The 4th Molecular Materials Meeting (M3) @ Singapore
Yun Zong A C and T. S. Andy Hor A B CA Institute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), 3 Research Link, Singapore 117602, Republic of Singapore.
B Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543, Republic of Singapore.
C Corresponding authors. Email: y-zong@imre.a-star.edu.sg; andyhor@imre.a-star.edu.sg

Yun Zong is co-Head of Technology Development in the Director's Office at the Institute of Materials Research and Engineering (IMRE) of the Agency for Science, Technology and Research (A*STAR), Singapore. He received his B.Sc. and M.Sc. degrees from Wuhan University (China), and his Ph.D. from Johannes-Gutenberg University of Mainz and Max-Planck Institute for Polymer Research, supported by a scholarship granted by the Katholischer-Akademischer Auslaender Dienst (KAAD), Germany. He was a post-doctoral research fellow at the National University of Singapore, a visiting scholar at Stanford University, and a technical advisor of Int.-Mat. Technologies. As a Senior Scientist in IMRE, he currently heads the Advanced Energy Storage Laboratory and the Molecular Materials Laboratory. He is also Programme Manager of the Advanced Energy Storage Programme at the Science and Engineering Research Council (SERC) of A*STAR. His current research focuses on the development of nanostructured porous materials, bifunctional catalysts, and ionic liquids for metal–air rechargeable battery applications. |

Professor Andy Hor is the Executive Director of the Institute of Materials Research and Engineering (IMRE) of the Agency for Science, Technology and Research (A*STAR) and Professor of Chemistry at the National University of Singapore. He is a Fellow of the Singapore National Academy of Science and the Royal Society of Chemistry, President of the Singapore National Institute of Chemistry, President of the Federation of Asian Chemical Societies, and Concurrent Professor of Fudan University (China). He is the founding chair of the Singapore National Academy of Science and A*STAR Young Scientist Award, and President of the Jury of L'Oréal for Women in Science (Singapore) in 2012 and 2013. His research interests are in heterometallic materials, organometallic catalysis, and supramolecular self-assembly, and he has published over 330 ISI papers with approximately 500 annual citations in recent years. He chaired the 15th Asian Chemical Congress and the 41st International Coordination Chemistry Conference, and is a member of the international advisory boards of Chemistry – An Asian Journal (VCH-Wiley), Dalton Transactions (RSC), Inorganic Chemistry Frontiers (RSC), and Inorganica Chimica Acta (Elsevier), as well as Associate Editor of the Australian Journal of Chemistry (CSIRO) and Editor of the Journal of Molecular & Engineering Materials (WS). |
Australian Journal of Chemistry 67(10) 1365-1369 https://doi.org/10.1071/CH14479
Published: 10 October 2014
The Molecular Materials Meeting (M3) @ Singapore is only four years old, but growing rapidly. Some 300 scientists and students from 20 countries gathered at Biopolis in Singapore from 14 to 16 January 2014 to exchange ideas on the latest developments of molecular materials research and technology (Fig. 1). The presentations at this M3 conference mainly focussed on the themes of Sustainable Technology, Energy and Additive Manufacturing (STEAM), Advanced Materials Technology (AMT), Materials for Healthcare and Lifestyle (MHL), as well as Food Science and Technology (FST). The three-day conference was concluded with a banquet dinner in a well-known Chinese restaurant in the downtown area with good food and wine. The five best poster presentations were selected by a dedicated panel comprising plenary speakers, editors, and senior professors, and prizes were presented to the winning junior researchers and students at the banquet. The evening provided an ideal networking opportunity for everyone.
The previous three commemorative editions of the M3@Singapore conferences in the Australian Journal of Chemistry were well-received by the scientific community with good citations. The first edition [Aust. J. Chem. 2011, 64 (9)] published papers covering diverse topics, e.g. atom transfer radical polymerization (ATRP)-based functional amphiphilic polymers synthesis,[1] temperature-driven self-organization of molecules,[2] metal–organic frameworks (MOFs),[3] aggregation-induced emission (AIE) bioimaging,[4] nanoparticle-catalyzed water-splitting,[5] dye-sensitized solar cells (DSSC),[6] etc. The second edition [Aust. J. Chem. 2012, 65 (9)] featured contributions on research in nanoparticles and organic semiconductor materials.[7] It covered the harnessing of nanotechnology for new applications, e.g. electro-responsive core-shell nanoparticles for rheology tuning,[8] chitosan-coated Au/Pd alloy nanoparticles and nanoclusters as catalysts for aerobic oxidative homocoupling reactions,[9] carbon nanotube-based materials to catalyze fuel cell reactions,[10] and CdTe-based hybrid fibres to enable low-voltage-driven electroluminescence devices.[11] In the area of organic semiconductor research, the edition focussed on the structure–property relationship that leads to design and synthesis of high performance molecular materials, e.g. heterocyclic dyes for efficient DSSCs,[12] phenyl-1H-pyrrole end-capped thiophenes for organic field-effect transistors (OFET),[13] and pyridine-bearing dihexylquaterthiophene as the blue emitter for organic light-emitting diodes (OLED).[14] The third edition [Aust. J. Chem. 2013, 66 (9)] introduced papers that illustrated various aspects of molecular materials research,[15] e.g. experimental revelation of heterocyclic triazole as a moiety that enhances magnetic property of dinuclear CuII complexes,[16] theoretical modelling resultant matching requirement of nuclear vibrations with the energy levels in rational molecular and material design for organic photovoltaics,[17] matching of optical band gaps with the electrochemical band gaps via tuning of the molecular structure of polymers to enhance redox stability,[18] polyurethane/clay nanocomposites with high mechanical strength and good thermal conductivity,[19] review and perspective of nanoparticles-hydrogel hybrid materials,[20] fabrication of highly sensitive SERS substrates at low cost for sensing-based analytical applications,[21] and a review on the combination of the design of molecular materials with physical structures (e.g. repeated strips with layered structures) for waveguide applications.[22] These are representative papers of the work presented at M3@Singapore.
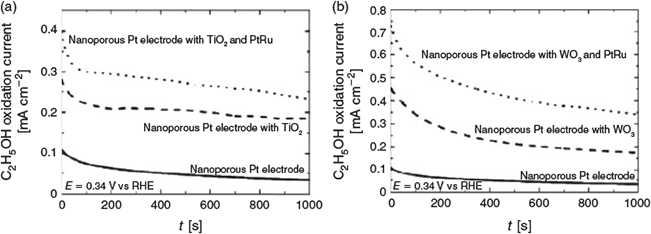
In this commemorative edition of the 4th M3@Singapore, we have selected 11 peer-reviewed papers that report or review the latest progress in molecular materials research. In nanostructured materials preparation, Rutkowska et al. (University of Warsaw, Poland) report on electrocatalytic metal oxide-supported noble metal nanoparticles on nanoporous platinum electrodes for electrooxidation of ethanol in which synergistic effects were observed.[23] The authors propose that the electrocatalytic activities were enhanced by the large population of hydroxyl groups on the surfaces of the nanostructured TiO2 and WO3, where the –OH groups induced oxidation of CO passivating intermediates (on Pt) and increased the activity of the Ru component. It was further noted that WO3 and TiO2 would undergo different mechanisms, and the oxidation reaction of ethanol was typically more efficient at the nanoporous Pt substrate covered by WO3-supported PtRu than at the Pt substrate with TiO2-supported PtRu on the surface (Fig. 2). Bai et al. (IMRE, Singapore) present the synthesis and catalytic activity of a magnetic Pd/Fe3O4 composite that was prepared by immobilizing triethoxysilane tethered tridentate pincer ligands onto the surfaces of pre-synthesized Fe3O4 nanoparticles followed by PdII complexation and in situ reduction.[24] The resultant hydrophilic Pd nanoparticles are 2–4 nm in size, and exhibit good thermal stability and excellent catalytic activity in aqueous phase reduction of 4-nitrophenol to 4-aminophenol. The simple synthetic procedure reported therein is comparable with the reported solvothermal (~5 nm Pd) and H2 reduction (~2 nm Pd) approaches which are generally more demanding in terms of reaction conditions and duration.
|
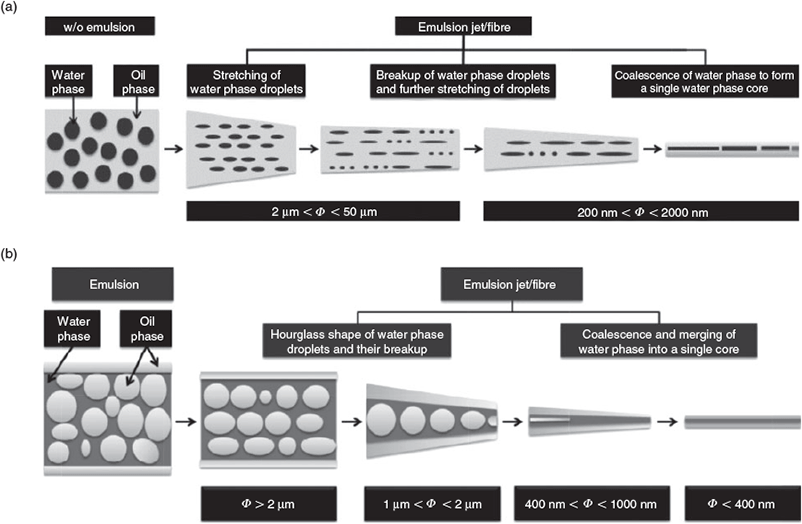
In nanostructured materials fabrication, Florence et al. (Mother Teresa Women’s University, India) prepared micropatterned ZnSe nanospheres arrays on silicon wafer-supported self-assembled sacrificial PS masks as anti-reflective coatings.[25] The reflectance off the surface of the as-prepared coatings was found to be reduced by nearly half in comparison to that of the ZnSe nanospheres fabricated on conventional gold-coated silicon substrate in the range of 300–800 nm. C. Wang and M. Wang (The University of Hong Kong, Hong Kong SAR, China) present a comparative study on the formation of core-shell structures in emulsion electrospun fibres using surfactants of different natures as stabilizers.[26] Their emulsions consisted of deionized water or phosphate buffer saline as the water phase, and a poly(lactic-co-glycolic acid) solution as the oil phase, and stabilized by non-ionic hydrophobic surfactant Span 80 or ionic hydrophilic surfactant sodium dodecyl sulfate (SDS), respectively. The authors found that the small amount of such surfactants significantly impacts the evolution of the core-shell structure of the emulsion electrospun fibres. In general, the droplets of water phase in the emulsion jet will undergo multi-stage stretching and recombination, and eventually forming the fibre core which can be discrete or continuous, varying notably if different surfactants are used in the emulsion (Fig. 3). The fibre diameter is dependent on polymer solution concentration, and the formation of more continuous core fibres requires higher water phase volume. Such core-shell nanofibres can be used to construct scaffolds of good wettability for tissue engineering applications.
|
In the popular area of 2D nanomaterials, Le Lay et al. (Aix-Marseille Université, CNRS, France) highlight the rise of elemental two-dimensional (2D) materials beyond the well-studied graphene (Fig. 4).[27] In this brief highlight, the authors cover single-layer silicene to multilayer silicene, germanene, and stanene/tinene, as well as phosphorene, which are all highly popular in non-graphene 2D material research. In conclusion, it was stressed that these non-traditional 2D materials possess significant advantages over graphene-based materials in terms of easier material transfer and integration. Some of them could be potentially used for quantum computing applications, thus promising a bright future for these materials.
|
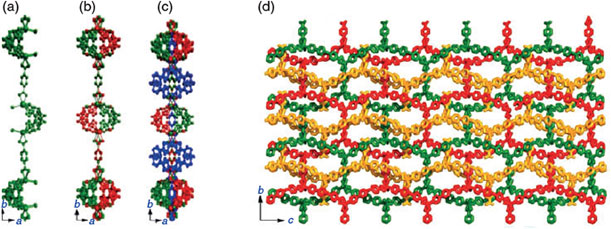
In fluorescence-related research, Low et al. (IMRE, Singapore) demonstrate a simple, fast, and sensitive method for the quantification of albumin.[28] In their approach, the authors used non-fluorescent fluorescamine to react with primary amines to form highly fluorescent products, which were proven valid for the detection of albumin with various fluorogenic dyes. Pan et al. (IMRE, Singapore) report on photoluminescent chitosan with low cytotoxicity produced via the reaction of the amines with CO2.[29] The concentration of carbamato anions that are believed to be the photoluminescence activators can be increased via CO2 bubbling of chitosan. The photoluminescence of this chitosan was found to be dependent on excitation wavelength, in that excitation with a longer wavelength generally leads to the red-shift of the emission wavelength. The low cytotoxicity of chitosan makes it a good candidate for fluorescent labels in bioimaging applications. For CO2 absoprtion, Chen et al. (Southern Medical University, China) present a Cd-based coordination polymer containing three integrated polymeric components that is porous and selectively adsorbs CO2.[30] This interpenetrated coordination polymer was constructed by two neutral and entangled 2D nets and one zwitterionic 1D chain with corner-sharing grids propagating along the c direction that lock the consecutive ligand struts of the nets (Fig. 5). The polymer can be readily isolated by a simple mixing of ligands, indicating good thermodynamic stability of such unusual interpenetrating motifs. As it is stabilized by strong ligand π–π stacking, the interpenetrated polymer structure remains robust even if the larger and softer bromides are substituted with chlorides. The authors showcase the use of water for the synthesis of water-stable PCPs/MOFs containing pyridinium carboxylate, paving the way for practical applications such as CO2 removal from fuel gas.[30]
|
For special application of polymers in oilfields, Deng et al. (Shandong University, China) report how electrolyte concentration, temperature, and shear rate could affect the rheological properties of hydrophobically associating polyacrylamide (HA-PAM) solution containing hydrophobically modified monomer and sodium 2-acrylamido-2-methylpropanesulfonic sulfonate.[31] It was found that the salt tolerance and shearing resistance of the HA-PAM polymer are much higher than that of the commonly used partially hydrolyzed polyacrylamide, promising great potential for use in tertiary oil recovery in oilfields with high salinity. The authors also propose the salt resistance mechanism of HA-PAM in solution based on the results obtained through both experimental methods and molecular simulation (Fig. 6), providing a useful guideline for the design and synthesis of high salt resistance, water-soluble polymers through the revealed structure–performance relationship.
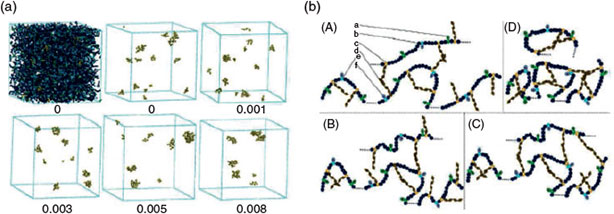
|
In food science and technology research, Liu et al. (National University of Singapore, Singapore) review the biocatalytic synthesis of flavor-active esters via lipase-mediated biocatalysis and the reaction mechanisms.[32] After elaboration on the characteristics of lipases and their applications in industries as enzymes for flavour and fragrance production, the authors summarize the synthesis of flavour esters through esterification or transesterification catalyzed by lipases, in solvent-free systems or in reaction media such as organic solvents, ionic liquids, supercritical fluids, and aqueous media, where the lipases were found to exhibit reaction media-dependent kinetic catalytic behaviours. The lipase catalytic mechanism is believed to be determined by such factors including substrate polarity, product, and reaction media. This understanding of enzyme properties and behaviours under different reaction conditions is crucial for production of esters on an industrial scale. From their perspective, the authors stress that interpreting the reaction mechanisms and the kinetic models of the lipase-catalyzed reactions in the complex media would help achieve in situ generation of flavours in food matrices containing water, which remains an attractive subject for future study. Finally, Chaumpluk and Suriyasomboon[33] report on a simple paper-based laboratory-on-a-chip method for the detection of a highly pathogenic strain of porcine reproductive and respiratory syndrome virus.
The M3@Singapore conference series provides a unique platform for the exchange of ideas, fostering collaboration and developing partnerships in the broad arena of molecular materials research and technologies. Known for its education focus, research investment, economic affluence, and highly capable R&D workforce, Singapore is the ideal meeting point between east and west, as well as south and north. With Singapore celebrating its 50th birthday in 2015 when M3@Singapore turns five years old, the 5th M3@Singapore, tentatively scheduled on 3–5 August 2015 (http://www.imre.a-star.edu.sg/), is expected to attract even more participants. We welcome all students, academics, researchers, and innovators to join us on this special occasion to review the past 50 years in molecular materials research while looking forward to the next 50 years.
References
[1] H. Hussain, E. Amado, J. Kressler, Aust. J. Chem. 2011, 64, 1183.| Crossref | GoogleScholarGoogle Scholar |
[2] J. M. MacLeod, F. Rosei, Aust. J. Chem. 2011, 64, 1299.
| Crossref | GoogleScholarGoogle Scholar |
[3] R. P. Davies, P. D. Lickiss, K. Robertson, A. J. P. White, Aust. J. Chem. 2011, 64, 1239.
| Crossref | GoogleScholarGoogle Scholar |
[4] Y. Hong, J. Wing Yip Lam, S. Chen, B. Z. Tang, Aust. J. Chem. 2011, 64, 1203.
| Crossref | GoogleScholarGoogle Scholar |
[5] K. J. Young, Y. Gao, G. W. Brudvig, Aust. J. Chem. 2011, 64, 1221.
| Crossref | GoogleScholarGoogle Scholar | 22140273PubMed |
[6] W. Zhang, R. Zhu, B. Liu, S. Ramakrishna, Aust. J. Chem. 2011, 64, 1282.
| Crossref | GoogleScholarGoogle Scholar |
[7] Y. Zong, T. S. A. Hor, Aust. J. Chem. 2012, 65, 1191.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWksb7I&md5=495cc284cbe18fc70a112e95e299e5ecCAS |
[8] Y. D. Liu, K. Zhang, W. L. Zhang, H. J. Choi, Aust. J. Chem. 2012, 65, 1195.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWksbzM&md5=b475d70bff11adb170386d7bdc1e0282CAS |
[9] O. Sophiphun, J. Wittayakun, R. N. Dhital, S. Haesuwannakij, A. Murugadoss, H. Sakurai, Aust. J. Chem. 2012, 65, 1238.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWksb%2FJ&md5=44e77e5be3af445f984d36df003bfacbCAS |
[10] J. Liu, L. Lai, N. G. Sahoo, W. Zhou, Z. Shen, S. H. Chan, Aust. J. Chem. 2012, 65, 1213.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWksbzJ&md5=79ba7bb9970d1a9cb99becb937ef1680CAS |
[11] M. Ando, C. Hosokawa, P. Yang, N. Murase, Aust. J. Chem. 2012, 65, 1257.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWksbzP&md5=a3c959fa44bbee6990c9af51b1309888CAS |
[12] Q. Li, Z. Jiang, J. Qin, Z. Li, Aust. J. Chem. 2012, 65, 1203.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWksbzF&md5=680652ae0c0b8c2a005c422a5befd448CAS |
[13] Y. Liu, J. Zhang, Y. Liu, G. Yu, Z. Ge, Aust. J. Chem. 2012, 65, 1252.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWksb3N&md5=b7923c90acbb083e705b3f9281b76425CAS |
[14] P. Sonar, S. G. Santamaria, T. T. Lin, A. Sellinger, H. Bolink, Aust. J. Chem. 2012, 65, 1244.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWksbzN&md5=be0f3d17579ed6889e05c4ebfb6cc9d9CAS |
[15] Y. Zong, T. S. A. Hor, Aust. J. Chem. 2013, 66, 993.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVektrfM&md5=e9aee9576483d99657af9b00e9ecd2a6CAS |
[16] S. Q. Bai, L. Jiang, J. L. Zuo, C. H. Yan, T. S. A. Hor, Aust. J. Chem. 2013, 66, 1029.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVektrfL&md5=708fa7fe9a325e046459f5d1733defb0CAS |
[17] S. Manzhos, Aust. J. Chem. 2013, 66, 1021.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVekt77I&md5=927e6d21aa47cf9efee11e6d1af2dc40CAS |
[18] S. Z. H. Lim, W. T. Neo, C. M. Cho, X. Wang, A. Y. X. Tan, H. S. O. Chan, J. Xu, Aust. J. Chem. 2013, 66, 1048.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVekt77O&md5=e0164dea666fb68079d57072c1bec28cCAS |
[19] S. L. Sin, J. N. Kumar, H. R. Tan, C. He, Y. Liu, J. Xu, Aust. J. Chem. 2013, 66, 1039.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVekt77J&md5=baddf0c1c780c34b7c016dbe8b8fc663CAS |
[20] E. Ye, X. J. Loh, Aust. J. Chem. 2013, 66, 997.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVekt77P&md5=aadc8cf60cc13491a87b2eab1473f0e4CAS |
[21] S. Dinda, F. L. Yap, V. Suresh, R. K. Gupta, D. Das, S. Krishnamoorthy, Aust. J. Chem. 2013, 66, 1034.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVektrfK&md5=b95baf2e13ba7c77abf4faba90076757CAS |
[22] M. C. Tan, D. J. Naczynski, P. V. Moghe, R. E. Riman, Aust. J. Chem. 2013, 66, 1008.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVektrfF&md5=ea0738fc722ad56ea5d37de1e726914fCAS |
[23] I. A. Rutkowska, M. D. Koster, G. J. Blanchard, P. J. Kulesza, Aust. J. Chem. 2014, 67, 1414.
| Crossref | GoogleScholarGoogle Scholar |
[24] S.-Q. Bai, L. Jiang, S.-L. Huang, M. Lin, S.-Y. Zhang, M.-Y. Han, J. Xu, Y. Lu, G.-X. Jin, T. S. A. Hor, Aust. J. Chem. 2014, 67, 1387.
| Crossref | GoogleScholarGoogle Scholar |
[25] S. S. Florence, P. Sachan, R. K. Gupta, R. John, U. Mahalingam, Aust. J. Chem. 2014, 67, 1427.
| Crossref | GoogleScholarGoogle Scholar |
[26] C. Wang, M. Wang, Aust. J. Chem. 2014, 67, 1403.
| Crossref | GoogleScholarGoogle Scholar |
[27] G. Le Lay, E. Salomon, P. De Padova, J.-M. Layet, T. Angot, Aust. J. Chem. 2014, 67, 1370.
| Crossref | GoogleScholarGoogle Scholar |
[28] M. Low, K. Y. Win, E. Ye, S. Liu, S. H. Ng, X. Zhou, M.-Y. Han, Aust. J. Chem. 2014, 67, 1382.
| Crossref | GoogleScholarGoogle Scholar |
[29] X. Pan, W. Ren, L. Gu, G. Wang, Y. Liu, Aust. J. Chem. 2014, 67, 1422.
| Crossref | GoogleScholarGoogle Scholar |
[30] J.-X. Chen, N.-N. Ding, M. Chen, W.-H. Chen, D. J. Young, W.-H. Zhang, T. S. A. Hor, Aust. J. Chem. 2014, 67, 1391.
| Crossref | GoogleScholarGoogle Scholar |
[31] Q. Deng, H. Li, Y. Li, X. Cao, Y. Yang, X. Song, Aust. J. Chem. 2014, 67, 1396.
| Crossref | GoogleScholarGoogle Scholar |
[32] J. Sun, L. W. W. Lee, S. Q. Liu, Aust. J. Chem. 2014, 67, 1373.
| Crossref | GoogleScholarGoogle Scholar |
[33] P. Chaumpluk, A. Suriyasomboon, Aust. J. Chem. 2014, 67, 1434.
| Crossref | GoogleScholarGoogle Scholar |



