Relationships of phosphorus fractions to organic carbon content in surface soils in mature subtropical forests, Dinghushan, China
Enqing Hou A B , Chengrong Chen B C , Dazhi Wen A C and Xian Liu BA Key Laboratory of Vegetation Restoration and Management of Degraded Ecosystems, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou 510650, China.
B Environmental Futures Centre, Griffith School of Environment, Griffith University, Nathan, Qld 4111, Australia.
C Corresponding authors. Email: c.chen@griffith.edu.au (CC); dzwen@scbg.ac.cn (DW)
Soil Research 52(1) 55-63 https://doi.org/10.1071/SR13204
Submitted: 10 July 2013 Accepted: 28 September 2013 Published: 5 February 2014
Abstract
Exploring the relationship between the accumulation of soil organic carbon (C) and the form and availability of soil phosphorus (P) is important for improved understanding of soil P availability and its regulation of C storage in forest ecosystems. Here, we investigated the relationships among soil organic C, sequentially extracted P fractions and P sorption index in 32 surface soils (0–0.15 m depth) across eight mature subtropical forests (80–400 years) in Dinghushan, China. Results showed that soil organic P (Po) accounted for 40–63% (mean 54%) of soil total P. Soil organic C was significantly positively correlated with both the content and the percentage of soluble inorganic P (Pi), Al-Po and Fe-Po fractions and the content of the Al-Pi fraction. The content of soil total Po increased significantly with soil organic C, whereas the percentage of soil total Po tended to increase with soil organic C only when soil organic C was low (<30 Mg/ha) but was relatively stable when soil organic C was high (≥30 Mg/ha). Moreover, soil organic C was highly correlated with P sorption index. Our results suggest that accumulation of organic C may increase, rather than decrease, the availability of P in surface soil in mature subtropical forests.
Additional keywords: organic carbon, organic phosphorus, phosphorus sorption index, soil phosphorus fractionation, subtropical forest.
Introduction
Phosphorus (P) is one of the important nutrients determining the function and primary productivity of terrestrial ecosystems (Aerts and Chapin 1999; Elser et al. 2007). Despite the typically large P stock present in soils (Smil 2000; Imai et al. 2010), soil P supply is rarely adequate in meeting the P demands of plants in terrestrial ecosystems (Aerts and Chapin 1999; Elser et al. 2007). This is largely due to multiple forms of P existing in the soils, which differ in their availability for plant uptake across time scales (Hedley et al. 1982; Chen et al. 2003). Soil P fractions determined by sequential fractionation methods can provide useful information on the availability and dynamics of soil P and its responses to various environmental or anthropogenic factors in terrestrial ecosystems, and thus they have been widely used to date (Chang and Jackson 1957; Hedley et al. 1982; Yang and Post 2011).
The important role of soil organic matter in maintaining soil availability of nutrients (including P) in terrestrial ecosystems has long been recognised (Tiessen et al. 1994; Johnson et al. 2003). Soil organic matter provides an important source of P that can be utilised by plants and soil microbes following mineralisation (Tiessen et al. 1994; Kirkby et al. 2011), as well as energy reserves and carbon (C) substrates used in microbial activities such as the production of enzymes involved in P mineralisation (Allison and Vitousek 2005; Allison et al. 2007). It can also maintain soil P availability by providing binding sites (Kang et al. 2009). However, some studies have suggested that an accumulation of soil organic C (or organic matter) may decrease soil P availability due to the inclusion of P in organic form (Huang et al. 2012; Schlesinger et al. 1998). For example, Schlesinger et al. (1998) proposed that P was limiting plant growth in soil at an early stage of development (110 years) on Rakata Island, Indonesia, perhaps as a result of the observed rapid accumulation of organic P. Wang et al. (2006) found that soil organic C concentration was positively related to the concentrations of soil soluble organic-P fractions, whereas it was negatively related to the concentrations of soil soluble inorganic-P fractions in depressional and riparian freshwater wetlands in North-east China. Further studies on the relationships between soil organic C and P fractions are needed to clarify the relationship between soil organic C accumulation and soil P availability, which has important implications in understanding the limitation P places on the storage of C in terrestrial ecosystems (Wang et al. 2010; Kirkby et al. 2011).
China has 0.97 million km2 of subtropical and tropical forests, representing 62% of its total forested area and 72% of its total forest net primary productivity (Zhao et al. 2004). A long-term monitoring study reported that soil organic C stock in the top 0.2 m soil layer had accumulated at an average rate of 0.61 Mg C/ha.year in an old-growth (>400 years) subtropical forest in Dinghushan, China, between 1979 and 2003 (Zhou et al. 2006). Recently, Huang et al. (2012) proposed that the continuous accumulation of soil organic C might have occluded P in its organic form, thereby decreasing soil P availability in forest ecosystems in this area, as the site with a relatively higher soil organic matter pool possessed a relatively lower soil soluble P pool.
To test this hypothesis, we sampled 32 surface mineral soils (0–0.15 m depth) from eight mature subtropical forests in Dinghushan that varied in topographic conditions, plant communities and land use history. Soil organic C content, P fractions extracted by a sequential extraction procedure and P sorption index were determined. Surface soil was investigated, as it is usually the major source of nutrients for plant uptake. It also features the highest quantities of organic C in soil profiles, and consequently, the effects of organic C accumulation on soil P forms and availability was expected to be the most significant. We hypothesised that the amounts of soil organic-P fractions might be positively related to soil organic C content, whereas the amounts of soil inorganic-P fractions might be negatively related to it. General characteristics of soil P fractions from the studied forests were also explored to advance our knowledge of soil P fractions in strongly weathered and acidified subtropical forest soils.
Materials and methods
Site description
The study was conducted in Dinghushan Biosphere Reserve, in the middle of Guangdong province in China (112°31′–112°34′E, 23°09′–23°12′N). The Reserve covers an area of 1155 ha, and has a typical subtropical monsoon climate. Mean annual temperature in the Reserve is 21°C, and mean annual precipitation is ~1900 mm. Nearly 80% of the precipitation falls in the wet season (April–September) and 20% in the dry season (October–March). Elevation ranges from 10 to 1000 m above sea level. Soil thickness mostly varies from 0.3 to 0.9 m. Surface soils are Ferralsols (FAO-UNESCO 1974), and have developed on sandstone during the early Holocene (Shen et al. 2001).
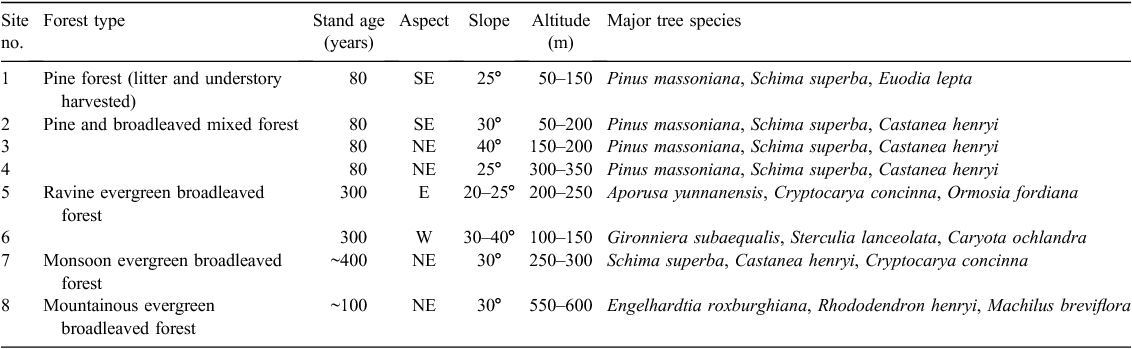
Basic information of the eight selected forest sites is summarised in Table 1. The eight forest sites represent the major vegetation types and topographical conditions in Dinghushan, and also represent five of the major vegetation types as well as the major soil type and geological conditions of natural subtropical forests in south China. All forests are mature-growth (≥80 years) and have been well protected since their establishment, except for the forest at site 1, which had been disturbed by the harvest of understory vegetation and litter by local residents between the 1950s and 1990s (Mo et al. 1995).
|
Sampling and sample preparation
Field sampling was conducted in October 2010. At each forest plot, four subplots (20 m by 20 m) were randomly set up with a distance of at least 10 m between them. At each subplot, three sampling points were randomly selected with the constraints that they were 1–2 m away from the nearest tree (diameter at breast height ≥0.05 m) and at least 5 m from its nearest neighbour. After forest floor materials were removed, a soil profile was excavated in the bare soil area. Mineral soil at the 0–0.15 m depth was sampled by three successive cutting rings (height 0.05 m, volume 100 cm3) from top to bottom (0.05 m depth per cutting ring). Nine soils (3 cutting rings per area × 3 areas) of each subplot were bulked together as one composite soil sample. In total, 32 composite soil samples were collected. By using a cutting ring to sample soil, the bulk density at the time of soil sampling was also determined. Soil C and nutrient pools investigated in this way should be more accurate than if separate samplings of soil for bulk density and soil C and nutrient concentration measurements were performed. Large roots and stones were separated from the sample and weighed during sieving (4-mm mesh) in the laboratory. Soil was air-dried for 2 weeks, and then ground and passed through 0.15-mm mesh for soil property determination.
Analytical methods
Total C and total N concentrations were determined using an Isoprime isotope ratio mass spectrometer with an elemental analyser (Isoprime-Euro EA 3000; Eurovector, Milan, Italy). Since surface soils are strongly acidic in the study area (pH 3.6–4.2; Liu et al. 2010a), the measured soil total C should be mainly in the organic form and, thus, is used to represent soil organic C throughout the text. Total P concentration was measured using a nitric acid–perchloric acid digestion, followed by a colourimetric analysis (Murphy and Riley 1962) using a UV-Vis spectrophotometer (UV1800; Shimadzu, Kyoto, Japan).
As suggested by Wu et al. (2000), NH4F is preferable to NaHCO3 in extracting readily available P fractions in acidic soils. A modified procedure of McDowell and Condron (2000) that included an NH4F extraction procedure (3.0 g air-dried soil) was thus adopted in the present study to extract soil P fractions. The protocol was as follows:
-
Soluble P [NH4Cl]: 30 mL 1.0 m NH4Cl, shaken for 30 min, centrifuged (relative centrifugal force, RCF, 10 000, 10 min), supernatant filtered (<0.45 mm);
-
Aluminium (Al)-associated P [NH4F]: 30 mL 0.1 m NH4F, shaken for 16 h, centrifuged (RCF 10 000, 10 min), supernatant filtered (<0.45 mm);
-
Wash: 30 mL distilled H2O, shaken 30 min, centrifuged (RCF 10 000, 10 min), supernatant discarded;
-
Iron (Fe)-associated P [NaOH]: 30 mL 0.1 m NaOH, shaken for 16 h, centrifuged (RCF 10 000, 10 min), supernatant filtered (<0.45 mm);
-
Wash: 30 mL distilled H2O, shaken 30 min, centrifuged (RCF 10 000, 10 min), supernatant discarded;
-
Calcium (Ca)-associated P [H2SO4]: 30 mL 0.5 m H2SO4 (H2SO4 I), shaken for 16 h, centrifuged (RCF 10 000, 10 min), supernatant filtered (<0.45 mm);
-
Wash: 30 mL distilled H2O, shaken 30 min, centrifuged (RCF 10 000, 10 min), supernatant discarded;
-
Residual P: residue was equally divided into two subsamples, one subsample directly extracted with 15 mL 0.5 m H2SO4 (H2SO4 II), the other subsample extracted with 15 mL 0.5 m H2SO4 after being ashed at 550°C for 2 h (H2SO4 III); the extraction procedure was the same as for step 6.
Inorganic P concentration in the extracts of 0.1 m NH4F (Al-Pi), 0.1 m NaOH (Fe-Pi), 0.5 m H2SO4 I (Ca-Pi), and 0.5 m H2SO4 II and III was directly measured using the molybdate blue method (Murphy and Riley 1962). Inorganic P concentration in the 1.0 m NH4Cl extract (soluble Pi) was below the detection limit by the molybdate blue method, and therefore reanalysed using the malachite green method (Ohno and Zibilske 1991). Organic P in the 0.1 m NH4F (Al-Po) and 0.1 m NaOH (Fe-Po) extracts was calculated as the difference between total P determined after persulfate digestion (Ormaza-González and Statham 1996) and inorganic P. Residual organic P (residual Po) was calculated as the difference of inorganic P between the H2SO4 II and III extracts. Residual inorganic P (residual Pi) was calculated as the difference between total P and the sum of soluble Pi, Al-Pi and -Po, Fe-Pi and -Po, Ca-Pi, and residual Po. Total inorganic P (total Pi) was calculated as the sum of soluble Pi, Al-Pi, Fe-Pi, Ca-Pi and residual Pi, whereas total organic P (total Po) was calculated as the sum of Al-Po, Fe-Po and residual Po. Soil organic C, total N, and total P and P fractions are all presented on an area basis (Mg/ha or kg/ha), and soil P fractions are also presented as a percentage of soil total P content.
Phosphorus sorption index was determined following the procedure of Kovar and Pierzynski (2009). The amount of P sorbed, x (mg P/kg), from an addition of 1.5 g P/kg soil (as KH2PO4 in 0.01 m CaCl2), was determined after shaking for 18 h at a soil weight : solution volume ratio of 1 : 20. The P sorption index (L/kg) was calculated as the quotient x/log C, where C is the solution concentration in the filtrate (mg/L).
Statistical analyses
The Pearson correlation method (P < 0.05, Bonferroni-corrected) was adopted to investigate the relationships among the contents of P fractions and P sorption index in the soils using SPSS 16.0 (SPSS Inc., Chicago). Relationships of organic C content with the contents and percentages of P fractions and P sorption index in the soils were investigated visually by plotting on an x–y scatter by using SigmaPlot 11.0 (Systat Software Inc., San Jose, CA).
Results
Basic soil properties
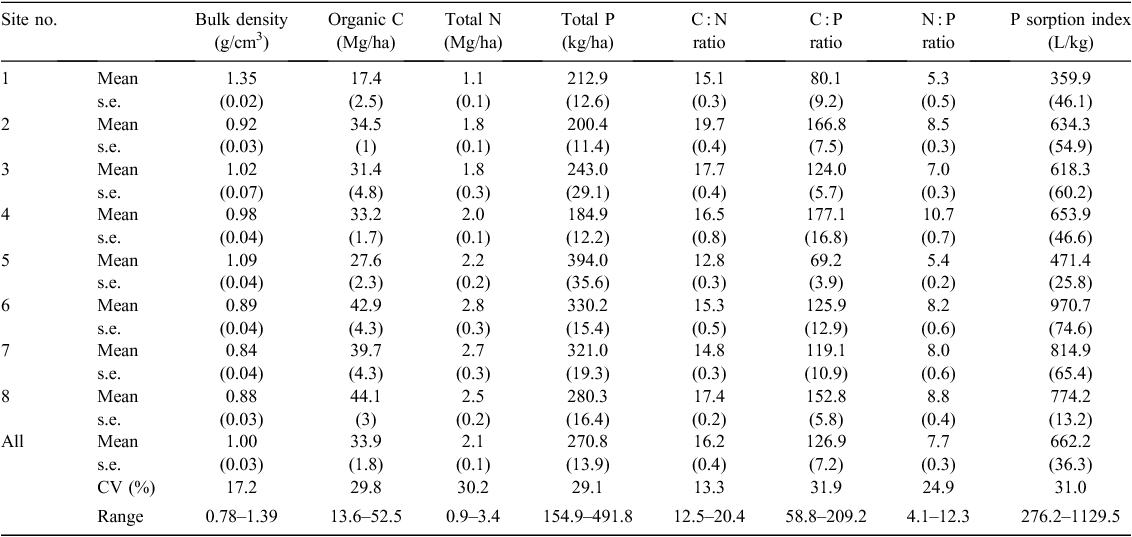
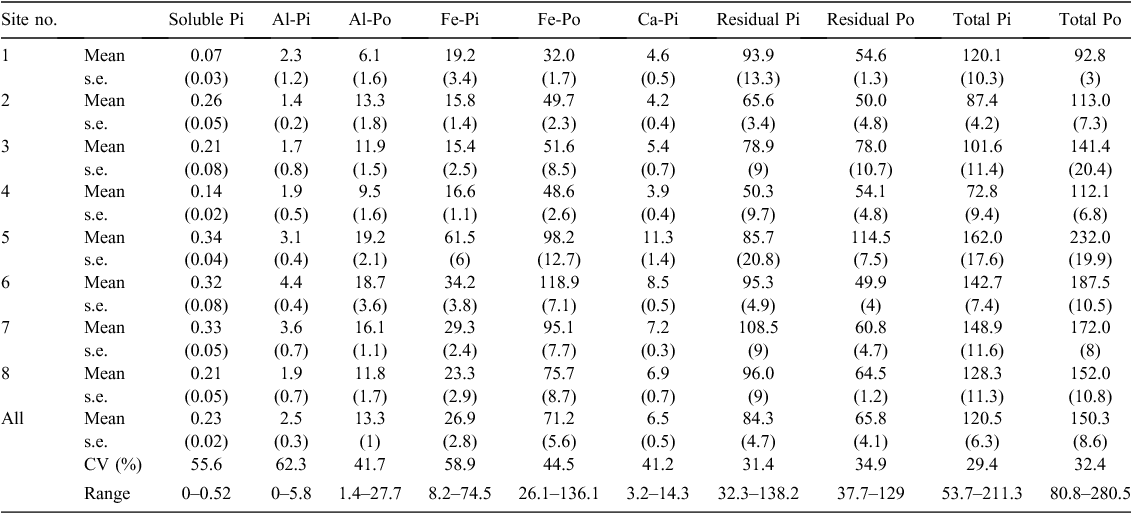
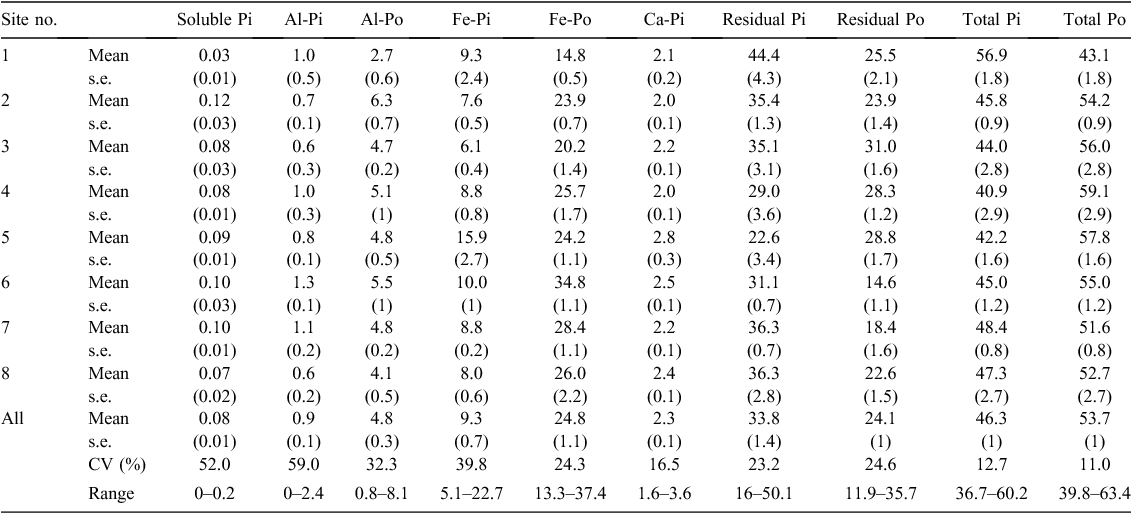
Soil bulk density varied between 0.78 and 1.39 (mean 1.00) g/cm3 across all 32 soils (Table 2). Soil organic C content was 13.6–52.5 (mean 33.9) Mg/ha and soil total N content 0.9–3.4 (mean 2.1) Mg/ha (Table 2). Soil organic C and total N contents were both markedly lower at site 1 than at all other sites (Table 2). Soil total P content ranged between 155 and 492 (mean 271) kg/ha, and was highest at sites 5–7 (321–394 kg/ha), intermediate at site 8 (281 kg/ha), and lowest at sites 1–4 (185–243 kg/ha) (Table 2). Phosphorus sorption index ranged between 276 and 1130 (mean 662) L/kg. Soil C : N, C : P and N : P mass ratios ranged from 12.5 to 20.4 (mean 16.2), from 58.8 to 209.2 (mean 126.9) and from 4.1 to 12.3 (mean 7.7), respectively.
|
Soil P fractions
Similar to soil total P content, the contents of all soil P fractions were generally higher at sites 5–7 than at other sites (Table 3). Soil organic P accounted for 40–64% (mean 54%) of soil total P (Table 4). Of the soil organic P, about half was present in the residues, represented by soil residual Po (mean 24% in soil total P, range 12–36%) (Table 4). Among the extractable organic P fractions, Fe-Po was the dominant fraction, accounting for 13–37% (mean 25%) of soil total P, whereas Al-Po accounted for only 0.8–8.1% (mean 4.8%) of soil total P (Table 4). The averaged percentages of soil soluble Pi, Al-Pi, Ca-Pi and Al-Po fractions were 0.08%, 0.9%, 2.3% and 4.8%, respectively (Table 4). The coefficient of variance of the percentage was smaller than that of the content for all soil P fractions (Tables 3 and 4).
|
|
Relationships between soil P fractions, P sorption index and soil organic C
The contents of soil Ca-Pi, Fe-Pi and Fe-Po fractions were significantly correlated with each other (r = 0.60–0.76, P < 0.05; Table 5). Soil residual Po content was significantly correlated with soil Fe-Pi and Ca-Pi fraction contents (r = 0.65–0.68, P < 0.05; Table 5). The contents of all soil P fractions except for soil soluble Pi, Al-Pi and residual Pi fractions were significantly correlated with soil total Po and total P contents (r = 0.50–0.95, P < 0.05; Table 5). All of these correlations were positive.
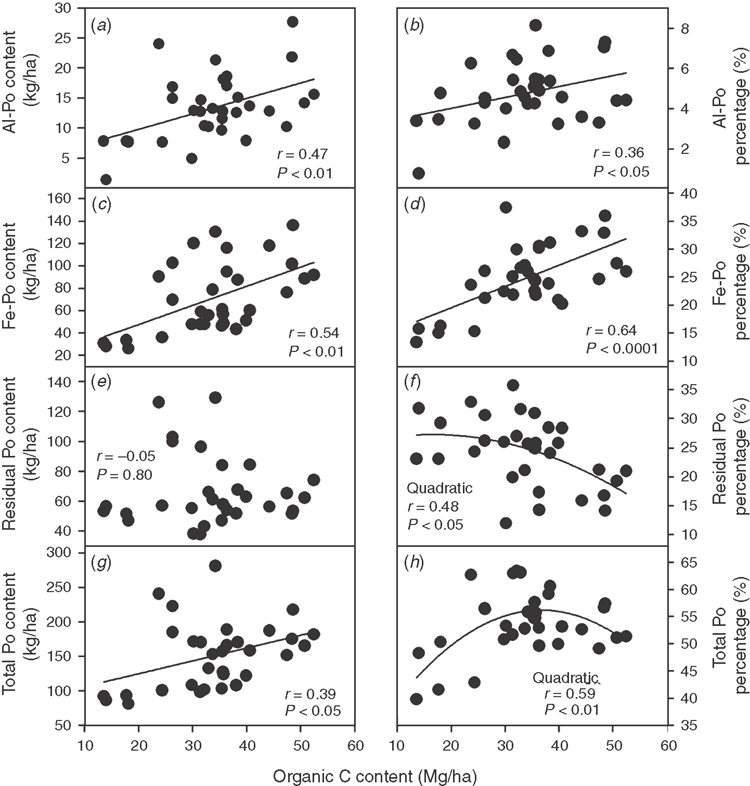
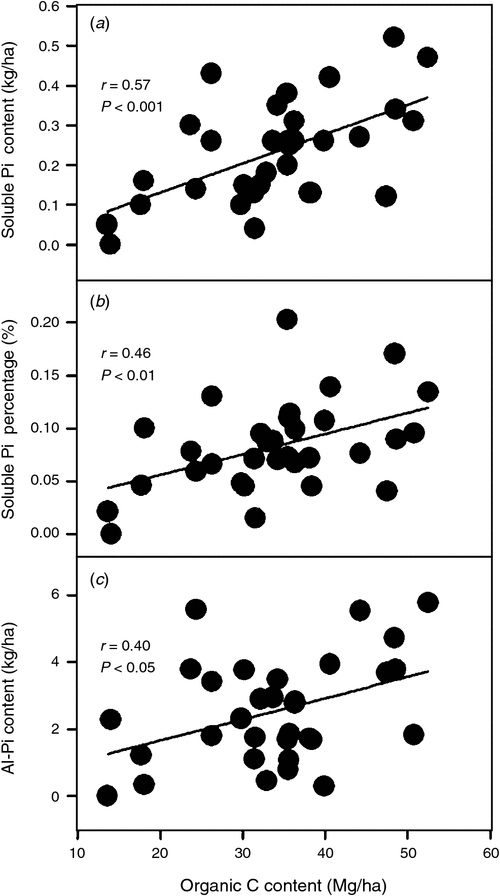
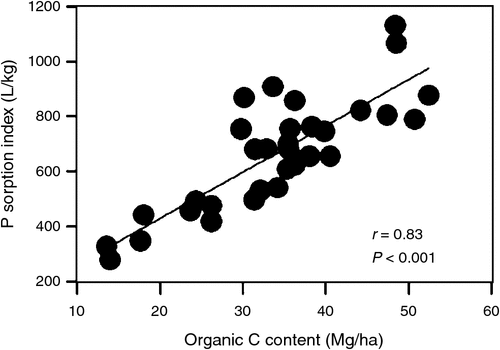
Soil organic C content was significantly positively correlated with both the contents and percentages of soil Al-Po and Fe-Po fractions (r = 0.36–0.64, P < 0.05; Fig. 1a–d). By contrast, the content of soil residual Po fraction did not correlate with soil organic C content (r = –0.05, P = 0.80; Fig. 1e) and the percentage of soil residual Po fraction tended to decrease with an increase in soil organic C content when soil organic C content was >30 Mg/ha (quadratic curve, r = 0.48, P < 0.05; Fig. 1f). The content of soil total Po fraction significantly increased with soil organic C content (r = 0.39, P < 0.05; Fig. 1g), while the percentage of soil total Po fraction tended to increase with soil organic C content only when soil organic C content was <30 Mg/ha (quadratic curve, r = 0.59, P < 0.01; Fig. 1h). For inorganic P fractions, both the content and the percentage of the soil soluble Pi fraction and the content of soil Al-Pi fraction were significantly and positively correlated with soil organic C content (r = 0.46–0.57, P < 0.05; Fig. 2). Neither the content nor the percentage of other inorganic P fractions was significantly correlated with soil organic C content (data not shown). Moreover, P sorption index was highly correlated with soil organic C content (r = 0.83, P < 0.001; Fig. 3).
|
|
Discussion
General characteristics of soil properties and soil P fractions
The markedly lower soil organic C and total N contents at site 1 than at the other sites could be mainly explained by the long-term litter and understory harvest at this site, which had removed a substantial proportion of litterfall from the soil surface (Mo et al. 1995). However, the impact of litterfall harvest on soil total P content seems small (Table 2). Although stand age at sites 5–7 was much greater than at the other sites (300–400 v. 80–100 years), soil organic C content did not reveal much difference between them (Table 2). This result seems inconsistent with the study by Zhou et al. (2006), which reported that surface-soil organic C stock accumulated rapidly at site 5 between 1979 and 2003. However, it is possible that soil organic C content at all study sites had accumulated at a high rate, possibly due to the continuously high atmospheric N deposition (~40 kg N/ha.year) (Mo et al. 2008; Huang et al. 2012) rather than to the stand development. Gleixner et al. (2009) reviewed soil C stock changes with stand development in many biomes and found that chronosequence estimates of soil organic C accumulation (–4.5 to 17.6 g C/m2.year) were generally much lower than the estimates based on repeated sampling (32–165 g C/m2.year).
As expected, soil organic P accounted for more than half of the soil total P in the study forests (Table 4). Half of the organic P existed as soil Al-Po and Fe-Po fractions, both of which are potentially available for plant uptake after mineralisation (Chen et al. 2002; Hamer et al. 2013). Inorganic P fractions associated with Al (Al-Pi) and Fe (Fe-Pi) were only one-tenth to half of the corresponding organic P fractions (Al-Po and Fe-Po), suggesting that mineralisation of soil organic P might be a limiting process of soil P cycling in the study forests (Huang et al. 2013). As expected, the percentage of soil Ca-Pi fraction (1.6–3.6%) was low, because surface soils in the study area have developed over thousands of years (Shen et al. 2001) and are strongly acidified (Liu et al. 2010a). High annual temperature (21°C) and rainfall (1900 mm) in the study area promote soil weathering. It is notable that more than half of the soil total P in the study soils existed within the soil residual P (Pi + Po) fractions, which are bound with Al and Fe oxides and are either very weakly available or unavailable for plant uptake (Chang and Jackson 1957; Chen et al. 2003; Wang et al. 2006). This result suggests that the large proportion of soil P occluded with Al and Fe oxides may be an important factor in the low P availability and P limitation on the growth of plants and microbes in the study area (Hou et al. 2012; Liu et al. 2012), in addition to the high atmospheric N deposition level and the low-P parent rock (Hou et al. 2012).
Relationship between soil organic C content and soil P fractions
As expected, increases in both the absolute and relative amounts of soil Al-Po and Fe-Po fractions with soil organic C content were found in this study (Fig. 1a–d), which is consistent with previous studies on the relationships between soil organic C and extractable organic P fractions in forest ecosystems (Schlesinger et al. 1998; Dieter et al. 2010). In contrast to the extractable organic P fractions, the content of soil residual Po fraction did not correlate with soil organic C content (Fig. 1e). The result was consistent with the study by Brandtberg et al. (2010), which found that the concentration of soil non-occluded Po fractions (NaHCO3-Po and first NaOH-Po) was significantly and positively correlated with soil organic C concentration, whereas the concentration of soil occluded P fraction (second NaOH-Po) was uncorrelated with organic C in surface forest soils in New Zealand. A negative relationship between soil organic C content and occluded Po percentage (Fig. 1f) corresponded to the positive relationships between soil organic C content and the percentages of soil Al-Po and Fe-Po fractions (Fig. 1b, d).
Soil total Po percentage increased with soil organic C content only when soil organic C content was low (<30 Mg/ha in the 0–0.15 m soil) but was relatively stable when soil organic C content was high (≥30 Mg/ha) (Fig. 1h). This result fits well with the notion that the soil organic P pool would increase with stand development or soil organic C accumulation and finally reach a steady-state characterised by an equilibrium between the build-up and decomposition of organic P in forest ecosystems (De Schrijver et al. 2012). Increases in the activity of enzymes participating in soil organic P mineralisation in line with increases in soil organic C content have been reported by many studies (Allison and Vitousek 2005; Sinsabaugh et al. 2008). Increases in soil organic C content can provide more energy and C-structure materials for microbes, leading to elevated production of phosphatase enzymes involved in the mineralisation of soil organic P (Allison and Vitousek 2005; Sinsabaugh and Moorhead 1994). Soil organic matter can also provide binding sites for phosphatases to protect them from degradation (Tabatabai et al. 2002).
Our test of the relationships between soil organic C content and soil inorganic P fractions revealed that soil soluble and labile Pi contents would increase with soil organic C accumulation (Fig. 2). These relationships may be partly because that labile P is largely derived from the decomposition of organic matter (Tiessen et al. 1994; Johnson et al. 2003). Soil organic matter can also absorb labile Pi via poorly crystalline or non-crystalline Al and Fe (Kang et al. 2009), as supported by the strong correlation between soil organic C content and P sorption index in our study (Fig. 3). Similarly, Johnson et al. (2003) found strong relationships between soil organic C and resin-P contents (r = 0.73–0.96, P < 0.05) at sites without a history of recent agriculture. Frizano et al. (2002) studied the change in soil organic C and soil P fractions during the early soil succession stage (1–55 years) on landslide scars in the Luquillo mountains, Puerto Rico; they also found a significant relationship between soil organic C and resin-P contents (r = 0.82, P < 0.01) and a significant relationship between soil organic C and labile P (resin-Pi + HCO3-Pi + HCO3-Po; defined by Hedley method) contents (r = 0.87, P < 0.01).
Our integrated results of the relationships between soil organic C content and soil P fractions suggest that P availability in surface soils may increase with soil organic C accumulation in mature subtropical forests. The results did not support the hypothesis that continuous accumulation of soil organic C could essentially lead to binding of the P pool, thus reducing soil P availability and leading to P limitation on plant growth (Schlesinger et al. 1998; Huang et al. 2012). Soil extractable Po (Al-Po and Fe-Po) contents might linearly increase with the accumulation of soil organic C, but the increased soil extractable Po was not transferred from soil extractable Pi (soluble Pi, Al-Pi and Fe-Pi) or residual Pi or Po fraction in the surface soil. Instead, it might be, at least partly, transferred from subsoil by root-uplifting of P. Plants can assimilate P and other mineral nutrients in both topsoil and subsoil by roots and return C, P and other nutrients onto the topsoil through litterfall (Jobbágy and Jackson 2001). In the long term, P can continuously accumulate in the topsoil with forest development (Jobbágy and Jackson 2001, 2004). The observed trend of increase in soil total P content with stand age (Tables 1 and 2) supported this hypothesis. We did not investigate organic C content and P fractions in the subsoil. However, a previous study on soil total P concentration in soil profiles at sites 1, 2 and 5 in the study area (Liu et al. 2010b) supports this hypothesis. Soil total P concentration tends to increase, or not change, with soil depth at sites 1 and 2 (stand age 80 years), while markedly decreasing with soil depth at site 5 (stand age 400 years). Moreover, the increase in soil organic C content can enhance soil P sorption capacity (Fig. 3); therefore, the retention capacity of P input from litterfall, the atmosphere or rock weathering may be relatively higher in soil with relatively higher organic C content (Frizano et al. 2002).
Conclusion
It has been suggested that organic C had continuously accumulated at a high rate in the surface soil in mature subtropical forests in south China during the past three decades (Zhou et al. 2006). Our results suggest that this accumulation of soil organic C might have enhanced, rather than reduced, P availability in surface soil in mature subtropical forests in China. Further study is warranted on the whole-ecosystem cycling of P and its relationship with the cycling and storage of C in natural subtropical forests in south China.
Acknowledgements
We thank two anonymous reviewers for their helpful comments on previous draft of the manuscript, Marijke Heenan for her experimental preparation and language improvement work, and Xuejin Wang and Yujin Zhang for field sampling assistance. This study was supported by Strategic Priority Research Program ‘Climate Change: Carbon Budget and Relevant Issues’ of the Chinese Academy of Sciences (No. XDA05050205), the Australian Research Council (FT0990547), National Natural Science Foundation of China (No. 31070409) and South China Botanical Garden Fund (201307).
References
Aerts R, Chapin FS (1999) The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Advances in Ecological Research 30, 1–67.| The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns.Crossref | GoogleScholarGoogle Scholar |
Allison SD, Vitousek PM (2005) Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biology & Biochemistry 37, 937–944.
| Responses of extracellular enzymes to simple and complex nutrient inputs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhvVSltb0%3D&md5=d3390c9338755165c9f0a43f652967d5CAS |
Allison VJ, Condron LM, Peltzer DA, Richardson SJ, Turner BL (2007) Changes in enzyme activities and soil microbial community composition along carbon and nutrient gradients at the Franz Josef chronosequence, New Zealand. Soil Biology & Biochemistry 39, 1770–1781.
| Changes in enzyme activities and soil microbial community composition along carbon and nutrient gradients at the Franz Josef chronosequence, New Zealand.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXkslOis7o%3D&md5=7eee8a8d2c5e9fcf8860bc2e3fd1c902CAS |
Brandtberg PO, Davis MR, Clinton PW, Condron LM, Allen RB (2010) Forms of soil phosphorus affected by stand development of mountain beech (Nothofagus) forests in New Zealand. Geoderma 157, 228–234.
| Forms of soil phosphorus affected by stand development of mountain beech (Nothofagus) forests in New Zealand.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXntVOqtrY%3D&md5=bfb07bca2cae30e2cd7a2c68d2528827CAS |
Chang SC, Jackson ML (1957) Fractionation of soil phosphorus. Soil Science 84, 133–144.
| Fractionation of soil phosphorus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG1cXislSrtQ%3D%3D&md5=538eb189e4bcf94950cde0936ec2cd53CAS |
Chen C, Condron L, Davis M, Sherlock R (2002) Phosphorus dynamics in the rhizosphere of perennial ryegrass (Lolium perenne L.) and radiata pine (Pinus radiata D. Don.). Soil Biology & Biochemistry 34, 487–499.
| Phosphorus dynamics in the rhizosphere of perennial ryegrass (Lolium perenne L.) and radiata pine (Pinus radiata D. Don.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xis1eiu7s%3D&md5=afbf2b9cc1b06bd7882e0e4ce1d5718eCAS |
Chen CR, Sinaj S, Condron LM, Frossard E, Sherlock RR, Davis MR (2003) Characterization of phosphorus availability in selected New Zealand grassland soils. Nutrient Cycling in Agroecosystems 65, 89–100.
| Characterization of phosphorus availability in selected New Zealand grassland soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmvFY%3D&md5=4f0d3453351d5ba4369e18fba7ef5c7fCAS |
De Schrijver A, Vesterdal L, Hansen K, De Frenne P, Augusto L, Achat DL, Staelens J, Baeten L, De Keersmaeker L, De Neve S, Verheyen K (2012) Four decades of post-agricultural forest development have caused major redistributions of soil phosphorus fractions. Oecologia 169, 221–234.
| Four decades of post-agricultural forest development have caused major redistributions of soil phosphorus fractions.Crossref | GoogleScholarGoogle Scholar | 22120703PubMed |
Dieter D, Elsenbeer H, Turner BL (2010) Phosphorus fractionation in lowland tropical rainforest soils in central Panama. Catena 82, 118–125.
| Phosphorus fractionation in lowland tropical rainforest soils in central Panama.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXoslynt74%3D&md5=343b91c02a62573d18b59b52896ee4d9CAS |
Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters 10, 1135–1142.
| Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems.Crossref | GoogleScholarGoogle Scholar | 17922835PubMed |
FAO-UNESCO (1974) ‘FAO-UNESCO Soil map of the world. Vol. 1. Legend.’ (UNESCO: Paris)
Frizano J, Johnson AH, Vann DR, Scatena FN (2002) Soil phosphorus fractionation during forest development on landslide scars in the Luquillo Mountains, Puerto Rico. Biotropica 34, 17–26.
Gleixner G, Tefs C, Jordan A, Hammer M, Wirth C, Nueske A, Telz A, Schmidt UE, Glatzel S (2009) Soil carbon accumulation in old-growth forests. In ‘Old-growth forests’. Vol. 207. (Eds C Wirth, G Gleixner, M Heimann) pp. 231–266. (Springer: Berlin-Heidelberg)
Hamer U, Potthast K, Burneo JI, Makeschin F (2013) Nutrient stocks and phosphorus fractions in mountain soils of Southern Ecuador after conversion of forest to pasture. Biogeochemistry 112, 495–510.
| Nutrient stocks and phosphorus fractions in mountain soils of Southern Ecuador after conversion of forest to pasture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjsFKqt7o%3D&md5=1aa043d93f443b1889252cdaf0260032CAS |
Hedley MJ, Stewart JWB, Chauhan BS (1982) Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Science Society of America Journal 46, 970–976.
| Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXjvFCl&md5=5f235b82d644fa1e6d40c58ac170d82bCAS |
Hou E, Chen C, McGroddy ME, Wen D (2012) Nutrient limitation on ecosystem productivity and processes of mature and old-growth subtropical forests in China. PLoS ONE 7, e52071
| Nutrient limitation on ecosystem productivity and processes of mature and old-growth subtropical forests in China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXlsVCqug%3D%3D&md5=ab380388c400ceb55cf1c5523dd4e7c4CAS | 23284873PubMed |
Huang WJ, Zhou GY, Liu JX (2012) Nitrogen and phosphorus status and their influence on aboveground production under increasing nitrogen deposition in three successional forests. Acta Oecologica 44, 20–27.
| Nitrogen and phosphorus status and their influence on aboveground production under increasing nitrogen deposition in three successional forests.Crossref | GoogleScholarGoogle Scholar |
Huang W, Liu J, Wang YP, Zhou G, Han T, Li Y (2013) Increasing phosphorus limitation along three successional forests in southern China. Plant and Soil 364, 181–191.
| Increasing phosphorus limitation along three successional forests in southern China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXit1Gksrg%3D&md5=8bcd7e7211a544d7ced3b98b8bf2d10aCAS |
Imai N, Kitayama K, Titin J (2010) Distribution of phosphorus in an above-to-below-ground profile in a Bornean tropical rain forest. Journal of Tropical Ecology 26, 627–636.
| Distribution of phosphorus in an above-to-below-ground profile in a Bornean tropical rain forest.Crossref | GoogleScholarGoogle Scholar |
Jobbágy E, Jackson R (2001) The distribution of soil nutrients with depth: Global patterns and the imprint of plants. Biogeochemistry 53, 51–77.
| The distribution of soil nutrients with depth: Global patterns and the imprint of plants.Crossref | GoogleScholarGoogle Scholar |
Jobbágy EG, Jackson RB (2004) The uplift of soil nutrients by plants: Biogeochemical consequences across scales. Ecology 85, 2380–2389.
| The uplift of soil nutrients by plants: Biogeochemical consequences across scales.Crossref | GoogleScholarGoogle Scholar |
Johnson AH, Frizano J, Vann DR (2003) Biogeochemical implications of labile phosphorus in forest soils determined by the Hedley fractionation procedure. Oecologia 135, 487–499.
Kang J, Hesterberg D, Osmond DL (2009) Soil organic matter effects on phosphorus sorption: A path analysis. Soil Science Society of America Journal 73, 360–366.
| Soil organic matter effects on phosphorus sorption: A path analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjt12rtrs%3D&md5=87ca4762082d8a8ed32656a6b8fbc089CAS |
Kirkby CA, Kirkegaard JA, Richardson AE, Wade LJ, Blanchard C, Batten G (2011) Stable soil organic matter: A comparison of C:N:P:S ratios in Australian and other world soils. Geoderma 163, 197–208.
| Stable soil organic matter: A comparison of C:N:P:S ratios in Australian and other world soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnt1amsr0%3D&md5=cd221a719b653c19772210ce8a10e901CAS |
Kovar JL, Pierzynski GM (2009) ‘Methods of phosphorus analysis for soils, sediments, residuals, and waters.’ Southern Cooperative Series Bulletin No. 408. (Virginia Technical University: Blacksburg, VA)
Liu KH, Fang YT, Yu FM, Liu QA, Li FR, Peng SL (2010a) Soil acidification in response to acid deposition in three subtropical forests of subtropical China. Pedosphere 20, 399–408.
| Soil acidification in response to acid deposition in three subtropical forests of subtropical China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpslOru7Y%3D&md5=7c5363431e1723dcb5d3f6b902d064f4CAS |
Liu XZ, Zhou GY, Zhang DQ, Liu SZ, Chu GW, Yan JH (2010b) N and P stoichiometry of plant and soil in lower subtropical forest successional series in southern China. Chinese Journal of Plant Ecology 34, 64–71. [in Chinese]
Liu L, Gundersen P, Zhang T, Mo JM (2012) Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biology & Biochemistry 44, 31–38.
| Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China.Crossref | GoogleScholarGoogle Scholar |
McDowell RW, Condron LM (2000) Chemical nature and potential mobility of phosphorus in fertilized grassland soils. Nutrient Cycling in Agroecosystems 57, 225–233.
| Chemical nature and potential mobility of phosphorus in fertilized grassland soils.Crossref | GoogleScholarGoogle Scholar |
Mo JM, Brown S, Lenart M, Kong GH (1995) Nutrient dynamics of a human impacted pine forest in a MAB reserve of subtropical China. Biotropica 27, 290–304.
| Nutrient dynamics of a human impacted pine forest in a MAB reserve of subtropical China.Crossref | GoogleScholarGoogle Scholar |
Mo JM, Zhang W, Zhu WX, Gundersen PER, Fang YT, Li DJ, Wang H (2008) Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Global Change Biology 14, 403–412.
| Nitrogen addition reduces soil respiration in a mature tropical forest in southern China.Crossref | GoogleScholarGoogle Scholar |
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27, 31–36.
| A modified single solution method for the determination of phosphate in natural waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF38XksVyntr8%3D&md5=17ea13f1f165a05bfa49825c9e608460CAS |
Ohno T, Zibilske LM (1991) Determination of low concentrations of phosphorus in soil extracts using malachite green. Soil Science Society of America Journal 55, 892–895.
| Determination of low concentrations of phosphorus in soil extracts using malachite green.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXltlCqsb4%3D&md5=738b3946eabd89c79dc8d9c279609f01CAS |
Ormaza-González FI, Statham PJ (1996) A comparison of methods for the determination of dissolved and particulate phosphorus in natural waters. Water Research 30, 2739–2747.
| A comparison of methods for the determination of dissolved and particulate phosphorus in natural waters.Crossref | GoogleScholarGoogle Scholar |
Schlesinger WH, Bruijnzeel LA, Bush MB, Klein EM, Mace KA, Raikes JA, Whittaker RJ (1998) The biogeochemistry of phosphorus after the first century of soil development on Rakata Island, Krakatau, Indonesia. Biogeochemistry 40, 37–55.
| The biogeochemistry of phosphorus after the first century of soil development on Rakata Island, Krakatau, Indonesia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXhtlSnsb8%3D&md5=4f947a080cc59301c8a112c4faa0d460CAS |
Shen CD, Yi WX, Sun YM, Xing CP, Yang Y, Yuan C, Li ZA, Peng SL, An ZS, Liu TS (2001) Distribution of 14C and 13C in forest soils of the Dinghushan Biosphere Reserve. Radiocarbon 43, 671–678.
Sinsabaugh R, Moorhead D (1994) Resource allocation to extracellular enzyme production: a model for nitrogen and phosphorus control of litter decomposition. Soil Biology & Biochemistry 26, 1305–1311.
| Resource allocation to extracellular enzyme production: a model for nitrogen and phosphorus control of litter decomposition.Crossref | GoogleScholarGoogle Scholar |
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw CC, Alexandra R, Cusack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Waldrop MP, Wallenstein MD, Zak DR, Zeglin LH (2008) Stoichiometry of soil enzyme activity at global scale. Ecology Letters 11, 1252–1264.
Smil V (2000) Phosphorus in the environment: Natural flows and human interferences. Annual Review of Energy and the Environment 25, 53–88.
| Phosphorus in the environment: Natural flows and human interferences.Crossref | GoogleScholarGoogle Scholar |
Tabatabai M, Dick W, Burns R, Dick R (2002) Enzymes in soil: research and developments in measuring activities. In ‘Enzymes in the environment: activity, ecology, and applications’. (Eds RG Burns, RP Dick) pp. 567–596. (CRC Press: New York)
Tiessen H, Cuevas E, Chacon P (1994) The role of soil organic matter in sustaining soil fertility. Nature 371, 783–785.
| The role of soil organic matter in sustaining soil fertility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXmvF2qtrw%3D&md5=1170bc2b353bd6b2ea124ca5d10d9b77CAS |
Wang GP, Liu JS, Wang JD, Yu JB (2006) Soil phosphorus forms and their variations in depressional and riparian freshwater wetlands (Sanjiang Plain, Northeast China). Geoderma 132, 59–74.
| Soil phosphorus forms and their variations in depressional and riparian freshwater wetlands (Sanjiang Plain, Northeast China).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xjt1Kgs7s%3D&md5=11b8f701b6550a2c77bf1b6f6bc87d96CAS |
Wang YP, Law RM, Pak B (2010) A global model of carbon, nitrogen and phosphorus cycles for the terrestrial biosphere. Biogeosciences 7, 2261–2282.
| A global model of carbon, nitrogen and phosphorus cycles for the terrestrial biosphere.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1agurvN&md5=2f7d21140f689d883901c8e20fd53f7eCAS |
Wu J, He ZL, Wei WX, O’Donnell A, Syers J (2000) Quantifying microbial biomass phosphorus in acid soils. Biology and Fertility of Soils 32, 500–507.
| Quantifying microbial biomass phosphorus in acid soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXoslWjt7g%3D&md5=ddee03cc14c6ba6080a2e62d22fb0ed7CAS |
Yang X, Post WM (2011) Phosphorus transformations as a function of pedogenesis: A synthesis of soil phosphorus data using Hedley fractionation method. Biogeosciences 8, 2907–2916.
| Phosphorus transformations as a function of pedogenesis: A synthesis of soil phosphorus data using Hedley fractionation method.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XisFSnurY%3D&md5=50d270cc3051b4747519c6d415798e45CAS |
Zhao T, Ouyang Z, Zheng H, Wang X, Miao H (2004) Forest ecosystem services and their valuation in China. Journal of Natural Resources 19, 480–491. [in Chinese]
Zhou G, Liu S, Li Z, Zhang D, Tang X, Zhou C, Yan J, Mo J (2006) Old-growth forests can accumulate carbon in soils. Science 314, 1417
| Old-growth forests can accumulate carbon in soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1CntrvP&md5=353d715490eaef8e2552d8a3717d149eCAS | 17138894PubMed |