Spermatogonia: origin, physiology and prospects for conservation and manipulation of the male germ line
Jens Ehmcke A , Karin Hübner B , Hans R. Schöler B and Stefan Schlatt A CA University of Pittsburgh School of Medicine, Department of Cell Biology and Physiology, Center for Research in Reproductive Biology, W952 Biomedical Science Tower, 3500 Terrace Street, Pittsburgh, PA 15261, USA.
B Max Planck Institute for Molecular Biomedicine, Mendelstrasse 7, D-48149 Münster, Germany.
C Corresponding author. Email: schlatt@pitt.edu
Reproduction, Fertility and Development 18(2) 7-12 https://doi.org/10.1071/RD05119
Submitted: 21 September 2005 Accepted: 21 September 2005 Published: 14 December 2005
Abstract
In recent years, the scientific community has become increasingly interested in spermatogonia. Methodological breakthroughs, such as germ cell transplantation and spermatogonial culture combined with novel germ line transfection strategies, have provided interesting new opportunities for studying the physiology of spermatogonial stem cells and their interaction with the stem cell niche. Furthermore, intense research into pluripotent and adult stem cells has generated new insight into the differentiation pathway of germ line stem cells and has opened new perspectives for stem cell technologies. The present review briefly introduces the physiology of spermatogonial stem cells and discusses future directions of basic research and practical approaches applicable to livestock maintenance and animal reproduction.
Origin of spermatogonia in ontogenesis
Primordial germ cells can be detected at a very early stage of ontogenesis owing to morphological and biochemical differences between somatic and germ cells. Primordial germ cells can be first detected in embryo-associated but extra-embryonic tissues during early ontogenesis. In mice, the primordial germ cells are located in the yolk sac and, only after nearly all other vital tissues of the embryo have been formed, the primordial germs cells start to migrate into the area of the genital ridge (McLaren 2003; Fig. 1). After migration into the undifferentiated gonads, the primordial germs cells differentiate into female or male germ cell precursors, depending on the sexual gonadal differentiation (Capel 2000). A non-reversible decision is made when these cells either enter meiosis to become oocytes or when they arrest mitotically while the formation of the seminiferous cord is initiated to form a testis. Cord formation appears to be the most decisive event allowing primordial germ cells to differentiate into male germ line stem cells. The germ cells resume proliferative activity at later stages of testicular development and give rise to millions of differentiating germ cells throughout adulthood.

|
Initiation of spermatogenesis from stem cells
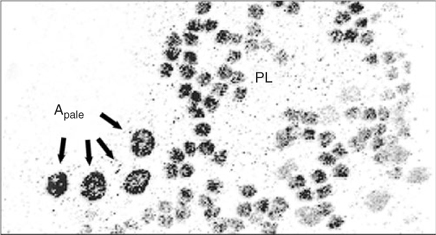
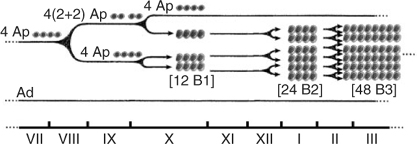
In the testis of adult mammalian species, spermatogonial stem cells maintain their numbers by self-renewal and give rise to differentiating germ cells. Most of the diploid germ cells are differentiating spermatogonia undergoing several rounds of mitotic divisions before entering meiotic prophase. In all mammals, undifferentiated spermatogonia maintain a stable population and derive differentiating spermatogonia, which finally enter meiosis and give rise to spermatocytes. Basic features of spermatogonial subtypes and their proliferation have been described for most species. These data were obtained mainly from histological cross-sections. The behaviour of spermatogonial stem cells has been analysed in whole mounts of testicular tissue. In non-human primates, seven different types of spermatogonia have been identified (Clermont and Leblond 1959; Clermont 1969; Clermont and Antar 1973; Kluin et al. 1983; Fouquet and Dadoune 1986; Zhengwei et al. 1997): the reserve stem cell Adark spermatogonium, the renewing stem cell Apale spermatogonium, the intermediate Atransition spermatogonium and four generations of B spermatogonia, namely B1, B2, B3 and B4. Although the morphological descriptions of undifferentiated and differentiating spermatogonia have long been presented, only our own recent studies in non-human primates have demonstrated unequivocally that proliferating spermatogonia are arranged in clones that are fully synchronised and connected by cytoplasmic bridges (Fig. 2). Every 10.5 days, the renewing Apale spermatogonia form small clones that split after this initial mitotic event (Ehmcke et al. 2005a). In contrast, the clones of differentiating spermatogonia maintain their clonal integrity during the subsequent divisions. Thus, premeiotic germ cells form increasingly larger clones. Finally, a large number of clonally arranged preleptotene spermatocytes enters meiosis (Ehmcke et al. 2005a, 2005b; Fig. 3). These data show that the expansion of stem cells proceeds in a clonal growth pattern that cannot be easily explored in tissue sections. So far, the description of spermatogonial stem cells, as well as the proliferative pattern of premeiotic germ cells, of domestic species has been described almost exclusively in tissue sections. Therefore, more basic research is needed to answer some of the relevant questions, including: what are the exact mechanisms of premeiotic germ cells expansion in domestic species? How often do the differentiating spermatogonia divide? How often do stem cells turnover? Which cell type is the true stem cell and which is the proliferating precursor? Answering these questions is difficult because astonishing differences exist between even closely related species, indicating that the mechanisms of stem cell turnover and the kinetics of pre- and post-meiotic germ cell development are quite adaptable for each species.
|
|
Spermatogonia: target for animal conservation?
As a clinical diagnosis, complete depletion of testicular stem cells is defined as Sertoli cell only syndrome (SCO). A partial depletion of testicular stem cells induces a focal SCO. Spermatogenic recovery after irradiation follows an all-or-nothing pattern in primates (de Rooij et al. 2002; Schlatt et al. 2002a; Kamischke et al. 2003). This finding indicates a critical role for spermatogonial stem cells in the restoration process. Functional proof for this is provided from germ cell transplantation experiments in mice and monkeys revealing that continuous recolonisation of remaining or reintroduced testicular stem cells leads to reinduction of spermatogenesis (Nagano and Brinster 1998; de Rooij et al. 2002; Kamischke et al. 2003). Therefore, spermatogonia are considered the selective target cells for the preservation of testis tissue and function, as well as for the development of novel options for fertility preservation in oncological patients (Nordhoff and Schlatt 2003; Orwig and Schlatt 2005). In the future, spermatogonial stem cells may be reintroduced into the testis of oncological patients to re-induce spermatogenesis (Radford et al. 1999; Radford 2003; Wallace and Thomson 2003). However, in domestic species, the prime interest with regard to testicular stem cell technology is not a cure of infertility of breeding stock or to explore the effects of environmental factors on male fertility. It is of prime importance to develop novel strategies to conserve valuable germ line cells and to introduce genomic changes in the male germ line (Hill and Dobrinski 2006).
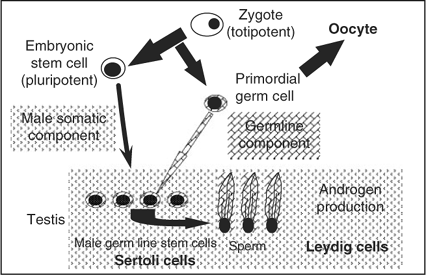
The clonal expansion of differentiating male germ cells and the recolonisation of spermatogenesis from stem cells indicates that the testicular stem cell and not the differentiating germ cells can be the target cell population when the male germ line is to be conserved or when genomic changes are inserted in the germ line. However, it may be possible to also target other potentially pluripotent cells and/or germ line cells, because it was shown recently in the mouse that even primordial germ cells isolated from the embryonic epiblast or teratocarcinoma cells have the potential to colonise the testis and generate male germ cells (Chuma et al. 2005; Nayernia et al. 2005). These findings indicate that the testicular microenvironment offers unique niches to germ line cells. Exposure of competent cell types of various origins appears to initiate morphogenetic changes guiding the responsive cells into the male differentiation pathway. Obviously, one of the most important steps in this differentiation pathway is the generation of a new type of stem cell: the spermatogonium. It appears that only after germ line cells have passed the spermatogonial stem cell stage are they able to generate male progeny entering meiosis and differentiating into sperm. The stem cell step is also necessary to colonise the seminiferous tubules and thereby form sufficient progenitors for the generation of high quantities of male gametes. The testicular microenvironment is unique in allowing germ line cells to enter this differentiation pathway. Figure 4 shows the dependence of the male germ line on entering the testicular microenvironment to initiate the male differentiation pathway.
|
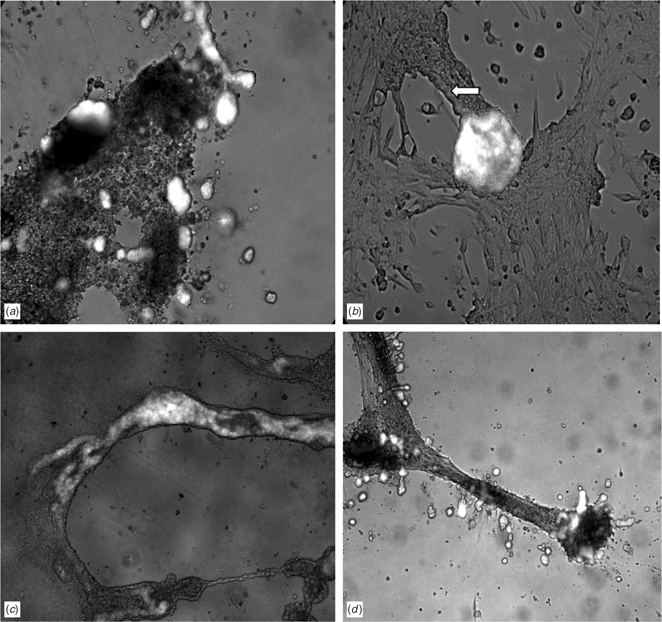
As was shown previously, primordial germ cells in culture dishes are capable of forming oogonia and follicle-like structures (Hubner et al. 2003). Interestingly, in vitro maintenance of premeiotic male germ cells alone or in coculture with feeder cells does not lead to generation of elongating male gametes, although, in some studies, several markers indicate some degree of meiotic progression (Creemers et al. 2002; Feng et al. 2002; van Pelt et al. 2002; Nagano et al. 2003; Geijsen et al. 2004). However, transplantation of these germ cells back into the testis allows the generation of male gametes (Nagano et al. 2003; Toyooka et al. 2003). We conclude from these findings that a variety of different totipotent and germ line cells are capable to enter meiosis under in vitro conditions but that male germ cell differentiation occurs exclusively in the intact testicular microenvironment. Preliminary experiments using mouse embryonic germ cells that were carrying the green fluorescent protein (GFP) transgene under control of the Oct4 promoter (the same Oct4-positive cells that were used in the study of Hubner et al. 2003) in coculture with immature mouse Sertoli cells revealed that these undifferentiated germ cells can obtain a male phenotype (Fig. 5). Initially, the embryonic germ cells only survive in contact with the Sertoli cells under conditions of serum-free and growth factor-free media. The germ cells grow in small clusters, often located on top of the Sertoli cell feeder layer (Fig. 5a). After several days, a few GFP-positive germ cells migrate from these clusters into the cord-like structures that are formed by Sertoli cells after prolonged culture periods (Fig. 5b; Schlatt et al. 1996). After the embryonic germ cells have migrated into the cord-like structures, they colonise large areas of the culture dish (Fig. 5c). When the culture dishes are coated with extracellular matrix, the embryonic germ cells show a different migration and localisation pattern. Under these culture conditions, they are observed exclusively at the periphery of the cord-like structures (Fig. 5d). We interpret these findings to indicate the ability of embryonic germ cells to respond to a new microenvironment. Interestingly, when these Sertoli cell–germ cell fragments were grafted into nude mice after several weeks of culture, we found that they generated teratocarcinomas, indicating that the transformation of cells into spermatogonia has not been completed in all cells or that the pluripotent differentiation pathway has not been terminated under these culture conditions. We conclude from these experiments that Sertoli cells provide specific niches to which a subfraction of embryonic germ cells can respond. The responsive cells then differentiate into spermatogonial-like cells. Once the germ cells pass through the bottleneck of recognising the new microenvironment, they can expand and colonise all niches offered in this tissue.
It remains to be investigated which other types of germ line and pluripotent cells other than spermatogonia (primordial germ cells, embryonic stem cells, embryonic germ cell, teratocarcinoma stem cells) are able to initiate spermatogenesis in the testicular microenvironment. Recent findings in the female mouse indicate that even adult stem cells isolated from bone marrow may be competent to enter the germ line and generate gametes (Johnson et al. 2005). In the future, the isolation of germ line-competent cells from sources other than the testis may render it possible to even use female cell preparations as donor cell preparations for germ cell transplantation and the re-initiation of spermatogenesis. If this becomes a valid new option, a selective generation of female offspring may be achievable from a breeding male that carries exclusively XX-containing germ line stem cells.
As an alternative to germ cell transplantation, testicular grafting has been shown to be a successful approach for the initiation of spermatogenesis and to generate sperm after dissection of testicular tissue from immature donors (Honaramooz et al. 2002; Schlatt et al. 2002b, 2003). This technique has already been successfully combined with prior cryopreservation of immature testicular tissue (Schlatt et al. 2002b; Shinohara et al. 2002; Orwig and Schlatt 2005). Testicular grafting is applicable to domestic species and provides an interesting option for conservation of the male germ line (Dobrinski et al. 2000; Dobrinski 2005). In principle, this technique offers a preservation of the male germ line stem cell together with its niche. Because the tissue is able to recover in the host up to the level of full spermatogenesis, grafting offers an easy tool to maintain the germ line of valuable livestock before it reaches puberty or generates sperm.
In summary, spectacular new findings on pluripotent cells and their capacity to transform and differentiate into different cells of the germ line offer unexpected new opportunities for germ line conservation and manipulation. In the near future, these findings will be combined with optimised cell culture and transfection strategies that may lead to new pathways for the introduction of genes into the germ line and improved strategies for the selection of targeted germ line cells. New strategies, such as germ cell transplantation and testicular grafting, generate a wide spectrum of scenarios by which gonadal cells and germ line cells can be preserved and restimulated to generate sperm. Although the safety and implications of any procedure that converts the DNA obtained from somatic cells or pluripotent stem cells into gametes has to be explored, these tools open completely novel scenarios for the generation of gametes and may have enormous impact on the future development of animal production.
Acknowledgments
The authors’ work presented in this review was funded by grants and fellowships from the Deutsche Forschungsgemeinschaft (Schl 394/6, Schl 394/3), start up funds from the University of Pittsburgh School of Medicine and a National Institutes of Health grant (1RO1 HD050617-01).
Capel, B. (2000). The battle of the sexes. Mech. Dev. 92, 89–103.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Fouquet, J. P. , and Dadoune, J. O. (1986). Renewal of spermatogonia in the monkey (Macaca fascicularis). Biol. Reprod. 35, 199–207.
| PubMed |

Geijsen, N. , Horoschak, M. , Kitai, K. , Gribnau, J. , Eggan, K. , and Daley, G. Q. (2004). Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature 427, 148–154.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hill, J. R. , and Dobrinski, I. (2006). Male germ cell transplantation in lifestock. Reprod. Fertil. Dev. 18, 13–18.

Honaramooz, A. , Snedaker, A. , Boiani, M. , Scholer, H. , Dobrinski, I. , and Schlatt, S. (2002). Sperm from neonatal mammalian testes grafted in mice. Nature 418, 778–781.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hubner, K. , Fuhrmann, G. , Christenson, L. K. , Kehler, J. , Reinbold, R. , De La Fuente, R. , Wood, J. , Strauss,, J. F. , Boiani, M. , and Scholer, H. R. (2003). Derivation of oocytes from mouse embryonic stem cells. Science 300, 1251–1256.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Johnson, J. , Bagley, J. , Skaznik-Wikiel, M. , Lee, H. J. , and Adams, G. B. , et al. (2005). Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell 122, 303–315.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kamischke, A. , Kuhlmann, M. , Weinbauer, G. F. , Luetjens, C. M. , Yeung, C. H. , Kronholz, H. L. , and Nieschlag, E. (2003). Gonadal protection from radiation by GnRH antagonist or recombinant human FSH: a controlled trial in a male nonhuman primate (Macaca fascicularis). J. Endocrinol. 179, 183–194.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kluin, P. M. , Kramer, M. F. , and de Rooij, D. G. (1983). Testicular development in Macaca irus after birth. Int. J. Androl. 6, 25–43.
| PubMed |

McLaren, A. (2003). Primordial germ cells in the mouse. Dev. Biol. 262, 1–15.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Nagano, M. , and Brinster, R. L. (1998). Spermatogonial transplantation and reconstitution of donor cell spermatogenesis in recipient mice. APMIS 106, 47–57.
| PubMed |

Nagano, M. , Ryu, B. Y. , Brinster, C. J. , Avarbock, M. R. , and Brinster, R. L. (2003). Maintenance of mouse male germ line stem cells in vitro. Biol. Reprod. 68, 2207–2214.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Nayernia, K. , Drabent, B. , Meinhardt, A. , Adham, I. M. , Schwandt, I. , Muller, C. , Sancken, U. , Kleene, K. C. , and Engel, W. (2005). Triple knockouts reveal gene interactions affecting fertility of male mice. Mol. Reprod. Dev. 70, 406–416.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Nordhoff, V. , and Schlatt, S. (2003). Present and future options for the preservation of testis tissue and function. Endocr. Dev. 5, 136–155.
| PubMed |

Orwig, K. E. , and Schlatt, S. (2005). Cryopreservation and transplantation of spermatogonia and testicular tissue for preservation of male fertility. J. Natl Cancer Inst. Monogr. 34, 51–56.
| PubMed |

Radford, J. (2003). Restoration of fertility after treatment for cancer. Horm. Res. 59(Suppl. 1), 21–23.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Radford, J. A. , Shalet, S. M. , and Lieberman, B. A. (1999). Fertility after treatment for cancer. BMJ 319, 935–936.
| PubMed |

Schlatt, S. , Loveland, K. , and deKretser, D. M. (1996). Discriminative analysis of Sertoli and peritubular cells and their proliferation in vitro: evidence for FSH-mediated contact inhibition of Sertoli cell mitosis. Biol. Reprod. 55, 227–235.
| PubMed |

Schlatt, S. , Foppiani, L. , Rolf, C. , Weinbauer, G. F. , and Nieschlag, E. (2002a). Germ cell transplantation into X-irradiated monkey testes. Hum. Reprod. 17, 55–62.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Schlatt, S. , Kim, S. , and Gosden, R. (2002b). Spermatogenesis and steroidogenesis in mouse, hamster and monkey testicular tissue after cryopreservation and heterotropic grafting. Reproduction 124, 323–329.
| PubMed |

Schlatt, S. , Honaramooz, A. , Boiani, M. , Schöler, H. R. , and Dobrinski, I. (2003). Progeny from sperm obtained after ectopic grafting of neonatal mouse testes. Biol. Reprod. 68, 2331–2335.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Shinohara, T. , Inoue, K. , Ogonuki, N. , Kanatsu-Shinohara, M. , and Miki, H. , et al. (2002). Birth of offspring following transplantation of cryopreserved immature testicular pieces and in-vitro microinsemination. Hum. Reprod. 17, 3039–3045.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Toyooka, Y. , Tsunekawa, N. , Akasu, R. , and Noce, T. (2003). Embryonic stem cells can form germ cells in vitro. Proc. Natl Acad. Sci. USA 100, 11 457–11 462.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

van Pelt, A. M. , Roepers-Gajadien, H. L. , Gademan, I. S. , Creemers, L. B. , de Rooij, D. G. , and van Dissel-Emiliani, F. M. (2002). Establishment of cell lines with rat spermatogonial stem cell characteristics. Endocrinology 143, 1845–1850.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wallace, W. H. , and Thomson, A. B. (2003). Preservation of fertility in children treated for cancer. Arch. Dis. Child. 88, 493–496.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zhengwei, Y. , McLachlan, R. I. , Bremner, W. J. , and Wreford, N. G. (1997). Quantitative (stereological) study of the normal spermatogenesis in the adult monkey (Macaca fascicularis). J. Androl. 18, 681–687.
| PubMed |