Search for the vulnerable giants: the presence of giant guitarfish and wedgefish in the Karimunjawa National Park and adjacent waters
Faqih Akbar Alghozali A , Muhammad Wiralaga Dwi Gustianto
A , Muhammad Wiralaga Dwi Gustianto  A , Ashma Hanifah
A , Ashma Hanifah  A , Maula Nadia
A , Maula Nadia  A , Widyastuti
A , Widyastuti  C , Lufni Fauzil Adhim D , Khansa Alifa Nurhaliza E , Hollie Booth
C , Lufni Fauzil Adhim D , Khansa Alifa Nurhaliza E , Hollie Booth  F G , Muhammad Ichsan
F G , Muhammad Ichsan  H I * , Andhika Prasetyo
H I * , Andhika Prasetyo  J and Nesha Ichida K
J and Nesha Ichida K
A
B
C
D
E
F
G
H
I
J
K
Abstract
Giant guitarfish (Family: Glaucostegidae) and wedgefish (Family: Rhinidae) (Critically Endangered, IUCN Red List and CITES Appendix II) are highly exploited throughout their distribution because of their highly valued fins in the international market. Both are commonly caught as bycatch or secondary valuable catch in the Java Sea, including in Karimunjawa National Park, Central Java, Indonesia.
Assess the presence and relative abundance of giant guitarfish and wedgefish species in Karimunjawa National Park and adjacent waters.
Data were collected using baited remote underwater video (BRUV) surveys across 40 sites, covering multiple zonation areas and depth ranges. All species were identified to the species level and their relative abundance was tested with one-way PERMANOVA based on sites, zonation areas and depths.
Two target species, Glaucostegus typus and Rhynchobatus australiae, were present in the study area with a maximum number of 3 and 6 and relative abundance of 0.0048 and 0.0096 respectively, over 477 BRUVs and 623.9 h of videos. Their presence during the study was not affected by sites, zonations or depth.
The presence and relative abundance of both G. typus and R. australiae were low, which may be a result of decades of overfishing, and have provided the first information to the urgency of managing the species in the areas.
Keywords: BRUV, elasmobranch, giant guitarfish, Indonesia, Karimunjawa, presence, relative abundance, wedgefish.
Introduction
The cartilaginous fishes (Class Chondricthyes) is an ancient and diverse group of species, including sharks, rays, skates and chimera (Ebert et al. 2021), which is now one of the world’s most threatened taxonomic groups (Dulvy et al. 2021). Of this group, giant guitarfish (Family: Glaucostegidae) and wedgefish (Family: Rhinidae) are among the most threatened, with the majority of species having recently been assessed as Critically Endangered by the IUCN Red List in 2018, because of extensive exploitation as target and valuable secondary catch (Kyne et al. 2019a, 2019b). These taxa are highly exploited throughout their distribution and have some of the highest-valued fins in the international market (Suzuki 2002; Dent and Clarke 2015; Moore 2017; Jabado 2018; Kyne et al. 2020; Haque et al. 2021). In 2019, both taxa were listed on the Convention on International Trade of Endangered Species (CITES) Appendix II at the CITES Conference of the Parties 18, which stipulates that any international trade in these taxa should be compatible with their survival in the wild (i.e. sustainable).
Indonesia is a global priority for conservation of giant guitarfish and wedgefish, because it is a hotspot of species diversity and also the world’s largest shark and ray fishing nation (Dent and Clarke 2015). Giant guitarfish and wedgefish are extensively caught and utilised in many regions, including Aceh, West Kalimantan, East Lombok and the northern coast of Java (Faizah and Chodrijah 2020; Simeon et al. 2020; Yuwandana et al. 2020; Booth et al. 2023a; Hermansyah et al. 2022). This creates a challenge for successful implementation of CITES, which was ratified by the Government of Indonesia in 2022 under the Minitrial Decree of the Ministry of Maritime Affairs and Fisheries Number 12 Year 2022 (recommends the catch quota and minimum catch size of 180 cm for giant guitarfish, Glaucostegus spp., and 170 cm for wedgefish, Rhynchobatus spp.) and Number 61 Year 2018 (concerning utilisation of protected or CITES listed fish species).
The Java Sea in northern Java is a priority location for giant guitarfish and wedgefish management in Indonesia, as it experiences intense fishing pressure, which creates a threat to these taxa; yet, it is also home to an important marine protected area (MPA), Karimunjawa National Park (KJNP), which offers a potential opportunity for improved fisheries management and conservation. Northern Java commercial fisheries frequently capture large giant guitarfish and wedgefish, particularly in vessels that use bottom longlines, gill-nets and trawls (Ministry of Maritime Affairs and Fisheries 2019; Yuwandana et al. 2020). The most commonly caught species are the giant guitarfish (Glaucostegus typus) and the bottlenose wedgefish (Rhynchobatus australiae) (Yuwandana et al. 2020). Both species have conservative life-history strategies, being slow-growing and long-lived (White 2007; Last and Stevens 2009; White et al. 2014a; Last et al. 2016). KJNP is located near the main fishing grounds of northern Java’s fishing fleets and may serve as an important mating and nursery ground for giant guitarfish and wedgefish, on the basis of their ecology and breeding behaviour (Kyne et al. 2019a, 2019b). KJNP is a multi-use MPA, which is still home to four traditional fishing villages that are permitted to conduct small-scale fishing activities within the traditional fishing zones of KJNP, whereas commercial fishing is restricted. Local fishing within KJNP comprises small-scale fisheries (SSFs) that utilise handlines, gill-nets, fish traps and spearguns (Elasmobranch Project Indonesia, EPI, unpubl. data), and giant guitarfish are ocassionally caught (Elasmobranch Project Indonesia 2019). A whole giant guitarfish and wedgefish (locally known as ‘kekeh’ and ‘junjunan’ respectively) can fetch up to IDR15 000 and R50 000 kg−1 respectively for a large individual (~100 kg), or the equivalent of ~US$100 and ~$320 fish−1 at the time of writing (2022) if sold in KJNP (EPI, unpubl. data).
Despite the need and opportunity for giant guitarfish and wedgefish conservation and fisheries management in the Java Sea and KJNP, there is a lack of data describing the presence, distribution, status and local uses of these taxa, which hinders species-specific management and implementation of CITES. This study aims to fill this gap by providing baseline information on giant guitarfish–wedgefish and other elasmobranch species presence, distribution, and relative abundance in KJNP and adjacent waters, by using baited remote underwater videos (BRUVs). This is the first study of its kind in the Java Sea, and offers potential recommendations for area-based and fisheries management in and around KJNP.
Materials and methods
Study site
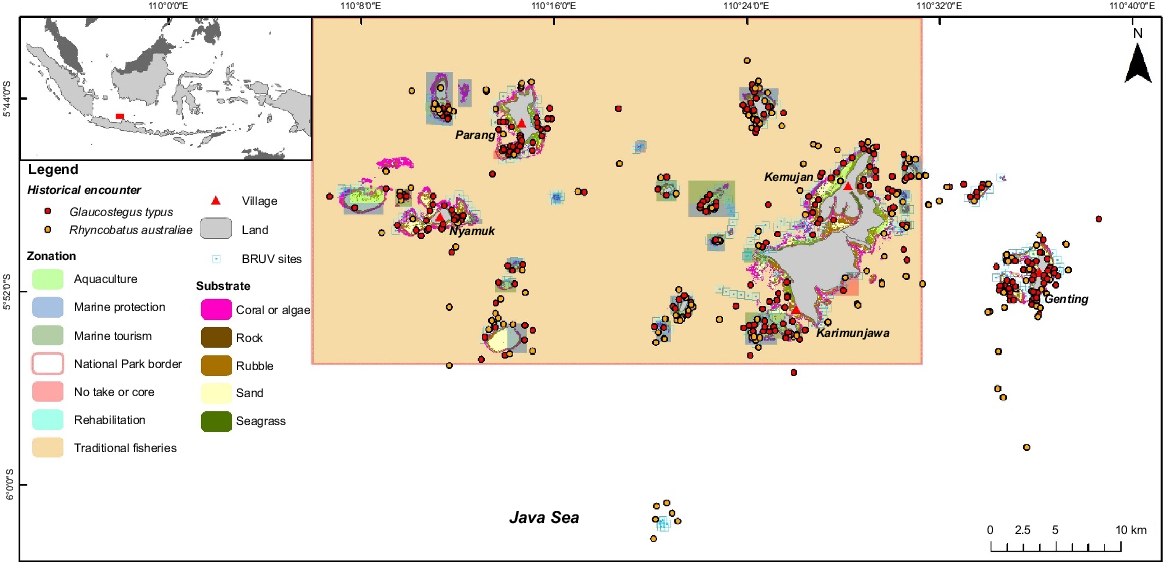
The study was conducted in the waters of KJNP (5°48′58.45″S, 110°28′07.04″E) in central Java Province, Indonesia (Fig. 1), which was declared as a Marine Protection Area (MPA) in 2001 (Direktorat Jenderal Perlindungan Hutan dan Konservasi Alam 2012), as well as the five islands east of the park (Cendikian, Gundul, Sambangan, Seruni and Genting). KJNP is a multi-use MPA, and since 2009, nine zones have been established, including the core (no-take), marine protection (no-take), marine utilisation, marine culture, traditional fisheries, forest, land utilisation, rehabilitation, and religion, culture and history zones. The core and traditional fisheries zone cover an area of 4446.29 and 1 028 992.49 km2 respectively (see Balai Taman Nasional Karimunjawa (BTNKJ), Profil Kawasan Taman Nasional Karimunjawa at https://tnkarimunjawa.id/profil/index).
Baited remote underwater video
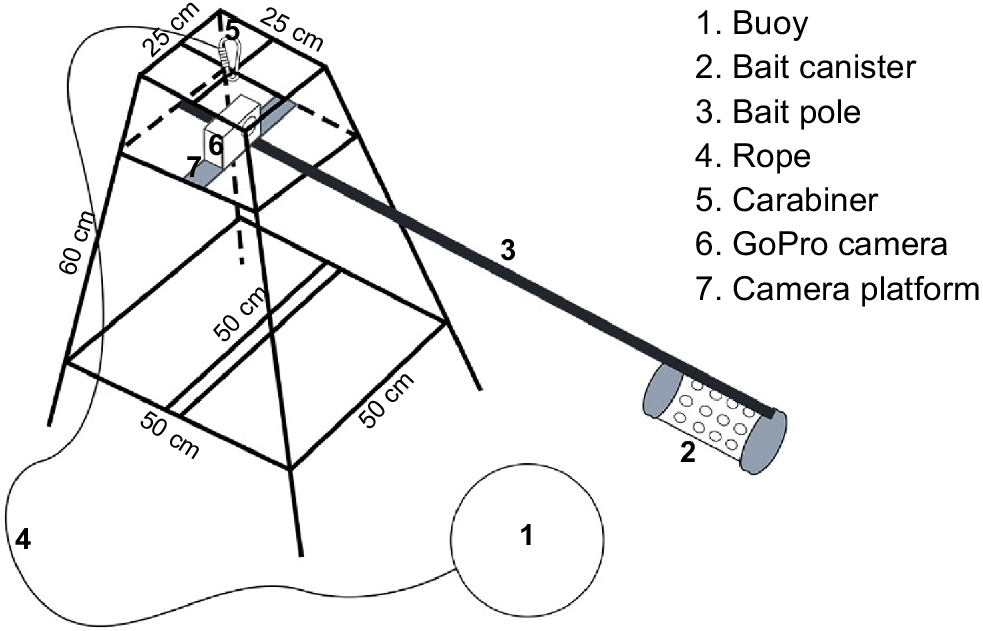
Baited remote underwater video surveys were conducted between August and October 2022 in 40 sites, which were selected on the basis of fishers’ historical encounters with giant guitarfish and wedgefish in the national park (Corbett 2009) that were collected by the EPI team between April and June 2022 (EPI, unpubl. data) (Fig. 1). The survey period was chosen because it has the calmest and best weather conditions between the East and West Monsoon seasons. Depending on the region, both monsoon seasons have rough weather, including unusually strong wind and waves. During West Monsoon season, especially in KJNP, most fishers will not go fishing unless weather is good. The BRUV units were distributed in the following four park zones: marine tourism, marine protection, traditional fisheries and core zones. Six BRUV units were used with a modified structure following the design used by Phenix et al. (2019), namely, a pyramid steel frame with dimensions of 50 × 50 cm (base) × 25 × 25 cm (top) × 60 cm (slant height) and 30 × 30 cm plus shaped camera platform in the middle of the frame (Fig. 2). Each BRUV unit was equipped with a 100 cm long, 1″ (~2.5 cm) diameter PVC bait pole and a 30 cm long, 3″ (~7.6 cm) diameter PVC bait canister attached at the end. The bait used was 1 kg of tuna-like species of Auxis thazard, Euthynnus affinis or Thunnus tonggol (Harvey et al. 2007) for each deployment. These species are oilier than are reef fishes, crustaceans and squids, which is perfect for elasmobranch species, and will last longer during soaking. This helped prevent bias because the BRUV recording went closer to the 1–1.5-h mark (standard duration for elasmobranch study with BRUVs). GoPro (Hero Black 7 and 8) cameras with underwater housing and a setting of 1080p, 60 frames s−1 and a linear view were used and attached to the camera platform facing the bait canister. A buoy fixed to a rope was attached to the frame to mark the BRUV unit post-deployment. The technical details of the BRUV survey followed a modified design by Beer (2015), Bond et al. (2012) and Rizzari et al. (2014).
In total, 489 BRUV units were deployed across all 27 islands and 8 reef flats or sandbars within and adjacent to KJNP. Most of the deployments were at a depth of 20–30 m (40.7%) and the most common substrate where BRUV units were deployed was plain sand (68.8%) (Table 1). In terms of zones, BRUV units were mainly deployed in traditional fisheries (39.2%), marine tourism (20.3%) and marine protection (16.8%) zones, and 15.3% were deployed outside of the national park area. Surveys were conducted between 08:00 and 16:00 hours and ~12 BRUV units were deployed per day with a soak time of 70–80 min each. The depth range was within 1–40 m, with each deployment distancing between 300 and 1000 m to avoid sighting replication and overlapping bait plumes. Each BRUV unit was deployed carefully from a boat to the seafloor with the coordinates and depth was taken using a GPS and depth sounder.
| Depth range (m) | n | %n | Substrate | n | %n | Zonation | n | %n | |
|---|---|---|---|---|---|---|---|---|---|
| 0–10 | 33 | 6.9 | Sand | 328 | 68.8 | Aquaculture | 11 | 2.3 | |
| 10.1–20 | 132 | 27.7 | Sand, coral | 29 | 6.1 | Traditional fisheries | 187 | 39.2 | |
| 20.1–30 | 194 | 40.7 | Sand, rubble | 84 | 17.6 | Marine protection | 80 | 16.8 | |
| 30.1–40 | 118 | 24.7 | Sand, rubble, coral | 17 | 3.6 | Marine tourism | 97 | 20.3 | |
| Other | 18 | 3.8 | No-take or core | 29 | 6.1 | ||||
| Outside National Park | 73 | 15.3 |
Video review
All BRUV recordings were reviewed and analysed in real time using available media players (e.g. MPC-HC, VLC, Windows Media Player). The analysis of each video duration started once the BRUV unit fully settled on the seafloor (mark zero) and went on until the BRUV unit was pulled up or the battery has died. We recorded the duration length and substrate recorded during each BRUV drop. We excluded videos from BRUV units that fell with the camera facing the surface.
All giant guitarfish, wedgefish and all other elasmobranch were identified and recorded. Giant guitarfish is easily identified by its morphological differences, such as the snout, head shape and number of large thorns on the ventral side, whereas wedgefish is more difficult to distinguish owing to its similar white spot patterns within its species complex (Jabado 2019). We also recorded encounters of all other shark and ray species recorded by the BRUV units. This is because fisher encounters suggest that giant guitarfish and wedgefish are likely to be low in abundance in KJNP, and additional data on other shark and ray taxa allowed us to contextualise abundance of giant guitarfish and wedgefish relative to other species, and explore any patterns in spatial co-occurrence. The maximum number (nmax) of individuals of each species in each video was then recorded. The relative abundance of each species was then calculated for their nmax per survey hour. Information on depth, substrate and deployment time were assigned against the species composition (Willis et al. 2000; Cappo et al. 2007a, 2007b; Harvey et al. 2007).
Data analysis
To understand any significant differences in distribution of giant guitarfish and wedgefish throughout the surveyed sites, one-way permutational ANOVA (PERMANOVA) with 9999 permutations and Euclidean similarity index were used to test differences in nmax, comparing west, east and outside of national park; in zonation, comparing no-take (core and marine protection), open access (rest) zone and outside of national park; and in depth, comparing 0–10, 10.1–20, 20.1–30 and 30.1–40 m (Beer 2015).
Ethical statement
The study was conducted under the research permit from BTNKJ (permit numbers 1567/T.34/TU/SIMAKSI/05/2022 and 1596/T.34/TU/SIMAKSI/08/2022) and Badan Riset dan Sumberdaya Manusia Kelautan dan Perikanan (BRSDMKP) (permit number 223/BRSDM/III/2022). No research ethic was legally required in 2021 for conducting research that involves human or wildlife and the permits issued by BTNKJ and BRSDMKP were sufficient to deem that the proposed research method and design were accepted by both authorities. The research did not perform any invasive activity to any wildlife in its process because of the research design of passive data collection and sandbed area as the targeted substrate.
Results
Of the 489 BRUV units that were deployed, 12 videos were excluded from the analysis because of falling backwards from strong currents, leaving a total of 477 videos (of 623.9 h) for inclusion in the study.
Species encountered and their relative abundance
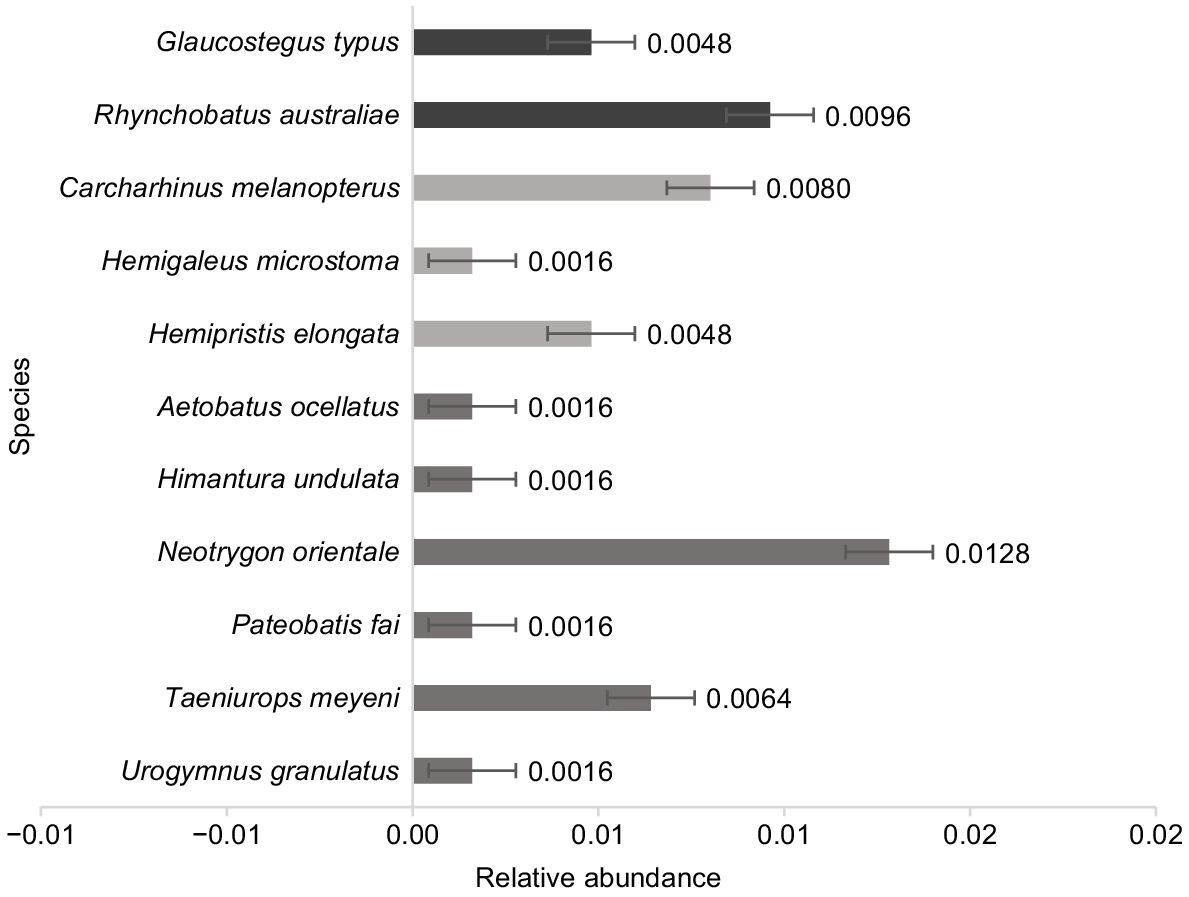
In total, three encounters and three nmax were recorded for G. typus (Family: Glaucostegidae), whereas three encounters and six nmax were recorded for R. australiae (Family: Rhinidae). The remaining nmax recorded for target species are three for sharks and nine for rays (Fig. 3). The G. typus and R. australiae both exhibited a relative abundance of 0.0048 and 0.0096 nmax h−1 respectively (Fig. 3). These values are moderate relative to other species recorded during the study (Fig. 3). Species with the lowest relative abundance were the sicklefin weasel shark (Hemigaleus microstoma), leopard whipray (Himantura undulata), pink whipray (Pateobatis fai) and mangrove whipray (Urogymnus granulatus), whereas the highest was the oriental bluespotted maskray (Neotrygon orientale) (Fig. 3).
Spatial and depth distribution
All sharks and rays, including G. typus and R. australiae, were recorded at depths below 10 m (Table 2). Most shark and ray species were recorded at depths of 20.1–30 m, with a maximum depth of 35.5 m for blacktip reef shark (Carcharhinus melanopterus), 37.2 m for snaggletooth shark (Hemipristis elongata) and 22.8 m for the only encountered H. microstoma. All species were mostly encountered within the traditional fisheries, marine protection, marine tourism zones and outside of the national park area. Although some shark and ray species encountered were reef species, they were present in the observed substrate of pure sandbed and sandbed mixed with corals or rubbles. We did not find statistically significant differences in the nmax of G. typus, R. australiae and all shark and ray species combined across site, national park zonation and depth (P > 0.05) on the basis of the one-way PERMANOVA test (Table S1 of the Supplementary material).
| Item | nmax | ||||
|---|---|---|---|---|---|
| G. typus | R. australiae | Sharks | Rays | ||
| Depth range (m) | |||||
| 0–10 | – | – | – | – | |
| 10.1–20 | 1 | – | – | 5 | |
| 20.1–30 | 2 | 4 | 7 | 12 | |
| 30.1–40 | – | 2 | 2 | 2 | |
| Substrate | |||||
| Sand | 2 | 6 | 6 | 10 | |
| Sand, coral | – | – | 1 | – | |
| Sand, rubble | 1 | – | 1 | 4 | |
| Sand, rubble, coral | – | – | – | 2 | |
| Other | – | – | – | 3 | |
| Zonation | |||||
| Aquaculture | – | – | – | – | |
| Traditional fisheries | 1 | 4 | 2 | 7 | |
| Marine protection | – | 1 | 2 | 4 | |
| Marine tourism | – | 1 | – | 6 | |
| No-take or core | 1 | – | – | – | |
| Outside National Park | 1 | – | 5 | 2 | |
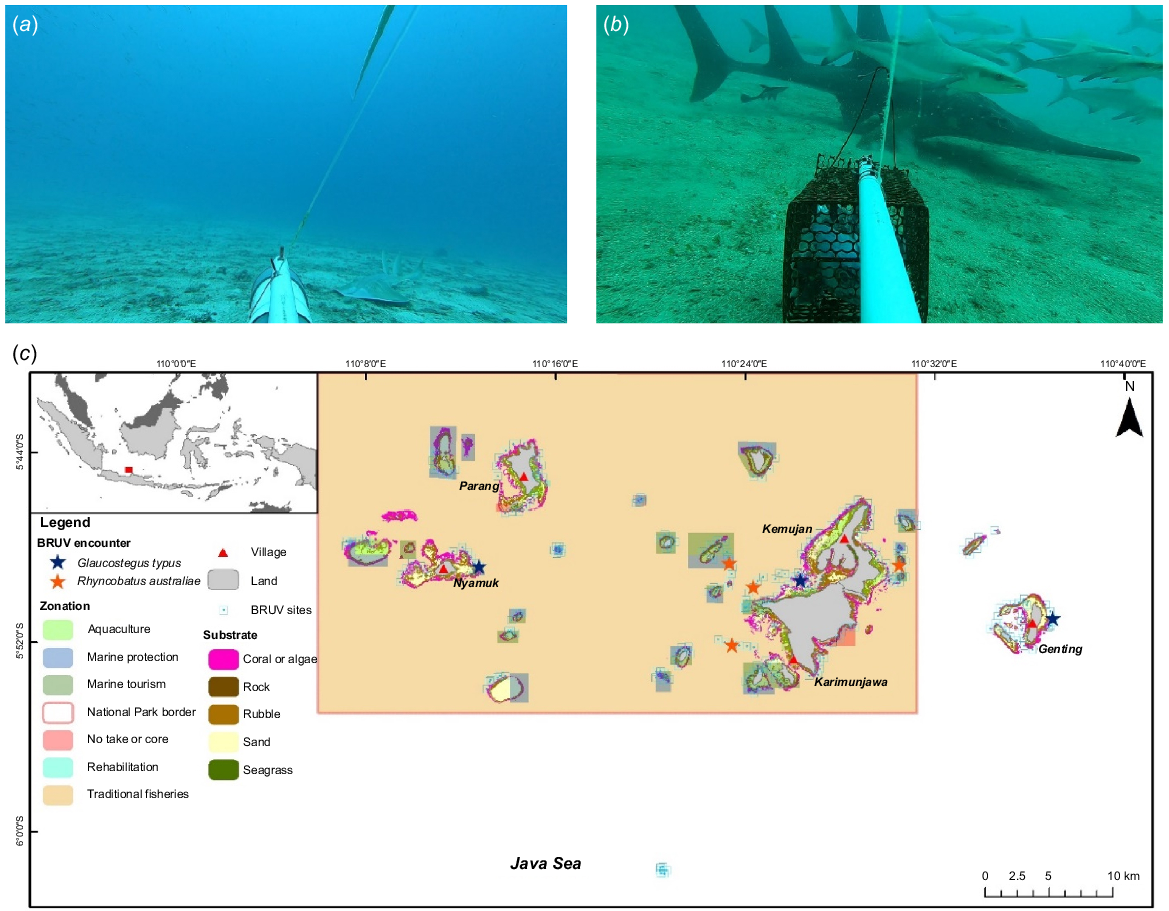
Both G. typus and R. australiae encounters happened in the pure sandbed area, with one encounter of the G. typus having a mixture of sand and rubbles (Table 3, Fig. 4). The encounters were recorded at a depth of 15.6–35.6 m for G. typus and 22.2–35.6 m for R. australiae. The encounters showed that live sharksucker (Echneis naucrates) is a symbiont for both species. However, R. australiae was recorded to also have the common remora (Remora remora) and cobia (Rachycentron canadum) as its symbiont, although R. canadum appeared in massive numbers (12 and 24 individuals) in two R. australiae (both had only 1 nmax) encounters. The R. australiae individuals were all attracted to the baits on the BRUV units, compared with the G. typus, with only one encounter showing attraction of the species to the bait.
| Species | nmax | Location | Depth (m) | Substrate | Behaviour | Symbiont | nmax | BRUV unit drop time | |
|---|---|---|---|---|---|---|---|---|---|
| G. typus | 1 | Malang Reef | 15.6 | Sand | Passing | Echneis naucrates | 3 | 8:34:00 hours | |
| 1 | East Genting | 26.8 | Sand | Passing | Echneis naucrates | 1 | 9:03:00 hours | ||
| 1 | East Nyamuk | 35.6 | Sand, rubble | Attracted | – | – | 9:30:00 hours | ||
| R. australiae | 1 | Tengah Island | 22.2 | Sand | Attracted | Rachycentron canadum | 12 | 10:12:00 hours | |
| Echneis naucrates | 2 | ||||||||
| 1 | Waka Reef | 34.2 | Sand | Attracted | Rachycentron canadum | 1 | 10:37:00 hours | ||
| Echneis naucrates | 4 | ||||||||
| 1 | Cemara Sandbar | 35.6 | Sand | Attracted | Rachycentron canadum | 24 | 1:15:00 hours | ||
| Echneis naucrates | 2 | ||||||||
| Remora remora | 1 | ||||||||
| 3 | Alang-Alang | 27.6 | Sand | Attracted | Rachycentron canadum | 2 | 1:51:00 hours | ||
| Echneis naucrates | 1 | ||||||||
| Remora remora | 1 |
Discussion
This study deployed BRUV units in areas where fishers had historical encounters with G. typus and R. australiae in KJNP and its adjacent waters, to gather data on their contemporary presence and distribution. Our data have provided up-to-date information on the presence, status and ecology of guitarfish, wedgefish and other elasmobranchs in and around KJNP, which can be used to inform management.
On the basis of the IUCN Red List and government fisheries data, populations of wedgefish and giant guitarfish are declining globally and in Indonesia (Directorate General of Capture Fisheries 2015, 2017; Kyne et al. 2019a, 2019b). This is supported by the low value of relative abundance for both G. typus (nmax = 3; relative abundance = 0.0048) and R. australiae (nmax = 6; relative abundance = 0.0096) in KJNP and nearby waters, compared with other studies that recorded similar or higher value with lower sampling efforts (<100 deployments) such as in the Arabian Gulf (Jabado et al. 2021), Mozambique (O’Connor and Cullain 2021) and Western Australia (Schramm et al. 2020). Although there is no comparable BRUV data from a previous period, historic fisher encounters in comparison with this low relative abundance suggest their population may be declining as well.
Threats such as bycatch or valuable secondary catch from local artisanal fishers with gill-nets, handlines and spearfishing with compressor diving (EPI, unpubl. data, 2022), and commercial fishers from northern Java region who are known to fish within and nearby the national park waters (Yuwandana et al. 2020), may worsen both species population in either KJNP or Java Sea. However, further research is needed to confirm the more accurate trends in the population in KJNP.
Despite statistical analysis showing that the presence of G. typus and R. australiae were not affected by sites, national park zonations and depths, the low relative abundance value exhibited by G. typus and R. australiae in this study may reflect the true condition in the location where they were encountered, considering their possible low mobility shown in some studies of their sister species. A number of studies on a wedgefish species movement in Madagascar, Tanzania, Mozambique, and South Africa stated that the species showed residency to an area they inhabit, although some did large-scale coastal movements between South Aftrica and Mozambique (Bennett et al. 2021; Jordaan et al. 2021). Furthermore, another study in South Africa showed that most R. australiae individuals (stated as R. djiddensis) stayed within 5-km radius in a catch–recapture study (Jordaan et al. 2021). Additionally, a study on G. typus in Australia showed that the species moved only between 1 and 3 km in the span of 5 days (Crook 2020). These studies may also indicate that the low relative abundance of both species in this study may mean that both species are residents (do not travel far) of where they were found because both species are assumed to have a lower mobility than that of highly mobile shark species (e.g. Carcharhinus amboinensis and C. sorrah; Knip et al. 2011, 2012), and a higher mobility than that of disc-shaped rays (e.g. Dasyatis lata and Urobatis helleri; Vaudo and Lowe 2006; Cartamil et al. 2010; White et al. 2014b). However, further research with different approaches is needed to confirm this in KJNP because species may not have been encountered because of the limited BRUV unit deployment duration.
The presence of other elasmobranch species with a similar or higher trophic level (TL) (R. Froese and D. Pauly, FishBase, see www.fishbase.org) in the same habitat, including the C. melanopterus (TL 3.9), H. elongata (TL 4.3), H. microstoma (TL 4.2), P. fai (TL 3.7), blotched fantail ray (Taeniurops meyeni) (TL 4.2) and U. granulatus (TL 4.1), indicates other meso- or top-predators occurring in the same sandbed habitat as G. typus (TL 3.6) and R. australiae (TL 3.5). Additionally, the presence of other predators, such as G. javanicus (TL 3.9), other piscivorous (fish eater) and durophagous (crustacean or hard-shelled invertebrate eater) moray eel species (Table S2 of the Supplementary material; Mehta 2009) and S. barracuda (TL 4.5), was also recorded at a high number (Table S2) during this study, suggesting the possibility of predatory competition with G. typus and R. australiae for similar prey items (Vaudo and Heithaus 2011; Purushottama et al. 2020, 2022; Sreekanth et al. 2022), such as crustaceans and small fishes (Hiatt and Strasburg 1960; Hansen 2015). Predatory competition may worsen a species population assumed to be depleting (Hollowell 2013), especially for G. typus and R. australiae, considering that both are Critically Endangered (IUCN Red List). Further research with various approaches, including BRUV survey using baits of giant guitarfish and wedgefish preferred prey, deployment in night-time and at deeper depth of >40 m is needed to ascertain this assumption further. The present result described species encounters only in each national park zonation and further research will be needed to analyse the correlation or implication of the current zonation area with giant guitarfish and wedgefish presence, especially in areas with high human activity.
The single individuals recorded in each encounter of G. typus and R. australiae in this study, with the exception of one encounter with three individuals of R. australiae (as stated by local fishers that sometimes the species was found in a fever of 2–3), differs with the aggregation characteristic of some sister species (~50 individuals of Pseudobatis horkelii; Anderson et al. 2021; ~6 of Glaucostegus cemiculus; Chaikin et al. 2020; ~3 of Glaucostegus halavi; Michael 1993). However, there may be differences between examples used as a comparison with G. typus and R. australiae in terms of aggregation that may not be recorded during the study period.
All encounters with G. typus and R. australiae showed that at least the species was accompanied by at least a symbiont, commonly known as hitchhiker species, because they performed commensalism symbiosis with their host, including the E. naucrates, R. remora and R. canadum. Both E. naucrates and R. remora are common hitchhikers for large marine animals, including shark and ray (Curtis et al. 2015). An exception for E. naucrates is that it can often be found with no host animal in shallow inshore waters and near coral reefs (Collette et al. 2015a); hence, its nmax was not recorded outside of their presence with both G. typus and R. australiae. As for R. canadum, it is also a common hitchhiker on some sharks and rays (Michael 1993), although notably seen with reef manta rays (Mobula alfredi), oceanic manta rays (Mobula birostris) (Nicholson-Jack et al. 2021) and whale sharks (Rhincodon typus) (Dove and Pierce 2022). In this study, R. canadum was sighted as symbiont only for R. australiae, with one encounter of one individual accompanied by 24 of R. canadum. The presence of these symbionts with G. typus and R. australiae is the same as with any other host marine species because they benefit from eating the host’s parasites as well as food scraps off the host (Curtis et al. 2015; Collette et al. 2015a, 2015b).
Limitations of the study
The limitation of the GoPro cameras as the main recording tools used in the BRUV structure may or may not have influenced the low encounter number of giant guitarfish and wedgefish. The limitation of GoPro usage in dark surroundings limits the quality of pictures or videos taken; hence, the study was performed during the day and may have created a bias, in that the species may have exhibited a higher relative abundance value if the study were performed during the night. The limited survey temporal period (August–October 2022) may also have affected the relative abundance value, because the presence of giant guitarfish and wedgefish may differ seasonally. However, high trophic consumers (>3) such as the giant guitarfish and wedgefish are assumed to be active during both the day and night opportunistically (Du Preez et al. 1988; Hammerschlag et al. 2017; Sreekanth et al. 2022). Therefore, the low relative abundance exhibited may strengthen the assumption that it reflects the true condition of both species at where they were encountered. Nonetheless, longer duration surveys and comparison studies conducted during the night are still needed to confirm this argument as well as to look at differences spatiotemporally.
Management implications
Given the extensive exploitation of giant guitarfish (Family: Glaucostegidae) and wedgefish (Family: Rhinidae) in both the Java Sea (Yuwandana et al. 2020) and Indonesia in general (Kyne et al. 2019a, 2019b), effective management will be needed to prevent local population decline or extinction (Dulvy et al. 2017). On the basis of the results and available knowledge, authors consider KJNP (see BTNKJ, Profil Kawasan Taman Nasional Karimunjawa at https://tnkarimunjawa.id/profil/index) as one of the last strongholds (White et al. 2017; MacKeracher et al. 2019) for both groups of species in Java Sea.
To maximise the effectiveness of giant guitarfish and wedgefish management in KJNP, we have several recommendations, including the following: strengthen research and monitoring, encourage management inclusivity and develop a scheme for fishers to minimise species mortality. These fisheries management actions are necessary for both small-scale fishers operating within traditional use zones of KJNP and commercial vessels from northern Java. This could be supported with species-specific data collection for both taxa, to fully understand ecology, exploitation levels and trade. This could include fisheries-dependent research such as catch-landing records in relation to fishing efforts (Yulianto et al. 2018), including understanding fishing efforts of fishers from Jepara region who have been said to fish for giant guitarfish and wedgefish in KJNP waters (Marganita et al. 2021). Moreover, fisheries-independent research (such as identification of critical habitat through live-specimen research) will be crucial to better understand the spatial and temporal movement of these species (Speed et al. 2010; Williamson et al. 2019). The combination of these types of research, and by additionally understanding the perspective of fishers in both species as a commodity, will provide robust evidence that can help inform an effective species management planning process alongside local fishers.
Second, it is important to acknowledge that ecological research alone will not reduce threats to sharks and rays in KJNP, because this will ultimately require a change in fisher behaviour (Booth et al. 2019). As such, this study could be complimented with socio-economic research to understand the underlying drivers and socio-economic importance of shark and ray fishing within KJNP and adjacent waters. This research could then help inform locally appropriate campaigns and interventions. Crucially, management planning where all actors, especially fishers, are represented in the decision-making process must be encouraged to improve inclusivity, transparency and minimise future conflicts in marine resource use (Gupta et al. 2020, Giareta et al. 2021; Booth et al. 2023b). In the long run, involving local fishers and communities will help KJNP Agency as the local authority to manage the species efficiently.
Last, we recommend that managers and other stakeholders develop a management scheme with the main objective of minimising giant guitarfish and wedgefish mortality in KJNP, while also considering local fishers’ economy, as the target conservation species that have high economic value. This will be crucial for the scheme to be implemented sustainably and supported by local fishers. Some examples include the use of incentive schemes (e.g. using positive incentives where fishers are rewarded for not catching or releasing the species) or exploring alternative fisheries. Such schemes need careful planning, such that they align with locally accepted norms, fisheries characteristics, species survivability and financing sources (Gupta et al. 2020; Booth et al. 2023b), and we encourage more interdisciplinary research as a key next step for securing KJNP as a potential sanctuary for giant guitarfish and wedgefish in Indonesia.
Conclusions
This study provides the first baseline information on giant guitarfish and wedgefish presence and their ecological characteristics in KJNP and nearby waters by using a fisheries-independent method. Although future studies are needed to show a trend, the low nmax and relative abundance recorded in the study may be a reflection of the declining population in the Java Sea and Indonesia, as assessed for both species’ declining populations nationally and globally (Kyne et al. 2019a, 2019b). However, further fisheries-dependent and non-dependent research is needed to better understand their population in KJNP and nearby waters, including fisheries threats from the nearby northern Java coastal area. Although both species are listed as critically endangered on the IUCN Red List, the information provided by this study urges stakeholders in KJNP and nearby waters to prioritise giant guitarfish and wedgefish conservation and management. In future, good stakeholder engagement and participatory planning will be essential to co-design solutions for reducing mortality while maintaining the important role of fisheries in the North Java Sea.
Data availability
The data that support this study will be shared upon reasonable request to and approval from the corresponding author.
Declaration of funding
This study was performed with the funding support of MCAF Award 22-01 from the Marine Conservation Action Fund–New England Aquarium and Small Grant SOSF 560 from the Save Our Seas Foundation. This study was also performed with several GoPro cameras and equipment donated by IDEA WILD. All funding support was received by Faqih Akbar Alghozali from Elasmobranch Project Indonesia (EPI).
Acknowledgements
We thank the Marine Conservation Action Fund–New England Aquarium, Save Our Seas Foundation and IDEA WILD for funding and donation of equipment that enabled us to do this study. We also thank the Guitarfish Project advisors, H. Booth, M. Ichsan, A. Prasetyo and N. Ichida, and Elasmobranch Project Indonesia Elasmo-Peers and volunteers for their continuous support during the study.
References
Anderson AB, Fiuza TMJ, Araujo GS, Canterle AM, Canto LMC, Freitas RHA, Gadig OBF, Floeter SR (2021) A safe haven for potential reproductive aggregations of the critically endangered Brazilian guitarfish (Pseudobatos horkelii). Journal of Fish Biology 99(6), 2030-2034.
| Crossref | Google Scholar | PubMed |
Bennett R, van Beuningen D, Markovina M, Razafindrakoto C, Sitoe J, Sidat N, Fernando S, da Silva I, Costa H, Bernard A, Dames V, Daly R, Mann B, Jordaan G, van der Merwe AB, Price A, Asbrury T (2021) Western Indian Ocean wedgefishes (Rhinidae): ecological knowledge, fisheries and management. In ‘Report on the American Elasmobranch Society Global Wedgefish & Guitarfish Symposium 2021’. (Eds DA Ebert, P Carlson, RM Aitchison, BL Huerta-Beltran, PM Kyne) Abstract, p. 9. (Moss Landing Marine Laboratories, San Jose State University: San Jose, CA, USA)
Bond ME, Babcock EA, Pikitch EK, Abercrombie DL, Lamb NF, Chapman DD (2012) Reef Sharks exhibit site-fidelity and higher relative abundance in marine reserves on the mesoamerican barrier reef. PLoS ONE 7(3), e32983.
| Crossref | Google Scholar | PubMed |
Booth H, Squires D, Milner-Gulland EJ (2019) The neglected complexities of shark fisheries, and priorities for holistic risk-based management. Ocean & Coastal Management 182, 104994.
| Crossref | Google Scholar |
Booth H, Ichsan M, Hermansyah RF, Rohmah LN, Naira KB, Adrianto L, Milner-Gulland EJ (2023a) A socio-psychological approach for understanding and managing bycatch in small-scale fisheries. People and Nature 5(3), 968-980.
| Crossref | Google Scholar |
Booth H, Ramdlan MS, Hafizh A, Wongsopatty K, Mourato S, Pienkowski T, Adrinato L, Milner-Gulland EJ (2023b) Designing locally appropriate conservation incentives for small-scale fishers. Biological Conservation 277, 109821.
| Crossref | Google Scholar |
Cappo M, De’ath G, Speare P (2007a) Inter-reef vertebrate communities of the Great Barrier Reef Marine Park determined by baited remote underwater video stations. Marine Ecology Progress Series 350, 209-221.
| Crossref | Google Scholar |
Cappo M, Harvey E, Shortis M (2007b) Counting and measuring fish with baited video techniques. An overview. In ‘Cutting-Edge Technologies in Fish and Fisheries Science, Australian Society for Fish Biology Workshop Proceedings’, 28–29 August 2006, Hobart, Tas., Australia. (Eds JM Lyle, DM Furlani, CD Buxton) Vol. 1, pp. 101–114. (Australian Society for Fish Biology: Hobart, Tas., Australia)
Cartamil D, Wegner NC, Kacev D, Ben-aderet N, Kohin S, Graham JB (2010) Movement patterns and nursery habitat of juvenile thresher sharks Alopias vulpinus in the Southern California Bight. Marine Ecology Progress Series 404, 249-258.
| Crossref | Google Scholar |
Chaikin S, Belmaker J, Barash A (2020) Coastal breeding aggregations of threatened stingrays and guitarfish in the Levant. Aquatic Conservation: Marine and Freshwater Ecosystems 30(6), 1160-1171.
| Crossref | Google Scholar |
Collette B, Curtis M, Williams JT, Smith-Vaniz WF, Pina Amargos F (2015a) Live sharksucker Echeneis naucrates. In ‘The IUCN Red List of Threatened Species 2015’. e.T190393A115317934. (International Union for Conservation of Nature and Natural Resources) Available at https://www.iucnredlist.org/species/190393/115317934 [Erratum version published in 2017]
Collette B, Curtis M, Williams JT, Smith-Vaniz WF, Pina Amargos F (2015b) Cobia Rachycentron canadum. In ‘The IUCN Red List of Threatened Species 2015’. e.T190190A70036823. (International Union for Conservation of Nature and Natural Resources) Available at https://www.iucnredlist.org/species/190190/70036823
Corbett J (2009) Good practices in participatory mapping: a review prepared for the International Fund for Agricultural Development (IFAD). (IFAD) Available at http://www.ifad.org/pub/map/PM_web.pdf
Crook KA (2020) Assessing the functional roles of rays in coastal sandflats. PhD Thesis, James Cook University, Qld, Australia. doi:10.25903/rq54%2D5813
Curtis M, Williams JT, Collette B, Smith-Vaniz WF, Pina Amargos F (2015) Common remora Remora remora. In ‘The IUCN Red List of Threatened Species 2015’. e.T198651A115343508. (International Union for Conservation of Nature and Natural Resources) Available at https://www.iucnredlist.org/species/198651/115343508 [Erratum version published in 2017]
Dent F, Clarke S (2015) State of the global market for shark products. FAO Fisheries and Aquaculture Technical Paper 590. (Food and Agriculture Organization of the United Nations: Rome, Italy) Available at http://www.fao.org/3/a-i4795e.pdf
Direktorat Jenderal Perlindungan Hutan dan Konservasi Alam (2012) Keputusan Direktur Jenderal Perlindungan Hutan dan Konservasi Alam. Tentang zonasi taman Nasional Karimunjawa, Direktur Jenderal Perlindungan Hutan dan Konservasi Alam. Nomor: SK.28/IV-SET/2012. (Kementerian Kehutanan) Available at https://tnkarimunjawa.id/assets/filepublikasi/3/dokpublik_1501472150.pdf [In Indonesian]
Du Preez HH, Mclachlan A, Marais JFK (1988) Oxygen consumption of two nearshore marine elasmobranchs, Rhinobatos annulatus (Muller & Henle, 1841) and Myliobatus aquila (Linnaeus, 1758). Comparative Biochemistry and Physiology – A. Physiology 89(2), 283-294.
| Crossref | Google Scholar |
Dulvy NK, Simpfendorfer CA, Davidson LNK, Fordham SV, Bräutigam A, Sant G, Welch DJ (2017) Challenges and priorities in shark and ray conservation. Current Biology 27(11), R565-R572.
| Crossref | Google Scholar | PubMed |
Dulvy NK, Pacoureau N, Rigby CL, Pollom RA, Jabado RW, Ebert DA, Finucci B, Pollock CM, Cheok J, Derrick DH, Herman KB, Sherman CS, VanderWright WJ, Lawson JM, Walls RHL, Carlson JK, Charvet P, Bineesh KK, Fernando D, Ralph GM, Simpfendorfer CA (2021) Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Current Biology 31(21), 4773-4787.e8.
| Crossref | Google Scholar |
Elasmobranch Project Indonesia (2019) Giant Guitarfish (Glaucostegus typus). (EPI) Available at https://elasmobranch.id/contributor/observation/19/ [Verified 23 October 2023]
Faizah R, Chodrijah U (2020) Size distribution and population parameter of white-spotted wedgefish (Rhynchobatus Australiae Whitley, 1939) from the eastern Indian Ocean, Indonesia. IOP Conference Series: Earth and Environmental Science 584(1), 012034.
| Crossref | Google Scholar |
Giareta EP, Prado AC, Leite RD, Padilha É, Santos IHd, Wosiak CDCDL, Wosnick N (2021) Fishermen’s participation in research and conservation of coastal elasmobranchs. Ocean & Coastal Management 199, 105421.
| Crossref | Google Scholar |
Gupta T, Booth H, Arlidge W, Rao C, Manoharakrishnan M, Namboothri N, Shanker K, Milner-Gulland EJ (2020) Mitigation of elasmobranch bycatch in trawlers: a case study in indian fisheries. Frontiers in Marine Science 7, 571.
| Crossref | Google Scholar |
Hammerschlag N, Skubel RA, Calich H, Nelson ER, Shiffman DS, Wester J, Macdonald CC, Cain S, Jennings L, Enchelmaier A, Gallagher AJ (2017) Nocturnal and crepuscular behavior in elasmobranchs: a review of movement, habitat use, foraging, and reproduction in the dark. Bulletin of Marine Science 93(2), 355-374.
| Crossref | Google Scholar |
Hansen NR (2015) Feeding ecology and habitat utilization of the great barracuda Sphyraena barracuda (Edwards 1771) in southeast Florida. MSc(MarBiol) Thesis, Nova Southeastern University, FL, USA. Available at https://nsuworks.nova.edu/occ_stuetd/32/
Haque AB, Washim M, D’Costa NG, Baroi AR, Hossain N, Nanjiba R, Hasan SJ, Khan NA (2021) Socio-ecological approach on the fishing and trade of rhino rays (Elasmobranchii: Rhinopristiformes) for their biological conservation in the Bay of Bengal, Bangladesh. Ocean & Coastal Management 210, 105690.
| Crossref | Google Scholar |
Harvey ES, Cappo M, Butler JJ, Hall N, Kendrick GA (2007) Bait attraction affects the performance of remote underwater video stations in assessment of demersal fish community structure. Marine Ecology Progress Series 350, 245-254.
| Crossref | Google Scholar |
Hermansyah RF, Adrianto L, Zulfikar A, Booth H (2022) Karakteristik biologi ikan pari kekeh (Rhynchobatus spp.) sebagai tangkapan sampingan di perairan aceh jaya. bawal widya riset perikanan tangkap. Biological characteristics of wedgefish (Rhynchobatus spp.) as by-catch in Aceh Jaya Waters. Bawal 14(2), 95-103 Available at http://ejournal-balitbang.kkp.go.id/index.php/bawal/article/view/11639/8270 [In Indonesian, also with English title and abstract].
| Google Scholar |
Hiatt RW, Strasburg DW (1960) Ecological relationships of the fish fauna on coral reefs of the Marshall Islands. Ecological Monographs 30(1), 65-127.
| Crossref | Google Scholar |
Hollowell KC (2013) A review of predator–prey interactions within marine ecosystems with a focus on top predator influences on ecosystem stability and fisheries management implications. MSc(MarBiol) Thesis, Oceanographic Center, Nova Southeastern University, USA. Available at https://nsuworks.nova.edu/cgi/viewcontent.cgi?referer=&httpsredir=1&article=1136&context=cnso_stucap
Jabado RW (2018) The fate of the most threatened order of elasmobranchs: shark-like batoids (Rhinopristiformes) in the Arabian Sea and adjacent waters. Fisheries Research 204, 448-457.
| Crossref | Google Scholar |
Jabado RW (2019) Wedgefishes and Giant Guitarfishes: a guide to species identification. (Wildlife Conservation Society) Available at www.wcs.org/our-work/wildlife/sharks-skates-rays
Jabado RW, Antonopoulou M, Möller M, Al Suweidi AS, Al Suwaidi AMS, Mateos-Molina D (2021) Baited remote underwater video surveys to assess relative abundance of sharks and rays in a long standing and remote marine protected area in the Arabian Gulf. Journal of Experimental Marine Biology and Ecology 540, 151565.
| Crossref | Google Scholar |
Jordaan GL, Mann BQ, Daly R, Dunlop SW, Cowley PD (2021) Movement patterns and growth rate of the whitespotted wedgefish Rhynchobatus djiddensis in southern Africa based on tag–recapture data. African Journal of Marine Science 43(2), 201-213.
| Crossref | Google Scholar |
Knip DM, Heupel MR, Simpfendorfer CA, Tobin AJ, Moloney J (2011) Ontogenetic shifts in movement and habitat use of juvenile pigeye sharks Carcharhinus amboinensis in a tropical nearshore region. Marine Ecology Progress Series 425, 233-246.
| Crossref | Google Scholar |
Knip DM, Heupel MR, Simpfendorfer CA (2012) Habitat use and spatial segregation of adult spottail sharks Carcharhinus sorrah in tropical nearshore waters. Journal of Fish Biology 80(4), 767-784.
| Crossref | Google Scholar | PubMed |
Kyne PM, Rigby CL, Dharmadi, Jabado RW (2019a) Bottlenose wedgefish Rhynchobatus australiae. In ‘The IUCN Red List of Threatened Species 2019’. e.T41853A68643043. (International Union for Conservation of Nature and Natural Resources) Available at https://www.iucnredlist.org/species/41853/68643043
Kyne PM, Rigby CL, Dharmadi, Gutteridge AN, Jabado RW (2019b) Giant guitarfish Glaucostegus typus. In ‘The IUCN Red List of Threatened Species 2019’. e.T104061138A68623995. (International Union for Conservation of Nature and Natural Resources) Available at https://www.iucnredlist.org/species/104061138/68623995
Kyne PM, Jabado RW, Rigby CL, Dharmadi, Gore MA, Pollock CM, Herman KB, Cheok J, Ebert DA, Simpfendorfer CA, Dulvy NK (2020) The thin edge of the wedge: extremely high extinction risk in wedgefishes and giant guitarfishes. Aquatic Conservation: Marine and Freshwater Ecosystems 30(7), 1337-1361.
| Crossref | Google Scholar |
MacKeracher T, Diedrich A, Simpfendorfer CA (2019) Sharks, rays and marine protected areas: a critical evaluation of current perspectives. Fish and Fisheries 20(2), 255-267.
| Crossref | Google Scholar |
Marganita D, Hatmoro CK, Alghozali FA, Gustianto MWD, Putri NP, Putri OK, Putra PR, Danil R, Samosir TOE (2021) Komposisi hasil tangkapan elasmobranchii berdasarkan alat tangkap dan kondisi nelayan di Demaan, Jepara. In ‘Simposium Hiu & Pari di Indonesia ke-3’, 7–8 April 2021, Jakarta, Indonesia. (Eds Kusdiantoro, H Rahayu, IM Zainuddin, Fahmi, S Koeshendrajana, NN Wiadnyana) pp. 382–390. (Pusat Riset dan Perikanan, Badan Riset dan Sumber Daya Manusia Kelautan dan Perikanan: Jakarta, Indonesia)
Mehta RS (2009) Ecomorphology of the moray bite: relationship between dietary extremes and morphological diversity. Physiological and Biochemical Zoology 82(1), 90-103.
| Crossref | Google Scholar |
Michael SW (1993) Reef sharks and rays of the world: a guide to their identification, behaviour, and ecology. Journal of the Marine Biological Association of the United Kingdom 73(4), 987.
| Crossref | Google Scholar |
Moore A (2017) Are guitarfishes the next sawfishes? Extinction risk and an urgent call for conservation action. Endangered Species Research 34, 75-88.
| Crossref | Google Scholar |
Nicholson-Jack AE, Harris JL, Ballard K, Turner KME, Stevens GMW (2021) A hitchhiker guide to manta rays: patterns of association between Mobula alfredi, M. birostris, their symbionts, and other fishes in the Maldives. PLOS ONE 16(7), e0253704.
| Crossref | Google Scholar | PubMed |
O’Connor B, Cullain N (2021) Distribution and community structure of at-risk and data deficient elasmobranchs in Zavora Bay, Mozambique. African Journal of Marine Science 43(4), 521-532.
| Crossref | Google Scholar |
Phenix LM, Tricarico D, Quintero E, Bond ME, Brandl SJ, Gallagher AJ (2019) Evaluating the effects of large marine predators on mobile prey behavior across subtropical reef ecosystems. Ecology and Evolution 9(24), 13740-13751.
| Crossref | Google Scholar | PubMed |
Purushottama GB, Raje SG, Das T, Akhilesh KV, Kizhakudan SJ, Zacharia PU (2020) Reproductive biology and diet composition of Rhynchobatus laevis (Bloch and Schneider, 1801) (Rhinopristiformes: Rhinidae) from the northern Indian Ocean. Indian Journal of Fisheries 67(4), 13-23.
| Crossref | Google Scholar |
Purushottama GB, Thomas S, Kizhakudan SJ, Zaharia PU (2022) Catch composition, reproductive biology and diet of the bowmouth guitarfish Rhina ancylostomus Bloch and Shneider, 1801 (Batoidea: Rhinidae) in the eastern Arabian Sea, India. Indian Journal of Fisheries 69(2), 1-11.
| Crossref | Google Scholar |
Rizzari JR, Frisch AJ, Connolly SR (2014) How robust are estimates of coral reef shark depletion? Biological Conservation 176, 39-47.
| Crossref | Google Scholar |
Schramm KD, Marnane MJ, Elsdon TS, Jones C, Saunders BJ, Goetze JS, Driessen D, Fullwood LAF, Harvey ES (2020) A comparison of stereo-BRUVs and stereo-ROV techniques for sampling shallow water fish communities on and off pipelines. Marine Environmental Research 162, 105198.
| Crossref | Google Scholar | PubMed |
Speed CW, Field IC, Meekan MG, Bradshaw CJA (2010) Complexities of coastal shark movements and their implications for management. Marine Ecology Progress Series 408, 275-293.
| Crossref | Google Scholar |
Sreekanth GB, Jaiswar AK, Akhliesh KV (2022) Feeding ecology of Giant Guitarfish, Glaucostegus cf. granulatus (Glaucostegidae: Rhinopristiformes) from eastern Arabian Sea. National Academy Science Letters 45(1), 19-24.
| Crossref | Google Scholar |
Suzuki T (2002) Development of shark fisheries and shark fin export in Indonesia: case study of Karangsong Village, Indramayu, West Java. In ‘Elasmobranch Biodiversity, Conservation and Management: Proceedings of the International Seminar and Workshop’, July 1997, Sabah, Malaysia. (Eds SL Fowler, TM Reed, FA Dipper) pp. 149–157. (IUCN SSC Shark Specialist Group: Cambridge, UK)
Vaudo JJ, Heithaus M (2011) Dietary niche overlap in a nearshore elasmobranch mesopredator community. Marine Ecology Progress Series 425, 247-260.
| Crossref | Google Scholar |
Vaudo JJ, Lowe CG (2006) Movement patterns of the round stingray Urobatis halleri (Cooper) near a thermal outfall. Journal of Fish Biology 68(6), 1756-1766.
| Crossref | Google Scholar |
White WT (2007) Species and size compositions and reproductive biology of rays (Chondrichthyes, Batoidea) caught in target and non-target fisheries in eastern Indonesia. Journal of Fish Biology 70(6), 1809-1837.
| Crossref | Google Scholar |
White J, Simpfendorfer CA, Tobin AJ, Heupel MR (2014a) Age and growth parameters of shark-like batoids: biology of shark-like batoids. Journal of Fish Biology 84(5), 1340-1353.
| Crossref | Google Scholar | PubMed |
White J, Simpfendorfer CA, Tobin AJ, Heupel MR (2014b) Spatial ecology of shark-like batoids in a large coastal embayment. Environmental Biology of Fishes 97(7), 773-786.
| Crossref | Google Scholar |
White TD, Carlisle AB, Kroodsma DA, Block BA, Casagrandi R, De Leo GA, Gatto M, Micheli F, McCauley DJ (2017) Assessing the effectiveness of a large marine protected area for reef shark conservation. Biological Conservation 207, 64-71.
| Crossref | Google Scholar |
Williamson MJ, Tebbs EJ, Dawson TP, Jacoby DMP (2019) Satellite remote sensing in shark and ray ecology, conservation and management. Frontiers in Marine Science 6, 135.
| Crossref | Google Scholar |
Willis TJ, Millar RB, Babcock RC (2000) Detection of spatial variability in relative density of fishes: comparison of visual census, angling, and baited underwater video. Marine Ecology Progress Series 198, 249-260.
| Crossref | Google Scholar |
Yulianto I, Booth H, Ningtias P, Kartawijaya T, Santos J, Sarmintohadi, Kleinertz S, Campbell SJ, Palm HW, Hammer C (2018) Practical measures for sustainable shark fisheries: lessons learned from an Indonesian targeted shark fishery. PLoS ONE 13(11), e0206437.
| Crossref | Google Scholar | PubMed |
Yuwandana DP, Agustina S, Haqqi MB, Simeon BM (2020) Studi Awal Perikanan Pari Kekeh (Rhynchobatus sp.) dan Pari Kikir (Glaucostegus sp.) di Perairan Utara Jawa Tengah. Akuatika Indonesia 5(1), 1.
| Crossref | Google Scholar |