Synchrotron X-ray distinction of seasonal hydrological and temperature patterns in speleothem carbonate
Peter M. Wynn A I , Ian J. Fairchild B , Christoph Spötl C , Adam Hartland D , Dave Mattey E , Barbara Fayard F G and Marine Cotte F HA Lancaster Environment Centre, University of Lancaster, Lancaster, LA1 4YQ, UK.
B School of Geography, Earth and Environmental Sciences, University of Birmingham, Birmingham, Edgbaston, B15 2TT, UK.
C Institut für Geologie und Paläontologie, Leopold-Franzens-Universität Innsbruck, Innrain 52, A-6020 Innsbruck, Austria.
D Chemistry Department and Environmental Research Institute, Faculty of Science and Engineering, Environmental Research Institute, University of Waikato, Hamilton 3240, New Zealand.
E Department of Earth Sciences, Royal Holloway University of London, Egham, Surrey, TW20 0EX, UK.
F European Synchrotron Radiation Facility, F-38043 Grenoble cedex, France.
G Laboratoire de Physique des Solides, UMR 8502, Bât 510, Université Paris Sud, F-91405 Orsay cedex, France.
H UPMC Univ. Paris 06, UMR 8220, Laboratory of Molecular and Structural Archeology – LAMS, Paris, France.
I Corresponding author. Email: p.wynn@lancaster.ac.uk
Environmental Chemistry 11(1) 28-36 https://doi.org/10.1071/EN13082
Submitted: 23 April 2013 Accepted: 9 October 2013 Published: 30 January 2014
Environmental context. Speleothem chemical records are used to reconstruct environmental change on a broad range of timescales. However, one of the biggest challenges is to link the records contained within speleothems at the sub-annual timescale to changing meteorological conditions. Seasonal infiltration patterns and cave ventilation dynamics are reconstructed through high resolution analysis of speleothem trace element content by synchrotron radiation, building towards proxy records of hydrological variability and winter duration as indices of recent climatic change beyond the instrumental period.
Abstract. Synchrotron micro-X-ray fluorescence (µXRF) spectrometry is used to reveal trace element patterns within speleothem calcite at the sub-annual scale and provide one of the first calibrations to prevailing meteorological conditions. Mapping of Zn and SO42– within speleothem calcite was performed at the European Synchrotron Radiation Facility over three annual cycles (1977–1979). Peaks in µXRF Zn concentrations occur on an annual basis, although banding of lower XRF intensity reveals multiple events at the sub-annual scale. The delivery of Zn to the speleothem was found to be dependent upon the presence of a water excess, the condition of any overlying snowpack and the pH of the soil solution as controlled by microbial activity. This generated a pattern of Zn event laminae that documented increasing concentrations from winter through to the following autumn and complies with existing models inferring surface-active trace metals are delivered to the point of speleothem growth in association with natural organic matter (referred to as NOM–metal complexes). Minimum and maximum concentrations of speleothem SO42– coincide with winter and summer respectively, in contrast to the near constant SO42– concentrations of the drip water. Fluctuations in speleothem SO42– levels closely follow changes in cave external temperatures, thereby validating existing models of sulfate incorporation into carbonate minerals thought to be driven by cave ventilation dynamics and internal cave atmospheric pCO2 (partial pressure). At the current resolution of analysis, this represents some of the first evidence linking event-based meteorological (temperature and precipitation) records to the trace element content of speleothem calcite, building towards reconstruction of indices of climatic change beyond the instrumental period.
Introduction
Speleothem chemical records have been employed in the reconstruction of environmental change on a broad range of timescales. Although some of the oldest records provide environmental information on the multi-millennial timescale and are ultimately limited by accuracy of dating techniques, speleothems that grew within the period of meteorological records are now being analysed at sub-annual resolution.[1–6] Sub-annual records are, to date, scarce, but it is here that one of the research frontiers resides, aiming to provide a modern calibration between speleothem proxy and meteorological conditions, enabling reconstruction of recent environmental change beyond the instrumental period at a hitherto unprecedented level of analysis. At the highest-resolution, sub-annual analyses of speleothems have utilised secondary ionisation mass spectrometry,[7,8] laser-ablation inductively coupled plasma mass spectrometry,[1,5] or micro-X-ray fluorescence (µXRF) spectrometry stimulated by synchrotron radiation (SR).[9] The unparalleled sensitivity and spatial resolution of µXRF have also led to important insights in understanding the potential for seasonal hydrology to control the trace element record of surface-active species (i.e. those transported in complexes with natural organic matter (NOM)) in speleothem calcite.[4,6] Here, we use SR-µXRF at high resolution (1-µm pixel) over three annual growth bands of speleothem calcite to reveal the detailed seasonality of trace element incorporation. Previous work has established the principles whereby SO42–, Pb and Zn levels within speleothems can record seasonal phenomena.[4,6,9] Two distinct modes of incorporation control the Zn and SO42– records retained within speleothems. Whereas many trace metals (including Zn) rely upon seasonal hydrology for transfer and incorporation into speleothem calcite, patterns of SO42– incorporation are known to depend upon ambient cave air CO2 concentrations driven by cave ventilation and external temperature dynamics. Here we provide a demonstration study that realises over a short (3-year) time frame the ultimate potential for linking these variations to meteorological records to calibrate archived signals to modes of trace element incorporation. This is the initial phase of work aiming to develop records of seasonal infiltration patterns and external temperature variability, building towards proxy records of winter duration and seasonal infiltration patterns as indices of climatic change. The following sections review the current model of understanding for trace metal and SO42– incorporation into speleothem calcite, before applying these assertions to link patterns of synchrotron µXRF at the sub-annual scale to meteorological records.
Current understanding: review of environmental conditions determining Pb and Zn concentrations in speleothem carbonate
Transport of surface-active trace metals (including Pb and Zn) through karstic systems is recognised to occur predominantly through complexation reactions with NOM.[10–12] Although studies of this process in caves are few, published data[10] are concordant with experimental findings from studies of particle transport in fractured rock aquifers.[13,14] Size fractions of NOM range from the nominally dissolved to the colloidal-to-particulate fractions. The behaviour of each within fractured rock aquifers can be classified on the basis of their tendency to diffuse into micro-fractures, with the smallest materials diffusing into micro-fractures to a greater extent than the coarser materials, affecting transit times,[15] and leading to the classification of ‘high-flux’ and ‘low-flux’ metals.[10] These classifications are not mutually exclusive (e.g. some Cu and Zn are also bound by colloids and nominally dissolved organic acids), but are intended to help conceptualise the connection between groups of metals and their hydrological significance.
Although particulate NOM may be more readily influenced by hydrodynamic factors for transport through soil and epikarst, release of fine colloids from the soil zone is also dependent upon physicochemical processes associated with overcoming electrostatic adhesion between colloid and soil particulates.[16,17] Such binding affinities are readily compensated through changes to solution pH or ionic strength. Mobilisation of colloidal materials can therefore occur during periods of enhanced microbial respiration and CO2 production and at peak effective rainfall intensity.[18] The presence of NOM-associated trace metals in speleothem calcite is therefore dependent upon a range of factors including trace metal binding affinity to NOM, the hydrodynamic properties of each size fraction, prevailing hydrology and mechanisms of release from the soil zone of production.
At the Obir cave site, Austria, calcite is shown to strongly favour the co-precipitation of Pb and Zn.[6] The hydrological supply of these ore-associated metals is thought to dictate the primary pattern of their subsequent preservation in speleothem calcite.[6] Correlations between Zn, Pb and UV fluorescence in Obir stalagmites provides further compelling evidence for the accelerated delivery of these metals by NOM, possibly corresponding to the high-flux concept.[10] These hydrological controls are deemed responsible for the orders of magnitude change in concentration centred on µXRF event laminae.[6] Trace element partitioning into the calcite is further dependent upon NOM binding affinities and dissociation kinetics, speleothem growth rate and drip rate, which will all affect absolute concentrations during partitioning into the crystal lattice, albeit of secondary importance to event-based hydrological delivery.[19] However, observed patterns of trace metal concentrations within speleothem calcite at the micrometre scale have never been matched to seasonal changes in surface meteorological records as drivers of the above models for trace metal delivery. The current study uses meteorological data recorded during the period of speleothem growth to evaluate the role of hydrological conditions in determining the characteristics of the speleothem trace metal archive.
Current understanding: review of environmental conditions determining SO42– concentrations in speleothem carbonate
Speleothems contain carbonate-associated sulfate (CAS), which characterises the changing dynamics of S loading to the atmosphere.[9,20] Sulfur inputs from atmospheric pollution, volcanic and marine emissions have distinct end-member compositions. This allows identification of trends in anthropogenic pollution,[21] potentially high-resolution volcanic emission inventories[22] and records of atmospheric circulation dynamics. However, capture of these end-member signals is dependent upon the degree of modification in the soil and epikarst. Typically this comprises a three-stage model of signal modification based on soil and vegetative cycling, hydrology and redox status.[23] Long-term changes in all of these parameters serve to determine dripwater and speleothem SO42– content. At much shorter (annual) timescales, dripwater SO42– concentrations and isotopes remain constant.[24] However, despite all evidence from long-term drip water time series indicating that SO42– concentrations in cave dripwaters do not change on an annual timescale, analysis undertaken by SR[9] and secondary ionisation mass spectrometry[6] have identified SO42– concentrations within speleothem carbonate that demonstrate cyclicity appearing to be of an annual nature. The source of this cyclicity has been attributed to seasonality in the degree of SO42– partitioning between drip water and speleothem carbonate, driven by cave ventilation dynamics and modulation of the pH to which drip waters de-gas during carbonate precipitation. Cave ventilation exhibits seasonal variations in air exchange, driven by external temperatures.[24] When the latter fall below the (constant) internal cave temperature, pressure gradients dictate that air flows into the cave system. This lowers the CO2 partial pressure (pCO2) of the internal cave atmosphere, forces enhanced de-gassing of drip waters and controls the pH to which the water degases and consequently precipitates calcium carbonate. Low pCO2 (air) conditions result in high rates of degassing, generating a greater proportion of CO32– and driving pH to higher values. The greater proportion of CO32– in turn lowers the SO42–/CO32– ratio in solution and drives a lower CAS incorporation into calcite.[6,9,25] However, despite the available evidence suggesting sulfate incorporation follows this annual cycle of pCO2 modulation, there has not yet been any linkage between SO42– concentration and external temperature regimes to validate this model. As cave ventilation is ultimately driven by the difference in temperature between the cave exterior and interior, the severity of a winter season should be reflected in the morphology (peak shape and amplitude) of the annual SO42– cycle as a record extending back beyond the instrumental period. Here, we present high-resolution time series of SO42– and trace metal concentrations in speleothem carbonate, to demonstrate the precise annual cycle of SO42– partitioning. Meteorological data collected at daily resolution over the period of interest are used to determine the cave ventilation behaviour.
Methods
Site and sample description
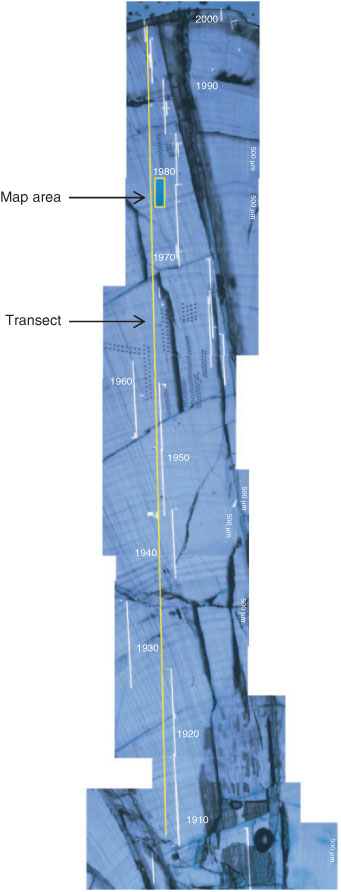
Obir cave (46°30′36″N, 14°32′24″E) is situated in south-east Austria and formed within the Obir Massif of the northern Karawanken Mountains along the Austrian–Slovenian border.[6,24] The cave consists of a complex network of sub-horizontal galleries and shafts that formed in Middle Triassic limestones. The studied cave chamber is located at ~1100 m above sea level (ASL). The overlying terrain is forested with mixed beech and spruce trees, underlain by a brown forest soil ~30 cm deep. Several meteorological stations are located in the area.[24] Meteorological records used as a part of this study were compiled from Loibl-Tunnel, located 24 km west-south-west and at the same altitude as the cave system (1098 m ASL). Annual precipitation in the region ranges between 1100 and 1600 mm and the average annual temperature outside the cave entrance is +6.2 °C.[24] Temperatures are below freezing between November and March with extensive snow cover during this period. Effective precipitation calculated using the Thornthwaite formula[26] shows peak water excess in both spring and autumn. The cave system was discovered during mining activities for Pb and Zn sulfide ores and is accessible today by an adit extending ~200 m into the hillside. Several of the cave chambers within the system have been extensively monitored for hydrological, atmospheric and speleothem characteristics.[6,24] The carbonate system is precisely regulated by cave ventilation, such that seasonality in directional air movement alters the pCO2 of the cave air, drip water degassing and the final pH of the drip waters. This process is reflected in the cave air composition, and isotope geochemistry of the speleothems.[24] Three stalagmites were collected from the Säulenhalle chamber (Obi12; Obi55; Obi84) and have been studied extensively in terms of petrology and geochemistry.[6] Sample Obi 84[27] was collected from the Säulenhalle chamber (see the map in Spötl et al.[24]) in December 2002 and was active at the time of removal. Säulenhalle is at a depth of ~70 m below the surface and has a temperature of 5.7 ± 0.1 °C. Hydrological characteristics of the drip feeding stalagmite Obi84 show pronounced inter-annual variation (in the range of 15–100 mL h–1, based on spot measurements). Stalagmite Obi 84 shows a consistent structure of visible laminae in the top few centimetres, which have been demonstrated to be annual based on laminae counting, trace element patterns and 14C dating.[27] The visible laminae within each annual growth band are approx. 15–20 µm wide, representing only a small portion of the total annual growth (total annual growth comprises 100–200-µm thickness of carbonate deposition). Enrichments of H, P, Na, Pb, Zn, F, Br and to a lesser extent Mg, across each visible lamina are coincident with decreases in Sr concentrations.[6,27] Discrete chemical enrichments in these annual, optically visible laminae were interpreted to reflect autumnal seasonality of infiltration events.[4] Analysis by hard X-ray SR revealed further multiple enrichments in chemical elements within this annual visible laminae, referred to here as ‘event laminae’. Unfortunately, the depth-penetrating nature of the hard X-rays (up to 110 μm deep dependent upon the element) prevented clear reconstruction of these individual fine bands.[6] Sulfate concentrations analysed by an ion microprobe were noted to demonstrate seasonal variability, with winter low concentrations consistent with enhanced ventilation regimes, although low resolution of analysis prevented further interpretation.[6]
Synchrotron radiation
Analysis was undertaken at the European Synchrotron Radiation Facility at beamline ID21. The X-ray microscope operates in the range 2–9 keV, the beam can be focussed using different optics (here a zone plate, offering a beam of 0.3 × 1.1 µm2), and the XRF signal can be collected using different detectors (here a large Silicon Drift Diode Detector). The sample is mounted vertically, with an angle of 60° with respect to the incoming beam and is raster scanned in order to generate two-dimensional maps. Experiments were carried out under vacuum, using the same sections of stalagmite Obi84 as those analysed by Fairchild et al.[6] Material for analysis was presented as thin wafers of stalagmite (<1 mm thick; 22 × 19 mm2), with polished upper surfaces and mounted to sample holders (3-cm diameter) using double sided tape. The previous analysis at ID21 had employed excitation at 2.9 keV, which had the advantage of facilitating analysis of several elements. However, the high Pb content of these samples presented a problem for the detection of S with the coincident excitation of M-lines by Pb with the K-S. The Pb signal is much stronger and affected the accuracy and the sensitivity of the detection of S. On returning to ID21, different excitations were employed to resolve the Pb and S interferences. For each area, XRF maps were acquired with a monochromatic beam at 2.482 keV where all S species are excited, with an over excitation of SO42–.[28] X-Ray absorption near-edge fine structure (XANES) spectra confirmed that sulfur is mainly present as sulfate (Supporting information, Fig. S1). Setting the energy of the incoming beam to the white line position of sulfates allows the absorption coefficient to be a factor of 3 or 4 higher than at higher energies, thereby enabling maximum detection of this species. Zn can be detected through the emission of characteristic L lines at ~1 keV. Use of the software PyMCA was effective in separating the different emission and scattering lines, in particular the Pb M and S Kα XRF emission using fitting algorithms, and was also used for image processing and collapsing into average profiles of non-equivalent count rates for each element of interest.[29]
Results
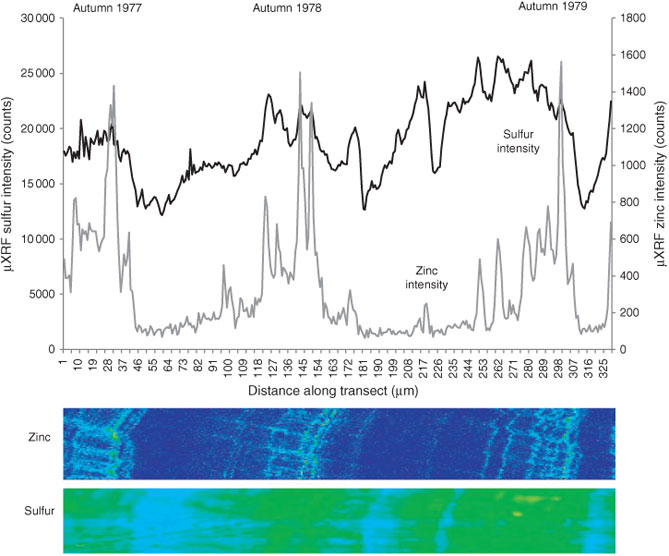
Analysis was undertaken as a series of nested images, building towards a high-resolution image revealing elemental structure at the sub-annual scale. Fig. 1 shows the location of the µXRF scans discussed in this paper, superimposed on thin section imagery of speleothem petrography. Secular changes to SO42– and Zn levels were obtained through the line transect, acquiring data using 10-µm pixels (dwell time 830 ms). A high-resolution map was raster scanned over three event laminae (1977–1979) and focussed to a pixel size of 1 µm (dwell time 830 ms). Co-incident variations in Pb, Mg, Na and P corroborate the patterns of concentration change previously noted in this speleothem.[6] The variability of Pb produces near-identical, but slightly less resolved results than Zn and so we only present new results for Zn.
|
Long-term changes in SO42– and Zn concentrations within speleothem Obi84 are shown in Fig. 2. The distance from the growing tip of the speleothem (mm) is converted into age (years) using the scale provided in Fairchild et al.[6] and dated 2000 to 1910 AD. Clear cycles in Zn concentrations are characteristic of the annual laminae identified,[4,6] and attributed to flushing of NOM–metal complexes during autumn season infiltration maxima.[10] Trends in SO42– concentration show an increase, which peak in the 1980s and subsequently fall, reflecting changing S emissions to the atmosphere. A decrease in δ34S for this speleothem confirms the origin of elevated S levels to be from industrial SO2 emissions.[21] Offsets between the timing of changes to S emissions and isotopic values were attributed to changing isotopic signatures of pollution.[21] The peak SO42– concentration in the speleothem calcite lags compared to the peak S emissions to the atmosphere, evident both here and in an analogous cave site at Ernesto, north-east Italy. This attenuation in SO42– can be explained by the delayed recovery of the soil ecosystem as a result of storage of S in the biogeochemical cycle.[21,23]
The relationship between the SO42– and Zn cycles within speleothem carbonate from Obi84 is considered at high resolution from 1977 to 1979 AD (Fig. 3; resolution 1-µm pixel size). The possibility of using enrichments of NOM-complexed elements as markers of water infiltration events places an autumnal timing constraint on the Zn peaks.[4,6] Cycling in SO42– content demonstrates a winter minimum and summer maximum according to the SO42– partitioning model controlled through degassing and pH (cave ventilation).[6]
Discussion
Characterising trace metal sub-event laminae
Peaks in Zn concentration in Fig. 3 have been proven to be annual on the basis of 14C dating[27] and placed within an autumnal timing constraint thought to be driven by peak water excess.[4,6] The presence of multiple event laminae between each autumnal peak in Zn concentration is also consistent with identification of multiple UV fluorescent laminae in a Romanian stalagmite within one annual cycle,[30] and the observations in Shihua Cave, Beijing, China, of multiple pulses of organic C during the wet season driven by infiltration events.[31,32] However, attribution of the main Zn pulse in each annual cycle to modelled peak water excess specific to 1977–1979 AD shows very little coincidence (Fig. 4). The bi-modal distribution of peak water excess demonstrates water availability in both spring and autumn, but the autumnal peak of water availability is frequently of secondary magnitude to that produced in spring.
Meteorological activity and event laminae
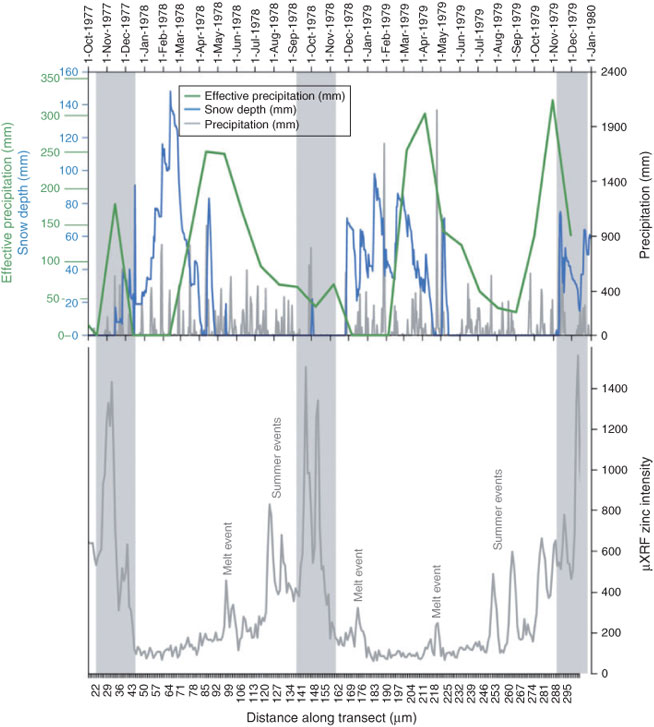
As Zn concentrations in the recipient stalagmite are known to peak during the autumn period,[6] limited correlation between the presence of annual laminae and peak water excess values indicates a more complex model of trace metal–NOM production, transport and incorporation than just flushing according to monthly effective precipitation. This challenge to the traditional hypothesis of trace metal incorporation in event laminae linked to seasonality of water excess at Obir cave is reconciled here with further knowledge of snowpack properties and inferred soil conditions. Local meteorological data is presented at a daily resolution from 1 October 1977 until 30 December 1979 in Fig. 4. Snow depth data demonstrate the onset of snowpack development in November and December each year. The main snowpack is continuously present until April, albeit punctuated by episodic melt events. Late snowpack recurrence in April and May is only short-lived. Precipitation occurs throughout the year, but only that which exceeds the soil moisture deficit contributes to soil and aquifer recharge and cave drip water production. Water excess is calculated using monthly rainfall totals from the Loibl-Tunnel station using the Thornthwaite formula, but only represents monthly average values, thereby omitting the significance of key events within any month and does not account for water availability during periods of snowmelt. This builds towards a three-component model for understanding the presence and relative significance of event laminae in annual calcite deposition. Of over-riding importance is the presence of a water excess; of secondary importance are snowpack dynamics that de-couple water availability from calculations of water excess; third, inferred soil conditions also play a role, i.e. they regulate the production of microbial CO2, pH and the availability of NOM. The relationship between snowpack depth, precipitation and effective precipitation in Fig. 4 are used to explain the seasonality of the Zn record discussed below.
Seasonality of trace metal enrichment within speleothem carbonate
Autumn
The most prominent event laminae have been suggested to coincide with autumnal water excess,[6] although in two of the three years presented (1977 and 1978) calculated autumnal water excess was low. However, such monthly calculations are misleading, because effective precipitation in autumn is mixed with early snowpack development and melting. Hydrological flushing thereby appears to be controlled in part by effective rainfall, and in part by snowpack melting. This occurs at a time when microbiological activity and pCO2 values in the soils will be close to the annual optimum, generating soil conditions conducive to NOM release[10] and seasonal release of elements through microbial cycling.[8] Acidification of the soil may also be enhanced through the ionic pulse generated during the initial stages of snowpack melt,[33] which enhances the release of NOM and colloids from the soil profile. These combined processes may be represented by peaks in Zn and Pb levels during November through December 1977, October 1978 and November through December 1979.
Winter and spring
Calculations of water excess during many winter months are presented as zero as a result of negative temperatures and the presence of precipitation as accumulated snowfall. The presence of a snowpack above the cave site thereby decouples effective winter precipitation from hydrological flushing through the soil and epikarst. Physical properties of the snowpack, including depth, temperature and water content will determine the extent to which surface melt is able to establish hydraulic connectivity with underlying soils and epikarst. Not every melt event will therefore result in full connectivity between the snowpack surface and cave drip, resulting in many melt events and periods of snowpack thinning not recorded in the speleothem trace metal record. Meteorological data in Fig. 4 are used to identify key periods of snowmelt and are matched up with Zn-rich horizons in the speleothem record. December 1977 to April 1978 presented rapid accumulation of a surface snowpack, interrupted by several periods of snowpack thinning. Only melt events at the start and end of the winter season, however, may be recorded as prominent event laminae. During winter 1979 (December 1978 to April 1979), the snowpack was thinner and accumulation was punctuated by prolonged melt events that appear in the speleothem Zn record as possible event laminae in December and April after which the snowpack completely disappeared. Winter and spring peaks in Zn levels are apparent in the speleothem record as being of consistently lower intensity compared to those formed later in the hydrological year.
Summer
After the period of spring snowmelt, calculations of monthly water excess provide a broad indication of effective rainfall events. Limited representation of the spring and summer months in the speleothem record compared to autumn and winter is attributable to the slower growth rates encountered as a result of higher pCO2 levels in the cave chamber. Despite declining amounts of water excess throughout the summer season, overall increasing Zn concentrations are observed throughout the spring and summer season. Therefore, an additional control, other than the effective precipitation or the number of individual synoptic events, must be regulating the abundance of Zn within speleothem calcite. Controls on colloid and NOM production in the soil zone are therefore central to understanding their production and release. Changes of pH through biogenic CO2 production are suggested to lead to enhanced mobilisation of colloids into soil solution during summer periods.[10] This would appear to be consistent with increasing concentrations of Zn in event laminae through the spring and autumn season. This is despite a declining monthly water excess and hydrodynamic agent to drive mobilisation.
Seasonality of SO42– presence within speleothem carbonate
The cycling of speleothem S concentration can be understood as a product of drip water pH. Ultimately, drip water pH is determined by the equilibrium between CO2(aq) and CO2(g).[9,25] At the annual scale, the key control of the build-up of CO2 within the cave system depends upon the ventilation regime, driven by external temperature fluctuations across the boundary of the cave internal temperature.[19] Breaching of this threshold causes ventilation driven by air pressure differences, such that the winter regime at Obir cave allows ingress of external air (low pCO2) into the cave system, whereas the summer regime encourages exhalation of cave air, drawing air rich in CO2 from the soil zone, down into the cave chambers.[24] Associated changes in δ13CCO2 of the cave air support such a model,[24] although the direct linkage of speleothem SO42– concentration to meteorological temperature variability has not been achieved thus far. This mechanism of pH control should lead to low winter and high summer concentrations of SO42– contained within speleothem carbonate and may be termed ventilation cycles. Fig. 5 shows variability in SO42– concentration across three annual cycles. Periods of external temperature transgression across the boundary of the average internal cave temperature (Säulenhalle chamber: +5.7 °C) are mapped onto Fig. 5, relative to assumed timings of Zn peaks shown in Fig. 4. A broad annual pattern to the S concentration depicts summer maxima and winter minima, as expected from the cave ventilation hypothesis. Where the external temperature remains below the internal threshold temperature of 5.7 °C, SO42– incorporation is at a minimum. Where external winter temperatures fluctuate persistently across the internal threshold, SO42– incorporation is very sensitive, manifesting as peaks and troughs throughout the winter period. The relatively greater representation of winter growth within the speleothem record (low sulfate incorporation), is a reflection of the faster growth rates during this time. A close association between proximity of external temperature to the cave threshold value and the transition of SO42– concentrations between maximum and minimum values demonstrates the importance of cave external temperatures in driving internal atmospheric dynamics and speleothem chemical composition in seasonally ventilated caves such as Obir.
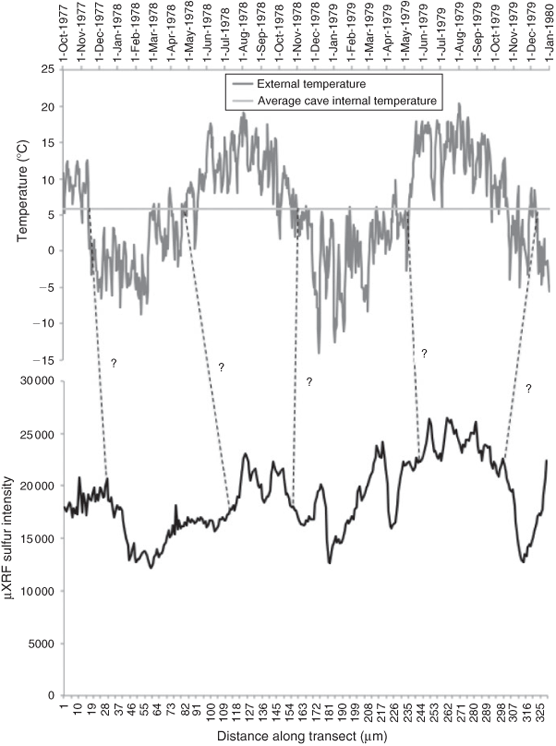
|
Time-series of SO42– and Zn obtained from simultaneous µXRF scans are overlain in Fig. 3. The distinct drivers controlling Zn (elemental flux) and SO42– concentrations (partition coefficient of incorporation) ensure that the long-term changes in Zn and SO42– are not correlated. However, on a sub-annual scale, the detailed architecture of elemental concentrations shows a closely coupled association between the timing of peaks and troughs in each element. This strong association points to a common driver influencing element dynamics. Although annual cycles in SO42– appear to be driven by pCO2 reversals forced by external temperature changes, short-term (sub-monthly) changes in SO42– incorporation appear linked to the Zn record. We suggest this may be attributable to CO2 addition to the cave atmosphere during times of high drip rates and degassing (forcing internal to the cave system) (compare to Frisia et al.[34]). This may coincide with high-flux events (transporting Zn and Pb) while enhancing SO42– incorporation through pCO2 and pH control.
Conclusion
SR-µXRF has been used to identify variability in the concentration of trace elements Zn and S during the annual growth cycle of speleothems. The spectroscopic tunability of the synchrotron X-ray beam was exploited to acquire elemental maps at the energy of the sulfate K-edge white line, increasing the sensitivity to sulfate. Indeed, at this energy, the absorption coefficent of sulfate is about 3–4 times higher, and the emission of overlapping and more intense Pb M-lines is avoided. The characteristic autumnal peak in surface-reactive trace metals[6] is complemented by a sub-annual architecture of XRF banding that increases in intensity towards autumnal peak concentrations. Mechanisms of NOM–metal delivery depend upon a range of hydrological (effective precipitation, snow depth and infiltration) and chemical factors (pH, ionic strength). By comparison with daily meteorological records of precipitation, snow depth, temperature and estimates of monthly precipitation excess, peaks in Zn levels can be matched to synoptic events. Incorporation of SO42– does not depend on delivery to the speleothem, but relies instead on cave atmospheric conditions. The proximity of external temperature to the cave internal threshold temperature drives cave ventilation. This regulates the pH to which drip waters degas, thereby determining the proportions of SO42– to carbonate ions in solution and hence speleothem calcite. This is manifest as a characteristic annual pattern of low winter, high summer concentrations (ventilation cycles), which verify the autumnal timing of the main Zn-bearing laminae. Despite differences in the controls of incorporation, the synchronicity between SO42– and Zn at the sub-annual scale suggests a common driver to both elements. This likely reflects the influence of enhanced dripping on pCO2 evolution in cave air, affecting SO42– incorporation and corresponding to higher NOM–metal fluxes. Through calibration of controls on trace element incorporation to external meteorological events, this should now enable reconstruction of indices of climatic change, both within and beyond the instrumental period.
Supporting material
XANES spectrum at the sulfur K-edge, acquired on speleothem Obi84. This information is available from the journal online (see http://www.publish.csiro.au/?act=view_file&file_id=EN13082_AC.pdf).
Acknowledgements
The authors thank the European Synchrotron Radiation Facility for funding this work (Experiment EC710). CS acknowledges the late R. Böhm (ZAMG, Vienna) for providing meteorological data.
References
[1] P. Treble, J. M. G. Shelley, J. Chappell, Comparison of high resolution sub-annual records of trace elements in a modern (1911–1992) speleothem with instrumental climate data from southwest Australia. Earth Planet. Sci. Lett. 2003, 216, 141.| Comparison of high resolution sub-annual records of trace elements in a modern (1911–1992) speleothem with instrumental climate data from southwest Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXos1ajurw%3D&md5=d91d02361ff0feec33e58361c29d43c7CAS |
[2] P. Treble, J. Chappell, J. M. G. Shelley, Complex speleothem growth processes revealed by trace element mapping and scanning electron microscopy of annual layers. Geochim. Cosmochim. Acta 2005, 69, 4855.
| Complex speleothem growth processes revealed by trace element mapping and scanning electron microscopy of annual layers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFOgs73N&md5=39fd3a88e05ac7fd1e78a20ffd316171CAS |
[3] K. R. Johnson, H. Chaoyong, N. S. Belshaw, G. M. Henderson, Seasonal trace-element and stable-isotope variations in a Chinese speleothem: The potential for high-resolution paleomonsoon reconstruction. Earth Planet. Sci. Lett. 2006, 244, 394.
| Seasonal trace-element and stable-isotope variations in a Chinese speleothem: The potential for high-resolution paleomonsoon reconstruction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjtVOntLk%3D&md5=4cb7859902497544eaf6eb9348a7fe07CAS |
[4] A. Borsato, S. Frisia, I. J. Fairchild, A. Somogyi, J. Susini, Trace element distribution in annual stalagmite laminae mapped by micrometer resolution X-ray fluorescence: implications for incorporation of environmentally significant species. Geochim. Cosmochim. Acta 2007, 71, 1494.
| Trace element distribution in annual stalagmite laminae mapped by micrometer resolution X-ray fluorescence: implications for incorporation of environmentally significant species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXis1Gjs7w%3D&md5=c737172d76f449816b43d5e7a8c5871fCAS |
[5] D. Mattey, D. Lowry, J. Duffet, R. Fisher, E. Hodge, S. Frisia, A 53 year seasonally resolved oxygen and carbon isotope record from a modern Gribraltar speleothem: Reconstructed drip water and relationship to local precipitation. Earth Planet. Sci. Lett. 2008, 269, 80.
| A 53 year seasonally resolved oxygen and carbon isotope record from a modern Gribraltar speleothem: Reconstructed drip water and relationship to local precipitation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlsFClsLw%3D&md5=1b1fedc479a65299674846322cd15765CAS |
[6] I. J. Fairchild, C. Spötl, S. Frisia, A. Borsato, J. Susini, P. M. Wynn, J. Cauzid, EIMF. Petrology and geochemistry of annually laminated stalagmites from an alpine cave (Obir, Austria): seasonal cave physiology, in Tufas and speleothems: unravelling the microbial and physical controls. Geological Society of London Special Publication (Eds H. M. Pedley, M. Rogerson), 2010, Vol. 336, pp. 295–321 (The Geological Society: London).
[7] M. S. Roberts, P. Smart, A. Baker, Annual trace element variations in a Holocene speleoethem. Earth Planet. Sci. Lett. 1998, 154, 237.
| Annual trace element variations in a Holocene speleoethem.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXhtFyhsrw%3D&md5=4099b19fc0c01a00c172bf56a47441e5CAS |
[8] I. J. Fairchild, A. Baker, A. Borsato, S. Frisia, R. W. Hinton, F. McDermott, A. F. Tooth, High resolution, multiple trace element variation in speleothems. J. Geol. Soc. London 2001, 158, 831.
| High resolution, multiple trace element variation in speleothems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXotl2ltbk%3D&md5=de00870f10ac5c4df18aa7311a0a1208CAS |
[9] S. Frisia, A. Borsato, I. J. Fairchild, J. Susini, Variations in atmospheric sulphate recorded in stalagmites by synchrotron micro-XRF and XANES analyses. Earth Planet. Sci. Lett. 2005, 235, 729.
| Variations in atmospheric sulphate recorded in stalagmites by synchrotron micro-XRF and XANES analyses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlvVCrt7g%3D&md5=6823646fceb52952d09bee338201575eCAS |
[10] A. Hartland, I. J. Fairchild, J. R. Lead, A. Borsato, A. Baker, S. Frisia, M. Baalousha, From soil to cave: Transport of trace metals by natural organic matter in dripwaters. Chem. Geol. 2012, 304–305, 68.
| From soil to cave: Transport of trace metals by natural organic matter in dripwaters.Crossref | GoogleScholarGoogle Scholar |
[11] A. Hartland, Colloidal geochemistry of speleothem-forming groundwaters 2011, Ph.D. thesis, University of Birmingham.
[12] A. Hartland, I. J. Fairchild, J. R. Lead, H. Zhang, M. Baalousha, Size, speciation and lability of NOM–metal complexes in hyperalkaline cave dripwater. Geochim. Cosmochim. Acta 2011, 75, 7533.
| Size, speciation and lability of NOM–metal complexes in hyperalkaline cave dripwater.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVylsrjP&md5=ce1862b98abfd57375ebb4d4971f2b03CAS |
[13] J. F. McCarthy, L. Shevenell, Processes controlling colloid composition in a fractured and karstic aquifer in eastern Tennessee, USA. J. Hydrol. 1998, 206, 191.
| Processes controlling colloid composition in a fractured and karstic aquifer in eastern Tennessee, USA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXjtVKrtb4%3D&md5=bbbfbc4477eb91f0d18274ec0abe2cdaCAS |
[14] N. Goppert, N. Goldscheider, Solute and colloid transport in karst conduits under low- and high-flow conditions. Ground Water 2008, 46, 61.
| 18181865PubMed |
[15] J. F. McCarthy, L. D. McKay, Colloid transport in the subsurface: past, present and future challenges. Vadose Zone J. 2004, 3, 326.
| 1:CAS:528:DC%2BD2cXptF2mtLw%3D&md5=e7dbf2b61d949b6b5d4a218a5b6b385cCAS |
[16] L. Shevenell, J. F. McCarthy, Effects of precipitation events on colloids in a karst aquifer. J. Hydrol. 2002, 255, 50.
| Effects of precipitation events on colloids in a karst aquifer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmtVWksA%3D%3D&md5=2a86b6acf277d793ff8e8c220b491700CAS |
[17] T. Kanti Sen, K. C. Khilar, Review on subsurface colloids and colloid associated contaminant transport in saturated porous media. Adv. Colloid Interface Sci. 2006, 119, 71.
| Review on subsurface colloids and colloid associated contaminant transport in saturated porous media.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhslKgur8%3D&md5=b8961080882b579a31f7ed6ba8d3282aCAS | 16324681PubMed |
[18] F. W. Cruz, I. Karmann, G. B. Magdaleno, N. Coichev, O. Viana, Influence of hydrological and climatic parameters on spatial-temporal variability of fluorescence intensity and DOC of karst percolation waters in the Santana Cave System, southeastern Brazil. J. Hydrol. 2005, 302, 1.
| Influence of hydrological and climatic parameters on spatial-temporal variability of fluorescence intensity and DOC of karst percolation waters in the Santana Cave System, southeastern Brazil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtFGhsL7I&md5=7e3ea8d83c17fd1c797dc1b00e3130adCAS |
[19] I. J. Fairchild, A. Baker, Speleothem Science: from Process to Past Environments 2012 (Wiley–Blackwell: Sussex, UK).
[20] P. M. Wynn, I. J. Fairchild, A. Baker, S. Frisia, A. Borsato, J. Baldini, F. McDermott, Isotopic archives of sulphur in speleothems. Geochim. Cosmochim. Acta 2008, 72, 2465.
| Isotopic archives of sulphur in speleothems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlvFKlsrw%3D&md5=47be104a9847bf422bc44235a364f8f3CAS |
[21] P. M. Wynn, I. J. Fairchild, S. Frisia, C. Spötl, A. Baker, A. Borsato, EIMF, High-resolution sulphur isotope analysis of speleothem carbonate by secondary ionisation mass spectrometry. Chem. Geol. 2010, 271, 101.
| EIMF, High-resolution sulphur isotope analysis of speleothem carbonate by secondary ionisation mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXivVyrur4%3D&md5=caf3e3f2e57e9672ea053f5708764199CAS |
[22] S. Frisia, A. Borsato, J. Susini, Synchrotron radiation applications to past volcanism archived in speleothems: an overview. J. Volcanol. Geotherm. Res. 2008, 177, 96.
| Synchrotron radiation applications to past volcanism archived in speleothems: an overview.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1KmtbnK&md5=585d9717d2dbcd96b8a32bdb7881f156CAS |
[23] P. M. Wynn, A. Borsato, A. Baker, S. Frisia, R. Miorandi, I. J. Fairchild, Biogeochemical cycling of sulphur in karst and transfer into speleothem archives at grotta di ernesto, Italy. Biogeochemistry 2013, 114, 255.
| Biogeochemical cycling of sulphur in karst and transfer into speleothem archives at grotta di ernesto, Italy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtVSmtbzO&md5=4c27e9bc4b2f83dc5349393f56ec2dcfCAS |
[24] C. Spötl, I. J. Fairchild, A. F. Tooth, Cave air control on dripwater geochemistry, Obir caves (Austria): implications for speleothem deposition in dynamically ventilated caves. Geochim. Cosmochim. Acta 2005, 69, 2451.
| Cave air control on dripwater geochemistry, Obir caves (Austria): implications for speleothem deposition in dynamically ventilated caves.Crossref | GoogleScholarGoogle Scholar |
[25] E. Busenberg, L. N. Plummer, Kinetic and thermodynamic factors controlling the distribution of SO42– and Na+ in calcites and selected aragonites. Geochim. Cosmochim. Acta 1985, 49, 713.
| Kinetic and thermodynamic factors controlling the distribution of SO42– and Na+ in calcites and selected aragonites.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXhs12qs7Y%3D&md5=9f7cbe09c76d5160e70ac53aee9dbb24CAS |
[26] C. W. Thornthwaite, An approach toward a rational classification of climate. Geogr. Rev. 1948, 38, 55.
| An approach toward a rational classification of climate.Crossref | GoogleScholarGoogle Scholar |
[27] C. L. Smith, I. J. Fairchild, C. Spötl, S. Frisia, A. Borsato, S. G. Moreton, P. M. Wynn, Chronology-building using objective identification of annual signals in trace element profiles of stalagmites. Quat. Geochronol. 2009, 4, 11.
| Chronology-building using objective identification of annual signals in trace element profiles of stalagmites.Crossref | GoogleScholarGoogle Scholar |
[28] M. Cotte, E. Checroun, J. Susini, P. Walter, Micro-analytical study of interactions between oil and lead compounds in paintings. Appl. Phys., A Mater. Sci. Process. 2007, 89, 841.
| Micro-analytical study of interactions between oil and lead compounds in paintings.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFyksrnM&md5=790ef69c26b6557648332a329d729706CAS |
[29] V. A. Solé, E. Papillon, M. Cotte, P. Walter, J. Susini, A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim. Acta B 2007, 62, 63.
| A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra.Crossref | GoogleScholarGoogle Scholar |
[30] Y. Y. Shopov, D. C. Ford, H. P. Schwarcz, Luminescent microbanding in speleothems: high resolution chronology and palaeoclimate. Geology 1994, 22, 407.
| Luminescent microbanding in speleothems: high resolution chronology and palaeoclimate.Crossref | GoogleScholarGoogle Scholar |
[31] M. Tan, A. Baker, D. Genty, C. Smith, J. Esper, B. G. Cai, Applications of stalagmite laminae to palaeoclimate reconstructions: comparison with dendrochronology/climatology. Quat. Sci. Rev. 2006, 25, 2103.
| Applications of stalagmite laminae to palaeoclimate reconstructions: comparison with dendrochronology/climatology.Crossref | GoogleScholarGoogle Scholar |
[32] F. Ban, G. Pan, J. Zhu, B. Cai, M. Tan, Temporal and spatial variations in the discharge and dissolved organic carbon of drip waters in Beijing Shihua Cave, China. Hydrol. Processes 2008, 22, 3749.
| Temporal and spatial variations in the discharge and dissolved organic carbon of drip waters in Beijing Shihua Cave, China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFyltrnI&md5=7c3265a05a53b43bfb9c269e09f1ca05CAS |
[33] M. W. Williams, J. M. Melack, Solute chemistry of snowmelt and runoff in an alpine basin, Sierra Nevada. Water Resour. Res. 1991, 27, 1575.
| Solute chemistry of snowmelt and runoff in an alpine basin, Sierra Nevada.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXmtl2js7c%3D&md5=c29f0ba901266ed25c09653991182b68CAS |
[34] S. Frisia, I. J. Fairchild, J. Fohlmeister, R. Miorandi, C. Spötl, A. Borsato, Carbon mass-balance modelling and carbon isotope exchange processes in dynamic caves. Geochim. Cosmochim. Acta 2011, 75, 380.
| Carbon mass-balance modelling and carbon isotope exchange processes in dynamic caves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFyjurzF&md5=841db66f38e079f5b6acd58597fc7908CAS |