Foreword to Professor Athelstan L. J. Beckwith Special Issue
Peter J. Duggan AA CSIRO Materials Science and Engineering, Private Bag 10, Clayton South, Vic. 3169, Australia. Email: peter.duggan@csiro.au

|

Peter Duggan is Principal Research Scientist at CSIRO Material Science and Engineering. He gained his BSc(Hons) from Flinders University in 1985 and PhD from ANU in 1990, under the direction of Prof. Beckwith. Peter held post doctoral positions at Columbia University, New York and the University of Cambridge. Prior to joining CSIRO in 2004, he held academic appointments at James Cook University and Monash University. His current research interests lie in the applications of non-natural amino acids, peptides and peptidomimetics in biology, and the applications of boronic acids and organoborates. |
Australian Journal of Chemistry 64(4) 355-357 https://doi.org/10.1071/CH11098
Published: 18 April 2011
Professor Athelstan Beckwith, FRACI, FAA, FRS, AO passed away on 15th May 2010. He was a giant of Australian Chemistry and his contributions to the field of physical organic chemistry and free radical research have had major impacts worldwide. Athel published over 200 papers, which have received more than 9900 citations. He was a great supporter of the Australian Journal of Chemistry, publishing more articles in our journal than anywhere else. This Special Issue of the Australian Journal of Chemistry is dedicated to him.
Athel grew up in Perth[1] and at the age of 13 suffered a severe and initially undiagnosed case of osteomyelitis, from which he was lucky to survive. He was confined to bed for more than 20 months and the disease left him with a fused knee and a characteristic gait. This was a very formative experience for Athel and made him determined to enjoy life to the full. As an adult he had many interests away of science including a great love of music, and he and his wife Kaye led very busy social lives.
Athel attended Perth Modern School and obtained his Bachelor of Science with Honours from the University of Western Australia in 1953. At UWA Athel was exposed to techniques, concepts and philosophies that greatly influenced the approach he took to science for the next half century. Doug White appears to have been particularly influential on the young Beckwith. White emphasized his belief that the most interesting chemical insights come from attempts to understand unexpected results and ‘failed’ experiments. Many of the most important discoveries and theories later published by Athel and his coworkers have resulted from the thorough investigation of unexpected results observed in the laboratory or found in the literature. At UWA, Athel was also exposed to the then, very new, electronic approach to organic chemistry just developed by Robinson and Ingold, and brought to the Department by Joe Miller, and to the new techniques of infrared spectroscopy and chromatography.
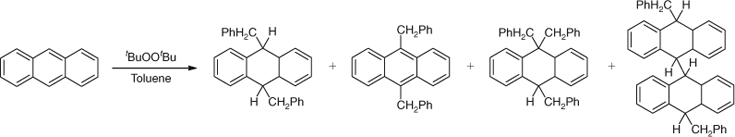
This was a different era in Australian science and in this regard, the nation was still maturing. The PhD degree was not awarded at most Australian Universities. So upon graduating with an Honours degree, Athel began to study for a Masters degree while working as a graduate assistant at UWA. During this time he began to investigate the mechanisms of some reactions of diazonium salts. This work again proved to be highly influential on Athel’s future scientific career. Instead of subsequently taking up an offer of a scholarship with Derek Barton in London, Athel then accepted a junior lectureship at the University of Adelaide. He soon decided, however, that if he intended to continue with academic research, he really did need to go overseas to gain a PhD and subsequently obtained a CSIRO overseas scholarship. After some telling advice from Ian Wark, the then Chief of the CSIRO Division of Industrial Chemistry, Athel decided to study for a DPhil with W. A. Waters at Oxford in the new field of free radical chemistry. This was a very brave move as at the time there was still vigorous debate about the involvement of free radicals in chemical reactions. We now know from the work of a vanguard of free radical chemists, which included Athel, that radical intermediates are widespread in synthetic, biological and polymer chemistry. In the mid 1950s, however, very few chemists believed this. At Oxford, Athel studied the mechanism of free radical aromatic substitution[2] and with the help of his newly developed expertise in chromatography, completed his DPhil in two years, the degree being conferred in 1956. An example of the type of chemistry investigated by Beckwith and Waters is shown in Scheme 1.
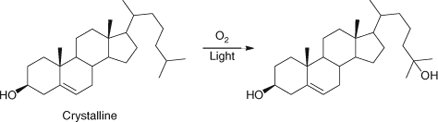
Dr Beckwith returned to Australia and began to search for commercial applications of wool wax at CSIRO in Melbourne. It was the study of the lanosterol present in wool wax that led him to investigate an unusual oxidation product of the related cholesterol. He concluded that this unusual product, 25-hydroxycholesterol (Scheme 2), resulted from the hydrogen abstraction by oxygen-centred radicals from crystalline cholesterol.[3] This was an important conclusion as it revealed one of the great advantages of free radicals – their ability to functionalize unactivated hydrocarbons through hydrogen abstraction. Athel pined for the freedoms of academic research though, and so after a relatively brief stint at CSIRO, returned to the University of Adelaide in early 1958, this time as a fully tenured member of staff. By 1965, and at the tender age of 35, he had been promoted to Professor and Head of the Department of Organic Chemistry at that university.
|
During his time at Adelaide, Athel kept a close eye on new developments in the field and took up several overseas study fellowships in order to learn about them first-hand. In 1961, Athel finally had the opportunity to study with Sir Derek Barton, taking up a Nuffield Foundation grant to spend a year’s study leave at Imperial College in London. Athel learnt a great deal from the highly impressive Barton, particularly regarding the new concept of stereochemistry. Later, in 1968, he took a year’s study leave at York to learn about electron spin resonance and secured an ESR spectrometer in Adelaide the following year. Soon after his trip to York, Athel took up a Carnegie Fellowship which supported an extensive lecture tour of North America. It was on this tour that he first met Cheves Walling and Keith Ingold, two giants of free radical chemistry with whom he stayed in close contact for many years. Indeed, Athel worked closely with Keith Ingold for the rest of his scientific career. They published several papers together and an important review on the rearrangements of excited states.[4]
In the late 1960s and early 1970s, the Beckwith group began to study a reaction that Athel would ultimately become best known for – the cyclization of the hex-5-enyl radical (Scheme 3). The Beckwith group’s investigation of this reaction is the subject of an engaging review by Algi Serelis, later in this issue, and will only be mentioned briefly here. A series of guidelines that predict the regioselectivity, relative rates and stereochemical outcomes of radical cyclization reactions were developed, which were to become known as the ‘Beckwith Rules’.[5] Athel’s group began to construct increasingly more complex ring systems by radical methods and it was a source of immense pride for Athel that from 1982 the great natural products chemist, Gilbert Stork, began use free radical cyclizations extensively. Many complex organic compounds have since been synthesized using free radical cyclizations and Curran’s synthesis of triquinanes by radical methods has been featured in the renowned reference book for synthetic organic chemists, ‘Classics in Total Synthesis’ by Nicolaou and Sorensen.[6]
|
|
In 1981, Professor Beckwith moved to the Research School of Chemistry at ANU to take up a Chair made available by the retirement of the great Arthur Birch. Here, Athel soon established a vibrant research group working on several aspects of organic free radical research. The availability of more extensive facilities, larger numbers of support staff and Australian and international students and post docs accelerated the output from the group. Many types of radical reactions were studied, some of which had previously been studied at Adelaide, whereas some were newly examined at ANU. These included the cyclization of alkoxyl[7] and aminyl[8] radicals, and the free radical chemistry of organosulfur compounds relevant to the biosynthesis of penicillin.[9] A free radical ester migration that appears to operate by an unusual spectrum of mechanisms ranging from one that passes through an open shell pericyclic transition state to one that has significant radical cationic character was examined in detail.[10] The applications of organic free radicals in enantioselective synthesis is the holy grail of the field and Athel pursued this with some success in his later years at ANU.[11] Techniques used by the group were wide ranging and included organic synthesis, careful reaction mixture analysis, the measurement of reaction kinetics, computational chemistry, ESR and NMR spectroscopy, isotopic labelling studies and radical trapping methods.[12] Research problems were assigned to individuals rather than teams. This undoubtedly reduced publication rates but provided excellent training as each group member had to become highly proficient with a wide range of techniques.
Athel accepted many invitations to give plenary lectures at local and international conferences and was a regular at international conferences devoted to free radicals including the Bürgenstock Meetings and the Gordon Conferences on Free Radicals. The high esteem with which Athel was held by the international organic chemistry community was aptly captured by Gerry Pattenden when he wrote; ‘Few would argue with the leading position that Athel L. J. Beckwith occupies in the line of pioneers of the applications of free radical reactions in contemporary organic synthesis. The plethora of citations and publications from his laboratory are ample evidence of the ingenuity and creativity of his contributions in the area.’[13] Athel was the recipient of many awards and medals including the Rennie Memorial Medal, the H. G. Smith Memorial Medal and the Organic Division Medal (later to be renamed the Birch Medal) from the RACI and the Centenary Medal from the Royal Society of Chemistry. He was elected Fellow of the RACI, the Australian Academy of Science and the Royal Society. The lectureship for recently appointed staff awarded by the Organic Division of the RACI has recently been named the Beckwith Lectureship in Athel’s honour. Professor Beckwith was also recognised by the wider Australian community through the award of a Centenary Medal in 2001 and was made an Officer of the Order of Australia in 2004.
It is worth considering the origins of Athel’s successes. He certainly benefited from an upbringing that was conducive to academic endeavours and the education that he received at Perth Modern School and the University of Western Australia was of a very high quality. This cannot fully explain his rise to become one of the pre-eminent Australian Chemists of his time, however. Rather, Athel possessed a powerful intellect, a tremendous self confidence and sense of importance of his research, and his clear and entertaining lecture style attracted quality collaborators. His enthusiasm for the unexpected and the ‘why?’ was infectious. He also had a record of adopting new techniques and theories at early stages. These characteristics were supplemented by Athel’s high integrity, congenial demeanour and highly developed social skills. He showed great stamina in his ability to endure regular trips to many parts of the world. This placed him on the world stage of organic chemistry and opened innumerable doors for his former group members that followed. All of these qualities combined to make Athel a major international player in his field.
On a more human level, Athel and his wife Kaye provided tremendous support and were genuinely concerned for the welfare of the Beckwith group members and their families. The hospitality shown by Athel and Kaye at the many group gatherings is legendary. Athel would be particularly pleased with the naming of the Beckwith Lectureship because he was also highly supportive of early career chemists around Australia. Athel grew up with several famous people, and was exposed to politicians from various arms of government as a young adult. This, combined with Kaye’s involvement in local government, made him a scientist who was unusually comfortable dealing and socializing with politicians. Athel was an advocate for science at the highest levels of Australian politics, especially after the move to the ANU and, together with Kaye, was also involved in the fight for indigenous rights. Athel was, and Kaye still is, greatly concerned with the plight of the indigenous people of Australia.
In 1995, the Australian Journal of Chemistry published a special issue dedicated to Professor Beckwith to mark the occasion of his 65th birthday (http://www.publish.csiro.au/nid/52/issue/2936.htm). It was a measure of the esteem in which Athel was held that the list of authors contained a roll call of outstanding Australian organic chemists and big-name international free radical chemists.
The list of contributors to this issue consist of former Beckwith group members, collaborators and colleagues who are united in their desire to celebrate the life of a great scientist and friend. We hope that it will provide a fitting tribute to a man who will be sorely missed.
References
[1] For a more full account of Prof. Beckwith’s life and philosophies, see a transcript of a 2003 interview conducted by Professor Bob Crompton for the Australian Academy of Science; http://www.science.org.au/scientists/interviews/b/ab.html.[2] (a) A. L. J. Beckwith, W. A. Waters, J. Chem. Soc. 1956, 1108.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG2sXhs1Ol&md5=043aec6c2f41ac594a8b456dcd8630a9CAS |
(b) A. L. J. Beckwith, W. A. Waters, J. Chem. Soc. 1957, 1001.
| Crossref | GoogleScholarGoogle Scholar |
(c) A. L. J. Beckwith, W. A. Waters, J. Chem. Soc. 1957, 1665.
| Crossref | GoogleScholarGoogle Scholar |
(d) A. L. J. Beckwith, R. O. C. Norman, W. A. Waters, J. Chem. Soc. 1958, 171.
| Crossref | GoogleScholarGoogle Scholar |
[3] A. L. J. Beckwith, Proc. Chem. Soc. London 1958, 194.
[4] A. L. J. Beckwith, K. U. Ingold, in Rearrangements in Ground and Excited States (Ed. P. de Mayo) 1980, Vol. 1. Ch. 4, pp. 161–310 (Academic Press: New York, NY).
[5] A.L.J. Beckwith, C. J. Easton, A. K. Serelis, J. Chem. Soc., Chem. Comm. 1980, 482.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXls1Oht78%3D&md5=dd0587a42de9c26fa0743d0448f07bd5CAS |
[6] K. C. Nicolaou, E. J. Sorensen, Classics in Total Synthesis 1996, Ch. 23, pp. 381–419 (VCH: Weinheim, Germany).
[7] (a) A. L. J. Beckwith, B. P. Hay, J. Am. Chem. Soc. 1988, 110, 4415.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXkvFajt7o%3D&md5=b2d5768ac56ff3ed632674e16863f6b2CAS |
(b) A. L. J. Beckwith, B. P. Hay, G. M. Williams, J. Chem. Soc. Chem. Commun. 1989, 1202.
| Crossref | GoogleScholarGoogle Scholar |
[8] A. L. J. Beckwith, B. J. Maxwell, J. Tsanaktsidis, Aust. J. Chem. 1991, 44, 1809.
| Crossref | GoogleScholarGoogle Scholar |
[9] (a) A. L. J. Beckwith, D. R. Boate, J. Chem. Soc. Chem. Commun. 1986, 189.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28XksVWqu7k%3D&md5=78f2765d71552d183f7c5051ff9c39b6CAS |
(b) A. L. J. Beckwith, S. A. M. Duggan, J. Chem. Soc., Perkin Trans. 2 1994, 1509.
| Crossref | GoogleScholarGoogle Scholar |
[10] (a) A. L. J. Beckwith, P. J. Duggan, J. Am. Chem. Soc. 1996, 118, 12838.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XntFSgur0%3D&md5=5688ba602d58fe7098e4780370c16ac2CAS |
(b) A. L. J. Beckwith, D. Crich, P. J. Duggan, Q. W. Yao, Chem. Rev. 1997, 97, 3273.
| Crossref | GoogleScholarGoogle Scholar |
[11] (a) A. L. J. Beckwith, C. L. L. Chai, J. Chem. Soc. Chem. Commun. 1990, 1087.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXntFar&md5=fc13e9c568f0a849b7aef269690ee2aeCAS |
(b) A. L. J. Beckwith, J. R. Axon, J. Chem. Soc. Chem. Commun. 1995, 549.
(c) G. A. Adamson, A. L. J. Beckwith, C. L. L. Chai, Aust. J. Chem. 2004, 57, 629.
| Crossref | GoogleScholarGoogle Scholar |
[12] (a) A. L. J. Beckwith, J. S. Poole, J. Am. Chem. Soc. 2002, 124, 9489.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XltlWrsr4%3D&md5=aef967945a301eac48f61f842cd3b221CAS | 12167045PubMed |
(b) A. L. J. Beckwith, V. W. Bowry, K. U. Ingold, J. Am. Chem. Soc. 1992, 114, 4983.
| Crossref | GoogleScholarGoogle Scholar |
(c) A. L. J. Beckwith, V. W. Bowry, G. Moad, J. Org. Chem. 1988, 53, 1632.
| Crossref | GoogleScholarGoogle Scholar |
[13] G. J. Hollingworth, G. Pattenden, D. J. Schulz, Aust. J. Chem. 1995, 48, 381.
| 1:CAS:528:DyaK2MXktFGjs7g%3D&md5=b3db9e69ebf2542068ded558a5d0baffCAS |