Natural Products Isolated from Endemic Tasmanian Vascular Plants*
Bianca J. Deans A , Miguel de Salas B C , Jason A. Smith A D and Alex C. Bissember A D
A D
A School of Natural Sciences – Chemistry, University of Tasmania, Hobart, Tas. 7001, Australia.
B School of Natural Sciences – Biology, University of Tasmania, Hobart, Tas. 7001, Australia.
C Tasmanian Herbarium, Tasmanian Museum and Art Gallery, Hobart, Tas. 7000, Australia.
D Corresponding authors. Email: jason.smith@utas.edu.au; alex.bissember@utas.edu.au

Bianca Deans obtained her B.Sc. (Hons, First Class) in chemistry from the University of Tasmania in 2014. Her honours research with Associate Professor Jason Smith and Associate Professor Robert Shellie concerned the bioprospecting of Australian native plants employing pressurised hot water extraction (PHWE), as well as the isolation of major components of Australian Xanthorrhoea resin. In 2015, Bianca commenced Ph.D. research with Professor Smith and Dr Alex Bissember. Her research project is focused on natural products isolation studies of various endemic Tasmanian plants using PHWE and aims to enhance the chemotaxonomic understanding of these under-explored species. |

Miguel de Salas is a plant taxonomist and Senior Curator at the Tasmanian Herbarium in Hobart. His research interests encompass most of Tasmania’s native vascular flora, but especially carnivorous plants, daisies, and violets. In a previous life, he was a molecular phylogenist working on harmful marine phytoplankton. |

Jason (Alfred) Smith is a graduate of the Flinders University of South Australia where he obtained both his B.Sc. (Hons) and Ph.D. degrees, the latter under the supervision of Professor Rolf Prager. He has carried out post-doctoral research at The Texas A&M University (with Professor Sir Derek H. R. Barton) and at The Australian National University (with Professor Martin G. Banwell). In 2001, he joined the School of Chemistry at The University of Tasmania where his research activities focus on the development of new synthetic methodologies and their application to the synthesis of biologically active and/or structurally complex molecules, including the exploitation of pyrrole as a molecular template for the assembly of alkaloids and the application of pressurised hot water extraction (PHWE) for bioprospecting of natural products. In 2005, he received The Royal Australian Chemical Institute’s Athel Beckwith Lectureship. |

Alex Bissember received his Ph.D. in chemistry in 2010 from the Australian National University under the supervision of Professor Martin G. Banwell. He subsequently undertook post-doctoral research with Professor Gregory C. Fu at the Massachusetts Institute of Technology and the California Institute of Technology (2010–2013). In 2013, he commenced his independent career at the University of Tasmania where he is currently Senior Lecturer in chemistry. His research interests span areas of organic, organometallic, inorganic, and natural products chemistry, and chemical education. |
Australian Journal of Chemistry 71(10) 756-767 https://doi.org/10.1071/CH18283
Submitted: 8 June 2018 Accepted: 11 July 2018 Published: 8 August 2018
Abstract
Tasmania is the south-eastern island state of Australia. It is geographically isolated and is recognised for both its rich diversity of plant species and high degree of endemism. Although 530 endemic Tasmanian vascular plant species are known, natural products have only been isolated from 27 of these species (~5.1 %), representing 3 classes (Dicotyledonae, Monocotyledonae, and Gymnospermae), 12 families, and 14 genera. Terpenoids, flavonoids, and alkaloids are the major classes of compound that have been isolated from these species. This report provides the first review of the natural products isolated from endemic Tasmanian plant species and covers ~70 years of research in this area.
Introduction
Following the geographical separation of Tasmania from mainland Australia ~14000 years ago,[1] Tasmania has developed rich levels of plant diversity and endemism.[2] Accordingly, Tasmania is regarded as a ‘hotspot’ of global importance for paleoendemic plants,[3] which are particularly ancient clades that are geographically restricted typically owing to selection pressures causing extinction in other areas.[4] The geographical isolation of species can result in genetic and morphological divergence, as well as the evolution of unique and distinctive phytochemistry. Thus, the distinct phytochemical profiles of endemic Tasmanian plants may represent a source of novel and interesting natural products with relevance to drug discovery and significant chemotaxonomic value.
The most recent study, completed in 2017, identified 530 extant species of endemic Tasmanian vascular plants. These include: 363 species of Dicotyledons (dicots), 150 Monocotyledons (monocots), 9 Gymnosperms, and 8 Pteridophytes.[5] No reviews concerning natural products isolation studies of endemic Tasmanian plants have been published to date and this article presents and discusses all of the relevant research in this area. A large number of reported natural products studies on such plant species, the earliest of which date from the mid-twentieth century, employed only qualitative analytical methods (TLC analysis) and/or mass spectrometric analysis of extracts. In these cases, pure compounds were typically not isolated and structural assignment was undertaken without utilising key techniques such as NMR spectroscopy. Consequently, this review is restricted to studies reporting the isolation of natural products featuring comprehensive characterisation data or unambiguous evidence supporting assigned structures.
Natural products isolation studies featuring NMR spectroscopic analysis have only been undertaken on 27 (~5.1 %) of the 530 extant species of endemic Tasmanian vascular plants over a span of ~70 years. An overview of this research, grouped by the class of vascular plant (dicots, monocots, and gymnosperms), is provided in Table 1. Natural product isolation studies have focused heavily on dicots (Table 1), which is the most species-rich class, featuring 363 extant species. Asteraceae (the daisies, with 74 species) is the largest dicot family, and also represents the most heavily studied group of endemic Tasmanian vascular plants with regards to phytochemistry. In contrast, the second largest family in the monocots (Orchidaceae, with 72 endemic Tasmanian species) has not been the subject of a single isolation study. Despite possessing 150 extant endemic species, the monocots are highly under-represented in phytochemical investigations. Indeed, only two species have been examined from two separate genera. Finally, a high proportion of natural products isolation studies have been conducted on gymnosperms (four of the nine extant species have been investigated). This probably derives from this group forming the basis of traditional forestry and timber industries in Tasmania. Interestingly, no reported natural product isolation studies have focused on endemic Tasmanian fern species (class Pteridophyta).

|
Class Dicotyledoneae
Family Asteraceae
The daisy family in Tasmania is the richest in endemic taxa, with a total of 74 species that are not found anywhere else in the world. Natural products isolation studies have been performed on eight species studied from four genera within this family.
Bedfordia
Bedfordia salicina is the only endemic Tasmanian member of this genus from which natural products (mainly terpenoids) have been reported. Specifically, sesquiterpenoids represent the majority of compounds isolated from B. salicina, with several compounds first reported from this species. These include furano-eremophilane derivative 1 and ligularenolide (2), as well as the dimeric eremophilane sesquiterpene 9β,9′β-bis-1,8-dihydroligularenolide (3a), which were isolated from the roots and aerial parts of B. salicina. (Fig. 1).[6] Dimer 3b was also isolated from the roots of this plant. Diene 4, the p-methoxycinnamate derivative 5a, and acids 6–10 were also obtained from the aerial parts of B. salicina, whereas clerodane diterpenoids bedfordiaditerpenalcohol (11) and isobedfordiaditerpenalcohol (12), pentadec-1-ene, chromanone 13, and compound 5b, the methyl ester of caffeic acid, were all isolated from the roots and aerial parts of B. salicina.[6] Another cinnamate ester was found in B. salicina roots.[6] To date, acids 8 and 9, as well as the diterpenoid 11 are unique to B. salicina, with acids 6 and 7 unique to the family Asteraceae.
Odixia
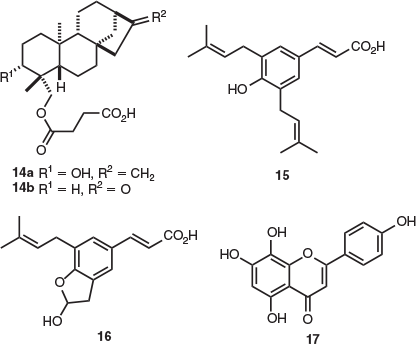
Natural product isolation studies have been reported on both endemic Tasmanian members of the genus Odixia, namely Odixia achlaena and Odixia angusta. Five ent-kaurene diterpenoids, including representative compound 14a, and nor-ent-kaurane derivative 14b were isolated from the aerial parts of O. angusta.[7] All of these compounds were obtained as their respective methyl esters after derivatisation. Both methyl esters derived from p-coumaric acid 15 and odixia acid (16) were also isolated from O. angusta.[7] Acid 16 is unique to this species. In addition, the two flavones isoscutellarein (17) and isoscutellarein-4′-methyl ester were isolated from the combined leaf and inflorescence material of both O. achlaena and O. angusta (Fig. 2).[8]
|
Ozothamnus
Natural products have been isolated from 4 of 13 endemic Tasmanian Ozothamnus species (O. hookeri, O. ledifolius, O. scutellifolius, and O. lycopodioides). The ent-kaurane diterpenoids represent the most common class of compounds isolated from these Ozothamnus species. This includes (–)-ent-16α-kauranol (18a), which was obtained from the aerial parts of both O. hookeri[8,9] and O. scutellifolius,[8,10] and the diol derivative ent-kauran-16α,19-diol (18b), which was reported from the aerial parts of O. ledifolius[11] and O. hookeri (Fig. 3).[9] In addition, the triol derivatives 16α,17,19-trihydroxy-ent-kaurane and 16β,17,19-trihydroxy-ent-kaurane were both isolated from the aerial parts of O. hookeri.[9] Furthermore, acetate derivatives 18c and 18e were found in the aerial parts of O. scutellifolius,[10] as well as dimethyl acetal-containing structures 19 (O. hookeri).[9] ent-Kaurane aldehyde 18d and acids 20a–c were isolated primarily from the aerial parts of O. scutellifolius.[10]
Numerous flavonoids have been isolated from endemic Tasmanian Ozothamnus members. For example, galangin derivative 21a was isolated from the aerial parts of O. ledifolius[8] and related flavonoid 21b was present in the aerial parts of O. hookeri.[8] Chalcones pinocembrin and pinocembrin-7-methyl ether were reported to be the major components obtained from the aerial parts of O. ledifolius; however, no spectroscopic data were disclosed.[8] The major flavone found in the aerial part of O. hookeri was compound 21d, while analogue 21c was obtained from the aerial parts of O. lycopodioides.[8] Other flavonoids isolated from the aerial parts of O. lycopodioides include linderoflavone B (22a) and eupalestin (22b), and similar compounds, such as 5,6,7,8,3′,4′-hexamethoxyflavone, nobiletin, and 5′-methoxynobiletin.[12]
Sesquiterpenoids have been found in Ozothamnus species. Specifically, the eudesmane derivative 3-oxocostusic acid (23) was identified as the major component from the aerial parts of O. ledifolius.[11] Three bisabolane sesquiterpenoids, bisabol-l-one (24a), 12-acetoxybisabol-l-one (24b), and 12-hydroxybisabol-l-one (24c), were isolated as minor constituents from the same species.[11] In addition, the triterpenoids betulinic acid (25) and morolic acid (26) were also isolated O. ledifolius.[11] Coumarin 27 was isolated from the aerial parts of O. lycopodioides and this represents the only coumarin that has been isolated from the Ozothamnus genus.[12] In addition, the long-chain fatty acid docosanoic acid, the novel phenolic ester derivative 3-(4-hydroxyphenyl)propyl docosanoate, and phenol 3-(4-hydroxyphenyl)propan-l-ol were also isolated from the aerial parts of O. hookeri.[9]
Olearia
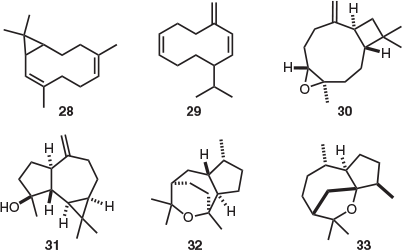
Of the 10 endemic Tasmanian species in the genus Olearia, O. phlogopappa is the only one from which natural products have been isolated and extensively characterised. After analysis and isolation of the essential oil components of O. phlogopappa, the major compound identified was bicyclogermacrene (28), in addition to germacrene-D (29), caryophyllene oxide (30), spathulenol (31), guaiane sesquiterpenoid kessane (32), and liguloxide (33) (Fig. 4).[13] The stereochemistry of terpenoids 28 and 29 was not specified by the authors.
|
Family Elaeocarpaceae
Aristotelia
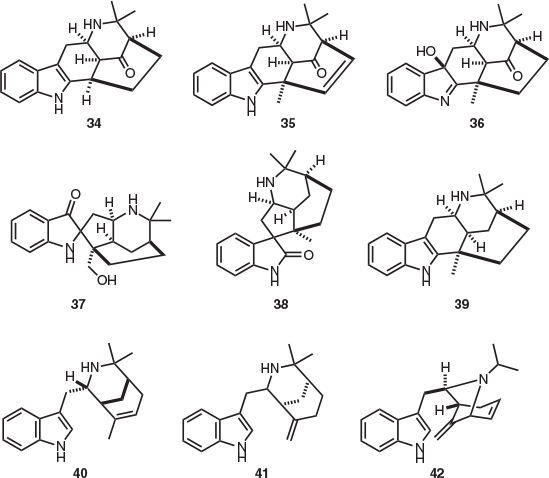
Aristotelia peduncularis is the only endemic Tasmanian member of the family Elaeocarpaceae. This species contains a large number of structurally diverse indole alkaloids. This includes aristoserratine (34),[14] the structure of which was secured by X-ray crystallography,[15] peduncularistine (35), triabunnine (36), aristolarine (37),[16] tasmanine (38), aristoteline (39),[17] hobartine (40), and sorelline (41) (Fig. 5).[18] The isolation of peduncularine (42)[19] prompted several spectroscopic, degradative,[20] and synthetic investigations[21] to elucidate its structure. Another proposed alkaloid ‘isopeduncularine’ was later reported from A. peduncularis;[22] however, it was later established that this natural product was the hydrochloride salt of heterocycle 42.[23] Alkaloids 34–42 are all unique to the genus Aristotelia.
|
Family Escalloniaceae
Anopterus
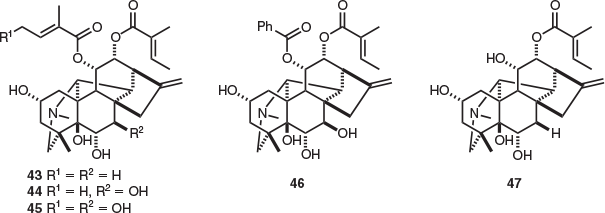
Of the two endemic Tasmanian species within the family Escalloniaceae, natural products isolation studies have only been undertaken on Anopterus glandulosus (native laurel). A range of alkaloids belonging to the ent-kaurene diterpenoid class were isolated from this species, with all of these alkaloids unique to the genus Anopterus. These include the alkaloids anopterine (43) and 7β-hydroxyanopterine (44), which were isolated from the leaves, twigs[24] and bark of A. glandulosus[25] (Fig. 6). In addition, other alkaloids isolated from A. glandulosus leaves and twigs include 4′,7β-dihydroxyanopterine (45), 7β-hydroxyanopteryl 11α-benzoate 12α-tiglate (46), and anopteryl 12α-tiglate (47).[24]
|
Family Myrtaceae
Twenty-nine species from four genera of the family Myrtaceae are endemic to Tasmania. Of these, the majority (21 species) belong to the genus Eucalyptus, and this is the only Myrtaceae genus from which isolation studies have been conducted.
Eucalyptus
Natural products have been isolated and extensively characterised from only 4 of the 21 endemic Tasmanian Eucalyptus species: E. delegatensis, E. risdonii, E. urnigera, and E. viminalis. 4-Phenylbutan-2-one was isolated from E. delegatensis leaves after derivatisation as the corresponding the 2,4-dinitrophenylhydrazone.[26] Eucalyptin (48a) and 11,12-dehydroursolic lactone acetate (49) were isolated from E. risdonii leaves.[27] E. urnigera leaves also contained compounds 48a and 49, in addition to 5-hydroxy-7,4′-dimethoxy-6-methylflavone (48b).[28,29]
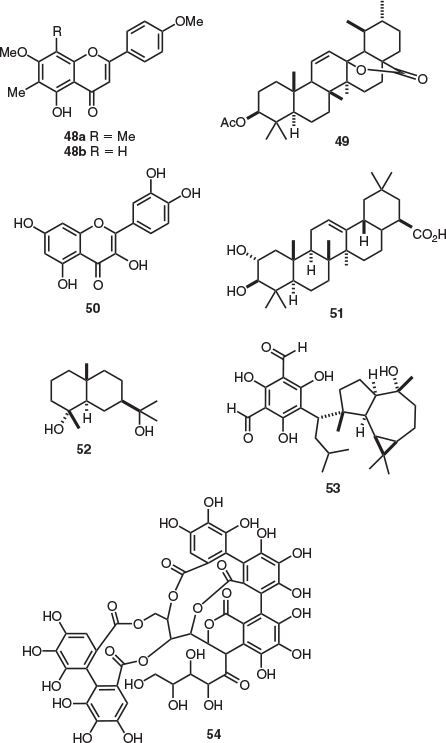
Several flavonoids have been isolated from the leaves of E. viminalis, including the C-methyl flavones eucalyptin (48a)[27,29] and flavonoid 6,8-dimethyl-5,7-dihydroxy-4′-methoxyflavone.[29] Glycoside-containing quercetin (50) derivatives, including quercitrin (quercetin 3-α-l-rhamnopyranoside), hirsutrin (quercetin 3-β-d-glucopyranoside), and rutin (quercetin 3-rutinoside) were also present.[30] Terpenoids have been obtained from E. viminalis leaves including the oleanane natural product maslinic acid (51),[31] lactone 49,[32] and cryptomeridiol (52).[31] Furthermore, the rare phenol aldehyde euvimal-1 (53) was also isolated from E. viminalis, which represents the first and only report of this meroterpene.[33] The C-glycosyl ellagitannin grandinin (54) and the dimeric ellagitannin oenothein B were isolated from the leaves of E. viminalis (Fig. 7).[34,35] Interestingly, there are few reports of C-glycosyl ellagitannins isolated from the genus Eucalyptus. The ellagitannin pterocarinin A represents the only reported natural product isolated from the bark of E. viminalis.[34]
|
Family Pittosporaceae
Billardiera
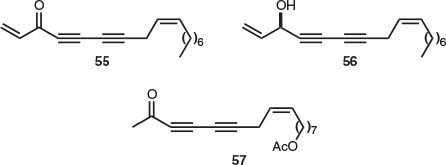
All of the four endemic Tasmanian species of the family Pittosporaceae belong to the genus Billardiera. The only natural product isolations study on endemic Tasmanian Billardiera was published in 1975 and this research focused on the vine B. longiflora. Four polyyne natural products were found. Falcarinone (55) was isolated from the roots and aerial parts, (–)-falcarinol (56) was present in the roots, and aerial sections also contained acetate 57 (Fig. 8).[36] However, in 2004, B. longiflora was reclassified into four individual species (B. longiflora, B. macrantha, B. nesophila, and B. viridiflora).[37] Phytochemical studies of these reclassified species are yet to be reported. Thus, the true identity of the Billardiera specimen featured in this study is unclear.
|
Family Proteaceae
Tasmania is home to 20 endemic Proteaceae species belonging to nine genera.[5] Three of the endemic genera are monotypic (Agastachys, Bellendena, and Cenarrhenes), making the entire genus endemic. Some of these species are highly paleoendemic – notably, Bellendena montana and Cenarrhenes nitida. Both of these represent the only living members of their respective clades, which have been dated as ~85 and ~30 million years old respectively.[38]
Agastachys
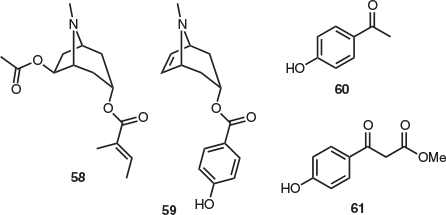
The only natural product isolation study undertaken on the leaves and stems of Agastachys odorata provided tropane alkaloids 58 and 59, p-hydroxyacetophenone (60), and methyl(p-hydroxybenzoyl)acetate (61) (Fig. 9). This represents the first and only reported isolation of tropane alkaloid 59. However, recent studies indicate that compounds 60 and 61 are likely artefacts of isolation (see below). Although the authors noted the presence of two additional compounds, which were suspected to be alkaloids, these were not isolated or characterised.[39]
|
Bellendena
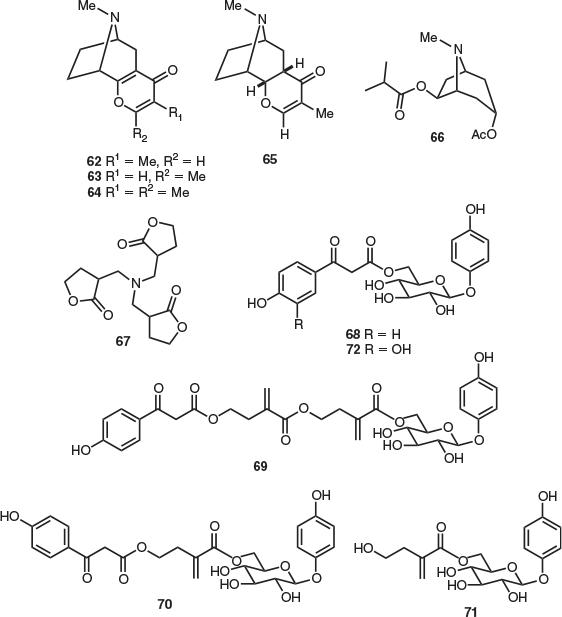
In comparison with other endemic Tasmanian Proteaceae members, the monotypic alpine shrub Bellendena montana has been extensively studied. Isolated alkaloids include the γ-pyrano-tropane bellendine (62), which was extracted from the inflorescences of B. montana (Fig. 10).[40] The structure of bellendine was secured by X-ray crystallography.[41] A separate phytochemical investigation indicated that bellendine (62) was the major alkaloid in B. montana flowers, and its isomer isobellendine (63) represented the major alkaloid of the ‘other parts of the plant’ examined.[42] An extraction of the upper leaves, flowers, and flowering stems of B. montana provided related alkaloids 62 and 63, darlingine (64), and the pyrono-tropane alkaloids 5,11-dihydroisobellendine (65), 2,3-dihydrobellendine, 2,3-epidihydrobellendine, 2,3-dihydrodarlingine, (±)-3α-acetoxy-6β-isobutoxytropane (66), (±)-6β-acetoxy-3α-isobutoxytropane, and 3α-acetoxy-6β-hydroxytropane.[42] Each of these isolated alkaloids is unique to the family Proteaceae. In 1979, four alkaloids were isolated from B. montana, which were not characterised.[42] On further study, one of these alkaloids was subsequently identified as the γ-lactone 67.[43] However, alkaloid 67 is likely an artefact of isolation, potentially formed by the reaction of ammonia and the later-identified arbutin derivative 71 during the extraction process (see below). In addition, benzoyl acetate 61, which was also present in the endemic Proteaceae A. odorata, was found in the flowers of B. montana,[40] However, this is also likely artefact of isolation (see below).
|
A broad phytochemical survey of 120 Proteaceae species that employed quantitative gas–liquid chromatography (GLC) to analyse leaf polyol sugars was reported in 2005 by Bieleski and Briggs.[44] In their study, which featured B. montana, C. nitida, and nine Persoonia species, the authors observed that the respective polar extracts obtained from these plants contained numerous unidentified polar constituents. Significantly, Bieleski and Briggs stated, ‘These additional peaks were almost always characteristic of a genus, and they clearly indicate the presence in the leaf extracts of compounds other than sugars or polyols that could have taxonomic value.’ This prompted a recent study that sought to investigate these observations. This research was facilitated by exploiting a novel pressurised hot-water extraction (PHWE) method.[45a–f,46] This technique facilitated the isolation of several novel glycosides from B. montana (and Cenarrhenes nitida and Persoonia gunnii; see below).
The authors determined that B. montana contained the phenolic glycoside arbutin and four derivatives.[46] This included new glucosides 68 (2.0 % w/w isolated from leaves and 0.32 % w/w from flowers), 69, and 70 (0.27 and 0.03 % w/w from flowers respectively), and known compound 71 (3.4 % w/w from leaves and flowers).[46] We suggest that in Bick’s earlier study,[43] the reaction of molecule 71 with ammonia during the extraction process provided alkaloid 67. Glucoside 71 was previously obtained from the leaves of the native New Zealand Proteaceae Toronia toru (Fig. 10).[47] The flavonoid glycoside polifolioside was also isolated from the leaves of B. montana (0.67 % w/w).[46] Interestingly, the aglycone analogue of molecule 68, methyl ester 61, which was isolated from B. montana in 1971[40] was not observed in this recent PHWE study.[46] The former work employed an extraction solvent mixture containing a high proportion of methanol and transesterification may have occurred during the isolation process. Thus, it is possible that methyl ester 61 is an artefact of isolation.
Cenarrhenes
The only natural products isolation study on the monotypic genus Cenarrhenes genus was reported in 2018 (also employing the PHWE technique).[46] In this way, significant quantities of the previously unreported glycoside 72 (9.1 % w/w) were obtained from Cenarrhenes nitida leaves. Compound 72 is structurally related to the aforementioned arbutin derivatives isolated from B. montana (Fig. 10).[46]
Persoonia
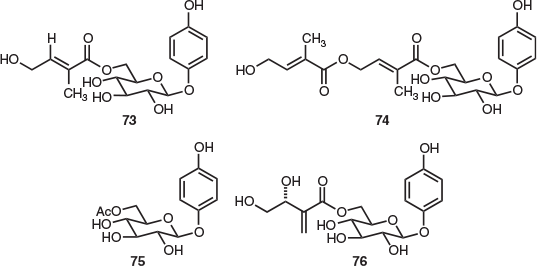
Of the five Tasmanian Persoonia species, the only reported natural products isolation study was undertaken on Persoonia gunnii leaves. This work also used PHWE to provide several arbutin derivatives.[46] This included previously unreported, novel tiglic acid ester glycosides 73 (2.9 % w/w) and 74 (0.45 % w/w) (both structures were secured by X-ray crystallography), the germane glycosides arbutin (6.2 % w/w) and pyroside (75) (1.5 % w/w), and compound 76 (1.6 % w/w) (Fig. 11).[46] Glycoside 76 was previously isolated from the endemic Australian species Persoonia linearis × pinifolia.[48]
|
Lomatia
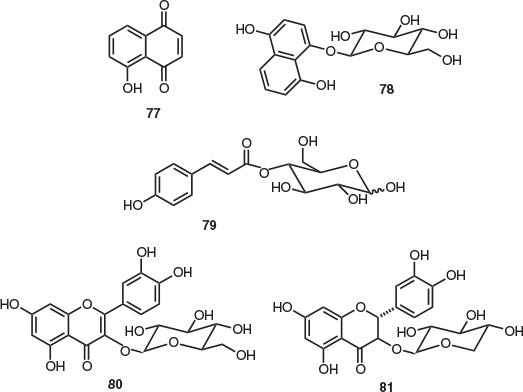
The only extensive natural products isolation study of the endemic Tasmanian Lomatia genus was conducted in 2018.[49] This work featured an investigation of all three extant species (L. tasmanica, L. polymorpha, and L. tinctoria). Both diethyl ether maceration and PHWE of the leaves of the rare, endangered L. tasmanica provided the napthoquinone pigment juglone (77) (Fig. 12).[49] This molecule is a known phytotoxin with allelophathic activity[50] and has demonstrated a capacity to stunt the growth of competing plants to provide a chemical ecological advantage.[51] Juglone (77) was also isolated from the leaves of both L. polymorpha and L. tinctoria (via both diethyl ether maceration and PHWE),[49] and, in 1973, this compound was isolated from the seeds, bark, and wood of L. tinctoria.[52]
|
As part of the same study, several glycosides were isolated following the PHWE of L. polymorpha leaves, including the glycoside precursor of juglone 1,4,8-trihydroxynaphthalene-1-O-β-d-glucose (78), 4-O-p-coumaroyl-d-glucose (79), quercetin 3-O-β-d-glucose (80), and dihydroquercetin 3-O-β-d-xyloside (81). The leaves of each of the species (L. tasmanica, L. polymorpha, and L. tinctoria) afforded α- and β-glucose as major saccharide components, which were isolated as their respective pentaacetate derivatives following peracetylation.[49]
Family Rutaceae
There are a total 20 species from 7 genera within the family Rutaceae that are endemic to Tasmania. Of these, the shrubby Acradenia represents the only genus from which natural product isolation studies have been reported.
Acradenia
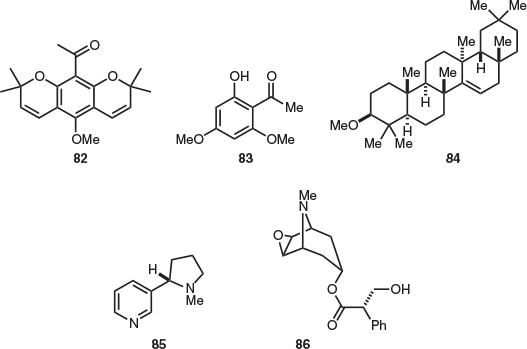
Natural products isolation studies on the essential oil from the leaves and terminal branchlets of Acradenia frankliniae were undertaken in 1961,[53] providing the structurally distinct pyranochromene franklinone (82), in addition to two chromene derivatives that were identified by comparison of melting points with known standards (Fig. 13). Subsequent studies also provided 82,[54,55] in addition to xanthoxylin (83)[55] and the triterpenoid crusgallin (84).[54] Gas chromatography–mass spectrometry (GC-MS) analysis of A. frankliniae leaf oil also identified numerous essential oils; however, these were not isolated.[55]
|
Family Solanaceae
Cyphanthera
The sole endemic Tasmanian member of the Solanaceae family, the shrub Cyphanthera tasmanica (previously Anthocercis tasmanica) has been the focus of previous isolation studies. As a result of this work, nicotine (85) and the tropane alkaloid hyoscine (86) were isolated from the aerial parts of C. tasmanica (Fig. 13).[56] In addition, it was noted that several minor alkaloids were also present; however, these were not characterised.[56]
Class Monocotyledoneae
Family Haemodoraceae
Haemodorum
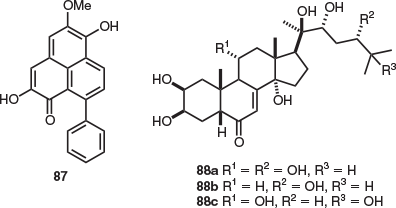
Haemodorum distichophyllum, a small grass from Tasmania’s south-west, is the only endemic Tasmanian species in the Haemodoraceae family. The sole natural products isolation study of H. distichophyllum identified the phenylanone pigment haemodorin (87) from leaf material (Fig. 14).[57]
|
Family Liliaceae
Ten endemic Tasmanian Liliaceae species from five genera are currently in existence. To date, natural product isolation studies have only been performed on the genus Blandfordia.
Blandfordia
Blandfordia punicea (Tasmanian Christmas bells), the rhizomatous herb, represents the only endemic Tasmanian Liliaceae member that has been subject to an extensive natural products isolation study. Seven phytoecdysteroids were isolated from B. punicea seeds, namely punisterone (88a), pterosterone (88b), and turkesterone (88c) in addition to the structurally related ecdysone 20-hydroxyecdysone, 5β,20-dihydroxyecdysone, and ponasterone C (Fig. 14).[58] It is suggested that these molecules serve as plant defence mechanisms and deter predation by phytophagous insects.[58]
Class Gymnospermae
Family Cupressaceae
Five extant Cupressaceae species from three genera are endemic to Tasmania; however, natural products isolation studies have only been conducted on the genus Athrotaxis. This genus is endemic to Tasmania.
Athrotaxis
Investigations on the three endemic Tasmanian Athrotaxis species (A. cupressoides (pencil pine) and A. selaginoides (King Billy pine) as well as their hybrid, A. × laxifolia (intermediate pine)) have been undertaken.[59,60] This led to the isolation of the homoerythrina alkaloids taxodine (89a), 3-epi-schelhammericine (90), homoerythratine, 2-hydroxytaxodine (91a), 2-hydroxyisotaxodine (homoerysotine) (91b), and athrocupressine (89b) from the aerial parts of all three species (Fig. 15). In addition, 2-acetoxytaxodine (91c) and 2-acetoxyisotaxodine were isolated from the aerial parts of both A. cupressoides[59] and A. selaginoides.[61] Structurally related alkaloids 2-epi-2-hydroxyisotaxodine, O-methylathrocupressine (91d), and 2-epi-homoerythratine were isolated from the aerial parts of A. cupressoides.[59] To date, alkaloids 89a–89b, 91a, 91c, 2-acetoxyisotaxodine, and 2-epi-2-hydroxyisotaxodine have not been found in plant species outside of the genus Athrotaxis.
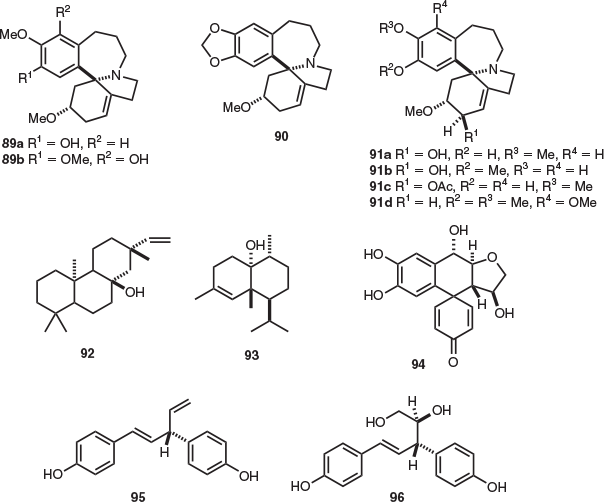
|
8-β-Hydroxyisopimarene (92)[62] was present in the leaf essential oil extracts obtained from all three Athrotaxis species. However, the authors did not specify whether diterpenoid 92 belonged to either the ent- or normal diterpenoid series and the specific rotation of this compound was not determined. In addition, the essential oil composition of both seeds and leaves of A. cupressoides and A. selaginoides provided the methyl ester derivative of the unsaturated fatty acid all-cis 5,11,14,17-eicosatetraenoic acid.[63] Sesquiterpenes cubenol (93) and epicubenol were isolated from A. selaginoides wood,[64] and the spiro-lignan athrotaxin (94) was present in the heartwood (the structures were secured by X-ray crystallography).[65,66] The phenolic compounds hinokiresinol (95) and agatharesinol (96) were also isolated from the heartwood of A. selaginoides in 1972.[65]
Family Podocarpaceae
Of the four extant Podocarpaceae species endemic to Tasmania, the genus Lagostrobos is the only one from which natural product isolation studies have been published.
Lagostrobos
Previous natural products isolation studies on Lagostrobos franklinii (previously known as Dacrydium franklinii) focused on investigating the essential oil and heartwood composition of this rare, extremely slow-growing species. These include the isolation of the phenolic natural products dacriniol (97a), zanthoxylol (97b), and dacrinial (98) found in the L. franklinii heartwood (Fig. 16).[67] In addition, several reports have identified the phenylpropene methyl eugenol (6.6 % w/w) as a major essential oil component of the L. franklinii heartwood. This was determined via GC-MS analysis,[68] and methyl eugenol and elemicin were also identified in heartwood volatile extracts by GLC.[67]
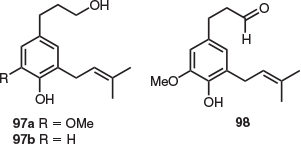
|
Conclusion
We have completed the first literature survey of natural products isolation studies of endemic Tasmanian vascular plants found in this geographically isolated island state of Australia. The paucity of natural products isolation studies on such plants (only 27 species; ~5.1 % of 530 known extant species) indicates a critical lack of knowledge regarding the fundamental chemotaxonomy of these plants. This review certainly emphasises the need for more extensive investigations of all families of endemic Tasmanian vascular plants. For example, only three of the four classes of vascular plants (Dicotyledonae, Monocotyledonae, and Gymnospermae) have been the subject of natural product isolation studies. Reports concerning the phytochemistry of Tasmanian fern species are conspicuously absent from the literature and such plants should be the subject of natural products isolation studies in future. Furthermore, the present review highlights that natural products isolation studies of key families, such as the Orchidaceae family (72 endemic Tasmanian species) are yet to be reported, despite being the largest family within class Monocotyledonae.
The key role that natural products have played (and continue to play) in leading drug discovery and the development of new pharmaceutical drugs is well established.[69] A high proportion of the some 27 species of endemic Tasmanian plants from which natural products have been isolated feature novel and structurally complex organic molecules. Thus, more extensive phytochemical studies are required to enhance our knowledge of the fundamental chemotaxonomy of these species. Furthermore, we suggest that endemic Tasmanian plants represent a valuable and untapped source of novel and significant compounds of potential therapeutic value that are yet to be discovered.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
The authors gratefully acknowledge CSIRO for financial support. B.J.D. thanks the Australian government for a Research Training Program Scholarship and the Tasmanian Museum and Art Gallery for the 2017 Wilson Bequest Bursary.
References
[1] K. Lambeck, J. Chappell, Science 2001, 292, 679.| Crossref | GoogleScholarGoogle Scholar |
[2] (a) J. B. Kirkpatrick, M. J. Brown, Bot. J. Linn. Soc. 1984, 88, 165.
| Crossref | GoogleScholarGoogle Scholar |
(b) M. D. Crisp, S. Laffan, H. P. Linder, A. Monro, J. Biogeogr. 2001, 28, 183.
| Crossref | GoogleScholarGoogle Scholar |
[3] R. M. Kooyman, M. Rossetto, H. Sauquet, S. W. Laffan, PLoS One 2013, 8, e80685.
| Crossref | GoogleScholarGoogle Scholar |
[4] G. J. Jordan, P. A. Harrison, J. R. P. Worth, G. J. Williamson, J. B. Kirkpatrick, Glob. Ecol. Biogeogr. 2016, 25, 127.
| Crossref | GoogleScholarGoogle Scholar |
[5] M. F. de Salas, M. L. Baker, A Census of the Vascular Plants of Tasmania, Including Macquarie Island 2017 (Tasmanian Herbarium, Tasmanian Museum and Art Gallery: Hobart). Available at: www.tmag.tas.gov.au (accessed 19 July 2018).
[6] F. Bohlmann, N. G. le Van, Phytochemistry 1978, 17, 1173.
| Crossref | GoogleScholarGoogle Scholar |
[7] C. Zdero, F. Bohlmann, A. Anderberg, Phytochemistry 1991, 30, 2703.
| Crossref | GoogleScholarGoogle Scholar |
[8] E. Wollenweber, M. Dörr, M. Beyer, J. N. Roitman, C. F. Puttock, Z. Naturforsch. C 1997, 52, 571.
[9] A. Rumbero, F. J. Arriaga-Giner, E. Wollenweber, Z. Naturforsch. C 2000, 55, 318.
[10] F. J. Arriaga-Giner, A. Rumbero, E. Wollenweber, Z. Naturforsch. C 1999, 54, 602.
[11] F. J. Arriaga-Giner, A. Rumbero, E. Wollenweber, Z. Naturforsch. C 1998, 53, 286.
[12] A. Rumbero, F. J. Arriga-Giner, E. Wollenweber, Z. Naturforsch. C 2000, 55, 1.
[13] C. Dragar, V. A. Dragar, R. C. Menary, J. Essent. Oil Res. 1993, 5, 507.
| Crossref | GoogleScholarGoogle Scholar |
[14] M. A. Hai, N. W. Preston, R. Kyburz, E. Schopp, I. R. C. Bick, M. Hesse, Helv. Chim. Acta 1980, 63, 2130.
| Crossref | GoogleScholarGoogle Scholar |
[15] I. R. C. Bick, M. A. Hai, V. A. Patrick, A. H. White, Aust. J. Chem. 1983, 36, 1037.
| Crossref | GoogleScholarGoogle Scholar |
[16] R. Kyburz, E. Schoepp, M. Hesse, Helv. Chim. Acta 1984, 67, 804.
| Crossref | GoogleScholarGoogle Scholar |
[17] R. Kyburz, E. Schoepp, I. R. C. Bick, M. Hesse, Helv. Chim. Acta 1981, 64, 2555.
| Crossref | GoogleScholarGoogle Scholar |
[18] R. Kyburz, E. Schoepp, I. R. C. Bick, M. Hesse, Helv. Chim. Acta 1979, 62, 2539.
| Crossref | GoogleScholarGoogle Scholar |
[19] I. R. C. Bick, J. B. Bremner, N. W. Preston, I. C. Calder, J. Chem. Soc. D 1971, 1155.
| Crossref | GoogleScholarGoogle Scholar |
[20] H.-P. Ros, R. Kyburz, N. W. Preston, R. T. Gallagher, I. R. C. Bick, M. Hesse, Helv. Chim. Acta 1979, 62, 481.
| Crossref | GoogleScholarGoogle Scholar |
[21] W. J. Klaver, H. Hiemstra, W. N. Speckamp, J. Am. Chem. Soc. 1989, 111, 2588.
| Crossref | GoogleScholarGoogle Scholar |
[22] I. R. C. Bick, M. A. Hai, N. W. Preston, Tetrahedron 1985, 41, 3127.
| Crossref | GoogleScholarGoogle Scholar |
[23] C. Dragar, I. R. C. Bick, Phytochemistry 1992, 31, 3601.
| Crossref | GoogleScholarGoogle Scholar |
[24] M. E. Wall, M. C. Wani, B. N. Meyer, H. Taylor, J. Nat. Prod. 1987, 50, 1152.
| Crossref | GoogleScholarGoogle Scholar |
[25] N. K. Hart, S. R. Johns, J. A. Lamberton, H. Suares, R. I. Willing, Aust. J. Chem. 1976, 29, 1295.
| Crossref | GoogleScholarGoogle Scholar |
[26] D. J. Boland, J. J. Brophy, T. M. Flynn, E. V. Lassak, Phytochemistry 1982, 21, 2467.
| Crossref | GoogleScholarGoogle Scholar |
[27] D. H. S. Horn, J. A. Lamberton, Aust. J. Chem. 1964, 17, 477.
| Crossref | GoogleScholarGoogle Scholar |
[28] J. A. Lamberton, Aust. J. Chem. 1964, 17, 692.
| Crossref | GoogleScholarGoogle Scholar |
[29] E. N. Zhukovich, L. M. Bobrenko, M. Yu. Semenova, S. Yu. Bokareva, Pharm. Chem. J. 2014, 48, 323.
| Crossref | GoogleScholarGoogle Scholar |
[30] N. M. Dzhanashiya, V. I. Glyzin, Chem. Nat. Compd. 1970, 6, 777.
[31] A. A. Savina, T. A. Sokol’skaya, D. A. Fesenko, Chem. Nat. Compd. 1983, 19, 114.
[32] A. A. Savina, T. A. Sokol’skaya, V. F. Zakharov, Chem. Nat. Compd. 1988, 24, 253.
[33] A. A. Savina, V. F. Zakharov, N. S. Tsybul’ko, Chem. Nat. Compd. 1991, 27, 696.
[34] G. Nonaka, K. Ismaru, R. Azuma, M. Ishimatsu, I. Nishioka, Chem. Pharm. Bull. 1989, 37, 2071.
| Crossref | GoogleScholarGoogle Scholar |
[35] S. C. Santos, P. G. Waterman, Fitoterapia 2001, 72, 95.
| Crossref | GoogleScholarGoogle Scholar |
[36] F. Bohlmann, C. Zdero, Chem. Ber. 1975, 108, 2541.
| Crossref | GoogleScholarGoogle Scholar |
[37] A. M. Buchanan, A Census of the Vascular Plants of Tasmania & Index to The Student’s Flora of Tasmania 2004 (Tasmanian Herbarium, Tasmanian Museum & Art Gallery: Hobart). Available at: www.tmag.tas.gov.au (accessed 19 July 2018).
[38] H. Sauquet, P. H. Weston, C. L. Anderson, N. P. Barker, D. J. Cantrill, A. R. Mast, V. Savolainen, Proc. Natl. Acad. Sci. USA 2009, 106, 221.
| Crossref | GoogleScholarGoogle Scholar |
[39] I. R. C. Bick, J. W. Gillard, H.-M. Leow, N. W. Preston, Aust. J. Chem. 1979, 32, 2071.
| Crossref | GoogleScholarGoogle Scholar |
[40] I. R. C. Bick, J. B. Bremner, J. W. Gillard, Phytochemistry 1971, 10, 475.
| Crossref | GoogleScholarGoogle Scholar |
[41] W. S. D. Motherwell, W. Isaacs, O. Kennard, I. R. C. Bick, J. B. Bremner, J. W. Gillard, J. Chem. Soc. D 1971, 133.
| Crossref | GoogleScholarGoogle Scholar |
[42] I. R. C. Bick, J. W. Gillard, H.-M. Leow, Aust. J. Chem. 1979, 32, 1827.
| Crossref | GoogleScholarGoogle Scholar |
[43] I. Ralph, C. Bick, J. W. Gillard, H.-M. Leow, Phytochemistry 1986, 25, 972.
| Crossref | GoogleScholarGoogle Scholar |
[44] R. L. Bieleski, B. G. Briggs, Aust. J. Bot. 2005, 53, 205.
| Crossref | GoogleScholarGoogle Scholar |
[45] (a) J. Just, B. J. Deans, W. J. Olivier, B. Paull, A. C. Bissember, J. A. Smith, Org. Lett. 2015, 17, 2428.
| Crossref | GoogleScholarGoogle Scholar |
(b) J. Just, T. B. Jordan, B. Paull, A. C. Bissember, J. A. Smith, Org. Biomol. Chem. 2015, 13, 11200.
| Crossref | GoogleScholarGoogle Scholar |
(c) J. Just, G. L. Bunton, B. J. Deans, N. L. Murray, A. C. Bissember, J. A. Smith, J. Chem. Educ. 2016, 93, 213.
| Crossref | GoogleScholarGoogle Scholar |
(d) B. J. Deans, A. C. Bissember, J. A. Smith, Aust. J. Chem. 2016, 69, 1219.
| Crossref | GoogleScholarGoogle Scholar |
(e) B. J. Deans, J. Just, J. Chhetri, L. K. Burt, J. N. Smith, N. L. Kilah, M. de Salas, N. Gueven, A. C. Bissember, J. A. Smith, ChemistrySelect 2017, 2, 2439.
| Crossref | GoogleScholarGoogle Scholar |
(f) B. J. Deans, W. J. Olivier, D. Girbino, A. C. Bissember, J. A. Smith, Fitoterapia 2018, 126, 65.
| Crossref | GoogleScholarGoogle Scholar |
(g) B. J. Deans, B. Skierka, B. W. Karagiannakis, D. Vuong, E. Lacey, J. A. Smith, A. C. Bissember, Aust. J. Chem. 2018, 71,
| Crossref | GoogleScholarGoogle Scholar |
[46] B. J. Deans, N. L. Kilah, G. J. Jordan, A. C. Bissember, J. A. Smith, J. Nat. Prod. 2018, 81, 1241.
| Crossref | GoogleScholarGoogle Scholar |
[47] N. B. Perry, N. J. Brennan, J. Nat. Prod. 1997, 60, 623.
| Crossref | GoogleScholarGoogle Scholar |
[48] J. K. MacLeod, H. B. Rasmussen, A. C. Willis, J. Nat. Prod. 1997, 60, 620.
| Crossref | GoogleScholarGoogle Scholar |
[49] B. J. Deans, L. Tedone, A. C. Bissember, J. A. Smith, Phytochemistry 2018, 153, 74.
| Crossref | GoogleScholarGoogle Scholar |
[50] E. F. Davis, Am. J. Bot. 1928, 15, 620.
[51] S. Topal, I. Kocacalistan, O. Arslan, A. Z. Tel, Phyton 2006, 46, 259.
[52] M. Moir, R. H. Thompson, Phytochemistry 1973, 12, 1351.
| Crossref | GoogleScholarGoogle Scholar |
[53] M. E. Baldwin, I. R. C. Bick, A. A. Komzak, J. R. Price, Tetrahedron 1961, 16, 206.
| Crossref | GoogleScholarGoogle Scholar |
[54] A. Quader, J. A. Armstrong, A. I. Gray, T. G. Hartley, P. G. Waterman, Biochem. Syst. Ecol. 1991, 19, 171.
| Crossref | GoogleScholarGoogle Scholar |
[55] J. J. Brophy, R. J. Goldsack, P. I. Forster, I. A. Southwell, J. Essent. Oil Res. 2001, 13, 136.
| Crossref | GoogleScholarGoogle Scholar |
[56] I. R. C. Bick, J. B. Bremner, J. W. Gillard, K. N. Winzenberg, Aust. J. Chem. 1974, 27, 2515.
| Crossref | GoogleScholarGoogle Scholar |
[57] I. R. C. Bick, A. J. Blackman, Aust. J. Chem. 1973, 26, 1377.
| Crossref | GoogleScholarGoogle Scholar |
[58] S. D. Sarker, L. N. Dinan, J.-P. Girault, J. Nat. Prod. 1996, 59, 789.
| Crossref | GoogleScholarGoogle Scholar |
[59] S. Panichanun, I. R. C. Bick, Tetrahedron 1984, 40, 2677.
| Crossref | GoogleScholarGoogle Scholar |
[60] S. Panichanun, I. R. C. Bick, Tetrahedron 1984, 40, 2685.
| Crossref | GoogleScholarGoogle Scholar |
[61] I. R. C. Bick, J. B. Bremner, A. Razak, L. V. Thuc, Experientia 1980, 36, 1135.
| Crossref | GoogleScholarGoogle Scholar |
[62] Personal correspondence with J. J. Brophy on 7 December 2017 in relation to: J. J. Brophy, R. J. Goldsack, C. J. R. Fookes, A. C. Rozefelds, J. Essent. Oil Res. 2002, 14, 109.
| Crossref | GoogleScholarGoogle Scholar |
[63] J. R. Vickery, F. B. Whitfield, G. L. Ford, B. H. Kennett, J. Am. Oil Chem. Soc. 1984, 61, 573.
| Crossref | GoogleScholarGoogle Scholar |
[64] A. Talvitie, A.-K. Borg-Karlson, Finn. Chem. Lett 1979, 3, 93.
[65] P. Daniels, H. Erdtman, K. Nishimura, T. Norin, J. Chem. Soc. Chem. Commun. 1972, 246.
| Crossref | GoogleScholarGoogle Scholar |
[66] A.-M. Pilotti, Acta Crystallogr. Sect. B 1972, 28, 2123.
| Crossref | GoogleScholarGoogle Scholar |
[67] K. H. Baggaley, H. Erdtman, N. J. McLean, T. Norin, G. Eriksson, Acta Chem. Scand. 1967, 21, 2247.
| Crossref | GoogleScholarGoogle Scholar |
[68] M. G. Fitzgerald, M. A. Line, Holzforschung 1990, 44, 335.
| Crossref | GoogleScholarGoogle Scholar |
[69] D. J. Newman, G. M. Cragg, J. Nat. Prod. 2016, 79, 629.
| Crossref | GoogleScholarGoogle Scholar |
* Alex C. Bissember is the recipient of the 2017 RACI Organometallic Award.




