Relationship between changes in core body temperature in lambs and post-slaughter muscle glycogen content and dark-cutting
D. G. Pighin A D G , W. Brown B , D. M. Ferguson E , A. D. Fisher F and R. D. Warner CA Instituto Tecnología de Alimentos, Instituto Nacional de Tecnología Agropecuaria – INTA, De Los Reseros y Las Cabañas, Morón, Argentina.
B Department of Primary Industries, 600 Sneydes Road, Werribee, Vic. 3030, Australia.
C CSIRO Animal, Food and Health Sciences, 671 Sneydes Road, Werribee, Vic. 3030, Australia.
D Consejo Nacional de Investigaciones Científica y Técnicas – CONICET, Av. Rivadavia 1917, Buenos Aires, Argentina.
E Livestock Welfare, CSIRO Livestock Industries, Locked Bag 1, Armidale, NSW 2350, Australia.
F Faculty of Veterinary Science and Animal Welfare Science Centre, The University of Melbourne, Werribee, Vic. 3030, Australia.
G Corresponding author. Email: dpighin@cnia.inta.gov.ar
Animal Production Science 54(4) 459-463 https://doi.org/10.1071/AN12379
Submitted: 2 November 2012 Accepted: 7 March 2013 Published: 21 May 2013
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
Pre-slaughter stress may decrease muscle glycogen content, a key element for a suitable low ultimate pH and prevention of dark-cutting meat. Body temperature monitoring is a tool used in research on animal stress, as an indicator of stress events. Possible relationships between body temperature of sheep and post-mortem muscle glycogen were investigated in this study. Body temperature was measured with intravaginal loggers inserted into each animal at 3 days pre-slaughter, to record body temperature every 3 min over a period of 3 days. Blood samples were collected from each animal at exsanguination for measurement of glucose and lactic acid concentrations. The muscle content of glycogen and lactic acid were determined in samples of M. longissimus collected at the level of the 13th rib, at 1 h post-slaughter. A plot of body temperature versus time showed a rise in body temperature from all animals during events such as mustering, loading onto the truck, unloading at the abattoir, during pre-slaughter handling and at slaughter. Pearson’s correlation coefficients were determined between (1) the main temperature increments occurring between farm and slaughter; and (2) post-slaughter muscle glycogen and lactate levels. A significant negative correlation was detected between elevation in core body temperature due to physical stress of sheep and muscle glycogen levels at slaughter. A low correlation was detected between body temperature and blood glucose or lactate concentrations. Further research should examine the relationship between core body temperature and meat quality in order to better understand the complex relationship between animal stress and meat quality.
Introduction
It is well known that muscle glycogen concentration at the time of slaughter is a key factor involved in the post-mortem anaerobic glycolysis of muscle cells (Lawrie 1958). Adequate muscle glycogen content will lead to a suitable pH decline in the conversion of muscle into meat, which in turn is related to the overall acceptability of meat (Tarrant 1989a). In contrast, low levels of muscle glycogen will limit pH decline, leading to high ultimate pH (pHu) of meat, also known as dark-cutting. Dark-cutting meat generally has less desirable flavour, tenderness, shelf-life and overall acceptability (Shorthose 1989; Purchas and Aungsupakorn 1993). The storage of muscle glycogen and the rate of post-mortem pH decline also depend on several factors including diet, exercise, muscle fibre composition, stress and muscle temperature (Tarrant 1989b; Zhu et al. 2011).
Pre-slaughter stress may decrease muscle glycogen content through activation of the nervous system, mediated by catecholamines (Kuchel 1991), and/or the endocrine system, mediated by glucocorticoids (Dantzer 1994). Several biochemical, physiological and behavioural measurements have been previously used to measure potential effects of animal stress (Moberg 2000). However, most of these measures are invasive or not well established, requiring expert staff and being time consuming.
In recent years, body temperature has started to be examined more closely as an indicator of animal stress. It is known that an animal’s temperature is a constant balance between heat gain and heat loss. To maintain body temperature at a constant point, several key mechanisms are involved, known collectively as the thermoregulatory system (Robertshaw 1985). Moreover, core body temperature can also be dramatically increased with muscular activity, nervous and hormonal factors (such as sympathetic nervous activity), catecholamines, and thyroid hormones (King 2004). At present, the capacity to continuously log body temperature of freely behaving animals has become easy, non-invasive and cost effective (Bluett et al. 2000; Lea et al. 2008). Moreover, an interesting relationship between body temperature and the level of stress, either physical and/or psychological, at several stages of animal handling has been also shown (Takakazu et al. 2001; Ferguson 2003). As temperature logging is a method that significantly lacks stress associated with animal handling, it may be a useful tool in animal stress physiology research.
The aim of the present study was to investigate the possible relationships between the body temperature of sheep and post-mortem muscle glycogen concentration, in order to predict dark-cutting meat.
Materials and methods
Animals
Forty cross-bred female lambs [first-cross: Merino × Border Leicester (BL), and second-cross: Merino × BL × Poll Dorset], ~6 months old were used in the study. Animals were raised in the fields of the experimental farm at DPI Rutherglen (Vic., Australia) and were fed with a pasture diet (lucerne, cereal regrowth and cereal stubble). The experiment was conducted in May 2009, when daily temperatures ranged in average between 0.8 and 15.0°C.
Animal handling and experimental procedures were approved by the Agricultural Research and Extension Animal Ethics Committee of Victorian Department of Primary Industries, Australia.
Temperature logging
Temperature data loggers (Dallas Thermocron iButton, DS1921 H, Maxim Integrated Products, Sunnyvale, CA, USA) were programmed to start logging on Sunday at 2.00 p.m. and to end logging on Tuesday at 7.00 p.m., when slaughter was planned to be completed, with individual temperatures recorded every 3 min. Loggers were coupled to the plastic wings of progesterone-free ovine intravaginal progesterone releasing devices (CIDR Interag, Hamilton, New Zealand) as described by Lea et al. (2008). The devices were washed in disinfectant solution and were inserted intravaginally in each animal 2 days before starting to log, using an applicator lubricated in the same disinfectant solution.
Temperature monitoring included a period of 16 h of grazing on the farm, 20 min of mustering before transport, 6 h of yarding, 1 h of pre-transport weighing, 13 min of truck loading, 6 h of transport, 5 min of truck unloading, ~16 h at the abattoir awaiting slaughter in lairage pens, and finally, the assembly before slaughter (5–10 min). Temperature loggers were removed at ~5 min post-slaughter, while the carcass was hanging upside-down. One data logger was not able to be located and retrieved from a carcass and was recorded as missing. Data was downloaded to a PC using the iButton receptor connected to a 1-Wire adaptor (Dallas Thermocron, DS1402D-DR8 iButton receptor and DS9490R USB 1-Wire adaptor, Maxim Integrated Products). Data was displayed and analysed using the software provided by Dallas Thermocron (Maxim Integrated Products).
Samples and pH measurement
Blood samples were collected from each animal at exsanguination into tubes containing heparin as anticoagulant and immediately placed on ice. Samples were centrifuged (3000g, 10 min, 4°C) within 5 h post-slaughter, plasma was separated and then stored at −20°C until analysis for glucose and lactic acid concentrations.
Muscle samples were removed from the M. longissimus lumborum (LL) at 1 h post-slaughter. Samples of LL were immediately frozen in liquid nitrogen until processing for glycogen and lactic acid levels. The pHu of the M. semitendinosus (ST) and LL was measured at 24 h post-mortem, using a Micrometer pH Vision Model 6007 (Jenco Instruments, San Diego, CA, USA) with a direct pH probe (Ionode Model IJ42).
Laboratory methods
Plasma glucose and lactic acid concentrations were measured using enzymatic commercial kits (Sigma-Aldrich Pty, St Louis, MO, USA and Randox Laboratories Ltd, Crumlin, Co. Antrim, UK, respectively).
Muscle glycogen concentration was determined according to Dreiling et al. (1987). Briefly, muscle homogenates were incubated in amyloglucosidase solution during 90 min to convert glycogen to glucose. The concentration of glucose was determined spectrophotometrically after 30 min incubation in GOD/POD solution. Muscle L-lactic acid level was determined in the same muscle homogenates as described by Noll (1985). Glycolytic potential was calculated from muscle glycogen and lactic acid levels according to Monin and Sellier (1985).
Statistical and data analyses
Basal temperature from each animal was calculated as the mean of the four lowest temperature values recorded in order to obtain a more representative lineal body temperature nadir. Peak maximum temperatures were used to evaluate changes in core body temperature associated to animal handling. Thus, main peak increments from each animal were calculated as the difference between the maximum temperature and the corresponding basal temperature and were expressed as percentage of individual basal temperature (%). Results obtained are expressed as mean ± standard deviation. Pearson’s linear regression coefficient was calculated in order to determine correlations between the different parameters assayed.
Results and discussion
The mean core body temperature of sheep recorded from yarding, Day 1, to the time of slaughter, Day 3, is shown in Fig. 1. Main temperature increments are marked with arrows. As can be seen, core body temperature reflected the presumed stress associated with some key events. In accordance with previous observations (Bluett et al. 2000), results obtained in the present study successfully show the application of intravaginal loggers to measure core body temperature.
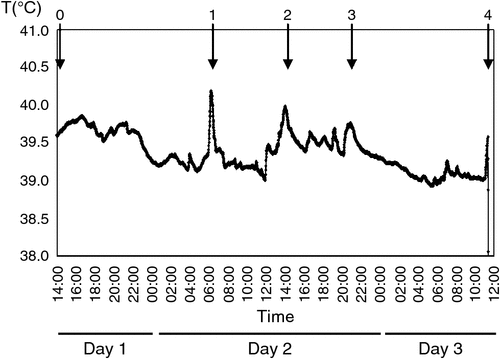
|
Mustering, truck loading and pen change were the most representative events reflected in core body temperature increases. All these procedures involved physical stress, and thereby, physical activity. The calculated temperature increments, expressed as percentages, corresponding to these peaks are shown in Table 1. As can be seen, peak increments belonging to mustering and truck loading were the greatest contributors to the total peak increment observed during the experimental period.
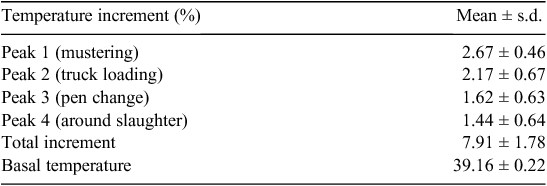
|
Zimerman et al. (2011) have recently reported a significant increase in blood cortisol concentration in castrated kids exposed to physical exercise (moved at 3 km/h) during 30 min before slaughter, comparable to cortisol concentration found in animals exposed to psychological stress. The authors have proposed that fear to human presence during the assay may be responsible for this finding. However, in the present study, it would be possible that the temperature increase recorded was consequence of both physical exercise and psychological stress. Both mechanisms would lead to the consumption of stored energy in the muscle.
Results of biochemical parameters assayed in blood and muscle collected at slaughter are shown in Table 2. Blood glucose was detected slightly increased in comparison with normal resting values previously published by other authors (Ross and Kitts 1969), possibly reflecting stress and fear of the animals immediately before slaughter. Nevertheless, lactic acid concentrations were detected within values previously published (Cottrell et al. 2008; Pighin et al. 2012; Ponnampalam et al. 2012). This finding does not agree with recent studies which suggested that excitation or fear sharply increases the blood concentrations of lactic acid in pigs (Edwards et al. 2010) and cattle (Warner et al. 2007). A possible explanation could be the different stress susceptibility of animal species and the level of stress involved in the different studies. Nevertheless, taking into account the metabolite levels, it is possible to believe that relatively low stress took place during the slaughter period in the present study. On the other hand, the standard deviation observed also suggests an important animal variability associated with stress susceptibility.

|
Glycogen and lactic acid levels in the LL were within a normal range (Cottrell et al. 2008), which was reflected in pHu of both ST and LL muscles (Table 2). Consequently, no dark-cutting meat was observed in the present study. Muscle glycogen content at 1 h post-slaughter ranged within levels corresponding to well-fed animals (Apple et al. 1995). This finding would agree with the concept that some loss of glycogen in vivo – above the threshold of 40–57 µmol/g – can be accommodated without deleterious effects on meat quality (Howard 1963; Tarrant 1989a).
The Pearson’s correlation coefficients between blood and muscle biochemical parameters, pHu for the ST and LL and major temperature peak and total increment of temperature are shown in Table 3. As shown, the highest correlation coefficient obtained was between temperature peak 1 and the LL muscle glycogen level or glycolytic potential (r = –0.455, –0.419, respectively; P < 0.01 for both). There was also a significant correlation between total temperature increase and muscle glycogen or glycolytic potential (P < 0.05 for both).
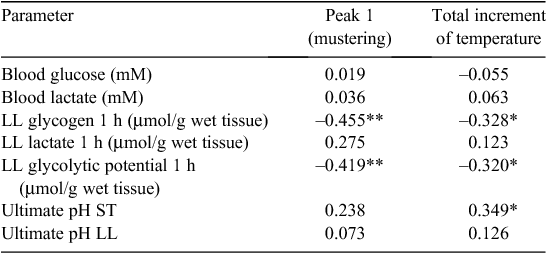
|
In the present study, none of the carcasses were defined as dark-cutting (pHu >6.0), and therefore it would be expected that the relationship between pHu and temperature would not be strong. Nevertheless, pHu of ST muscle showed a significant negative correlation with the total temperature increase. Since the ST muscle is more involved with body movements than the LL, this finding could be explained by means of the different fibre composition of these muscles.
Core body temperature depends on the heat load, which in turn depends on the environment’s heat and the temperature associated to food oxidation (Yousef 1985). Since regulation of body temperature in mammals is inextricably linked to hydration state (Simon et al. 1986), climatic conditions, exercise and diet would be key issues to take into account in temperature monitoring. Recently, McKinley et al. (2009) have noted it that rehydration plays a major role in core body temperature and regulation.
Bearing in mind that dark-cutting meat would involve greater impact of stressors on body biochemistry and, consequently, on body temperature, it is suggested that the inverse correlation found between body temperature peaks and muscle glycogen storage levels, means that temperature monitoring may be a useful tool to predict the probability of altered meat quality. Further studies should also focus attention on the effect of climatic and dietary conditions on stress-mediated body temperature increases.
Conclusion
A significant negative correlation was found between changes in core body temperature due to physical stress of sheep and muscle glycogen levels at slaughter. There was little relationship between muscle pHu and changes in body temperature but this is most likely because there was no dark-cutting high pH meat in the lamb carcasses.
Thus, changes in body temperature pre-slaughter in lambs show an interesting potential for enabling prediction of muscle glycogen levels and dark-cutting post-slaughter, and to propose changes in protocols of animal handling that contribute to avoid such meat quality alteration.
Acknowledgements
The financial support from INTA (Argentina) and DPI (Australia) is gratefully acknowledged. Authors express their gratitude to Matthew Kerr, Athula Naththarampatha and Greg Seymour for their interest and technical assistance.
References
Apple JK, Dikeman ME, Minton JE, McMurphy RM, Fedde MR, Leith DE, Unruh JA (1995) Effects of restraint and isolation stress and epidural blockade on endocrine and blood metabolite status, muscle glycogen metabolism, and incidence of dark-cutting longissimus muscle of sheep. Journal of Animal Science 73, 2295–2307.Bluett SJ, Fisher AD, Waugh CD (2000) Heat challenge of diary cows in the Waikato: a comparison of spring and summer. Proceedings of the New Zealand Society of Animal Production 60, 226–229.
Cottrell JJ, Mc Donagh MB, Dunshea FR, Warner RD (2008) Inhibition of nitric oxide release pre-slaughter increases post-mortem glycolysis and improves tenderness in ovine muscles. Meat Science 80, 511–521.
| Inhibition of nitric oxide release pre-slaughter increases post-mortem glycolysis and improves tenderness in ovine muscles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVagu73N&md5=5ab487d307bea8f8b6c61e0c7d4b82a9CAS | 22063360PubMed |
Dantzer R (1994) Animal welfare methodology and criteria. Revue Scientifique et Technique del Office International des Epizooties 13, 291–302.
Dreiling CE, Brown DE, Casale L, Kelly L (1987) Muscle glycogen: comparison of iodine binding and enzyme digestion assays and application to meat samples. Meat Science 20, 167–177.
| Muscle glycogen: comparison of iodine binding and enzyme digestion assays and application to meat samples.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXitVKhtQ%3D%3D&md5=9715fb2568869bb2865c0ea85311ee2eCAS | 22054495PubMed |
Edwards LN, Grandin T, Engle TE, Porter SP, Ritter MJ, Sosnicki AA, Anderson DB (2010) Use of exsanguinations blood lactate to assess the quality of pre-slaughter pig handling. Meat Science 86, 384–390.
| Use of exsanguinations blood lactate to assess the quality of pre-slaughter pig handling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptVejsLs%3D&md5=e0d9d45bb81a93de93de6c847cc60464CAS | 20566249PubMed |
Ferguson DM (2003) Regulation of post mortem glycolysis in ruminant muscle. PhD thesis, The University of New England, Armidale.
Howard A (1963) The relation between physiological stress and meat quality. In ‘Carcass composition and appraisal of meat animals’. (Ed. DE Tribe) pp. 11–21. (CSIRO Publishing: Melbourne)
King J (2004) Thermoregulation: physiological responses and adaptations to exercise in hot and cold environments. Journal of Hyperplasia Research 4(3). Available at http://www.abcbodybuilding.com/magazine04/thermoregulation.htm [verified 26 April 2013]
Kuchel O (1991) Stress and catecholamines. In ‘Stress revisited 1. Neuroendocrinolgy of stress’. (Eds G Jasmin, M Cantin) pp. 80–103. (Karger: Basel, Switzerland)
Lawrie RA (1958) Physiological stress in relation to dark cutting beef. Journal of the Science of Food and Agriculture 9, 721–727.
| Physiological stress in relation to dark cutting beef.Crossref | GoogleScholarGoogle Scholar |
Lea JM, Niemeyer DDO, Reed MT, Fisher AD, Ferguson DM (2008) Development and validation of a simple technique for logging body temperature in free-ranging cattle. Australian Journal of Experimental Agriculture 48, 741–745.
| Development and validation of a simple technique for logging body temperature in free-ranging cattle.Crossref | GoogleScholarGoogle Scholar |
McKinley MJ, Weissenborn F, Mathai ML (2009) Drinking-induced thermoregulatory panting in rehydrated sheep: influences of oropharyngeal/esophageal signals, core temperature, and thirst satiety. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 296, R1881–R1888.
| Drinking-induced thermoregulatory panting in rehydrated sheep: influences of oropharyngeal/esophageal signals, core temperature, and thirst satiety.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnsF2lu7s%3D&md5=f65150d75b4cad86fb3d5eb03378bae6CAS | 19297542PubMed |
Moberg GP (2000) Biological response to stress: implications for animal welfare. In ‘The biology of animal stress – basic principles and implications for animal welfare’. (Eds GP Moberg, JA Mench) pp. 1–22. (CABI Publishing: Oxon)
Monin G, Sellier P (1985) Pork of low technological quality with a normal rate of muscle pH fall in the immediate post-mortem period: the case of the Hampshire breed. Meat Science 13, 49–63.
| Pork of low technological quality with a normal rate of muscle pH fall in the immediate post-mortem period: the case of the Hampshire breed.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvFWjuw%3D%3D&md5=5716d4d7ed22255f506e19ec13d18ac7CAS | 22055445PubMed |
Noll F (1985) L+ lactate determination. In ‘Methods of enzymatic analysis’. (Ed. HU Bergmeyer) pp. 583–588. (VCH Verlagsgesellschaft: Weinheim, Germany)
Pighin DG, Cunzolo SA, Zimerman M, Domingo E, Pazos AA, Grigioni G (2012) Peri mortem muscle biochemistry in an animal model of acute stress. In ‘Proceedings of 58th international congress of meat science and technology, August 2012, Montreal, Canada’. (CD-ROM, Paper ID: ICoMST2012paper150.pdf )
Ponnampalam EN, Warner RD, Dunshea FR (2012) Basal and hormone metabolism in lambs varies with breed and diet quality. Domestic Animal Endocrinology 42, 94–102.
| Basal and hormone metabolism in lambs varies with breed and diet quality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XmtFCqtg%3D%3D&md5=239ffd3b5223953b4760c920d7398343CAS | 22119112PubMed |
Purchas RW, Aungsupakorn R (1993) Further investigations into the relationship between ultimate pH and tenderness for beef samples from bulls and steers. Meat Science 34, 163–178.
| Further investigations into the relationship between ultimate pH and tenderness for beef samples from bulls and steers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXksVOqsL0%3D&md5=45743c70851dee7313343f5a33f961adCAS | 22060661PubMed |
Robertshaw D (1985) Heat loss of cattle. In ‘Volume 1: stress physiology in livestock. Basic principles’. (Ed. MK Yousef) pp. 55–66. (CRC Press: Boca Raton, FL)
Ross JP, Kitts WD (1969) Concentration of certain blood metabolites in obese pregnant and non pregnant ewes. Canadian Journal of Animal Science 49, 91–95.
| Concentration of certain blood metabolites in obese pregnant and non pregnant ewes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1MXktVWhurk%3D&md5=78b12e1e8683304a383a2ba6d89c216aCAS |
Shorthose WR (1989) Dark-cutting in beef and sheep carcasses under the different environments in Australia. In ‘Dark-cutting in cattle and sheep’. (Eds SU Fabiansson, WR Shorthose, RD Warner) pp. 68–73. (Australian Meat and Livestock Research and Development Corporation: Sydney)
Simon E, Pierau KF, Taylor DCM (1986) Central and peripheral thermal control of effectors in homeothermic temperature regulation. Physiological Reviews 66, 235–300.
Takakazu O, Oka K, Tetsuro H (2001) Mechanisms and mediators of psychological stress-induced rise in core temperature. Psychosomatic Medicine 63, 476–486.
Tarrant PV (1989a) Animal behaviour and environment in the dark-cutting condition in beef – a review. International Journal of Food Science & Technology 13, 1–21.
Tarrant PV (1989b) Animal behaviour and environment in the dark-cutting condition. In ‘Dark-cutting in cattle and sheep’. (Eds SU Fabiansson, WR Shorthose, RD Warner) pp. 8–18. (Australian Meat and Livestock Research and Development Corporation: Sydney)
Warner RD, Ferguson DM, Cottrell JJ, Knee BW (2007) Acute stress induced by the pre-slaughter use of electric prodders causes tougher beef meat. Australian Journal of Experimental Agriculture 47, 782–788.
| Acute stress induced by the pre-slaughter use of electric prodders causes tougher beef meat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXnsV2gsb0%3D&md5=f3cf2957ea88e227aa16fb97db86c1aaCAS |
Yousef MK (1985) Thermoneutral zone. In ‘Stress physiology in livestock – Volume I. Basic principles’. (Ed. MK Yousef) pp. 67–75. (CRC Press: Boca Raton, FL)
Zhu X, Ruusunenb M, Gusellac M, Zhoua G, Puolanne E (2011) High post-mortem temperature combined with rapid glycolysis induces phosphorylase denaturation and produces pale and exudative characteristics in broiler Pectoralis major muscles. Meat Science 89, 181–188.
| High post-mortem temperature combined with rapid glycolysis induces phosphorylase denaturation and produces pale and exudative characteristics in broiler Pectoralis major muscles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXntlGhs7w%3D&md5=fcc36942088e665dfd6064de0f6af1d9CAS | 21663805PubMed |
Zimerman M, Grigioni G, Taddeo H, Domingo E (2011) Physiological stress responses and meat quality traits of kids subjected to different pre-slaughter stressors. Small Ruminant Research 100, 137–142.
| Physiological stress responses and meat quality traits of kids subjected to different pre-slaughter stressors.Crossref | GoogleScholarGoogle Scholar |


