Influence of calcium on the toxicity of saline solutions to the mayfly, Austrophlebioides sp. AV11
Vinitha Nanjappa A C * , Sue Vink A , Jason Dunlop A D , Matt N. Krosch A E and Reinier Mann B
A C * , Sue Vink A , Jason Dunlop A D , Matt N. Krosch A E and Reinier Mann B
A Centre for Water in the Mineral Industry, Sustainable Minerals Industry, The University of Queensland, Saint Lucia, Qld 4072, Australia.
B Department of Environment and Science, Queensland Government, Dutton Park, Qld 4102, Australia.
C Present address: School of Civil and Environmental Engineering, Queensland University of Technology, Brisbane, Qld 4000, Australia.
D Present address: Office of the Queensland Mine Rehabilitation Commissioner, Queensland Government, Brisbane, Qld 4001, Australia.
E Present address: Forensic Services, Queensland Police Service, Queensland Government, Brisbane, Qld 4000, Australia.
Marine and Freshwater Research 73(12) 1499-1509 https://doi.org/10.1071/MF22001
Submitted: 1 January 2022 Accepted: 10 September 2022 Published: 18 October 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Although calcium may provide a means to reduce toxicity of saline waters, the relationship between them is not well understood.
Aim: To investigate whether increasing calcium may result in a proportional reduction in toxicity.
Methods: Acute toxicity tests were conducted using an artificial mine-affected water (AMW) and the same AMW with increased calcium on the mayfly species, Austrophlebioides sp. AV11.
Results: Results demonstrated that there was a reduction in toxicity when calcium concentration (added as both calcium chloride and calcium sulfate together) was increased by both two-fold (+143 mg L−1) and four-fold (+272 mg L−1) compared with the AMW test solution (161 mg L−1). A further increase by up to eight-fold (+718 mg L−1) did not substantially change the toxicity of the AMW solution.
Conclusion: The toxicity did not reduce when calcium chloride and calcium sulfate salts were used independently. This study suggests that adding calcium (as calcium chloride and calcium sulfate) to a saline solution reduces toxicity upto a maximum threshold, beyond which no further benefit is achieved.
Implications: Increased calcium can have diminishing effect on toxicity or reach a maximum threshold beyond which no further reduction in toxicity is achieved. Improved understanding of this relationship is needed to inform the management of saline mine waters.
Keywords: Austrophleioides sp. AV11, calcium, ecotoxicology, freshwater, insects, invertebrates, mayfly, salinity.
Introduction
Diverse and healthy aquatic invertebrate communities are crucial to the functioning of aquatic and some terrestrial food webs and many ecosystem services (National Park Service 2015; Hauer and Resh 2017). Common major cations (Ca2+, Mg2+, Na+ and K+) and anions (Cl−, HCO3− and SO42–) are essential components of natural waters as well as living organisms. Anthropogenic activities such as processes in coal mining (Kennedy et al. 2003; Gozzard et al. 2009), oil industries (Boelter et al. 1992), application of salts on the roads for de-icing in temperate climates (Evans and Frick 2001) and agricultural practices (Hart et al. 1990) can result in water discharges that are high in salts. These sources not only increase the salinity but vary dramatically in ionic composition and alter the ionic composition in the receiving water (Hart et al. 1990). Consequently, this interferes with osmoregulation and ion regulation (Mount et al. 2016) in organisms, which may lead to adverse effects or, sometimes, death (Hart et al. 1991). The loss of sensitive species as a result of osmotic stress from increased salinity (James et al. 2003) can lead to changes in community structure and biodiversity of an ecosystem (Nielsen et al. 2003). Understanding the mechanism of toxicity and the relative importance of anthropogenic salinity in relation to natural freshwater composition is essential for effective management and regulation.
Several studies have shown that the toxicity of a saline solution is dependent on the types of ion present in the solution (Mount et al. 1997; van Dam et al. 2010; Mount et al. 2016; Soucek et al. 2018). For example, sodium sulfate was more toxic with a 96-h LC50 (lethal concentration for 50% of population in 96 h of exposure to the toxicant) of 2 mS cm−1 (1360 mg L−1) than was sodium chloride with a 96-h LC50 of 4–8 mS cm−1 (2720–5440 mg L−1) on a mayfly Tricorythus sp. when tested in a range of solutions with similar electrical conductivities (EC; Goetsch and Palmer 1997). An extensive suite of toxicity tests on all the major ions conducted by Mount et al. (1997) reported varying levels of toxicity on the cladoceran, Ceriodaphnia dubia, the anomopod, Daphnia magna, and the cyprinid, Pimephales promelas, exposed to different ions. From this study, potassium was the most toxic ion and sulfate was the least toxic ion. However, the toxicity of potassium was found to be reduced in the presence of sodium, indicating that one ion can influence the toxicity of another ion in the solution. A similar finding of the toxicity of potassium to C. dubia was reported in Mount et al. (2016).
A study by Kunz et al. (2013) described the effect of ionic composition on C. dubia, the unionid mussel Lampsilis siliquoidea, the amphipod Hyalella azteca and the mayfly Centroptilium triangulifer, using three different reconstituted waters based on coal mine drainage. Two compositions were representative of alkaline mine drainage with elevated magnesium, calcium, potassium, sulfate and bicarbonate and the third was representative of neutralised mine drainage with elevated sodium, potassium, sulfate and bicarbonate. The tests were conducted with equal percentage dilutions of the reconstituted waters. The survival in a 35-day exposure for C. triangulifer showed toxic effects for the two alkaline mine drainage compositions with NOEC of <33%, <50% and no toxic effect with NOEC of >100% for the neutral mine drainage composition. The two compositions were toxic to amphipod, mussel and mayfly, whereas the neutralised mine drainage composition was toxic to amphipod and mussel but not to mayfly. Hence, the toxicity of saline solutions is not only due to salinity (i.e. ionic strength), but can also be influenced by the ionic composition of the solution.
Studies have reported that increased calcium concentration decreases the toxicity of saline solutions (Davies and Hall 2007; Soucek 2007; Soucek et al. 2011). An increase in the Ca:Mg ratio from 0.7 to 7.0, while maintaining a hardness of 100 mg L−1 in toxicity tests with amphipod H. azteca decreased the toxicity of sulfate from 96-h LC50 of 2100 to 2720 mg L−1 (Davies and Hall 2007). In the same study, the same change in Ca:Mg ratio resulted in a decrease in sulfate toxicity among D. magna from a 48-h LC50 of 1190 to 1980 mg L−1. A study by van Dam et al. (2010) evaluated the effect of magnesium toxicity with an increase in calcium concentration as Mg:Ca mass ratio. This was shown to result in a reduction in the toxicity of magnesium sulfate to the duckweed Lemna aequinoctialis, the cnidarian Hydra viridissima and the gastropod Amerianna cumingi. Although these studies all agree that calcium may decrease the toxicity of a solution, the physiological mechanisms by which calcium reduces toxicity are not well understood. Possible explanations are that calcium can increase permeability of cell membranes to water and decrease the permeability to other ions in freshwater organisms (Cuthbert and Maetz 1972).
The majority of published studies that have examined the effects of individual cations or anions were conducted with single (e.g. NaCl) or double (e.g. NaCl + KCl) salts. However, natural waters usually consist of a wide range of major ions. Few studies have attempted to explain the differences in toxicity of complex natural waters with different ionic profiles. Scheibener et al. (2017) investigated the response of five mayfly species to sodium concentration associated with sulfate. The survival of the mayflies increased when sodium concentration was increased from 16.1 to 250.7 mg L−1 (0.7–10.9 mM), but the survival decreased when sodium concentration was increased further to 501.4 mg L−1 (21.8 mM), with the associated sulfate concentration of 1996.8 mg L−1 (20.8 mM). This suggests that all ions in the solution play a vital role in determining the survival of organisms.
The toxicity of major ions in complex ionic mixtures to mayfly has been found to vary. Prasad et al. (2014) and Dunlop et al. (2016) reported toxicities of waters that varied in the proportion of ions present. The waters tested in those studies represented either streams (Dunlop et al. 2016) or dam waters associated with mine sites (Prasad et al. 2014) of the Fitzroy River Basin, Queensland, Australia. The study by Prasad et al. (2014) reported a difference in toxicity among mine dam waters and attributed the difference in toxicity to mayflies to the proportion of calcium to major ions present in the solutions (Prasad et al. 2014). To evaluate further the potential influence of calcium on toxicity, the authors of the present study plotted the toxicity data from the two studies against the proportion of calcium (% meq) to major ions present in test waters (Supplementary Fig. S1). The toxicities did not correspond to the proportion of calcium as expected. An analysis of these data showed that the solutions with similar toxicity had different calcium proportions (n = 2), and solutions with similar calcium proportions had different toxicities (n = 2). Although it was postulated that calcium present in a solution can have an ameliorative effect, as observed for calcium concentration, this effect was not evident in the data of the above-mentioned studies. Because these studies were not specifically designed to test this affect, it is difficult to ascertain with any confidence what the actual influence of varying the proportion and concentration of calcium is on mayfly.
The order Ephemeroptera (mayflies) occurs in abundance in most freshwaters around Australia and other parts of the world (Williams 1980), and is known to be sensitive to salinity (Kefford et al. 2003) as well as ionic composition (Prasad et al. 2014). Mayflies are widely used as a bio-indicator to assess the water quality of a stream (Hubbard and Peters 1978; Kefford 2000; Chessman 2003; Sibley et al. 2018). Studies on macroinvertebrates by Kefford et al. (2003) have shown that mayflies are the taxa most sensitive to salinity and the abundance and richness of these taxa has also been used to assess potential salinity impacts in freshwater streams. Additionally, previous studies have used mayfly species for toxicity testing and have found them to be sensitive to a complex mixture of major ions (Prasad et al. 2014; Dunlop et al. 2016; Soucek et al. 2018). Thus, a mayfly species was used in the present study to allow comparison with studies of Prasad et al. (2014) and Dunlop et al. (2016).
The species targeted in the present study was Austrophlebioides sp. AV11 from the family Leptophlebiidae (identified by Dr Phil Suter, LaTrobe University, Wodonga, Vic., Australia), which is endemic to mainland Australia and Tasmania. Leptophlebiidae is one of the families of Ephemeroptera that is dominant worldwide and in Australia (Australia 1988) and south-eastern Queensland.
The present study was designed to improve our understanding of the role of an increased calcium concentration in the toxicity of saline waters. Specifically, the hypothesis tested was that increased calcium concentration decreases the toxicity of a saline solution to a species of Ephemeroptera, Austrophlebioides sp. AV11. Experiments were developed whereby toxicity was assessed when (1) the calcium concentration was increased proportionally to major ions in a solution by two-fold, four-fold and eight-fold, by using a combination of calcium chloride and calcium sulfate salts, and (2) calcium concentration was increased using either calcium chloride or calcium sulfate salts. The effect of calcium concentration was tested in formulated water that simulated waters containing all the ions.
Materials and methods
Collection of mayfly nymphs
Ephemeroptera nymphs were collected from Mount Barney creek, a reference site listed in the Queensland Water Quality Guidelines (Department of Environment and Resource Management 2009). It is situated 150 km south-west of Brisbane, Queensland, Australia, at a latitude −28.2372°S and longitude 152.7427°E. The catchment area upstream of the collection site is in the headwaters of the Mount Barney National Park, a conserved region and natural environment (Fig. 1) that is unaffected by urban runoff (Department of Environment and Resource Management 2009).
|
|
The nymphs were collected by washing them off rocks in a bucket of water. Nine collections were made across the duration of the study. The first two collections were range-finding tests for toxicant solutions and a definitive test for the reference toxicant respectively. The remaining seven collections were definitive tests (Supplementary Table S1). Although it was not possible to distinguish the instars or measure the size of the nymphs, individuals with approximately similar size (3–4 mm long excluding cerci) were selected live in plastic sorting trays in the field. Selected nymphs were transferred to containers supplied with aeration by using a battery-powered diaphragm pump. One or two decaying leaves were introduced into the container as food until the beginning of the experiments. The organisms were transported to the laboratory with aeration and were stored in a temperature-controlled room (20°C ± 2 s.d.) until the start of the exposure experiments. The experiments were conducted the following day within 24 h of the time of collection. The survival of organisms in the controls were >90%. Thus, the mortality 24 h prior to the test was not significant.
Water quality
Although salinity is commonly measured as electrical conductivity (EC, μS cm−1), some studies have reported total dissolved salts (TDS). To provide a consistent basis for comparison, in this study we present both EC and TDS after converting between units using the formula 0.68 × EC (μS cm−1) described in Hart et al. (1991).
Field water quality
Electrical conductivity (EC), dissolved oxygen (DO), pH and temperature were recorded at the collection site immediately before collecting organisms during each collection time. Creek water was sampled for analysis of major cations and anions. Water samples were stored in the laboratory at 4°C until the analyses were conducted.
Test-water quality
In situ EC, DO, pH and temperature were recorded at the beginning, 48 and 96 h during each test. All the test solutions including the diluted solutions were sampled at the beginning of each test for analysis of major cations and anions. All solutions were stored at 4°C along with the creek water until analyses.
Analysis of major anions and cations was performed at the School of Agricultural and Food Sciences, The University of Queensland, in accordance with Standard method 200.7 (United States Enironmental Protection Agency 1994) and 3120 (American Public Health Association 2005). Calcium, potassium, sodium, magnesium, and sulfate as sulfur were analysed by inductively coupled plasma–optical emission spectroscopy (Varian Vista Pro USA). Chloride was analysed by flow injection analysis (AQ2+ Automated Discrete Analyser, Seal Analytical Inc. USA). Alkalinity was analysed by Gran titration (Metrohm 902 Titrando, Metrohm, USA). Quality assurance and quality control protocols included a standard and method blank for 5% of the samples and a duplicate for 10% of the samples for each analyte. The EC and pH were measured using TPS Model 901-CP.
Preparation of test solutions
Reference toxicant
Analytical grade sodium chloride (Merck) was dissolved in MilliQ ultrahigh-purity (UHP) water to prepare a stock solution with nominal EC of 20 mS cm−1 (13 600 mg L−1). The stock solution was diluted using UHP water to obtain test solutions with EC of 0.3, 0.6, 1.0, 5.0, 7.0 mS cm−1 (TDS of 240, 408, 680, 3400, 4760 mg L−1). To avoid the potential for pH-related toxicity, pH was maintained above pH 6 by addition of 25 mg L−1 of sodium hydroxide and 272 mg L−1 of monobasic phosphate buffer to each test solution (Adelman and Smith 1976).
Toxicant solutions
Artificial Mine Water 1 from Prasad et al. (2014) was used as the base test solution. This solution is hereafter referred to as AMW. AMW is a formulated saline solution based on a typical mine-water ion composition for the Queensland coal fields and used for subsequent toxicity tests (Mann et al. 2014).
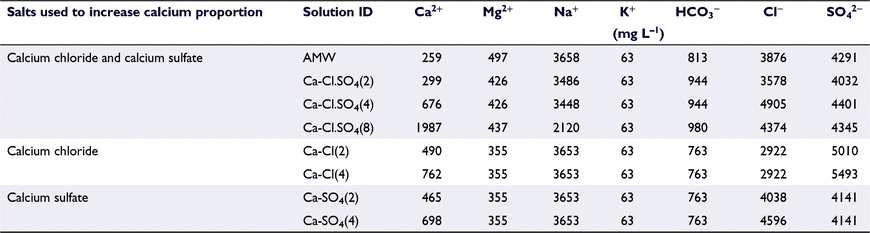
Eight stock solutions were prepared (Table 1) for the toxicity tests. The nominal concentrations of ions described in Table 1 were calculated as percentage milliequivalent of the ions.
|
Stock solutions were prepared 2 days prior to the experiments and stored in the laboratory at room temperature (20°C ± 1). The measured concentrations of the eight stock solutions are provided in the supplementary material (Table S2). Each stock solution was prepared by dissolving calculated amount of analytical-grade salts (Merck) in UHP water. For each toxicant solution, a stock solution at the highest test EC of ∼14 mS cm−1 (10 240 mg L−1) was prepared. The stock solution was mixed overnight and then filtered through 0.45-μM Millipore Durapore membrane filters by vacuum filtration.
Six individual test solutions were prepared as dilutions of the stock solutions ranging from 1.2 to 14 mS cm−1 (850–10 240 mg L−1 TDS). The dilutions were based on the range-finding tests with observed partial mortality in all the treatments. The dilutions prepared were ∼1.2, 4.0, 6.0, 8.0, 10 and 14 mS cm−1 (850, 2700, 4120, 5360, 6700 and 10 240 mg L−1). Diluting each stock solution ensured that the relative proportion of ions remained constant in the test solutions. Calcium concentration was increased proportionally above the AMW toxicant solution. The first set of solutions with an increase in the calcium concentration was prepared by adding both calcium chloride and calcium sulfate salts. Calcium concentration was increased proportionally using twofold Ca-Cl.SO4(2), fourfold Ca-Cl.SO4(4) and eightfold Ca-Cl.SO4(8) increases above that of the proportion of calcium in AMW. A second and third sets of solutions were prepared to further verify the effect of anions. For these solutions, calcium concentration was increased by adding either calcium chloride or calcium sulfate separately. Calcium was increased as two-fold Ca-Cl(2), Ca-SO4(2) and four-fold Ca-Cl(4), Ca-SO4(4). As calcium was increased proportionally, the proportion and, therefore, the concentration of sodium ion decreased in the saline solutions.
Previous work has reported difficulties in preparing saline test solutions with particular ion compositions or ratios (Mount et al. 2016; Vink et al. 2016). One of the challenges of our study was the potential for oversaturation of solutions, particularly with respect to carbonate species that precipitated out of solution. Although every effort (including investigations of bubbling with CO2) was made to ensure complete dissolution of all ions, the intended nominal concentrations were not always achieved. For this reason, all results are presented as the measured values for solutions and not nominal concentrations.
Toxicity-test procedure
Acute static non-renewal toxicity tests were conducted according to standard methods (United States Enironmental Protection Agency 2002), with slight modifications. The duration of exposure was 96 h. The experiments were conducted in a temperature- and light-controlled room with a 12:12 h light:dark cycle. The temperature was maintained at 20°C ± 1 during all experiments. Tests were conducted in food-grade plastic containers. Each container was 1000 mL in size and 500 mL of solution was added. The containers were aerated and had DO at 95–100% saturation.
Each test consisted of a set of triplicate test containers for each saline solution and control solutions (unfiltered and undiluted Mount Barney Creek water) and a reference toxicant (NaCl) series. One or two toxicant solutions were used for toxicity tests run during each sampling period, along with a reference toxicant. In all, 8–12 individuals were introduced into each test container by using disposable plastic pipette and plastic spoon. There were no substrata introduced into the containers. The organisms were not fed during the experiments. Observations of mortality were conducted at 24-h intervals. Individuals were considered dead when there was no response to gentle prodding. If individuals had emerged or were missing, this was also recorded. Emerged or missing organisms (frequency of one in three or four treatments) were deducted from the total number of individuals exposed to the treatments. At the end of the toxicity test, individuals used in the experiments were preserved in 90% ethanol for identification to species level.
Data analyses
The 96-h LC50 values were derived by fitting probit regression using Toxcalc (ver. 5.01, Tidepool Scientific LLC, USA) for the reference toxicant (n = 7) and CETIS (Tidepool Scientific LLC) for the saline solutions (n = 6). Each LC50 was calculated from one toxicity test with seven treatments including the controls and three replicates. The replicates were pooled in the calculation of LC50.
The toxicity of each solution with an increased calcium concentration was compared with the toxicity of AMW. Significant difference was assessed using F-test and log-regression models (Motulsky and Christopoulos 2003) implemented in Graphpad prism (ver. 9.4.1, Graphpad Software, USA). The F-test uses sums of squares to compare the variability of two sets of data and thus forms a robust and useful analysis for comparison. The model provides regression curves for the comparison between the regression used to derive 96-h LC50 values and allows comparison between the dose–response curves.
The differences in toxicity between the solutions with increased calcium and AMW were assessed using F-tests, on the basis of 96-h LC50 values and the overall dose–response data. To do so, log-regression models were used to compare the 96-h LC50 values and dose–response data. For the comparison of 96-h LC50 values between AMW and solution with an increased calcium, a single-parameter log-regression model was used. For the comparison of the dose–response data, a four-parameter log-regression model was used. However, for the four-parameter log-regression model, the bottom of the curve was constrained to 0 to fit the criteria of the model. The output of the comparsions provide the f-ratio. The f-ratio was used to calculate the P-value, where P < 0.05 was used to evaluate the significant difference. In addition to the P-value, the model provided a graphical output for the comparisons of 96-h LC50 and dose–response data. When there was a statistical difference, there were two distinct curves for each solution and when there was no statistical difference, there was an overlay of curves for both solutions. Comparison of both 96-h LC50 values and the dose–response curves enhances the evaluation of the effect of the addition of calcium.
Results
Field water quality
Stream water EC varied between 55.3 and 106.3 μS cm−1, DO concentrations varied between 104 and 119%, temperature varied between 12.9 and 22.6°C and pH varied between 7.37 and 8.54 (Supplementary Table S3). These changes in stream conditions reflect typical seasonal changes observed in south-eastern Queensland (Bureau of Meteorology 2014). The major ion composition showed that the waters were relatively low in salinity and invariant (Table S1). The standard deviation was between 0.2 and 1.3 mg L−1 for the major ions, except bicarbonate, which had a standard deviation of 18.8 mg L−1 (Supplementary Table S4). The conductivity of the stream has been recorded between 60 and 113 μS cm−1 since 2003 (Healthy Land and Water, pers. comm., 2019). This indicates that organisms were not previously exposed to high levels of salinity either throughout the study period or prior to collection.
Toxicant solutions
Laboratory test-water quality: DO was maintained between 7.75 and 8.5 mg L−1 (90–100% saturation). The pH was maintained between pH 6.11 and 8.80, and, therefore, within the test criteria of pH 6–9 (United States Enironmental Protection Agency 2002), for all solutions throughout each test duration.
The distribution of ions in the solutions is shown in Table 2. The percentage of calcium was higher than that in AMW for Ca-Cl.SO4(2), Ca-Cl.SO4(4) and Ca-Cl.SO4(8), as postulated. The Ca-Cl(2) solution did not have the expected amount of calcium. The calcium concentration was higher than that in AMW for Ca-Cl(4), Ca-SO4(2) and Ca-SO4(4).

|
Reference toxicant
In total, seven toxicity tests (n = 7) were performed, each of which included a test with the reference toxicant (n = 7). The survival of test organisms in the control was >90% in the reference toxicant tests and, therefore, all tests met the acceptability criteria (United States Enironmental Protection Agency 2002). The mean 96-h LC50 value for the seven toxicity tests was 142.2 mg L−1 with a standard deviation of 47 and 32.9% CV (Fig. S2).
Comparison between the toxicity of AMW and the toxicity of solutions with increased calcium concentrations
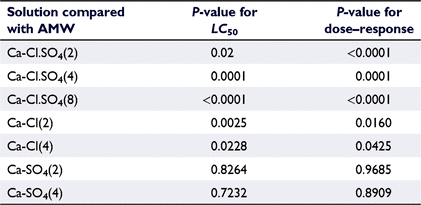
There was a 100% survival of organisms in the controls in all toxicity tests. The 96-h LC50 of AMW was 4072 mg L−1 (Fig. 2). There was a significantly lower toxicity than that of AMW of two of the solutions with an increased calcium concentration using both chloride and sulfate salts, namely, Ca-Cl.SO4(2), with 96-h LC50 of 4527 mg L−1, and Ca-Cl.SO4(4), with 96-h LC50 of 5134 mg L−1, whereas Ca-Cl.SO4(8) with 96-h LC50 of 4241 mg L−1 was marginally different from AMW. There was a greater toxicity to the solution Ca-Cl(2) with 96-h LC50 of 3101 mg L−1, and Ca-Cl(4) with 96-h LC50 of 3436 mg L−1, than the toxicity of AMW. There was also a greater toxicity to the solution Ca-SO4(2) than to AMW, with 96-h LC50 of 3466 mg L−1, and no significant difference in the toxicity of the solutions Ca-SO4(4) and AMW, with 96-h LC50 of 4203 mg L−1.
|
|
Examination of the dose–response curves supported these conclusions. When there was a statistically significant difference (i.e. P < 0.05), two distinct curves were observed for each solution and, conversely, when there was no significant difference (i.e. P ≥ 0.05), there was substantial overlay of curves.
The solutions Ca-Cl.SO4(2), Ca-Cl.SO4(4) and Ca-Cl.SO4(8) showed statistically significant (P < 0.05) differences from AMW for both 96-h LC50 and dose–response curve (Table 3). Whereas Ca-Cl.SO4(2), Ca-Cl.SO4(4) and Ca-Cl.SO4(8) were lower in toxicity than was AMW, the toxicity of Ca-Cl.SO4(8) showed little difference and the corresponding LC50 value did not show a distinct reduction in toxicity as did that for a two-fold and four-fold increase in calcium. This indicates that a high proportion of calcium when present as both calcium chloride and calcium sulfate does not necessarily reduce toxicity (Fig. S3).
|
For the solution Ca-Cl(2), both the 96-h LC50 and dose–response curve suggested a statistically significant (P < 0.05) difference from AMW. The toxicity of this solution was higher than that of AMW. This could be a consequence of a lower calcium concentration than expected in this solution. For the Ca-Cl(4) solution, the 96-h LC50 was significantly different from that of AMW (P < 0.05), whereas the dose–response curve did not show any significant difference. The comparison of 96-h LC50 values showed two distinct curves, whereas comparison of the dose–response curves showed a single curve (Fig. S4).
No significant difference was observed between Ca-SO4(2) and AMW for either 96-h LC50 values (P = 0.09) or dose–response data (P = 0.7; Fig. S4e, f). Similarly, there was no significant difference between Ca-SO4(4) and AMW for either 96-h LC50 values (P = 0.05) or dose–response data (P = 0.8; Fig. S4g, h), indicating that there was no difference between the toxicities of these solutions and that of AMW.
Discussion
It is well established that an increase in calcium concentration can reduce toxicity; however, it is unclear whether there is an upper limit beyond which no further reduction in toxicity is achievable. This study tested the hypothesis that an increase in calcium would decrease the toxicity of a saline solution. An increase in calcium (using calcium chloride and calcium sulfate together) did decrease the toxicity of the saline solution for a two-fold and four-fold increase in calcium, but there was very slight difference in toxicity for an eight-fold increase. An exception to this was that when calcium is present with chloride as Ca-Cl(2), there was an increase in toxicity compared with AMW and there was no significant difference in toxicity for Ca-Cl(4) or any test solution where calcium was increased using calcium sulfate. This indicates that chloride may affect the influence of calcium on reducing the toxicity.
Comparison of the results of the present study and those of previous studies by Prasad et al. (2014) and Dunlop et al. (2016)
Toxicity results from the present study were compared with the data from previous studies by Prasad et al. (2014) and Dunlop et al. (2016) (Fig. 3). Solutions AMW, Ca-SO4(4), and Ca-Cl.SO4(8) all have similar toxicity, but the proportion of calcium is different. Fitzroy composition (FC) had a higher proportion of calcium than did Ca-Cl.SO4(8), but the toxicity was lower than that of Ca-Cl.SO4(8). In contrast, marine salt (MS) had the lowest toxicity with a similar proportion of calcium compared with AMW. This suggests that there may be an interaction among all the ions that influence the toxicity of solutions.
|
|
Comparison of results with other studies
Previous studies that have examined the effect of increased calcium concentration in simple saline mixtures of one or two salts have demonstrated the protective effect of calcium. Davies and Hall (2007), Dwyer et al. (1992) and van Dam et al. (2010) reported an ameliorative effect of calcium on the toxicity of solutions to freshwater organisms. Davies and Hall (2007) used 16–36 mg L−1 calcium in their study on the toxicity of sodium sulfate on H. azteca and D. magna. The toxicity of sodium sulfate decreased with an increase in calcium concentration. Dwyer et al. (1992) reported the lowest toxicity to striped bass in the solution with a high concentration of calcium (280 mg L−1). Similarly, van Dam et al. (2010) reported a decrease in toxicity of Mg with an increase of calcium concentration. In this study, there was a complete reduction in toxicity at the inhibitory concentration for 50% of population (IC50) for L. aequinoctialis and A. cumingi and there was a slight reduction in toxicity for H. viridissima. The calcium concentrations used in the solutions were between 0.02 and 8 mg L−1. In all these studies, it was reported that calcium has an ameliorative effect on the toxicity of solutions.
The concentration of calcium in the studies of Dwyer et al. (1992) was ≤280 mg L−1. Even in the present study, solutions with a calcium concentration of <280 mg L−1 have shown an ameliorative effect. In the present study, at the LC50 concentrations for the Ca-Cl.SO4(2) and Ca-Cl.SO4(4) salt mixtures, the calcium concentrations were 127 mg L−1 (3.2 mM) and 172 mg L−1 (4.3 mM) respectively. However, the 96-h LC50 for Ca-Cl.SO4(8) was 397 mg L−1 (9.9 mM), with a very low difference in toxicity from that of AMW. The solutions wherein calcium was increased either with calcium chloride or calcium sulfate did not show any ameliorative effect. The ameliorative effect of calcium on the toxicity of saline waters that has been proposed in the literature (Dwyer et al. 1992) was not demonstrated in the present study. It is notable that the effect of calcium has been characterised in simple saline mixtures of one or two salts (Mount et al. 1997; van Dam et al. 2010). However, in the current study, the solutions were a mixture of all major ions, all of which may contribute to either toxicity or the ameliorative effects of the mixture.
Effect of anions on the toxicity of saline solutions
Additionally, it could be the anions that influence the toxicity. It has often been observed that bicarbonate is the anion that is most toxic to a range of organisms. For example, Mount et al. (1997) reported that bicarbonate was the most toxic anion among the other anions and sulfate the least toxic in acute toxicity tests on C. dubia, D. magna and P. promelas. This was similar in chronic toxicity tests for anions reported by Lasier and Hardin (2010), the toxicity expressed as milliequivalents per litre (meq L−1) showed that bicarbonate was the most toxic anion and sulfate was the least toxic anion to C. dubia. Mount et al. (1997) also reported that the toxicity of sodium and calcium was due to the associated anions and not merely related to these cations. The use of calcium chloride or calcium sulfate separately to increase the calcium in the present study did not show a decrease in toxicity. This could be due to the change in the anion ratio. It has been found that the concentration of chloride and bicarbonate ions influence the Cl−/HCO3− exchange across the epithelia of gills in crabs (Towle 1993). Further changes to the anion ratio can influence the acid–base balance regulated by the gill epithelia in freshwater fish (Perry et al. 2003). The hypothesis tested here was that increased calcium concentration decreases the toxicity of a saline solution to a species of Ephemeroptera, Austrophlebioides sp. AV11. Therefore, toxicity studies on bicarbonate are required to further characterise and improve understanding of the toxic effect of anions.
Rationale for difference in observed toxicity among the toxicant solutions
An explanation for why the protective effect of calcium is lost at higher calcium concentrations may lie in the physiological role that calcium plays in cell membrane permeability and stability of the membrane. High concentrations of free calcium can cause lysis of the cell and, eventually, cell death (Rubin et al. 1985). In cellular physiology, calcium is reported to promote cellular adhesion and cellular activity; however, high concentrations of free calcium ions have been found to inhibit this function (Rubin et al. 1985). Calcium has been found to have a high affinity to bind to negative charges on the surface of the cells and influences the excitability and the permeability of the cell membranes. Calcium is involved in the excitation of cells where excitation refers to the contraction of muscle fibre. The stimulus from a neuron to muscle fibre influences the concentration of calcium inside and outside the cell membrane that changes the electrical potential across the cell membrane. This assists in the contraction of muscle fibre (Sperelakis 2012). The influence of calcium concentration on membrane potential was illustrated by McWilliams and Potts (1978) in the brown trout, Salmo trutta. They found that the trans-epithelial membrane potential (TEP) was negative in a calcium-free media, but was shifted to positive with a calcium concentration of 80 mg L−1. There was no significant change above this concentration. This could provide an explanation for the absence of a decrease in toxicity observed for the solution with high calcium tested here; however, further evaluation of TEP is required.
Conclusions
An increase in the calcium was found to decrease the toxicity of a saline solution to Austrophlebioides sp. AV11 when both calcium chloride and calcium sulfate were used together to increase calcium. However, the same effect was not produced when using calcium chloride or calcium sulfate alone to increased calcium. A decrease in toxicity was observed for solutions with an increase of calcium of 1.8%meq (+143 mg L−1 or 3.6 mM) and 3.8% meq (+272 mg L−1 or 6.8 mM) above the calcium of 2% meq (161 mg L−1 or 4 mM) of the base toxicant AMW. Toxicity was decreased by 15% with the two-fold increase and 35% with a four-fold increase of calcium above that of AMW. Although calcium had an ameliorative effect on toxicity, further increases in calcium up to 11.3% meq (+718 mg L−1 or 17.9 mM) did not reduce toxicity. An increase in toxicity was observed for one solution in which only calcium chloride was used to increase calcium; however, this solution had a lower proportion of calcium than intended, which may explain this result.
The ionic composition in freshwater varies and it is necessary to evaluate the effect of specific compositions, particularly where saline discharges to aquatic ecosystems result in changes to the ionic composition in the receiving environment. The findings of this study have shown that the concentration of calcium can influence toxicity and, as a result, calcium requires consideration in the regulation of saline discharges. The influence of calcium on the toxicity of saline solutions as observed here may vary with other ionic compositions and the effect may differ among organisms. Further investigation of the interactions of major ions and with the anions are therefore required to improve understanding of the factors that influence the toxicity of complex saline solutions. Additionally, because the anions present also seem to influence the toxicity, further investigations on anion toxicity are required to understand the toxicity of saline solutions. Hence, it is essential to understand the mechanisms of toxicity and the relative importance of anthropogenic salinity in relation to natural freshwater composition for water quality management programs and effective legislation.
Supplementary material
Supplementary material is available online.
Data availability
Data will be made available upon reasonable request.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of funding
There was no defined funding for this project.
References
Adelman, IR, and Smith, LL (1976). Fathead Minnows (Pimephales promelas) and Goldfish (Carassius auratus) as standard fish in bioassays and their reaction to potential reference toxicants. Journal of the Fisheries Research Board of Canada 33, 209–214.| Fathead Minnows (Pimephales promelas) and Goldfish (Carassius auratus) as standard fish in bioassays and their reaction to potential reference toxicants.Crossref | GoogleScholarGoogle Scholar |
American Public Health Association (2005) ‘Standard methods for the examination of water & wastewater’, 21st edn. (American Public Health Association. American Water Works Association. Water Environment Federation)
Australia Bureau of Flora Fauna (1988) ‘Ephemeroptera, Megaloptera, Odonata, Plecoptera, Trichoptera.’ (Australian Government Public Service)
Boelter, AM, Lamming, FN, Farag, AM, and Bergman, HL (1992). Environmental effects of saline oil-field discharges on surface waters. Environmental Toxicology and Chemistry 11, 1187–1195.
| Environmental effects of saline oil-field discharges on surface waters.Crossref | GoogleScholarGoogle Scholar |
Bureau of Meteorology (2014) Climate Glossary. Available at http://www.bom.gov.au/climate/glossary/seasons.shtml [Verified May 2014]
Chessman B (2003) SIGNAL 2. A scoring system for macroinvertebrates (‘water bugs’) in Australian waters. Monitoring River Health Initiative Technical Report Number 31, Commonwealth of Australia, Canberra, ACT, Australia.
Cuthbert, AW, and Maetz, J (1972). The effects of calcium and magnesium on sodium fluxes through gills of Carassius auratus, L. Journal of Physiology-London 221, 633–643.
| The effects of calcium and magnesium on sodium fluxes through gills of Carassius auratus, L.Crossref | GoogleScholarGoogle Scholar |
Davies, TD, and Hall, KJ (2007). Importance of calcium in modifying the acute toxicity of sodium sulphate to Hyalella azteca and Daphnia magna. Environmental Toxicology and Chemistry 26, 1243–1247.
| Importance of calcium in modifying the acute toxicity of sodium sulphate to Hyalella azteca and Daphnia magna.Crossref | GoogleScholarGoogle Scholar |
Department of Environment and Resource Management (2009) ‘Queensland water quality guidelines 2009.’ (DERM, Queensland Government)
Dunlop, JE, Mann, RM, Hobbs, D, Smith, REW, Nanjappa, V, Vardy, S, and Vink, S (2016). Considering background ionic proportions in the development of sulfate guidelines for the Fitzroy River Basin. Australasian Bulletin of Ecotoxicology and Environmental Chemistry 3, 1–10.
Dwyer, FJ, Burch, SA, Ingersoll, CG, and Hunn, JB (1992). Toxicity of trace element and salinity mixtures to striped bass (Morone saxatilis) and Daphnia magna. Environmental Toxicology and Chemistry 11, 513–520.
| Toxicity of trace element and salinity mixtures to striped bass (Morone saxatilis) and Daphnia magna.Crossref | GoogleScholarGoogle Scholar |
Evans M, Frick C (2001) The effects of road salts on aquatic ecosytems. WSTD Contribution Number 02-308. (Environment Canada) Available at https://vegvesen.brage.unit.no/vegvesen-xmlui/bitstream/handle/11250/193946/the_effects_road_salts.pdf?sequence=1&isAllowed=y
Goetsch, P-A, and Palmer, CG (1997). Salinity tolerances of selected macroinvertebrates of the Sabie River, Kruger National Park, South Africa. Archives of Environmental Contamination and Toxicology 32, 32–41.
| Salinity tolerances of selected macroinvertebrates of the Sabie River, Kruger National Park, South Africa.Crossref | GoogleScholarGoogle Scholar |
Gozzard, E, Vink, S, Nanjappa, V, and Moran, CJ (2009). Salt dissolution dynamics on surface mine spoils. Mining Technology 118, 177–184.
Hart, BT, Bailey, P, Edwards, R, Hortle, K, James, K, McMahon, A, Meredith, C, and Swadling, K (1990). Effects of salinity on river, stream and wetland ecosystems in Victoria, Australia. Water Research 24, 1103–1117.
| Effects of salinity on river, stream and wetland ecosystems in Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Hart, BT, Bailey, P, Edwards, R, Hortle, K, James, K, McMahon, A, Meredith, C, and Swadling, K (1991). A review of the salt sensitivity of the Australian freshwater biota. Hydrobiologia 210, 105–144.
| A review of the salt sensitivity of the Australian freshwater biota.Crossref | GoogleScholarGoogle Scholar |
Hauer FR, Resh VH (2017) Chapter 15 – Macroinvertebrates. In ‘Methods in Stream Ecology, Volume 1: Ecosystem Structure’, 3rd edn. (Eds FR Hauer, GA Lamberti) pp. 297–319. (Academic Press)
| Crossref |
Hubbard MD, Peters WL (1978) Environmental requirements and pollution tolerance of Ephemeroptera. EPA 600/4-78-061, US Environmental Protection Agency, Washington, DC, USA.
James, KR, Cant, B, and Ryan, T (2003). Responses of freshwater biota to rising salinity levels and implications for saline water management: a review. Australian Journal of Botany 51, 703–713.
| Responses of freshwater biota to rising salinity levels and implications for saline water management: a review.Crossref | GoogleScholarGoogle Scholar |
Kefford, BJ (2000). The effect of saline water disposal: implications for monitoring programs and management. Environmental Monitoring and Assessment 63, 313–327.
| The effect of saline water disposal: implications for monitoring programs and management.Crossref | GoogleScholarGoogle Scholar |
Kefford, BJ, Papas, PJ, and Nugegoda, D (2003). Relative salinity tolerance of macroinvertebrates from the Barwon River, Victoria, Australia. Marine and Freshwater Research 54, 755–765.
| Relative salinity tolerance of macroinvertebrates from the Barwon River, Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Kennedy, AJ, Cherry, DS, and Currie, RJ (2003). Field and laboratory assessment of a coal processing effluent in the Leading Creek Watershed, Meigs County, Ohio. Archives of Environmental Contamination and Toxicology 44, 324–331.
| Field and laboratory assessment of a coal processing effluent in the Leading Creek Watershed, Meigs County, Ohio.Crossref | GoogleScholarGoogle Scholar |
Kunz, JL, Conley, JM, Buchwalter, DB, Norberg-King, TJ, Kemble, NE, Wang, N, and Ingersoll, CG (2013). Use of reconstituted waters to evaluate effects of elevated major ions associated with mountaintop coal mining on freshwater invertebrates. Environmental Toxicology and Chemistry 32, 2826–2835.
| Use of reconstituted waters to evaluate effects of elevated major ions associated with mountaintop coal mining on freshwater invertebrates.Crossref | GoogleScholarGoogle Scholar |
Lasier, PJ, and Hardin, IR (2010). Observed and predicted reproduction of Ceriodaphnia dubia exposed to chloride, sulfate, and bicarbonate. Environmental Toxicology and Chemistry 29, 347–358.
| Observed and predicted reproduction of Ceriodaphnia dubia exposed to chloride, sulfate, and bicarbonate.Crossref | GoogleScholarGoogle Scholar |
McWilliams, PG, and Potts, WTW (1978). The effects of pH and calcium concentrations on gill potentials in the brown trout, Salmo trutta. Journal of Comparative Physiology 126, 277–286.
| The effects of pH and calcium concentrations on gill potentials in the brown trout, Salmo trutta.Crossref | GoogleScholarGoogle Scholar |
Mann, RM, Vink, S, Micevska, T, Hobbs, D, and Smith, REW (2014). Are variations in ionic proportions important for the derivation of trigger values for saline mine discharge waters? Australasian Bulletin of Ecotoxicology & Environmental Chemistry 1, 1–11.
Motulsky HJ, Christopoulos A (2003) ‘Fitting models to biological data using linear and non linear regression. A practical guide to curve fitting.’ (Graphpad Software Inc.: San Diego, CA, USA)
Mount, DR, Gulley, DD, Hockett, JR, Garrison, TD, and Evans, JM (1997). Statistical models to predict the toxicity of major ions to Ceriodaphnia dubia, Daphnia magna and Pimephales promelas (fathead minnows). Environmental Toxicology and Chemistry 16, 2009–2019.
| Statistical models to predict the toxicity of major ions to Ceriodaphnia dubia, Daphnia magna and Pimephales promelas (fathead minnows).Crossref | GoogleScholarGoogle Scholar |
Mount, DR, Erickson, RJ, Highland, TL, Hockett, JR, Hoff, DJ, Jenson, CT, Norberg-King, TJ, Peterson, KN, Polaske, ZM, and Wisniewski, S (2016). The acute toxicity of major ion salts to Ceriodaphnia dubia: I. Influence of background water chemistry. Environmental Toxicology and Chemistry 35, 3039–3057.
| The acute toxicity of major ion salts to Ceriodaphnia dubia: I. Influence of background water chemistry.Crossref | GoogleScholarGoogle Scholar |
National Park Service (2015) Aquatic macroinvertebrates – ecological role. (US Department of Interior) Available at https://www.nps.gov/articles/aquatic-macroinvertebrates-ecological-role.htm [Verified 14 May 2022]
Nielsen, DL, Brock, MA, Rees, GN, and Baldwin, DS (2003). Effects of increasing salinity on freshwater ecosystems in Australia. Australian Journal of Botany 51, 655–665.
| Effects of increasing salinity on freshwater ecosystems in Australia.Crossref | GoogleScholarGoogle Scholar |
Perry, SF, Shahsavarani, A, Georgalis, T, Bayaa, M, Furimsky, M, and Thomas, SLY (2003). Channels, pumps, and exchangers in the gill and kidney of freshwater fishes: their role in ionic and acid-base regulation. Journal of Experimental Zoology – A. Comparative Experimental Biology 300A, 53–62.
| Channels, pumps, and exchangers in the gill and kidney of freshwater fishes: their role in ionic and acid-base regulation.Crossref | GoogleScholarGoogle Scholar |
Prasad, R, Vink, S, and Nanjappa, V (2014). Impact of salinity and ionic composition on freshwater macroinvertebrates in the Fitzroy River catchment, Central Qld, Australia. Australasian Bulletin of Ecotoxicology and Environmental Chemistry 1, 12–29.
Rubin RP, Weiss GB, Putney JW Jr (1985) ‘Calcium in biological systems.’ (Plenum Press)
| Crossref |
Scheibener, S, Conley, JM, and Buchwalter, D (2017). Sulfate transport kinetics and toxicity are modulated by sodium in aquatic insects. Aquatic Toxicology 190, 62–69.
| Sulfate transport kinetics and toxicity are modulated by sodium in aquatic insects.Crossref | GoogleScholarGoogle Scholar |
Sibley P, Raby M, Wirtz J, McCoole M, Lagadic L, Soucek D, Norberg-King T, Roessink I (2018) Evaluating the relative sensitivity of the ‘P&T’ in EPT: implications for standardized toxicity testing. (US EPA). Available at https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NHEERL&TIMSType=&count=10000&dirEntryId=343208&searchAll=&showCriteria=2&simpleSearch=0 [Verified 1 January 2020]
Soucek, DJ (2007). Comparison of hardness- and chloride-regulated acute effects of sodium sulfate on two freshwater crustaceans. Environmental Toxicology and Chemistry 26, 773–779.
| Comparison of hardness- and chloride-regulated acute effects of sodium sulfate on two freshwater crustaceans.Crossref | GoogleScholarGoogle Scholar |
Soucek, DJ, Linton, TK, Tarr, CD, Dickinson, A, Wickramanayake, N, Delos, CG, and Cruz, LA (2011). Influence of water hardness and sulfate on the acute toxicity of chloride to sensitive freshwater invertebrates. Environmental Toxicology and Chemistry 30, 930–938.
| Influence of water hardness and sulfate on the acute toxicity of chloride to sensitive freshwater invertebrates.Crossref | GoogleScholarGoogle Scholar |
Soucek, DJ, Mount, DR, Dickinson, A, and Hockett, JR (2018). Influence of dilution water ionic composition on acute major ion toxicity to the mayfly Neocloeon triangulifer. Environmental Toxicology and Chemistry 37, 1330–1339.
| Influence of dilution water ionic composition on acute major ion toxicity to the mayfly Neocloeon triangulifer.Crossref | GoogleScholarGoogle Scholar |
Sperelakis N (2012) ‘Cell Physiology Source Book: Essentials of Membrane Biophysics’, 3rd edn. (Elsevier Science)
Towle, DW (1993). Ion transport systems in membrane vesicles isolated from crustacean tissues. Journal of Experimental Zoology 265, 387–396.
| Ion transport systems in membrane vesicles isolated from crustacean tissues.Crossref | GoogleScholarGoogle Scholar |
United States Enironmental Protection Agency (1994) Method 200-7, revision 4.4: determination of metals and trace metals in water and wastes by inductively coupled plasma–atomic emission spectrometry. (US EPA) Available at https://www.epa.gov/sites/production/files/2015-08/documents/method_200-7_rev_4-4_1994.pdf
United States Enironmental Protection Agency (2002) ‘Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms’, EPA-821-R-02-012, 5th edn. (US EPA, Office of Water)
van Dam, RA, Hogan, AC, McCullough, CD, Houston, MA, Humphrey, CL, and Harford, AJ (2010). Aquatic toxicity of magnesium sulfate, and the influence of calcium, in very low ionic concentration water. Environmental Toxicology and Chemistry 29, 410–421.
| Aquatic toxicity of magnesium sulfate, and the influence of calcium, in very low ionic concentration water.Crossref | GoogleScholarGoogle Scholar |
Vink S, Nanjappa V, Wolhuter A, Henderson A (2016) Assessing the toxicity of saline solutions with different ionic compositions on freshwater organisms. (ACARP) Available at https://www.acarp.com.au/abstracts.aspx?repId=C23010
Williams WD (1980) ‘Australian freshwater life: the invertebrates of Australian inland waters’, 2nd edn. (Macmillan)


