Mycoplasma vaccination responses in immunodepressed weanling pigs supplemented with S. cerevisiae boulardii
I. P. Oswald A D , A. P. F. L. Bracarense B and N. Schwerin CA INRA ToxAlim, Toulouse, France.
B University of Londrina, Brazil.
C Lallemand Pty. Ltd, Maroochydore, QLD 4558.
D Corresponding author. Email: isabelle.oswald@toulouse.inra.fr
Animal Production Science 55(12) 1528-1528 https://doi.org/10.1071/ANv55n12Ab074
Published: 11 November 2015
Mycotoxins are known for their immunodepressive properties in animals by affecting non-specific and acquired immunity (Oswald et al. 2005). As a consequence, immunity acquired through vaccination is also impaired by mycotoxin ingestion. Saccharomyces cerevisiae boulardii (SCB) is extensively documented for its immune-modulatory benefits in animals and in man (Kelesidis and Pothoulakis 2012). This study focused on the interaction between mycotoxin and yeast by examining in particular the vaccination response and small intestinal histomorphometry as ways of assessing pigs’ responses to the challenge.
Twenty-four castrated 6-week-old pigs (13.6 ± 1.80 kg; mean ± SD) were involved in a 4-week study. Pigs were individually housed and randomly allocated to one of four diets: Control (C); Fumonisin B1 (FB1) at 12 ppm; SCB CNCM I-1079 at 5x109 cfu/kg feed; and FB1 + SCB. At d 0 and 8, the pigs were vaccinated using a commercial vaccine allowing subsequent specific immunoglobulin (Ig) titration as a model vaccine (Mycoplasma hyopneumoniae, Stellamune mono-injection; Pfizer, France). Weekly blood samples were taken for measurement of Ig content by ELISA (Bethyl, TX, USA for IgA, IgG, IgM; Kit IDVET for M. hyponeumoniae specific Ig). Pigs were necropsied at the completion of the study and samples of jejunum and ileum processed for morphometry (Bracarense et al. 2012). Data were analysed per time point by ANOVA and differences between means separated by Tukey’s post-hoc test (XLSTAT®; USA).
No treatment effect (P > 0.05) was depicted for IgA, IgM and IgG. However, specific Ig levels against Mycoplasma increased from d 15 in C whereas it was still minimal for FB1 (Fig. 1). Interestingly, FB1-SCB reached a similar Ig titer than SCB after 29 d, suggesting an inhibition of a deleterious effect from FBI. Histologically, the intestine was affected by FB1 and addition of SCB restored (P < 0.05) intestinal lining in the ileum compared to FB1 alone (12.0, 9.7, 15.3, 11.0 for C, SCB, FB1 and FB1-SCB, respectively). Villous height increased (P < 0.05) in both jejunum and ileum for FB1-SC vs FB1 to become comparable to C (342, 354, 280, 345 µm for C, SCB, FB1 and FB1-SCB, respectively). No treatment effect (P > 0.05) was found for crypt depth.
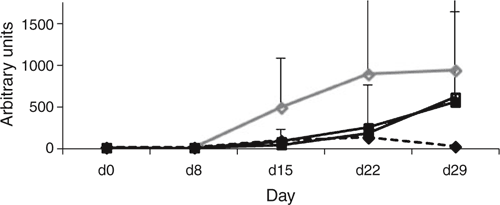
|
In conclusion, the FB1 immune challenge model given to pigs notably reduced the specific antibody response. The use of SCB (strain I-1079) increased the challenged pigs’ vaccination response to M. hyopneumoniae. Measures of small intestinal morphometry were positively improved with SCB reaching villi similar in height to non-challenged animals. However, the findings of this pilot study require confirmation in larger scale studies to assess the impact on animal performance and lungs lesions.
References
Bracarense APFL, Lucioli J, Grenier B, Moll WD, Schatzmayr G, Oswald IP (2012) British Journal of Nutrition 107, 1776–1786.| Crossref | GoogleScholarGoogle Scholar |
Kelesidis T, Pothoulakis C (2012) Therapeutic Advances in Gastroenterology 5, 111–125.
| Crossref | GoogleScholarGoogle Scholar |
Oswald IP, Marin DE, Bouhet DE, Pinton P, Taranu L, Accensi F (2005) Food Additives and Contaminants 22, 354–360.
| Crossref | GoogleScholarGoogle Scholar |
Acknowledgements are given to J. D. Baily for fumonisin production and to A. M. C. Cossalter and J. Laffitte for technical help.


