Flutamide induces uterus and ovary damage in the mouse via apoptosis and excessive autophagy of cells following triggering of the unfolded protein response
Haiming Yu A * , Xiaoqing Zhou B C * , Yujing Zhang B , Kexin Wen B D , Zhengli Yan B , Hu Fu B and Yongfei Zhu B E
B E
A Department of Critical Medicine, The First Affiliated Hospital of Hunan Normal University (The People’s Hospital of Hunan Province), Changsha 410002, PR China.
B Key Laboratory of Study and Discovery of Small Targeted Molecules of Hunan Province, Medical School, Hunan Normal University, Changsha 410013, PR China.
C Department of Infection Control, The Eighth Hospital of Xi’An/Shanxi Provincial Infectious Disease Hospital, Xi’An 710061, PR China.
D Changsha Center for Disease Control and Prevention of Hunan Province, Changsha 410004, PR China.
E Corresponding author. Email: njzhu70@126.com
Reproduction, Fertility and Development 33(7) 466-475 https://doi.org/10.1071/RD20287
Submitted: 1 November 2020 Accepted: 9 March 2021 Published: 1 April 2021
Abstract
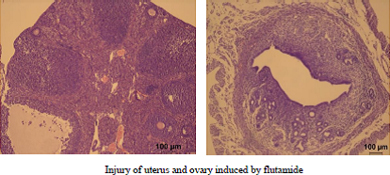
Intrauterine exposure to flutamide not only causes abnormal development of the reproductive organs in male offspring, but also damages ovaries and uteri. The unfolded protein response (UPR) is believed to play an important role in embryo development and teratogenic processes. In the present study, pregnant mice were administered either flutamide (300 mg kg−1 day−1, p.o.) on an equivalent volume of soybean oil (control) on Days 12–18 of gestation. Eight weeks after birth, female offspring in the flutamide-treated group had a lower bodyweight and lower ovarian and uterine weights, but there was no significant difference in uterine and ovarian weights normalised by bodyweight between the flutamide-treated and control groups. Furthermore, histopathological changes were observed in all uteri and ovaries in the flutamide-treated group, with fewer and less-developed follicles in the ovaries. In both the uteri and ovaries, flutamide increased the expression of UPR members, although the expression of cell cycle-related genes remained unchanged compared with the control group. Flutamide increased the expression of all autophagy- and apoptosis-related genes evaluated in the uterus, as well as some in the ovary. The results suggest that the in utero exposure of mice to flutamide may contribute to uterine and ovarian damage in the offspring, with endoplasmic reticulum stress possibly triggered by the UPR leading to the induction of excessive autophagy and apoptosis.
Keywords: damage, flutamide, ovary, unfolded protein response, uterus.
References
B’chir, W., Maurin, A. C., Carraro, V., Averous, J., Jousse, C., Muranishi, Y., Parry, L., Stepien, G., Fafournoux, P., and Bruhat, A. (2013). The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 41, 7683–7699.| The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression.Crossref | GoogleScholarGoogle Scholar | 23804767PubMed |
Bohnert, K. R., McMillan, J. D., and Kumar, A. (2018). Emerging roles of ER stress and unfolded protein response pathways in skeletal muscle health and disease. J. Cell. Physiol. 233, 67–78.
| Emerging roles of ER stress and unfolded protein response pathways in skeletal muscle health and disease.Crossref | GoogleScholarGoogle Scholar | 28177127PubMed |
Bouty, A., Ayers, K. L., Pask, A., Heloury, Y., and Sinclair, A. H. (2015). The Genetic and Environmental Factors Underlying Hypospadias. Sex Dev. 9, 239–259.
| The Genetic and Environmental Factors Underlying Hypospadias.Crossref | GoogleScholarGoogle Scholar | 26613581PubMed |
Bretin, A., Carrière, J., Dalmasso, G., Bergougnoux, A., B’chir, W., Maurin, A. C., Müller, S., Seibold, F., Barnich, N., Bruhat, A., Darfeuille-Michaud, A., and Nguyen, H. T. (2016). Activation of the EIF2AK4–EIF2A/eIF2α-ATF4 pathway triggers autophagy response to Crohn disease-associated adherent-invasive Escherichia coli infection. Autophagy 12, 770–783.
| Activation of the EIF2AK4–EIF2A/eIF2α-ATF4 pathway triggers autophagy response to Crohn disease-associated adherent-invasive Escherichia coli infection.Crossref | GoogleScholarGoogle Scholar | 26986695PubMed |
Cheng, Y., Feng, Y., Jansson, L., Sato, Y., Deguchi, M., Kawamura, K., and Hsueh, A. J. (2015). Actin polymerization-enhancing drugs promote ovarian follicle growth mediated by the Hippo signaling effector YAP. FASEB J. 29, 2423–2430.
| Actin polymerization-enhancing drugs promote ovarian follicle growth mediated by the Hippo signaling effector YAP.Crossref | GoogleScholarGoogle Scholar | 25690654PubMed |
Cybulsky, A. V. (2017). Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat. Rev. Nephrol. 13, 681–696.
| Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases.Crossref | GoogleScholarGoogle Scholar | 28970584PubMed |
Dhanasekaran, D. N., and Reddy, E. P. (2008). JNK signaling in apoptosis. Oncogene 27, 6245–6251.
| JNK signaling in apoptosis.Crossref | GoogleScholarGoogle Scholar | 18931691PubMed |
Durlej, M., Kopera, I., Knapczyk-Stwora, K., Hejmej, A., Duda, M., Koziorowski, M., Slomczynska, M., and Bilinska, B. (2011). Connexin 43 gene expression in male and female gonads of porcine offspring following in utero exposure to an anti-androgen, flutamide. Acta Histochem. 113, 6–12.
| Connexin 43 gene expression in male and female gonads of porcine offspring following in utero exposure to an anti-androgen, flutamide.Crossref | GoogleScholarGoogle Scholar | 19853283PubMed |
Fernández, A., Ordóñez, R., Reiter, R. J., González-Gallego, J., and Mauriz, J. L. (2015). Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis. J. Pineal Res. 59, 292–307.
| Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis.Crossref | GoogleScholarGoogle Scholar | 26201382PubMed |
Fischer, H., Koenig, U., Eckhart, L., and Tschachler, E. (2002). Human caspase 12 has acquired deleterious mutations. Biochem. Biophys. Res. Commun. 293, 722–726.
| Human caspase 12 has acquired deleterious mutations.Crossref | GoogleScholarGoogle Scholar | 12054529PubMed |
Flaws, J. A., Hirshfield, A. N., Hewitt, J. A., Babus, J. K., and Furth, P. A. (2001). Effect of bcl-2 on the primordial follicle endowment in the mouse ovary. Biol. Reprod. 64, 1153–1159.
| Effect of bcl-2 on the primordial follicle endowment in the mouse ovary.Crossref | GoogleScholarGoogle Scholar | 11259262PubMed |
Gellersen, B., and Brosens, J. J. (2014). Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 35, 851–905.
| Cyclic decidualization of the human endometrium in reproductive health and failure.Crossref | GoogleScholarGoogle Scholar | 25141152PubMed |
Gordon, J. W., Shaw, J. A., and Kirshenbaum, L. A. (2011). Multiple facets of NF-κB in the heart: to be or not to NF-κB. Circ. Res. 108, 1122–1132.
| Multiple facets of NF-κB in the heart: to be or not to NF-κB.Crossref | GoogleScholarGoogle Scholar | 21527742PubMed |
Guo, C., Yeh, S., Niu, Y., Li, G., Zheng, J., Li, L., and Chang, C. (2017). Targeting androgen receptor versus targeting androgens to suppress castration resistant prostate cancer. Cancer Lett. 397, 133–143.
| Targeting androgen receptor versus targeting androgens to suppress castration resistant prostate cancer.Crossref | GoogleScholarGoogle Scholar | 28323036PubMed |
Hetz, C., and Papa, F. R. (2018). The Unfolded Protein Response and Cell Fate Control. Mol. Cell 69, 169–181.
| The Unfolded Protein Response and Cell Fate Control.Crossref | GoogleScholarGoogle Scholar | 29107536PubMed |
Hunt, T. (1989). Maturation promoting factor, cyclin and the control of M-phase. Curr. Opin. Cell Biol. 1, 268–274.
| Maturation promoting factor, cyclin and the control of M-phase.Crossref | GoogleScholarGoogle Scholar | 2576632PubMed |
Iguchi, T., Tamada, S., Kato, M., Yasuda, S., Yamasaki, T., and Nakatani, T. (2019). Enzalutamide versus flutamide for castration-resistant prostate cancer after combined androgen blockade therapy with bicalutamide: study protocol for a multicenter randomized phase II trial (the OCUU-CRPC study). BMC Cancer 19, 339.
| Enzalutamide versus flutamide for castration-resistant prostate cancer after combined androgen blockade therapy with bicalutamide: study protocol for a multicenter randomized phase II trial (the OCUU-CRPC study).Crossref | GoogleScholarGoogle Scholar | 30971225PubMed |
Ji, Y. K., Lee, G. S., Choi, K. C., and Jeung, E. B. (2006). Anti-progestogenic effect of flutamide on uterine expression of calbindin-D9k mRNA and protein in immature mice. Reprod. Toxicol. 22, 694–701.
| Anti-progestogenic effect of flutamide on uterine expression of calbindin-D9k mRNA and protein in immature mice.Crossref | GoogleScholarGoogle Scholar | 16777378PubMed |
Kang, R., Zeh, H. J., Lotze, M. T., and Tang, D. (2011). The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 18, 571–580.
| The Beclin 1 network regulates autophagy and apoptosis.Crossref | GoogleScholarGoogle Scholar | 21311563PubMed |
Kania, E., Pająk, B., and Orzechowski, A. (2015). Calcium homeostasis and ER stress in control of autophagy in cancer cells. BioMed Res. Int. 2015, 352794.
| Calcium homeostasis and ER stress in control of autophagy in cancer cells.Crossref | GoogleScholarGoogle Scholar | 25821818PubMed |
Kimura, M., Ichimura, S., Sasaki, K., Masuya, H., Suzuki, T., Wakana, S., Ikegawa, S., and Furuichi, T. (2015). Endoplasmic reticulum stress-mediated apoptosis contributes to a skeletal dysplasia resembling platyspondylic lethal skeletal dysplasia, Torrance type, in a novel Col2a1 mutant mouse line. Biochem. Biophys. Res. Commun. 468, 86–91.
| Endoplasmic reticulum stress-mediated apoptosis contributes to a skeletal dysplasia resembling platyspondylic lethal skeletal dysplasia, Torrance type, in a novel Col2a1 mutant mouse line.Crossref | GoogleScholarGoogle Scholar | 26545783PubMed |
Kirkpatrick, B. W., Arias, J. A., and Rutledge, J. J. (1988). Effects of prenatal and postnatal fraternity size on long-term reproduction in mice. J. Anim. Sci. 66, 62–69.
| Effects of prenatal and postnatal fraternity size on long-term reproduction in mice.Crossref | GoogleScholarGoogle Scholar | 3366718PubMed |
Knapczyk-Stwora, K., Durlej, M., Bilinska, B., and Slomczynska, M. (2011). Immunohistochemical studies on the proliferative marker Ki-67 and estrogen receptor alpha (ERα) in the uterus of neonatal and immature pigs following exposure to flutamide. Acta Histochem. 113, 534–541.
| Immunohistochemical studies on the proliferative marker Ki-67 and estrogen receptor alpha (ERα) in the uterus of neonatal and immature pigs following exposure to flutamide.Crossref | GoogleScholarGoogle Scholar | 20598360PubMed |
Knapczyk-Stwora, K., Nynca, A., Ciereszko, R. E., Paukszto, L., Jastrzebski, J. P., Czaja, E., Witek, P., Koziorowski, M., and Slomczynska, M. (2019). Flutamide-induced alterations in transcriptional profiling of neonatal porcine ovaries. J. Anim. Sci. Biotechnol. 10, 35.
| Flutamide-induced alterations in transcriptional profiling of neonatal porcine ovaries.Crossref | GoogleScholarGoogle Scholar | 30988948PubMed |
Lin, T., Lee, J. E., Kang, J. W., Shin, H. Y., Lee, J. B., and Jin, D. I. (2019). Endoplasmic Reticulum (ER) Stress and Unfolded Protein Response (UPR) in Mammalian Oocyte Maturation and Preimplantation Embryo Development. Int. J. Mol. Sci. 20, 409.
| Endoplasmic Reticulum (ER) Stress and Unfolded Protein Response (UPR) in Mammalian Oocyte Maturation and Preimplantation Embryo Development.Crossref | GoogleScholarGoogle Scholar |
Mariño, G., Niso-Santano, M., Baehrecke, E. H., and Kroemer, G. (2014). Self-consumption: the interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 15, 81–94.
| Self-consumption: the interplay of autophagy and apoptosis.Crossref | GoogleScholarGoogle Scholar | 24401948PubMed |
Nemer, M., and Horb, M. E. (2007). The KLF family of transcriptional regulators in cardiomyocyte proliferation and differentiation. Cell Cycle 6, 117–121.
| The KLF family of transcriptional regulators in cardiomyocyte proliferation and differentiation.Crossref | GoogleScholarGoogle Scholar | 17245133PubMed |
Nishitoh, H., Matsuzawa, A., Tobiume, K., Saegusa, K., Takeda, K., Inoue, K., Hori, S., Kakizuka, A., and Ichijo, H. (2002). ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 16, 1345–1355.
| ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats.Crossref | GoogleScholarGoogle Scholar | 12050113PubMed |
Paradisi, R., Fabbri, R., Battaglia, C., and Venturoli, S. (2013). Ovulatory effects of flutamide in the polycystic ovary syndrome. Gynecol. Endocrinol. 29, 391–395.
| Ovulatory effects of flutamide in the polycystic ovary syndrome.Crossref | GoogleScholarGoogle Scholar | 23327685PubMed |
Parent, A. S., Franssen, D., Fudvoye, J., Pinson, A., and Bourguignon, J. P. (2016). Current Changes in Pubertal Timing: Revised Vision in Relation with Environmental Factors Including Endocrine Disruptors. Endocr. Dev. 29, 174–184.
| Current Changes in Pubertal Timing: Revised Vision in Relation with Environmental Factors Including Endocrine Disruptors.Crossref | GoogleScholarGoogle Scholar | 26680578PubMed |
Polański, Z., Homer, H., and Kubiak, J. Z. (2012). Cyclin B in mouse oocytes and embryos: importance for human reproduction and aneuploidy. Results Probl. Cell Differ. 55, 69–91.
| Cyclin B in mouse oocytes and embryos: importance for human reproduction and aneuploidy.Crossref | GoogleScholarGoogle Scholar | 22918801PubMed |
Qi, Z., and Chen, L. (2019). Endoplasmic Reticulum Stress and Autophagy. Adv. Exp. Med. Biol. 1206, 167–177.
| Endoplasmic Reticulum Stress and Autophagy.Crossref | GoogleScholarGoogle Scholar | 31776985PubMed |
Rengasamy, P. (2017). Congenital Malformations Attributed to Prenatal Exposure to Cyclophosphamide. Anticancer. Agents Med. Chem. 17, 1211–1227.
| Congenital Malformations Attributed to Prenatal Exposure to Cyclophosphamide.Crossref | GoogleScholarGoogle Scholar | 27924730PubMed |
Sano, R., and Reed, J. C. (2013). ER stress-induced cell death mechanisms. Biochim. Biophys. Acta 1833, 3460–3470.
| ER stress-induced cell death mechanisms.Crossref | GoogleScholarGoogle Scholar | 23850759PubMed |
Senft, D., and Ronai, Z. A. (2015). UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem. Sci. 40, 141–148.
| UPR, autophagy, and mitochondria crosstalk underlies the ER stress response.Crossref | GoogleScholarGoogle Scholar | 25656104PubMed |
Shaheen, A. (2018). Effect of the unfolded protein response on ER protein export: a potential new mechanism to relieve ER stress. Cell Stress Chaperones 23, 797–806.
| Effect of the unfolded protein response on ER protein export: a potential new mechanism to relieve ER stress.Crossref | GoogleScholarGoogle Scholar | 29730847PubMed |
Sharma, R. P., Schuhmacher, M., and Kumar, V. (2017). Review on crosstalk and common mechanisms of endocrine disruptors: Scaffolding to improve PBPK/PD model of EDC mixture. Environ. Int. 99, 1–14.
| Review on crosstalk and common mechanisms of endocrine disruptors: Scaffolding to improve PBPK/PD model of EDC mixture.Crossref | GoogleScholarGoogle Scholar | 27697394PubMed |
Sinclair, A. W., Cao, M., Pask, A., Baskin, L., and Cunha, G. R. (2017). Flutamide-induced hypospadias in rats: A critical assessment. Differentiation 94, 37–57.
| Flutamide-induced hypospadias in rats: A critical assessment.Crossref | GoogleScholarGoogle Scholar | 28043016PubMed |
Smith, M., and Wilkinson, S. (2017). ER homeostasis and autophagy. Essays Biochem. 61, 625–635.
| ER homeostasis and autophagy.Crossref | GoogleScholarGoogle Scholar | 29233873PubMed |
Suh, D. H., Kim, M. K., Kim, H. S., Chung, H. H., and Song, Y. S. (2012). Unfolded protein response to autophagy as a promising druggable target for anticancer therapy. Ann. N. Y. Acad. Sci. 1271, 20–32.
| Unfolded protein response to autophagy as a promising druggable target for anticancer therapy.Crossref | GoogleScholarGoogle Scholar | 23050960PubMed |
Tiwari, M., Prasad, S., Pandey, A. N., Premkumar, K. V., Tripathi, A., Gupta, A., Chetan, D. R., Yadav, P. K., Shrivastav, T. G., and Chaube, S. K. (2017). Nitric oxide signaling during meiotic cell cycle regulation in mammalian oocytes. Front. Biosci. (Schol. Ed.) 9, 307–318.
| Nitric oxide signaling during meiotic cell cycle regulation in mammalian oocytes.Crossref | GoogleScholarGoogle Scholar | 28410121PubMed |
Wang, K. (2015). Autophagy and apoptosis in liver injury. Cell Cycle 14, 1631–1642.
| Autophagy and apoptosis in liver injury.Crossref | GoogleScholarGoogle Scholar | 25927598PubMed |
Wang, G., Huang, W. Q., Cui, S. D., Li, S., Wang, X. Y., Li, Y., Chuai, M., Cao, L., Li, J. C., Lu, D. X., and Yang, X. (2015). Autophagy is involved in high glucose-induced heart tube malformation. Cell Cycle 14, 772–783.
| Autophagy is involved in high glucose-induced heart tube malformation.Crossref | GoogleScholarGoogle Scholar | 25738919PubMed |
Yang, J., and Yao, S. (2015). JNK-Bcl-2/Bcl-xL-Bax/Bak Pathway Mediates the Crosstalk between Matrine-Induced Autophagy and Apoptosis via Interplay with Beclin 1. Int. J. Mol. Sci. 16, 25744–25758.
| JNK-Bcl-2/Bcl-xL-Bax/Bak Pathway Mediates the Crosstalk between Matrine-Induced Autophagy and Apoptosis via Interplay with Beclin 1.Crossref | GoogleScholarGoogle Scholar | 26516844PubMed |
Yoneda, T., Imaizumi, K., Oono, K., Yui, D., Gomi, F., Katayama, T., and Tohyama, M. (2001). Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J. Biol. Chem. 276, 13935–13940.
| Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress.Crossref | GoogleScholarGoogle Scholar | 11278723PubMed |
Yu, H., Wen, K., Zhou, X., Zhang, Y., Yan, Z., Fu, H., Zhu, J., and Zhu, Y. (2020). Role of unfolded protein response in genital malformation/damage of male mice induced by flutamide. Hum. Exp. Toxicol. 39, 1690–1699.
| Role of unfolded protein response in genital malformation/damage of male mice induced by flutamide.Crossref | GoogleScholarGoogle Scholar | 32662666PubMed |
Yuan, Y. M., Zhang, C., Bai, C., Zheng, M., and Zhou, X. J. (2013). [Expression of insulin-like factor 3 in the testis of flutamide-induced cryptorchidism mice and its significance]. Zhonghua Nan Ke Xue 19, 968–971.
| 24341087PubMed |
Zhang, Y., Li, F., Liu, L., Jiang, H., Jiang, X., Ge, X., Cao, J., Wang, Z., Zhang, L., and Wang, Y. (2018). Salinomycin-induced autophagy blocks apoptosis via the ATG3/AKT/mTOR signaling axis in PC-3 cells. Life Sci. 207, 451–460.
| Salinomycin-induced autophagy blocks apoptosis via the ATG3/AKT/mTOR signaling axis in PC-3 cells.Crossref | GoogleScholarGoogle Scholar | 29966607PubMed |
Zhou, X., Wen, K., Yin, H., Yang, J., and Zhu, Y. (2018). [Effects of intrauterine flutamide exposure on uterus and ovary development and on oxidative stress among female offspring]. Aibian. Jibian. Tubian 30, 384–388.
Zhu, Y., Zhu, Y., Yin, H., Zhou, H., Wan, X., Zhu, J., and Zhang, T. (2012). All-trans-retinoic acid induces short forelimb malformation during mouse embryo development by inhibiting chondrocyte maturation rather than by evoking excess cell death. Toxicol. Lett. 211, 172–186.
| All-trans-retinoic acid induces short forelimb malformation during mouse embryo development by inhibiting chondrocyte maturation rather than by evoking excess cell death.Crossref | GoogleScholarGoogle Scholar | 22498432PubMed |


