Mammalian membrane block to polyspermy: new insights into how mammalian eggs prevent fertilisation by multiple sperm
Allison J. Gardner A and Janice P. Evans A BA Department of Biochemistry and Molecular Biology, Division of Reproductive Biology, Johns Hopkins University, Bloomberg School of Public Health, Baltimore, MD 21205, USA.
B Corresponding author. Email: jpevans@jhsph.edu
Reproduction, Fertility and Development 18(2) 53-61 https://doi.org/10.1071/RD05122
Submitted: 21 September 2005 Accepted: 21 September 2005 Published: 14 December 2005
Abstract
To inhibit fertilisation by more than one sperm (a condition known as polyspermy), eggs have developed preventative mechanisms known as blocks to polyspermy. The block at the level of the egg extracellular coat (the zona pellucida in mammals, the vitelline envelope in non-mammals) has been well characterised in many different animal species and the block at the level of the egg plasma membrane is understood in some non-mammalian species. However, virtually nothing is known about the membrane block to polyspermy in mammalian eggs, despite data dating back 50–90 years that provide evidence for its existence. In the present review, we will discuss the background on blocks to polyspermy used by animal eggs and then focus on the membrane block to polyspermy in mammalian eggs. This will include a summary of classical studies that provide evidence for this block in mammalian eggs, assays used to study the mammalian membrane block and what has been elucidated from recent experimental studies about the cellular signalling events that lead to membrane block establishment and the mechanism of how the membrane block may prevent additional fertilisation.
Polyspermy in mammals and its consequences
Polyploidy will generally be fatal to an embryo and has been detected in approximately 10–20% of spontaneously aborted human conceptuses (Jacobs et al. 1978; Hassold et al. 1980; Michelmann et al. 1986). Polyploid embryos can result from either digyny (due to errors in meiosis I during oocyte maturation or meiosis II during egg activation) or diandry (due to fertilisation by a diploid sperm or by two sperm). The majority of triploid human embryos appear to be the result of polyspermic fertilisation, with two sperm fertilising an egg (Jacobs et al. 1978; Zaragoza et al. 2000). Mammals use several mechanisms to prevent polyspermy (addressed below), although, even with these in place, the incidence of polyspermy is 1–2% when mating occurs around the time of ovulation. A variety of factors can contribute to increased rates of polyspermy in different species; these have been the subject of a recent review (Wang et al. 2003). For example, the incidence of polyspermy can increase, to up to 30% or even higher, if mating is delayed after ovulation until slightly later in the oestrous cycle (Austin 1961; Hunter 1991a, 1991b; Wang et al. 2003). There are also some species, most notably the pig, that have increased rates of polyspermy, in vivo to some extent and especially with in vitro inseminations (Hunter 1991a; Wang et al. 2003).
Triploid embryos die at various times after conception. Although most triploid embryos die early in embryogenesis, it is possible for some to survive into post-implantation development; studies of human embryos have found that a subset of triploid embryos survived well into gestation (average approximately 111 days in one study of 1000 abortions; Hassold et al. 1980). A small number of triploid embryos has survived into the third trimester or to full term, but died shortly after birth (Jacobs et al. 1978; Hassold et al. 1980). It is thought that survival rates depend on the extent of mosaicism or what proportion of cells are diploid (Michelmann et al. 1986).
Blocks to polyspermy
As noted above, mammals use a variety of mechanisms to reduce the incidence of polyspermy. It is thought that capacitation, sperm transit through regions of the female tract (e.g. cervix, uterotubal junction) and sperm oviducal reservoirs serve to regulate the number of sperm that reach the site of fertilisation in a variety of species (Yanagimachi 1994; Suarez 1999). In the pig, in particular, exposure of gametes to oviducal epithelial cells and/or oviducal secretions can reduce polyspermy (Hunter 1991a; Wang et al. 2003). In addition to these processes involving the female reproductive tract, the eggs themselves mount blocks to polyspermy to prevent fertilisation by additional sperm after the egg has already been fertilised. Blocks to polyspermy occur primarily at two levels on the egg: the egg plasma membrane and the egg coat (the zona pellucida (ZP) in mammals and the vitelline envelope in non-mammals).
The egg coat block to polyspermy has been studied extensively in both non-mammalian and mammalian animals. The egg coat block (also called the ‘slow’ block) in many animal species involves the exocytosis of cortical granules (CGs) from the cortex of the egg. In echinoderms and anuran amphibians, CG exocytosis results in the elevation of the vitelline envelope from the plasma membrane of the egg and the loss of the ability of the vitelline envelope to support sperm binding (Wolf 1974; Jaffe and Gould 1985; Hedrick and Hardy 1991). The block to polyspermy at the ZP of mammalian eggs is very similar to this, with the exocytosis of CGs causing conversion of the ZP to a form that cannot support sperm binding (Yanagimachi 1994; Abbott and Ducibella 2001).
In contrast with the egg coat block, membrane block to polyspermy differs significantly between mammalian and non-mammalian eggs. In non-mammals (such as sea urchins and frogs, two of the species most extensively studied), the membrane block involves a rapid and transient depolarisation of the egg plasma membrane potential so that sperm penetration of the egg is not favoured (although not completely inhibited; Jaffe and Gould 1985). Post-fertilisation membrane depolarisation occurs within a few seconds of fertilisation (and, thus, is also referred to as the ‘fast block to polyspermy’), lasts up to several minutes and is observed in sea urchins, starfish, the marine worm Urechis, some ascidians, molluscs, annelids, nemerteans and anuran amphibians (Jaffe and Gould 1985). In contrast, significant membrane depolarisation has not been observed in mouse, hamster or rabbit eggs (Miyazaki and Igusa 1981; Igusa et al. 1983; Jaffe et al. 1983; McCulloh et al. 1983). Thus, the mechanism of the mammalian membrane block does not appear to be mediated by a change in membrane potential. It should also be noted that the mammalian membrane block is established in approximately the same time frame as the ZP block (0.5–1 h), whereas in non-mammalian species the electrical membrane block occurs within seconds of fertilisation and the egg coat block takes 30–60 s (sea urchin) to several minutes (frogs; Jaffe and Gould 1985). Thus, the mammalian blocks are distinguished spatially (membrane or ZP), whereas the non-mammalian blocks are distinguished spatially and temporally (fast or slow).
As noted above, a variety of factors can contribute to increased polyspermy rates, one of these being delayed mating leading to conception occurring at times later than ovulation (Austin 1961; Hunter 1991a, 1991b; Wang et al. 2003). We have shown recently that aged eggs have a reduced ability to establish a membrane block to polyspermy (Wortzman and Evans 2005), suggesting that this may be one cause of increased polyspermy in aged eggs. Post-ovulatory ageing may also affect CG exocytosis (and, thus, the ZP block to polyspermy), although in different ways. Aged eggs in a variety of species (mouse, rabbit, rat, hamster) have been reported to undergo spontaneous CG loss (i.e. without fertilisation; Szollosi 1967; Longo 1974a, 1974b; Xu et al. 1997). It is not clear that aged eggs have a reduced ability to exocytose CGs if fertilised, although this is a possibility because sperm-induced Ca2+ oscillations are abnormal in aged mouse eggs compared with eggs collected near the time of ovulation (Igarashi et al. 1997; Gordo et al. 2002) and this abnormal Ca2+ signalling may affect egg activation responses, such as CG exocytosis.
Historical perspective: evidence for the membrane block to polyspermy in mammalian eggs
Little is known about the molecular or mechanistic basis of the membrane block in mammalian eggs, although evidence for its existence comes from several findings that date back several decades. Classic studies of fertilised, monospermic eggs recovered from natural matings found that there are extra sperm in the perivitelline space (PVS; between the ZP and the plasma membrane) that are apparently unable to fertilise the egg, despite being right next to the membrane (see Lewis and Wright 1935; Odor and Blandau 1949; Austin 1961). Numbers of supernumerary perivitelline sperm vary by species. The eggs of some species (rabbit, pika, pocket gopher, mole) have tens to hundreds of sperm in the PVS, suggestive of a highly effective membrane block and very little ZP block. In eggs from other species (dog, sheep, field vole), PVS sperm are rare, suggestive of a highly effective ZP block. Most species (mouse, human, rat, guinea-pig, cat, ferret, pig, cattle) appear to use both blocks to polyspermy; in these eggs, approximately one to 10 supernumerary sperm are found in the PVS, suggestive of an effective membrane block and also a ZP block to limit the number of sperm reaching the PVS (Lewis and Wright 1935; Odor and Blandau 1949; Austin 1961; Hunter 1990; Sengoku et al. 1995; Hunter et al. 1998). With regard to human eggs, there is one retrospective analysis of clinical data from subzonal inseminations that concluded that human eggs lack a membrane block (Wolf et al. 1997), but data from experimental studies provide convincing evidence that human eggs use a membrane block in addition to a ZP block to prevent polyspermy (Sengoku et al. 1995).
Complementing these data from studies of in vivo-fertilised eggs are data from in vitro fertilisation (IVF) experiments. These experiments use ZP-free eggs, allowing one to distinguish between effects at the level of the ZP and at the plasma membrane. Studies of eggs from a variety of mammalian species (e.g. mouse, hamster, human) have shown that ZP-free fertilised eggs are penetrated by few, if any, additional sperm when challenged with a second insemination, suggesting that the zygote membrane is somehow different from the membrane of the unfertilised egg and is unable to support sperm interactions (Wolf 1978; Zuccotti et al. 1991; Horvath et al. 1993; Maluchnik and Borsuk 1994; Sengoku et al. 1995). Furthermore, the number of sperm that fuse with ZP-free eggs plateaus (rather than increases steadily) when the eggs are cocultured continuously with sperm for several hours (Wolf 1978; Binor et al. 1982; McAvey et al. 2002), raising the possibility that the egg membrane changes in some way that then prevents sperm penetration at later time-points after insemination.
Experimental assessments of the membrane block to polyspermy in mammalian eggs
Assays used to study the membrane block to polyspermy are based on classical studies that provided evidence for use of this block by mammalian eggs. Because a molecular marker for the establishment of the membrane block has not yet been identified, functional assays are used to assess the membrane block under various experimental conditions. Experiments to evaluate the membrane block are performed with ZP-free eggs in order to eliminate the contribution of the ZP block in preventing polyspermy. Two types of designs used to investigate the membrane block will be discussed here: (1) sperm incorporation over time; and (2) re-insemination. The end-point for both experiments is the average number of sperm fused per egg, as determined by staining fixed eggs with a DNA dye, such as 4′,6′-diamidino-2-phenylindole (DAPI), to visualise decondensing sperm heads in the egg cytoplasm (McAvey et al. 2002).
Assay 1: sperm incorporation over time
When a normal membrane block is established, the number of sperm that fuse with an egg plateaus with time and, thus, examining sperm incorporation over time is a method for studying the membrane block. Figure 1 shows the data from a sample of this type of experiment, assessing sperm incorporation over time in mouse eggs inseminated with two different sperm concentrations. As shown in Fig. 1, eggs inseminated with a high sperm concentration (100 000 sperm mL−1) may become slightly polyspermic (an average of approximately two sperm per egg), likely due to the fact that more sperm–egg contacts are occurring with a higher sperm concentration than with a low sperm concentration, particularly before the full establishment of the membrane block. Nevertheless, the plateau in the number of sperm that fuse with ZP-free eggs suggests that the egg membrane is modified in some way, resulting in a zygote membrane that does not favour sperm penetrations at time-points after insemination. In a typical assay of sperm incorporation over time, relative levels of establishment of the membrane block are monitored by comparing the extent of sperm fusion between different experimental groups (see McAvey et al. 2002).
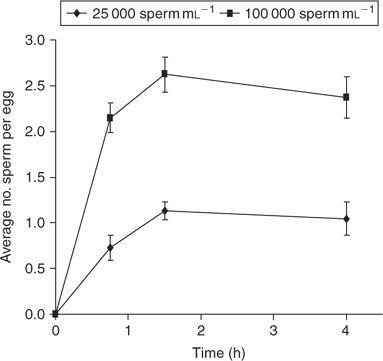
|
Assay 2: re-insemination
A second type of bioassay for the membrane block is a re-insemination experiment, which is outlined in Fig. 2. In this type of experiment, eggs that have been fertilised or experimentally manipulated are challenged with a second round of insemination at various times after the first insemination and the ability of the eggs to prevent further fertilisation is assessed in each of the groups. The extent of sperm penetration after this second insemination is indicative of the effectiveness of the membrane block. An important consideration for this assay is a means to distinguish the two populations of fertilising sperm. If the recovery period between the first and second insemination is long enough (>3 h), sperm from the first IVF will be forming male pronuclei and, thus, can be distinguished morphologically from sperm from the second insemination, which will still be decondensing (Wortzman and Evans 2005). In addition, labelling the sperm to be used in one of the inseminations is another means of distinguishing sperm from the first and second inseminations; this method can be used when the recovery period is less than 3 h or when a particular treatment disrupts male pronuclear formation. In our studies of mouse fertilisation, we have labelled sperm for the second insemination with the membrane-permeable dye MitoTracker Green (Invitrogen, Carlsbad, CA, USA) (A. J. Gardner and J. P. Evans, unpublished observations). This dye has also been used to label bovine sperm (Sutovsky et al. 1997). MitoTracker Green selectively accumulates in mitochondria, which are localised in the mid-piece of the sperm.
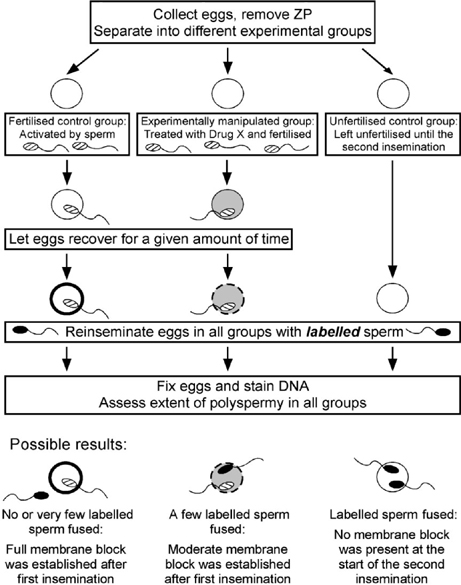
|
It should be noted that it is essential to include appropriate controls in the second insemination step of these re-insemination assays. At one end of the spectrum are unfertilised ‘naïve’ eggs; these eggs indicate the extent of sperm fusion with eggs that have not yet established a membrane block. At the other end of the spectrum are fertilised eggs, namely eggs that were fertilised and activated in the first insemination step. These eggs will have established the membrane block and, thus, will indicate the extent to which penetration by sperm from the second insemination can be prevented by the membrane block. Through the inclusion of these controls, the extent of membrane block establishment in experimentally manipulated eggs can be assessed relative to normally fertilised eggs that have established a normal membrane block and to unfertilised eggs that have not established a membrane block.
Recent experimental insight into the mechanisms underlying the mammalian membrane block
Since the classical studies of in vivo fertilised eggs provided evidence that mammalian eggs use a membrane block to prevent polyspermy, more recent investigations have used the assays described above and other experimental approaches to examine the mammalian membrane block and how it is established following fertilisation. The section below summarises some of these more recent findings, including some of our own unpublished work. Unless otherwise noted, the majority of the work mentioned in this section uses the mouse as an experimental model system.
Calcium signalling and the membrane block to polyspermy
Shortly after fertilisation takes place, Ca2+ release activity is delivered from the sperm into the egg cytoplasm which triggers transient increases, or oscillations, in intracellular Ca2+ levels in the fertilised egg (Kurokawa et al. 2004). These Ca2+ oscillations give rise to a set of events known collectively as egg activation, which includes CG exocytosis, giving rise to the ZP block to polyspermy (see above), and cell cycle resumption (i.e. exit from meiotic arrest and progression to embryonic interphase). The Ca2+ oscillations triggered either by fertilisation or parthenogenetic activation are disrupted by the intracellular Ca2+ chelator BAPTA-AM (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid acetoxymethyl ester) and lead to the inhibition of downstream egg activation events (Kline and Kline 1992). In addition, mouse eggs treated with 10 µm BAPTA-AM to completely abolish all post-fertilisation Ca2+ oscillations (Kline and Kline 1992) become increasingly polyspermic over time compared with control eggs, placing membrane block establishment among the Ca2+-dependent egg activation events (McAvey et al. 2002).
Although treatment of mouse eggs with 10 µm BAPTA-AM abolishes sperm-induced Ca2+ oscillations completely, treatment of eggs with lower concentrations of BAPTA-AM leads to the attenuation of sperm-induced Ca2+ signalling; eggs that experienced these moderated Ca2+ signals undergo partial egg activation responses (Kline and Kline 1992; A. J. Gardner, C. J. Williams and J. P. Evans, unpublished observations). Similar results have been obtained from studies using electropermeabilisation to activate mouse eggs parthenogenetically by inducing controlled pulses of Ca2+ influx, thus mimicking post-fertilisation Ca2+ oscillations (Ducibella et al. 2002). This work shows that there are distinct Ca2+ requirements for the initiation and subsequent completion of specific egg activation responses (Ducibella et al. 2002). These studies did not examine Ca2+ dependence of the membrane block and so our laboratory has investigated how the treatment of eggs with a range of concentrations of BAPTA-AM to attenuate sperm-induced Ca2+ signalling impacts the ability of eggs to establish a membrane block. We have found that, as post-fertilisation Ca2+ signalling is gradually attenuated with increasing concentrations of BAPTA-AM, eggs become increasingly polyspermic when inseminated (A. J. Gardner, C. J. Williams and J. P. Evans, unpublished data). These results suggest that the membrane block is a graded response, rather than an all-or-none event where the membrane is either completely receptive or completely unreceptive to sperm.
Parthenogenesis and the membrane block to polyspermy
Eggs can be induced to activate in the absence of sperm by treatment with SrCl2 or Ca2+ ionophore, a phenomenon known as parthenogenesis. The SrCl2 and Ca2+ ionophore cause an increase in [Ca2+]cyt that is sufficient to induce low to moderate extents of egg activation events, such as CG exocytosis and cell cycle resumption, but it remains unclear whether parthenogenetically activated eggs are able to establish a membrane block to polyspermy. Although there are previous reports that ZP-free eggs activated parthenogenetically with SrCl2 or the Ca2+ ionophore A23187 did not establish a membrane block to polyspermy (Wolf et al. 1979; Horvath et al. 1993), there are caveats to aspects of these studies. First, some experiments did not include fertilised eggs as a control. This control is crucial (discussed above), particularly in light of our finding that the membrane block is not an on/off response and that, instead, there are varying levels of robustness. Second, these studies did not take the post-ovulatory age of the eggs into account. At later post-ovulatory ages, eggs have improved abilities to undergo egg activation responses in response to parthenogenetic stimuli (Fulton and Whittingham 1978; Nagai 1987; Collas et al. 1989; Ware et al. 1989; Fissore and Robl 1992; Abbott et al. 1998). Therefore, our laboratory has examined the question of whether parthenogenetic activation with SrCl2 or the Ca2+ ionophore A23187 can induce the establishment of membrane block in mouse eggs, testing eggs that were collected 13 and 17 h after human chorionic gonadotrophin (hCG), the latter of which have been reported to undergo greater extents of cell cycle resumption in response to inositol 1,4,5-trisphosphate injection (Xu et al. 1997). Our studies show that sperm penetrated SrCl2- and A23187-activated eggs to similar extents as unfertilised eggs, whereas virtually no sperm penetrated eggs that had been activated by a fertilising sperm. The results were similar with eggs collected at 13 and 17 h after hCG, indicating that parthenogenetic activation with SrCl2 and A23187 did not induce membrane block establishment and that post-ovulatory age did not affect the ability of mouse eggs to establish a membrane block in response to these stimuli (A. J. Gardner and J. P. Evans, unpublished observations).
Intracytoplasmic sperm injection and the membrane block to polyspermy
Eggs of many (although not all) species can be fertilised by injection of a sperm into the egg cytoplasm, a procedure known as intracytoplasmic sperm injection (ICSI; Bedford et al. 2004; Yanagimachi 2005). Intracytoplasmic sperm injection-fertilised eggs undergo activation events, such as CG exocytosis and cell cycle resumption, and give rise to live offspring in several species (for a review, see Yanagimachi 2005), but evidence from studies of mouse and human eggs suggests that ICSI does not induce establishment of a membrane block to polyspermy (Maleszewski et al. 1996; Sengoku et al. 1999). We have re-examined this issue because advances in mouse ICSI techniques that improve post-ICSI Ca2+ signalling have been made since 1996, when the first studies of the membrane block in ICSI-fertilised mouse eggs were performed (Kurokawa and Fissore 2003). However, even with various modifications to the ICSI protocols to improve Ca2+ signalling, we find that ICSI-fertilised mouse eggs incorporate just as many sperm as do unfertilised eggs (G. B. Wortzman, M. Kurokawa, R. A. Fissore and J. P. Evans, unpublished observations), suggesting that ICSI did not trigger the establishment of a membrane block to polyspermy.
Taken together, these data raise the question of what is different between conventionally fertilised eggs and eggs that are fertilised by ICSI, as well as eggs that are activated parthenogenetically (discussed above), and how do these differences contribute to the failure of ICSI-fertilised or parthenogenetically activated eggs to establish a membrane block to polyspermy. One candidate is Ca2+ signalling. It is known that Ca2+ oscillations generated by ICSI or parthenogenesis differ from those generated by conventional fertilisation. Calcium imaging studies of eggs fertilised in vitro show that the first Ca2+ oscillation begins 1–4 min after sperm–egg fusion and originate at the sperm entry point, with later oscillations beginning in the vegetal pole (Lawrence et al. 1997; Deguchi et al. 2000), whereas the timing and/or spatial organisation of Ca2+ signals induced by ICSI, SrCl2 or Ca2+ ionophore differ from these induced during conventional IVF (Kline and Kline 1992; Nakano et al. 1997; Sato et al. 1999; Kurokawa and Fissore 2003). As noted above, our data indicate that Ca2+ signalling plays a role in the establishment of the membrane block (McAvey et al. 2002; A. J. Gardner, C. J. Williams and J. P. Evans, unpublished observations). With this in mind, it seems possible that there are qualitative differences between the signalling triggered when eggs are activated in the absence of sperm and the signalling triggered by bona fide fertilisation. We have tested the hypothesis that more ‘fertilisation-like’ oscillations are required for the establishment of the membrane block by examining whether injection of an extract of soluble sperm proteins would trigger the establishment of membrane block in mouse eggs. Injection of sperm extract is known to induce Ca2+ oscillations with spatial and temporal characteristics very similar to those induced by a fertilising sperm (Wu et al. 1998a, 1998b; Oda et al. 1999). However, we found that sperm extract-injected eggs did not appear to establish a membrane block to polyspermy, because just as many sperm penetrated these eggs as fused with unfertilised ‘naïve’ control eggs (G. B. Wortzman, M. Kurokawa, R. A. Fissore and J. P. Evans, unpublished observations). Therefore, it is likely that additional signalling from the sperm contributes to the initiation of the membrane block after fertilisation.
Another candidate is sperm-induced remodelling of the egg cortical cytoskeleton, which appears to be abnormal in ICSI-fertilised eggs (G. B. Wortzman, M. Kurokawa, R. A. Fissore and J. P. Evans, unpublished observations) and is lacking is parthenogenetically activated eggs. It is interesting to note that treating eggs with cytochalasin D, a drug that disrupts actin microfilaments and, thus, inhibits cortical remodelling, leads to a moderate increase in the extent of polyspermy (McAvey et al. 2002). Based on these observations, post-fertilisation rearrangement of the cortical cytoskeleton could be a contributing factor to the post-fertilisation modifications in the egg that culminate in a membrane block to polyspermy. Finally, an additional candidate is the sperm–egg membrane interaction. Sperm–egg membrane interaction is a step-wise process including: (1) sperm–egg binding (which may include a signalling event triggered by the binding of an extracellular sperm ligand to an egg receptor); and (2) membrane fusion between the gametes. Experiments are underway in our laboratory to examine how these numerous possibilities may play a role in the establishment of membrane block.
Differences in the membranes of unfertilised and fertilised eggs
In addition to the question of how the fertilising sperm triggers the establishment of membrane block is the issue of how the plasma membranes of unfertilised and fertilised eggs differ and how these differences render the membrane of a fertilised egg unable to be penetrated by additional sperm. Relatively little is known in this area. The membrane block appears to be established approximately 1–2 h after insemination (Wolf 1978; Maluchnik and Borsuk 1994; Sengoku et al. 1995; Redkar and Olds-Clarke 1999; McAvey et al. 2002) and persists for many hours, or even days, into the early embryonic cleavage-stage embryos (Zuccotti et al. 1991; Maleszewski and Bielak 1993; Sengoku et al. 1995, 1999; Redkar and Olds-Clarke 1999). The failure of additional sperm to penetrate already fertilised eggs may be due to a decrease in sperm adhesion to the egg plasma membrane or perhaps sperm detachment after brief or weak adhesion (Wolf and Hamada 1979; Horvath et al. 1993; Sengoku et al. 1995; Redkar and Olds-Clarke 1999). Other than this, little is known about the molecular differences between unfertilised and fertilised egg membranes. Experiments using fluorescent tags to track certain membrane lipids in mouse eggs suggest that there are fertilisation-induced changes in the organisation of membrane lipids (Wolf et al. 1981). Similar techniques were used to track the diffusion of two labelled membrane proteins in mouse eggs; it was observed that the fluidity of one labelled protein in the membrane increased after fertilisation, whereas the fluidity of the other tagged protein did not change (Wolf and Ziomek 1983). Other than these bits of data, little is known about the differences in the organisation or composition of protein and lipid domains of membranes in unfertilised and fertilised eggs. A better understanding of the changes in membrane dynamics that occur after fertilisation should lead to the identification of a molecular marker(s) for the establishment of the membrane block and facilitate the development of additional assays for the membrane block to complement the functional assays examining whether eggs can prevent subsequent sperm penetration.
Perspectives and future directions
Although there is clearly much we still have to learn about the mammalian membrane block to polyspermy, there are some interesting implications from what we do know. The finding that ICSI does not trigger the establishment of the membrane block suggests that although ICSI can induce several egg activation events and support embryonic development, it does not induce all egg activation events, namely the membrane block. Although this has few ramifications in the clinic (because ICSI-fertilised eggs are not going to be exposed to additional sperm), it does indicate that ICSI is not the full equivalent of conventional IVF. The different utilisation of ZP and membrane blocks to polyspermy by different mammalian species is also a point of interest. These differences could be exploited as a means to perform a comparative study of fertilisation in different species, as well as to gain insights into molecular mechanisms underlying the membrane block. Finally, approaches such as proteomics and analyses of knockout mice with abnormalities in egg activation should allow us to gain insights into the molecular players that contribute to the membrane block.
Acknowledgments
Work in our laboratory is supported by grants from the National Institute of Child Health and Human Development in the National Institutes of Health (HD037696, HD045671) and the March of Dimes (6-FY04–59) to JPE. AJG has been supported by a training grant from the National Institute of Child Health and Human Development (HD 07276).
Abbott, A. L. , and Ducibella, T. (2001). Calcium and the control of mammalian cortical granule exocytosis. Front. Biosci. 6, D792–D806.
| PubMed |
Bedford, S. J. , Kurokawa, M. , Hinrichs, K. , and Fissore, R. A. (2004). Patterns of intracellular calcium oscillations in horse oocytes fertilized by intracytoplasmic sperm injection: possible explanations for the low success of this assisted reproduction technique in the horse. Biol. Reprod. 70, 936–944.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Horvath, P. M. , Kellom, T. , Caulfield, J. , and Boldt, J. (1993). Mechanistic studies of the plasma membrane block to polyspermy in mouse eggs. Mol. Reprod. Dev. 34, 65–72.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Hunter, R. H. , Vajta, G. , and Hyttel, P. (1998). Long-term stability of the bovine block to polyspermy. J. Exp. Zool. 280, 182–188.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Jaffe, L. A. , Sharp, A. P. , and Wolf, D. P. (1983). Absence of an electrical polyspermy block in the mouse. Dev. Biol. 96, 317–323.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Sutovsky, P. , Oko, R. , Hewitson, L. , and Schatten, G. (1997). The removal of the sperm perinuclear theca and its association with the bovine oocyte surface during fertilization. Dev. Biol. 188, 75–84.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Yanagimachi, R. (2005). Intracytoplasmic injection of spermatozoa and spermatogenic cells: its biology and applications in humans and animals. Reprod. Biomed. Online 10, 247–288.
| PubMed |

Zaragoza, M. V. , Surti, U. , Redline, R. W. , Millie, E. , Chakravarti, A. , and Hassold, T. J. (2000). Parental origin and phenotype of triploidy in spontaneous abortions: predominance of diandry and association with the partial hydatidiform mole. Am. J. Hum. Genet. 66, 1807–1820.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zuccotti, M. , Yanagimachi, R. , and Yanagimachi, H. (1991). The ability of hamster oolemma to fuse with spermatozoa: its acquisition during oogenesis and loss after fertilization. Development 112, 143–152.
| PubMed |



