Effects of LH and FSH on androgen and oestrogen release in the myometrium of pigs during the oestrous cycle and early pregnancy
Ewa M. Waszkiewicz A , Agata Zmijewska
A , Agata Zmijewska  A , Wiktoria Kozlowska A and Anita Franczak
A , Wiktoria Kozlowska A and Anita Franczak  A B
A B
A Department of Animal Anatomy and Physiology, Faculty of Biology and Biotechnology, University of Warmia and Mazury in Olsztyn, Oczapowskiego 1A, 10-718 Olsztyn, Poland.
B Corresponding author. Email: anitaf@uwm.edu.pl
Reproduction, Fertility and Development 32(14) 1200-1211 https://doi.org/10.1071/RD20148
Submitted: 5 June 2020 Accepted: 17 August 2020 Published: 2 October 2020
Abstract
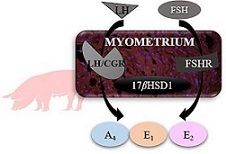
The porcine myometrium possesses steroidogenic activity. LH and FSH are hypothesised to regulate the myometrial production of androstenedione (A4), testosterone (T), oestrone (E1) and 17β-oestradiol (E2). In this study, we used myometrium collected from cycling (n = 15) and pregnant (n = 15) pigs on Days 10–11, 12–13 and 15–16 of the oestrous cycle or pregnancy to determine: (1) the abundance of LH and FSH receptor (LH/choriogonadotrophin receptor (CGR) and FSHR) mRNA and protein; (2) activity of 17β-hydroxysteroid dehydrogenase 1 (17βHSD1); and (3) A4, T, E1 and E2 release in response to LH and FSH treatment, used at doses 10 or 100 ng mL−1 for 6 h. In results, the myometrium possesses LH/CGR and FSHR with minor alterations in their expression in the course of the oestrous cycle or early pregnancy. 17βHSD1 activity was the highest on Days 12–13 of the oestrous cycle and the lowest on Days 15–16 of the oestrus cycle and pregnancy, when compared to the other studied days of the oestrous cycle or pregnancy. The LH and FSH treatment increased A4 release on Days 12–13 of the oestrous cycle, and E1 and E2 release on Days 15–16 of the oestrous cycle. Moreover, on Days 12–13 E2 release was increased in response to FSH treatment (100 ng mL−1) in cycling pigs and in response to LH (100 ng mL−1) in pregnant pigs. In conclusion, the myometrium of pregnant and non-pregnant pigs expresses LH/CGR and FSHR and has 17βHSD1 activity. In addition, the amount of A4, E1, and E2 release from the myometrium is altered in response to LH and FSH, especially in cycling pigs. LH and FSH appear to be important regulators of myometrial oestrogen release in pigs mostly during luteolysis.
Keywords: enzyme, steroid hormone, uterus.
References
Akins, E. L., and Morrissette, M. C. (1968). Gross ovarian changes during estrous cycle of swine. Am. J. Vet. Res. 29, 1953–1957.| 5692889PubMed |
Bazer, F. W., and Thatcher, W. W. (1977). Theory of maternal recognition of pregnancy in swine based on estrogen controlled endocrine versus exocrine secretion of prostaglandin F2α by the uterine endometrium. Prostaglandins 14, 397–401.
| Theory of maternal recognition of pregnancy in swine based on estrogen controlled endocrine versus exocrine secretion of prostaglandin F2α by the uterine endometrium.Crossref | GoogleScholarGoogle Scholar | 897228PubMed |
Blitek, A., and Ziecik, A. J. (2005). Effect of LH on prostaglandin F2α and prostaglandin E2 secretion by cultured porcine endometrial cells. Reproduction 130, 105–112.
| Effect of LH on prostaglandin F2α and prostaglandin E2 secretion by cultured porcine endometrial cells.Crossref | GoogleScholarGoogle Scholar | 15985636PubMed |
Bustin, S. A., Benes, V., Garson, J. A., Hellemans, J., Huggett, J., Kubista, M., Mueller, R., Nolan, T., Pfaffl, M. W., Shipley, G. L., Vandesompele, J., and Wittwer, C. T. (2009). The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622.
| The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments.Crossref | GoogleScholarGoogle Scholar | 19246619PubMed |
Chandran, S., Cairns, M. T., O’Brien, M., and Smith, T. J. (2014). Transcriptomic effects of estradiol treatment on cultured human uterine smooth muscle cells. Mol. Cell. Endocrinol. 393, 16–23.
| Transcriptomic effects of estradiol treatment on cultured human uterine smooth muscle cells.Crossref | GoogleScholarGoogle Scholar | 24942541PubMed |
Ciereszko, R. (1999). Radioimmunoassay of steroid hormones in biological fluids. In ‘Animal Physiology Demonstrations and Methods’ [in Polish]. (Eds J. Przala.) pp. 157–163. (University of Warmia and Mazury Press: Olsztyn.)
Conley, A. J., and Ford, S. P. (1989). Direct luteotrophic effect of oestradiol-17β on pig corpora lutea. J. Reprod. Fertil. 87, 125–131.
| Direct luteotrophic effect of oestradiol-17β on pig corpora lutea.Crossref | GoogleScholarGoogle Scholar | 2621687PubMed |
Franczak, A. (2008). Endometrial and myometrial secretion of androgens and estrone during early pregnancy and luteolysis in pigs. Reprod. Biol. 8, 213–228.
| Endometrial and myometrial secretion of androgens and estrone during early pregnancy and luteolysis in pigs.Crossref | GoogleScholarGoogle Scholar | 19092984PubMed |
Franczak, A., and Kotwica, G. (2008). Secretion of estradiol-17β by porcine endometrium and myometrium during early pregnancy and luteolysis. Theriogenology 69, 283–289.
| Secretion of estradiol-17β by porcine endometrium and myometrium during early pregnancy and luteolysis.Crossref | GoogleScholarGoogle Scholar | 17977590PubMed |
Franczak, A., and Kotwica, G. (2010). Androgens and estradiol-17β production by porcine uterine cells: in vitro study. Theriogenology 73, 232–241.
| Androgens and estradiol-17β production by porcine uterine cells: in vitro study.Crossref | GoogleScholarGoogle Scholar | 19880166PubMed |
Franczak, A., Wojciechowicz, B., and Katwica, G. (2013). Novel aspects of cytokine action in porcine uterus – endometrial and myometrial production of estrone (E1) in the presence of interleukin 1β (Il1β), interleukin 6 (Il6) and tumor necrosis factor (TNFα) – in vitro study. Folia Biol. (Krakow) 61, 253–261.
| Novel aspects of cytokine action in porcine uterus – endometrial and myometrial production of estrone (E1) in the presence of interleukin 1β (Il1β), interleukin 6 (Il6) and tumor necrosis factor (TNFα) – in vitro study.Crossref | GoogleScholarGoogle Scholar | 24279177PubMed |
Franczak, A., Wojciechowicz, B., Kolakowska, J., and Kotwica, G. (2014). The effect of interleukin-1β, interleukin-6, and tumor necrosis factor-α on estradiol-17β release in the myometrium: the in vitro study on the pig model. Theriogenology 81, 266–274.
| The effect of interleukin-1β, interleukin-6, and tumor necrosis factor-α on estradiol-17β release in the myometrium: the in vitro study on the pig model.Crossref | GoogleScholarGoogle Scholar | 24139936PubMed |
Geisert, R. D., Ross, J. W., Ashworth, M. D., White, F. J., Johnson, G. A., and DeSilva, U. (2006). Maternal recognition of pregnancy signal or endocrine disruptor: the two faces of oestrogen during establishment of pregnancy in the pig. Soc. Reprod. Fertil. Suppl. 62, 131–145.
| 16866314PubMed |
Grazul-Bilska, A. T., Reyaz, A., Valkov, V., Dorsam, S. T., and Redmer, D. A. (2018). Follicle stimulating hormone receptor protein is expressed in ovine uterus during the estrous cycle and utero-placenta during early pregnancy: an immunohistochemical study. Acta Histochem. 120, 420–428.
| Follicle stimulating hormone receptor protein is expressed in ovine uterus during the estrous cycle and utero-placenta during early pregnancy: an immunohistochemical study.Crossref | GoogleScholarGoogle Scholar | 29754696PubMed |
Gry, M., Rimini, R., Strömberg, S., Asplund, A., Pontén, F., Uhlén, M., and Nilsson, P. (2009). Correlations between RNA and protein expression profiles in 23 human cell lines. BMC Genomics 10, 365.
| Correlations between RNA and protein expression profiles in 23 human cell lines.Crossref | GoogleScholarGoogle Scholar | 19660143PubMed |
Hascalik, S., Celik, O., Tagluk, M. E., Yildirim, A., and Aydin, N. E. (2010). Effects of highly purified urinary FSH and human menopausal FSH on uterine myoelectrical dynamics. Mol. Hum. Reprod. 16, 200–206.
| Effects of highly purified urinary FSH and human menopausal FSH on uterine myoelectrical dynamics.Crossref | GoogleScholarGoogle Scholar | 19720661PubMed |
Hu, J., Braileanu, G. T., and Mirando, M. A. (2003). Effect of ovarian steroids on basal and oxytocin-induced prostaglandin F2α secretion from pig endometrial cells. Reprod. Fertil. Dev. 15, 197–205.
| Effect of ovarian steroids on basal and oxytocin-induced prostaglandin F2α secretion from pig endometrial cells.Crossref | GoogleScholarGoogle Scholar | 12921694PubMed |
Kaczmarek, M. M., Blitek, A., Schams, D., and Ziecik, A. J. (2008). The effect of insulin-like growth factor-I, relaxin and luteinizing hormone on vascular endothelial growth factor secretion by cultured endometrial stromal cells on different days of early pregnancy in pigs. Reprod. Biol. 8, 163–170.
| The effect of insulin-like growth factor-I, relaxin and luteinizing hormone on vascular endothelial growth factor secretion by cultured endometrial stromal cells on different days of early pregnancy in pigs.Crossref | GoogleScholarGoogle Scholar | 18677403PubMed |
Kaczmarek, M. M., Blitek, A., Schams, D., and Ziecik, A. J. (2010). Effect of luteinizing hormone and tumour necrosis factor-alpha on VEGF secretion by cultured porcine endometrial stromal cells. Reprod. Domest. Anim. 45, 481–486.
| Effect of luteinizing hormone and tumour necrosis factor-alpha on VEGF secretion by cultured porcine endometrial stromal cells.Crossref | GoogleScholarGoogle Scholar | 20586953PubMed |
Kaczynski, P., Bauersachs, S., Baryla, M., Goryszewska, E., Muszak, J., Grzegorzewski, W. J., and Waclawik, A. (2020). Estradiol-17β-induced changes in the porcine endometrial transcriptome in vivo. Int. J. Mol. Sci. 21, 890.
| Estradiol-17β-induced changes in the porcine endometrial transcriptome in vivo.Crossref | GoogleScholarGoogle Scholar |
Kaminski, T., Smolinska, N., Kiezun, M., Dobrzyn, K., Szeszko, K., and Maleszka, A. (2018). Effect of orexin B on CYP17A1 and CYP19A3 expression and oestradiol, oestrone and testosterone secretion in the porcine uterus during early pregnancy and the oestrous cycle. Animal 12, 1921–1932.
| Effect of orexin B on CYP17A1 and CYP19A3 expression and oestradiol, oestrone and testosterone secretion in the porcine uterus during early pregnancy and the oestrous cycle.Crossref | GoogleScholarGoogle Scholar | 29366436PubMed |
Kibbe, W. A. (2007). OligoCalc: an online oligonucleotide properties calculator. Nucleic Acids Res. 35, W43–W46.
| OligoCalc: an online oligonucleotide properties calculator.Crossref | GoogleScholarGoogle Scholar | 17452344PubMed |
Kiezun, M., Smolinska, N., Dobrzyn, K., Szeszko, K., Rytelewska, E., and Kaminski, T. (2017). The effect of orexin A on CYP17A1 and CYP19A3 expression and on oestradiol, oestrone and testosterone secretion in the porcine uterus during early pregnancy and the oestrous cycle. Theriogenology 90, 129–140.
| The effect of orexin A on CYP17A1 and CYP19A3 expression and on oestradiol, oestrone and testosterone secretion in the porcine uterus during early pregnancy and the oestrous cycle.Crossref | GoogleScholarGoogle Scholar | 28166959PubMed |
Kisielewska, K., Rytelewska, E., Gudelska, M., Kiezun, M., Dobrzyn, K., Szeszko, K., Bors, K., Wyrebek, J., Kaminski, T., and Smolinska, N. (2019). The effect of orexin B on steroidogenic acute regulatory protein, P450 side-chain cleavage enzyme, and 3β-hydroxysteroid dehydrogenase gene expression, and progesterone and androstenedione secretion by the porcine uterus during early pregnancy and the est. J. Anim. Sci. 97, 851–864.
| The effect of orexin B on steroidogenic acute regulatory protein, P450 side-chain cleavage enzyme, and 3β-hydroxysteroid dehydrogenase gene expression, and progesterone and androstenedione secretion by the porcine uterus during early pregnancy and the est.Crossref | GoogleScholarGoogle Scholar | 30508170PubMed |
Krzymowski, T., and Stefańczyk-Krzymowska, S. (2004). The oestrous cycle and early pregnancy – a new concept of local endocrine regulation. Vet. J. 168, 285–296.
| The oestrous cycle and early pregnancy – a new concept of local endocrine regulation.Crossref | GoogleScholarGoogle Scholar | 15501146PubMed |
Li, M., Jia, Y., Ling, Y., Chen, Y., Zhang, L., Luo, D., Lai, L., Guo, M., Zhang, D., Ren, M., Xu, H., and Kuang, H. (2017). Reduced expression of follicle stimulating hormone receptor mRNA and protein in pregnancies complicated by pre-eclampsia. Mol. Med. Rep. 16, 367–372.
| Reduced expression of follicle stimulating hormone receptor mRNA and protein in pregnancies complicated by pre-eclampsia.Crossref | GoogleScholarGoogle Scholar | 28534997PubMed |
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25, 402–408.
| Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method.Crossref | GoogleScholarGoogle Scholar | 11846609PubMed |
Makieva, S., Saunders, P. T. K., and Norman, J. E. (2014). Androgens in pregnancy: roles in parturition. Hum. Reprod. Update 20, 542–559.
| Androgens in pregnancy: roles in parturition.Crossref | GoogleScholarGoogle Scholar | 24643344PubMed |
Messinis, I. E., Messini, C. I., and Dafopoulos, K. (2010). The role of gonadotropins in the follicular phase. Ann. NY Acad. Sci. 1205, 5–11.
| The role of gonadotropins in the follicular phase.Crossref | GoogleScholarGoogle Scholar | 20840246PubMed |
Payne, A. H., and Hales, D. B. (2004). Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 25, 947–970.
| Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones.Crossref | GoogleScholarGoogle Scholar | 15583024PubMed |
Ryan, D. P., Yaakub, H., Harrington, D., and Lynch, P. B. (1994). Follicular development during early pregnancy and the estrous cycle of the sow. Theriogenology 42, 623–632.
| Follicular development during early pregnancy and the estrous cycle of the sow.Crossref | GoogleScholarGoogle Scholar | 16727568PubMed |
Rytelewska, E., Kisielewska, K., Gudelska, M., Kiezun, M., Dobrzyn, K., Bors, K., Wyrebek, J., Kaminska, B., Kaminski, T., and Smolinska, N. (2020). The effect of orexin a on the StAR, CYP11A1 and HSD3B1 gene expression, as well as progesterone and androstenedione secretion in the porcine uterus during early pregnancy and the oestrous cycle. Theriogenology 143, 179–190.
| The effect of orexin a on the StAR, CYP11A1 and HSD3B1 gene expression, as well as progesterone and androstenedione secretion in the porcine uterus during early pregnancy and the oestrous cycle.Crossref | GoogleScholarGoogle Scholar | 31733930PubMed |
Schwanhäusser, B., Busse, D., Li, N., Dittmar, G., Schuchhardt, J., Wolf, J., Chen, W., and Selbach, M. (2011). Global quantification of mammalian gene expression control. Nature 473, 337–342.
| Global quantification of mammalian gene expression control.Crossref | GoogleScholarGoogle Scholar | 21593866PubMed |
Shemesh, M. (2001). Actions of gonadotrophins on the uterus. Reproduction 121, 835–842.
| Actions of gonadotrophins on the uterus.Crossref | GoogleScholarGoogle Scholar | 11373169PubMed |
Shimizu, T., Krebs, S., Bauersachs, S., Blum, H., Wolf, E., and Miyamoto, A. (2010). Actions and interactions of progesterone and estrogen on transcriptome profiles of the bovine endometrium. Physiol. Genomics 42A, 290–300.
| 20876846PubMed |
Simpson, E. R., Clyne, C., Speed, C., Rubin, G., and Bulun, S. (2001). Tissue-specific estrogen biosynthesis and metabolism. Ann. N. Y. Acad. Sci. 949, 58–67.
| Tissue-specific estrogen biosynthesis and metabolism.Crossref | GoogleScholarGoogle Scholar | 11795380PubMed |
Sirotkin, A. V., Chrenek, P., Darlak, K., Valenzuela, F., and Kuklová, Ž. (2008). Some endocrine traits of transgenic rabbits. II. Changes in hormone secretion and response of isolated ovarian tissue to FSH and ghrelin. Physiol. Res. 57, 745–751.
| 17949242PubMed |
Smolinska, N., Dobrzyn, K., Kiezun, M., Szeszko, K., Maleszka, A., and Kaminski, T. (2016). Effect of adiponectin on the steroidogenic acute regulatory protein, P450 side chain cleavage enzyme and 3β-hydroxysteroid dehydrogenase gene expression, progesterone and androstenedione production by the porcine uterus during early pregnancy. J. Physiol. Pharmacol. 67, 443–456.
| 27512005PubMed |
Staszkiewicz, J., Skowronski, M. T., Siawrys, G., Kaminski, T., Krazinski, B. E., Plonka, K. J., Wylot, B., Przala, J., and Okrasa, S. (2007). Expression of proopiomelanocortin, proenkephalin and prodynorphin genes in porcine luteal cells. Acta Vet. Hung. 55, 435–449.
| Expression of proopiomelanocortin, proenkephalin and prodynorphin genes in porcine luteal cells.Crossref | GoogleScholarGoogle Scholar | 18277703PubMed |
Stepien, A., Shemesh, M., and Ziecik, A. J. (1999). Luteinising hormone receptor kinetic and LH-induced prostaglandin production throughout the oestrous cycle in porcine endometrium. Reprod. Nutr. Dev. 39, 663–674.
| Luteinising hormone receptor kinetic and LH-induced prostaglandin production throughout the oestrous cycle in porcine endometrium.Crossref | GoogleScholarGoogle Scholar | 10619173PubMed |
Stepien, A., Derecka, K., Gawronska, B., Bodek, G., Zwierzchowski, L., Shemesh, M., and Ziecik, A. J. (2000). LH/hCG receptors in the porcine uterus – a new evidence of their presence in the cervix. J. Physiol. Pharmacol. 51, 917–931.
| 11220499PubMed |
Stilley, J. A. W., and Segaloff, D. L. (2018). FSH actions and pregnancy: looking beyond ovarian FSH receptors. Endocrinology 159, 4033–4042.
| FSH actions and pregnancy: looking beyond ovarian FSH receptors.Crossref | GoogleScholarGoogle Scholar |
Stilley, J. A. W., Christensen, D. E., Dahlem, K. B., Guan, R., Santillan, D. A., England, S. K., Al-Hendy, A., Kirby, P. A., and Segaloff, D. L. (2014). FSH receptor (FSHR) expression in human extragonadal reproductive tissues and the developing placenta, and the impact of its deletion on pregnancy in mice. Biol. Reprod. 91, 74.
| FSH receptor (FSHR) expression in human extragonadal reproductive tissues and the developing placenta, and the impact of its deletion on pregnancy in mice.Crossref | GoogleScholarGoogle Scholar |
Stilley, J. A. W., Guan, R., Santillan, D. A., Mitchell, B. F., Lamping, K. G., and Segaloff, D. L. (2016). Differential regulation of human and mouse myometrial contractile activity by FSH as a function of FSH receptor density. Biol. Reprod. 95, 36.
| Differential regulation of human and mouse myometrial contractile activity by FSH as a function of FSH receptor density.Crossref | GoogleScholarGoogle Scholar |
Szafrańska, B., Ziecik, A., and Okrasa, S. (2002). Primary antisera against selected steroids or proteins and secondary antisera against gamma-globulins – an available tool for studies of reproductive processes. Reprod. Biol. 2, 187–204.
| 14666157PubMed |
Tarleton, B. J., Braden, T. D., Wiley, A. A., and Bartol, F. F. (2003). Estrogen-induced disruption of neonatal porcine uterine development alters adult uterine function. Biol. Reprod. 68, 1387–1393.
| Estrogen-induced disruption of neonatal porcine uterine development alters adult uterine function.Crossref | GoogleScholarGoogle Scholar | 12606348PubMed |
Waszkiewicz, E. M., Kozlowska, W., Zmijewska, A., and Franczak, A. (2020). Expression of insulin-like growth factor 1 (IGF-1) and epidermal growth factor (EGF) receptors and the effect of IGF-1 and EGF on androgen and estrogen release in the myometrium of pigs – in vitro study. Animals (Basel) 10, 915.
Wiesak, T., Przala, J., Muszynska, A., and Hunter, M. G. (1990). Effect of catecholamines and FSH on progesterone secretion by pig granulosa cells. Endocrinol. Exp. 24, 449–456.
| 1965717PubMed |
Wojciechowicz, B., Kotwica, G., Kolakowska, J., and Franczak, A. (2013). The activity and localization of 3β-hydroxysteroid dehydrogenase/Δ5–Δ4 isomerase and release of androstenedione and progesterone by uterine tissues during early pregnancy and the estrous cycle in pigs. J. Reprod. Dev. 59, 49–58.
| 23095516PubMed |
Wojciechowicz, B., Kotwica, G., Kołakowska, J., Zglejc, K., Martyniak, M., and Franczak, A. (2016). The alterations in endometrial and myometrial transcriptome at the time of maternal recognition of pregnancy in pigs. Agri Gene 2, 5–10.
| The alterations in endometrial and myometrial transcriptome at the time of maternal recognition of pregnancy in pigs.Crossref | GoogleScholarGoogle Scholar |
Wojciechowicz, B., Kotwica, G., Zglejc, K., Waszkiewicz, E., and Franczak, A. (2017). Expression of 17β-hydroxysteroid dehydrogenase and the effects of LH, FSH and prolactin on oestrone and 17β-oestradiol secretion in the endometrium of pigs during early pregnancy and the oestrous cycle. Reprod. Fertil. Dev. 29, 975–984.
| Expression of 17β-hydroxysteroid dehydrogenase and the effects of LH, FSH and prolactin on oestrone and 17β-oestradiol secretion in the endometrium of pigs during early pregnancy and the oestrous cycle.Crossref | GoogleScholarGoogle Scholar | 28442048PubMed |
Yung, Y., Aviel-Ronen, S., Maman, E., Rubinstein, N., Avivi, C., Orvieto, R., and Hourvitz, A. (2014). Localization of luteinizing hormone receptor protein in the human ovary. Mol. Hum. Reprod. 20, 844–849.
| Localization of luteinizing hormone receptor protein in the human ovary.Crossref | GoogleScholarGoogle Scholar | 24874553PubMed |
Ziecik, A., Tilton, J. E., Weigl, R., and Williams, G. L. (1983). Plasma luteinizing hormone during pregnancy in the pig. Anim. Reprod. Sci. 5, 213–218.
| Plasma luteinizing hormone during pregnancy in the pig.Crossref | GoogleScholarGoogle Scholar |
Ziecik, A. J., Stanchev, P. D., and Tilton, J. E. (1986). Evidence for the presence of luteinizing hormone/human chorionic gonadotropin-binding sites in the porcine uterus. Endocrinology 119, 1159–1163.
| Evidence for the presence of luteinizing hormone/human chorionic gonadotropin-binding sites in the porcine uterus.Crossref | GoogleScholarGoogle Scholar | 3015568PubMed |
Ziecik, A. J., Derecka, K., Gawronska, B., Stepien, A., and Bodek, G. (2001). Nongonadal LH/hCG receptors in pig: functional importance and parallels to human. Semin. Reprod. Med. 19, 19–30.
| Nongonadal LH/hCG receptors in pig: functional importance and parallels to human.Crossref | GoogleScholarGoogle Scholar | 11394200PubMed |
Ziecik, A. J., Bodek, G., Blitek, A., Kaczmarek, M., and Waclawik, A. (2005). Nongonadal LH receptors, their involvement in female reproductive function and a new applicable approach. Vet. J. 169, 75–84.
| Nongonadal LH receptors, their involvement in female reproductive function and a new applicable approach.Crossref | GoogleScholarGoogle Scholar | 15683766PubMed |
Zuker, M. (2003). Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415.
| Mfold web server for nucleic acid folding and hybridization prediction.Crossref | GoogleScholarGoogle Scholar | 12824337PubMed |


