Ovulation mitigates fatty liver associated with reproductive suppression and oxidative stress in Damaraland mole-rats (Fukomys damarensis)
Christina M. Schmidt A C , Sandra Arbi B and Nigel C. Bennett A
A C , Sandra Arbi B and Nigel C. Bennett A
A Department of Zoology and Entomology, University of Pretoria, Private Bag x 20, Hatfield, Gauteng, 0028, South Africa.
B Department of Anatomy, University of Pretoria, Private Bag x 20, Hatfield, Gauteng, 0028, South Africa.
C Correspondimg author. Email: cschmidt@wells.edu
Reproduction, Fertility and Development 32(10) 923-928 https://doi.org/10.1071/RD20049
Submitted: 16 February 2020 Accepted: 18 May 2020 Published: 5 June 2020
Abstract
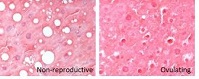
Oxidative damage is often linked to reproduction; however, reproducing female Damaraland mole-rats (Fukomys damarensis) exhibit a reduction in oxidative damage relative to their non-reproductive, anovulatory, cohorts. Specifically, liver concentrations of malondialdehyde, a biomarker for lipid peroxidation, are significantly lower in reproducing females. We examined liver histology in reproductive, anovulatory and recently ovulating non-reproductive females, demonstrating an accumulation of lipid droplets only in the livers of anovulatory females and no fibrosis, cell death or inflammatory infiltrates in any group. Our observations suggest that anovulatory females experience a form of non-alcoholic fatty liver disease, which is reversed once they commence ovulation. We propose hormonal interactions that may underlie our observations.
Additional keywords: histology, hormones, lipid peroxidation, NAFLD.
References
Arbi, S., Eksteen, E. C., Oberholzer, H. M., Taute, H., and Bester, M. J. (2015). Premature collagen fibril formation, fibroblast–mast cell interactions and mast cell-mediated phagocytosis of collagen in keloids. Ultrastruct. Pathol. 39, 95–103.| Premature collagen fibril formation, fibroblast–mast cell interactions and mast cell-mediated phagocytosis of collagen in keloids.Crossref | GoogleScholarGoogle Scholar | 25569098PubMed |
Aydin, M., Oktar, S., Yonden, Z., Ozturk, O. H., and Yilmaz, B. (2010). Direct and indirect effects of kisspeptin on liver oxidant and antioxidant systems in young male rats. Cell Biochem. Funct. 28, 293–299.
| Direct and indirect effects of kisspeptin on liver oxidant and antioxidant systems in young male rats.Crossref | GoogleScholarGoogle Scholar | 20517893PubMed |
Bennett, N. C. (1994). Reproductive suppression in social Cryptomys damarensis colonies – a lifetime of socially-induced sterility in males and females (Rodentia: Bathyergidae). J. Zool. (Lond.) 234, 25–39.
| Reproductive suppression in social Cryptomys damarensis colonies – a lifetime of socially-induced sterility in males and females (Rodentia: Bathyergidae).Crossref | GoogleScholarGoogle Scholar |
Bennett, N. C. (2011). Teasing apart socially-induced infertility in non-reproductive female Damaraland mole-rats, Fukomys damarensis (Rodentia: Bathyergidae). Integr. Zool. 6, 311–320.
| Teasing apart socially-induced infertility in non-reproductive female Damaraland mole-rats, Fukomys damarensis (Rodentia: Bathyergidae).Crossref | GoogleScholarGoogle Scholar | 22182323PubMed |
Bennett, N. C., and Faulkes, C. G. (2000). ‘African Mole-Rats: Ecology and Eusociality’. (Cambridge University Press: Cambridge.)
Bennett, N. C., Jarvis, J. U. M., Faulkes, C. G., and Millar, R. P. (1993). LH responses to single doses of exogenous GnRH by freshly captured Damaraland mole-rats. J. Reprod. Fertil. 99, 81–86.
| LH responses to single doses of exogenous GnRH by freshly captured Damaraland mole-rats.Crossref | GoogleScholarGoogle Scholar | 8283457PubMed |
Bennett, N. C., Jarvis, J. U. M., Millar, R. P., Sasano, H., and Ntshinga, K. V. (1994). Reproductive suppression in eusocial Cryptomys damarensis colonies: socially-induced infertility in females. J. Zool. (Lond.) 233, 617–630.
| Reproductive suppression in eusocial Cryptomys damarensis colonies: socially-induced infertility in females.Crossref | GoogleScholarGoogle Scholar |
Bennett, N. C., Faulkes, C. G., and Molteno, A. J. (1996). Reproductive suppression in subordinate, non-breeding female Damaraland mole-rats: two components to a lifetime of socially induced infertility. Proc. Biol. Sci. 263, 1599–1603.
| Reproductive suppression in subordinate, non-breeding female Damaraland mole-rats: two components to a lifetime of socially induced infertility.Crossref | GoogleScholarGoogle Scholar | 8952096PubMed |
Bhatia, A. J., and Wade, G. N. (1991). Effects of pregnancy and ovarian steroids on fatty acid synthesis and uptake in Syrian hamsters. Am. J. Physiol. 260, R153–R158.
| Effects of pregnancy and ovarian steroids on fatty acid synthesis and uptake in Syrian hamsters.Crossref | GoogleScholarGoogle Scholar | 1992816PubMed |
Biondo-Simões, M. d. L. P., Rolf Erdmann, T. I., Ossamu Ioshii, S., Eduardo Fouto Matias, J. I., Leonardo Guaita Calixto, H. V., José Schebelski, I. D. V., and Professor, A. (2009). The influence of estrogen on liver regeneration. An experimental study in rats. Acta Cir. Bras. 24, 3–6.
| The influence of estrogen on liver regeneration. An experimental study in rats.Crossref | GoogleScholarGoogle Scholar |
Blount, J. D., Vitikainen, E. I. K., Stott, I., and Cant, M. A. (2016). Oxidative shielding and the cost of reproduction. Biol. Rev. Camb. Philos. Soc. 91, 483–497.
| Oxidative shielding and the cost of reproduction.Crossref | GoogleScholarGoogle Scholar | 25765468PubMed |
Chuffa, L. G. A., Seiva, F. R. F., Fávaro, W. J., Teixeira, G. R., Amorim, J. P. A., Mendes, L. O., Fioruci, B. A., Pinheiro, P. F. F., Fernandes, A. A. H., Franci, J. A. A., Delella, F. K., Martinez, M., and Martinez, F. E. (2011). Melatonin reduces LH, 17 beta-estradiol and induces differential regulation of sex steroid receptors in reproductive tissues during rat ovulation. Reprod. Biol. Endocrinol. 9, 108.
| Melatonin reduces LH, 17 beta-estradiol and induces differential regulation of sex steroid receptors in reproductive tissues during rat ovulation.Crossref | GoogleScholarGoogle Scholar |
Cichoż-Lach, H., and Michalak, A. (2014). Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 20, 8082–8091.
| Oxidative stress as a crucial factor in liver diseases.Crossref | GoogleScholarGoogle Scholar | 25009380PubMed |
Dapson, R. W., Fagan, C., Kiernan, J. A., and Wickersham, T. W. (2011). Certification procedures for sirius red F3B (CI 35780, Direct red 80). Biotech. Histochem. 86, 133–139.
| Certification procedures for sirius red F3B (CI 35780, Direct red 80).Crossref | GoogleScholarGoogle Scholar | 21417582PubMed |
Edens, N. K., and Wade, G. N. (1983). Effects of estradiol on tissue distribution of newly-synthesized fatty acids in rats and hamsters. Physiol. Behav. 31, 703–709.
| Effects of estradiol on tissue distribution of newly-synthesized fatty acids in rats and hamsters.Crossref | GoogleScholarGoogle Scholar | 6665058PubMed |
Fang, X., Seim, I., Huang, Z., Gerashchenko, M. V., Xiong, Z., Turanov, A. A., Zhu, Y., Lobanov, A. V., Fan, D., Yim, S. H., Yao, X., Ma, S., Yang, L., Lee, S.-G., Kim, E. B., Bronson, R. T., Šumbera, R., Buffenstein, R., Zhou, X., Krogh, A., Park, T. J., Zhang, G., Wang, J., and Gladyshev, V. N. (2014). Adaptations to a subterranean environment and longevity revealed by the analysis of mole rat genomes. Cell Rep. 8, 1354–1364.
| Adaptations to a subterranean environment and longevity revealed by the analysis of mole rat genomes.Crossref | GoogleScholarGoogle Scholar | 25176646PubMed |
Garratt, M., Vasilaki, A., Stockley, P., McArdle, F., Jackson, M., and Hurst, J. L. (2011). Is oxidative stress a physiological cost of reproduction? An experimental test in house mice. Proc. Biol. Sci. 278, 1098–1106.
| Is oxidative stress a physiological cost of reproduction? An experimental test in house mice.Crossref | GoogleScholarGoogle Scholar | 20926440PubMed |
Gray, J. M., and Greenwood, M. R. C. (1982). Time course of effects of ovarian hormones on food intake and metabolism. Am. J. Physiol. 243, E407–E412.
| Time course of effects of ovarian hormones on food intake and metabolism.Crossref | GoogleScholarGoogle Scholar | 7137344PubMed |
Haass, C. L., and Eness, P. G. (1984). Bovine fatty liver syndrome. Iowa State Univ. Vet. 46, 7.
Hart, C. G., Voelz, B. E., Brockus, K. E., and Lemley, C. O. (2018). Hepatic steroid inactivating enzymes, hepatic portal blood flow and corpus luteum blood perfusion in cattle. Reprod. Domest. Anim. 53, 751–758.
| Hepatic steroid inactivating enzymes, hepatic portal blood flow and corpus luteum blood perfusion in cattle.Crossref | GoogleScholarGoogle Scholar | 29542193PubMed |
Junqueira, L. C. U., Bignolas, G., and Brentani, R. R. (1979). Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 11, 447–455.
| Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections.Crossref | GoogleScholarGoogle Scholar |
Kenagy, R., Weinstein, I., and Heimberg, M. (1981). The effects of 17α-estradiol and progesterone on the metabolism of free fatty acid by perfused livers from normal female and ovariectomized rats. Endocrinology 108, 1613–1621.
| The effects of 17α-estradiol and progesterone on the metabolism of free fatty acid by perfused livers from normal female and ovariectomized rats.Crossref | GoogleScholarGoogle Scholar | 7215287PubMed |
Kim, H.-J., and Kalkhoff, R. K. (1975). Sex steroid influence on triglyceride metabolism. J. Clin. Invest. 56, 888–896.
| Sex steroid influence on triglyceride metabolism.Crossref | GoogleScholarGoogle Scholar | 1159092PubMed |
Kim, H.-J., and Kalkhoff, R. K. (1978). Altered apolipoproteins in sex steroid-treated rats. Metabolism 27, 571–587.
| Altered apolipoproteins in sex steroid-treated rats.Crossref | GoogleScholarGoogle Scholar | 205760PubMed |
Koek, G. H., Liedorp, P. R., and Bast, A. (2011). The role of oxidative stress in non-alcoholic steatohepatitis. Clin. Chim. Acta 412, 1297–1305.
| The role of oxidative stress in non-alcoholic steatohepatitis.Crossref | GoogleScholarGoogle Scholar | 21514287PubMed |
Matsuzawa, N., Takamura, T., Kurita, S., Misu, H., Ota, T., Ando, H., Yokoyama, M., Honda, M., Zen, Y., Nakanuma, Y., Miyamoto, K., and Kaneko, S. (2007). Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology 46, 1392–1403.
| Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet.Crossref | GoogleScholarGoogle Scholar | 17929294PubMed |
Molteno, A. J., and Bennett, N. C. (2000). Anovulation in non-reproductive female Damaraland mole-rats (Cryptomys damarensis). J. Reprod. Fertil. 119, 35–41.
| Anovulation in non-reproductive female Damaraland mole-rats (Cryptomys damarensis).Crossref | GoogleScholarGoogle Scholar | 10864811PubMed |
Monaghan, P., Metcalfe, N. B., and Torres, R. (2009). Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol. Lett. 12, 75–92.
| Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation.Crossref | GoogleScholarGoogle Scholar | 19016828PubMed |
Morita, M., Ishida, N., Uchiyama, K., Yamaguchi, K., Itoh, Y., Shichiri, M., Yoshida, Y., Hagihara, Y., Naito, Y., Yoshikawa, T., and Niki, E. (2012). Fatty liver induced by free radicals and lipid peroxidation. Free Radic. Res. 46, 758–765.
| Fatty liver induced by free radicals and lipid peroxidation.Crossref | GoogleScholarGoogle Scholar | 22468959PubMed |
Ołdakowski, L., Wasiluk, A., Sadowska, E. T., Koteja, P., and Taylor, J. R. E. (2015). Reproduction is not costly in terms of oxidative stress. J. Exp. Biol. 218, 3901–3910.
| Reproduction is not costly in terms of oxidative stress.Crossref | GoogleScholarGoogle Scholar | 26519508PubMed |
Pecquery, R., Leneveu, M. C., and Giudicelli, Y. (1986). Estradiol treatment decreases the lipolytic responses of hamster white adipocytes through a reduction in the activity of the adenylate cyclase catalytic subunit. Endocrinology 118, 2210–2216.
| Estradiol treatment decreases the lipolytic responses of hamster white adipocytes through a reduction in the activity of the adenylate cyclase catalytic subunit.Crossref | GoogleScholarGoogle Scholar | 3009154PubMed |
Pérez-Tamayo, R., and Montfort, I. (1980). The susceptibility of hepatic collagen to homologous collagenase in human and experimental cirrhosis of the liver. Am. J. Pathol. 100, 427–442..
| 6157326PubMed |
Ramirez, I. (1980). Relation between estrogen-induced hyperlipemia and food intake and body weight in rats. Physiol. Behav. 25, 511–518.
| Relation between estrogen-induced hyperlipemia and food intake and body weight in rats.Crossref | GoogleScholarGoogle Scholar | 7208648PubMed |
Reid, I. M., Roberts, C. J., and Manston, R. (1979). Reduced fertility associated with fatty liver in high-yielding dairy cows. Vet. Sci. Commun. 3, 231–236.
| Reduced fertility associated with fatty liver in high-yielding dairy cows.Crossref | GoogleScholarGoogle Scholar |
Repetto, M. G., Ossani, G., Monserrat, A. J., and Boveris, A. (2010). Oxidative damage: the biochemical mechanism of cellular injury and necrosis in choline deficiency. Exp. Mol. Pathol. 88, 143–149.
| Oxidative damage: the biochemical mechanism of cellular injury and necrosis in choline deficiency.Crossref | GoogleScholarGoogle Scholar | 19913531PubMed |
Repetto, M., Semprine, J., and Boveris, A. (2012). Lipid peroxidation: chemical mechanism, biological implications and analytical determination. In ‘Lipid Peroxidation’. (Ed. Angel Catala.) pp. 3–30. (IntechOpen: London)
Rolo, A. P., Teodoro, J. S., and Palmeira, C. M. (2012). Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 52, 59–69.
| Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis.Crossref | GoogleScholarGoogle Scholar | 22064361PubMed |
Schmidt, C. M., Jarvis, J. U. M., and Bennett, N. C. (2013). The long-lived queen: reproduction and longevity in female eusocial Damaraland mole-rats (Fukomys damarensis). Afr. Zool. 48, 193–196.
| The long-lived queen: reproduction and longevity in female eusocial Damaraland mole-rats (Fukomys damarensis).Crossref | GoogleScholarGoogle Scholar |
Schmidt, C. M., Blount, J. D., and Bennett, N. C. (2014). Reproduction is associated with a tissue-dependent reduction of oxidative stress in eusocial female Damaraland mole-rats (Fukomys damarensis). PLoS One , .
| Reproduction is associated with a tissue-dependent reduction of oxidative stress in eusocial female Damaraland mole-rats (Fukomys damarensis).Crossref | GoogleScholarGoogle Scholar | 25068591PubMed |
Smith, J. T., Clifton, D. K., and Steiner, R. A. (2006). Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction 131, 623–630.
| Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling.Crossref | GoogleScholarGoogle Scholar | 16595713PubMed |
Snyman, P. C., Jackson, C. R., and Bennett, N. C. (2006). Do dispersing non-reproductive female Damaraland mole-rats, Cryptomys damarensis (Rodentia: Bathyergidae) exhibit spontaneous or induced ovulation? Physiol. Behav. 87, 88–94.
| Do dispersing non-reproductive female Damaraland mole-rats, Cryptomys damarensis (Rodentia: Bathyergidae) exhibit spontaneous or induced ovulation?Crossref | GoogleScholarGoogle Scholar | 16209879PubMed |
Stier, A., Reichert, S., Massemin, S., Bize, P., and Criscuolo, F. (2012). Constraint and cost of oxidative stress on reproduction: correlative evidence in laboratory mice and review of the literature. Front. Zool. 9, .
| Constraint and cost of oxidative stress on reproduction: correlative evidence in laboratory mice and review of the literature.Crossref | GoogleScholarGoogle Scholar | 23268929PubMed |
Sumida, Y., Niki, E., Naito, Y., and Yoshikawa, T. (2013). Involvement of free radicals and oxidative stress in NAFLD/NASH. Free Radic. Res. 47, 869–880.
| Involvement of free radicals and oxidative stress in NAFLD/NASH.Crossref | GoogleScholarGoogle Scholar | 24004441PubMed |
Sun, H., Wang, X., Chen, J., Song, K., Gusdon, A. M., Li, L., Bu, L., and Qu, S. (2016). Melatonin improves non-alcoholic fatty liver disease via MAPK-JNK/P38 signaling in high-fat-diet-induced obese mice. Lipids Health Dis. 15, 202.
| Melatonin improves non-alcoholic fatty liver disease via MAPK-JNK/P38 signaling in high-fat-diet-induced obese mice.Crossref | GoogleScholarGoogle Scholar | 27876064PubMed |
Van Thiel, D. H., and Gavaler, J. S. (1987). Pregnancy-associated sex steroids and their effects on the liver. Semin. Liver Dis. 7, 1–7.
| Pregnancy-associated sex steroids and their effects on the liver.Crossref | GoogleScholarGoogle Scholar | 3589711PubMed |
Velidandla, S., Gaikwad, P., Ealla, K. K. R., Bhorgonde, K. D., Hunsingi, P., and Kumar, A. (2014). Histochemical analysis of polarizing colors of collagen using Picrosirius Red staining in oral submucous fibrosis. J. Int. Oral Health 6, 33–38..
| 24653600PubMed |
Voigt, C., and Bennett, N. C. (2018). Reproductive status-dependent kisspeptin and RFamide-related peptide (Rfrp) gene expression in female Damaraland mole-rats. J. Neuroendocrinol. 30, e12571.
| Reproductive status-dependent kisspeptin and RFamide-related peptide (Rfrp) gene expression in female Damaraland mole-rats.Crossref | GoogleScholarGoogle Scholar | 29345030PubMed |
Wade, G. N., and Schneider, J. E. (1992). Metabolic fuels and reproduction in female mammals. Neurosci. Biobehav. Rev. 16, 235–272.
| Metabolic fuels and reproduction in female mammals.Crossref | GoogleScholarGoogle Scholar | 1630733PubMed |
Young, B., O’Dowd, G., and Woodford, P. (2013). ‘Wheater’s Functional Histology: a Text and Colour Atlas’. (Churchill Livingstone: London.)
Zhang, J.-J., Meng, X., Li, Y., Zhou, Y., Xu, D.-P., Li, S., and Li, H.-B. (2017). Effects of melatonin on liver injuries and diseases. Int. J. Mol. Sci. 18, .
| Effects of melatonin on liver injuries and diseases.Crossref | GoogleScholarGoogle Scholar | 28737710PubMed |
Zhou, S., Holmes, M. M., Forger, N. G., Goldman, B. D., Lovern, M. B., Caraty, A., Faulkes, C. G., and Coen, C. W. (2010). Release from socially-induced reproductive suppression in eusocial naked mole-rats (Heterocephalus glaber) is marked by increased kisspeptin-immunoreactive cell bodies in the hypothalamic anteroventral periventricular nucleus. In ‘Proceedings of the 7th International Congress of Neuroendocrinology, Rouen’. p. 220. (Rouen, France.) Available at https://prodinra.inra.fr/?locale=en#!ConsultNotice:41186 [Verified 22 May 2020].
Zhou, H., Du, W., Li, Y., Shi, C., Hu, N., Ma, S., Wang, W., and Ren, J. (2018). Effects of melatonin on fatty liver disease: The role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. J. Pineal Res. 64, e12450.
| Effects of melatonin on fatty liver disease: The role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy.Crossref | GoogleScholarGoogle Scholar | 29363153PubMed |


