Emergence phenology of Tasmanian mayflies (Ephemeroptera) in a first-order creek and comparison with northern hemisphere confamilials
Ronald E. Thresher A B *
A B *
A CSIRO Oceans & Atmosphere, GPO Box 1538, Hobart, Tas. 7001, Australia.
B Present address: SF Tech, 50 Bramble Street, Ridgeway, Tas. 7054, Australia.
Marine and Freshwater Research 73(12) 1489-1498 https://doi.org/10.1071/MF22131
Submitted: 9 July 2022 Accepted: 5 September 2022 Published: 17 October 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Australian mayflies are hypothesised to differ from those in the Northern Hemisphere by having longer, more variable emergence seasons that overlap more widely among taxa.
Aim: To test this hypothesis by comparing the behaviour of related northern and southern hemisphere species in similar habitats and at similar spatial scales.
Methods: Emergence dates were recorded fortnightly over two consecutive years, one ‘warm’ and one ‘cool’, in a rocky creek in south-eastern Tasmania, and at a coarser scale for Tasmania as whole, spanning an ∼20-year period. The results are compared with emergence patterns at two sites in the northern hemisphere climatically similar to Tasmania.
Key results: Durations of emergence seasons in Tasmania did not differ significantly at either the single site or whole of island scales from those in the northern hemisphere, but unlike in the latter, the start of emergence does not appear to be temperature-dependent.
Conclusions: Apparent regional differences are likely to result primarily from climatically inappropriate comparisons rather than from fundamental differences in behaviour.
Implications: Differences in the factor(s) that cue emergence suggest that the life histories of mayflies in Tasmania, and possibly elsewhere in Australia, are determined less by physiology than by aquatic ecology.
Keywords: Australia, Baetidae, Britain, climate, emergence, habitat, Leptophlebiidae, quiescence, temperature-dependence, USA.
Introduction
Mayflies (order Ephemeroptera) have a life cycle involving two profoundly different stages, namely, a long aquatic nymph (larval or naid) stage that can last from months to years, followed by a legendarily brief aerial adult phase. Duration of the latter is typically a few days, but ranges among taxa from hours to a few weeks (Brittain 1982). The transition between the aquatic and aerial stages involves nymphs crawling, floating or swimming to the water surface, where they moult into a winged subadult form (the subimago) that, subsequently, moults again into the reproductive adult (imago). The nymph-to-subadult transition is referred to as emergence (‘hatching’ in angler’s parlance) and is a critical point in the insect’s life history (Edmunds and Edmunds 1980). Among many species, emergence is highly synchronised, emergence periods are short, and they often differ among congeneric species. This sequencing is a dominant feature of northern hemisphere mayflies in particular, with the timing of ‘hatches’ being abundantly illustrated in flyfishing books (e.g. Arbona 1980) as guides to the best ‘terminal tackle’ for flyfishers at a given time and location.
Whether or not this scenario holds for southern hemisphere mayfly taxa is not clear. In northern New Zealand, emergence periods of stream-dwelling leptophlebiid mayflies inferred from nymphal size-frequency distributions overlap extensively and are ‘weakly seasonal and non-seasonal’ (Towns 1983). This apparent contrast with northern hemisphere mayflies was suggested to reflect a longer period of food availability (fine organic particulates) in New Zealand than that of the coarse organics that apparently support northern hemisphere species (Winterbourn et al. 1981; Towns 1983). Similar arguments have been made for Australian mayflies. Campbell (1986), in reviewing life histories of Australian species, suggested that they featured long emergence periods, poor synchronisation and geographic and inter-annual variability within species, which is largely consistent with the New Zealand data. However, in addition, he concluded that these features also characterised ‘moderate temperate’ northern hemisphere species, as defined by Clifford et al. (1973). The apparent differences between the Australian and northern hemisphere taxa, therefore, were hypothesised to reflect a climatically inappropriate comparison based on species in ‘cold temperate’ northern hemisphere locations. Against this, a number of ecological hypotheses has been put forward to explain apparent hemispheric difference in seasonality, framed against differences in the river systems (Lake et al. 1985). These include (1) low and more variable flow regimes in Australia, (2) a greater input of terrestrial organic material in summer, during periods of low flow, than in autumn in North America, a period of usually higher flow, and (3) more woody debris in Australian streams, constituting a food resource available for longer periods during the year.
Tasmania is the overall coolest of the Australian states, and possibly closest to the definition of a cold-temperate climate. Published data on emergence seasonality of Tasmanian mayflies are sparse (although see Thresher, in press, for state-wide compilations), with most of the data on Australian species being based on studies in Victoria and New South Wales. To help fill this gap, the present study examines in detail emergence seasonality of mayflies over a 2-year period in a small headwater stream in south-eastern Tasmania (Dunns Creek), extends the analysis state-wide for a common species reliably identifiable as a subadult and adult in field studies, and then, at two spatial scales, compares the emergence patterns of Tasmanian mayflies with those of related taxa in the northern hemisphere. First, emergence durations of Tasmanian species pooled state-wide are compared with similarly aggregated data for taxa in Britain, a northern hemisphere island with a climate similar to that of Tasmania; for example, average daily temperatures in London range from 6.8 to 20°C, as compared with 8.5–17.5° in Hobart. The second analysis compares emergence patterns at Dunns Creek with those at Fourmile Creek, Pennsylvania. The latter is a habitat similar to Dunns Creek and the southern-most site in North America, for which I could find detailed data on species confamilial with those in the Tasmanian creek.
Materials and methods
From 2 July 2002 to 17 June 2004, almost every fortnight the same ∼70-m section of Dunns Creek was searched for subadult and adult mayflies. Dunns Creek is a shallow, free-flowing and rocky tributary of the Browns River that runs mainly through undeveloped, forested areas below Kunyani–Mount Wellington. The sampling location was the bridge crossing and up-stream of it on Summerleas Road. Almost all sampling was conducted in early afternoon. At each sample, water temperature, weather, river height (subjectively assessed on a scale of 0, essentially no flow, to 5, flood) and species seen and their numbers were recorded. Digital photographs were taken and specimens that could not be unambiguously identified in the field were preserved in reagent-grade ethanol. Laboratory identification involved the use of dissecting and compound microscopes, and was based on the sparse published literature (e.g. Tillyard 1936), Thresher (in press), and advice from J. Dean and P. Suter. Each sampling event took from 1 to 2 h, and consisted of examining bridge supports and stream-side vegetation for mayflies, turning over and replacing rocks in the river looking for crawling-out emergers, and searching for swarming adults. Eleven specimens that were seen, but neither collected nor unambiguously identified in the field, were recorded as unknowns and included in the counts of the number of mayflies present.
Intraspecific variability in emergence seasons focussed on the Black Spinner, Atalophlebia albiterminata. A. albiterminata is a widely distributed habitat generalist that is unambiguously identifiable on the basis of its large size, colour pattern and behaviour (see Thresher, in press). Compilations of its emergences and those of other Tasmanian mayflies are based on the dates and locations where I observed, photographed or collected subadults or adults from 2002 to 2019, inclusive (see Thresher, in press). In most years, sampling trips around the state occurred year-round, but most effort was from early September through to late May, bracketing the emergence period of the species.
Temporally coarse emergence dates (months) for British mayflies are from Elliott and Humpesch (1983). The geographic coverage other than ‘Britain’ is not specified. For the comparison, the Tasmanian data were aggregated into months, to match the resolution of the British data. Emergences for Fourmile Creek (Pennsylvania) are detailed by Grant et al. (1997) for 1989 and 1990. Early spring temperatures in 1990 were ‘unseasonab(ly) warm’ compared with those in 1989, which parallels to an extent interannual differences in water temperatures at Dunns Creek (see below).
The Tasmanian data were plotted and analysed on the basis of day number each fiscal year, starting with 1 July as 1, and the northern hemisphere data calendar year days starting on 1 January, so as to span winter-to-winter periods for each. Analyses involved both parametric and non-parametric statistics, as appropriate, and were undertaken using Statview (Abacus Corporation, Baltimore, MD, USA).
Results
Dunns Creek
Over the 2-year period, fortnightly water temperatures ranged from 5.0 to 18.7°C, the creek flow index from 0 to 5 and the number of mayflies recorded from 0 to 22. Water temperatures averaged 2.1°C higher and creek flow lower in 2002–2003 than in 2003–2004 (average 11.3°C (range 6.0–18.7°C) v. 9.2°C (5.0–15.5°C), and 2.6°C (0–5°C) v. 3.1°C (2–5°C) respectively). Over the 2 years and in each year independently, water temperature and flow were highly, negatively correlated (for years pooled, Spearman rank rho = −0.45, P < 0.002). In the first sampling year, the creek nearly dried up over the summer, with water temperatures peaking over 18°C, whereas in Year 2, flow rates were variable, but showed no strong seasonality, and summer water temperatures remained moderate (Fig. 1).
Ten nominal ephemeropteran species were recorded during the 2-year sampling, namely, eight leptophlebiids in four genera, one baetid and one caenid. Five species are formally described; for the remaining three, see Thresher (in press). The sole specimen of Tasmanocoenis tonnoiri was collected on 14 November 2002. In total, there were 317 records over the 2-year period, dominated by the leptophlebiid genus Nousia (194 records). The mean number of subadults and adults per sample was higher in 2003–2004 than in 2002–2003 (7.2 (0–22) v. 4.9 (0–22); Fig. 1), but the mean number of taxa seen was similar (1.7 v. 1.8). In the first year, emergence occurred essentially year-round, with at least one subadult or adult found on all but one early winter (late June) sample. In Year 2, specimens were found in all but four early to mid-winter (mid-June to mid-July) samples (Fig. 1). Reflecting this winter depression, the gross pattern of mayfly numbers was similar in the 2 years (Fig. 1), namely, low in winter and early spring, then peaking in the summer months (December through February) before declining, but remaining high through autumn. As a result of this seasonal cycle, there was a positive relationship between water temperature and the number of mayflies seen, independently significant in both years (rho = 0.48, P < 0.02, and 0.66, P < 0.001, for Years 1 and 2 respectively). There was also a negative relationship between creek height–flow rate and mayfly numbers, significant in 2003–2004 (rho = −0.43, P < 0.02), but not 2002–2003 (rho = −0.25, n.s.). However, in a step-down multiple regression, in both years, only water temperature contributed significantly to predicting mayfly numbers.
Statistical comparisons were precluded by the unpaired nature of the data sets between years (fortnightly sampling for both, but on different dates). However, inspection of the time series showed emergence periods that were almost identical between years for four leptophlebiid species (Nousia delicatula, Nousia sp. 3, A. albiterminata and Koorrnonga brunnea; Fig. 2). Mean emergence dates were similar between years in Offadens hickmani (Baetidae) and the bicoloured Nousia (see below), but, in both taxa, winter and early spring emergence was evident in 2002–2003, but not in 2003–2004 (Fig. 2). Emergence by the leptophlebiid Tillyardophlebia tristis started at approximately the same date in both years, but the species was recorded too infrequently to draw strong conclusions.
Analysis of the bicoloured Nousia data is confounded by difficulties in species identification. Three bicoloured morphotypes were found at Dunns Creek, identifiable thus far only on the basis of the shape of the adult male genitalia (see Thresher, in press). None has been formally described. Most specimens were the ‘Dunns Creek’ morphotype. However, in 2003–2004, males slightly different from the ‘Halfmoon Creek’ morphotype were collected on 30 August and 27 September, and two similar to the ‘Condominium Creek/Wedge River’ morphotype on 3 February and 7 March. Because of its distinctive genital morphology and a winter–spring emergence phenology that differed markedly from other bicoloured Nousia morphotypes (typically summer, see Thresher, in press), the spring-emerging taxon was treated separately from other bicolours. The data for the summer-emerging taxa were pooled because of uncertain species attribution, particularly for females and subadults; however, in 2003–2004 at least, it is likely that they comprised two similar-appearing species.
Numbers of individuals recorded differed between years only for the bicoloured Nousia (Mann Whitney U25,28 = 241, P < 0.025), being higher in Year 2 than in Year 1 (Fig. 2). During the major emergence period (November–May), more than four times as many were recorded in the second year than the first (mean per sample 7.1 v. 1.6).
State-wide analysis of A. albiterminata
For the state as a whole, dates on which subadult and adult A. albiterminata were observed were stationary from 2002 to 2019 (slope of detection dates against year t = −1.12, n.s.) and did not differ significantly among years (ANOVA F18,253 = 0.65, n.s.; Fig. 3a). However, emergence seasons differed among sites. For 11 sites with at least 10 records each, differences among sites were significant (P < 0.002; F1,163 = 10.4; Fig. 3b). Pair-wise post hoc analysis (Fisher’s PLSD) clustered the sites into the following two groups: Group 1 consisted of the Break O’Day River, the lower South Esk River, the middle and upper reaches of the Macquarie River and Bronte Lagoon; Group 2 consisted of the upper South Esk River, the Ouse, Styx and Arve rivers and Dunns Creek. Within both groups, there were no significant (P < 0.05) pair-wise differences. However, 20 of 25 paired comparisons between groups were significant. The lower Macquarie, consisting of the river at Cressy and Brumbies Creek, bracketed the two groups, not significantly different from sites in either.
Comparison with northern hemisphere confamilials
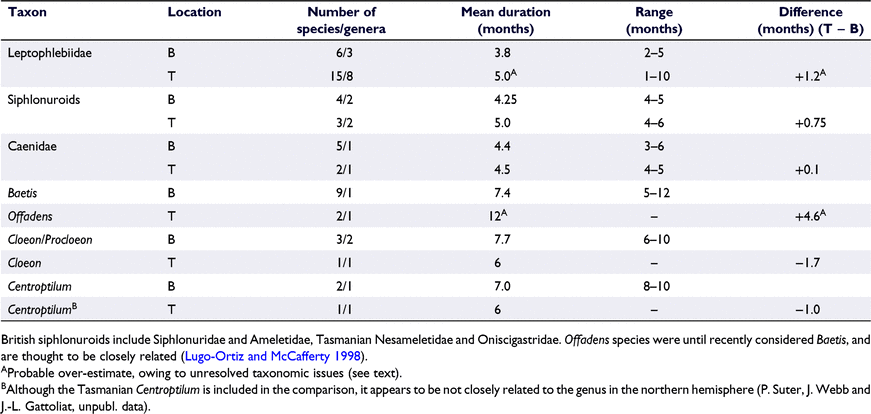
Comparative data for Britain and Tasmania are available for the superfamily Siphlonuroidea, three families (Leptophlebiidae, Caenidae and Baetidae) and, within Baetidae, three genus pairs (Table 1). Within taxa, differences among the sites in emergence durations ranged from 1.7 months shorter for Tasmania to 4.6 months longer. The mean difference across taxa was 0.66 months longer in Tasmania. Sample sizes for most taxa were too small to warrant statistical analysis; however, for the group with the largest sample sizes (leptophlebiids), the difference between Britain and Tasmania was not significant (U6,15 = 34, n.s.). Even so, taxonomic issues, suggest that the apparent difference across some groups could be over-estimates. The Tasmanian leptophlebiid data exclude several undescribed species collected only rarely as adults or subadults during a 30-year sampling effort, as well as the taxonomically poorly defined bicoloured Nousia. Rarity could reflect short emergence periods, whereas most ‘bicolours’ appeared to have emergence periods ranging from 1 to 5 months, which, if included, would reduce the mean for the family as a whole. The mean and range of durations for Offadens could also be an over-estimate, because the genus in Tasmania includes at least two undescribed species and probably a suite of cryptic species currently pooled as the year-long emerging O. hickmani (Webb and Suter 2011; P. Suter, pers. comm.).
Comparisons between Dunns Creek and Fourmile Creek are shown in Fig. 4, split by ‘warm’ and ‘cool’ years for each. The average duration at Dunns Creek during the warm year, for seven leptophlebiid taxa, was 79 days (range 28–119) and, for the cooler second year, 110 days (40–161) for six taxa. Emergence periods of four leptophlebiid species at Fourmile Creek averaged 93.5 days (range 15–122 days) during the warm year and 89 days (52–134) during the cool one. For years pooled, differences among sites were not significant (U8,11 = 43.5, n.s.). For baetids, the parallel numbers were (warm year) 161 days (one species) at Dunns Creek and 125 days (two species, 105 and 145) at Fourmile Creek, and for the cool years, 113 v. 135 days (102 and 168). Sample sizes were too small to warrant statistical analysis. Emergence seasons for five of six species, both leptophlebiids and baetids, started earlier at Fourmile Creek in the warm year than in the cool one (mean difference 43 days, ranging from 20 to 66 days). The exception was Paraleptophlebia debilis, which started emerging 10 days later in the warm year. Interannual differences in the duration of emergence periods ranged widely, from 51 days shorter in the warm year to 37 days longer. In both years, emergence seasons for three of the four leptophlebiids and both baetids overlapped extensively. By contrast, only two of six leptophlebiids at Dunns Creek started earlier in the warm year (mean difference 3.2 days, range −10 to 24 days); however, durations were shorter for four of the five species (excluding the sparsely sampled T. tristis), ranging from 42 days shorter to 29 days longer (mean 17.2 days shorter). Otherwise, the main difference between years at Dunns Creek was the emergence of a bicoloured Nousia in early spring, which was seen only in the warm year, and a 100-day earlier start to emergence in the warm year by the baetid, O. hickmani. Duration of emergence by O. hickmani was 48 days longer in the warm year than in the cool one. Emergence seasons for four of the six leptophlebiids overlapped extensively in both years, although peak periods were non-overlapping among the three Nousia taxa (two species and the bicoloured morphotypes) and between O. hickmani and most leptophlebiids.
Discussion
Sample sizes at Dunns Creek were small, but, for most taxa (the exception being T. tristis), emergence seasons that are consistent among years or well defined within years suggest that the apparent phenology is robust. However, for most species, emergence seasons at Dunns Creek were much shorter than those for the same species Tasmania-wide (for the latter, see Thresher, in press); for N. delicatula, 69 (maximum of 2 years) v. 259 days, K. brunnea, 103 v. 203 days, O. hickmani, 166 v. 348 days, and A. albiterminata, 118 v. 280 days. The exceptions were Nousia sp. 3, for which emergence at Dunns Creek spanned the entire season elsewhere in the state (134 v. 109 days), and probably the summer-emerging bicoloured Nousia, which state-wide emerges from November through to at least late April.
Emergence seasons of leptophlebiids at Dunns Creek were also similar to those at single-site studies elsewhere in southern Australia, on the basis of a sparse comparative literature. The mean duration for six nominally different leptophlebiid species in Victoria, on the basis of Marchant et al. (1984), Campbell and Holt (1984) and Campbell et al. (1990), was 4.1 months (range 3–6), which is only slightly longer than 3.1 months (1.5–7) at Dunns Creek (durations rounded to nearest half month and entered separately for each year). Within genera, two Nousia in the La Trobe River, Victoria, had durations of 3 and 4 months (Marchant et al. 1984), which matched a 3.5-month mean for four species at Dunns Creek. The average for A. albiterminata at Dunns Creek was slightly shorter than that of A. australasica in a South Australian creek (3–4 months v. 5), but that of K. brunnea was much shorter (2.5–3 months) than the 10 months of the South Australian Koorrnonga inconspicua (reported as Nousia inconspicua; Suter and Bishop 1980). However, the latter emerges overwinter rather than in spring–summer, which appears to be the norm for the genus is Tasmania (Thresher, in press). Reported durations interstate for Offadens (as Baetis) are also long relative to O. hickmani at Dunns Creek (mean 7.5 months v. 4.5). There are no data for specimens at Dunns Creek, but interstate reports for caenids (2–6 months; Suter and Bishop 1980; Marchant et al. 1984) and the long emergence durations reported for siphlonuroids in Victoria (mean 7.3 months, range 2–10; Campbell 1986) are similar to those for Tasmania as a whole.
State-wide analysis of A. albiterminata suggested that at least part of the discrepancy between Dunns Creek and the state as a whole, and possibly between Tasmanian and southern mainland sites for some species, reflects site-specific differences in emergence seasonality. For A. albiterminata, emergence in one group of sites starts in September–October and ends in March–April; in a second, emergence was rarely detected prior to November. The two groups differ markedly in habitat; the first is dominated by large, slow-flowing and deep water (e.g. the broadwaters of the Macquarie River), whereas the second consists of five shallow, fast-flowing, rocky sites. The distinction is illustrated in the South Esk River; at the shallow upstream sites near Mathinna, the earliest detection of an adult was on 15 December 2017, but below Avoca, where the river slows and forms large pools, it was 14 October 2008. Emergence dates that are stationary across years and early season sampling of all sites suggested that the differences were not the result of temporally biased sampling. The lower Macquarie and Brumbies Creek appear to have a third emergence phenology, starting as early as those in the still-water sites but with a late season peak approximately coinciding with the timing of the shallow rocky habitats. The area is also unique among the frequently sampled sites as a tailwater, with intermittently very fast flow, but soft-bottomed and heavily vegetated. Size-frequency data of subadults and adults suggested that for the state as a whole, A. albiterminata is bivoltine (Thresher, in press), but its shorter emergence seasons in rocky streams and rivers could imply that the species is univoltine in that habitat.
Apparent differences among infrequently sampled sites could also result from interannual differences in emergence seasons, which Campbell (1986) suggested to be characteristic of Australian mayflies. Four of the seven siphlonuroid species he examined showed large differences among years, as did a mainland leptophlebiid, Kirrara procera (Campbell and Holt 1984). By contrast, Marchant et al. (1984) found large interannual differences in one species of Offadens (as Baetis) in the La Trobe River, but not in a second species, in two caenids or in three leptophlebiids. In Tasmania, interannual differences in emergence seasons of A. albiterminata are not significant, but this could be due to homogenisation across sites in the pooled data. At Dunns Creek, emergence seasons of only O. hickmani differed substantially between years, occurring 100 days earlier and being 48 days longer in 2002–2003 than in 2003–2004. The start of the emergence season for six leptophlebiids recorded on both years was essentially identical between years (mean 3.5 days later in 2003–2004 than in 2002–2003, ranging from 23 days earlier to 36 days later), but the duration was, on average, longer in the second year (100 v. 83 days, excluding the spring emerging bicolour Nousia, which was not recorded in year 2). Campbell (1986) did not speculate on the causes for the different emergence seasons, but throughout implied the effects of water temperature on nymph development rates. A temperature effect on development could account for the average longer emergence durations of leptophlebiids at Dunns Creek during the cooler second year of observations, but appeared to have little effect on when they started.
This could bear on the perceived differences in emergence phenology of mayflies between the northern hemisphere and Australia. Campbell (1986) suggested that the differences reflected a climatically inappropriate comparison between cold temperate northern sites (short, warm summers bracketed by cold winters) and uniformly, relatively warm sites in southern Australia. This hypothesis is supported by the current analyses. Even given the probable over-estimates of durations for Tasmanian leptophlebiids and baetids owing to taxonomic uncertainties, emergence seasons average less than a month longer in Tasmania than they do in climatically similar Britain. For siphlonuroids and caenids, they are essentially identical; for leptophlebiids, the taxon with the largest sample sizes, differences between Britain and Tasmania were not significant. Nor were emergence seasons at Dunns Creek consistently longer than those at Fourmile Creek, Pennsylvania. For leptophlebiids, emergence seasons at Dunns Creek were in fact longer than they were in Pennsylvania when ‘warm’ years were compared, but shorter when the ‘cool’ years were compared. Differences in both comparisons were small (19 and 21 days respectively) and, when pooled at each site, not significant. For baetids, the average duration at Fourmile Creek was 22 days longer than that of O. hickmani at Dunns Creek in the cool years, but 36 days shorter in the ‘warm’ ones. Neither geographic comparison supported the hypothesis that Australian (Tasmanian) emergence periods are inherently or consistently long relative to those of northern hemisphere confamilials.
What does differ between Dunns Creek and Fourmile Creek is the apparent effect of temperature on the onset of emergence. At Fourmile Creek, a warmer than usual spring coincided with emergence in five of six species that started over a month earlier than in the cool year. This shift is consistent with the results of other northern hemisphere studies that have shown emergence times within sites being earlier in warm years (Illies and Masteller 1977; Peters et al. 1987; Finn and Poff 2008), with widespread recognition of the phenomenon among fishers (e.g. Arbona 1980), and with equally widespread reports of temperature-dependent emergence seasons for northern hemisphere mayflies in general (Brittain 1982; Ward and Stanford 1982; Newbold et al. 1994). Newbold et al. (1994) successfully modelled latitudinal variations in North American emergence dates for several mayflies, including Leptophlebia cupida, on the basis of geographic differences in dates of species-specific temperature thresholds. These results contrast strikingly with the behaviour of leptophlebiids at Dunns Creek. Despite an average difference in temperatures between years in excess of 2°, emergence in all six species, spanning four genera, started on almost identical dates in both years. However, duration of the emergence seasons were slightly longer in the cool year for most species, suggesting a disconnect between whatever cues the onset of emergence seasonally and the factors that determine its duration. The latter is widely suggested to be a function of nymphal development rates, both overseas and in Australia (Ward and Stanford 1982; Campbell 1986). The leptophlebiid patterns also contrast strikingly with that of O. hickmani, which started to emerge more than 3 months earlier in the warm year and hence superficially appears to follow the ‘northern hemisphere norm’. However, the winter–early spring emergence of it and a bicolour morphotype that year started at a time when water temperatures and water levels did not differ markedly between the warm and cool years at Dunns Creek (Fig. 1). Why both were detected in 2002–2003, but not 2003–2004 is not clear. Tasmania-wide temperatures in 2002 averaged approximately the same as in 2003 (Cooper 2019), suggesting that the mid-calendar year emergences of O. hickmani and the Nousia morphotype in 2002–2003 cannot easily be attributed to a carry-over of warm conditions from early 2002 as a similar carry-over should have been evident in 2003–2004.
Temperatures at first detection for six leptophlebiid taxa (treating the summer-emerging bicolour Nousia as distinct from the winter-emerging morphotype) at Dunns Creek averaged 3.0°C higher (range 0.6–6.7°C) in 2002–2003 than in the following year. Estimating degree-days is difficult because of the fortnightly sampling regime and two spring samples in 2003–2004 for which no temperatures were recorded. However, if the gap is filled by linear interpolation, then the average difference between the 2 years is 510 degree-days more (range 122–1211) in the first year for five summer emerging taxa. Differences between years of these magnitudes are difficult to reconcile with the temperature-dependence paradigm. The habitat-correlated differences among sites for A. albiterminata also fit the paradigm poorly. Although consistent differences could be expected between shallow, rocky streams and deep, slow-flowing ones, temperatures would likely rise more rapidly each year in the former, suggesting that emergence by A. albiterminata should start earlier in small streams than in the large rivers and lakes, which is contrary to observations. The habitat-correlated differences also appear to rule out day length or a similar seasonal metric as a globally acting cue for emergence in the species. Rather, the observations and the reasonably fixed dates of emergence at Dunns Creek suggest two testable hypotheses.
The first is that emergence dates primarily reflect a seasonally aggregated hatching of eggs. The eggs of Australian mayflies, like those overseas, develop more slowly as water temperatures decline (Elliott 1978; Friesen et al. 1979; Suter and Bishop 1990; Parnrong and Campbell 2003). In at least two Australian leptophlebiids, namely Atalophlebia australis and K. inconspicua, development in low temperatures stops at a reasonably advanced stage, but then proceeds to hatching once conditions warm up (Suter and Bishop 1990). Low temperatrure ‘quiescence’ could result in similar emergence periods among years if rising water temperatures in spring and early summer are similar even if peak summer temperatures differ markedly. This was at least partly the case at Dunns Creek; in both years, temperatures rose from a mid-winter low of ∼6°C to reach 12°C in early November. Thereafter, however, 15°C was reached nearly 2 months earlier in 2002–2003 than 2003–2004. The data therefore do not falsify a ‘quiescence hypothesis’; however, detailed information on species-specific threshold temperatures are required for it to be tested rigorously. The hypothesis also implies that post-hatch temperature-dependent growth rates by nymphs have a minimal effect on the start of the emergence season, if not its duration; are not immediately consistent with the habitat-correlated differences in emergence dates by A. albiterminata, noted above; and may not apply to species, such as Nousia sp 3, that emerge in late autumn and winter.
A second hypothesis is that the start of emergence is determined by ecological constraints on the nymphs, possibly along the lines of food quality or availability as suggested elsewhere for southern hemisphere mayflies (Towns 1983; Lake et al. 1985). A. albiterminata nymphs, for example, preferentially feed on leaves of the aquatic reed, Triglochin procerum (Watson and Barmuta 2011). Possibly coincidentally, the reed is abundant in slower-moving habitats, including the lower Macquarie, where emergence starts early in the season, but uncommon in rocky streams, where it starts later. Possibly, the development rate of Tasmanian nymphs is determined more by dietary constraints than water temperature.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The author declares that they have no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
I thank John Dean and Phil Suter for their advice regarding species identification and providing copies of relevant grey literature, A. R. Griffin for the lending of a compound microscope, and R. Marchant for suggesting egg development be considered as a possible factor affecting the timing of emergence.
References
Arbona FL (1980) ‘Mayflies: the angler and the trout.’ (Winchester Press: Piscataway, NJ, USA)Brittain, JE (1982). Biology of mayflies. Annual Review of Entomology 27, 119–147.
| Biology of mayflies.Crossref | GoogleScholarGoogle Scholar |
Campbell, IC (1986). Life histories of some Australian siphlonurid and oligoneurid mayflies (Insecta: Ephemeroptera). Australian Journal of Marine and Freshwater Research 37, 261–288.
| Life histories of some Australian siphlonurid and oligoneurid mayflies (Insecta: Ephemeroptera).Crossref | GoogleScholarGoogle Scholar |
Campbell IC, Holt MK (1984) The life history of Kirrara procera Harker in two southern Australian rivers. In ‘Proceedings of the IVth international conference on Ephemeroptera’, 4–10 September 1983, Bechynĕ, Czechoslovakia. (Eds V Landa, T Soldán, M Tonner) pp. 299–305. (Czechoslovak Academy of Sciences: Ceske Budejovice, Czechoslovakia)
Campbell IC, Duncan MJ, Swadling KM (1990) Life histories of some Ephemeroptera from Victoria, Australia. In ‘Mayflies and stoneflies, life histories and biology’. (Ed. IC Campbell) pp. 81–84. (Kluwer Academic Press: Dordrecht, Netherlands)
Clifford HF, Robertson MR, Zelt KA (1973) Life cycle patterns of mayflies (Ephemeroptera) from some streams of Alberta, Canada. In ‘Proceedings of the 1st international conference on Ephemeroptera’, 17–20 August 1970, Tallahassee, FL, USA. (Eds WL Peters, JG Peters) pp. 122–131. (E.J. Brill: Leiden, Netherlands)
Cooper E (2019) How much has Tasmania’s climate changed in the 100 years to now? In ABC News, 26 July 2019. (Australian Broadcasting Corporation) Available at https://www.abc.net.au/news/2019-07-26/climate-change-measurable-impacts-in-tasmania/11338326 [Verified 26 July 2019]
Edmunds GF Jr, Edmunds CH (1980) Predation, climate, and emergence and mating of mayflies. In ‘Advances in ephemeroptera biology’. (Eds JF Flanngan, KE Marshall) pp. 277–285. (Springer: Boston, MA, USA)
Elliott, JM (1978). Effect of temperature on the hatching time of eggs of Ephemerella ignita (Poda) (Ephemeroptera:Ephemerellidae). Freshwater Biology 8, 51–58.
| Effect of temperature on the hatching time of eggs of Ephemerella ignita (Poda) (Ephemeroptera:Ephemerellidae).Crossref | GoogleScholarGoogle Scholar |
Elliott JM, Humpesch UH (1983) ‘A key to the adults of the British Ephemeroptera with notes on their ecology.’ Scientific Publications of the Freshwater Biological Association 47. (Freshwater Biological Association)
Finn, DS, and Poff, NLR (2008). Emergence and flight activity of alpine stream insects in two years with contrasting winter snowpack. Arctic, Antarctic, and Alpine Research 40, 638–646.
| Emergence and flight activity of alpine stream insects in two years with contrasting winter snowpack.Crossref | GoogleScholarGoogle Scholar |
Friesen, MK, Flannagan, JF, and Lawrence, SG (1979). Effects of temperature and cold storage on development time and viability of eggs of the burrowing mayfly Hexagenia rigida (Ephemeroptera: Ephemeridae). The Canadian Entomologist 111, 665–673.
| Effects of temperature and cold storage on development time and viability of eggs of the burrowing mayfly Hexagenia rigida (Ephemeroptera: Ephemeridae).Crossref | GoogleScholarGoogle Scholar |
Grant, P, Burrian, SK, and Masteller, EC (1997). Emergence of mayflies (Ephemeroptera) from streams of Erie Co., Pa. Journal of the Pennsylvania Academy of Science 70, 105–112.
Illies, J, and Masteller, EC (1977). A possible explanation of emergence patterns of Baetis vernus Curtis (Ins. Ephemeroptera) on the Britenbach. Schlitz studies on productivity, Nr. 22. Internationale Revue der gesampten Hydrobiologie 62, 315–321.
| A possible explanation of emergence patterns of Baetis vernus Curtis (Ins. Ephemeroptera) on the Britenbach. Schlitz studies on productivity, Nr. 22.Crossref | GoogleScholarGoogle Scholar |
Lake, PS, Barmuta, LA, Boulton, AJ, Campbell, IC, and St Clair, RM (1985). Australian streams and Northern Hemisphere stream ecology: comparisons and problems. Proceedings of the Ecological Society of Australia 14, 61–82.
Lugo-Ortiz, CR, and McCafferty, WP (1998). Offadens, a new genus of small minnow mayflies (Ephemeroptera, Baetidae) from Australia. Proceedings of the Entomological Society of Washington 100, 306–309.
Marchant, R, Graesser, A, Metzeling, L, Mitchell, P, Norris, R, and Suter, P (1984). Life histories of some benthic insects from the La Trobe River, Victoria. Australian Journal of Freshwater and Marine Research 35, 793–806.
| Life histories of some benthic insects from the La Trobe River, Victoria.Crossref | GoogleScholarGoogle Scholar |
Newbold, JD, Sweeney, BW, and Vannote, RL (1994). A model for seasonal synchrony in stream mayflies. Journal of the North American Benthological Society 13, 3–18.
| A model for seasonal synchrony in stream mayflies.Crossref | GoogleScholarGoogle Scholar |
Parnrong S, Campbell IC (2003) The effects of temperature on egg hatching of the mayfly Austrophlebioides marchanti (Ephemeroptera: Leptophlebiidae). In ‘Research update on Ephemeroptera and Plecoptera’. (Ed. E. Gaino) pp. 189–193 (University of Perugia Press: Perugia, Italy)
Peters, JG, Peters, WL, and Fink, TJ (1987). Seasonal synchronization of emergence in Dolania americana (Ephemeroptera: Behningiidae). Canadian Journal of Zoology 65, 3177–3185.
| Seasonal synchronization of emergence in Dolania americana (Ephemeroptera: Behningiidae).Crossref | GoogleScholarGoogle Scholar |
Suter PJ, Bishop JE (1980) The effect of mesh size on the interpretation of the life history of two mayflies from South Australia. In ‘Advances in ephemeroptera biology’. (Eds JF Flannagan, KE Marshall) pp. 381–403. (Plenum Press: New York, NY, USA)
Suter PJ, Bishop JE (1990) Post-oviposition development of eggs of South Australian mayflies. In ‘Mayflies and stoneflies: life history and biology’. (Ed. I. C. Campbell) pp. 85–94. (Kluwer Academic Press: Dordrecht, Netherlands)
Thresher RE (in press) ‘Tasmanian mayflies.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Towns, DR (1983). Life history patterns of six sympatric species of Leptophlebiidae (Ephemeroptera) in a New Zealand stream and the role of interspecific competition in their evolution. Hydrobiologia 99, 37–50.
| Life history patterns of six sympatric species of Leptophlebiidae (Ephemeroptera) in a New Zealand stream and the role of interspecific competition in their evolution.Crossref | GoogleScholarGoogle Scholar |
Tillyard, RJ (1936). The trout-food insects of Tasmania. Part II. A monograph of the mayflies of Tasmania. Papers and Proceedings of the Royal Society of Tasmania 1935, 23–59.
Ward, JV, and Stanford, JA (1982). Thermal responses in the evolutionary ecology of aquatic insects. Annual Review of Entomology 27, 97–117.
| Thermal responses in the evolutionary ecology of aquatic insects.Crossref | GoogleScholarGoogle Scholar |
Watson, A, and Barmuta, LA (2011). Feeding-preference trials confirm unexpected stable isotope analysis results: freshwater macroinvertebrates do consume macrophytes. Marine and Freshwater Research 62, 1248–1257.
| Feeding-preference trials confirm unexpected stable isotope analysis results: freshwater macroinvertebrates do consume macrophytes.Crossref | GoogleScholarGoogle Scholar |
Webb, JM, and Suter, PJ (2011). Identification of larvae of Australian Baetidae. Museum Victoria Science Reports 15, 1–24.
| Identification of larvae of Australian Baetidae.Crossref | GoogleScholarGoogle Scholar |
Winterbourn, MJ, Rounick, JS, and Cowie, B (1981). Are New Zealand stream ecosystems really different? New Zealand Journal of Marine and Freshwater Research 15, 321–328.
| Are New Zealand stream ecosystems really different?Crossref | GoogleScholarGoogle Scholar |