Weight gain and enteric methane production of cattle fed on tropical grasses
D. Korir A D , S. Marquardt A , R. Eckard D , A. Sanchez B , U. Dickhoefer B , L. Merbold A E , K. Butterbach-Bahl A C , C. Jones
A D , S. Marquardt A , R. Eckard D , A. Sanchez B , U. Dickhoefer B , L. Merbold A E , K. Butterbach-Bahl A C , C. Jones  A , M. Robertson-Dean F and J. Goopy
A , M. Robertson-Dean F and J. Goopy  A D *
A D *
A Mazingira Centre, International Livestock Research Institute (ILRI), PO Box 30709, 00100 Nairobi, Kenya.
B Animal Nutrition and Rangeland Management in the Tropics and Subtropics, Institute of Agricultural Sciences in the Tropics, University of Hohenheim, 1, 70599 Stuttgart, Germany.
C Institute of Meteorology and Climate Research, Atmospheric Environmental Research (IMK-IFU), Karlsruhe Institute of Technology (KIT), Garmisch-Partenkirchen, Germany.
D Faculty of Agriculture and Veterinary Sciences, University of Melbourne, Carlton, Vic., Australia.
E Agroscope, Research Division Agroecology and Environment, Reckenholzstrasse 191, 8046 Zurich, Switzerland.
F University of New England, Department of Mathematics and Statistics, Armidale, NSW, Australia.
Animal Production Science 63(2) 120-132 https://doi.org/10.1071/AN21327
Submitted: 28 June 2021 Accepted: 6 August 2022 Published: 10 October 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Planted grasses are becoming an increasingly important feed resource for tropical smallholder ruminant production; yet, limited research has been conducted to quantify productivity or enteric methane (CH4) production of animals consuming these grasses.
Aim: An experiment was conducted to assess yields and nutritional attributes of the following three tropical grasses: Cenchrus purpureus var. Kakamega 1 (Napier), Chloris gayana var. Boma (Rhodes) and Urochloa brizantha var. Xaeres (Brachiaria), and quantify enteric CH4 production of cattle fed on them.
Methods: Yearling Boran steers (n:18; initial liveweight 216 ± 5.8 kg (mean ± s.e.m.) were allocated to one of three grasses, in a completely randomised design and fed ad libitum for two feeding periods, each period lasting for 70 days. Intake, liveweight (LW), apparent total-tract digestibility and enteric CH4 production were assessed. The grasses used were grown on site and biomass yields were monitored over a 2-year period. Animal growth was also simulated to a final weight of 350 kg, and the amount of feed and size of land required to produce, and days to reach final weight, were estimated.
Key results: Mean voluntary dry-matter intake (DMI) and ADG were higher (P < 0.05) in Period 2 than in Period 1, but did not differ among treatments (P > 0.05) within period. Methane yield (MY; CH4 g/DMI kg) was similar among treatments (26.7–28.5, P = 0.26) but Napier had a higher CH4 conversion factor [Ym; CH4 (MJ)/gross energy intake (MJ)] than did Rhodes and Brachiaria (0.0987 vs 0.0873 and 0.0903 respectively; P = 0.013). Our modelling indicated that steers consuming Rhodes took at least 30 more days to reach the target LW, required larger land area for feed production and produced more enteric CH4 than did the other two diets.
Conclusion: Even though animal performance and MY among treatments did not differ, the animals had higher MY and Ym than currently estimated by the Intergovernmental Panel on Climate Change.
Implication: The three grasses supported similar animal growth rate, implying that growing of higher-yielding grasses such as Napier offers an opportunity to optimise land productivity in the tropics. However, suitable feeding practices such as protein supplementation need to be explored to enhance ruminant production and reduce enteric CH4 production.
Keywords: beef, biomass yield, East Africa, feed requirement, live weight gain, methane yield, respiration chambers.
Introduction
Sown pastures and natural rangelands form the world’s largest land-use system, occupying up to 43% of terrestrial ice-free earth surface and supporting domestic and wild ruminant populations (Gibson 2009). A growing human population and increasing demand for animal protein is putting pressure on this land to support higher animal production despite the adverse effects of climate change on its productivity and increased competition for land for other land use (Thornton 2010). In Sub-Saharan Africa where up to 40% of the cattle population is kept in mixed crop–livestock systems (Cecchi et al. 2010), fodder cultivation is increasingly being practised as land ownership decreases, leading to intensification of livestock systems (Thornton 2010) to optimise land productivity. Tropical (C4) grasses are the main fodder used in these systems, yet these have attracted limited research attention in terms of their nutritional value to ruminants (Parr et al. 2014). However, what is evident in the available literature is that animal productivity on (at least some) C4 grasses is lower than that on temperate (C3) grass species (Caswell et al. 1973; Barbehenn et al. 2004). Tropical grasses grow faster, yielding high biomass, but in the process deposit more structural carbohydrate, such as neutral detergent fibre (NDF) and lignin to support plant stature (Caswell et al. 1973). Low crude protein (CP) concentration that is also typical of tropical grasses, together with high NDF concentration decrease their intake and nutrient digestibility by animals and thus lower animal productivity (Buxton 1996; Gibson 2009), compared with animals fed on better-quality diets. Minimal variations in fodder quality among tropical grass species grown in the same environmental conditions are expected on the basis of their growth patterns, but management practices such as soil amendment and age at harvesting (Pinares-Patiño et al. 2003; Lee et al. 2017; Bedaso et al. 2021), as well as climatic factors such as precipitation and temperature, have been shown to have a more pronounced effect (Lee et al. 2017; Habte et al. 2020).
Studies assessing growth rate in cattle when fed tropical forages are limited and have largely been conducted outside Africa, such as in Australia (average daily gain, ADG: 0.4–0.5 kg: Kurihara et al. 1999; Tomkins et al. 2011) and Brazil (ADG: 0.5 kg: Paciullo et al. 2011; Lima et al. 2019). Few available measurements from East Africa align well with these findings, with Kariuki et al. (1998) reporting ADG of 0.5 kg while feeding Cenchrus purpureus to growing Friesian heifers and Kariuki et al. (1999) reported 0.41 kg on growing Friesian × Sahiwal heifers fed on the same grass.
Ruminants produce methane (CH4) during their normal fermentative digestion in the rumen and this is a problem because of the impact of CH4 on climate change and accounts for up to 12% loss of gross energy intake (Johnson and Johnson 1995), negatively affecting animal production. Nutritional parameters including quantity, feed type and quality are the main determinants of the level of enteric CH4 production (Broucek 2014; Charmley et al. 2016). Low-quality diets (higher CP and lower fibre content) are associated with longer rumen retention time and alter fermentation pathway to more acetate production, with these two phenomena being associated with a higher CH4 yield (MY: g CH4/kg DMI; Beauchemin and McGinn 2006; Janssen 2010). Enteric CH4 measurements from cattle fed tropical grasses seems to vary a lot (Table 1) but there is a general trend that MY and CH4 conversion factor (Ym: CH4 (MJ)/GEI (MJ)) are negatively related with diet quality and intake.

|
The observed differences in MY and Ym within tropical diets highlight the limitation of applying universal equations in predicting enteric CH4 emissions and emphasise the need for more context-specific measurements, using local feed resources and local animal phenotypes to improve the accuracy of enteric CH4 calculation purposes and to inform development of more suitable mitigation strategies.
Napier and Rhodes are the dominant grasses cultivated in East Africa. Napier grass is predominantly cultivated in cut and carry systems, whereas Rhodes is commonly grown for hay. Napier grass is grown extensively in East Africa because of its high biomass yield (up to 60 t DM/ha.year), wide ecological adaptation, ease of propagation and persistence (Anindo and Potter 1994). However, in recent years, its productivity has been compromised by smut and stunt diseases that reduce yields by up to 60% (Farrell et al. 2001; Jones et al. 2004). This has led to researchers looking into suitable alternative species that could perform similarly in the region. Recently, improved Brachiaria grass varieties, (originally native to Africa) have been re-introduced to East Africa from South America (Djikeng et al. 2014; Ghimire et al. 2015), where they are being used extensively in beef and dairy production (Holmann et al. 2004). Initial experimental work on the introduced varieties in East Africa has focussed mainly on agronomic aspects and laboratory-based feed measurements (Ondiko et al. 2016). Longer-term studies comparing biomass yield, chemical composition, animal performance, enteric CH4 production and land requirements of the different locally available grasses relative to the newly introduced Brachiaria grass varieties are needed to fill these knowledge gaps.
The objectives of the present study were to quantify biomass yield of two commonly grown tropical grasses in East Africa, in comparison to newly introduced Brachiaria, and to evaluate growth and enteric CH4 production in cattle fed ad libitum on the three grass species. We evaluated the agronomic performance of each grass by using our experimental results to model pasture resource requirements and enteric CH4 production of animals fed to reach a pre-determined weight. Because the three tested grasses were grown and managed under the same conditions, we expected minimal differences in the fodder quality and, therefore, we hypothesised that (1) voluntary intake, total-tract digestibility and ADG would not differ among the treatment groups, (2) daily enteric CH4 production, MY and Ym do not differ among treatments, and (3) the amount of land required to grow fodder needed to feed growing Boran steers to a predetermined weight gain, and cumulative enteric CH4 produced do not differ for the three grass treatments.
Materials and methods
Study site and climate
This study consisted of two parts:
-
An agronomic trial to assess growth and composition of three locally available tropical grasses undertaken over a 2-year period, and
-
An animal feeding experiment to evaluate growth and enteric CH4 production from steers fed solely on each of the cultivated grasses.
Both trials were conducted concurrently at the Mazingira Centre of the International Livestock Research Institute (ILRI) Nairobi, Kenya (1.27102359S, 36.72329510E; 1795 m above sea level). The area receives about 980 mm of rain annually and mean annual temperature of 17.5°C (Pelster et al. 2016). Temperature and rainfall data were recorded from an on-site weather station described by Zhu et al. (2018). The agronomic trial was conducted from March 2018 to March 2020 and the animal experiment between May and November 2018 (Table 2).

|
Grass establishment and biomass yield measurements
The three experimental grasses, namely, Cenchrus purpureus var. Kakamega 1 (Napier grass, NG), Chloris gayana var. Boma (Rhodes grass, RG) and Urochloa brizantha var. Xaeres (Brachiaria grass, BG) were established on newly cultivated land that had previously been used for intermittent grazing for 12 years, colonised mainly by Cynodon plectostachyus and RG. The field was prepared 3 months prior to planting of the experimental grasses by disc ploughing to a depth of 0.3 m and was harrowed 1 month later. The field was then divided along the slope into three plots, two measuring 15 000 m2 each where RG and BG were established, and the third 10 000 m2 where NG was established. Rhodes grass seeds were sown in furrows 3 cm deep, with inter-row spacing of 0.4 m, whereas NG was planted in rows using cuttings with a spacing of 0.5 m between plants and 1 m between rows. Brachiaria seedlings were planted in rows with a spacing of 0.3 m between plantings and 0.8 m between rows. A buffer zone of 1 m was maintained in between the plots. The grasses were initially established in May 2017 and gap-filling using stolons was undertaken in March 2018 because of high plant mortality in the three grasses (>50%), after which biomass yield measurements were started, continuously for 24 months. The grasses relied mainly on rainfall and were irrigated only during drier than average periods to simulate average precipitation to prevent undue water stress. The RG and BG plots were divided into five equal blocks each, whereas the NG was divided into four, along the contour. The initial cut for the three grasses was then conducted in a staggered manner, with a 14-day interval from one block to another to ensure that the grass forages harvested during the trial were at a similar stage of growth throughout the animal experiment. Each block provided fodder for six steers for 14 days in the respective treatment groups. Napier grass was harvested and fed to the animals at 56 days of regrowth, whereas RG and BG were harvested at 70 day cycles because NG grew faster than did the other two grasses and attained the recommended harvesting height (∼1 m) earlier. Grasses were harvested manually using either a machete or brush cutter (RG).
For the assessment of biomass yield, three-quadrats measuring 1.5 × 2 m, 1.2 × 2.4 m and 1 × 1 m for NG, BG and RG treatments respectively, were selected randomly within the respective blocks during each round of harvests and the aboveground biomass down to 2 cm above the soil harvested. Fresh biomass was weighed with a digital mini crane scale (Model: HOS-02, Zhejiang Haoyu Industry & Trade Co. Ltd, Zhejiang, China) and the weights were recorded in a field notebook. The fodder was homogenised by quadrat and 1 kg of the harvested material sampled and chopped to 3–5 cm lengths. This was again homogenised and a subsample of ∼300 g was picked and packed into paper bags prior to transferring to the laboratory for drying at 50°C for 72 h. Dried samples were then ground using a hammer mill (MF 10 basic, IKA, Werke GmbH & Co. KG, Staufen, Germany) fitted with 1 mm sieve and dried again at 105°C for 24 h in an air-forced oven to obtain the true DM. Biomass yield per quadrat on a DM basis was determined as follows:
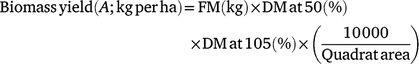
where FM is total fresh forage weight as harvested; DM at 50 is the percentage DM obtained after drying samples at 50°C and DM at 105 is the percentage DM obtained after dying the pre-dried samples at 105°C. Biomass yields were extrapolated to per hectare basis by multiplying the output by 10 000 m2 (1 ha) divided by the area of the quadrat harvested in square metres.
During each harvest, the yields obtained in the three quadrats (A) were averaged to estimate biomass yield in each block (B; n = 4 for Napier, and n = 5 for Rhodes and Brachiaria), these being replicates at plot level (grass) but biased on time. At plot level (C), yields in each harvest cycle (n = 6 for Napier and n = 5 for Rhodes and Brachiaria per year) were obtained by averaging block yields (B) over each harvest cycle. Total biomass yields (D) for Year 1 and Year 2 of measurements were obtained by summing yields (C) in the harvest cycles in each year, and the mean annual yield over the 2 years was obtained by summing the yields from the 2 years and dividing by two.
Experimental animals and housing
The experimental design was reviewed and approved by the institutional animal care and use committee of ILRI (approval no: IACUC-RC2017-15). Boran steers [n = 18; initial liveweight (LW): 216 ± 5.8 kg; age: 18 ± 2 months mean ± s.e.m.)] that were sourced from ILRI Kapiti Research Station located about 60 km south-east of Nairobi were used. On arrival at the experimental facility, the animals were treated with a pour on acaricide (Bayticol® Pour-On, Bayer New Zealand Ltd), drenched with albendazole anthelmintic (Albafas 10%, Norbrook Kenya Ltd, Nairobi, Kenya) and vaccinated against foot-and-mouth disease. The animals were ear-tagged and kept in a quarantine facility for 1 month before being released into paddocks where they were grazed on pastures dominated by Cynodon plectostachyus, for 8 months. Twenty-eight days prior to the commencement of the trial, the steers were moved to the experimental facility and housed in individual pens, initial LWs were taken, and the animals were drenched as previously described (see above) and placed on a pre-trial diet consisting of ad libitum chaffed RG hay with continuous access to clean water and mineral block (Afya Bora Ready Block, Unga Farm Care Ltd, Nairobi Kenya; elemental composition (%): calcium: 18.8; phosphorus: 2.8; sodium chloride: 31.2; magnesium: 0.09; manganese: 0.084 zinc: 0.17; iodine: 0.0068; copper: 0.055; iron: 0.115; cobalt: 0.0009, and selenium: 0.0004).
Steers were stratified by LW then randomly allocated to three groups (A, B, C), each with six animals. The animal groups were then allocated randomly to either RG, NG or BG dietary treatment and the steers remained in the same treatments in both experimental periods. Two animals from each treatment group were allocated into one of the three management groups (Groups 1, 2, and 3), with animals in the same management group going through each measurement protocol at the same time. At any given time, two management groups were housed in a partitioned open animal yard covered with sailcloth, in individual pens measuring 1.90 m by 2.87 m, while a third was housed in a fully enclosed and partitioned animal unit, with individual pens measuring 1.0 m by 2.0 m, where total collections of faeces and urine were undertaken. Each group was transitioned through the enclosed unit for apparent total-tract digestibility studies at an interval of 21 days.
The feeding trial consisted of two feeding periods, with each feeding period lasting for 70 days and a wash-out period of 28 days in between where the animals were fed on the pre-trial diet (chaffed Rhodes grass hay). The period effect was designed to demonstrate the effect of season on pasture growth and quality, with the first feeding period coinciding with a rainy and, the second, a dry spell. One animal from the BG group was diagnosed with anaplasmosis 2 weeks prior to the end of Period 1 and was euthanised 1 week later because the animal continued to lose body condition even after treatment. As the disease compromised mean intake, the animal was excluded from the statistical analyses and treated as a missing value and a replacement animal was introduced during the second experimental period. Also, an animal from NG group contracted lumpy skin disease and was euthanised 2 days before the end of Period 2, but the animal’s data were included in the analyses because there was no evidence that the intake was affected.
Feeding and data collection
Steers were fed on fresh grass harvested from the experimental plots as described earlier in the text. Grasses were harvested each afternoon, transported to the animal facility, and chaffed to 2–4 cm the following morning prior to feeding. Daily feed intake throughout each period was determined as the difference between the grass offered and the refusals collected the following morning. Feed was offered at 110% of the net intake in the previous day in three equal tranches (0900 hours, 1200 hours, and 1600 hours) to minimise wastage from the feeding troughs. Orts were collected, weighed and recorded, then pooled by treatment, and samples were taken (∼300 g) and stored in zip-lock polythene bags at −20°C. Samples of the feed offered (∼300 g) were also taken on alternate days, put in polythene bags and stored as described for the refusals. Stored samples were thawed every 14 days, pooled by treatment for rations and refusals separately and a representative subsample (∼300 g) was transferred to labelled paper bags. Fresh weights were recorded then dried in an air-forced oven (Genlab Oven, Genlab Ltd, UK) at 50°C for 96 h before the final weight was recorded. Steers’ LW measurements were taken twice weekly before morning feeding, by using a digital animal-weighing scale (Gallagher Weigh Scale W210, Australia; precision = 0.5 kg).
Total faeces and urine collection
Total collection of urine and faeces was conducted the week after the enteric-CH4 measurement, for each animal management group in each period. Faeces were collected from each pen at least every 2 h and aggregated every 24 h in labelled buckets for each animal. Total faeces from each animal were weighed every morning at 0900 hours, homogenised by hand and a subsample of about 400 g (with the actual weight recorded) was transferred to labelled and pre-weighed aluminum foil trays for oven-drying at 50°C for 96 h. The dried samples were then packed in zip-lock polythene bags and stored at room temperature pending further processing and analysis. Urine was collected continuously using urine collection bags over 7 days for N-balance calculations (data not presented).
Enteric CH4 measurements
Three open-circuit respiratory chambers (No Pollution Industries, Edinburgh, UK) were used to conduct measurement of daily CH4 production (DMP; g/day) of the steers. Construction and operation of the chambers has previously been described in Goopy et al. (2020). In brief, chambers (3.08 m L × 1.50 m W × 2.00 m H) had an internal volume of 8.90 m3 and were equipped with individual environmental-control units. The internal chamber environment was set at 22°C and 50% relative humidity with internal air circulation of 220 L/s and exhaust were set at ∼18 L/s. A cavity ringdown laser absorption spectrometer (Picarro G2508 Analyzer, Santa Clara, CA, USA) was used to measure CH4 concentration (μg/g) of the inlet and exhaust airstreams of the chambers, with the instrument taking a sample from each chamber in succesion, every 12 min. Air flow rate (L/s) was measured using a venturi apparatus with differential pressure transducers (Model DP 2500-R8-AZ, Johnson Controls Inc.). Daily CH4 production was calculated as total volume of air flow through the chamber multiplied by the net mean CH4 concentration over each measurement event. Enteric CH4 production was determined over ∼22.5 h extrapolated to 24 h by applying the mean concentration of CH4 and air flow over the last 3 h of the measurement applied to the final 1.5 h. Measurements were conducted on three separate, alternate days for each animal in both feeding periods, blocked by chamber. Recoveries for CH4 were measured for each chamber at the start and at the end of every measurement period by injecting known amounts of CH4 into the chamber by using a gas-phase titration unit (Environics 4020, Environics Inc., Tolland, USA) and measuring CH4 concentration in the exhaust air at equilibrium. Recoveries were 96 ± 2.6% during the first phase of the experiment and 76 ± 0.49% during the second period (due to deterioration of the rotor seal in the 16-port distribution manifold of the analytical instrument). Recovery losses were corrected for arithmetically.
Sample analyses
Dried feed and faecal samples were ground through a hammer mill fitted with 1 mm sieve and analysed as follows: true DM was determined at 105°C for 24 h in an air-forced oven, while ash was determined by combustion in a muffle furnace at 550°C for 4 h (Heraeus M110 muffle furnace, Heraeus Holding GmbH, Hanau, Germany) according to AOAC methods (Association of Official Analytical Chemists (AOAC) 1990, Method no. 924.05). Feed and refusal samples were analysed for neutral detergent fibre (NDF) and acid detergent fibre (ADF; Association of Official Analytical Chemists (AOAC) 1990, Method no. 6.5.1 and Method no. 6.5.2 respectively), by using an Ankom200 fibre analyser (Ankom Technology Cooperation, Fairport, USA) with alfa-amylase enzyme. Total nitrogen (N) content in the samples was determined by the Kjeldahl procedure (Association of Official Analytical Chemists (AOAC) 1990, Method no. 988.05) using selenium catalyst tablets, and CP was deemed to be total N multiplied by a factor of 6.25. Gross-energy content of feed was determined by bomb calorimetry (Par 6300, Par Instruments). Apparent total-tract digestibility of the diets (%) for DM, organic matter (OM), NDF, ADF and CP was calculated by subtracting the total amount of nutrients excreted in faeces from the respective amounts ingested and dividing the difference by the net nutrient intake.
Estimation of land requirements for fodder production and enteric CH4 production
The data from grass production, plus intake, LW gain and DMP measured during the animal experiment were used to estimate land-area requirements for fodder production and total enteric CH4 production during the period required to bring animals to a pre-determined market weight for each of the three experimental grasses. These estimates were then used as indicators of resource use efficiency. Assuming an initial LW of 216 kg and a market weight of 350 kg, we used the mean ADG of each treatment group to estimate days on feed required to reach market weight. For the estimation of DMP, LW on any given day was deemed to be the ADG plus the LW in the previous day (Eqn 1) and was used to set daily DMI where this was estimated according to Eqn 2 below. Daily CH4 production was deemed as the product of DMI and mean MY (Eqn 3) for the respective grasses. Total calculated feed intake was increased by 10% as an allowance for wastage, in calculating total biomass requirement.
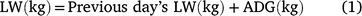
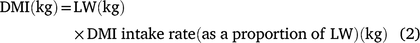
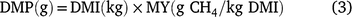
where LW is estimated daily LW; ADG is the ADG per treatment; DMI is estimated daily DM intake and MY is methane yield.
Land needed to produce the amount of feed required for each fodder type was estimated by Eqn 4, as follows:
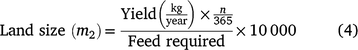
where yield is the amount of annual biomass yield per hectare for each grass species; n is the number of days needed by the animals per treatment to reach final LW and feed required is the net DMI adjusted for 10% losses.
Total enteric CH4 production was calculated as being the sum of DMP over the number of days required to reach market weight. Methane intensity was defined as total CH4 produced divided by the LW gain (g CH4/kg weight gain).
Data analyses
Statistical analyses were performed using R 3.5.3 (R Development Core Team, USA). All dependent variable data were first checked for normality by plotting normal quantile–quantile plots in R. For the agronomic trial data, a linear mixed model was fitted, as follows:
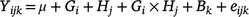
where Yijk is annual biomass yield, μ is the overall mean biomass yield, G is grass species (i = 1–3), H is year of harvest (j = 1–2), Gi × Hj grass species by year interaction, Bk is the random effect of block (j = 1–5) and eijk is the residual error. The chemical composition in the diets was analysed by fitting the following model:
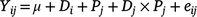
where Yij is the dependent variable, μ is the overall mean of the variable, D is diet (i = 1–3), P is period (j = 1–2), Di × Pj is diet by period interaction, and eijk is the residual error.
For the animal trial measured parameters (intake, apparent total-tract digestibility, ADG, DMP, MY and Ym), a linear mixed model was used, as follows:
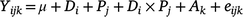
where Yijk is annual biomass yield, μ is the overall mean biomass yield, D is diet (i = 1–3), P is period (j = 1–2), Di × Pj is Diet × Period interaction, Ak is the random effect of animal identity (j = 1–12) and eijk is the residual error. ANOVA Type 3 analysis was then used to test the effect of fixed factors in the models fitted and where interaction was not significant, this was dropped from the model. Least square means were calculated using ‘lsmeans’ package (Lenth and Lenth 2018) and Tukey’s method was used to separate means employing ‘Multicompview’ package (Graves et al. 2015) in R. Level of significance was determined at P = 0.05.
Results
Grass composition and biomass yield
Chemical composition of the three grasses is presented in Table 3. The DM concentration of all grasses was higher in Period 2 than Period 1 (P < 0.001), with NG consistently having lower (P < 0.001) DM than the other two grasses over the two periods. Napier grass also had lower (P < 0.001) OM and GE concentration in both feeding periods than did the other two diets that did not differ from each other. Fibre concentration in all the grasses was higher (NDF: P = 0.006) and ADF P = 0.0035) in Period 1 than in Period 2, with RG having a higher NDF concentration than the other two grasses in both periods.
Rhodes grass yielded the lowest (P < 0.001) mean cumulative DM biomass per annum compared with NG and BG, which did not differ from each other (Table 3). Biomass yields for all the three grasses were higher (P < 0.001) during the first year than in the second year of measurements (data not shown).
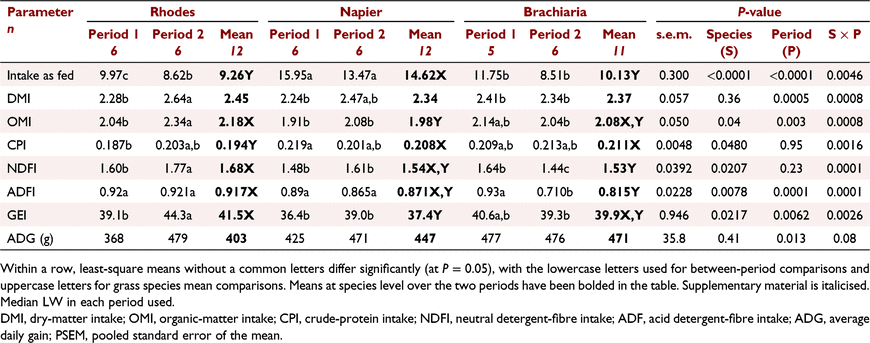
Feed intake and weight gain
There was a significant (P < 0.001) interaction between species and period with respect to mean DMI (over the 65 days of measurement; Table 4) and the interaction persisted even when expressed on a NDF basis (P = 0.0226). Thus, all further results are reported by period. DMI did not differ (P = 0.27) among dietary groups in Period 1 but animals fed on RG had a higher (P = 0.0055) intake than did those fed on BG, but not NG, in Period 2. Grass species influenced CP intake significantly (P = 0.0093) in Period 1, with animals fed on RG having a lower intake than for steers fed on NG. However, the CP intakes in Period 2 were similar (P = 0.17) across the three dietary groups. The OM, NDF and ADF intakes by animals fed on RG were higher (P < 0.05) than those of animals fed on the two other diets in Period 2, but there was no difference among the diets in Period 1 (Table 4). Whereas there was no difference (P = 0.1) among the diets in the GE intake in Period 1, RG animals had a higher (P = 0.0011) intake than did those on the two other diets in Period 2. The ADG of animals fed on RG tended to be lower (P = 0.09) than that of animals fed on NG and BG in Period 1, but ADG did not differ (P = 0.99) among the diets in Period 2 (Table 4). Animals fed on RG had a higher ADG in Period 2 than in Period 1 (P = 0.02). Initial LWs were 214 ± 6.9 versus 242 ± 7.7 kg, while the final weights were 243 ± 9.0 versus 276 ± 9.5 kg for Periods 1 and 2 respectively.
Apparent total-tract nutrient digestibility
The period and period by species interaction effects on DM, OM, CP, NDF and ADF nutrient digestibility were not significant (P > 0.05) and, as such, the results are presented as a mean for the two periods (Table 5). The DM and OM apparent total-tract digestibility of steers fed on RG was lower (P < 0.05) than that of animals fed on NG, but not different from that of animals fed on BG (Table 5). Feeding of RG also resulted in a lower (P < 0.05) CP digestibility than did feeding of NG and BG, with no difference between the latter two diets.
Enteric methane production
Steers fed on NG treatment had higher (P > 0.05) DMP, CH4 per OM intake and Ym than did those fed on RG, but were not different from those fed on BG (Table 6). However, when CH4 was expressed per unit DMI (P = 0.26), DOMI (P = 0.77) or weight gain, there were no differences among the three diets. There was a grass species by period interaction effect (P = 0.021) and period effect (P = 0.048) on DMP (CH4 g/day), with emissions in Period 2 being higher than those in Period 1 in RG and NG diets.
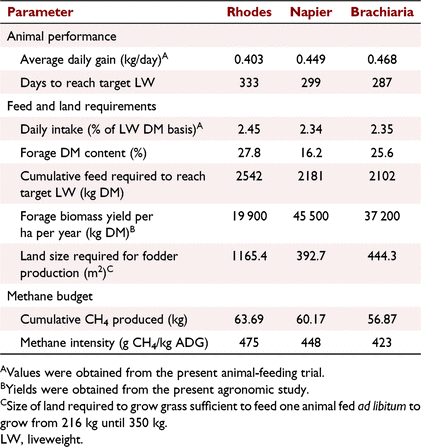
Land requirements for fodder production and enteric methane production
Steers fed on RG took more days to reach target LW than did the steers fed on either NG or BG diets (46 and 34 days respectively; Table 7). The amount of RG fodder required to feed a growing animal over the estimated growth period was about 20% higher than the amount needed for BG and NG diets over the same growth phase. Because of its low biomass yield per unit area among the three grasses, RG needed at least twice the size of land compared with that of the other two grasses to produce enough fodder that is needed. Feeding RG diet was also predicted to produce numerically the highest cumulative CH4 (kg) and CH4 intensity (g CH4 kg LW change) over the growth period, while the BG diet had the lowest estimates for the two parameters (Table 7).
|
Discussion
Grass biomass yield and fodder chemical composition
Biomass yield of the three grass species under identical growing conditions varied dramatically, with RG in particular achieving only about half the yield of the other two species. Reasons for the difference were not investigated, but all grasses were grown under high-rainfall conditions, with supplementary irrigation provided to reduce water stress and help maintain a harvesting schedule. It is well recognised that RG is adapted to conditions of lower precipitation and is more tolerant of water stress (Cook et al. 2005) and thus it seems reasonable to postulate that the relative performance of the three species may differ considerably under different climatic conditions. Yields in all the three grasses were higher during the first year than in the second year and this could have been caused by the grasses being established in plots that had just been cultivated after 12 years of use as grazing field. Ploughing is known to stimulate soil OM (SOM) mineralisation (Sainju et al. 2007) and this could have led to increased nutrient availability for grass growth during the first year. Since no soil amendments were applied, the drop in yield in the second year could be an indication of slowdown of SOM mineralisation.
The results of chemical composition reported here for the three experimental grasses were similar to those in other published studies in both chemical composition (Tran et al. 2009; Archimède et al. 2018) and biomass yield (Kariuki et al. 1999; Arshad et al. 2016; Kifuko-Koech et al. 2016; Njarui et al. 2016). The higher fibre concentration in the grasses fed to the animals in Period 1 that coincided with the wet season (May–July 2018) compared with Period 2 (September–November), which was relatively dry, was consistent with the findings of Habte et al. (2020) where they also observed higher NDF and ADF contents in different genotypes of Napier grass harvested during wet than in the dry season. Because plant growing conditions were more favourable during the wet season, the grasses grew faster, indicated by higher biomass (Fig. 1) and taller height (data not reported), and therefore were likely to have deposited more fibre to support their stature (Pembleton et al. 2017). The lower CP in Period 2 could have been a result of a possible lower available N and moisture level in the soil than in Period 1, compromising uptake by the grasses (Agehara and Warncke 2005).
Effect of grass species on intake
Dry-matter intakes for the three grasses in Period 1 were similar and this was expected because there were minimal quality differences among the three diets offered to the animals. The higher NDF concentration in the diets fed to the animals in Period 1 than in Period 2 could have prolonged rumen retention time during this period and, hence, lowered voluntary intake (Ketelaars and Tolkamp 1992). However, in Period 2, despite RG having a marginally higher NDF concentration than did the other two diets, animals fed on this diet had higher DMI and OMI, which was unexpected. With the mean DMI of about 2.3% and 2.5% of LW reported in Periods 1 and 2 respectively, the present results were consistent with intakes reported elsewhere where cattle were fed ad libtum on tropical grasses of comparable quality (Kurihara et al. 1999; Tomkins et al. 2011; Kennedy and Charmley 2012; Perry et al. 2017).
Apparent total-tract nutrient digestibility and weight gain
The lower DM and OM apparent total-tract digestibility observed for RG group than that for NG could be attributed to the lower CP digestibility in the forage fed to the animals, as DMI and fibre digestibility were not influenced by the treatment. Even though BG and RG were harvested at the same chronological stage, it is possible that harvested RG fodder was already more mature physiologically as evident by the higher NDF content in fodder harvested both in Period 1 and Period 2, and this could explain low protein digestibility (Sarmadi et al. 2016). Advancement in physiological age in fodder is associated with an increased proportion of CP being bound to structural carbohydrates and this compromises its degradability and availability for microbial use in the rumen (Stone 1994).
The similar ADG in all three treatment groups despite possible differences in CP degradability could be because energy and not CP was the first limiting nutrient to higher animal performance. At CP levels of 8–10% reported in the tropical grasses tested in present study, it is well documented that the first limiting nutrient to animal productivity on tropical grasses is often energy availability because of low digestibility (Moran 2005). It is also possible that the higher CP availability in NG and BG did not result in a significantly higher nutrient availability to support notably higher animal growth. Average daily weight gain of about 0.5 kg observed in the present experiment agrees with findings from other studies reported in literature where growing animals were also fed on similar tropical grasses without any supplementation (Kariuki et al. 1998, 1999).
Enteric methane production
The treatment effect on DMP between periods was accounted for by the differences in DMI because the emissions were not different when expressed as MY and Ym. Animals fed on RG diet had a lower DMP than did those on the other two diets and this could be related to lower OM apparent total-tract digestibility, affecting substrate availability for microbial conversion to CH4. The similar MY among the three diets despite the differences in digestibility was in agreement with findings by Pinares-Patiño et al. (2003) who observed no difference in MY of cows grazing timothy grass at different phenotypic stages with varying OM digestibility (56.3–77.6%). However, this finding was contrary to the findings by Kennedy and Charmley (2012) and Montenegro et al. (2016) who observed a negative correlation (−0.21 and −0.89 respectively) between MY and DM digestibility. However, it should be noted that unlike the latter two studies where the better-digested diets resulted in an increased DMI, this was not the case in the present study, suggesting similar rumen retention time among the three diets. The lower DMP and Ym by animals fed on RG, therefore, could have been a result of a lower amount of substrate per unit DMI for methanogens to convert to CH4. Our reported mean MYs (RG: 26.7 (24.7–28.8); NG: 28.5 (26.5–30.6); BG: 27.5 (25.4–29.7) [mean (range) g CH4/kg DMI] and Ym [RG: 0.0873 (0.0806–0.0940); NG: 0.0987 (0.0920–0.1054); BG: 0.0903 (0.0833–0.0973)] agree with values reported elsewhere in literature where cattle were fed on tropical grasses (Kurihara et al. 1999; Tomkins et al. 2011; Chaokaur et al. 2015; Archimède et al. 2018; Goopy et al. 2020). However, these figures were higher than what has been reported in some studies (Kennedy and Charmley 2012: MY: 19.0–21.67; Ym: 0.055–0.069; Perry et al. 2017: MY: 19.5, Ym: 0.058) where animals were also fed tropical grasses of comparable quality to those used in the present study. Even though the explanation for the poor agreement of these parameters (YM and Ym) in animals fed on poor tropical grasses, diet-characteristic effects on rumen passage rate and fermentation pathways remain the most plausible source of these variations (Kennedy and Charmley 2012) and warrant further investigation. Our results were also higher than the current Intergovernmental Panel on Climate Change (IPCC) recommendations for cattle feeding on high-forage diets (MY: 23.3 g/kg DMI and Ym: 7.0%; Gavrilova et al. 2019) and this could be a result of animal and diet differences in the two contexts (Kurihara et al. 1999). It is worth noting that temperate breeds of cattle used in the development of IPCC equations have been shown to have at least 14% higher maintenance-energy requirements than for tropical cattle (Kurihara et al. 1999; Goopy et al. 2020). This, together with the higher gross-energy content in temperate grasses, could possibly lead to over-estimation of DMI in the tropical context and, hence, the lower MY and Ym than what we observed.
Land requirements for fodder production and enteric CH4 production
The numerically lower ADG of steers fed on RG than that of steers fed on NG and BG resulted in animals fed on this diet taking a longer time period to reach the target LW when animals were simulated to grow from 216 to 350 kg. Because of the longer animal growth period, the amount of feed and the size of land required were also higher than those for the other two diets. Because of the low biomass yield per unit area of RG compared with the other two grasses reported in the present study, this further confounded the bigger land-size requirements for the diet. The higher CH4 emissions and emission intensity for animals fed on RG diet equally can be attributed to the longer duration it takes for the animals to reach the target weight. The findings of this work suggested that more land is required to gain the same LW in growing cattle fed on RG than that required for those on NG and BG diets that were comparable. In terms of GHG emissions, feeding of RG diet would produce at least 5% more enteric CH4 than would the other two diets.
Conclusions
Feeding cattle improved Brachiaria grass species did not improve animal performance over the other commonly grown tropical grasses in East Africa, namel Rhodes and Napier, in terms of voluntary intake and weight gain. This suggests that the opportunity to improve livestock productivity in smallholder systems in East Africa, where feed and nutrient scarcity is often the main limiting factor, could lie in better management of available fodder resources and not necessarily in improved grass varieties and cultivars. More compelling from our findings were the high MY and Ym across the three grasses studied, notwithstanding that the animals were fed at production level. If this effect is confirmed, it could mean about a 20% underestimate of enteric-CH4 emissions from local cattle breeds in East Africa. More similar studies using local breeds of cattle and local feed resources are required to confirm these findings. Improved ruminant feeding practices that would lead to better utilisation of tropical grasses, better animal productivity and reduced enteric-CH4 emissions need to be explored to make smallholder cattle production more sustainable and to increase profit margins for the farmers.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
Funding for this study was provided by the German Federal Ministry for Economic Cooperation and development (BMZ) and the German Technical Cooperation (GIZ) under the project ‘In situ assessment of GHG emissions from two livestock systems in East Africa – determining current status and quantifying mitigation options’ (Grant no. 5 521 914) and further support was given by the International Fund for Agricultural Development (IFAD) funded project ‘Climate-smart dairy systems in East Africa through improved forages and feeding strategies: enhancing productivity and adaptive capacity while mitigating GHG emissions’ (Grant no. 20 000 001 002). The authors also acknowledge the CGIAR Fund Council, Australia (ACIAR), Irish Aid, European Union, International Fund for Agricultural Development (IFAD), Netherlands, New Zealand, UK, USAID and Thailand for funding to the CGIAR Research Program on Livestock.
Acknowledgements
The authors are grateful to Dr Alice Onyango, Victor Omondi, George Wanyama and Francis Njenga for conducting the laboratory analyses of our samples. We also sincerely thank Dr Sonja Leitner who provided us with the weather data that they collected over the period of our experiments. We acknowledge the ILRI farm management, particularly Dr Ilona Gluecks, for providing us with part of their property to grow the grasses that were used in the experiments.
References
Agehara S, Warncke DD (2005) Soil moisture and temperature effects on nitrogen release from organic nitrogen sources. Soil Science Society of America Journal 69, 1844–1855.| Soil moisture and temperature effects on nitrogen release from organic nitrogen sources.Crossref | GoogleScholarGoogle Scholar |
Anindo DO, Potter HL (1994) Seasonal variation in productivity and nutritive value of Napier grass at Muguga, Kenya. East African Agricultural and Forestry Journal 59, 177–185.
| Seasonal variation in productivity and nutritive value of Napier grass at Muguga, Kenya.Crossref | GoogleScholarGoogle Scholar |
Archimède H, Rira M, Eugène M, Fleury J, Lastel ML, Periacarpin F, Silou-Etienne T, Morgavi DP, Doreau M (2018) Intake, total-tract digestibility and methane emissions of Texel and Blackbelly sheep fed C4 and C3 grasses tested simultaneously in a temperate and a tropical area. Journal of Cleaner Production 185, 455–463.
| Intake, total-tract digestibility and methane emissions of Texel and Blackbelly sheep fed C4 and C3 grasses tested simultaneously in a temperate and a tropical area.Crossref | GoogleScholarGoogle Scholar |
Arshad I, Ali W, Khan ZA, Bhayo WA (2016) Effect of water stress on the growth and yield of Rhodes grass (Chloris gayana. L. Kunth.). PSM Biological Research 1, 58–61.
Association of Official Analytical Chemists (AOAC) (1990) Official methods of analysis. Vol. II, 15th edn. Sec. 985.29. The Association of Official Analytical Chemists: Washington DC.
Barbehenn RV, Chen Z, Karowe DN, Spickard A (2004) C3 grasses have higher nutritional quality than C4 grasses under ambient and elevated atmospheric CO2. Global Change Biology 10, 1565–1575.
| C3 grasses have higher nutritional quality than C4 grasses under ambient and elevated atmospheric CO2.Crossref | GoogleScholarGoogle Scholar |
Beauchemin KA, McGinn SM (2006) Enteric methane emissions from growing beef cattle as affected by diet and level of intake. Canadian Journal of Animal Science 86, 401–408.
| Enteric methane emissions from growing beef cattle as affected by diet and level of intake.Crossref | GoogleScholarGoogle Scholar |
Bedaso NH, Bezabih M, Kelkay TZ, Adie A, Khan NA, Jones CS, Mekonnen K, Wolde-meskel E (2021) Effect of fertilizer inputs on productivity and herbage quality of native pasture in degraded tropical grasslands. Agronomy Journal 114, 216–227.
| Effect of fertilizer inputs on productivity and herbage quality of native pasture in degraded tropical grasslands.Crossref | GoogleScholarGoogle Scholar |
Broucek J (2014) Production of methane emissions from ruminant husbandry: a review. Journal of Environmental Protection 5, 1482–1493.
| Production of methane emissions from ruminant husbandry: a review.Crossref | GoogleScholarGoogle Scholar |
Buxton DR (1996) Quality-related characteristics of forages as influenced by plant environment and agronomic factors. Animal Feed Science and Technology 59, 37–49.
| Quality-related characteristics of forages as influenced by plant environment and agronomic factors.Crossref | GoogleScholarGoogle Scholar |
Caswell H, Reed F, Stephenson SN, Werner PA (1973) Photosynthetic pathways and selective herbivory: a hypothesis. The American Naturalist 107, 465–480.
| Photosynthetic pathways and selective herbivory: a hypothesis.Crossref | GoogleScholarGoogle Scholar |
Cecchi G, Wint W, Shaw A, Marletta A, Mattioli R, Robinson T (2010) Geographic distribution and environmental characterization of livestock production systems in eastern Africa. Agriculture, Ecosystems & Environment 135, 98–110.
| Geographic distribution and environmental characterization of livestock production systems in eastern Africa.Crossref | GoogleScholarGoogle Scholar |
Chaokaur A, Nishida T, Phaowphaisal I, Sommart K (2015) Effects of feeding level on methane emissions and energy utilization of Brahman cattle in the tropics. Agriculture, Ecosystems & Environment 199, 225–230.
| Effects of feeding level on methane emissions and energy utilization of Brahman cattle in the tropics.Crossref | GoogleScholarGoogle Scholar |
Charmley E, Williams SRO, Moate PJ, Hegarty RS, Herd RM, Oddy VH, Reyenga P, Staunton KM, Anderson A, Hannah MC (2016) A universal equation to predict methane production of forage-fed cattle in Australia. Animal Production Science 56, 169–180.
| A universal equation to predict methane production of forage-fed cattle in Australia.Crossref | GoogleScholarGoogle Scholar |
Cook BG, Pengelly BC, Brown S, Donnelly J, Eagles D, Franco M, Hanson J, Mullen BF, Partridge I, Peters M (2005) ‘Tropical forages: an interactive selection tool.’ (CSIRO, DPI&F(Qld), CIAT and ILRI: Brisbane, Qld, Australia)
Djikeng A, Rao IM, Njarui D, Mutimura M, Caradus J, Ghimire SR, Johnson L, Cardoso JA, Ahonsi M, Kelemu S (2014) Climate-smart Brachiaria grasses for improving livestock production in East Africa. Tropical Grasslands – Forrajes Tropicales 2, 38–39.
| Climate-smart Brachiaria grasses for improving livestock production in East Africa.Crossref | GoogleScholarGoogle Scholar |
Farrell G, Simons SA, Hillocks RJ (2001) Aspects of the biology of Ustilago kamerunensis, a smut pathogen of Napier grass (Pennisetum purpureum). Journal of Phytopathology-Phytopathologische Zeitschrift 149, 739–744.
| Aspects of the biology of Ustilago kamerunensis, a smut pathogen of Napier grass (Pennisetum purpureum).Crossref | GoogleScholarGoogle Scholar |
Gavrilova O, Leip A, Dong H, MacDonald JD, Gomez Bravo CA, Amon B, Barahona Rosales R, Prado AD, de Lima MA, Oyhantcabal W, Van der Weerden, TJ (2019). Emissions from livestock and manure management. In ‘2019 Refinement to the 2006 IPCC guidelines for national greenhouse gas inventories: Agriculture, forestry and other land use’. vol. 4. (Eds E Calvo Buendia, K Tanabe, A Kranic, J Baasansuren, M Fukuda, S Ngarize, A Osako, Y Pyrozhenko, P Shermanau, S Federici) pp. 10.49–10.73. (IPCC: Geneva, Switzerland)
Ghimire SR, Njarui D, Mutimura M, Cardoso J, Johnson L, Gichangi E, Teasdale S, Odokonyero K, Caradus J, Rao IM (2015) Climate-smart Brachiaria for improving livestock production in East Africa: emerging opportunities. In ‘Proceedings of the 23rd international grassland congress’, New Delhi, India, 20–24 November 2015. pp. 361–370. (Range Management Society of India)
Gibson DJ (2009) ‘Grasses and grassland ecology.’ (Oxford University Press)
Goopy JP, Korir D, Pelster D, Ali AIM, Wassie SE, Schlecht E, Dickhoefer U, Merbold L, Butterbach-Bahl K (2020) Severe below-maintenance feed intake increases methane yield from enteric fermentation in cattle. British Journal of Nutrition 123, 1239–1246.
| Severe below-maintenance feed intake increases methane yield from enteric fermentation in cattle.Crossref | GoogleScholarGoogle Scholar |
Graves S, Piepho H-P, Selzer ML, Dorai-Raj S (2015) Package “multcompView”. Visualizations of Paired Comparisons. Available at https://cran.r-project.org/web/packages/multcompView/multcompView.pdf [accessed June 13, 2018].
Habte E, Muktar MS, Abdena A, Hanson J, Sartie AM, Negawo AT, Machado JC, Ledo FJdS, Jones CS (2020) Forage performance and detection of marker trait associations with potential for Napier grass (Cenchrus purpureus) improvement. Agronomy 10, 542
| Forage performance and detection of marker trait associations with potential for Napier grass (Cenchrus purpureus) improvement.Crossref | GoogleScholarGoogle Scholar |
Holmann F, Rivas L, Argel P, Pérez E (2004) Impact of the adoption of Brachiaria grasses: Central America and Mexico. Livestock Research for Rural Development 16, 98
Hunter RA (2007) Methane production by cattle in the tropics. British Journal of Nutrition 98, 657
| Methane production by cattle in the tropics.Crossref | GoogleScholarGoogle Scholar |
Janssen PH (2010) Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Animal Feed Science and Technology 160, 1–22.
| Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics.Crossref | GoogleScholarGoogle Scholar |
Johnson KA, Johnson DE (1995) Methane emissions from cattle. Journal of Animal Science 73, 2483–2492.
| Methane emissions from cattle.Crossref | GoogleScholarGoogle Scholar |
Jones P, Devonshire BJ, Holman TJ, Ajanga S (2004) Napier grass stunt: a new disease associated with a 16SrXI group phytoplasma in Kenya. Plant Pathology 53, 519
| Napier grass stunt: a new disease associated with a 16SrXI group phytoplasma in Kenya.Crossref | GoogleScholarGoogle Scholar |
Kariuki JN, Gachuiri CK, Gitau GK, Tamminga S, van Bruchem J, Muia JMK, Irungu KRG (1998) Effect of feeding Napier grass, lucerne and sweet potato vines as sole diets to dairy heifers on nutrient intake, weight gain and rumen degradation. Livestock Production Science 55, 13–20.
| Effect of feeding Napier grass, lucerne and sweet potato vines as sole diets to dairy heifers on nutrient intake, weight gain and rumen degradation.Crossref | GoogleScholarGoogle Scholar |
Kariuki JN, Gitau GK, Gachuiri CK, Tamminga S, Muia JMK (1999) Effect of supplementing Napier grass with desmodium and lucerne on DM, CP and NDF intake and weight gains in dairy heifers. Livestock Production Science 60, 81–88.
| Effect of supplementing Napier grass with desmodium and lucerne on DM, CP and NDF intake and weight gains in dairy heifers.Crossref | GoogleScholarGoogle Scholar |
Kennedy PM, Charmley E (2012) Methane yields from Brahman cattle fed tropical grasses and legumes. Animal Production Science 52, 225–239.
| Methane yields from Brahman cattle fed tropical grasses and legumes.Crossref | GoogleScholarGoogle Scholar |
Ketelaars JJMH, Tolkamp BJ (1992) Toward a new theory of feed-intake regulation in ruminants 1. Causes of differences in voluntary feed-intake: critique of current views. Livestock Production Science 30, 269–296.
| Toward a new theory of feed-intake regulation in ruminants 1. Causes of differences in voluntary feed-intake: critique of current views.Crossref | GoogleScholarGoogle Scholar |
Kifuko-Koech MN, Ndung’u-Magiroi KW, Kamidi M (2016) The effects of cutting frequency on the dry matter yield and nutritive quality of Brachiaria grass cultivars in acidic soils of western Kenya. In ‘Climate smart Brachiaria grasses for improving livestock production in East Africa – Kenya experience. Proceedings of the workshop held in Naivasha, Kenya, Nairobi, Kenya, 15 September 2016’. pp. 90–101. (Kenya Agricultural and Livestock Research Organization)
Kurihara M, Magner T, Hunter RA, McCrabb GJ (1999) Methane production and energy partition of cattle in the tropics. British Journal of Nutrition 81, 227–234.
| Methane production and energy partition of cattle in the tropics.Crossref | GoogleScholarGoogle Scholar |
Lee MA, Davis AP, Chagunda MGG, Manning P (2017) Forage quality declines with rising temperatures, with implications for livestock production and methane emissions. Biogeosciences 14, 1403–1417.
| Forage quality declines with rising temperatures, with implications for livestock production and methane emissions.Crossref | GoogleScholarGoogle Scholar |
Lenth R, Lenth MR (2018) Package ‘lsmeans’. The American Statistician 34, 216–221.
Lima MA, Paciullo DSC, Morenz MJF, Gomide CAM, Rodrigues RAR, Chizzotti FHM (2019) Productivity and nutritive value of Brachiaria decumbens and performance of dairy heifers in a long-term silvopastoral system. Grass and Forage Science 74, 160–170.
| Productivity and nutritive value of Brachiaria decumbens and performance of dairy heifers in a long-term silvopastoral system.Crossref | GoogleScholarGoogle Scholar |
Montenegro J, Barrantes E, DiLorenzo N (2016) Methane emissions by beef cattle consuming hay of varying quality in the dry forest ecosystem of Costa Rica. Livestock Science 193, 45–50.
| Methane emissions by beef cattle consuming hay of varying quality in the dry forest ecosystem of Costa Rica.Crossref | GoogleScholarGoogle Scholar |
Moran J (2005) ‘Tropical dairy farming: feeding management for small holder dairy farmers in the humid tropics.’ (CSIRO Publishing) https://doi.org/10.1071/9780643093133
Njarui DM, Gichangi EM, Ghimire SR, Muinga RW (2016) Effect of season and cutting interval on productivity and nutritive value of Brachiaria grass cultivars in semi-arid tropical Kenya. Climate smart Brachiaria grasses for improving livestock production in East Africa – Kenya experience. In ‘Proceedings of the workshop held in Naivasha, Kenya, 14–15 September 2016’. pp. 298. (Kenya Agricultural and Livestock Research Organization)
Ondiko CN, Njunie MN, Njarui DMG, Auma E, Ngode L (2016) Effects of cutting frequency on forage production and nutritive value of Brachiaria grass cultivars in coastal lowlands of Kenya. In ‘Climate smart Brachiaria grasses for improving livestock production in East Africa – Kenya experience. Proceedings of the workshop held in 660 Naivasha, Kenya, Nairobi, Kenya, 14–15 September 2016’. pp. 271. (Kenya Agricultural and Livestock Research Organization: Nairobi, Kenya)
Paciullo DSC, de Castro CRT, Gomide CAdM, Maurício RM, Pires MdFÁ, Müller MD, Xavier DF (2011) Performance of dairy heifers in a silvopastoral system. Livestock Science 141, 166–172.
| Performance of dairy heifers in a silvopastoral system.Crossref | GoogleScholarGoogle Scholar |
Parr CL, Lehmann CER, Bond WJ, Hoffmann WA, Andersen AN (2014) Tropical grassy biomes: misunderstood, neglected, and under threat. Trends in Ecology & Evolution 29, 205–213.
| Tropical grassy biomes: misunderstood, neglected, and under threat.Crossref | GoogleScholarGoogle Scholar |
Pelster DE, Gisore B, Goopy J, Korir D, Koske JK, Rufino MC, Butterbach-Bahl K (2016) Methane and nitrous oxide emissions from cattle excreta on an East African grassland. Journal of Environmental Quality 45, 1531–1539.
| Methane and nitrous oxide emissions from cattle excreta on an East African grassland.Crossref | GoogleScholarGoogle Scholar |
Pembleton KG, Rawnsley RP, Turner LR, Corkrey R, Donaghy DJ (2017) Quantifying the interactions between defoliation interval, defoliation intensity and nitrogen fertiliser application on the nutritive value of rainfed and irrigated perennial ryegrass. Crop and Pasture Science 68, 1100–1111.
| Quantifying the interactions between defoliation interval, defoliation intensity and nitrogen fertiliser application on the nutritive value of rainfed and irrigated perennial ryegrass.Crossref | GoogleScholarGoogle Scholar |
Perry LA, Al Jassim R, Gaughan JB, Tomkins NW (2017) Effect of feeding forage characteristic of wet- or dry-season tropical C4 grass in northern Australia, on methane production, intake and rumen outflow rates in Bos indicus steers. Animal Production Science 57, 2033–2041.
| Effect of feeding forage characteristic of wet- or dry-season tropical C4 grass in northern Australia, on methane production, intake and rumen outflow rates in Bos indicus steers.Crossref | GoogleScholarGoogle Scholar |
Pinares-Patiño CS, Baumont R, Martin C (2003) Methane emissions by Charolais cows grazing a monospecific pasture of timothy at four stages of maturity. Canadian Journal of Animal Science 83, 769–777.
| Methane emissions by Charolais cows grazing a monospecific pasture of timothy at four stages of maturity.Crossref | GoogleScholarGoogle Scholar |
Sainju UM, Lenssen A, Caesar-Thonthat T, Waddell J (2007) Dryland plant biomass and soil carbon and nitrogen fractions on transient land as influenced by tillage and crop rotation. Soil and Tillage Research 93, 452–461.
| Dryland plant biomass and soil carbon and nitrogen fractions on transient land as influenced by tillage and crop rotation.Crossref | GoogleScholarGoogle Scholar |
Sarmadi B, Rouzbehan Y, Rezaei J (2016) Influences of growth stage and nitrogen fertilizer on chemical composition, phenolics, in situ degradability and in vitro ruminal variables in amaranth forage. Animal Feed Science and Technology 215, 73–84.
| Influences of growth stage and nitrogen fertilizer on chemical composition, phenolics, in situ degradability and in vitro ruminal variables in amaranth forage.Crossref | GoogleScholarGoogle Scholar |
Stone BA (1994) Prospects for improving the nutritive value of temperate, perennial pasture grasses. New Zealand Journal of Agricultural Research 37, 349––363.
| Prospects for improving the nutritive value of temperate, perennial pasture grasses.Crossref | GoogleScholarGoogle Scholar |
Thornton PK (2010) Livestock production: recent trends, future prospects. Philosophical Transactions of the Royal Society B: Biological Sciences 365, 2853–2867.
| Livestock production: recent trends, future prospects.Crossref | GoogleScholarGoogle Scholar |
Tomkins NW, McGinn SM, Turner DA, Charmley E (2011) Comparison of open-circuit respiration chambers with a micrometeorological method for determining methane emissions from beef cattle grazing a tropical pasture. Animal Feed Science and Technology 166–167, 240–247.
| Comparison of open-circuit respiration chambers with a micrometeorological method for determining methane emissions from beef cattle grazing a tropical pasture.Crossref | GoogleScholarGoogle Scholar |
Tran H, Salgado P, Lecomte P (2009) Species, climate and fertilizer effects on grass fibre and protein in tropical environments. The Journal of Agricultural Science 147, 555–568.
| Species, climate and fertilizer effects on grass fibre and protein in tropical environments.Crossref | GoogleScholarGoogle Scholar |
Zhu Y, Merbold L, Pelster D, Diaz-Pines E, Wanyama GN, Butterbach-Bahl K (2018) Effect of dung quantity and quality on greenhouse gas fluxes from tropical pastures in Kenya. Global Biogeochemical Cycles 32, 1589–1604.
| Effect of dung quantity and quality on greenhouse gas fluxes from tropical pastures in Kenya.Crossref | GoogleScholarGoogle Scholar |