Environmental factors and predator abundance predict the distribution and occurrence of two sympatric urchin species at Ningaloo Reef, Western Australia
Emma L. Westlake A D , Cindy Bessey A B , Rebecca Fisher B C , Damian P. Thomson A and Michael D. E. Haywood A
A D , Cindy Bessey A B , Rebecca Fisher B C , Damian P. Thomson A and Michael D. E. Haywood A
A Commonwealth Scientific and Industrial Research Organisation, Oceans and Atmosphere, 35 Stirling Highway, Crawley, WA 6009, Australia.
B University of Western Australia, School of Plant Biology and the Oceans Institute, 35 Stirling Highway, Crawley, WA 6009, Australia.
C Australian Institute of Marine Science, 35 Stirling Highway, Crawley, WA 6009, Australia.
D Corresponding author. Email: emma.westlake@csiro.au
Marine and Freshwater Research 72(12) 1711-1721 https://doi.org/10.1071/MF21091
Submitted: 24 March 2021 Accepted: 13 July 2021 Published: 23 August 2021
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
Sea urchins can play a critical ecological role in the functioning of marine benthic ecosystems, mediating competitive interactions between corals and algae. Yet, little is known about factors affecting urchin distribution in intact coral reef systems. This study aims to determine the spatial distribution of two sympatric urchin species, Echinometra mathaei and Echinostrephus molaris, and potential factors contributing to this, within the intact coral reef system of Ningaloo Marine Park, north-western Western Australia. Benthic photographs and surveys were conducted on SCUBA at 126 sites across the Park to determine urchin presence, rugosity, substrate cover, water velocity, and fish predation for each site. Generalised additive models found that E. mathaei presence was positively related to algal cover, rugosity and non-sanctuary zones, suggesting that distribution may be driven by foraging behaviour, habitat complexity and predation. Echinostrephus molaris presence was positively related to habitat and region, suggesting its distribution may be largely driven by hydrodynamics, feeding strategy and regional variation. This study highlighted species-habitat associations and the complexities of these in structuring urchin communities. Although occupying similar niches, the predominantly non-overlapping feeding preferences, and morphological and behavioural differences between E. mathaei and E. molaris enable these species to coexist within the intact reef system of Ningaloo Marine Park.
Keywords: sea urchins, distribution, Echinometra mathaei, Echinostrephus molaris, predation, management zones.
Introduction
Sea urchins (Class Echinoidea) can play a critical role in the functioning of marine benthic ecosystems (Done et al. 1996; Peyrot-Clausade et al. 2000; Scheffer et al. 2001; Harborne et al. 2009). Within temperate reefs, high urchin densities have been responsible for the removal of vast stands of macroalgae (e.g. Lawrence 1975; Lawrence and Sammarco 1982; see also review by Sala et al. 1998), subsequently reducing primary productivity and food web complexity (Filbee-Dexter and Scheibling 2014). On tropical coral reefs, substantial increases in urchin densities have been implicated in driving phase shifts from coral- to algal-dominated environments (e.g. Sammarco 1980; Hughes et al. 1987; Lessios 1988; Hughes 1994; Dudgeon et al. 2010). In addition to their role in mediating competition between coral and algae (Edmunds and Carpenter 2001), urchins are crucial bioeroders (Scoffin et al. 1980; Bak 1990), actively eroding reef and dead coral substrata through their feeding and boring behaviours (Downing and El Zahr 1987; McClanahan and Kurtis 1991; Done et al. 1996).
The relationship between urchin abundance and spatial distribution on coral reefs is complex (e.g. McClanahan and Kurtis 1991; McClanahan 1998; Dumas et al. 2007; Burt et al. 2010; Graham and Nash 2013). The size, composition and distribution of urchin populations are affected by a diverse set of environmental variables, including structural complexity, coral and macroalgal cover, sedimentation, predation and disease (Lessios 1988; Feehan and Scheibling 2014) across multiple scales (Sánchez-Jérez et al. 2001; see review by Dumas et al. 2007). Additionally, urchin settlement and recruitment patterns (Watts et al. 1990), hydrodynamics (Russo 1977; Ogden et al. 1989), behavioural processes (Chabanet et al. 1997; Lawrence 2001; Dumas et al. 2007), and anthropogenic influences such as fishing (e.g. Hay 1984; McClanahan and Shafir 1990) may also play a role in determining urchin abundance and distribution across local scales. Of these, predation has been identified as a principal regulatory agent affecting urchin presence and density (e.g. Hughes 1994; Sala and Zabala 1996; Harborne et al. 2009; Gil Fernández et al. 2016).
Marine protected areas (MPAs) may, therefore, indirectly affect urchin distribution. Marine protected areas are management zones that provide varying levels of protection to species and ecosystems, conserve and maintain marine biodiversity and natural and associated cultural resources, and enhance the productivity of fish and marine invertebrate populations (Hoyt 2018). Research on MPAs has shown that a return to hypothetical former predation levels through the exclusion of fishing practices and subsequent restoration of predatory fish numbers may reduce urchin densities (e.g. McClanahan and Muthiga 1989; McClanahan and Shafir 1990) and minimise the negative effects of unregulated urchin populations (Harborne et al. 2009). Conversely, a decrease in the abundance of predatory fish, as a result of fishing, may increase sea urchin abundance (Hughes 1994).
Ningaloo Marine Park provides a model system in which to investigate factors affecting urchin distribution both within and outside of MPAs. Ningaloo Marine Park consists of a complex network of multiple-use zones varying in levels of environmental protection and permitted activities, from general use and recreation zones to the more protected special purpose and no-take sanctuary zones (Department of Biodiversity, Conservation and Attractions 2020). Ningaloo Marine Park is considered an intact coral reef system (e.g. Johansson et al. 2010, 2013) and is recognised for its outstanding universal value and significant natural habitats for the conservation of biological diversity (United Nations Educational, Scientific and Cultural Organization 2011). The Ningaloo Marine Park extends along ~300 km of coastline and across three degrees of latitude (Kobryn et al. 2013). As such, it is influenced by a broad range of environmental variables, making it an ideal location to investigate urchin distribution.
Nine species of herbivorous urchins commonly inhabit shallow coral reefs of the Indian Ocean (McClanahan 1998). Of these, Echinometra mathaei and Echinostrephus molaris are widely distributed within the Indo-West Pacific region (Ohgaki et al. 2019) and have been identified as common urchin species on the Ningaloo Reef. Both are small-bodied urchins (Cernohorsky et al. 2015) and, although considered modest bioeroders compared with other urchin species (Russo 1980; Bak 1990), are important in influencing the structure of the healthy coral reef ecosystem at Ningaloo Reef (Langdon et al. 2013). Whereas data on the bioerosion rates of E. molaris on the Reef are lacking, E. mathaei is considered a significant contributor to this process (Johansson et al. 2010; Langdon et al. 2013). Furthermore, E. mathaei algal grazing studies at Ningaloo Reef have demonstrated the important ecological role this species plays in mediating algal growth and competition (Langdon 2012; Langdon et al. 2013).
Although they are identified as sympatric urchin species, E. molaris and E. mathaei vary in their morphological and behavioural characteristics. Echinostrephus molaris is a very small echinometrid, reaching 40-mm test diameter (Campbell et al. 1973). It is considered a burrowing urchin, either boring directly into the reef structure or utilising and expanding pre-existing crevices using its spines, large peristome and Aristotle’s lantern (Edmundson 1946; Asgaard and Bromley 2008). Owing to its morphology, it has adapted to a near-complete existence within these. Its test is barrel-shaped, allowing it to rotate within its boring about its oral–aboral axis, and very short ambital spines along each side ensure secure anchorage within and along its boring. It is the only known echinoid genus adapted to 100% suspension feeding, using its long aboral spines to capture drifting algae and suspended particulates (Asgaard and Bromley 2008). By contrast, E. mathaei grows slightly larger, up to 50-mm test diameter (McClanahan and Muthiga 1989). It occupies pre-formed crevices and cup-shaped borings (Campbell et al. 1973; Clark 1976; Tsuchiya and Nishihira 1984) that it either creates or expands through feeding and spine abrasion (Campbell et al. 1973; Russo 1977, 1980; McClanahan 1988). Although being capable of suspension feeding, it is considered to be a grazing echinoid (Khamala 1971; Dart 1972), predominantly scraping the substratum of turf algae (Dart 1972) and augmenting its diet with floating algal particles under stronger hydrodynamic conditions (Campbell et al. 1973).
Despite the considerable literature available on urchins and their ecological importance, little is known of the factors affecting the distribution of both E. mathaei and E. molaris on intact coral reef systems. This study aims to determine the spatial variation and occurrence of two sympatric urchin species, namely E. mathaei and E. molaris, and the potential factors contributing to this, within the Ningaloo Marine Park. It tests the hypothesis that urchin occurrence is lower within sanctuary zones closed to fishing, owing to increased predation by fish species otherwise targeted by fishers.
Materials and methods
Study site
This study was conducted in the coral reef habitats throughout the Ningaloo Marine Park (Fig. 1), the largest fringing coral reef in Australia. Located along the north-western coast of Western Australia, Ningaloo Marine Park has both State and Commonwealth boundaries and extends latitudes of 21°47′S at Bundegi in the north to 24°00′S at Red Bluff in the south, to the high-water mark. Benthic communities were surveyed during May 2015 and 2016 throughout the northern end of the park on the outer reef slope (n = 74; ~8-m depth) and inner reef habitats (n = 52; includes bommies (n = 25), lagoon (n = 12), reef flat (n = 9) and inshore (n = 6); ~2-m depth). Potential differences in urchin presence among management zones were investigated, with benthic surveys also being conducted within sanctuary (Jurabi, Mangrove and Osprey) and adjacent non-sanctuary zones.
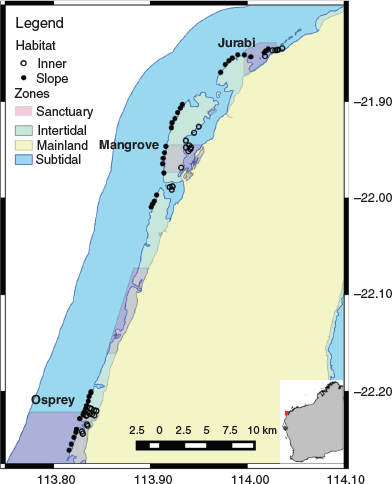
|
Benthic surveys
Urchin presence was determined by evaluating benthic photographs taken by SCUBA divers along a 25-m transect at each site. Photographs were taken parallel to, and at a height of ~0.3 m from, the substrate, at 0.5-m intervals along the transect. The transect start location was chosen haphazardly within the site and the tape laid out following the depth contour. A subset of 30 randomly selected photographs was used at each site to avoid any autocorrelation issues arising from analysing multiple consecutive images. The area represented by each photograph was estimated using the average length and width of photographs containing sections of transect tape and determined to be ~0.35 m2 (0.7 × 0.5 m). From each site, a total of 30 images, representing an area of 10.5 m2 per transect, were used to quantify the two most abundant sea urchin species, namely, Echinometra mathaei (Blainville, 1825) and Echinostrephus molaris (Blainville, 1825). Urchin presence was determined following the positive identification of a representative feature, such as a spine protruding from the burrow. If some part of the urchin was not visible, despite the presence of a characteristic urchin burrow, the presence of that urchin could not be confirmed and was therefore not included.
Structural complexity at each site was estimated using an index of rugosity obtained by laying a 10-m length of chain (chain link 22 × 12 mm) over the substrate alongside a transect tape. The chain was allowed to extend over coral and within the gaps and grooves of the substrate. The distance the chain extended along the transect tape was recorded, and the index of rugosity determined as 10 m divided by the recorded distance of the chain along the transect tape (modified from Risk 1972). Therefore, a high index of rugosity was associated with structurally complex habitat, whereas low values corresponded with smoother, less complex habitat.
Substrate cover (as a percentage) at each site was determined from 30 benthic photographs randomly selected from a total of 50 images. By using TransectMeasure (SeaGIS, Bacchus Marsh, Australia, see https://www.seagis.com.au/transect.html; Abdo et al. 2006), six points were overlaid on each image, and the broad cover class (algae, hard coral, soft coral, sponge, seagrass, abiotic, other, indeterminate, or unknown) beneath each point was recorded. Percentage cover of algae (turfing and macroalgae) was then used as an index of food availability for urchins, whereas percentage hard coral cover was used as an index of competition for space with corals.
Estimates of wave-induced water velocity were derived using ‘simulating waves near shore’ (SWAN) model predictions (Booij et al. 1999). These were based on the mean orbital seafloor velocity at each site on a 30- × 30-m grid encompassing the study area from 1 January to 31 December 2007.
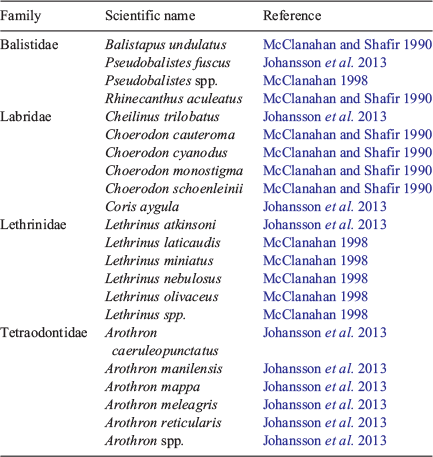
An index of fish predation on E. mathaei was determined using data from underwater visual fish surveys conducted by SCUBA divers along a 100 × 10-m belt transect at each site. These were conducted concurrently with benthic surveys. Teleost species observed along each transect were identified, quantified and the total length was estimated (Babcock et al. 2008). Predatory fish biomass was then determined for each transect by summing the biomass of all observed predators of E. mathaei (identified through the literature; see Table 1) that were ≥35 cm in total length. In total, 22 predatory fish species were included in this index (Table 1). Because little is known of predatory fish species of E. molaris, no index was determined for this species.
|
Statistical analysis
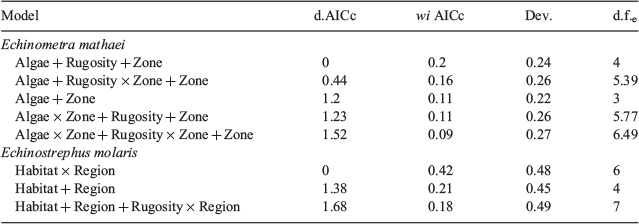
A full set of generalised additive models (GAMS) was constructed, fit and compared using the FSSgam R package (R. Fisher, see https://github.com/beckyfisher/FSSgam_package; Fisher et al. 2018), so as to evaluate which ecological predictors best explained sea urchin occurrence (response variable). These GAMs were fit using a binomial distribution on the presence or absence of urchins on each transect, with a logit link function. Presence or absence was modelled rather than abundance because the data were highly zero inflated, with zeros making up nearly 50% of observations at the transect level for both species (52 and 48% for E. mathaei and E. molaris respectively). Predictor variables included (i) habitat (slope and inner reef habitats, e.g. indicator of shelter, hydrodynamic effects), (ii) region (indicator of spatial distribution), (iii) zone (sanctuary and non-sanctuary, e.g. indicator of predation), (iv) rugosity (index of structural complexity), (v) percentage cover of algae (index of food availability), (vi) percentage cover of hard coral (index of competition for space with corals), and (vii) water velocity (e.g. indicator of food transport, sedimentation), as well as interaction terms. All models with pairs of variables with a Pearson correlation above a covariance cut-off limit of 0.28 were removed, and only models containing up to three predictors were allowed, ensuring that the resulting model fits remained ecologically interpretable (Fisher et al. 2018). The resulting 74 models for each species were compared using Akaike information criteria for small sample sizes (AICc), as described in Fisher et al. (2018). For brevity, only the top-performing models (within 2AICc) for each urchin species are presented, with plots constructed only for that with the lowest AICc. Complete model results for all models within 2AICc are contained in Table S1 of the Supplementary material.
To account for incidences of high predatory fish abundances, non-parametric statistics (Kruskal–Wallis test) were used to compare predatory fish biomass in sanctuary and non-sanctuary zones. All graphics and statistical analyses were run using R (ver. 4.0.0, R Foundation for Statistical Computing, Vienna, Austria, see https://www.R-project.org/).
Results
Generalised additive models of the presence or absence of urchins on transects showed that the predictor variables contained in the model with the lowest AICc value were different for each urchin species. The top-performing model for predicting E. mathaei occurrence contained the variables algal cover, management zone and rugosity, whereas only habitat and region were the variables in the top-performing model for E. molaris (Table 2). The deviance explained by the top-performing model of E. molaris was 0.48, compared with only 0.24 for the top-performing model of E. mathaei.
|
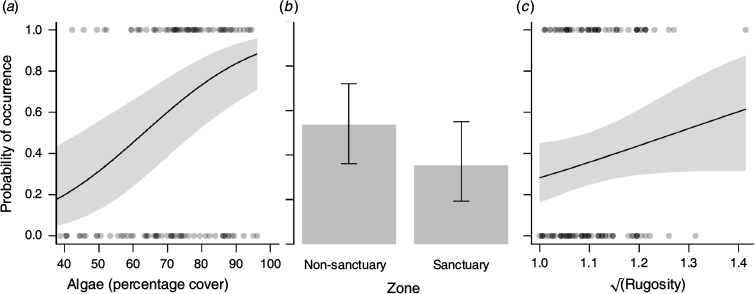
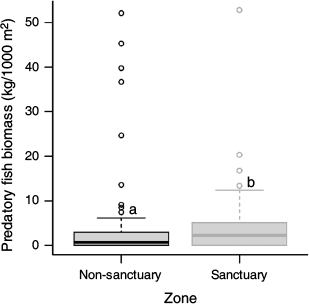
Echinometra mathaei occurrence had a positive relationship with both percentage cover of algae and rugosity (Fig. 2a, c) and were higher in non-sanctuary (2; median individuals per 10.5 m2) than in sanctuary (0; median individuals per 10.5 m2) zones (Fig. 2b). Interestingly, predatory fish biomass was significantly higher in sanctuary (2249; median kilograms per 1000 m2) than in non-sanctuary (698; Kruskal–Wallis χ2 = 5.68, d.f. = 1, P = 0.017) zones (Fig. 3).
|
|
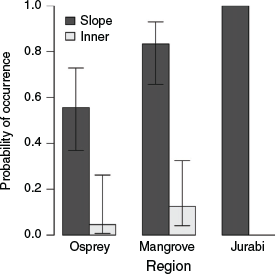
By contrast, E. molaris occurrence was highest on the outer reef slope (29; median individuals per 10.5 m2), with negligible urchins present in inner reef habitats (0; median individuals per 10 m2) across all regions (Fig. 4). For slope habitats, the most northern sites (Jurabi) contained the highest occurrence of E. molaris (29; median individuals per 10.5 m2), whereas the most southern sites (Osprey) contained the lowest (1; median individuals per 10.5 m2; Fig. 4). The interaction between rugosity and region was significant in the third-top model, although this was relatively weak and of lower weight than the model where this interaction is excluded (Table 2).
|
Discussion
This study highlighted the differing spatial distributions of two urchin species within Ningaloo Marine Park and the potential drivers of variation. Although E. mathaei and E. molaris are considered sympatric echinoids, the distribution of each species in this study can be associated with different variables. Echinometra mathaei occurrence typically increased with percentage algal cover and structural complexity and was lower in protected areas. Echinostrephus molaris, in contrast, showed greater presence within reef-slope habitats and northern sites.
Differences in the distribution of species are often related to their ecological niche, defined as the set of environmental conditions within which a species can survive and persist (Hutchinson 1957). Numerous studies have investigated the association among urchin distribution, abundance and habitat (e.g. McClanahan and Kurtis 1991; McClanahan 1998; Dumas et al. 2007; Burt et al. 2010; Graham and Nash 2013), each highlighting the complexities relating to urchin spatial distribution on coral reefs. Here, we discuss the unique factors that may contribute to the sympatry observed in these two urchin species.
Echinometra mathaei
Foraging behaviour
On Ningaloo Reef, the presence of E. mathaei was positively related to percentage algal cover. Echinometra mathaei is considered a grazing echinoid (Khamala 1971; Dart 1972) and systematically scrapes the substratum of turf algae (Dart 1972). Large fractions of calcium carbonate sediments have been identified in the gut contents of E. mathaei (Downing and El Zahr 1987; McClanahan and Kurtis 1991), supporting the conclusion that benthic epi- and endolithic algae are the major components of Echinometra spp. diet (McClanahan and Muthiga 2013). Furthermore, Echinometra spp. allocate energy resources to growth, reproduction and maintenance, depending on food availability (Gadgil and Bossert 1970; Muthiga 1996; Brown-Saracino et al. 2007), specifically algae. Observations of smaller urchin sizes under low algal-cover conditions (Brown-Saracino et al. 2007) and energy-based simulation models (McClanahan 1992, 1995) have highlighted the importance of turf algae as an energy source for E. mathaei. Over half of all benthic cover on Ningaloo Reef is composed of macroalgal and turfing algal communities (Kobryn 2018). This high algal cover and subsequent food availability in some areas of the Reef may, therefore, be a significant contributing factor to increased urchin presence.
Marine protected areas and predation
The presence of E. mathaei was higher in non-sanctuary areas than within sanctuary zones. Conversely, predatory fish abundance was greater within sanctuary zones, suggesting the role of predation in mediating urchin occurrence. Sanctuary zones and MPAs are created to protect and conserve biodiversity, and marine habitats, along with areas and species of high conservation value (Roberts et al. 2005; Edgar et al. 2007; Kirkman 2013). Although responses to protection are highly variable among fish taxa (Côté et al. 2001), MPAs have been shown to effectively increase abundance and biomass of larger fish species vulnerable to fishing practices (e.g. Russ 1985, 2002; García-Rubies and Zabala 1990; Harmelin et al. 1995; Letourneur 1996; Rakitin and Kramer 1996; Côté et al. 2001). This was observed at Ningaloo Reef, with predatory fish biomass being significantly higher in sanctuary than in non-sanctuary zones.
Yet, the increased presence of E. mathaei outside of sanctuary areas compared with that within sanctuary zones, adds support to suggestions that non-target species may respond negatively to protection, which has been attributed to greater predator pressure within sanctuary areas (Letourneur 1996; McClanahan et al. 1999).
Ningaloo Reef is a hotspot for recreational fishing, with the unusually narrow continental shelf and proximity to land making the oceanic waters, reef and coastal communities highly accessible to fishers. Of the >500 species of fish that inhabit the reef and deeper offshore waters (Department of Biodiversity, Conservation and Attractions 2019), many, including balistids, labrids and lethrinids, are identified as predatory species of urchins (McClanahan and Shafir 1990; McClanahan 1998; Johansson et al. 2013), with numerous species of the latter two families being targeted by recreational fishers (Ryan et al. 2017). Whereas conflicting reports of urchin abundances within MPAs at Ningaloo Reef exist (e.g. Westera 2003; Webster 2008; Langdon 2012), recreational line fishing alone may be sufficient to alter the composition of targeted fish (Westera et al. 2003) and, subsequently, urchin populations.
Marine protected areas that provide refuge for species of urchin predators have shown lower urchin densities than have reefs with depauperate predator populations (McClanahan and Muthiga 1989; McClanahan and Kurtis 1991; Hughes 1994; Sala and Zabala 1996; McClanahan et al. 1999; Carreiro-Silva and McClanahan 2001; Harborne et al. 2009; Bronstein and Loya 2014; Gil Fernández et al. 2016). Additionally, the size and biomass of predatory species within MPAs may also adversely affect urchin abundances (Gil Fernández et al. 2016). Larger-bodied fish are able to predate more successfully on urchins than are smaller individuals (Harborne et al. 2009; Johansson et al. 2013). Thus, a greater abundance of larger predatory fish may act as a regulatory agent affecting urchin presence, and account for lower urchin densities (McClanahan and Kurtis 1991; Westera et al. 2003), within sanctuary zones of Ningaloo Marine Park.
Habitat complexity
At Ningaloo Reef, E. mathaei presence was greater in areas of increased rugosity. Structural complexity and coral cover have been recognised as important factors influencing epifaunal mobile invertebrate communities, including urchin populations (Ogden 1976; Weil et al. 2005; Lee 2006; Dumas et al. 2007; Clemente and Hernández 2008; Labbé-Bellas et al. 2016). Additionally, small-scale habitat heterogeneity (e.g. crevices, pits, cracks) has been implicated in urchin recruitment and retention, morphology and behaviour (Hereu et al. 2005; Hernández and Russell 2010; Clemente et al. 2013). This is indicative of the importance of reef structure because of the services provided, including refuge from predation, shelter and food resources (Ogden 1976; McClanahan and Kurtis 1991; Benedetti-Cecchi and Cinelli 1995; McClanahan 1998).
Echinometra mathaei is highly susceptible to predation when outside of its burrow (McClanahan 1988). As such, the availability of shelter is a key factor in determining predator rates (e.g. Roberts and Ormond 1987; Beck 1995) and influencing urchin distribution and abundance (Carpenter 1984; McClanahan and Kurtis 1991; Sala et al. 1998). Echinometra mathaei lives in crevices and cup-shape borings (Campbell et al. 1973; Clark 1976; Tsuchiya and Nishihira 1984) that alone may not provide sufficient protection from predators. However, urchins are capable of accessing concealed microhabitats, burrowing directly into the reef matrix (Done et al. 1991; Bellwood et al. 2004; Glynn and Manzello 2015; Perry and Harborne 2016) and, subsequently, gaining shelter (Labbé-Bellas et al. 2016). Whereas some fishes, e.g. larger triggerfish, are highly efficient predators of concealed urchins (Fricke 1971), other fish families, including labrids and lethrinids, are restricted to predating on solitary exposed urchins.
The role of habitat complexity may also assist reproduction through reducing energy expenditure. Echinometra spp. have low energy requirements because of the reduced amount of organic matter within their bodies (McClanahan and Muthiga 2013). Much of this energy expenditure is dedicated to reproduction, allowing E. mathaei to dominate in low-predation, high environmental-stress conditions (McClanahan and Muthiga 2013). Although described as a burrowing echinoid, Gray (1990) suggested that burrowing behaviour is related to unfavourable environmental conditions, with energy being expended only on such when inhospitable conditions prevail. Lowering energetic demands by reducing the need for burrow formation may allow E. mathaei to direct energy, instead, to reproduction. As a result, an increase in rugosity and structural complexity may provide greater protection from predation and increase the availability of refuges (Johansson et al. 2013), limiting the energy expended on burrow formation.
Echinostrephus molaris
Site specificity
Echinostrephus molaris presence was higher on the outer reef slope than in other areas of the reef. Gradients in the physical environment, including exposure to wave action (Ebert 1982), sedimentation (Dumas et al. 2007; Sangil and Guzman 2016), and habitat structural complexity (Graham and Nash 2013), influence echinoid community structure, abundance and distribution in coral reef ecosystems (Andrew 1993; Clemente and Hernández 2008). The outer reef slope of Ningaloo Reef is structurally complex, being characterised by distinct spur and groove structures (Collins et al. 2011). Spur communities are dominated by encrusting coralline algae, robust-branching and tabular-branching coral assemblages and associated domal, arborescent, foliaceous and encrusting corals (Collins et al. 2011). It is subject to high wave energy, water movement and tidal run-off (Department of Environment, Water, Heritage and the Arts 2008), and decreased sedimentation.
Although introducing a level of stress, hydrodynamics may play an important role in influencing predation, foraging and sedimentation. Echinostrephus molaris has a well-adapted morphology that allows it to occupy longitudinal borings that provide a high level of protection from dislodgement and predation (Campbell et al. 1973). Individuals inhabiting borings have been found in habitats dominated by hydrodynamic forces, including groove structures dominated by offshore flow (Rogers et al. 2013; C. Bessey and D. Thomson, CSIRO, pers. comm.), whereas those within crevices or on bare substrates dominate more sheltered areas (Tsuchiya and Nishihira 1984; Prince 1995). This morphology and use of borings in habitats dominated by hydrodynamic forces, in addition to its preference to feed on drift algae, allow efficient exploitation of food resources and reduce competition from other organisms (Asgaard and Bromley 2008), making it ideally suited to the increased energy and water-flow conditions of the outer reef environment (Johansson et al. 2013).
Echinostrephus is the only known echinoid genus adapted completely to suspension feeding (Asgaard and Bromley 2008), using its spines to catch drifting algae (Campbell et al. 1973; Russo 1977, 1980). In sheltered inner reef flat areas, the quantity of drift algae is far lower than at exposed sites (Russo 1977), owing to decreased current speeds and an absence of macroalgae inhabiting this area (Russo 1977; Neill 1988). Thus, the greater current velocities on the outer exposed reef slope may increase the availability and accessibility of food (Bronstein and Loya 2014). The consumption of drift algae may be especially advantageous when mobile foraging behaviour is hazardous, such as, for example, where the risk of dislodgement or predation is high, enabling E. molaris to persist in otherwise stressful environments (Vanderklift et al. 2009).
Urchin abundance has previously been negatively correlated with increased sedimentation of fine particulates (Dumas et al. 2007; Sangil and Guzman 2016), with high sedimentation negatively affecting grazing rates (Traiger 2019), settlement and early life stages (Walker 2007; Traiger 2019). As a result, it is possible that lower suspended sediment loads resulting from the topographic and hydrodynamic characteristics of the reef slope, compared with sheltered lagoonal and back reef habitats (Browne et al. 2013; Fabricius et al. 2014; Goatley et al. 2016; Tebbett et al. 2017), may contribute to differences in E. molaris presence between these habitats.
Regional variation
The highest occurrence of E. molaris was found at northern sites and it decreased towards southerly sites. Variation in urchin densities has been observed across global, regional, local and within-reef scales (Pearse and Phillips 1968; Kelso 1970; Glynn et al. 1979; McClanahan 1988; McClanahan and Muthiga 2001; Dumas et al. 2007; Johansson et al. 2010). Ningaloo Marine Park extends for 300 km from Bundegi in the north to Red Bluff in the south. Mapping of the marine habitat of Ningaloo Reef has highlighted clear differences in biodiversity among regions (Kobryn 2018) with distinction between the northern and southern regions being attributed to local environmental conditions and the habitat preferences of specific organisms (Kobryn 2018). Within the northern region, extensive macroalgae and higher coral cover transition to sparse and patchy macroalgal and coral communities in the south beyond Yardie Creek (Kobryn et al. 2013; Kobryn 2018). Structurally, substrates transition from spur and groove structures in the north to limestone pavement in the south. As a result, increased E. molaris presence at northern sites may be due to a combination of preferred environmental factors, including greater food availability and increased protection from predation (Sheppard-Brennand et al. 2017) afforded by greater structural complexity.
Yet, the spatial patchiness displayed by urchin populations remains difficult to explain in terms of environmental factors alone (McClanahan and Muthiga 2001; Langdon 2012). Anthropogenic effects of fishing and area use may facilitate differences in urchin densities among regions. Ningaloo Reef, although remote, is easily accessed from the coast, with some areas of reef within 100 m of the shore. Boat ramps and main access points to Ningaloo Reef are located to the north, with the main ramps at Exmouth, Bundegi and Tantabiddi near the tip of the Exmouth Peninsula. The northern Jurabi sites surveyed in this study are located close to the Tantabiddi boat ramp, one of the largest boat ramps in the area, providing direct access to Ningaloo Reef. Sites further south, between Mandu and Osprey, are located on a more remote section of the Reef, further away from access points. Additionally, within this section of Ningaloo Reef, a complex system of marine protected zones and open access areas exists. No-take zones prohibiting fishing practices and special purpose zones are interspersed with recreation and general-purpose areas open to fishing. A large area prohibiting spearfishing extends from Tantabiddi Well, south of Jurabi, to Winderabandi Point, encompassing all southern sites (Mangrove and Osprey). Consequently, the higher occurrence of urchins present at the northern (Jurabi) sites may also be attributed to more extensive fishing areas, and increased access to and fishing of predatory species in this area.
Conclusions
This study has highlighted the related but differing abiotic and biotic factors controlling the distribution of two sympatric urchin species at a small spatial scale. By investigating urchin occurrence and distribution over a range of sites and reef zones, this study has highlighted the species–habitat associations, and emphasises the complexities of these, in determining urchin community structure.
Urchin species have been shown to coexist generally because of the variation in the niche of each species. The relationships between echinoderms and habitat are species-specific and most likely attributed to preferences in resources, predation risk and reproduction (Labbé-Bellas et al. 2016), food preferences, foraging behaviour and morphological adaptations (McClanahan 1988; Bonaviri et al. 2011).
Yet, understanding the mechanisms that control the distribution and abundance of different urchin populations is challenging. Different morphological adaptations, and spatial specialisations to avoid predation and competition (e.g. different burrow types and locations; McClanahan 1988), lead to differences in spatial resource utilisation (McClanahan and Muthiga 1989). Although occupying similar niches in reef communities, the predominantly non-overlapping feeding preferences, and morphological and behavioural differences between E. molaris and E. mathaei may enable these two species to coexist within the intact coral reef system of Ningaloo Marine Park.
Author contributions
E. L. Westlake interpreted data and wrote the paper; C. Bessey conceived the initial project idea and experimental design; C. Bessey and R. Fisher developed the experimental concept, conducted statistical analyses, generated graphics and contributed to manuscript writing; D. P. Thomson and M. D. E. Haywood contributed to data collection. All authors contributed to manuscript revisions.
Compliance with ethical standards
This work was undertaken before the inclusion of observational research in CSIRO ethics requirements. However, this work aligned with current CSIRO Wildlife, Livestock and Laboratory Animal Animal Ethics Committee (CWLLA AEC) approval for the use of free-living wildlife for research (current permit 2019-07).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This research was supported by the Ningaloo Outlook Marine Research Partnership. Ningaloo Outlook is a BHP–CSIRO Industry–Science Marine Research Partnership investing A$5.4 million over 5 years to gather new knowledge on the Ningaloo Reef and its important ecological values. BHP Billiton had no such involvement in the preparation of data, paper or the decision to submit for publication.
Acknowledgements
The authors recognise and thank those who have contributed to the research and preparation of this paper, particularly Jamie Small, Ryan Crossing, Margaret Miller, Anna Cresswell and Melanie Trapon for their logistical and technical assistance, Jim Gunson for hydrodynamic modelling support, and John Keesing, Mat Vanderklift, James McLaughlin and Shaun Wilson for their constructive suggestions and comments on the drafts of this manuscript. We recognise and acknowledge the Jinigudira people, the traditional custodians of the land and sea country on which this study was conducted.
References
Abdo, D. A., Seager, J. W., Harvey, E. S., McDonald, J. I., Kendrick, G. A., and Shortis, M. R. (2006). Efficiently measuring complex sessile epibenthic organisms using a novel photogrammetric technique. Journal of Experimental Marine Biology and Ecology 339, 120–133.| Efficiently measuring complex sessile epibenthic organisms using a novel photogrammetric technique.Crossref | GoogleScholarGoogle Scholar |
Andrew, N. L. (1993). Spatial heterogeneity, sea urchin grazing and habitat structure on reefs in temperate Australia. Ecology 74, 292–302.
| Spatial heterogeneity, sea urchin grazing and habitat structure on reefs in temperate Australia.Crossref | GoogleScholarGoogle Scholar |
Asgaard, U., and Bromley, R. G. (2008). Echinometrid sea urchins, their trophic styles and corresponding bioerosion. In ‘Current Developments in Bioerosion’. (Eds M. Wisshak and L. Tapanila.) Erlangen Earth Conference Series, pp. 279–304. (Springer-Verlag.)
| Crossref |
Babcock, R., Haywood, M., Vanderklift, M., Clapin, G., Kleczkowski, M., Dennis, D., Skewes, T., Milton, D., Murphy, N., Pillans, R., and Limbourn, A. (2008). Ecosystem impacts of human usage and effectiveness of zoning for biodiversity conservation: broad-scale fish census. Final analysis and recommendations 2007. CSIRO Marine and Atmospheric Research.
Bak, R. P. M. (1990). Patterns of echinoid bioerosion in two Pacific coral reef lagoons. Marine Ecology Progress Series 66, 267–272.
| Patterns of echinoid bioerosion in two Pacific coral reef lagoons.Crossref | GoogleScholarGoogle Scholar |
Beck, M. W. (1995). Size-specific shelter limitation in stone crabs: a test of the demographic bottleneck hypothesis. Ecology 76, 968–980.
| Size-specific shelter limitation in stone crabs: a test of the demographic bottleneck hypothesis.Crossref | GoogleScholarGoogle Scholar |
Bellwood, D. R., Hughes, T. P., Folke, C., and Nystrom, M. (2004). Confronting the coral reef crisis. Nature 429, 827–833.
| Confronting the coral reef crisis.Crossref | GoogleScholarGoogle Scholar | 15215854PubMed |
Benedetti-Cecchi, L., and Cinelli, F. (1995). Habitat heterogeneity, sea urchin grazing and the distribution of algae in littoral rock pools on the west coast of Italy (western Mediterranean). Marine Ecology Progress Series 126, 203–212.
| Habitat heterogeneity, sea urchin grazing and the distribution of algae in littoral rock pools on the west coast of Italy (western Mediterranean).Crossref | GoogleScholarGoogle Scholar |
Bonaviri, C., Vega Fernández, T. V., Fanelli, G., Badalamenti, F., and Gianguzza, P. (2011). Leading role of the sea urchin Arbacia lixula in maintaining the barren state in southwestern Mediterranean. Marine Biology 158, 2505–2513.
| Leading role of the sea urchin Arbacia lixula in maintaining the barren state in southwestern Mediterranean.Crossref | GoogleScholarGoogle Scholar |
Booij, N., Ris, R. C., and Holthuijsen, L. H. (1999). A third-generation wave model for coastal regions: 1. Model description and validation. Journal of Geophysical Research – Oceans 104, 7649–7666.
| A third-generation wave model for coastal regions: 1. Model description and validation.Crossref | GoogleScholarGoogle Scholar |
Bronstein, O., and Loya, Y. (2014). Echinoid community structure and rates of herbivory and bioerosion on exposed and sheltered reefs. Journal of Experimental Marine Biology and Ecology 456, 8–17.
| Echinoid community structure and rates of herbivory and bioerosion on exposed and sheltered reefs.Crossref | GoogleScholarGoogle Scholar |
Brown-Saracino, J., Peckol, P., Allen Curran, H., and Robbart, M. L. (2007). Spatial variation in sea urchins, fish predators, and bioerosion rates on coral reefs of Belize. Coral Reefs 26, 71–78.
| Spatial variation in sea urchins, fish predators, and bioerosion rates on coral reefs of Belize.Crossref | GoogleScholarGoogle Scholar |
Browne, N. K., Smithers, S. G., and Perry, C. T. (2013). Carbonate and terrigenous sediment budgets for two inshore turbid reefs on the central Great Barrier Reef. Marine Geology 346, 101–123.
| Carbonate and terrigenous sediment budgets for two inshore turbid reefs on the central Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Burt, J. A., Feary, D. A., Usseglio, P., Bauman, A., and Sale, P. F. (2010). The influence of wave exposure on coral community development on man-made breakwater reefs, with a comparison to a natural reef. Bulletin of Marine Science 86, 839–859.
| The influence of wave exposure on coral community development on man-made breakwater reefs, with a comparison to a natural reef.Crossref | GoogleScholarGoogle Scholar |
Campbell, A. C., Dart, J. K. G., Head, S. M., and Ormond, R. F. G. (1973). The feeding activity of Echinostrephus molaris (de Blainville) in the Central Red Sea. Marine Behaviour and Physiology 2, 155–169.
| The feeding activity of Echinostrephus molaris (de Blainville) in the Central Red Sea.Crossref | GoogleScholarGoogle Scholar |
Carpenter, R. C. (1984). Predator and population density control of homing behavior in the Caribbean echinoid Diadema antillarum. Marine Biology 82, 101–108.
| Predator and population density control of homing behavior in the Caribbean echinoid Diadema antillarum.Crossref | GoogleScholarGoogle Scholar |
Carreiro-Silva, M., and McClanahan, T. R. (2001). Echinoid bioerosion and herbivory on Kenyan coral reefs: the role of protection from fishing. Journal of Experimental Marine Biology and Ecology 262, 133–153.
| Echinoid bioerosion and herbivory on Kenyan coral reefs: the role of protection from fishing.Crossref | GoogleScholarGoogle Scholar | 11445084PubMed |
Cernohorsky, N. H., McClanahan, T. R., Babu, I., and Horsák, M. (2015). Small herbivores suppress algal accumulation on Agatti atoll, Indian Ocean. Coral Reefs 34, 1023–1035.
| Small herbivores suppress algal accumulation on Agatti atoll, Indian Ocean.Crossref | GoogleScholarGoogle Scholar |
Chabanet, P., Ralambondrainy, H., Amanieu, M., Faure, G., and Galzin, R. (1997). Relationships between coral reef substrata and fish. Coral Reefs 16, 93–102.
| Relationships between coral reef substrata and fish.Crossref | GoogleScholarGoogle Scholar |
Clark, A. M. (1976). Echinoderms of coral reefs. Chapter 3. In ‘Biology and Geology of Coral Reefs,’ vol. 3. (Eds O. A. Jones and R. Endean.) pp. 95–123. (Academic Press: New York, NY, USA.)
Clemente, S., and Hernández, J. C. (2008). Influence of wave exposure and habitat complexity in determining spatial variation of the sea urchin Diadema aff. Antillarum (Echinoidae: Diadematidae) populations and macroalgal cover (Canary Islands – eastern Atlantic Ocean). Revista de Biología Tropical 56, 229–254.
Clemente, S., Hernández, J. C., Montaño-Moctezuma, G., Russell, M. P., and Ebert, T. A. (2013). Predators of juvenile sea urchins and the effect of habitat refuges. Marine Biology 160, 579–590.
| Predators of juvenile sea urchins and the effect of habitat refuges.Crossref | GoogleScholarGoogle Scholar |
Collins, L. B., Twiggs, E. J., and Tecchiato, S. (2011). Ningaloo Marine Park – Characterisation of geomorphology and sedimentology. WAMSI 3.4.2 Final Report, Western Australian Marine Science Institution, Perth, WA, Australia.
Côté, I. M., Mosqueira, I., and Reynolds, J. D. (2001). Effects of marine reserve characteristics on the protection of fish populations: a meta-analysis. Journal of Fish Biology 59, 178–189.
| Effects of marine reserve characteristics on the protection of fish populations: a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Dart, J. K. G. (1972). Echinoids, algal lawn and coral recolonization. Nature 239, 50–51.
| Echinoids, algal lawn and coral recolonization.Crossref | GoogleScholarGoogle Scholar |
Department of Biodiversity, Conservation and Attractions (2019). Ningaloo Coast World Heritage Area visitor guide. (DBCA.) Available at https://parks.dpaw.wa.gov.au/sites/default/files/downloads/parks/Ningaloo%20Coast%20World%20Heritage%20Area%20Visitor%20Guide_0.pdf [Verified 31 July 2020].
Department of Biodiversity, Conservation and Attractions (2020). Ningaloo Coast World Heritage Area map and sanctuary zones 2020. (DBCA.) Available at https://parks.dpaw.wa.gov.au/sites/default/files/downloads/parks/Ningaloo%20Coast%20World%20Heritage%20Area%20Visitor%20map%20and%20sanctuary%20zones_0.pdf [Verified 28 May 2021].
Department of Environment, Water, Heritage and the Arts (2008). The North-West Marine Bioregional Plan – Bioregional Profile. (DEWHA.) Available at https://parksaustralia.gov.au/marine/pub/scientific-publications/archive/north-west-bioregional-plan.pdf [Verified 31 July 2020].
Done, T. J., Dayton, P. K., Dayton, A. E., and Steger, R. (1991). Regional and local variability in recovery of shallow coral communities: Moorea, French Polynesia and central Great Barrier Reef. Coral Reefs 9, 183–192.
| Regional and local variability in recovery of shallow coral communities: Moorea, French Polynesia and central Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Done, T. J., Ogden, J. C., Weibe, W. J., and Rosen, B. R. (1996). Biodiversity and ecosystem function of coral reefs. In ‘Functional Roles of Biodiversity: a Global Perspective’. (Eds H. A. Mooney, J. H. Cushman, E. Medina, O. E. Sala, and E-D. Schulze). pp. 393–429. (Wiley: Chichester, UK.)
Downing, N., and El Zahr, C. R. (1987). Gut evacuation and filling rates in the rock-boring sea urchin, Echinometra mathaei. Bulletin of Marine Science 41, 579–584.
Dudgeon, S. R., Aronson, R. B., Bruno, J. F., and Precht, W. F. (2010). Phase shifts and stable states on coral reefs. Marine Ecology Progress Series 413, 201–216.
| Phase shifts and stable states on coral reefs.Crossref | GoogleScholarGoogle Scholar |
Dumas, P., Kulbicki, M., Chifflet, S., Fichez, R., and Ferraris, J. (2007). Environmental factors influencing urchin spatial distributions on disturbed coral reefs (New Caledonia, South Pacific). Journal of Experimental Marine Biology and Ecology 344, 88–100.
| Environmental factors influencing urchin spatial distributions on disturbed coral reefs (New Caledonia, South Pacific).Crossref | GoogleScholarGoogle Scholar |
Ebert, T. A. (1982). Longevity, life history, and relative body wall size in sea urchins. Ecological Monographs 52, 353–394.
| Longevity, life history, and relative body wall size in sea urchins.Crossref | GoogleScholarGoogle Scholar |
Edgar, G. J., Russ, G. R., and Babcock, R. C. (2007). Marine protected areas. In ‘Marine Ecology’. (Eds S. D. Connell and B. M. Gillanders.) pp. 533–564. (Oxford University Press: Melbourne, Vic., Australia.)
Edmunds, P. J., and Carpenter, R. C. (2001). Recovery of Diadema antillarum reduces macroalgal cover and increases abundance of juvenile corals on a Caribbean reef. Proceedings of the National Academy of Sciences of the United States of America 98, 5067–5071.
| Recovery of Diadema antillarum reduces macroalgal cover and increases abundance of juvenile corals on a Caribbean reef.Crossref | GoogleScholarGoogle Scholar | 11274358PubMed |
Edmundson, C. H. (1946). ‘Reef and Shore Fauna of Hawaii’, 2nd edn. (Bernice P. Bishop Museum: Honolulu, HI, USA.)
Fabricius, K. E., Logan, M., Weeks, S., and Brodie, J. (2014). The effects of river run-off on water clarity across the central Great Barrier Reef. Marine Pollution Bulletin 84, 191–200.
| The effects of river run-off on water clarity across the central Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar | 24863415PubMed |
Feehan, C. J., and Scheibling, R. E. (2014). Effects of sea urchin disease on coastal marine ecosystems. Marine Biology 161, 1467–1485.
| Effects of sea urchin disease on coastal marine ecosystems.Crossref | GoogleScholarGoogle Scholar |
Filbee-Dexter, K., and Scheibling, R. E. (2014). Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Marine Ecology Progress Series 495, 1–25.
| Sea urchin barrens as alternative stable states of collapsed kelp ecosystems.Crossref | GoogleScholarGoogle Scholar |
Fisher, R., Wilson, S. K., Sin, T. M., Lee, A. C., and Langlois, T. J. (2018). A simple function for full-subsets multiple regression in ecology with R. Ecology and Evolution 8, 6104–6113.
| A simple function for full-subsets multiple regression in ecology with R.Crossref | GoogleScholarGoogle Scholar | 29988441PubMed |
Fricke, H. W. (1971). Fische als feinde tropischer seeigel. Marine Biology 9, 328–338.
| Fische als feinde tropischer seeigel.Crossref | GoogleScholarGoogle Scholar |
Gadgil, M., and Bossert, W. H. (1970). Life historical consequences of natural selection. American Naturalist 104, 1–24.
| Life historical consequences of natural selection.Crossref | GoogleScholarGoogle Scholar |
García-Rubies, A., and Zabala, M. (1990). Effects of total fishing prohibition on the rocky fish assemblages of Medes Islands marine reserve (NW Mediterranean). Scientia Marina 54, 317–328.
Gil Fernández, C., Paulo, D., Serrão, E. A., and Engelen, A. H. (2016). Limited differences in fish and benthic communities and possible cascading effects inside and outside a protected marine area in Sagres (SW Portugal). Marine Environmental Research 114, 12–23.
| Limited differences in fish and benthic communities and possible cascading effects inside and outside a protected marine area in Sagres (SW Portugal).Crossref | GoogleScholarGoogle Scholar | 26741737PubMed |
Glynn, P. W., and Manzello, D. P. (2015). Bioerosion and coral reef growth: a dynamic balance. In ‘Coral Reefs in the Anthropocene’. (Ed. C. Birkeland.) pp. 67–97. (Springer: Dordrecht, Netherlands.)
| Crossref |
Glynn, P. W., Wellington, G. M., and Birkeland, C. (1979). Coral reef growth in the Galápagos: limitation by sea urchins. Science 203, 47–49.
| Coral reef growth in the Galápagos: limitation by sea urchins.Crossref | GoogleScholarGoogle Scholar | 17840510PubMed |
Goatley, C. H. R., Bonaldo, R. M., Fox, R. J., and Bellwood, D. R. (2016). Sediments and herbivory as sensitive indicators of coral reef degradation. Ecology and Society 21, art29.
| Sediments and herbivory as sensitive indicators of coral reef degradation.Crossref | GoogleScholarGoogle Scholar |
Graham, N. A. J., and Nash, K. L. (2013). The importance of structural complexity in coral reef systems. Coral Reefs 32, 315–326.
| The importance of structural complexity in coral reef systems.Crossref | GoogleScholarGoogle Scholar |
Gray, C. M. (1990). Population densities of the sea urchin, Echinometra mathaei, in relation to defined habitat types in Diani Lagoon, Kenya. Technical Report. (Exeter University: Exeter, UK.) Available at https://aquadocs.org/handle/1834/8484
Harborne, A. R., Renaud, P. G., Tyler, E. H. M., and Mumby, P. J. (2009). Reduced density of the herbivorous urchin Diadema antillarum inside a Caribbean marine reserve linked to increased predation pressure by fishes. Coral Reefs 28, 783–791.
| Reduced density of the herbivorous urchin Diadema antillarum inside a Caribbean marine reserve linked to increased predation pressure by fishes.Crossref | GoogleScholarGoogle Scholar |
Harmelin, J. G., Bachet, F., and Garcia, F. (1995). Mediterranean marine reserves: fish indices as tests of protection efficiency. Marine Ecology (Berlin) 16, 233–250.
| Mediterranean marine reserves: fish indices as tests of protection efficiency.Crossref | GoogleScholarGoogle Scholar |
Hay, M. E. (1984). Patterns of fish and urchin grazing on Caribbean coral reefs: are previous results typical? Ecology 65, 446–454.
| Patterns of fish and urchin grazing on Caribbean coral reefs: are previous results typical?Crossref | GoogleScholarGoogle Scholar |
Hereu, B., Zabala, M., Linares, C., and Sala, E. (2005). The effects of predator abundance and habitat structural complexity on survival of juvenile sea urchins. Marine Biology 146, 293–299.
| The effects of predator abundance and habitat structural complexity on survival of juvenile sea urchins.Crossref | GoogleScholarGoogle Scholar |
Hernández, J. C., and Russell, M. P. (2010). Substratum cavities affect growth-plasticity, allometry, movement and feeding rates in the sea urchin Strongylocentrotus purpuratus. Journal of Experimental Marine Biology and Ecology 213, 520–525.
| Substratum cavities affect growth-plasticity, allometry, movement and feeding rates in the sea urchin Strongylocentrotus purpuratus.Crossref | GoogleScholarGoogle Scholar |
Hoyt, E. (2018). Marine Protected Areas. In ‘Encyclopedia of Marine Mammals’, 3rd edn. (Eds B. Würsig, J. G. M. Thewissen, and K. M. Kovacs.) pp. 569–580. (Academic Press.)
| Crossref |
Hughes, T. P. (1994). Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265, 1547–1551.
| Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef.Crossref | GoogleScholarGoogle Scholar | 17801530PubMed |
Hughes, T. P., Reed, D. C., and Boyle, M.-J. (1987). Herbivory on coral reefs: community structure following mass mortalities of sea urchins. Journal of Experimental Marine Biology and Ecology 113, 39–59.
| Herbivory on coral reefs: community structure following mass mortalities of sea urchins.Crossref | GoogleScholarGoogle Scholar |
Hutchinson, G. E. (1957). Concluding remarks. Cold Spring Harbor Symposia on Quantitative Biology 22, 415–427.
| Concluding remarks.Crossref | GoogleScholarGoogle Scholar |
Johansson, C. L., Bellwood, D. R., and Depczynski, M. (2010). Sea urchins, macroalgae and coral reef decline: a functional evaluation of an intact reef system, Ningaloo, Western Australia. Marine Ecology Progress Series 414, 65–74.
| Sea urchins, macroalgae and coral reef decline: a functional evaluation of an intact reef system, Ningaloo, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Johansson, C. L., Bellwood, D. R., Depczynski, M., and Hoey, A. S. (2013). The distribution of the sea urchin Echinometra mathaei (de Blainville) and its predators on Ningaloo Reef, Western Australia: the implication for top-down control in an intact reef system. Journal of Experimental Marine Biology and Ecology 442, 39–46.
| The distribution of the sea urchin Echinometra mathaei (de Blainville) and its predators on Ningaloo Reef, Western Australia: the implication for top-down control in an intact reef system.Crossref | GoogleScholarGoogle Scholar |
Kelso, D. P. (1970). Comparative morphological and ecological study of two species of the sea urchin genus Echinometra in Hawaii. Ph.D. Thesis, University of Hawaii, HI, USA. Available at http://hdl.handle.net/10125/12001.
Khamala, C. P. M. (1971). Ecology of Echinometra mathaeii (Echinoidea: Echinodermata) at Diani Beach, Kenya. Marine Biology 11, 167–172.
Kirkman, H. (2013). Choosing boundaries to marine protected areas and zoning the MPAs for restricted use and management. Ocean and Coastal Management 81, 38–48.
| Choosing boundaries to marine protected areas and zoning the MPAs for restricted use and management.Crossref | GoogleScholarGoogle Scholar |
Kobryn, T. H. (2018). Mapping marine and terrestrial habitat in Ningaloo Reef. (Ningaloo Collaboration Cluster.) Available at https://research.csiro.au/ningaloo/wp-content/uploads/sites/59/2018/09/20-Ningaloo_factsheet_Kobryn_marine-habitats_Final.pdf [Verified 31 July 2020].
Kobryn, H. T., Wouters, K., Beckley, L. E., and Heege, T. (2013). Ningaloo Reef: shallow marine habitats mapped using a hyperspectral sensor. PLoS One 8, e70105.
| Ningaloo Reef: shallow marine habitats mapped using a hyperspectral sensor.Crossref | GoogleScholarGoogle Scholar | 23922921PubMed |
Labbé-Bellas, R., Cordeiro, C. A. M. M., Floeter, S. R., and Segal, B. (2016). Sea urchin abundance and habitat relationships in difference Brazilian reef types. Regional Studies in Marine Science 8, 33–40.
| Sea urchin abundance and habitat relationships in difference Brazilian reef types.Crossref | GoogleScholarGoogle Scholar |
Langdon, M. W. (2012). The ecology of the grazing urchin Echinometra mathaei at Ningaloo Marine Park. Ph.D. thesis, Murdoch University, Perth, WA, Australia.
Langdon, M. W., van Keulen, M., and Paling, E. I. (2013). Distribution, abundance and bioerosion of the grazing sea urchin Echinometra mathaei at Ningaloo Marine Park, Western Australia. Journal of the Royal Society of Western Australia 96, 19.
Lawrence, J. M. (1975). On the relationships between marine plants and sea urchins. Oceanography and Marine Biology – an Annual Review 13, 213–286.
Lawrence, J. M. (2001). The edible sea-urchins. Developments in Aquaculture and Fisheries Science 32, 1–4.
| The edible sea-urchins.Crossref | GoogleScholarGoogle Scholar |
Lawrence, J. M., and Sammarco, P. W. (1982). Effects of feeding on the environment: Echinoidea. In ‘Echinoderm Nutrition’. (Eds M. Jangoux and J. M. Lawrence.) pp. 499–519. (CRC Press: Rotterdam, Netherlands.)
Lee, S. C. (2006). Habitat complexity and consumer-mediated positive feedbacks on a Caribbean coral reef. Oikos 112, 442–447.
| Habitat complexity and consumer-mediated positive feedbacks on a Caribbean coral reef.Crossref | GoogleScholarGoogle Scholar |
Lessios, H. A. (1988). Mass mortality of Diadema antillarum in the Caribbean: what have we learned? Annual Review of Ecology, Evolution, and Systematics 19, 371–393.
| Mass mortality of Diadema antillarum in the Caribbean: what have we learned?Crossref | GoogleScholarGoogle Scholar |
Letourneur, Y. (1996). Réponses des peuplements et populations de poisons aux reserves marines: le cas de l’île de Mayotte, Océan Indien occidental. Ecoscience 3, 442–450.
| Réponses des peuplements et populations de poisons aux reserves marines: le cas de l’île de Mayotte, Océan Indien occidental.Crossref | GoogleScholarGoogle Scholar |
McClanahan, T. R. (1988). Coexistence in a sea urchin guild and its implications to coral reef diversity and degradation. Oecologia 77, 210–218.
| Coexistence in a sea urchin guild and its implications to coral reef diversity and degradation.Crossref | GoogleScholarGoogle Scholar | 28310374PubMed |
McClanahan, T. R. (1992). Resource utilisation, competition, and predation: a model and example from coral reef grazers. Ecological Modelling 61, 195–215.
| Resource utilisation, competition, and predation: a model and example from coral reef grazers.Crossref | GoogleScholarGoogle Scholar |
McClanahan, T. R. (1995). Fish predators and scavengers of the sea urchin Echinometra mathaei in Kenyan coral-reef marine parks. Environmental Biology of Fishes 43, 187–193.
| Fish predators and scavengers of the sea urchin Echinometra mathaei in Kenyan coral-reef marine parks.Crossref | GoogleScholarGoogle Scholar |
McClanahan, T. R. (1998). Predation and the distribution and abundance of tropical sea urchin populations. Journal of Experimental Marine Biology and Ecology 221, 231–255.
| Predation and the distribution and abundance of tropical sea urchin populations.Crossref | GoogleScholarGoogle Scholar |
McClanahan, T. R., and Kurtis, J. D. (1991). Population regulation of the rock-boring sea urchin Echinometra mathaei (de Blainville). Journal of Experimental Marine Biology and Ecology 147, 121–146.
| Population regulation of the rock-boring sea urchin Echinometra mathaei (de Blainville).Crossref | GoogleScholarGoogle Scholar |
McClanahan, T. R., and Muthiga, N. A. (1989). Patterns of predation of a sea urchin, Echinometra methaei (de Blainville), on Kenyan coral reefs. Journal of Experimental Marine Biology and Ecology 126, 77–94.
| Patterns of predation of a sea urchin, Echinometra methaei (de Blainville), on Kenyan coral reefs.Crossref | GoogleScholarGoogle Scholar |
McClanahan, T. R., and Muthiga, N. A. (2001). The ecology of Echinometra. In ‘Edible Sea Urchins’. (Ed J. M. Lawrence). pp. 225–243. (Elsevier: Amsterdam, Netherlands.)
McClanahan, T. R., and Muthiga, N. A. (2013). Chapter 23: Echinometra. In ‘Developments in Aquaculture and Fisheries Science’, vol. 38. pp. 337–353. (Elsevier.)
| Crossref |
McClanahan, T. R., and Shafir, S. H. (1990). Causes and consequences of sea urchin abundance and diversity in Kenyan coral reef lagoons. Oecologia 83, 362–370.
| Causes and consequences of sea urchin abundance and diversity in Kenyan coral reef lagoons.Crossref | GoogleScholarGoogle Scholar | 28313008PubMed |
McClanahan, T. R., Muthiga, N. A., Kamukuru, A. T., Machano, H., and Kiambo, R. W. (1999). The effects of marine parks and fishing on coral reefs of northern Tanzania. Biological Conservation 89, 161–182.
| The effects of marine parks and fishing on coral reefs of northern Tanzania.Crossref | GoogleScholarGoogle Scholar |
Muthiga, N. A. (1996). The role of early life history strategies on the population dynamics of the sea urchin Echinometra mathaei (de Blainville) on reefs in Kenya. Ph.D. Thesis, University of Nairobi, Mombasa, Kenya.
Neill, J. B. (1988). Experimental analysis of burrow defense in Echinometra mathaei (de Blainville) on Indo-West Pacific reef flat. Journal of Experimental Marine Biology and Ecology 115, 127–136.
| Experimental analysis of burrow defense in Echinometra mathaei (de Blainville) on Indo-West Pacific reef flat.Crossref | GoogleScholarGoogle Scholar |
Ogden, J. C. (1976). Some aspects of herbivore-plant relationships on Caribbean reefs and seagrass beds. Aquatic Botany 2, 103–116.
| Some aspects of herbivore-plant relationships on Caribbean reefs and seagrass beds.Crossref | GoogleScholarGoogle Scholar |
Ogden, N. B., Odgen, J. C., and Abbott, I. A. (1989). Distribution, abundance and food of sea urchins on a leeward Hawaiian reef. Bulletin of Marine Science 45, 539–549.
Ohgaki, S.-I., Kato, T., Kobayashi, N., Tanase, H., Kumagai, N. H., Ishida, S., Nakano, T., Wada, Y., and Yusa, Y. (2019). Effects of temperature and red tides on sea urchin abundance and species richness over 45 years in southern Japan. Ecological Indicators 96, 684–693.
| Effects of temperature and red tides on sea urchin abundance and species richness over 45 years in southern Japan.Crossref | GoogleScholarGoogle Scholar |
Pearse, J., and Phillips, B. F. F. (1968). Continuous reproduction in the Indo-Pacific sea urchin Echinometra mathaei at Rottnest Island, Western Australia. Australian Journal of Marine and Freshwater Research 19, 161–172.
| Continuous reproduction in the Indo-Pacific sea urchin Echinometra mathaei at Rottnest Island, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Perry, C. T., and Harborne, A. R. (2016). Bioerosion on modern reefs: impacts and responses under changing ecological and environmental conditions. In ‘Coral Reefs at the Crossroads. Coral Reefs of the World’, vol. 6. (Eds D. K. Hubbard, C. S. Rogers, J. H. Lipps, and G. D. Stanley Jr.) pp. 69–101. (Springer: Dordrecht, Netherlands.)
| Crossref |
Peyrot-Clausade, M., Chabanet, P., Conand, C., Fontaine, M. F., Letourneur, Y., and Harmelin-Vivien, M. (2000). Sea urchin and fish bioerosion on La Réunion and Moorea reefs. Bulletin of Marine Science 66, 477–485.
Prince, J. (1995). Limited effects of the sea urchin Echinometra mathaei (de Blainville) on the recruitment of benthic algae and macroinvertebrates into intertidal rock platforms at Rottnest Island, Western Australia. Journal of Experimental Marine Biology and Ecology 186, 237–258.
| Limited effects of the sea urchin Echinometra mathaei (de Blainville) on the recruitment of benthic algae and macroinvertebrates into intertidal rock platforms at Rottnest Island, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Rakitin, A., and Kramer, D. L. (1996). Effect of a marine reserve on the distribution of coral reef fishes in Barbados. Marine Ecology Progress Series 131, 97–113.
| Effect of a marine reserve on the distribution of coral reef fishes in Barbados.Crossref | GoogleScholarGoogle Scholar |
Risk, M. J. (1972). Fish diversity on a coral reef in the Virgin Islands. Atoll Research Bulletin 153, 1–4.
| Fish diversity on a coral reef in the Virgin Islands.Crossref | GoogleScholarGoogle Scholar |
Roberts, C. M., and Ormond, R. F. G. (1987). Habitat complexity and coral reef fish diversity and abundance on Red Sea fringing reefs. Marine Ecology Progress Series 41, 1–8.
| Habitat complexity and coral reef fish diversity and abundance on Red Sea fringing reefs.Crossref | GoogleScholarGoogle Scholar |
Roberts, C. M., Hawkins, J. P., and Gell, F. R. (2005). The role of marine reserves in achieving sustainable fisheries. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 360, 123–132.
| The role of marine reserves in achieving sustainable fisheries.Crossref | GoogleScholarGoogle Scholar | 15713592PubMed |
Rogers, J. S., Monismith, S. G., Feddersen, F., and Storlazzi, C. D. (2013). Hydrodynamics of spur and groove formations on a coral reef. Journal of Geophysical Research – Oceans 118, 3059–3073.
| Hydrodynamics of spur and groove formations on a coral reef.Crossref | GoogleScholarGoogle Scholar |
Russ, G. (1985). Effects of protective management on coral reef fishes in the central Philippines. In ‘Proceedings of the 5th International Coral Reef Congress’, 27 May–1 June 1985, Tahiti. (Eds C. Gabrie, and B. Salvat.) Vol. 4, pp. 219–224. (Museum National d’Histoire Naturelle and Ecole Pratique des Hautes Etudes: Tahiti, French Polynesia.)
Russ, G. R. (2002). Yet another review of marine reserves as reef fishery management tools. In ‘Coral Reef Fishes. Dynamics and Diversity in a Complex Ecosystem’. (Ed. P. F. Sale.) pp. 421–443. (Academic Press: San Diego, CA, USA.)
| Crossref |
Russo, A. R. (1977). Water flow and the distribution and abundance of echinoids (genus Echinometra) on a Hawaiian Reef. Marine and Freshwater Research 28, 693–702.
| Water flow and the distribution and abundance of echinoids (genus Echinometra) on a Hawaiian Reef.Crossref | GoogleScholarGoogle Scholar |
Russo, A. R. (1980). Bioerosion by two rock boring echinoids (Echinometra mathaei and Echinostrephus aciculatus) on Enewetak Atoll, Marshall Islands. Journal of Marine Research 38, 99–110.
Ryan, K. L., Hall, N. G., Lai, E. K., Smallwood, C. B., Taylor, S. M., and Wise, B. S. (2017). Statewide survey of boat-based recreational fishing in Western Australia 2015/16. Fisheries Research report number 287, Department of Primary Industries and Regional Development, Perth WA, Australia.
Sala, E., and Zabala, M. (1996). Fish predation and the structure of the sea urchin Paracentrotus lividus populations in the NW Mediterranean. Marine Ecology Progress Series 140, 71–81.
| Fish predation and the structure of the sea urchin Paracentrotus lividus populations in the NW Mediterranean.Crossref | GoogleScholarGoogle Scholar |
Sala, E., Ribes, M., Hereu, B., Zabala, M., Alvà, V., Coma, R., and Garrabou, J. (1998). Temporal variability in abundance of the sea urchins Paracentrotus lividus and Arbacia lixula in the northwestern Mediterranean: comparison between a marine reserve and an unprotected area. Marine Ecology Progress Series 168, 135–145.
| Temporal variability in abundance of the sea urchins Paracentrotus lividus and Arbacia lixula in the northwestern Mediterranean: comparison between a marine reserve and an unprotected area.Crossref | GoogleScholarGoogle Scholar |
Sammarco, P. W. (1980). Diadema and its relationship to coral spat mortality: grazing, competition, and biological disturbance. Journal of Experimental Marine Biology and Ecology 45, 245–272.
| Diadema and its relationship to coral spat mortality: grazing, competition, and biological disturbance.Crossref | GoogleScholarGoogle Scholar |
Sánchez-Jérez, P., Cesar, A., Cortez, F. S., Pereira, C. D. S., and Silva, S. L. R. (2001). Spatial distribution of the most abundant sea urchin populations on the southeast coast of São Paulo (Brazil). Ciencias Marinas 27, 139–153.
| Spatial distribution of the most abundant sea urchin populations on the southeast coast of São Paulo (Brazil).Crossref | GoogleScholarGoogle Scholar |
Sangil, C., and Guzman, H. M. (2016). Assessing the herbivore role of the sea urchin Echinometra viridis: keys to determine the structure of communities in disturbed coral reefs. Marine Environmental Research 120, 202–213.
| Assessing the herbivore role of the sea urchin Echinometra viridis: keys to determine the structure of communities in disturbed coral reefs.Crossref | GoogleScholarGoogle Scholar | 27591516PubMed |
Scheffer, M., Carpenter, S., Foley, J. A., Folkes, C., and Walker, B. (2001). Catastrophic shifts in ecosystems. Nature 413, 591–596.
| Catastrophic shifts in ecosystems.Crossref | GoogleScholarGoogle Scholar | 11595939PubMed |
Scoffin, T., Stearn, C., Boucher, D., Frydl, P., Hawkins, C., Hunter, I. W., and Macgeachy, J. (1980). Calcium carbonate budget of a fringing reef on the West Coast of Barbados. Bulletin of Marine Science 30, 475–508.
Sheppard-Brennand, H., Dworjanyn, S. A., and Poore, A. G. B. (2017). Global patterns in the effects of predator declines on sea urchins. Ecography 40, 1029–1039.
| Global patterns in the effects of predator declines on sea urchins.Crossref | GoogleScholarGoogle Scholar |
Tebbett, S. B., Goatley, C. H. R., and Bellwood, D. R. (2017). Algal turf sediments and sediment production by parrotfishes across the continental shelf of the northern Great Barrier Reef. PLoS One 12, e0170854.
| Algal turf sediments and sediment production by parrotfishes across the continental shelf of the northern Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar | 28122042PubMed |
Traiger, S. B. (2019). Effects of elevated temperature and sedimentation on grazing rates of the green sea urchin: implications for kelp forests exposed to increased sedimentation with climate change. Helgoland Marine Research 73, 5.
| Effects of elevated temperature and sedimentation on grazing rates of the green sea urchin: implications for kelp forests exposed to increased sedimentation with climate change.Crossref | GoogleScholarGoogle Scholar |
Tsuchiya, M., and Nishihira, M. (1984). Distribution of two types of the sea urchin Echinometra mathaei (Blainville) on Okinawan reef flat. Galaxea 3, 131–143.
United Nations Educational, Scientific and Cultural Organization (2011). Natural properties – Ningaloo Coast (Australia). Decision: 35 COM 8B.7. In ‘Decisions adopted by the World Heritage Committee at its 35th session’. WHC-11/35.COM/20, pp. 174–177. (UNESCO: Paris, France.)
Vanderklift, M. A., Lavery, P. S., and Waddington, K. I. (2009). Intensity of herbivory on kelp by fish and sea urchins differs between inshore and offshore reefs. Marine Ecology Progress Series 376, 203–211.
| Intensity of herbivory on kelp by fish and sea urchins differs between inshore and offshore reefs.Crossref | GoogleScholarGoogle Scholar |
Walker, J. W. (2007). Effects of fine sediments on settlement and survival of the sea urchin Evechinus chloroticus in northeaster New Zealand. Marine Ecology Progress Series 331, 109–118.
| Effects of fine sediments on settlement and survival of the sea urchin Evechinus chloroticus in northeaster New Zealand.Crossref | GoogleScholarGoogle Scholar |
Watts, R. J., Johnson, M. S., and Black, R. (1990). Effects of recruitment on genetic patchiness in the urchin Echinometra mathaei in Western Australia. Marine Biology 105, 145–151.
| Effects of recruitment on genetic patchiness in the urchin Echinometra mathaei in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Webster, F. (2008). Coral reef resilience: balancing production and herbivory. Ph.D. Thesis, Murdoch University, Perth, WA, Australia.
Weil, E., Torres, J. L., and Ashton, M. (2005). Population characteristics of the sea urchin Diadema antillarum in La Parguera, Puerto Rico, 17 years after the mass mortality event. Revista de Biología Tropical 53, 219–231.
| 17471614PubMed |
Westera, M. (2003). The effect of recreational fishing on targeted fishes and trophic structure, in a coral reef marine park. Ph.D. Thesis, Edith Cowan University, Perth, WA, Australia. Available at https://ro.ecu.edu.au/theses/1499
Westera, M., Lavery, P., and Hyndes, G. (2003). Differences in recreationally targeted fishes between protected and fished areas of a coral reef marine park. Journal of Experimental Marine Biology and Ecology 294, 145–168.
| Differences in recreationally targeted fishes between protected and fished areas of a coral reef marine park.Crossref | GoogleScholarGoogle Scholar |


