Darling river system.
The absence of tributary-derived recruits in the Lower Darling River, in particular the 2014 and 2015 cohorts, suggests that in the time frame represented in this study (2007–18), golden perch originating in the Warrego or Culgoa rivers did not make a detectable contribution to the Lower Darling River. Furthermore, the distinctive Warrego–Culgoa 87Sr/86Sr signature has not previously been observed in fish collected from the River Murray and its tributaries (Zampatti et al. 2019). It may be that fish from these regions do not disperse widely, possibly due to local-scale adaptions and migration patterns, or that only low numbers of fish may disperse large distances (Comte and Olden 2018). Nevertheless, golden perch eggs, larvae and juveniles are known to disperse hundreds of kilometres downstream along river systems (Stuart and Sharpe 2020; Zampatti et al. 2021). Consequently, it is likely that sequential barriers to movement along the Darling River, including aspects of the MLSS, particularly the Menindee Main Weir, may inhibit the downstream movement of golden perch at various life stages. The MLSS does not form an impenetrable barrier to all life stages, as demonstrated by the collection of juvenile golden perch dispersing downstream from the lakes (e.g. Stuart and Sharpe 2020). However, the regulating structures associated with the MLSS, and those upstream, may constitute enough of a barrier to result in distinct age structures and recruitment sources between population contingents in the Lower and Mid-Darling River.
In the Lower Darling River in 2018, the golden perch age structure included prominent 2009 and 2016 cohorts. Fish from these cohorts demonstrated a Darling River natal origin and, in the case of the 2009 Darling-derived cohort, also contributed to populations in the River Murray (Zampatti et al. 2021). These age-classes, however, were largely absent from the Mid-Darling River. This was despite this region experiencing high flows in these years, like the Lower Darling River, and the collection of early life stage golden perch, with a proposed origin in the northern tributaries of the Barwon–Darling river system, in association with these flows (Stuart and Sharpe 2020). The fate of these cohorts in the Mid-Darling River remains unresolved, but anthropogenic fragmentation of the river system may be a contributing factor to the disparate population structures among regions.
In the Lower Darling River, there was also a minor influence of the River Murray as a recruitment source, but these fish were absent from the Mid-Darling River, again indicating that barriers such as the Menindee Main Weir may inhibit the upstream movement of golden perch. This effect is likely to be substantial in years of low to medium flow, which are frequent in the Darling River (Thoms et al. 2004), but may be ameliorated during occasional large floods when bidirectional connectivity is reinstated past major barriers in the system. Indeed, a study of golden perch genetics by Faulks et al. (2010) found that high-flow events in arid rivers promote recruitment and connectivity that increase effective population size and genetic diversity. Consequently, mitigating the effects of Menindee Main Weir and other weirs on fish movement presents a key management priority for the Barwon–Darling river system to promote greater age structure diversity and resilience in golden perch populations, and potentially other wide-ranging species, such as silver perch Bidyanus bidyanus (Thiem et al. 2021).
Integrating otolith chemistry and age enables consideration of the spatial and temporal provenance of cohorts and associated environmental conditions, including flow (Campana and Thorrold 2001). In the Mid- and Lower Darling River, distinct cohorts of golden perch were related to recruitment events in natal locations that coincided with overbank or in-channel flow pulses. These findings concur with the hydrological characteristics documented in a range of golden perch recruitment studies (Mallen-Cooper and Stuart 2003; Roberts et. al. 2008; Zampatti and Leigh 2013). Nevertheless, golden perch also exhibits life history flexibility across its geographic range and, in some intermittently flowing rivers of the MDB, recruitment has also been documented in cease-to-flow periods (Balcombe et al. 2006). In the present study, however, fish originating under this hydrological scenario did not appear to contribute to golden perch populations in the Darling River.
For fishes with periodic life histories, river regulation and climate (e.g. drought) can diminish age structure diversity and decrease population resilience (Winemiller 2005; Olden et al. 2006). In the present study, the year-class strength of golden perch was low when flows were low and stable or non-existent. In particular, age structures reflect the profound effect of the Millennium drought (2001–09), punctuated by one recruitment event in 2007, and the more recent drought (2013–19) in the Darling River catchment. However, in the Mid-Darling River, the effects of drought and flow regulation on age structure appear to have been partially buffered by cohorts derived from tributary rivers in association with in-channel flow pulses. These findings demonstrate that the preservation of even low-magnitude flow events in tributaries may confer improved population resilience to main-stem fish populations, if tributaries are hydrologically and physically connected to the main river. They also reinforce the need to protect the natural hydrological characteristics of arid, predominantly unregulated rivers like the Warrego to preserve ecological function (Kingsford 2000). From a management perspective, understanding the characteristics of flow associated with spawning, recruitment and movement in source tributaries could promote the efficacious use of finite environmental water resources (Poff et al. 2003).
Movement
This study indicates a diversity of lifetime movement patterns for golden perch in the Darling River with some variation between the mid- and lower reaches of the river. In the Mid-Darling River, immigration of YOY recruits from the Warrego or Culgoa rivers was a strong driver of population structure, but birth and lifelong residency in the Mid-Darling River was also prominent. Similarly, lifelong residency was commonplace in the Lower Darling River, particularly in fish collected in 2014. However, in 2018, a large proportion (46%) of fish exhibited a more complex lifetime movement pattern, where fish were born in the Darling River, moved downstream to the Lower Murray, commonly at age-0+ to age-1+, and then returned to the Lower Darling River later in life. The expression of alternative movement behaviours within a population has individual- and population-level impacts (Barthel et al. 2008). Movement may beneficially expose fish to suitable habitats and environmental conditions for growth and reproduction, but may also increase the incidence of individual mortality (Alerstam et al. 2003). For example, golden perch that move among rivers or regions of the MDB exhibit faster growth rates that potentially confer fitness benefits (Barrow et al. 2021). Ultimately, it is likely that a combination of retentive and dispersive behaviours will promote resilience in golden perch populations in variable environments (Secor 1999).
Ontogenetic variability in habitat use has previously been described for golden perch, particularly the use of inundated floodplain habitats as nurseries (Ebner et al. 2009; Rolls and Wilson 2010; Sharpe 2011). In the present study, ~40% of individuals sampled in the Lower Darling River in 2018 were born in the Darling River and moved downstream into the Murray, where they remained for several years before migrating back into the Darling River. Return movements into the Darling River occurred at ages when fish were likely to have been reproductively mature (i.e. >4 years; Mallen-Cooper and Stuart 2003). Whether these movements constitute a form of natal fidelity, which is common in diadromous and some potamodromous fishes (Dittman and Quinn 1996; Duponchelle et al. 2016), remains to be explored; nevertheless, this complex movement pattern presents a range of considerations for river management. The dispersal of fish from the Lower Darling River, either passively as eggs and larvae or actively as juveniles, requires hydrological and physical connectivity between the Darling and Murray rivers, including unimpeded lotic environments over hundreds to thousands of kilometres (Mallen-Cooper and Zampatti 2020; Stuart and Sharpe 2020). Subsequently, the return migrations of adults require similar considerations, but also the restoration of seasonal flow pulses to cue upstream movements (Koster et al. 2014; Mallen-Cooper and Zampatti 2018) and a need to mitigate instream barriers to passage with effective fishways (Baumgartner et al. 2014).
Management implications and conclusions
In the years preceding the 2018–19 fish kills, there was substantial variability in golden perch age structure, recruitment source and movement patterns between the Lower and Mid-Darling River. These differences were potentially affected by spatially discrete recruitment sources, distinct hydrology between the regions and interrupted longitudinal connectivity. The MLSS, particularly the Menindee Main Weir, appears to delineate the population contingents. Consequently, rehabilitation of golden perch populations would benefit from the reinstatement of bidirectional connectivity at this structure, and others throughout the Barwon–Darling river system, to facilitate the upstream migration of juveniles and adults and the downstream dispersal of eggs, larvae and YOY. For all life stages, movements may occur over hundreds to thousands of kilometres, thus requiring the restoration of hydrological, hydraulic and physical connectivity at these scales (Mallen-Cooper and Zampatti 2020; Stuart and Sharpe 2020; Zambaldi and Pompeu 2020).
Considering the ongoing effects of climate change and flow regulation, promoting greater age structure diversity of golden perch in the Darling River will help safeguard populations against environmental perturbations (Berkeley et al. 2004) and would improve spawning outcomes, recruitment and population growth (Secor 2000). Golden perch in the Darling River, and more broadly in the MDB, have the advantage of population and life history strategies that integrate spatial and temporal bet-hedging mechanisms (Slatkin 1974; Kraus and Secor 2004). These include a combination of a periodic life history, migratory and recruitment source diversity, life history flexibility and ontogenetic variation in habitat use. In conjunction, these traits produce a portfolio effect whereby a diverse array of life history ‘assets’ has a stabilising effect on meta-populations due to differential responses of contingents to environmental variability (Schindler et al. 2010). In the case of golden perch in the Darling River, the resilience of this portfolio could be further improved by increasing the mosaic of recruitment and refuge habitats and improving the spatial processes that connect these (Kerr et al. 2010).
The 2018–19 fish kills in the Darling River spurred a renewed and urgent call to action for the rehabilitation of native fish populations in the MDB (Vertessy et al. 2019; MDBA 2020). The present study contributes to an understanding of pre-fish kill age demographics of golden perch in the Darling River, including how the spatial arrangement of recruitment sources, movement and connectivity influence population dynamics. Understanding population structure and causal mechanisms is fundamental to the rehabilitation of riverine fishes worldwide. This knowledge is key to establishing realistic rehabilitation objectives and targets, guiding restoration initiatives, such as mitigating barriers to movement and environmental flows, and ultimately measuring population response.
Conflicts of interest
Lee Baumgartner is an Associate Editor and Katherine Doyle is a Guest Editor of the ‘Fish Kills in Freshwaters’ special issue in Marine and Freshwater Research. Despite this relationship, they did not at any stage have Editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this Journal. Marine and Freshwater Research encourages its editors to publish in the Journal and they are kept totally separate from the decision-making process for their manuscripts. The authors declare that they have no further conflicts of interest.
Declaration of funding
The Australian Research Council funded A. Sadekov through the ARC Centre of Excellence for Coral Reef Studies (CE140100020 to Malcolm McCulloch). Aspects of this project were funded by the Murray–Darling Basin Authority and the Commonwealth Environmental Water Office and the New South Wales Recreational Fishing Trust Fund.
Acknowledgements
The authors thank Ian Magraith from SARDI Aquatic Sciences, who provided field support throughout the project. Thanks also to Stephen (Harry) Balcombe (Griffith University) and Jarod Lyon (Arthur Rylah Institute), who were generous with their knowledge on the ecology and population dynamics of golden perch in the MDB. The authors are grateful for support of analytical work at the Centre for Microscopy, Characterisation and Analysis at the University of Western Australia.
References
Alerstam, T., Hedenström, A., and Åkesson, S. (2003). Long-distance migration: evolution and determinants. Oikos 103, 247–260.| Long-distance migration: evolution and determinants.Crossref | GoogleScholarGoogle Scholar |
Anderson, J. R., Morison, A. K., and Ray, D. J. (1992). Validation of the use of thin-sectioned otoliths for determining age and growth of golden perch, Macquaria ambigua (Perciformes: Percichthyidae), in the Lower Murray–Darling Basin, Australia. Australian Journal of Marine and Freshwater Research 43, 1103–1128.
| Validation of the use of thin-sectioned otoliths for determining age and growth of golden perch, Macquaria ambigua (Perciformes: Percichthyidae), in the Lower Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Arnell, N. W., and Gosling, S. N. (2013). The impacts of climate change on river flow regimes at the global scale. Journal of Hydrology 486, 351–364.
| The impacts of climate change on river flow regimes at the global scale.Crossref | GoogleScholarGoogle Scholar |
Arthington, A. H., Bunn, S. E., Poff, N. L., and Naiman, R. J. (2006). The challenge of providing environmental flow rules to sustain river ecosystems. Ecological Applications 16, 1311–1318.
| The challenge of providing environmental flow rules to sustain river ecosystems.Crossref | GoogleScholarGoogle Scholar | 16937799PubMed |
Balcombe, S. R., Arthington, A. H., Foster, N. D., Thoms, M. C., Wilson, G. G., and Bunn, S. E. (2006). Fish assemblages of an Australian dryland river: abundance, assemblage structure and recruitment patterns in the Warrego River, Murray–Darling Basin. Marine and Freshwater Research 57, 619–633.
| Fish assemblages of an Australian dryland river: abundance, assemblage structure and recruitment patterns in the Warrego River, Murray–Darling Basin.Crossref | GoogleScholarGoogle Scholar |
Balme, J. (1995). 30,000 years of fishery in western New South Wales. Archaeology in Oceania 30, 1–21.
| 30,000 years of fishery in western New South Wales.Crossref | GoogleScholarGoogle Scholar |
Barrett, J. (2004). Introducing the Murray–Darling Basin Native Fish Strategy and initial steps towards demonstration reaches. Ecological Management & Restoration 5, 15–23.
| Introducing the Murray–Darling Basin Native Fish Strategy and initial steps towards demonstration reaches.Crossref | GoogleScholarGoogle Scholar |
Barrow, J. S., Yen, J. D. L., Koehn, J. D., Zampatti, B. P., Thiem, J. D., and Tonkin, Z. (2021). Lifetime movement history is associated with variable growth of a potamodromous freshwater fish. Journal of Animal Ecology , .
| Lifetime movement history is associated with variable growth of a potamodromous freshwater fish.Crossref | GoogleScholarGoogle Scholar |
Barthel, B. L., Cooke, S. J., Svec, J. H., Suski, C. D., Bunt, C. M., Phelan, F. J. S., and Philipp, D. P. (2008). Divergent life histories among smallmouth bass Micropterus dolomieu inhabiting a connected river–lake system. Journal of Fish Biology 73, 829–852.
| Divergent life histories among smallmouth bass Micropterus dolomieu inhabiting a connected river–lake system.Crossref | GoogleScholarGoogle Scholar |
Baumgartner, L. J., Reynoldson, N., and Gilligan, D. M. (2006). Mortality of larval Murray cod (Maccullochella peelii peelii) and golden perch (Macquaria ambigua) associated with passage through two types of low-head weirs. Marine and Freshwater Research 57, 187–191.
| Mortality of larval Murray cod (Maccullochella peelii peelii) and golden perch (Macquaria ambigua) associated with passage through two types of low-head weirs.Crossref | GoogleScholarGoogle Scholar |
Baumgartner, L., Zampatti, B., Jones, M., Stuart, I., and Mallen-Cooper, M. (2014). Fish passage in the Murray–Darling Basin, Australia: Not just an upstream battle. Ecological Management & Restoration 15, 28–39.
| Fish passage in the Murray–Darling Basin, Australia: Not just an upstream battle.Crossref | GoogleScholarGoogle Scholar |
Berkeley, S. A., Hixon, M. A., Larson, R. J., and Love, M. S. (2004). Fisheries sustainability via protection of age structure and spatial distribution of fish populations. Fisheries 29, 23–32.
| Fisheries sustainability via protection of age structure and spatial distribution of fish populations.Crossref | GoogleScholarGoogle Scholar |
Best, J. (2019). Anthropogenic stresses on the world’s big rivers. Nature Geoscience 12, 7–21.
| Anthropogenic stresses on the world’s big rivers.Crossref | GoogleScholarGoogle Scholar |
Brennan, S. R., and Schindler, D. E. (2017). Linking otolith microchemistry and dendritic isoscapes to map heterogeneous production of fish across river basins. Ecological Applications 27, 363–377.
| Linking otolith microchemistry and dendritic isoscapes to map heterogeneous production of fish across river basins.Crossref | GoogleScholarGoogle Scholar | 27875020PubMed |
Brennan, S. R., Torgersen, C. E., Hollenbeck, J. P., Fernandez, D. P., Jensen, C. K., and Schindler, D. E. (2016). Dendritic network models: Improving isoscapes and quantifying influence of landscape and in-stream processes on strontium isotopes in rivers. Geophysical Research Letters 43, 5043–5051.
| Dendritic network models: Improving isoscapes and quantifying influence of landscape and in-stream processes on strontium isotopes in rivers.Crossref | GoogleScholarGoogle Scholar |
Cadwallader, P. L. (1978). Some causes of the decline in range and abundance of native fish in the Murray–Darling River system. Proceedings of the Royal Society of Victoria 90, 211–224.
Campana, S. E., and Thorrold, S. R. (2001). Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations? Canadian Journal of Fisheries and Aquatic Sciences 58, 30–38.
| Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations?Crossref | GoogleScholarGoogle Scholar |
Close, P. G., Dobbs, R. J., Tunbridge, D. J., Speldewinde, P. C., Warfe, D. M., Toussaint, S., and Davies, P. M. (2014). Customary and recreational fishing pressure: large-bodied fish assemblages in a tropical, intermittent Australian river. Marine and Freshwater Research 65, 466–474.
| Customary and recreational fishing pressure: large-bodied fish assemblages in a tropical, intermittent Australian river.Crossref | GoogleScholarGoogle Scholar |
Comte, L., and Olden, J. D. (2018). Fish dispersal in flowing waters: a synthesis of movement- and genetic-based studies. Fish and Fisheries 19, 1063–1077.
| Fish dispersal in flowing waters: a synthesis of movement- and genetic-based studies.Crossref | GoogleScholarGoogle Scholar |
Cooke, S. J., Paukert, C., and Hogan, Z. (2012). Endangered river fish: factors hindering conservation and restoration. Endangered Species Research 17, 179–191.
| Endangered river fish: factors hindering conservation and restoration.Crossref | GoogleScholarGoogle Scholar |
Cooke, S. J., Martins, E. G., Struthers, D. P., Gutowsky, L. F., Power, M., Doka, S. E., Dettmers, J. M., Crook, D. A., Lucas, M. C., Holbrook, C. M., and Krueger, C. C. (2016). A moving target – incorporating knowledge of the spatial ecology of fish into the assessment and management of freshwater fish populations. Environmental Monitoring and Assessment 188, 239.
| A moving target – incorporating knowledge of the spatial ecology of fish into the assessment and management of freshwater fish populations.Crossref | GoogleScholarGoogle Scholar | 27004432PubMed |
Crook, D. A., O’Mahony, D. J., Gillanders, B. M., Munro, A. R., Sanger, A. C., Thurstan, S., and Baumgartner, L. J. (2016). Contribution of stocked fish to riverine populations of golden perch (Macquaria ambigua) in the Murray–Darling Basin, Australia. Marine and Freshwater Research 67, 1401–1409.
| Contribution of stocked fish to riverine populations of golden perch (Macquaria ambigua) in the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Dittman, A., and Quinn, T. (1996). Homing in Pacific salmon: mechanisms and ecological basis. The Journal of Experimental Biology 199, 83–91.
| Homing in Pacific salmon: mechanisms and ecological basis.Crossref | GoogleScholarGoogle Scholar | 9317381PubMed |
Douglas, G. B., Gray, C. M., Hart, B. T., and Beckett, R. (1995). A strontium isotopic investigation of the origin of suspended particulate matter (SPM) in the Murray–Darling River system, Australia. Geochimica et Cosmochimica Acta 59, 3799–3815.
| A strontium isotopic investigation of the origin of suspended particulate matter (SPM) in the Murray–Darling River system, Australia.Crossref | GoogleScholarGoogle Scholar |
Dufour, S., and Piégay, H. (2009). From the myth of a lost paradise to targeted river restoration: forget natural references and focus on human benefits. River Research and Applications 25, 568–581.
| From the myth of a lost paradise to targeted river restoration: forget natural references and focus on human benefits.Crossref | GoogleScholarGoogle Scholar |
Duponchelle, F., Pouilly, M., Pécheyran, C., Hauser, M., Renno, J. F., Panfili, J., Darnaude, A. M., García-Vasquez, A., Carvajal-Vallejos, F., García-Dávila, C., and Doria, C. (2016). Trans-Amazonian natal homing in giant catfish. Journal of Applied Ecology 53, 1511–1520.
| Trans-Amazonian natal homing in giant catfish.Crossref | GoogleScholarGoogle Scholar |
Ebner, B. C., Scholz, O., and Gawne, B. (2009). Golden perch Macquaria ambigua are flexible spawners in the Darling River, Australia. New Zealand Journal of Marine and Freshwater Research 43, 571–578.
| Golden perch Macquaria ambigua are flexible spawners in the Darling River, Australia.Crossref | GoogleScholarGoogle Scholar |
Farrell, J. W., Clemens, S. C., and Gromet, L. P. (1995). Improved chronostratigraphic reference curve of late Neogene seawater 87Sr/86Sr. Geology 23, 403–406.
| Improved chronostratigraphic reference curve of late Neogene seawater 87Sr/86Sr.Crossref | GoogleScholarGoogle Scholar |
Faulks, L. K., Gilligan, D. M., and Beheregaray, L. B. (2010). Islands of water in a sea of dry land: hydrological regime predicts genetic diversity and dispersal in a widespread fish from Australia’s arid zone, the golden perch (Macquaria ambigua). Molecular Ecology 19, 4723–4737.
| Islands of water in a sea of dry land: hydrological regime predicts genetic diversity and dispersal in a widespread fish from Australia’s arid zone, the golden perch (Macquaria ambigua).Crossref | GoogleScholarGoogle Scholar | 20887362PubMed |
Fausch, K. D., Torgersen, C. E., Baxter, C. V., and Li, H. W. (2002). Landscapes to riverscapes: bridging the gap between research and conservation of stream fishes: a continuous view of the river is needed to understand how processes interacting among scales set the context for stream fishes and their habitat. Bioscience 52, 483–498.
| Landscapes to riverscapes: bridging the gap between research and conservation of stream fishes: a continuous view of the river is needed to understand how processes interacting among scales set the context for stream fishes and their habitat.Crossref | GoogleScholarGoogle Scholar |
Forbes, J. P., Watts, R. J., Robinson, W. A., Baumgartner, L. J., Allen, M. S., McGuffie, P., Cameron, L. M., and Crook, D. A. (2015a). System-specific variability in Murray cod and golden perch maturation and growth influences fisheries management options. North American Journal of Fisheries Management 35, 1226–1238.
| System-specific variability in Murray cod and golden perch maturation and growth influences fisheries management options.Crossref | GoogleScholarGoogle Scholar |
Forbes, J. P., Watts, R. J., Robinson, W. A., Baumgartner, L. J., Steffe, A. S., and Murphy, J. J. (2015b). Recreational fishing effort, catch, and harvest for Murray cod and golden perch in the Murrumbidgee River, Australia. North American Journal of Fisheries Management 35, 649–658.
| Recreational fishing effort, catch, and harvest for Murray cod and golden perch in the Murrumbidgee River, Australia.Crossref | GoogleScholarGoogle Scholar |
Glencross, B. D., and Felsing, M. (2006). Influence of fish size and water temperature on the metabolic demand for oxygen by barramundi, Lates calcarifer (Bloch), in freshwater. Aquaculture Research 37, 1055–1062.
| Influence of fish size and water temperature on the metabolic demand for oxygen by barramundi, Lates calcarifer (Bloch), in freshwater.Crossref | GoogleScholarGoogle Scholar |
Gowan, C., and Fausch, K. D. (1996). Long-term demographic responses of trout populations to habitat manipulation in six Colorado streams. Ecological Applications 6, 931–946.
| Long-term demographic responses of trout populations to habitat manipulation in six Colorado streams.Crossref | GoogleScholarGoogle Scholar |
Grill, G., Lehner, B., Lumsdon, A. E., MacDonald, G. K., Zarfl, C., and Liermann, C. R. (2015). An index-based framework for assessing patterns and trends in river fragmentation and flow regulation by global dams at multiple scales. Environmental Research Letters 10, 015001.
| An index-based framework for assessing patterns and trends in river fragmentation and flow regulation by global dams at multiple scales.Crossref | GoogleScholarGoogle Scholar |
Humphries, P., and Winemiller, K. O. (2009). Historical impacts on river fauna, shifting baselines, and challenges for restoration. Bioscience 59, 673–684.
| Historical impacts on river fauna, shifting baselines, and challenges for restoration.Crossref | GoogleScholarGoogle Scholar |
Humphries, P., King, A. J., McCasker, N., Kopf, R. K., Stoffels, R., Zampatti, B. P., and Price, A. E. (2020). Riverscape recruitment: a conceptual synthesis of drivers of fish recruitment in rivers. Canadian Journal of Fisheries and Aquatic Sciences 77, 213–225.
| Riverscape recruitment: a conceptual synthesis of drivers of fish recruitment in rivers.Crossref | GoogleScholarGoogle Scholar |
Kail, J., Brabec, K., Poppe, M., and Januschke, K. (2015). The effect of river restoration on fish, macroinvertebrates and aquatic macrophytes: a meta-analysis. Ecological Indicators 58, 311–321.
| The effect of river restoration on fish, macroinvertebrates and aquatic macrophytes: a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Kerr, L. A., Cadrin, S. X., and Secor, D. H. (2010). The role of spatial dynamics in the stability, resilience, and productivity of an estuarine fish population. Ecological Applications 20, 497–507.
| The role of spatial dynamics in the stability, resilience, and productivity of an estuarine fish population.Crossref | GoogleScholarGoogle Scholar | 20405802PubMed |
King, A. J., Gwinn, D. C., Tonkin, Z., Mahoney, J., Raymond, S., and Beesley, L. (2016). Using abiotic drivers of fish spawning to inform environmental flow management. Journal of Applied Ecology 53, 34–43.
| Using abiotic drivers of fish spawning to inform environmental flow management.Crossref | GoogleScholarGoogle Scholar |
Kingsford, R. T. (2000). Protecting rivers in arid regions or pumping them dry? Hydrobiologia 427, 1–11.
| Protecting rivers in arid regions or pumping them dry?Crossref | GoogleScholarGoogle Scholar |
Kingsford, R. T. (2011). Conservation management of rivers and wetlands under climate change–a synthesis. Marine and Freshwater Research 62, 217–222.
| Conservation management of rivers and wetlands under climate change–a synthesis.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., King, A. J., Beesley, L., Copeland, C., Zampatti, B. P., and Mallen-Cooper, M. (2014). Flows for native fish in the Murray–Darling Basin: lessons and considerations for future management. Ecological Management & Restoration 15, 40–50.
| Flows for native fish in the Murray–Darling Basin: lessons and considerations for future management.Crossref | GoogleScholarGoogle Scholar |
Kondolf, G. M. (1995). Five elements for effective evaluation of stream restoration. Restoration Ecology 3, 133–136.
| Five elements for effective evaluation of stream restoration.Crossref | GoogleScholarGoogle Scholar |
Kopf, R. K., Finlayson, C. M., Humphries, P., Sims, N. C., and Hladyz, S. (2015). Anthropocene baselines: human-induced changes to global freshwater biodiversity restoration potential. Bioscience 65, 798–811.
| Anthropocene baselines: human-induced changes to global freshwater biodiversity restoration potential.Crossref | GoogleScholarGoogle Scholar |
Koster, W. M., Dawson, D. R., O’Mahony, D. J., Moloney, P. D., and Crook, D. A. (2014). Timing, frequency and environmental conditions associated with mainstem–tributary movement by a lowland river fish, golden perch (Macquaria ambigua). PLoS One 9, e96044.
| Timing, frequency and environmental conditions associated with mainstem–tributary movement by a lowland river fish, golden perch (Macquaria ambigua).Crossref | GoogleScholarGoogle Scholar | 24788137PubMed |
Koster, W. M., Dawson, D. R., Liu, C., Moloney, P. D., Crook, D. A., and Thomson, J. R. (2017). Influence of streamflow on spawning-related movements of golden perch Macquaria ambigua in south-eastern Australia. Journal of Fish Biology 90, 93–108.
| Influence of streamflow on spawning-related movements of golden perch Macquaria ambigua in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar | 27734494PubMed |
Koster, W. M., Stuart, I., Tonkin, Z., Dawson, D., and Fanson, B. (2021). Environmental influences on migration patterns and pathways of a threatened potamodromous fish in a regulated lowland river network. Ecohydrology 14, e2260.
| Environmental influences on migration patterns and pathways of a threatened potamodromous fish in a regulated lowland river network.Crossref | GoogleScholarGoogle Scholar |
Kraus, R. T., and Secor, D. H. (2004). Dynamics of white perch Morone americana population contingents in the Patuxent River estuary, Maryland, USA. Marine Ecology Progress Series 279, 247–259.
| Dynamics of white perch Morone americana population contingents in the Patuxent River estuary, Maryland, USA.Crossref | GoogleScholarGoogle Scholar |
Lake, P. S. (2005). Perturbation, restoration and seeking ecological sustainability in Australian flowing waters. Hydrobiologia 552, 109–120.
| Perturbation, restoration and seeking ecological sustainability in Australian flowing waters.Crossref | GoogleScholarGoogle Scholar |
Lintermans, M. (2007). Fishes of the Murray–Darling Basin: an Introductory Guide. (Murray–Darling Basin Authority: Canberra, ACT, Australia.)
Lucas, M. C., and Baras, E. (2001). Migration of Freshwater Fishes. (Blackwell Science: Oxford).
Mallen-Cooper, M., and Stuart, I. G. (2003). Age, growth and non-flood recruitment of two potamodromous fishes in a large semi-arid/temperate river system. River Research and Applications 19, 697–719.
| Age, growth and non-flood recruitment of two potamodromous fishes in a large semi-arid/temperate river system.Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M., and Zampatti, B. P. (2018). History, hydrology and hydraulics: Rethinking the ecological management of large rivers. Ecohydrology 11, e1965.
| History, hydrology and hydraulics: Rethinking the ecological management of large rivers.Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M., and Zampatti, B. P. (2020). Restoring the ecological integrity of a dryland river: why low flows in the Barwon–Darling River must flow. Ecological Management & Restoration 21, 218–228.
| Restoring the ecological integrity of a dryland river: why low flows in the Barwon–Darling River must flow.Crossref | GoogleScholarGoogle Scholar |
Murray–Darling Basin Authority (2014). Basin-wide environmental watering strategy. (Murray–Darling Basin Authority: Canberra, ACT, Australia.)
Murray–Darling Basin Authority (2020). Native fish recovery strategy. (Murray–Darling Basin Authority: Canberra, ACT, Australia.)
New South Wales Department of Primary Industries (2006). Reducing the Impact of Weirs on Aquatic Habitat – New South Wales Detailed Weir Review. Lower Murray Darling CMA region. NSW DPI, Sydney, NSW, Australia.
New South Wales Department of Primary Industries (2012). Fishway options for weirs of the Northern Murray Darling Basin. Report prepared for the Murray–Darling Basin Authority. NSW DPI, Sydney, NSW, Australia.
Normile, D. (2019). Massive fish die-off sparks outcry in Australia. Science 363, 331.
| Massive fish die-off sparks outcry in Australia.Crossref | GoogleScholarGoogle Scholar | 30679352PubMed |
Olden, J. D., Poff, N. L., and Bestgen, K. R. (2006). Life-history strategies predict fish invasions and extirpations in the Colorado River Basin. Ecological Monographs 76, 25–40.
| Life-history strategies predict fish invasions and extirpations in the Colorado River Basin.Crossref | GoogleScholarGoogle Scholar |
Paton, C., Hellstrom, J., Paul, B., Woodhead, J., and Hergt, J. (2011). Iolite: Freeware for the visualisation and processing of mass spectrometric data. Journal of Analytical Atomic Spectrometry 26, 2508–2518.
| Iolite: Freeware for the visualisation and processing of mass spectrometric data.Crossref | GoogleScholarGoogle Scholar |
Pearson, M. R., Reid, M. A., Miller, C., and Ryder, D. (2020). Comparison of historical and modern river surveys reveal changes to waterhole characteristics in an Australian dryland river. Geomorphology 356, 107089.
| Comparison of historical and modern river surveys reveal changes to waterhole characteristics in an Australian dryland river.Crossref | GoogleScholarGoogle Scholar |
Poff, N. L., Allan, J. D., Palmer, M. A., Hart, D. D., Richter, B. D., Arthington, A. H., Rogers, K. H., Meyer, J. L., and Stanford, J. A. (2003). River flows and water wars: emerging science for environmental decision making. Frontiers in Ecology and the Environment 1, 298–306.
| River flows and water wars: emerging science for environmental decision making.Crossref | GoogleScholarGoogle Scholar |
Poff, N. L., Olden, J. D., Merritt, D. M., and Pepin, D. M. (2007). Homogenization of regional river dynamics by dams and global biodiversity implications. Proceedings of the National Academy of Sciences of the United States of America 104, 5732–5737.
| Homogenization of regional river dynamics by dams and global biodiversity implications.Crossref | GoogleScholarGoogle Scholar | 17360379PubMed |
Pollux, B. J. A., Pollux, P. M. J., Korosi, A., Verberk, W. C. E. P., and Van der Velde, G. (2006). Reproduction, growth, and migration of fishes in a regulated lowland tributary: potential recruitment to the river Meuse. In ‘Living Rivers: Trends and Challenges in Science and Management’. pp. 105–120. (Springer: Dordrecht, Netherlands.)
Pracheil, B. M., McIntyre, P. B., and Lyons, J. D. (2013). Enhancing conservation of large-river biodiversity by accounting for tributaries. Frontiers in Ecology and the Environment 11, 124–128.
| Enhancing conservation of large-river biodiversity by accounting for tributaries.Crossref | GoogleScholarGoogle Scholar |
Puckridge, J. T., Sheldon, F., Walker, K. F., and Boulton, A. J. (1998). Flow variability and the ecology of large rivers. Marine and Freshwater Research 49, 55–72.
| Flow variability and the ecology of large rivers.Crossref | GoogleScholarGoogle Scholar |
Reid, D. D., Harris, J. H., and Chapman, D. J. (1997). ‘NSW Inland Commercial Fishery Data Analysis.’ (Fisheries Research & Development Corporation: Canberra, ACT, Australia.)
Reynolds, J. D., Webb, T. J., and Hawkins, L. A. (2005). Life history and ecological correlates of extinction risk in European freshwater fishes. Canadian Journal of Fisheries and Aquatic Sciences 62, 854–862.
| Life history and ecological correlates of extinction risk in European freshwater fishes.Crossref | GoogleScholarGoogle Scholar |
Rice, S. P., Kiffney, P., Greene, C., and Pess, G. R. (2008). The ecological importance of tributaries and confluences. In ‘River Confluences, Tributaries and the Fluvial Networks’. (Eds S. P. Rice, A. G. Roy and B. L. Rhoads.) pp. 209–242. (Wiley: Chichester, UK).
Roberts, D. T., Duivenvoorden, L. J., and Stuart, I. G. (2008). Factors influencing recruitment patterns of golden perch (Macquaria ambigua oriens) within a hydrologically variable and regulated Australian tropical river system. Ecology Freshwater Fish 17, 577–589.
| Factors influencing recruitment patterns of golden perch (Macquaria ambigua oriens) within a hydrologically variable and regulated Australian tropical river system.Crossref | GoogleScholarGoogle Scholar |
Rolls, R. J., and Wilson, G. G. (2010). Spatial and temporal patterns in fish assemblages following an artificially extended floodplain inundation event, northern Murray–Darling Basin, Australia. Environmental Management 45, 822–833.
| Spatial and temporal patterns in fish assemblages following an artificially extended floodplain inundation event, northern Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar | 20127088PubMed |
Rolls, R. J., Growns, I. O., Khan, T. A., Wilson, G. G., Ellison, T. L., Prior, A., and Waring, C. C. (2013). Fish recruitment in rivers with modified discharge depends on the interacting effects of flow and thermal regimes. Freshwater Biology 58, 1804–1819.
| Fish recruitment in rivers with modified discharge depends on the interacting effects of flow and thermal regimes.Crossref | GoogleScholarGoogle Scholar |
Schindler, D. E., Hilborn, R., Chasco, B., Boatright, C. P., Quinn, T. P., Rogers, L. A., and Webster, M. S. (2010). Population diversity and the portfolio effect in an exploited species. Nature 465, 609–612.
| Population diversity and the portfolio effect in an exploited species.Crossref | GoogleScholarGoogle Scholar | 20520713PubMed |
Schmutz, S., Jurajda, P., Kaufmann, S., Lorenz, A. W., Muhar, S., Paillex, A., Poppe, M., and Wolter, C. (2016). Response of fish assemblages to hydromorphological restoration in central and northern European rivers. Hydrobiologia 769, 67–78.
| Response of fish assemblages to hydromorphological restoration in central and northern European rivers.Crossref | GoogleScholarGoogle Scholar |
Secor, D. H. (1999). Specifying divergent migrations in the concept of stock: the contingent hypothesis. Fisheries Research 43, 13–34.
| Specifying divergent migrations in the concept of stock: the contingent hypothesis.Crossref | GoogleScholarGoogle Scholar |
Secor, D. H. (2000). Spawning in the nick of time? Effect of adult demographics on spawning behaviour and recruitment in Chesapeake Bay striped bass. ICES Journal of Marine Science 57, 403–411.
| Spawning in the nick of time? Effect of adult demographics on spawning behaviour and recruitment in Chesapeake Bay striped bass.Crossref | GoogleScholarGoogle Scholar |
Secor, D. H. (2007). The year-class phenomenon and the storage effect in marine fishes. Journal of Sea Research 57, 91–103.
| The year-class phenomenon and the storage effect in marine fishes.Crossref | GoogleScholarGoogle Scholar |
Shams, F., Dyer, F., Thompson, R., Duncan, R. P., Thiem, J. D., Enge, T. G., and Ezaz, T. (2020). Multiple lines of evidence indicate limited natural recruitment of golden perch (Macquaria ambigua) in the Highly Regulated Lachlan River. Water 12, 1636.
| Multiple lines of evidence indicate limited natural recruitment of golden perch (Macquaria ambigua) in the Highly Regulated Lachlan River.Crossref | GoogleScholarGoogle Scholar |
Sharpe, C. P. (2011). Spawning and recruitment ecology of golden perch (Macquaria ambigua Richardson 1845) in the Murray and Darling rivers. Ph.D. Thesis, Griffith University, Brisbane, Qld, Australia. Available at https://research-repository.griffith.edu.au/handle/10072/366211
Shenton, W., Bond, N. R., Yen, J. D., and Mac Nally, R. (2012). Putting the “ecology” into environmental flows: ecological dynamics and demographic modelling. Environmental Management 50, 1–10.
| Putting the “ecology” into environmental flows: ecological dynamics and demographic modelling.Crossref | GoogleScholarGoogle Scholar | 22543580PubMed |
Slatkin, M. (1974). Hedging one’s evolutionary bets. Nature 250, 704–705.
| Hedging one’s evolutionary bets.Crossref | GoogleScholarGoogle Scholar |
Stanford, J. A., Ward, J. V., Liss, W. J., Frissell, C. A., Williams, R. N., Lichatowich, J. A., and Coutant, C. C. (1996). A general protocol for restoration of regulated rivers. Regulated Rivers 12, 391–413.
| A general protocol for restoration of regulated rivers.Crossref | GoogleScholarGoogle Scholar |
Stuart, I. G., and Sharpe, C. P. (2020). Riverine spawning, long distance larval drift, and floodplain recruitment of a pelagophilic fish: a case study of golden perch (Macquaria ambigua) in the arid Darling River, Australia. Aquatic Conservation 30, 675–690.
| Riverine spawning, long distance larval drift, and floodplain recruitment of a pelagophilic fish: a case study of golden perch (Macquaria ambigua) in the arid Darling River, Australia.Crossref | GoogleScholarGoogle Scholar |
Thiem, J. D., Wooden, I. J., Baumgartner, L. J., Butler, G. L., Forbes, J. P., and Conallin, J. (2017). Recovery from a fish kill in a semi-arid Australian river: Can stocking augment natural recruitment processes? Austral Ecology 42, 218–226.
| Recovery from a fish kill in a semi-arid Australian river: Can stocking augment natural recruitment processes?Crossref | GoogleScholarGoogle Scholar |
Thiem, J. D., Baumgartner, L. J., Fanson, B., Tonkin, Z., and Zampatti, B. P. (2021). Contrasting natal origin and movement history informs recovery pathways for three lowland river species following a mass fish kill. Marine and Freshwater Research , .
| Contrasting natal origin and movement history informs recovery pathways for three lowland river species following a mass fish kill.Crossref | GoogleScholarGoogle Scholar |
Thompson, R. M., King, A. J., Kingsford, R. M., Mac Nally, R., and Poff, N. L. (2018). Legacies, lags and long-term trends: Effective flow restoration in a changed and changing world. Freshwater Biology 63, 986–995.
| Legacies, lags and long-term trends: Effective flow restoration in a changed and changing world.Crossref | GoogleScholarGoogle Scholar |
Thoms, M. C., and Sheldon, F. (2000). Water resource development and hydrological change in a large dryland river: the Barwon–Darling River, Australia. Journal of Hydrology 228, 10–21.
| Water resource development and hydrological change in a large dryland river: the Barwon–Darling River, Australia.Crossref | GoogleScholarGoogle Scholar |
Thoms, M. C., Sheldon, F., and Crabb, P. (2004). The hydrology of rivers in the Darling Basin. In ‘The Darling’. (Eds R. Breckwodt, R. Boden, and J. Andrew.) pp. 78–92. (Murray–Darling Basin Commission: Canberra, ACT, Australia.)
Tonkin, J. D., Poff, N. L. R., Bond, N. R., Horne, A., Merritt, D. M., Reynolds, L. V., Olden, J. D., Ruhi, A., and Lytle, D. A. (2019). Prepare river ecosystems for an uncertain future. Nature 570, 301–303.
| Prepare river ecosystems for an uncertain future.Crossref | GoogleScholarGoogle Scholar | 31213691PubMed |
van Dijk, A. I., Beck, H. E., Crosbie, R. S., de Jeu, R. A., Liu, Y. Y., Podger, G. M., Timbal, B., and Viney, N. R. (2013). The Millennium Drought in southeast Australia (2001–2009): natural and human causes and implications for water resources, ecosystems, economy, and society. Water Resources Research 49, 1040–1057.
| The Millennium Drought in southeast Australia (2001–2009): natural and human causes and implications for water resources, ecosystems, economy, and society.Crossref | GoogleScholarGoogle Scholar |
Vertessy, R., Barma, D., Baumgartner, L., Mitrovic, S., and Sheldon, F. (2019). Independent Assessment of the 2018–19 fish deaths in the lower Darling. Report to the Federal Government of Australia. (Australian Government: Canberra, ACT, Australia.) Available at https://www.mdba.gov.au/publications/mdba-reports/response-fish-deaths-lower-darling
Walker, K. F., Sheldon, F., and Puckridge, J. T. (1995). A perspective on dryland river ecosystems. Regulated Rivers 11, 85–104.
| A perspective on dryland river ecosystems.Crossref | GoogleScholarGoogle Scholar |
Ward, J. V., Tockner, K., Uehlinger, U., and Malard, F. (2001). Understanding natural patterns and processes in river corridors as the basis for effective river restoration. Regulated Rivers 17, 311–323.
| Understanding natural patterns and processes in river corridors as the basis for effective river restoration.Crossref | GoogleScholarGoogle Scholar |
Webber, P. A., Bestgen, K. R., and Haines, G. B. (2013). Tributary spawning by endangered Colorado River basin fishes in the White River. North American Journal of Fisheries Management 33, 1166–1171.
| Tributary spawning by endangered Colorado River basin fishes in the White River.Crossref | GoogleScholarGoogle Scholar |
Winemiller, K. O. (2005). Life history strategies, population regulation, and implications for fisheries management. Canadian Journal of Fisheries and Aquatic Sciences 62, 872–885.
| Life history strategies, population regulation, and implications for fisheries management.Crossref | GoogleScholarGoogle Scholar |
Winemiller, K. O., and Rose, K. A. (1992). Patterns of life-history diversification in North American fishes: implications for population regulation. Canadian Journal of Fisheries and Aquatic Sciences 49, 2196–2218.
| Patterns of life-history diversification in North American fishes: implications for population regulation.Crossref | GoogleScholarGoogle Scholar |
Wohl, E., Angermeier, P. L., Bledsoe, B., Kondolf, G. M., MacDonnell, L., Merritt, D. M., Palmer, M. A., Poff, N. L., and Tarboton, D. (2005). River restoration. Water Resources Research 41, W10301.
| River restoration.Crossref | GoogleScholarGoogle Scholar |
Woodhead, J., Swearer, S., Hergt, J., and Maas, R. (2005). In situ Sr-isotope analysis of carbonates by LA-MC-ICP-MS: interference corrections, high spatial resolution and an example from otolith studies. Journal of Analytical Atomic Spectrometry 20, 22–27.
| In situ Sr-isotope analysis of carbonates by LA-MC-ICP-MS: interference corrections, high spatial resolution and an example from otolith studies.Crossref | GoogleScholarGoogle Scholar |
Wright, D. W., Zampatti, B. P., Baumgartner, L. J., Brooks, S., Butler, G. L., Crook, D. A., Fanson, B. G., Koster, W., Lyon, J., Strawbridge, A., and Tonkin, Z. (2020). Size, growth and mortality of riverine golden perch (Macquaria ambigua) across a latitudinal gradient. Marine and Freshwater Research 71, 1651–1661.
| Size, growth and mortality of riverine golden perch (Macquaria ambigua) across a latitudinal gradient.Crossref | GoogleScholarGoogle Scholar |
Zambaldi, L., and Pompeu, P. S. (2020). Evaluation of river fragmentation and implications for the conservation of migratory fish in southeastern Brazil. Environmental Management 65, 702–709.
| Evaluation of river fragmentation and implications for the conservation of migratory fish in southeastern Brazil.Crossref | GoogleScholarGoogle Scholar | 32086549PubMed |
Zampatti, B. P. (2019). Ecology and population dynamics of golden perch in a fragmented, flow-impacted river: implications for conservation and management. Ph.D. thesis, University of Adelaide, Adelaide, SA, Australia.
Zampatti, B. P., and Leigh, S. J. (2013). Within-channel flows promote spawning and recruitment of golden perch, Macquaria ambigua ambigua, implications for environmental flow management in the River Murray, Australia. Marine and Freshwater Research 64, 618–630.
| Within-channel flows promote spawning and recruitment of golden perch, Macquaria ambigua ambigua, implications for environmental flow management in the River Murray, Australia.Crossref | GoogleScholarGoogle Scholar |
Zampatti, B. P., Strawbridge, A., Thiem, J. D., Tonkin, Z., Maas, R., Woodhead, J., and Fredberg, J. (2018). Golden perch (Macquaria ambigua) and silver perch (Bidyanus bidyanus) age demographics, natal origin and migration history in the River Murray, Australia. South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Zampatti, B., Fanson, B., Strawbridge, A., Tonkin, Z., Thiem, J., Butler, G., Balcombe, S., Koster, W., King, A., Crook, D., Woods, R., Brooks, S., Lyon, J., Baumgartner, L., and Doyle, K. (2019). Basin-scale population dynamics of Golden Perch and Murray Cod: relating flow to provenance, movement and recruitment in the Murray–Darling Basin. In ‘Murray–Darling Basin Environmental Water Knowledge and Research Project — Fish Theme Research Report’. (Eds A. Price, S. Balcombe, P. Humphries, A. King and B. Zampatti.) pp. 26–30. (Centre for Freshwater Ecology, La Trobe University: Wodonga, Vic., Australia.)
Zampatti, B. P., Leigh, S. J., Wilson, P. J., Crook, D. A., Gillanders, B. M., Maas, R., Macdonald, J. I., and Woodhead, J. (2021). Otolith chemistry delineates the influence of natal origin, dispersal and flow on the population dynamics of golden perch (Macquaria ambigua) in a regulated river. Marine and Freshwater Research 72, 1484–1495.
| Otolith chemistry delineates the influence of natal origin, dispersal and flow on the population dynamics of golden perch (Macquaria ambigua) in a regulated river.Crossref | GoogleScholarGoogle Scholar |


