Spermiation response to exogenous hormone therapy in hibernated and non-hibernated boreal toads (Anaxyrus boreas boreas)
Andrew J. Kouba A , Cecilia J. Langhorne B , Scott T. Willard B , Theodore Smith C and Carrie K. Kouba B *
B *
A Wildlife, Fisheries, and Aquaculture Department, Mississippi State University, Mississippi State, MS 39762, USA.
B Biochemistry, Molecular Biology, Entomology and Plant Pathology Department, Mississippi State University, Mississippi State, MS 39762, USA.
C Native Aquatic Species Restoration Facility, Colorado Parks and Wildlife, Alamosa, CO 81101, USA.
Reproduction, Fertility and Development 34(5) 453-460 https://doi.org/10.1071/RD21033
Published online: 1 November 2021
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Conservation programs for threatened high- elevation amphibian species rely on hibernation to trigger appropriate male reproductive behaviours and gametogenesis. Although common practice and anecdotal observations have supported the practice of hibernation, there is limited empirical evidence documenting the effects on reproduction in these species. In this study, the effect of hibernation on sperm quantity and quality was evaluated for the alpine species Anaxyrus boreas boreas. Hibernated (n = 19) and non-hibernated (n = 21) male toads were administered 10 IU g−1 body weight (BW) human chorionic gonadotropin (hCG) and spermic urine was collected over 24 h. Hibernation had no effect on the number of males undergoing spermatogenesis, but hibernated males produced sperm in higher concentrations. Sperm quality was measured in terms of total motility, forward progressive motility and quality of forward progression. Although there was no difference in the total sperm motility of samples from hibernated and non-hibernated toads, the percentage of sperm exhibiting forward progressive motility and the quality of forward progression was significantly greater from hibernated toads. These results support our hypothesis that hibernation impacts both sperm quantity and quality in male boreal toads. This study will better inform captive breeding management decisions for threatened alpine species, in imminent danger of extinction.
Keywords: alpine-species, anuran, assisted-reproduction, brumation, chorionic gonadotropin, hormone, over-wintering, spermatogenesis.
Introduction
Amphibian reproductive cues are strongly influenced by abiotic factors such as temperature, rainfall, snowmelt, humidity, photoperiod, barometric pressure and food availability (Corn 2005; Brizzi and Corti 2006; Mann et al. 2010; Rastogi et al. 2011). In particular, temperature has a major influence on reproductive patterns of high-elevation anuran species inhabiting seasonally extreme environments (Duellman and Trueb 1994; Rastogi et al. 2011). For example, wide swings in temperature extremes tend to influence annual migration between breeding and hibernating habitats (Hartel et al. 2007; Rastogi et al. 2011). In order to survive long periods of freezing temperatures, high- elevation anurans require a period of dormancy to circumvent limited food availability and reduce the energetic costs of maintaining their body temperature to avoid freezing (Pinder et al. 1992; Storey and Storey 1992; Storey 2000). This period of dormancy involves overwintering in a hibernacula below the frost-line.
One iconic alpine sub-species that hibernates for long periods of time is the southern Rocky Mountain boreal toad (Anaxyrus boreas boreas), which is the eastern clade of the more common western toad (Anaxyrus boreas). The boreal toad is the only high-elevation anuran species inhabiting the alpine and subalpine regions of the Rocky Mountains between 2300 and 2700 m and spends up to 8 months in hibernation, emerging from winter refugia to breed between May and July (Hammerson 1999; Loeffler et al. 2001). The boreal toad is currently a target species for restoration efforts since dramatic population declines have occurred at many breeding sites throughout its range due to chytrid fungus, habitat loss and inadequate regulatory mechanisms (Carey 1993). As part of its recovery efforts, the Colorado Parks and Wildlife division maintains over 600 adult animals as an insurance population for reintroduction efforts. All of the animals are located at the Colorado Native Aquatic Species Restoration Facility (NASRF). To mimic the natural life history traits, the entire colony undergoes approximately 5-months of indoor artificial hibernation prior to seasonal breeding operations. Currently, reproductive output is low, generating an insufficient number of animals to sustain the reintroduction program, while maintaining sustainability goals. Anecdotal reports attribute the low reproductive output to lack of amplexus by males, few females laying eggs, low egg numbers, poor fertilization rates and high mortality of embryos/neurulas in early stage development. In light of these observations, questions have arisen as to whether something is missing (e.g. one or more abiotic cues) from the artificial hibernacula compared to what is present in nature or if hibernation could be eliminated altogether and replaced with exogenous hormone treatments to control reproductive events.
Hibernation and environmental constraints for reintroduction with high alpine amphibian species can limit the window of opportunity for breeding with very little chance to repeat mating attempts within a season. If this period of dormancy can be circumvented or possibly reduced through the use of exogenous hormone stimulation for gamete production, the breeding window of seasonal species could potentially be extended and reproductive output increased. Maintaining a colony in an active state also eliminates the risk of hibernation-associated mortality and chronologically advances maturity. Hibernation can affect both the immune system and the gastrointestinal bacterial fauna, resulting in immuno-suppressed individuals with an increased susceptibility to infectious disease (Wright and Whitaker 2001). Furthermore, indoor simulated hibernation requires reliable refrigerators and regular monitoring to avoid desiccation, which can result in mortality (Wright and Whitaker 2001). Although there is some risk involved in creating an artificial hibernation environment in captivity, reproductive behaviours are strongly predicated upon hibernation for many temperate high elevation anuran species. Yet, few studies have investigated the impact of hibernation under controlled laboratory settings. Several studies have shown that hibernation impacts male amplexus in boreal toads (Roth et al. 2010), ovulation in female boreal toads (Calatayud et al. 2015) and vocal advertisement signalling, female receptivity, amplexus, and oviposition in mountain yellow-legged frogs (Rana muscosa) (Santana et al. 2015).
At NASRF, the boreal toad breeding colony loses an average of 20 individuals per year through hibernation, which equates to around 3% of the colony [T. Smith, pers. comm.] and Roth et al. (2010) reported two mortalities out of 200 hibernated boreal toads in their study. In large breeding populations, such a loss might be negligible but could pose a serious threat to small captive colonies. As such, breeding facilities may be disinclined to employ hibernation techniques that could potentially have serious consequences for small or rare populations. Therefore, in some circumstances it might be advantageous to avoid hibernation if there is no clear effect on reproductive output. Since hibernation is thought to play an important role in gonadal development and gamete maturity in amphibians (Tsai 2011), further investigation of its importance and whether hibernation could be circumvented while maintaining reproductive output, or lower mortality, is of great interest to conservation biologists. Several studies have begun evaluating the impacts of hibernation on male (Roth et al. 2010) or female (Roth et al. 2010; Calatayud et al. 2015) reproductive performance in the boreal toads to assess whether hibernation was needed, or could be modified, for breeding purposes. While the Roth et al. (2010) study established a good starting foundation for the effect of hibernation on male breeding behaviours, no sperm quantity or quality metrics were reported between hibernated and non-hibernated toads.
We hypothesised that if hibernation serves an important function in steroidogenesis and gametogenesis in male boreal toads, sperm quantity and quality would be higher in hibernated males than non-hibernated males. To test this hypothesis, we used human chorionic gonadotropin (hCG), previously shown to stimulate sperm production in this species (Langhorne et al. 2021), to collect spermic urine from hibernated and non-hibernated males. The goals of this study were to evaluate the following parameters between the two treatment groups including: (1) percentage of males producing sperm; (2) sperm concentration; (3) sperm motility; and (4) quality of sperm forward progression. Results from this study will clarify the importance of hibernation on spermatogenesis in this alpine species and will help inform captive breeding efforts so that they can weigh the risk vs reward of employing such strategies in their programs.
Materials and methods
Animals and treatment groups
All adult male boreal toads originated from egg masses collected in the southern Rocky Mountains or were captive reared at NASRF. Non-hibernated male toads (n = 21; 6–12 years old) were transferred from NASRF to Mississippi State University (MSU) and several hibernated males (n = 19; 6–14 years old) were maintained at NASRF for comparison purposes. Toads received at MSU were not hibernated for the subsequent breeding seasons, while toads maintained at NASRF remained on their annual hibernation regimen.
The MSU colony of non-hibernated boreal toads were housed in ventilated polycarbonate containers (30 cm × 46 cm × 66 cm) with access to water and shelter. Toads were maintained between 20 and 23°C and provided with standard fluorescent lights on a seasonal light cycle. Husbandry practices and environmental stimuli (light and temperature) for the MSU non-hibernated toads were similar to conditions for the NASRF toads (description below) when not in hibernation, with the exception of the enclosure size and water source (aged tap water vs well water). Single-sex groups of 4–5 individuals were housed per container with refugia and offered a variety of food items including crickets, wax worms, and mealworms, three times per week. Crickets were fed a Repashy SuperLoad nutrient-dense supplement (Repashy Ventures Inc., CA, USA) and dusted with Reptivite powder (ZooMed Laboratories, Inc., Costa Mesa, CA, USA) to prevent malnutrition, prior to being fed out. The average weight and snout vent length (SVL) of non-hibernated male boreal toads was 35.1 ± 0.9 g and 61.6 ± 0.7 mm, respectively.
The NASRF colony of hibernated boreal toads were housed indoors and maintained on a natural light cycle in large fiberglass tanks (121 cm × 60 cm × 30 cm) with a continuous supply of fresh well water. Toads were fed a variety of prey items three times per week, including gutloaded (BugBurger, Allen’s Repashy, La Jolla, CA, USA) crickets, mealworms, red-wiggler worms and waxworms. Crickets were dusted with Reptivite powder (ZooMed Laboratories, Inc., Costa Mesa, CA, USA) prior to being fed out. In December, toads were allocated 4–5/tub into ventilated plastic containers (33 cm × 13 cm × 15 cm) with a substrate of sand and sphagnum moss and placed into a refrigerator cabinet (Foster refrigerator, Corp., Hudson, NY, USA). Hibernacula temperatures ranged from 2 to 6°C. The toads were removed from hibernation 5 months later in May. The average weight and snout vent length (SVL) of hibernated male boreal toads was 47.9 ± 2.5 g and 64.1 ±1.1 mm, respectively. The morphometric measurements for hibernated toads reflect variables collected after hibernation, not prior to. All animal procedures were conducted following review and approval by the Mississippi State University Institutional Animal Care and Use Committee (IACUC #10-082).
Sperm quality and quantity between hibernated and non-hibernated toads
The goal of this study was to compare sperm quantity and quality between the two treatment groups, non-hibernated (n = 21) and hibernated (n = 19) male boreal toads, following exogenous hormone therapy. Boreal toads from the two treatment groups were administered an intra-peritoneal injection of 10 IU g−1 body weight (BW) human chorionic gonadotropin (hCG), previously found to be the optimal concentration for sperm production in this species (Langhorne et al. 2021). Urine samples were collected and analysed for the presence of sperm as described below. If sperm was present in a given sample at a given time-point, the individual was classified as a ‘responder’. Individuals not producing sperm at a given time-point were classified as ‘non-responders’ and were not included in any subsequent analysis. The percentage of animals that responded was then compared between non-hibernated and hibernated males.
For the duration of the hormone trial, male boreal toads were maintained in plastic containers (35 cm × 20 cm × 13 cm) holding aged tap water (2 cm depth) to ensure continuous urine production. Prior to hormone administration, toad weight and snout-vent length (SVL) were recorded and an initial urine sample (T0) was obtained from each toad to determine the presence or absence of sperm. To collect urine, males were gently removed from their holding containers and held above a 150 mm Petri dish, spreading the hind limbs apart by the thumb and index finger, until urination occurred (usually within 1 min).
Spermic urine was collected at 2, 3, 5, 7, 9, 12 and 24 h post-hormone administration (PA). Samples were analysed immediately post-collection by placing a 10 μL aliquot of spermic urine onto a glass slide under 400X objective on an Olympus CX41 phase-contrast microscope and counting 100 random spermatozoa for motility analysis, which is reported as a percent. Spermic urine variables measured included volume, percent sperm showing Forward Progressive Motility (FPM, proportion of sperm exhibiting forward motion), percent sperm showing Non-Progressive Motility (NPM, proportion of sperm exhibiting flagellar motion but are not moving forward), Quality of Forward Progressive Movement ([QFPM], a subjective scale from 0 (no movement) to 5 (very rapid forward movement)) and Total Motility (TM = FPM + NPM), as previously described for other bufonid species (Browne et al. 2006; Kouba et al. 2012; McDonough et al. 2016). Sperm concentration was measured by inactivating motility in a 1:10 dilution of PBS and counting on a Neubaeur haemocytometer to obtain an average sperm concentration mL−1.
Statistical analysis
Assumptions of normality and homogeneity of variance were tested using the Shapiro–Wilk and Levene’s tests, respectively. Body parameters (weight and SVL) and the number of male responders were compared between hibernated and non-hibernated groups using an independent samples t-test. Sperm parameters (Concentration, % Total Motility [%TM], % Forward Progressive Motility [%FPM] and Quality of FPM [QFPM]) were compared between hibernated and non-hibernated toads by a two-way repeated measures analysis of variance (ANOVA) using the General Linear Model procedure (GLM). A split-plot model was used to analyse the effect of hibernation on spermiation response across time (0, 2, 3, 5, 7, 9, 12 and 24 h PA) where time was the main plot factor and treatment (hibernation, non-hibernation) the sub-plot factor. Within the model, treatment and time were fixed factors, and sperm parameters (Concentration, %TM, %FPM and QFPM) were dependent factors. ‘Individual toad’ was nested within treatment to remove variation among individuals from the error term. Significant main effects were explored using Tukey-Kramer Honestly Significant Difference (HSD) post hoc tests. Percentage data were arcsine transformed using the transformation sin−1(√x) before further analysis. All values are expressed as mean ± s.e.m. and significance was established at P ≤ 0.05. All statistical analysis was performed in SAS Ver. 9.4 (Cary, NC, USA).
Results
Morphometrics
Male boreal toads that underwent a period of hibernation prior to exogenous hormone stimulation weighed significantly more (t23 = 4.30; P < 0.001) than non-hibernated toads (47.9 ± 2.5 g vs 35.1 ± 0.9, respectively). In contrast, mean SVL did not significantly differ (P = 0.12) between the hibernated and non-hibernated groups (64.1 ± 1.1 mm and 61.5 ± 0.7 mm, respectively).
Spermiation response
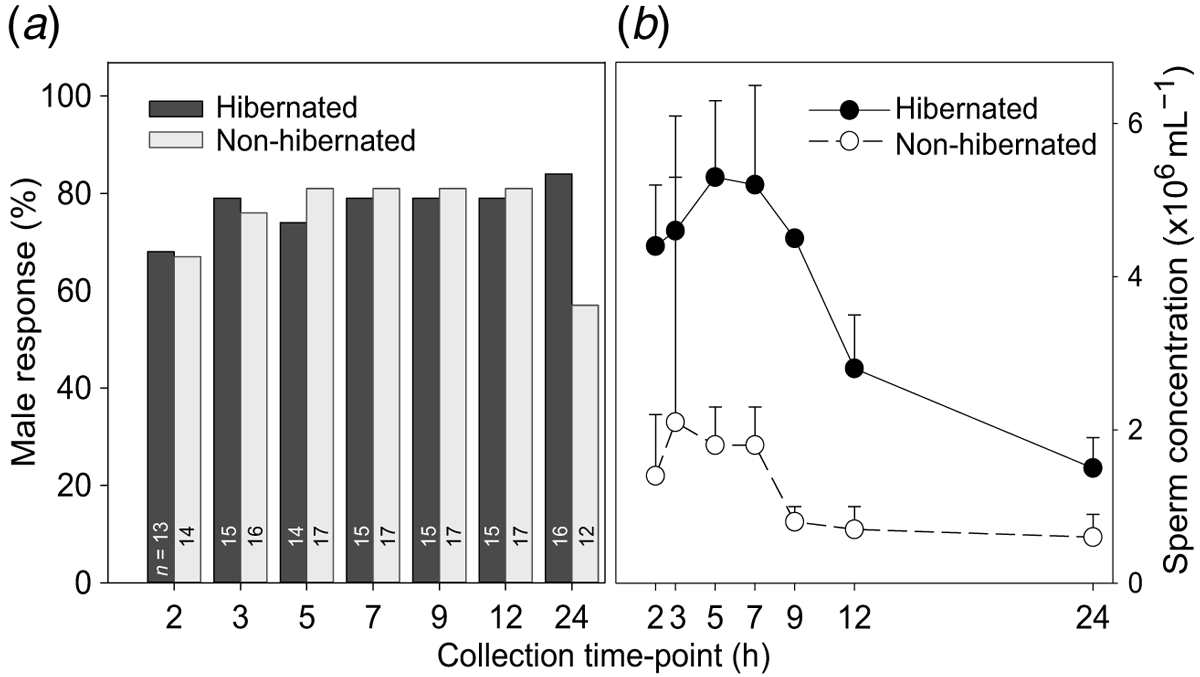
Urine samples were successfully collected from all of the males at each collection time-point (2, 3, 5, 7, 9, 12 and 24 h PA) for evaluation. All urine samples collected prior to hormone treatment (T0) were aspermic. A spermiation response was induced in >60% of males in both the hibernated and non-hibernated groups, beginning as early as 2 h post-hormone administration. There was no significant difference (t38 = 4.90; P > 0.05) in the number of males responding to hormone treatment between hibernated and non-hibernated toads (Fig. 1a). The spermiation response remained between 74 and 84% of males across the collection periods, regardless of treatment, with the exception of a decrease to 57% in non-hibernated males at the 24 h collection period (Fig. 1a). Overall, hibernation did not impact the percentage of animals producing sperm in response to hCG.
Sperm concentration
Sperm concentration of male boreal toads was significantly higher (F1,161 = 49.6; P < 0.001) in the hibernated male toad group, compared to the non-hibernated male group at all time points (Fig. 1b). Furthermore, sperm concentration varied according to different time-points following hCG administration within treatment (F6,161 = 3.81; P < 0.001). For example, sperm production was significantly higher (P < 0.001; Tukey’s HSD) in hibernated males between 2 and 9 h post-administration than at the two later collection time-points of 12 and 24 h (Fig. 1b). Peak sperm concentrations ranged from 4.3 to 5.2 × 106 mL−1 for hibernated toads compared to 1.5–1.8 × 106 mL−1, for non-hibernated toads. Sperm production was significantly depleted (P < 0.05) by the 24 h collection period with 0.6 ± 0.3 × 106 mL−1 and 1.7 ± 0.5 × 106 mL−1 for both non-hibernated and hibernated toads, respectively (Fig. 1b). There was no significant treatment by time interaction with regards to sperm concentration (F6,161 = 1.07; P = 0.38), indicating that the direction and general spermiation profile between both groups did not differ across time, even though concentration was higher in hibernated animals (Fig. 1b). Thus, although there was no difference in the number of animals spermiating or production profile over time, there was a significantly higher concentration of sperm produced when male toads were hibernated.
Sperm quality metrics
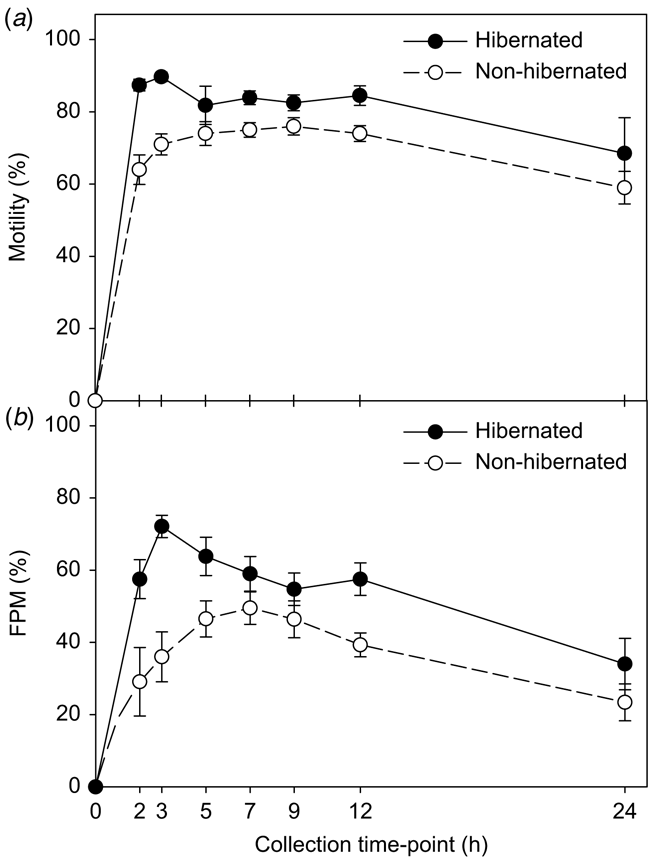
Fig. 2a shows that the general pattern of sperm total motility remained relatively constant over the 24 h collection period, ranging between 87.4 and 89.7% in hibernated toads and 59–76% in the non-hibernated toads. There was no significant difference (F6,131 = 1.48; P = 0.22) in the proportion of motile sperm released by hibernated and non-hibernated toads and no treatment by time interaction was observed (F6,131 = 1.11; P = 0.35). However, there was a significant effect of time (F6,131 = 3.11; P < 0.01), with higher proportions of motile sperm between 3 and 12 h following hCG administration, compared to the 24 h time point (P < 0.05; Tukey’s HSD).
|
Fig. 2b shows the proportion of FPM sperm released by hibernated and non-hibernated males over 24 h in response to hormone treatment. There was a significant treatment by time interaction (F6,127 = 3.43; P < 0.01), indicating that the proportion of forward motile spermatozoa and direction of response varied over the 24 h collection period between the hibernated and non-hibernated toad groups. Specifically, sperm FPM within the hibernated male group was significantly higher (P < 0.001; Tukey’s HSD) across all collection time-points, with the exception of the 24 h period. FPM in the hibernated group peaked at 3 h following hCG administration, with 72 ± 3.1% of sperm exhibiting forward motility (Fig. 2b). Furthermore, the proportion of FPM steadily decreased over time in hibernated toads from 72% at 3 h, 57% at 9 h, and finally to 34% by 24 h following hCG administration. In contrast, sperm collected from non-hibernated males was significantly lower and demonstrated a different pattern across time. FPM steadily increased between 2 and 7 h post-hormone administration with a peak of 49 ± 4.5% at 7 h (Fig. 2b). Subsequently, FPM steadily decreased by 24 h with a final rate of 23 ± 5.1% FPM.
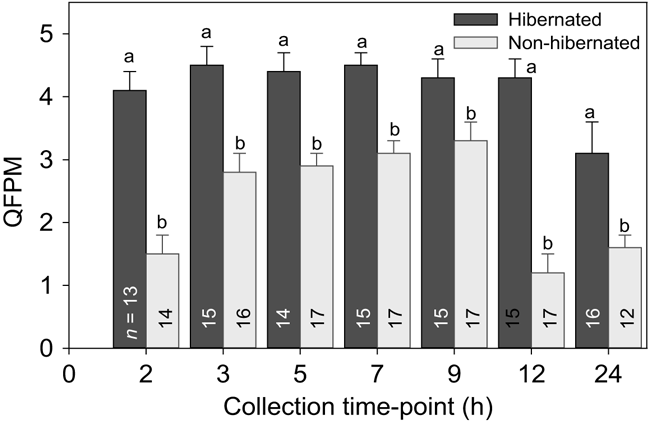
Consistent with sperm FPM, there was also an overall interaction effect of treatment × time (F6,127 = 2.15; P = 0.05) in the sperm QFPM released by hibernated and non-hibernated males (Fig. 3). Overall, hibernated male toads produced significantly (P < 0.05; Tukey’s HSD) more vigorous forward sperm movement with differences reflected at specific collection time points. For example, higher (P < 0.05; Tukey’s HSD) quality sperm movement was observed between 3 and 9 h following hCG administration in non-hibernated toads compared to the 2, 12 or 24 h collection times; whereas, the 2–12 h time period in hibernated toads produced similar movement patterns (Fig. 3). The QFPM ranged between 1.2 and 3.3 in the non-hibernated males, yet was much higher in the hibernated toads, ranging from 3.1 to 4.5.
Discussion
For high-elevation anurans, hibernation serves as a means to avoid challenges related to prolonged extreme cold and may also have an influential role in reproductive function (Tsai 2011). Unfortunately, there is little information on the underlying physiological mechanisms that occur during anuran hibernation for most species. Understanding life-history traits of high elevation anuran species under extreme thermal regimes will be paramount, as many appear to be more susceptible to the zoonotic disease chytrid fungus (Stevenson et al. 2020) and may require conservation management or breeding programs. Our hypothesis that hibernation serves an important function in gametogenesis in male boreal toads, appears to have been partially borne out with observed increases in sperm quantity and some metrics of higher sperm quality in hibernated males than non-hibernated males. However, the percentage of animals producing sperm was not different, nor was the overall total sperm motility. The general concept of whether hormone therapy of non-hibernated toads could be used to circumvent natural reproductive cues for the stimulation of spermatogenesis was supported; although sperm concentration and forward progression was lower in non-hibernated toads. These results build on previous work showing hibernated male boreal toads displayed stronger reproductive behaviours (i.e. amplexus) than non-hibernated males (Roth et al. 2010).
Interestingly, the percentage of hibernated and non-hibernated males responding to hormone therapy was similar (∼80%) across all time points, except for the 24 h sample. This population response, regardless of treatment, suggests that about 20% of the males will not produce sperm following hormone administration with hCG. Thus, artificial hibernation did not increase the responsiveness to hormone therapy over that of non-hibernated males. Previous work by Langhorne et al. (2021) on determining the optimal concentration of hCG to administer for spermiation in non-hibernated boreal toads showed that higher concentrations than administered here (15 vs 10 IU g/BW hCG) did not result in more animals spermiating. Hence, increasing the concentration of hormone presumably would not have produced a different response. We are uncertain why a small percentage of males do not spermiate in response to hCG administration. Similar to these results, work by Roth et al. (2010) found there was no difference in the percentage of male boreal toads undergoing spermiation regardless of hibernation or not, using gonadotropin releasing hormone (GnRH). Roth et al. (2010) found that overall 72% (26/36) of hormone treated animals produced sperm, on par with the 74–84% of males producing sperm using hCG in this study. Several potential speculative reasons why not all males produce sperm include: (1) a mis-match of the artificial hibernation and natural environmental conditions; (2) reproductive dysfunction in some proportion of the population; (3) in-breeding related suppression of reproductive potential; (4) individual variation in response to hormones; and or (5) neuro-endocrine blocking mechanisms.
While the percentage of males spermiating was similar between treatment groups, hibernation appeared to significantly increase sperm concentration. Hibernated toads at peak production yielded nearly four times the concentration of sperm released by non-hibernated toads (5.3 vs 1.5 × 106 mL−1). Previous work on male hibernated NASRF boreal toads induced to spermiate through hormone therapy 3 months after removal from hibernation (mid-August), showed a sperm concentration profile over time about half way between the two treatment groups studied here (Kouba and Vance 2009). Although the sperm concentration was lower at all respective time points for this hibernated group, compared to males that were only a couple weeks out of hibernation, the sperm concentrations were still higher than non-hibernated males. Hence, the benefits of hibernation on sperm concentration may decline the further animals are away from emergence and would need to be investigated. Although the absolute sperm concentration was very different between hibernated and non-hibernated males, the temporal patterns were very similar, suggesting that gamete production, release, and decline follow a conserved physiological pattern. Whereas, the magnitude of sperm production appears to be strongly influenced by environmental cues, one of which might have been temperature. These results suggest that a period of hibernation is beneficial for overall sperm production, which may impact fertilisation success. When the goal is to collect sperm for in vitro fertilisation or biobanking, having higher concentrations of sperm is beneficial, both for establishing long-term storage and for optimising sperm–egg ratios known to be important for optimal fertilisation rates.
Male toads are physiologically primed for breeding in response to environmental variables that cue emergence from hibernation (Lofts 1964). Consequently, the absence of certain environmental stimuli on non-hibernated males may disrupt the natural pattern of spermatogenic activity, resulting in overall reduced sperm production. The boreal toad is a discontinuous breeder and, as with other temperate seasonal reproducing species, spermatogenesis likely occurs directly following the breeding season. Spermatogenesis would be complete prior to the next season’s hibernation event, whereby immature sperm are stored in the seminiferous tubules until emergence, when final maturation is completed (Duellman and Trueb 1994; Rastogi et al. 2011). The amplitude of the annual testicular cycle is greatest in anurans with discontinuous cycles, in contrast to species with continuous spermatogenic cycles (i.e. tropical species) that show no signs of seasonal fluctuations (Lofts 1964). Thus, the amplitude change in sperm production between our two treatment groups may be explained by the evolutionary processes of sperm production in high altitudinal species relative to hibernation.
The impact of hibernation on sperm quality was mixed, with very little impact on total motility, although FPM was very different between the two groups. Thus, if a sperm sample was collected, regardless of treatment, motility was fairly high (∼75–80%) and similar for both groups. The only impact on motility was a slight decrease in both treatment groups at the 24 h time point, which led to an effect of time. While direct comparisons of sperm motility between hibernated and non-hibernated males are unavailable for other species, there are examples where motility was evaluated comparing different hormone treatments (Byrne and Silla 2010; Kouba et al. 2012; Silla et al. 2020; Langhorne et al. 2021). These studies all show similar results on sperm motility parameters, such that if a sample was collected, regardless of treatment, motility was typically high and not impacted by specific variations in hormone therapy or environmental cues. In contrast to sperm total motility, both FPM and QFPM were significantly higher in samples collected from hibernated males. The difference in sperm forward progression may be relevant to flagellum maturation and energy stores in the mitochondria during spermatogenesis. ATP accumulation in the axenome during spermatogenesis is required for strong forward movement in aquatic species including fish and amphibians (Bondarenko and Cosson 2019). If non-hibernated toads are kept active year-round, the testes could potentially contain a heterogeneous mixture of sperm cells at varying stages of development. The different levels of maturation in sperm cells, and thus ATP accumulation, might explain the significantly reduced sperm forward progressive motility observed in non-hibernated male toads. If hormonally-induced sperm, obtained from non-hibernated males, has a mixed proportion of mature and immature cells, then a period of hibernation may improve the quality of sperm obtained as they would represent a greater proportion of mature sperm.
The hibernated boreal toads weighed significantly more upon emergence than non-hibernated toads, even though they both originated from the same founder stock, were similar in age, weighed similar amounts when first acquired and both groups were originally housed at NASRF. Moreover, the toads were fed similar prey items on the same schedule at both institutions. Frogs and toads store their energetic supplies in abdominal fat bodies that can comprise several classes of lipids and proteins and can synthesise steroid hormones in vivo (Rastogi et al. 2011). Fat body mass is directly linked to reproductive success and there are indications that, in both sexes, fat bodies are necessary to support normal gonadal activity (Rastogi et al. 2011). In males for example, removal of fat bodies appear to cause degeneration of primary spermatocytes and testicular atrophy (Rastogi et al. 2005). Unfortunately, we cannot eliminate that the lack of comparable amounts of fat reserves, and by default weight, could be a contributory factor to the sub-optimal spermiation response of the non-hibernated males. Studies have shown that higher altitudinal amphibians have a greater accumulation of fat body reserves during the late summer and pre-hibernation months than con-specifics at lower altitudes (Elmberg 1991; Chen et al. 2011). As temperature decreases, the increased mass of fat bodies in high altitude species is believed to be an adaptation to prolonged winters in colder regions. Moreover, temperature extremes may also impact leptin secretion in Bufonids (Chen et al. 2021), with tadpoles exposed to low temperatures accumulating more body mass and size in response to changes in the leptin signaling pathway. The non-hibernated males were held at a constant room temperature over several seasons; thus, they did not experience any environmental cues simulating the need to increase fat body stores. The connection to fat body accumulation and gametogenesis in male boreal toads is unknown and worth further investigation given our findings.
The results presented here illustrate the positive influence of a period of hibernation on the seasonal sperm quantity and quality response of male boreal toads. While we did not see a difference in the percentage of animals producing sperm, it is difficult to separate the impact of exogenous hormone stimulation vs hibernation itself. We propose that if the goal of captive breeding facilities is to produce offspring for reintroduction efforts, a period of hibernation would be beneficial to maximise the chances of breeding and fertilisation. Unfortunately, we don’t yet have a clear understanding of how long the period of hibernation should be (e.g. 1–5 months) to stimulate a normal cycle and production of mature gametes. Is it necessary to replicate the natural hibernation cycle or could shortened periods be equally as effective, yet reduce health risks? If the goal were to collect as much sperm as possible to support genome banking for genetic management, then a period of hibernation may not be advisable. By not hibernating the males, multiple sperm collection efforts (∼8) could be achieved throughout the year with intermittent periods of 2–4 weeks between collections (McDonough et al. 2016), whereas that time is lost if animals are in hibernation. We believe the information presented herein will be valuable for the captive breeding management of other threatened high elevation anuran species that would naturally hibernate in the wild, depending on the goals set aside in the recovery or research programs for the species. Moreover, this work continues to build on knowledge related to the physiological processes of hibernation on gonadal development and sperm maturation in high elevation anuran species.
Data availability
Data are available from the corresponding author upon reasonable request.
Conflicts of interest
The authors have no competing interests to report.
Declaration of funding
This study was supported by an Institute of Museum and Library Services (IMLS) National Leadership Grant (LG-25-09-0064-09) for sustainability of threatened amphibian collections. The funding agency had no role in the project design, data collection, analysis, interpretation of results or construction of the written document. In addition, this manuscript is a contribution of the Mississippi Agricultural and Forestry Experiment Station and the material is based upon work supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch project under the accession number W3173. In addition, the study was partially supported by the USDA-ARS Biophotonics Initiative grant # 58-6402-018.
Author contributions
AJ Kouba – project conception, supervision, funding acquisition, resource procurement, data analysis, interpretation, final manuscript production. CJ Langhorne – project conception, experimental design, data collection, statistical analysis, data interpretation and initial manuscript draft. ST Willard – supervision, resources at MSU, data interpretation, editing. T Smith – supervision, resources at NASRF, animal care, editing. CK Kouba – project supervision, funding acquisition, data analysis, interpretation, curation, final manuscript production.
Acknowledgements
The authors are grateful to Natalie Calatayud for technical support relative to sperm collection in the boreal toads. Moreover, we thank Michael Robinson and Lindsay Bullock for their assistance with the husbandry and care of the MSU boreal toad colony during this study. We also thank NASRF staff and the Association of Zoos and Aquarium (AZA) Amphibian Taxonomic Advisory Group (ATAG) for help with logistics and support. The authors wish to thank the Colorado Division of Parks and Wildlife, in particular, Harry Crockett, Joe Marrinan, and all the staff at the Native Aquatic Species Restoration Facility for accommodating our research.
References
Bondarenko, V, and Cosson, J (2019). Structure and beating behavior of the sperm motility apparatus in aquatic animals. Theriogenology 135, 152–163.| Structure and beating behavior of the sperm motility apparatus in aquatic animals.Crossref | GoogleScholarGoogle Scholar | 31216506PubMed |
Brizzi R, Corti C (2006) Reproductive cycles of the European amphibians: a brief history of studies on the role of exogenous and endogenous factors. In ‘Herpetologia Bonnensis II: Proceedings of the 13th Congress of the Societas Europaea Herpetologica, 27 September–2 October 2005, Bonn, Germany’. (Eds M Vences, J Köhler, T Ziegler, W Böhme) Vol. 27, p. 30. (Societas Europaea Herpetologica, Alexander Koenig Museum: Bonn, Germany)
Browne, RK, Seratt, J, Vance, C, and Kouba, A (2006). Hormonal priming, induction of ovulation and in-vitro fertilization of the endangered Wyoming toad (Bufo baxteri. Reproductive Biology and Endocrinology 4, 34.
| Hormonal priming, induction of ovulation and in-vitro fertilization of the endangered Wyoming toad (Bufo baxteri.Crossref | GoogleScholarGoogle Scholar | 16790071PubMed |
Byrne, PG, and Silla, AJ (2010). Hormonal induction of gamete release, and in-vitro fertilisation, in the critically endangered Southern Corroboree Frog, Pseudophryne corroboree. Reproductive Biology and Endocrinology 8, 144.
| Hormonal induction of gamete release, and in-vitro fertilisation, in the critically endangered Southern Corroboree Frog, Pseudophryne corroboree.Crossref | GoogleScholarGoogle Scholar | 21114857PubMed |
Calatayud, NE, Langhorne, CJ, Mullen, AC, Williams, CL, Smith, T, Bullock, L, Kouba, AJ, and Willard, ST (2015). A hormone priming regimen and hibernation affect oviposition in the boreal toad (Anaxyrus boreas boreas. Theriogenology 84, 600–607.
| A hormone priming regimen and hibernation affect oviposition in the boreal toad (Anaxyrus boreas boreas.Crossref | GoogleScholarGoogle Scholar | 26025241PubMed |
Carey, C (1993). Hypothesis concerning the causes of the disappearance of Boreal Toads from the Mountains of Colorado. Conservation Biology 7, 355–362.
| Hypothesis concerning the causes of the disappearance of Boreal Toads from the Mountains of Colorado.Crossref | GoogleScholarGoogle Scholar |
Chen, W, Zhang, LX, and Lu, X (2011). Higher pre-hibernation energy storage in anurans from cold environments: a case study on a temperate frog Rana chensinensis along a broad latitudinal and altitudinal gradients. Annales Zoologici Fennici 48, 214–220.
| Higher pre-hibernation energy storage in anurans from cold environments: a case study on a temperate frog Rana chensinensis along a broad latitudinal and altitudinal gradients.Crossref | GoogleScholarGoogle Scholar |
Chen, X, Ren, C, Teng, Y, Shen, Y, Wu, M, Xiao, H, and Wang, H (2021). Effects of temperature on growth, development and the leptin signaling pathway of Bufo gargarizans. Journal of Thermal Biology 96, 102822.
| Effects of temperature on growth, development and the leptin signaling pathway of Bufo gargarizans.Crossref | GoogleScholarGoogle Scholar | 33627262PubMed |
Corn, PS (2005). Climate change and amphibians. Animal Biodiversity and Conservation 28, 59–67.
Duellman WE, Trueb L (1994) Introduction to the amphibia. In ‘Biology of amphibians’. (Eds WE Duellman, L Trueb). (John Hopkins University Press Ltd: London, UK/Baltimore, MD, USA)
Elmberg, J (1991). Ovarian cyclicity and fecundity in boreal common frogs Rana temporaria L. along a climatic gradient. Functional Ecology 5, 340–350.
| Ovarian cyclicity and fecundity in boreal common frogs Rana temporaria L. along a climatic gradient.Crossref | GoogleScholarGoogle Scholar |
Hammerson GS (1999) ‘Amphibians and reptiles in Colorado’. 2nd edn. p. 484. (University Press of Colorado and Colorado Division of Wildlife: Denver, CO, USA)
Hartel, T, Sas, I, Pernetta, AP, and Geltsh, IC (2007). The reproductive dynamics of temperate amphibians: a review. North-Western Journal of Zoology 3, 127–145.
Kouba, AJ, delBarco-Trillo, J, Vance, CK, Milam, C, and Carr, M (2012). A comparison of human chorionic gonadotropin and luteinizing hormone releasing hormone on the induction of spermiation and amplexus in the American toad (Anaxyrus americanus. Reproductive Biology and Endocrinology 10, 59.
| A comparison of human chorionic gonadotropin and luteinizing hormone releasing hormone on the induction of spermiation and amplexus in the American toad (Anaxyrus americanus.Crossref | GoogleScholarGoogle Scholar | 22905699PubMed |
Kouba, AJ, and Vance, CK (2009). Applied reproductive technologies and genetic resource banking for amphibian conservation. Reproduction, Fertility and Development 21, 719–737.
| Applied reproductive technologies and genetic resource banking for amphibian conservation.Crossref | GoogleScholarGoogle Scholar |
Langhorne, CJ, Calatayud, NE, Kouba, CK, Willard, ST, Smith, T, Ryan, PL, and Kouba, AJ (2021). Efficacy of hormone stimulation on sperm production in an alpine amphibian (Anaxyrus boreas boreas) and the impact of short-term storage on sperm quality. Zoology 146, 125912.
| Efficacy of hormone stimulation on sperm production in an alpine amphibian (Anaxyrus boreas boreas) and the impact of short-term storage on sperm quality.Crossref | GoogleScholarGoogle Scholar | 33743452PubMed |
Loeffler C (Ed.) (2001) ‘Conservation plan and agreement for the management and recovery of the southern Rocky Mountain population of the boreal toad (Bufo boreas boreas)’. p. 76 + appendices. (Boreal Toad Recovery Team, Colorado Division of Wildlife: Denver, CO, USA)
Lofts, B (1964). Seasonal changes in the functional activity of the interstitial and spermatogenetic tissues of the green frog, Rana esculenta. General and Comparative Endocrinology 4, 550–562.
| Seasonal changes in the functional activity of the interstitial and spermatogenetic tissues of the green frog, Rana esculenta.Crossref | GoogleScholarGoogle Scholar | 14217824PubMed |
Mann, RM, Hyne, RV, and Choung, CB (2010). Hormonal induction of spermiation, courting behavior and spawning in the Southern bell frog, Litoria raniformis. Zoo Biology 29, 774–782.
| Hormonal induction of spermiation, courting behavior and spawning in the Southern bell frog, Litoria raniformis.Crossref | GoogleScholarGoogle Scholar | 20549714PubMed |
McDonough, CE, Martin, MW, Vance, CK, Cole, JA, and Kouba, AJ (2016). Frequency of exogenous hormone therapy impacts spermiation in male Fowler’s toad (Bufo fowleri. Reproduction, Fertility and Development 28, 995–1003.
| Frequency of exogenous hormone therapy impacts spermiation in male Fowler’s toad (Bufo fowleri.Crossref | GoogleScholarGoogle Scholar |
Pinder AW, Storey KB, Uitsch GR (1992) Estivation and hibernation. In ‘Environmental physiology of the amphibians’. (Eds ME Feder, WW Burggren) pp. 250–274. (University of Chicago Press: Chicago, IL, USA)
Rastogi RK, Iela L, di Meglio M, Di Fiore MM, D’Aniello B, Pinelli C, et al. (2005) Hormonal regulation of reproductive cycles in amhibians. In ‘Amphibian biology. Volume 6: Endocrinology’. (Ed H Heatwole) pp. 2045–2177. (Surrey Beatty and Sons: Chipping Norton, NSW, Australia)
Rastogi RK, Pinelli C, Polese G, D’Aniello B, Chieffi-Baccari G (2011) Hormones and reproductive cycles in anuran amphibians. In ‘Hormones and reproduction of vertebrates. Volume 2: amphibians’. (Eds DO Norris, KH Lopez) pp. 171–185. (Academic Press: London, UK)
Roth, TL, Szymanski, DC, and Keyster, ED (2010). Effects of age, weight, hormones, and hibernation on breeding success in boreal toads (Bufo boreas boreas. Theriogenology 73, 501–511.
| Effects of age, weight, hormones, and hibernation on breeding success in boreal toads (Bufo boreas boreas.Crossref | GoogleScholarGoogle Scholar | 20004010PubMed |
Santana, FE, Swaisgood, RR, Lemm, JM, Fisher, RN, and Clark, RW (2015). Chilled frogs are hot: hibernation and reproduction of the Endangered mountain yellow-legged frog Rana muscosa. Endangered Species Research 27, 43–51.
| Chilled frogs are hot: hibernation and reproduction of the Endangered mountain yellow-legged frog Rana muscosa.Crossref | GoogleScholarGoogle Scholar |
Silla, AJ, McFadden, MS, and Byrne, PG (2020). Hormone-induced sperm-release in the critically endangered Booroolong frog (Litoria booroolongensis): effects of gonadotropin-releasing hormone and human chorionic gonadotropin. Conservation Physiology 7, coy080.
| Hormone-induced sperm-release in the critically endangered Booroolong frog (Litoria booroolongensis): effects of gonadotropin-releasing hormone and human chorionic gonadotropin.Crossref | GoogleScholarGoogle Scholar |
Stevenson, LA, Roznik, EA, Greenspan, SE, Alford, RA, and Pike, DA (2020). Host thermoregulatory constraints predict growth of an amphibian chytrid pathogen (Batrachochytrium dendrobatidis. Journal of Thermal Biology 87, 102472.
| Host thermoregulatory constraints predict growth of an amphibian chytrid pathogen (Batrachochytrium dendrobatidis.Crossref | GoogleScholarGoogle Scholar | 31999604PubMed |
Storey KB (Ed.) (2000) Turning down the fires of life: metabolic regulation of hibernation and estivation. In ‘Molecular mechanisms of metabolic arrest’. pp. 1–21. (BIOS Scientific Publishers: Oxford, UK)
Storey, KB, and Storey, JM (1992). Natural freeze tolerance in ectothermic vertebrates. Annual Review of Physiology 54, 619–637.
| Natural freeze tolerance in ectothermic vertebrates.Crossref | GoogleScholarGoogle Scholar | 1562185PubMed |
Tsai P-S (2011) Neuroendocrine control of reproduction in amphibians. In ‘Hormones and reproduction of vertebrates. Volume 2: amphibians’. (Eds DO Norris, KH Lopez) pp. 21–37. (Academic Press: London, UK)
Wright KM, Whitaker BR (2001) Reproduction. In ‘Amphibian medicine and captive husbandry’. pp. 285–299. (Kreiger Publishing Company: Malabar, FL, USA)