What eats a cauliflower coral? An assessment of predation on the endangered temperate soft coral, Dendronepthya australis
H. Finlay-Jones A , V. Raoult A C , D. Harasti B and T. F. Gaston A
A C , D. Harasti B and T. F. Gaston A
A School of Environmental and Life Sciences, University of Newcastle, 10 Chittaway Road, Ourimbah, NSW 2258, Australia.
B Port Stephens Fisheries Instititute, New South Wales Department of Primary Industries, Taylors Beach Road, Taylors Beach, NSW, 2316, Australia.
C Corresponding author. Email: vincent.raoult@newcastle.edu.au
Marine and Freshwater Research 73(3) 307-318 https://doi.org/10.1071/MF21155
Submitted: 31 May 2021 Accepted: 7 October 2021 Published: 16 November 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Temperate soft corals are found in many estuaries around the world and often form large habitats in these environments, yet the functional ecology of soft corals is poorly understood. To understand the functional role of a soft coral in temperate ecosystems, we examined the role of the endangered Dendronepthya australis cauliflower coral as habitat for fishes and invertebrates, and whether associated species used the soft coral as a food source. Using Bayesian stable isotope mixing models of δ13C and δ15N values of soft corals and a suite of potential invertebrate consumers, we found that five of eight soft-coral-associated invertebrates were all likely to be feeding almost exclusively on the soft corals. In situ feeding experiments conducted using baited remote underwater video systems (BRUVS) with soft coral cuttings as ‘bait’ did not identify any larger species as consumers. Fish assemblages studied using remote underwater video systems (RUVS) were diverse in the soft coral habitat and overlapped with assemblages of both sediment and seagrass environments. These results highlighted that these soft corals have a valuable trophic role in estuarine food webs through trophic transfer of nutrients via invertebrate consumers, and that soft coral habitats are used by commercially and recreationally important fishes.
Keywords: soft coral, functional ecology, estuary, habitat, trophic ecology, stable isotopes.
Introduction
Food webs are complex systems that include large numbers of species from many trophic levels, with complicated links and interactions among organisms (Briand et al. 2016). However, the role of some organisms within those food webs cannot always be clearly quantified. For example, keystone species have a disproportionate impact on an ecosystem relative to their abundance (Lu et al. 2001). Less abundant species may have specialised interactions that also make them equally important (Dobson 2009), or add redundancy within the food webs (Allesina et al. 2009). Trophic effects may also be non-consumptive and drive changes in behaviour as a result of the potential for predation (Hammerschlag et al. 2018; Lester et al. 2020). For this reason, all species within an ecosystem should be included in food web models, because they all provide functions within the ecosystem (Libralato et al. 2006) that may enhance resilience to anthropogenic pressures (Urrutia-Cordero et al. 2016).
Soft corals are often ignored in ecological studies, including in food web analyses (Steinberg et al. 2020). In part, this may be due to the evidence that in some systems, abundant soft coral habitats are indicators of habitat loss (Norström et al. 2009) and that they often contain natural toxins that deter predation (Coll et al. 1982; Fernando et al. 2017). However, there is growing evidence that soft coral habitats add value to, rather than subtract value from, marine ecosystems, especially as habitat for marine fishes, but also by producing metabolites that reduce stress in corals (Baillon et al. 2012; Poulos et al. 2013; Haydon et al. 2018; Epstein and Kingsford 2019). In some ecosystems, soft corals are regularly fed on by fishes (Garra et al. 2020), and thus may be key components of marine food webs. These traits question the apparent consensus that soft coral habitats have low value for marine ecosystems.
Temperate soft coral habitats often occur in developed areas exposed to a mix of anthropogenic pressures, which can increase the likelihood of the loss of soft coral habitats. In 2021, the cauliflower soft coral, Dendronephthya australis (family Nephtheidae), became the first species of soft coral to be listed as Threatened in Australia under threatened species legislation. It became listed as Endangered under both state (NSW Fisheries Management Act 1999) and national legislation (Environment Protection and Biodiversity Conservation Act 1999) because of the declines in distribution and abundance across its range. A recent study investigating the rate of decline in the Port Stephens estuary, where it was previously considered abundant, found a decline of ~70% over the past decade (Larkin et al. 2021). Declines in the soft coral abundance have been attributed to damage from boat anchoring, poorly installed boat moorings, sand inundation (Harasti 2016) and entanglement by fishing line (Smith and Edgar 2014), and this soft coral is susceptible to predation from opisthobranch species, with the level of predation difficult to assess (Davis et al. 2018).
Dendronephthya australis predominately occurs in estuarine environments from Port Stephens south to Jervis Bay in New South Wales, where it is known to occur in depths of 1–18 m (Davis et al. 2015; Poulos et al. 2016). Occurrence of D. australis within the Port Stephens estuary is influenced by the following four environmental parameters: bathymetry, slope of seabed, velocity of tidal currents and distance from estuary mouth (Poulos et al. 2016), and colony size changes with the relative current velocity (Davis et al. 2015). However, whether predation pressures affect D. australis distributions is currently unknown. It is also unclear what impacts on broader temperate ecosystems the loss of D. australis habitats would result in.
Soft coral habitats on the eastern coast of Australia appear to be used consistently by a wide variety of animals. Dendronephthya australis habitat in particular is used by a range of invertebrate and fish species (Davis et al. 2015, 2017; van Lier et al. 2017), particularly juvenile snapper (Chrysophrys auratus), an important recreational and commercial fish species (Poulos et al. 2013, 2016). Dendronephthya australis is also a preferred habitat for the Endangered white’s seahorse, Hippocampus whitei, with adults showing a preference for this habitat over other habitats such as seagrasses (Harasti et al. 2014b). It is unclear whether the associations of fishes with D. australis are driven by the structural habitat D. australis provides or through a direct consumptive interaction. In a recent assessment of the role of D. australis in the benthic food web, there was almost no evidence to suggest that D. australis was used as a direct food source by consumers (Corry et al. 2018). However, Corry et al. (2018) explored the functional role of D. australis in only one estuary (Port Stephens) where it typically occurs at depths of ~15 m, whereas the soft coral is also found in the shallower (<3 m) and more urbanised estuary of Brisbane Water, ~100 km south of Port Stephens. In addition, Corry et al. (2018), by using stable isotope analysis, examined the resource use of D. australis itself, and found little to no evidence of the corals being a secondary source for higher-order consumers. Given the conflicting evidence that suggests that soft corals can be predated on, it is possible that the functional role of D. australis differs among estuaries and is location dependent.
In the present study, we aimed to identify species associated with D. australis and whether the associated community was unique among estuary habitats within two New South Wales estuaries where D. australis occurs. We also assessed the species assemblage physically living on the soft corals. We aimed to identify the role of D. australis in estuarine food webs through use of predation experiments, by using remote underwater videos baited with soft coral clippings, and through stable isotope analyses. Developing a better understanding of the functional ecology of the endangered D. australis will allow informed management of this threatened habitat within these two estuaries that are under different anthropogenic pressures, and can also contribute to management in other estuaries where it may be found in the future.
Materials and methods
Study areas
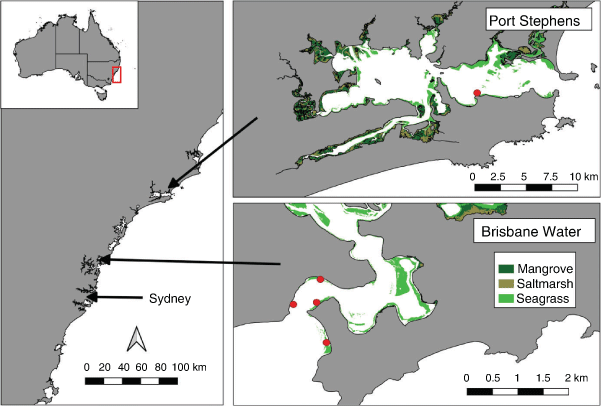
This study was conducted in two estuaries, namely, Brisbane Water estuary located on the Central Coast of New South Wales (NSW), Australia, ~50 km north of Sydney (33.52°S, 151.33°E), and Port Stephens estuary located ~100 km further north (32.69°S, 152.04°E). Brisbane Water estuary is an open wave-dominated barrier estuary system, has a river catchment area of 153 km2 and travels for 18 km towards the south, to the mouth at Broken Bay. The estuary contains many different habitats including saltmarsh, mangroves, seagrass, sponge and soft coral habitats. Wagstaffe Point has a high abundance of threatened seagrass habitat (Posidonia australis; Department of Primary Industries 2008). There are three known locations of D. australis colonies in Brisbane Water, namely, Lobster Beach, Ettalong beach and the foreshore of Ettalong (Houghton 2016). These locations are within 100 m of the shoreline and have a bottom depth of less than 4 m (Houghton 2016). These three locations had the highest observable abundances of colonies within the southern part of the estuary and were used as the sampling locations (Fig. 1). The Lobster Beach habitat is flatter and has little seagrass nearby. The Ettalong Beach and Ettalong Foreshore sites are surrounded by seagrasses (mostly Zostera sp. but also Halophila ovalis), with some seagrass growing among the colonies, and more varied bathymetry ranging from 1- to 5-m depth. The Wagstaffe Point seagrass area is covered by dense Zostera sp. and Posidonia australis and has a bathymetric profile similar to that of Lobster Beach.
|
The Port Stephens estuary is the largest drowned river valley in NSW (Roy et al. 2001) and consists of an eastern (49 km2) and western (85 km2) basin that are linked by a 1-km-wide channel at Soldiers Point. The eastern basin is a marine-dominated environment that contains extensive sponge, seagrass and rocky reef habitats (Davis et al. 2015). Several mapping studies of marine habitats in the estuary have found that D. australis predominantly occurs along the southern shoreline (Davis et al. 2015; Poulos et al. 2016; Larkin et al. 2021).
Food-web sample collection
Sampling was conducted under the approval of the NSW Department of Primary Industry Scientific Research Permit P01/0059(A)-4.0 and The University of Newcastle Animal Ethics Approval A-2012-238. Soft coral colonies were collected from Ettalong Beach, Ettalong Foreshore and Lobster Beach in Brisbane Water from May to July 2019, and from a site known as the Pipeline in Port Stephens (32°43′05″S, 152°08′29″E; Fig. 1). At each site, three coral colonies were removed by hand while snorkeling or diving, by placing a plastic bag around the whole colony and gently pulling the colony out of the sand by its base. Plastic bags were then sealed and placed in containers with ice and placed in a 4°C fridge until laboratory processing.
For processing, each colony was rinsed under fresh water over a white tray to remove organisms still inhabiting the colony. A more detailed specimen removal was then conducted as the swollen soft coral crabs (Calvactaea tumida) were found to live within the epidermal layer of the soft coral and had to be removed with a scalpel. Brittle stars (Ophionereis schayeri) were entangled in the polyps and had to be removed using forceps. Larger specimens were removed and collected with a 500-µm sieve then rinsed with distilled water. The colony sediment was filtered through a 500-µm sieve to identify macroinvertebrates living within the root tendrils of the corals. The sieved sediment was then observed under a dissecting microscope to identify all associated macroinvertebrates. All associated species were identified and frozen (–20°C) until stable isotope processing.
A total of 9 L of water were collected by boat in the Ettalong boat channel on both outgoing and incoming tides to collect particulate organic matter (POM). Three plankton tows with a 150-µm mesh net were conducted at 2 kn (~1.03 m s–1) for ~400 m in Ettalong boat channel on both outgoing and incoming tides to collect zooplankton samples for isotope analysis. These samples were collected only from one site because the POM and zooplankton species are unlikely to change among habitats because the salinity, water temperature (~16°C) and nutrient flows among sites will be similar (Winder and Jassby 2011).
Identifying associated species through remote underwater video systems
To observe the marine fauna associated within the soft coral, seagrass and sand habitats, remote underwater video systems (RUVS) were deployed within Brisbane Water estuary. Three different habitats were used to compare species, namely, seagrass, soft coral and sand. These habitats represent the dominant habitats within the southern area of Brisbane Water, where the soft coral is located. Lobster Beach and Ettalong Foreshore have high abundances of soft coral in shallow water (<3-m depth) and because of the separation between the sites (2 km), influence of breakwaters, navigational channel and possible sand dredging sites, both these locations were used to film associated species in the soft coral habitats. The location of the seagrass habitat was conducted at Wagstaffe Point, because it has a high abundance of Posidonia australis, which is an important habitat for bream, glassfish, pipefish and cardinal fishes (York et al. 2006). Wagstaffe Point is easily accessible by boat and has seagrass habitat in depths suitable for sampling (<4 m). The sand habitat chosen for sampling is located further south along Ettalong Beach, close to Umina Beach. This location is easily accessible by boat or from the shore and there were no visible signs of seagrass or soft coral within this coarse sand habitat.
The RUVS were made up of a GoPro camera attached to a 5-kg exercise plate and a ~50 × 3-cm diameter PVC pipe attached to the bottom where the camera was pointing towards the end of the pipe. A GoPro Hero 7 camera (www.gopro.com) was secured to each setup with a GoPro mount, with 15-m synthetic lines for deploying and retrieving the camera system and a floating buoy to allow clear identification at the surface. This simple design (commonly called mini-BRUVS/RUVS) are more compact for deployment from smaller vessels or while diving (Harasti et al. 2014a; Kiggins et al. 2018; Quaas et al. 2019), and by association also have less drag, which would otherwise be an issue given the high currents (>2 m s–1) that occur in these channels that can make retrieval difficult with larger style BRUVS.
The RUVS were deployed in sets of eight within each habitat, a minimum of 20 m apart, between depths of 2 and 4 m, and parallel to the shore to limit effects of depth. The minimum 20-m distance was chosen to allow sufficient replication within the spatially constrained habitats, and taking into account the typical low visibility (<5 m) in the estuary that meant recounting fishes observed in one RUVS was unlikely. There is little empirical evidence for minimum deployment distances for RUVS, unlike for BRUVS, which require greater intervals (>50 m) owing to possibility of overlapping bait plumes (Whitmarsh et al. 2017). We ensured that the habitat of interest (e.g. coral colonies) was included in the field of view if the habitat was patchy. The RUVS were left to film for 60 min, set to a linear view, and with 1080p resolution video at 24 frames per second. This was repeated three times at each location from May to August 2019, allowing for different tidal fluctuations (outgoing, incoming and slack tides) and seasonal variability to be included. Cameras were deployed at one site at a time because Brisbane Water is a heavily used waterway and there is a high risk of boat interactions and theft of cameras if unsupervised. Habitats to be sampled on a day were haphazardly chosen (because the cameras needed to be deployed in sets within a single habitat), and two or three habitats were sampled each day.
Predation experiment
To determine whether any larger consumers regularly feed on D. australis, we conducted a predation experiment to record whether this ever occurred. After the RUVS sampling was complete, the weighted cameras were adjusted to allow bait (D. australis) to be attached to the end of the PVC pipe. Predation experiments were conducted at Ettalong Beach, in the soft coral habitat, by using six cameras and sections of D. australis obtained the morning of deployments as bait. This was replicated at the Fly Point no-take marine reserve in Port Stephens because it is known for its abundance and diversity of fish life (Davis et al. 2016). Deployments were for 60 min and used the same camera settings as did the RUVS.
Stable isotope analysis
Because a similar study was conducted using stable isotopes in Port Stephens (Corry et al. 2018), we processed only samples obtained from Brisbane Water for stable isotopes. Sieves were used to separate the zooplankton samples into >500-µm and 250–500-µm size fractions. Particulate organic matter (POM) samples were filtered through a single 2-µm glass-fibre filter paper under a low vacuum. Frozen samples were thawed before muscle tissue extraction could commence. Epidermal tissue was extracted from branches of soft coral colonies, excluding any polyps in case any small brittle stars were still present. For fishes, white muscle tissue was extracted and placed into glass Petri dishes for analysis. Smaller decapods and crabs were analysed whole after the removal of internal organs and the stomach. Because of the high abundances of small associated species (amphipods, mysids, polychaetes) and the difficulty of removing muscle tissue, these small samples were processed whole.
Samples were rinsed with distilled water, placed in individual HCl-rinsed glass Petri dishes and dried to a constant weight at 60°C for 72 h. Dried samples were homogenised by grinding down to a fine powder with a Retsch MM200 ball mill for larger samples (soft coral and large arthropods) and by using a mortar and pestle for smaller samples (amphipods, mysids). Dissecting tools and apparatuses were cleaned between each sample with distilled water and ethanol to decrease potential of cross-contamination between samples.
Carbonates within calcified structures can alter δ13C values (Fry 2006); so, calcified tissues were removed where possible. However, it was not possible to dissect calcified tissues and separate them from muscle in small invertebrates, soft coral and zooplankton. To reduce the amounts of carbonates present within the samples, half of each dried powdered sample was saturated in 0.1-M hydrochloric acid for 1 h or until bubbling stopped, rinsed with distilled water and re-dried and reground as per Serrano et al. (2008). Acidification can lead to an enrichment of δ15N values (Serrano et al. 2008), so the other half of the powdered samples was not acidified, and acid washed and non-acid washed portions of samples were processed separately. Between 1 and 2 mg of powdered samples were then weighed in tin foil capsules and sent to Griffith University’s Stable Isotope Facility. After processing, some individual amphipod, mysid and polychaete samples were not large enough to obtain enough dried tissue for stable isotope analysis (SIA; minimum of 1.5 mg dried), so samples of smaller specimens were amalgamated and mixed before SIA. Typically, this was in groups of four if possible. Carbon (δ13C) and nitrogen (δ15N) stable isotope ratios were determined through a continuous-flow stable isotope ratio mass spectrometer (CF-IRMS), model Delta V Plus (Thermo Scientific Corporation, USA), interfaced with an elemental analyser (Thermo Fisher Flash 2000 HT EA, Thermo Electron Corporation, USA) at Griffith University Stable Isotope Laboratory (Brisbane, Qld, Australia). Analytical precision for both δ13C and δ15N values relative to the standards (Pee Dee Belemnite and atmospheric nitrogen) was 0.1‰.
Video analysis
Video footage was analysed through EventMeasure (ver. 5.43, see www.seagis.com.au). From the footage, all fish species were identified to their lowest taxonomic level (wherever possible), as well as the time the first fish of the species entered the field of view. These data, along with MaxN, can provide information on relative abundance, species diversity and species richness (Cappo et al. 2006). MaxN is the maximum number of individuals for a given species counted within the field of view at the same time and is a conservative estimate of fish abundance (Harvey et al. 2011; Lowry et al. 2011; Langlois et al. 2020). All interactions between fish assemblages and soft coral were noted.
Data analysis
All statistical analyses were conducted using R (ver. 3.4.4, R Foundation for Statistical Computing, Vienna, Austria, see https://www.r-project.org/) and RStudio (ver. 1.1.442, see https://rstudio.com/). A single-factor ANOVA was used to test whether there were significant differences between cumulative MaxN (additive MaxN for all species observed during a RUVS deployment) and species richness among habitats, and a q-q plot was used to check residual normality. To test whether there were differences among habitats, a permutational analysis of variance (PERMANOVA) was run using the vegan package (ver. 2.5-7, J. Oksanen, F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs, and H. Wagner, see https://cran.r-project.org/web/packages/vegan/index.html). A Bray–Curtis similarity coefficient was used to quantify the dissimilarity among the habitats and, from this, an analysis of similarity percentages (SIMPER) was run using the coefficient (Warton et al. 2012). SIMPER analysis identifies the species that contribute most to the observed patterns within habitats. The more abundant the species is within the habitat, the more it contributes to the similarity, whereas a species with a consistently high contribution to the dissimilarity is a recognised discriminating species (Heaven and Scrosati 2008; Warton et al. 2012). To test for differences between assemblages and habitat type, a Tukey’s post hoc HSD test was used.
Bayesian stable isotope mixing models in package MixSIAR (ver. 3.1.12, see https://cran.r-project.org/web/packages/MixSIAR/index.html; Stock et al. 2018) were run in R to calculate the contributions of each possible source to the diets of species associated with the soft corals. Bayesian isotope mixing models assume that all sources of the diet are included and that there is complete mixing of these sources in the system (Raoult et al. 2018). Consequently, all known primary producers that occur in the estuary were included in the model; saltmarshes, seagrasses and mangrove source values were obtained from (Hewitt et al. 2020) and samples of the soft coral D. australis. Although it is generally preferable to have consumer sample sizes >10 for these mixing models, many of the less numerous samples here were amalgamates of numerous individuals, so sample sizes >5 were deemed acceptable, provided these were amalgamates. High lipid content affects δ13C values, especially in cnidarians (Kiljunen et al. 2006), so all samples with C:N > 3.5 were mathematically lipid corrected to pure protein (St John Glew et al. 2019). The discrimination factors for the consumers were set to 0.3‰ for Δ13C and 2.2‰ for Δ15N because of the high abundances of crustaceans found within the colonies (deVries et al. 2015). Discrimination factors for organisms such as polychaetes are less well understood; so, we used the same discrimination factor as for crustaceans. The standard deviations of these enrichment factors were set to 1‰ for Δ13C and 1.5‰ for Δ15N, so as to account for uncertainties in the trophic levels and in diet–tissue discrimination factors (Abrantes and Sheaves 2009; Jennings and Van Der Molen 2015; Raoult et al. 2018) and because Bayesian stable isotope mixing models are sensitive to discrimination factor selection (Bond and Diamond 2011). Because Bayesian stable isotope mixing models are more reliable when fewer sources are included (Parnell et al. 2010), we grouped sources with similar stable isotope values by using a posteriori grouping that groups sources on the basis of their overlapping standard deviations and means, as suggested in Parnell et al. (2010). The distribution of consumers within the isoscape was then examined after trophic correction to ensure that the model assumptions were met (Smith et al. 2013). A multiplicative residual model was used to account for individual variation in diet preference (Brown et al. 2018). A generic prior was used as the diets of these consumers are not known. The models aimed at satisfying the Gelman and Geweke diagnostics (Cook et al. 2006). If this requirement was not satisfied, the models were run again, including longer runs.
Results
Species associated with soft coral habitats
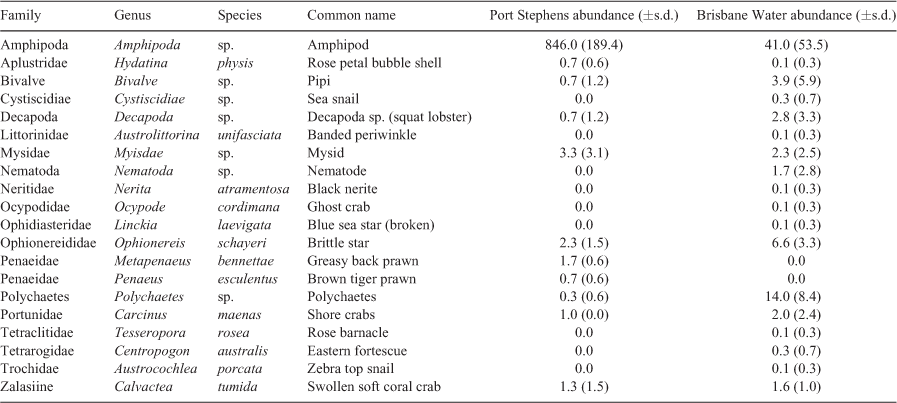
In total, 96 complete RUVS deployments across the three habitats (sand, seagrass and soft coral) were collected in Brisbane Water. The footage showed a total of 37 different species found within the three different habitats, including five species within the sand habitat, 21 species within the seagrass habitat and 25 within both soft coral habitats at Ettalong Foreshore (EF) and Lobster Beach (LB). Preliminary data exploration suggested that the two soft coral habitats were likely to have different fish assemblages, and so these were analysed separately. Some of the most abundant fishes were commercially and recreationally important fishery species Girella tricuspitata (Luderick), Acanthopagrus australis (yellowfin bream; Table 1). Notable species also included the Endangered Chelonia mydas (Green sea turtle) and Near Threatened estuary stingrays (Hemitrygon fluviorum).

|
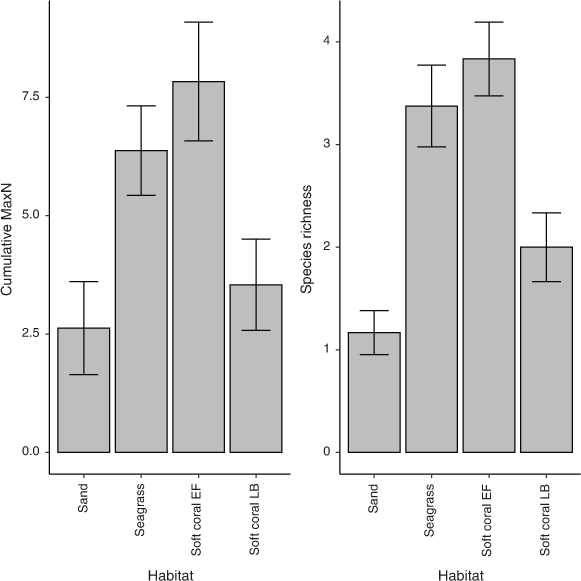
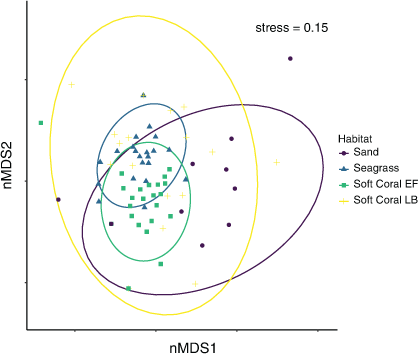
The mean cumulative species MaxN recorded across RUVS differed significantly among the habitats, with the soft coral habitat in EF having the highest mean MaxN of 7.8 ± 1.3 (Fig. 2). The soft coral located at LB had a significantly lower mean total MaxN (3.5 ± 1.0) than did the seagrass (6.4 ± 1.0); however, sand had the lowest at 2.63 ± 0.98 (Fig. 2). Species richness also differed among the habitats within Brisbane Water, with soft coral (EF) being associated with the highest mean species richness of 3.8 ± 0.4 (Fig. 2). The soft coral habitat on Lobster Beach had a lower species richness of 2.1 ± 0.3 than did the seagrass (3.4 ± 0.4), but higher than did the sand habitat (1.2 ± 0.4; Fig. 2). Assemblages of organisms recorded by BRUVS were significantly different among different habitat types (d.f. = 3, F = 7.12, P < 0.001), with the Ettalong soft coral and seagrass habitats being the most similar, whereas the Lobster Beach soft coral habitat overlapped with all other habitats (Fig. 3). Most organisms associated with soft coral colonies were invertebrates and were dominated by amphipods and polychaetes (Table 2).
|
|
|
Trophic role of soft corals
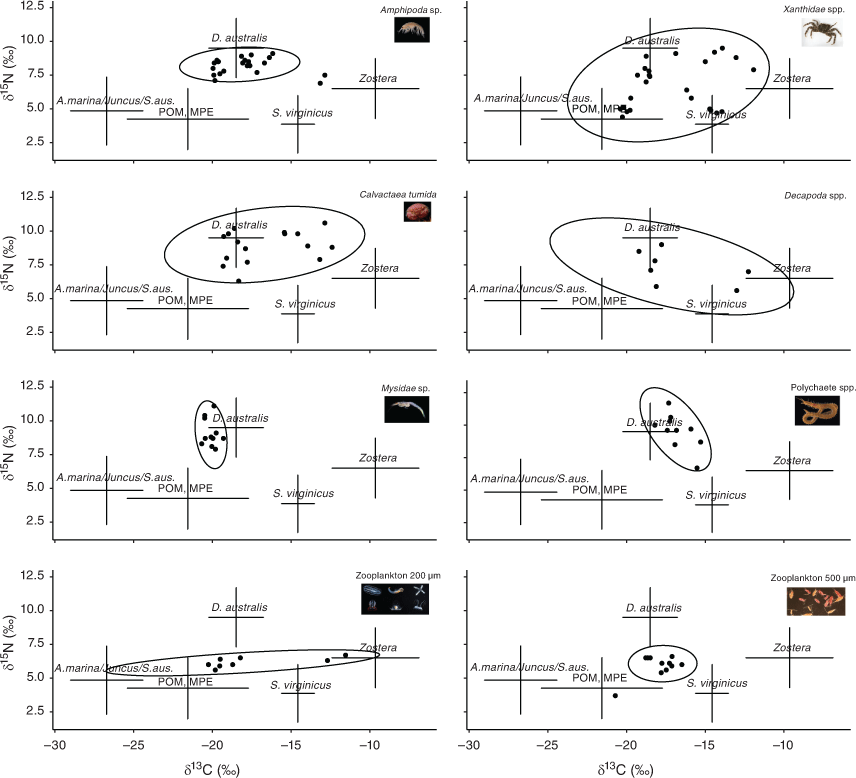
None of the BRUVS recorded any feeding on soft corals by any organism in either Port Stephens or Brisbane Water estuaries. Of the organisms collected from the soft corals in Brisbane Water, 144 consumer samples (some amalgamated) and 30 source samples were processed for stable isotopes. Amphipods, swollen soft-shelled crabs, mysids and polychaetes had trophic enrichment-corrected values that were similar to those of D. australis, whereas other crab species, decapods and zooplankton had broader values more closer to other primary producers in the ecosystem (Fig. 4). Seagrass was the most 13C-enriched source in the system, whereas mangroves and saltmarsh succulents were the most depleted. The range for δ15N values in the isoscape was approximately half that of δ13C values (10 v. 20‰). Very high (>12) C:N ratios of brittle stars (Ophionereis schayeri) were so high as to produce non-sensical results after mathematical lipid correction and were not included in the mixing models.
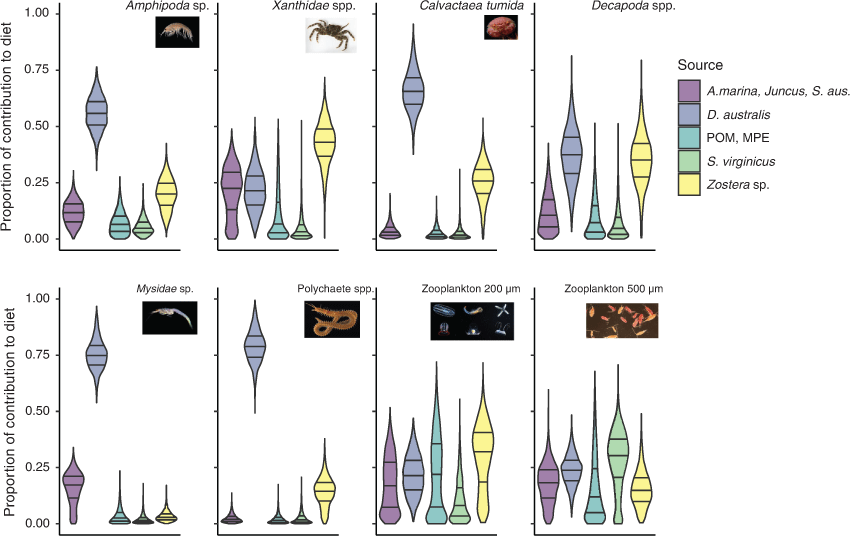
Bayesian stable isotope mixing models suggested that D. australis was the dominant contributor to the diets of five of the eight organisms associated with the soft corals (Fig. 5). The organisms that did not rely mainly on D. australis relied on Zostera sp. seagrass or salt couch (Sporobolus virginicus).
Discussion
The functional role of Dendronephyta australis appears to be that of an important resource and habitat for small invertebrates. Macro assemblages associated with soft coral habitats comprised commercially important and threatened species and these assemblages overlapped with sand and seagrass-dominated habitats. Although we observed no organisms feeding directly on the soft coral, there were diverse and numerous organisms that lived on or within the soft coral colonies. Stable isotope analyses showed that most organisms found on these soft corals also fed primarily on soft coral tissues, suggesting they are an important food resource for many benthic-associated invertebrates. Because many of the species that fed primarily on soft corals (e.g. mysids) are an important food source for many larger commercially important fishes such as Acanthopagrus australis, these results suggest that soft coral habitats have a unique functional ecological role that benefits estuarine ecosystems.
Predators of D. australis
Soft coral tissues were the dominant food source for five of the eight soft coral-associated species examined, indicating that the loss of soft coral ecosystems would likely have bottom-up effects on estuarine food webs (Frederiksen et al. 2006). Of the species with high contributions from soft corals, amphipods and mysid shrimp are a common food resource for commercially important fishes such as A. australis (Hadwen et al. 2007). Amphipods are also a food source for seahorses (Manning et al. 2019), of which threatened species such as White’s seahorse (Hippocampus whitei) often associate with D. australis (Harasti et al. 2014b). White’s seahorses are believed to be present in Brisbane Water; however, there have been no sightings of this species associated with D. australis in this estuary. Unlike zooplankton fractions that consumed a mixture of sources, mysids and amphipods appeared selective with very low contributions from other common estuarine producers. This suggests that soft corals are a critical part of the diets of these organisms, and that the loss of soft coral habitats would likely have bottom-up impacts on many commercially important fishes because of the loss of prey (Frederiksen et al. 2006). Unsurprisingly, symbiotic soft-coral crabs that live inside soft coral colony tissues had a high contribution of D. australis in their diets, similar to other crab symbionts that have demonstrated a diet based on the coral host (Glynn et al. 1985). One of the functional ecological roles of D. australis, therefore, is as a main food source for low-level consumers that may then be predated on by larger consumers.
Our results contrast with those of Corry et al. (2018) who found almost no consumption of the soft coral by small associated organisms in Port Stephens estuary. The most striking difference between the stable isotope food web in our study and that of Corry et al. (2018) is the more depleted (~4‰) δ13C values of D. australis in Brisbane Water. The soft corals in both estuaries appear to feed at similar trophic levels (δ15N values of ~10‰) but rely on different carbon pathways, with perhaps a greater contribution of seagrasses to D. australis diet in Port Stephens than in Brisbane Water. This is surprising because seagrasses from the genuses Zostera, Posidonia and Halophila are abundant near the soft coral habitats of Brisbane Water, so it is unclear why this difference exists. We used slightly different discrimination factors (0.3 for Δ 13C and 2.2 for Δ 15N) in our study than those used in Corry et al. (2018; 1‰ for Δ 13C and 2.9‰ for Δ 15N); however, we believe they were more appropriate because (1) they were specific for crustaceans rather than generic and (2) the species most likely to feed on D. australis (C. tumida found in the tissues of the soft coral) had posterior distributions that agree with this, suggesting, broadly, that our model parameters were appropriate. It is likely, therefore, that the role of D. australis and perhaps other temperate soft corals is plastic and highly location dependent.
No large consumers were recorded feeding directly on soft corals. Although the potential chemical defences produced by D. australis are not known, soft corals are often considered unpalatable to larger consumers because of toxic compounds that they produce (Hu et al. 2011). Soft corals from the genus Dendronephthya produce anti-fouling compounds that have toxic effects on fishes (Lee 2017) and may deter larger consumers. Some teleosts from genus Chaetodontidae have developed resistance to soft coral compounds and feed on them regularly (Pratchett 2005); however, none was seen during BRUVS and RUVS deployments in our experiments. Some Chaetodon spp. occur in these estuaries in the austral summer (Booth et al. 2007) and may predate on D. australis during those periods, but are not present during most of the year (autumn–spring) and, therefore, are unlikely to exhibit significant predation pressure on these soft corals. If there are no large predators of D. australis, it is likely that the factors limiting the spread of these corals are environmental factors such as sedimentation, heat stress or pollution, rather than direct predation.
Soft corals as a temperate habitat
Soft coral habitats harboured diverse fish assemblages resembling those of seagrasses and had similar species richness and fish abundance. Some overlap in fish assemblages between seagrass and soft corals was expected; seagrasses (Halophila ovalis, Zostera sp.) often occurred, albeit with a low cover, within or next to soft coral habitats. Intrinsically, soft corals provide similar habitat benefits as do seagrasses by having a vertical structure protruding from otherwise bare sediments. Seagrasses are widely agreed to be key nursery habitats for many estuarine species (Bloomfield and Gillanders 2005; Bertelli and Unsworth 2014; Unsworth et al. 2019). In contrast, soft coral habitats are generally considered an inferior habitat relative to scleratinian corals (Epstein and Kingsford 2019; Steinberg et al. 2020). Our results corroborated those of Corry et al. (2018) and Poulos et al. (2013), which highlights the value of soft coral habitats for fish assemblages, including for species of commercial and recreational value. As the results of three studies align on the importance of soft coral habitats for marine and estuarine fishes, it may be time to revisit the premise that soft coral habitats are inferior to other commonly encountered habitats such as seagrasses or corals.
Implications
As discussed, the Dendronephthya australis soft coral habitat is currently undergoing a rapid decline across its range in NSW (Larkin et al. 2021); hence, it has been recently listed as an Endangered species. This study demonstrated that the soft coral is an important habitat for a range of species that use it as a food source and to provide shelter, and that the loss of this species from an ecosystem would clearly have flow-on effects to the wider marine food web. Because this habitat is still under threat from anthropogenic pressures such as boat anchor and mooring installations (Harasti 2016) as well as from the impacts of sand movement (Larkin et al. 2021), it is imperative that remediation and management actions are implemented to ensure that this species does not become locally extinct and deleteriously affect local food webs.
Data availability
All data are available by contacting the corresponding author.
Conflicts of interest
The authors declare there are no conflicts of interest.
Declaration of funding
Part of this project was funded by a Central Coast Council Protection of the Environment Trust Project Grant 2017.
Acknowledgements
We thank all the volunteers and undergraduate students of the University of Newcastle that helped map the soft coral distribution and assisted H. Finlay-Jones with her sample processing.
References
Abrantes, K., and Sheaves, M. (2009). Food web structure in a near-pristine mangrove area of the Australian wet tropics. Estuarine, Coastal and Shelf Science 82, 597–607.| Food web structure in a near-pristine mangrove area of the Australian wet tropics.Crossref | GoogleScholarGoogle Scholar |
Allesina, S., Bodini, A., and Pascual, M. (2009). Functional links and robustness in food webs. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 364, 1701–1709.
| Functional links and robustness in food webs.Crossref | GoogleScholarGoogle Scholar | 19451121PubMed |
Baillon, S., Hamel, J.-F., Wareham, V. E., and Mercier, A. (2012). Deep cold‐water corals as nurseries for fish larvae. Frontiers in Ecology and the Environment 10, 351–356.
| Deep cold‐water corals as nurseries for fish larvae.Crossref | GoogleScholarGoogle Scholar |
Bertelli, C. M., and Unsworth, R. K. (2014). Protecting the hand that feeds us: seagrass (Zostera marina) serves as commercial juvenile fish habitat. Marine Pollution Bulletin 83, 425–429.
| Protecting the hand that feeds us: seagrass (Zostera marina) serves as commercial juvenile fish habitat.Crossref | GoogleScholarGoogle Scholar | 23998854PubMed |
Bloomfield, A., and Gillanders, B. (2005). Fish and invertebrate assemblages in seagrass, mangrove, saltmarsh, and non-vegetated habitats. Estuaries 28, 63–77.
| Fish and invertebrate assemblages in seagrass, mangrove, saltmarsh, and non-vegetated habitats.Crossref | GoogleScholarGoogle Scholar |
Bond, A. L., and Diamond, A. W. (2011). Recent Bayesian stable‐isotope mixing models are highly sensitive to variation in discrimination factors. Ecological Applications 21, 1017–1023.
| Recent Bayesian stable‐isotope mixing models are highly sensitive to variation in discrimination factors.Crossref | GoogleScholarGoogle Scholar | 21774408PubMed |
Booth, D., Figueira, W., Gregson, M., Brown, L., and Beretta, G. (2007). Occurrence of tropical fishes in temperate south-eastern Australia: role of the East Australian Current. Estuarine, Coastal and Shelf Science 72, 102–114.
| Occurrence of tropical fishes in temperate south-eastern Australia: role of the East Australian Current.Crossref | GoogleScholarGoogle Scholar |
Briand, M. J., Bonnet, X., Guillou, G., and Letourneur, Y. (2016). Complex food webs in highly diversified coral reefs: Insights from δ13C and δ15N stable isotopes. Food Webs 8, 12–22.
| Complex food webs in highly diversified coral reefs: Insights from δ13C and δ15N stable isotopes.Crossref | GoogleScholarGoogle Scholar |
Brown, C. J., Brett, M. T., Adame, M. F., Stewart-Koster, B., and Bunn, S. E. (2018). Quantifying learning in biotracer studies. Oecologia 187, 597–608.
| Quantifying learning in biotracer studies.Crossref | GoogleScholarGoogle Scholar | 29651662PubMed |
Cappo, M., Harvey, E., and Shortis, M. (2006). Counting and measuring fish with baited video techniques- an overview. In ‘AFSB Conference and Workshop: Cutting-edge Technologies in Fish and Fisheries Science’, 28–29 August 2006, Hobart, Tas., Australia. (Eds J. M. Lyle, D. M. Furlani, and C. D. Buxton.) pp. 101–114. (Australian Society for Fish Biology: Hobart, Tas., Australia.)
Coll, J. C., La Barre, S., Sammarco, P. W., Williams, W. T., and Bakus, G. J. (1982). Chemical defences in soft corals (Coelenterata: Octocorallia) of the great barrier reef: a study of comparative toxicities. Marine Ecology Progress Series 8, 271–278.
| Chemical defences in soft corals (Coelenterata: Octocorallia) of the great barrier reef: a study of comparative toxicities.Crossref | GoogleScholarGoogle Scholar |
Cook, S. R., Gelman, A., and Rubin, D. B. (2006). Validation of software for Bayesian models using posterior quantiles. Journal of Computational and Graphical Statistics 15, 675–692.
| Validation of software for Bayesian models using posterior quantiles.Crossref | GoogleScholarGoogle Scholar |
Corry, M., Harasti, D., Gaston, T., Mazumder, D., Cresswell, T., and Moltschaniwskyj, N. (2018). Functional role of the soft coral Dendronephthya australis in the benthic food web of temperate estuaries. Marine Ecology Progress Series 593, 61–72.
| Functional role of the soft coral Dendronephthya australis in the benthic food web of temperate estuaries.Crossref | GoogleScholarGoogle Scholar |
Davis, T. R., Harasti, D., and Smith, S. D. (2015). Extension of Dendronephthya australis soft corals in tidal current flows. Marine Biology 162, 2155–2159.
| Extension of Dendronephthya australis soft corals in tidal current flows.Crossref | GoogleScholarGoogle Scholar |
Davis, T. R., Harasti, D., Kelaher, B., and Smith, S. D. (2016). Diversity surrogates for estuarine fish assemblages in a temperate estuary in New South Wales, Australia. Regional Studies in Marine Science 7, 55–62.
| Diversity surrogates for estuarine fish assemblages in a temperate estuary in New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Davis, T. R., Harasti, D., Kelaher, B., and Smith, S. D. (2017). Spatial and temporal variation in subtidal molluscan diversity amongst temperate estuarine habitats. Marine Ecology 38, e12428.
| Spatial and temporal variation in subtidal molluscan diversity amongst temperate estuarine habitats.Crossref | GoogleScholarGoogle Scholar |
Davis, T. R., Harasti, D., and Smith, S. D. (2018). Responses of Dendronephthya australis to predation by Dermatobranchus sp. nudibranchs. Marine and Freshwater Research 69, 186–190.
| Responses of Dendronephthya australis to predation by Dermatobranchus sp. nudibranchs.Crossref | GoogleScholarGoogle Scholar |
Department of Primary Industries (2008). Brisbane water. Available at https://www.dpi.nsw.gov.au/content/research/areas/aquatic-ecosystems/estuarine-habitats-maps/IINSW_EstMac_map35.pdf
deVries, M. S., del Rio, C. M., Tunstall, T. S., and Dawson, T. E. (2015). Isotopic incorporation rates and discrimination factors in mantis shrimp crustaceans. PLoS One 10, e0122334.
| Isotopic incorporation rates and discrimination factors in mantis shrimp crustaceans.Crossref | GoogleScholarGoogle Scholar | 25835953PubMed |
Dobson, A. (2009). Food-web structure and ecosystem services: insights from the Serengeti. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 364, 1665–1682.
| Food-web structure and ecosystem services: insights from the Serengeti.Crossref | GoogleScholarGoogle Scholar | 19451118PubMed |
Epstein, H. E., and Kingsford, M. J. (2019). Are soft coral habitats unfavourable? A closer look at the association between reef fishes and their habitat. Environmental Biology of Fishes 102, 479–497.
| Are soft coral habitats unfavourable? A closer look at the association between reef fishes and their habitat.Crossref | GoogleScholarGoogle Scholar |
Fernando, I. S., Sanjeewa, K. A., Kim, H.-S., Kim, S.-Y., Lee, S.-H., Lee, W. W., and Jeon, Y.-J. (2017). Identification of sterols from the soft coral Dendronephthya gigantea and their anti-inflammatory potential. Environmental Toxicology and Pharmacology 55, 37–43.
| Identification of sterols from the soft coral Dendronephthya gigantea and their anti-inflammatory potential.Crossref | GoogleScholarGoogle Scholar |
Frederiksen, M., Edwards, M., Richardson, A. J., Halliday, N. C., and Wanless, S. (2006). From plankton to top predators: bottom‐up control of a marine food web across four trophic levels. Journal of Animal Ecology 75, 1259–1268.
| From plankton to top predators: bottom‐up control of a marine food web across four trophic levels.Crossref | GoogleScholarGoogle Scholar |
Fry, B. (2006). ‘Stable Isotope Ecology.’ (Springer-Verlag: New York, NY, USA.)
Garra, S., Hall, A., and Kingsford, M. J. (2020). The effects of predation on the condition of soft corals. Coral Reefs 39, 1329–1343.
| The effects of predation on the condition of soft corals.Crossref | GoogleScholarGoogle Scholar |
Glynn, P. W., Perez, M., and Gilchrist, S. L. (1985). Lipid decline in stressed corals and their crustacean symbionts. The Biological Bulletin 168, 276–284.
| Lipid decline in stressed corals and their crustacean symbionts.Crossref | GoogleScholarGoogle Scholar |
Hadwen, W. L., Russell, G. L., and Arthington, A. H. (2007). Gut content-and stable isotope-derived diets of four commercially and recreationally important fish species in two intermittently open estuaries. Marine and Freshwater Research 58, 363–375.
| Gut content-and stable isotope-derived diets of four commercially and recreationally important fish species in two intermittently open estuaries.Crossref | GoogleScholarGoogle Scholar |
Hammerschlag, N., Barley, S. C., Irschick, D. J., Meeuwig, J. J., Nelson, E. R., and Meekan, M. G. (2018). Predator declines and morphological changes in prey: evidence from coral reefs depleted of sharks. Marine Ecology Progress Series 586, 127–139.
| Predator declines and morphological changes in prey: evidence from coral reefs depleted of sharks.Crossref | GoogleScholarGoogle Scholar |
Harasti, D. (2016). Declining seahorse populations linked to loss of essential marine habitats. Marine Ecology Progress Series 546, 173–181.
| Declining seahorse populations linked to loss of essential marine habitats.Crossref | GoogleScholarGoogle Scholar |
Harasti, D., Gallen, C., Malcolm, H., Tegart, P., and Hughes, B. (2014a). Where are the little ones: distribution and abundance of the threatened serranid Epinephelus daemelii (Günther, 1876) in intertidal habitats in New South Wales, Australia. Journal of Applied Ichthyology 30, 1007–1015.
| Where are the little ones: distribution and abundance of the threatened serranid Epinephelus daemelii (Günther, 1876) in intertidal habitats in New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Harasti, D., Martin‐Smith, K., and Gladstone, W. (2014b). Ontogenetic and sex‐based differences in habitat preferences and site fidelity of White’s seahorse Hippocampus whitei. Journal of Fish Biology 85, 1413–1428.
| Ontogenetic and sex‐based differences in habitat preferences and site fidelity of White’s seahorse Hippocampus whitei.Crossref | GoogleScholarGoogle Scholar | 25098708PubMed |
Harvey, E. S., Mclean, D., Frusher, S., Haywood, M. D. E., Newman, S. J., and Williams, A. (Eds) (2011). The use of BRUVs as a tool for assessing marine fisheries and ecosystems: a review of the hurdles and potentials. The University of Western Australia.
Haydon, T. D., Seymour, J. R., and Suggett, D. J. (2018). Soft corals are significant DMSP producers in tropical and temperate reefs. Marine Biology 165, 109.
| Soft corals are significant DMSP producers in tropical and temperate reefs.Crossref | GoogleScholarGoogle Scholar |
Heaven, C. S., and Scrosati, R. A. (2008). Benthic community composition across gradients of intertidal elevation, wave exposure, and ice scour in Atlantic Canada. Marine Ecology Progress Series 369, 13–23.
| Benthic community composition across gradients of intertidal elevation, wave exposure, and ice scour in Atlantic Canada.Crossref | GoogleScholarGoogle Scholar |
Hewitt, D. E., Smith, T. M., Raoult, V., Taylor, M. D., and Gaston, T. F. (2020). Stable isotopes reveal the importance of saltmarsh-derived nutrition for two exploited penaeid prawn species in a seagrass dominated system. Estuarine, Coastal and Shelf Science 236, 106622.
| Stable isotopes reveal the importance of saltmarsh-derived nutrition for two exploited penaeid prawn species in a seagrass dominated system.Crossref | GoogleScholarGoogle Scholar |
Houghton, J. (2016). Mapping new populations and evaluating the biological value of a estuarine soft coral in NSW. B.Sc.(Hons) Thesis, University of Newcastle, Newcastle, NSW, Australia.
Hu, J., Yang, B., Lin, X., Zhou, X., Yang, X., Long, L., and Liu, Y. (2011). Chemical and biological studies of soft corals of the Nephtheidae family. Chemistry & Biodiversity 8, 1011–1032.
| Chemical and biological studies of soft corals of the Nephtheidae family.Crossref | GoogleScholarGoogle Scholar |
Jennings, S., and Van Der Molen, J. (2015). Trophic levels of marine consumers from nitrogen stable isotope analysis: estimation and uncertainty. ICES Journal of Marine Science 72, 2289–2300.
| Trophic levels of marine consumers from nitrogen stable isotope analysis: estimation and uncertainty.Crossref | GoogleScholarGoogle Scholar |
Kiggins, R., Knott, N. A., and Davis, A. R. (2018). Miniature baited remote underwater video (mini-BRUV) reveals the response of cryptic fishes to seagrass cover. Environmental Biology of Fishes 101, 1717–1722.
| Miniature baited remote underwater video (mini-BRUV) reveals the response of cryptic fishes to seagrass cover.Crossref | GoogleScholarGoogle Scholar |
Kiljunen, M., Grey, J., Sinisalo, T., Harrod, C., Immonen, H., and Jones, R. I. (2006). A revised model for lipid-normalizing δ13C values from aquatic organisms, with implications for isotope mixing models. Journal of Applied Ecology 43, 1213–1222.
| A revised model for lipid-normalizing δ13C values from aquatic organisms, with implications for isotope mixing models.Crossref | GoogleScholarGoogle Scholar |
Langlois, T., Goetze, J., Bond, T., Monk, J., Abesamis, R. A., Asher, J., Barrett, N., Bernard, A. T., Bouchet, P. J., and Birt, M. J. (2020). A field and video annotation guide for baited remote underwater stereo‐video surveys of demersal fish assemblages. Methods in Ecology and Evolution 11, 1401–1409.
| A field and video annotation guide for baited remote underwater stereo‐video surveys of demersal fish assemblages.Crossref | GoogleScholarGoogle Scholar |
Larkin, M. F., Davis, T. R., Harasti, D., Cadiou, G., Poulos, D. E., and Smith, S. D. (2021). The rapid decline of an endangered temperate soft coral species. Estuarine, Coastal and Shelf Science 255, 107364.
| The rapid decline of an endangered temperate soft coral species.Crossref | GoogleScholarGoogle Scholar |
Lee, S.-H. (2017). Developmental toxicity and anti-inflammatory effect of the soft coral Dendronephthya gigantea collected from Jeju Island in zebrafish model. Fisheries and Aquatic Sciences 20, 32.
| Developmental toxicity and anti-inflammatory effect of the soft coral Dendronephthya gigantea collected from Jeju Island in zebrafish model.Crossref | GoogleScholarGoogle Scholar |
Lester, E. K., Langlois, T. J., Simpson, S. D., McCormick, M. I., and Meekan, M. G. (2020). The hemisphere of fear: the presence of sharks influences the three dimensional behaviour of large mesopredators in a coral reef ecosystem. Oikos 129, 731–739.
| The hemisphere of fear: the presence of sharks influences the three dimensional behaviour of large mesopredators in a coral reef ecosystem.Crossref | GoogleScholarGoogle Scholar |
Libralato, S., Christensen, V., and Pauly, D. (2006). A method for identifying keystone species in food web models. Ecological Modelling 195, 153–171.
| A method for identifying keystone species in food web models.Crossref | GoogleScholarGoogle Scholar |
Lowry, M., Folpp, H., Gregson, M., and Mckenzie, R. (2011). A comparison of methods for estimating fish assemblages associated with estuarine artificial reefs. Brazilian Journal of Oceanography 59, 119–131.
| A comparison of methods for estimating fish assemblages associated with estuarine artificial reefs.Crossref | GoogleScholarGoogle Scholar |
Lu, Z., Ma, L., and Gou, Q. (2001). Concepts of keystone species and species importance in ecology. Journal of Forestry Research 12, 250–252.
| Concepts of keystone species and species importance in ecology.Crossref | GoogleScholarGoogle Scholar |
Manning, C., Foster, S., and Vincent, A. (2019). A review of the diets and feeding behaviours of a family of biologically diverse marine fishes (family Syngnathidae). Reviews in Fish Biology and Fisheries 29, 197–221.
| A review of the diets and feeding behaviours of a family of biologically diverse marine fishes (family Syngnathidae).Crossref | GoogleScholarGoogle Scholar |
Norström, A. V., Nyström, M., Lokrantz, J., and Folke, C. (2009). Alternative states on coral reefs: beyond coral–macroalgal phase shifts. Marine Ecology Progress Series 376, 295–306.
| Alternative states on coral reefs: beyond coral–macroalgal phase shifts.Crossref | GoogleScholarGoogle Scholar |
Parnell, A. C., Inger, R., Bearhop, S., and Jackson, A. L. (2010). Source partitioning using stable isotopes: coping with too much variation. PLoS One 5, e9672.
| Source partitioning using stable isotopes: coping with too much variation.Crossref | GoogleScholarGoogle Scholar | 20300637PubMed |
Poulos, D. E., Harasti, D., Gallen, C., and Booth, D. J. (2013). Biodiversity value of a geographically restricted soft coral species within a temperate estuary. Aquatic Conservation 23, 838–849.
| Biodiversity value of a geographically restricted soft coral species within a temperate estuary.Crossref | GoogleScholarGoogle Scholar |
Poulos, D. E., Gallen, C., Davis, T., Booth, D. J., and Harasti, D. (2016). Distribution and spatial modelling of a soft coral habitat in the Port Stephens–Great Lakes Marine Park: implications for management. Marine and Freshwater Research 67, 256–265.
| Distribution and spatial modelling of a soft coral habitat in the Port Stephens–Great Lakes Marine Park: implications for management.Crossref | GoogleScholarGoogle Scholar |
Pratchett, M. S. (2005). Dietary overlap among coral-feeding butterflyfishes (Chaetodontidae) at lizard island, northern great barrier reef. Marine Biology 148, 373–382.
| Dietary overlap among coral-feeding butterflyfishes (Chaetodontidae) at lizard island, northern great barrier reef.Crossref | GoogleScholarGoogle Scholar |
Quaas, Z., Harasti, D., Gaston, T., Platell, M., and Fulton, C. J. (2019). Influence of habitat condition on shallow rocky reef fish community structure around islands and headlands of a temperate marine protected area. Marine Ecology Progress Series 626, 1–13.
| Influence of habitat condition on shallow rocky reef fish community structure around islands and headlands of a temperate marine protected area.Crossref | GoogleScholarGoogle Scholar |
Raoult, V., Gaston, T. F., and Taylor, M. D. (2018). Habitat–fishery linkages in two major south-eastern Australian estuaries show that the C4 saltmarsh plant Sporobolus virginicus is a significant contributor to fisheries productivity. Hydrobiologia 811, 221–238.
| Habitat–fishery linkages in two major south-eastern Australian estuaries show that the C4 saltmarsh plant Sporobolus virginicus is a significant contributor to fisheries productivity.Crossref | GoogleScholarGoogle Scholar |
Roy, P. S., Williams, R. J., Jones, A. R., Yassini, I., Gibbs, P. J., Coates, B., West, R. J., Scanes, P. R., Hudson, J. P., and Nichol, S. (2001). Structure and function of south-east Australian estuaries. Estuarine, Coastal and Shelf Science 53, 351–384.
| Structure and function of south-east Australian estuaries.Crossref | GoogleScholarGoogle Scholar |
Serrano, O., Serrano, L., Mateo, M. A., Colombini, I., Chelazzi, L., Gagnarli, E., and Fallaci, M. (2008). Acid washing effect on elemental and isotopic composition of whole beach arthropods: implications for food web studies using stable isotopes. Acta Oecologica 34, 89–96.
| Acid washing effect on elemental and isotopic composition of whole beach arthropods: implications for food web studies using stable isotopes.Crossref | GoogleScholarGoogle Scholar |
Smith, S. D., and Edgar, R. J. (2014). Documenting the density of subtidal marine debris across multiple marine and coastal habitats. PLoS One 9, e94593.
| Documenting the density of subtidal marine debris across multiple marine and coastal habitats.Crossref | GoogleScholarGoogle Scholar | 24743690PubMed |
Smith, J. A., Mazumder, D., Suthers, I. M., and Taylor, M. D. (2013). To fit or not to fit: evaluating stable isotope mixing models using simulated mixing polygons. Methods in Ecology and Evolution 4, 612–618.
| To fit or not to fit: evaluating stable isotope mixing models using simulated mixing polygons.Crossref | GoogleScholarGoogle Scholar |
St John Glew, K., Graham, L. J., McGill, R. A. R., and Trueman, C. N. (2019). Spatial models of carbon, nitrogen and sulphur stable isotope distributions (isoscapes) across a shelf sea: an inla approach. Methods in Ecology and Evolution 10, 518–531.
| Spatial models of carbon, nitrogen and sulphur stable isotope distributions (isoscapes) across a shelf sea: an inla approach.Crossref | GoogleScholarGoogle Scholar |
Steinberg, R. K., Dafforn, K. A., Ainsworth, T., and Johnston, E. L. (2020). Know thy anemone: a review of threats to octocorals and anemones and opportunities for their restoration. Frontiers in Marine Science 7, 590.
| Know thy anemone: a review of threats to octocorals and anemones and opportunities for their restoration.Crossref | GoogleScholarGoogle Scholar |
Stock, B. C., Jackson, A. L., Ward, E. J., Parnell, A. C., Phillips, D. L., and Semmens, B. X. (2018). Analyzing mixing systems using a new generation of Bayesian tracer mixing models. PeerJ 6, e5096.
| Analyzing mixing systems using a new generation of Bayesian tracer mixing models.Crossref | GoogleScholarGoogle Scholar | 29942712PubMed |
Unsworth, R. K., Nordlund, L. M., and Cullen‐Unsworth, L. C. (2019). Seagrass meadows support global fisheries production. Conservation Letters 12, e12566.
| Seagrass meadows support global fisheries production.Crossref | GoogleScholarGoogle Scholar |
Urrutia-Cordero, P., Ekvall, M. K., and Hansson, L.-A. (2016). Local food web management increases resilience and buffers against global change effects on freshwaters. Scientific Reports 6, 29542.
| Local food web management increases resilience and buffers against global change effects on freshwaters.Crossref | GoogleScholarGoogle Scholar | 27386957PubMed |
van Lier, J. R., Harasti, D., Laird, R., Noble, M. M., and Fulton, C. J. (2017). Importance of soft canopy structure for labrid fish communities in estuarine mesohabitats. Marine Biology 164, 45.
| Importance of soft canopy structure for labrid fish communities in estuarine mesohabitats.Crossref | GoogleScholarGoogle Scholar |
Warton, D. I., Wright, T. W., and Wang, Y. (2012). Distance-based multivariate analyses confound location and dispersion effects. Methods in Ecology and Evolution 3, 89–101.
| Distance-based multivariate analyses confound location and dispersion effects.Crossref | GoogleScholarGoogle Scholar |
Whitmarsh, S. K., Fairweather, P. G., and Huveneers, C. (2017). What is Big BRUVver up to? Methods and uses of baited underwater video. Reviews in Fish Biology and Fisheries 27, 53–73.
| What is Big BRUVver up to? Methods and uses of baited underwater video.Crossref | GoogleScholarGoogle Scholar |
Winder, M., and Jassby, A. D. (2011). Shifts in zooplankton community structure: implications for food web processes in the upper San Francisco estuary. Estuaries and Coasts 34, 675–690.
| Shifts in zooplankton community structure: implications for food web processes in the upper San Francisco estuary.Crossref | GoogleScholarGoogle Scholar |
York, P. H., Booth, D. J., Glasby, T. M., and Pease, B. C. (2006). Fish assemblages in habitats dominated by Caulerpa taxifolia and native seagrasses in south-eastern Australia Marine Ecology Progress Series 312, 223–234.
| Fish assemblages in habitats dominated by Caulerpa taxifolia and native seagrasses in south-eastern AustraliaCrossref | GoogleScholarGoogle Scholar |