Environmental effects on arsenosugars and arsenolipids in Ectocarpus (Phaeophyta)
Ásta H. Pétursdóttir A B C D , Kyle Fletcher B , Helga Gunnlaugsdóttir C , Eva Krupp A , Frithjof C. Küpper B and Jörg Feldmann A DA TESLA – Trace Element Speciation Laboratory, Department of Chemistry, University of Aberdeen, Aberdeen, AB24 3UE, Scotland, UK.
B Oceanlab, University of Aberdeen, Newburgh, AB41 6AA, UK.
C Matis, Food Safety, Environment and Genetics Department, Vinlandsleid 12, IS-113 Reykjavik, Iceland.
D Corresponding authors. Email: astap@matis.is; j.feldmann@abdn.ac.uk
Environmental Chemistry 13(1) 21-33 https://doi.org/10.1071/EN14229
Submitted: 25 October 2014 Accepted: 2 March 2015 Published: 28 July 2015
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Environmental context. Arsenolipids, which are present in seaweed, can show high toxicity, emphasising the need for more information on these compounds. We investigated the effects of different stress factors on the arsenic compounds formed by cultures of brown algae, and compared the results with those from field-collected samples. We show that the arsenolipid and arsenosugar profiles differ depending on the experimental conditions, and that a deficiency in phosphate has a direct positive effect on the biosynthesis of arsenic-containing phospholipids.
Abstract. Seaweeds have recently been shown to contain a significant proportion of arsenic in the form of arsenolipids (AsLp). Three strains of the filamentous brown alga Ectocarpus species were grown in the laboratory with different simulations of environmental stress: control conditions (1/2 Provasoli-enriched seawater), low nitrate (30 % of the amount of nitrates in the control), low phosphate (30 % of the amount of phosphate in the control) and under oxidative stress levels (2 mM H2O2). Generally, the major AsLp was an arsenic-containing hydrocarbon, AsHC360 (50–80 %), but additionally, several arsenic-containing phospholipids (AsPL) were identified and quantified using high-performance liquid chromatography–inductively coupled plasma mass spectrometry and electrospray ionisation mass spectrometry (HPLC-ICP-MS/ESI-MS). The AsLps in cultures were compared with AsLps in Ectocarpus found in its natural habitat as well as with other brown filamentous algae. The AsLp and arsenosugar profiles differed depending on the experimental conditions. Under low phosphate conditions, a significant reduction of phosphorus-containing arsenosugars was noticed, and a significant increase of phosphate-containing AsLps was found when compared with the controls. Strains grown under oxidative stress showed a significant increase in AsLps as well as clear physiological changes.
Additional keywords: chloroplasts, cultures, HPLC-ESIMS, HPLC-ICP-MS, lipid-soluble arsenic, speciation.
Introduction
Arsenic is found in organic and inorganic forms and ~100 naturally occurring arsenic species have been identified.[1–3] Arsenic can be toxic and is known to be carcinogenic; however, the toxicity is species-dependent and varies between arsenic species.[4] A large quantity of naturally occurring organic forms of arsenic are formed from inorganic arsenic that is taken up from the environment. The total arsenic (totAs) concentration in many seaweed species is high and can reach more than 100 mg kg–1 dry weight (DW).[5] There is no evidence that elevated arsenic concentration in seawater due to pollution has an effect on the arsenic concentration in seaweed. Arsenic accumulates in seaweed because of the structural similarities of arsenate and phosphate. With phosphate being actively taken up by phosphate transporters, these transporters can mistake arsenate for phosphate owing to lack of specificity.[6,7] Phosphate availability is hypothesised to regulate AsV uptake in algae,[8] where phosphate exposure to marine phytoplankton Dunaliella tertiolecta has been shown to influence arsenic uptake as well as the distribution of arsenic between different fractions.[9] Other studies showed little difference in AsV uptake, metabolism and species formation in marine phytoplankton depending on phosphate availability.[10]
Generally, only a minor portion of arsenic in seaweed is in the form of the toxic arsenate taken up from the ocean.[4] The arsenic in seaweed is also not found in the form of arsenoesters as analogues to the phosphate esters in biomolecules such as phosphorylated proteins, deoxyribonucleic acid (DNA) or adenosine triphosphate (ATP), but mainly found as arsenosugars in macroalgae. Most arsenosugars are dimethylarsinoylribosides and most can be associated with just four compounds, although 20 naturally occurring arsenosugars have been identified.[4] Recently, it was established that besides arsenosugars, arsenic can form lipid-soluble dimethylarsinoyl species in seaweeds,[11,12] i.e. hydrocarbons (AsHC), fatty acids (AsFA) and phospholipids (AsPL), Fig. 1. It has been suggested that these arsenolipid (AsLp) species might have a functional role within the cell membrane, e.g. by using AsPLs to replace phospholipids.[11,13] Benson and coworkers[14] grew algal cultures in 74AsV and showed that the arsenate was taken up by the algae, where the first biotransformation intermediates were lipid-soluble As species, thought to be AsPL. The AsPLs might therefore be formed first where the arsenosugars (AsSugars) are subsequently a hydrolysed product of the AsPLs.[15] These arsenic species are closely related because AsPLs have the same AsSugar structure as one of the most common AsSugars, AsSugarPO4. The formation of lipids before sugars would be the opposite of the transformation scheme proposed by Edmonds et al.[16] (Fig. 1b).
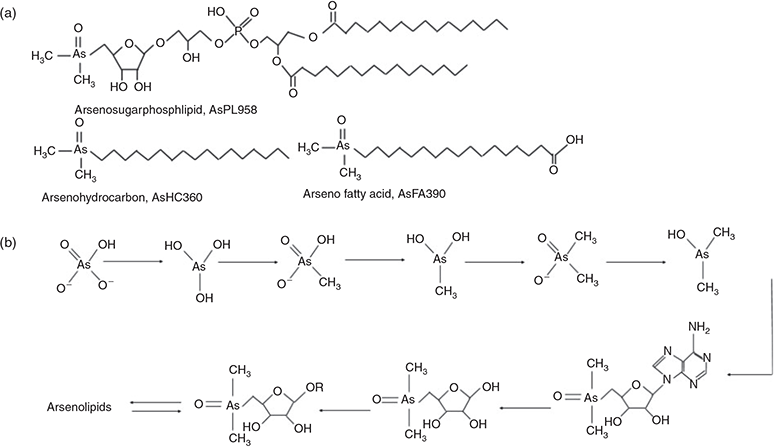
|
However, it is not clear whether the biosynthetic pathway actually takes place in the alga itself or whether these major arsenic species might be produced by bacteria, fungi or microalgae.[12,17,18]
Recent findings of Meyer et al.[19] have shown that AsHCs show different toxicity depending on the structure of the AsHC and can have similar cytotoxicity to inorganic arsenic (iAs). These new findings underline the relevance of accurately identifying AsLps but also the need to understand better how they are formed and which factors influence their formation.
Ectocarpus siliculosus has been adopted as a general model organism for brown algae, partly owing to its long history in research[20] but also because its life cycle can be completed in 3 months in Petri dishes.[21] Ectocarpus can be cultivated from a single cell and maintained easily[22] and its entire genome has been sequenced and annotated,[23] although this has not yet revealed new insights about its arsenic metabolism.
The objectives of the present study were to investigate whether arsenic uptake and transformation in seaweed, using Ectocarpus, depend on environmental conditions – with the aim of shedding light on whether the arsenic is potentially used by the organism and may therefore have a physiological function.
Materials and methods
Chemical and standards
All chemicals used were of analytical grade or better unless otherwise stated and double-distilled water was used for all sample preparation. Formic acid (methanoic acid, HCO2H) and the chemicals for Provasoli-enriched sea water (β-glycerophosphate disodium salt hexahydrate (C3N7Na2O6P·5H2O), sodium nitrate (NaNO3), ammonium iron sulfate hexahydrate ((NH4)2Fe(SO4)2·6H2O), sodium EDTA, vitamin B12, thiamine, biotin, boric acid (H3BO3), manganese sulfate·4H2O (MnSO4·4H2O), zinc sulfate·7H2O (ZnSO4·7H2O), cobalt sulfate·7H2O (CoSO4·7H2O) were supplied by Sigma–Aldrich (UK). Sodium dimethylarsinic acid (98 % DMA) used for quantification was obtained from ChemService (USA). For calibration of totAs and for spiking Ectocarpus cultures, a 1002-mg As L–1 certified As stock solution (as H3AsO4 in 0.5 M HNO3) was supplied by Merck (UK). Hexane, hydrogen peroxide (H2O2, 30 %, laboratory reagent grade (LR)), potassium hydroxide (KOH, LR), hydrochloric acid (HCl), dichloromethane (DCM), nitric acid (HNO3 69 %) and methanol (MeOH) were supplied by Fisher Scientific (UK). Germanium (Ge) used as internal standard for speciation was obtained from Sigma–Aldrich and rhodium used as an internal standard for totAs from Specpure, Alfa Aesar (Germany). Certified reference material SPS-WW2 (waste water, Spectrapure Standards, Norway) was used for monitoring the performance of the Agilent 8800 inductively coupled plasma mass spectrometry (ICP-MS) on the days when totAs was measured.
Samples
Ectocarpus sp., Elachista fucicola, Hincksia sp. and Pylaiella littoralis (all Ectocarpales) were collected in their natural habitats. Ectocarpus and Pylaiella were collected at Grótta beach, Reykjavík, Iceland, in August 2013. The samples were transported in a tub of seawater to Aberdeen. Ectocarpus was additionally collected at Aberdeen Beach, UK, and Cruden Bay, UK (August 2013). Elachista was collected in Newburgh, UK (July 2013). Each individual tuft of filamentous algae was confirmed to be the assigned species by looking at portions of each tuft under a microscope (Fig. S1). The samples were cleaned by manually removing all other algae tangled with them, and rinsed both in seawater and in MilliQ water before freeze-drying. All concentrations are reported on a freeze-dried basis. The samples were subsequently ground using a pestle and mortar.
Unialgal Ectocarpus cultures were obtained from the Culture Collection of Algae and Protozoa (CCAP, Oban, UK) and are abbreviated as strains 1–3 (St1–St3) St1: EC-Oban (CCAP 1310/300 – E. crouaniorum), St2: EC-007–04 (CCAP 1310/13 – E. fasciculatus) and St3: EC-022–10 (CCAP1310/214 – E. siliculosus). A phylogenetic tree can be found in Fig. S4. By visual assessment, the cultures were shown to contain a small amount of bacteria, but the algal biomass was far in excess of bacterial biomass because bacteria were kept at minimum by using sterile equipment if possible and autoclaving everything else before use. The experiment is therefore not suited to concluding whether the arsenic species are generated by bacteria or not. When harvested, the cultures were rinsed in MilliQ water before freeze-drying and the samples ground to a fine powder before extraction.
Experimental design
Three cultures of of Ectocarpus strains were grown in half-strength Provasoli-enriched sea water (PES)[24,25] in Petri dishes (12 °C, 12 h light/12 h dark). The media were changed every 2 weeks and each culture split into three plates when needed. Once sufficient starting material was available, modified half-strength PES was prepared, where the stock solutions were prepared to have only 30 % of the normal amount of either phosphates or nitrates. Generally, seawater levels range from ~10 to 40 µM nitrates and 1 to 3 µM phosphates,[26] whereas the filtered seawater used here was lower in nutrients. The concentrations used are listed in Table 1. A total of 25 L of seawater was filtered and kept under cold conditions to use throughout the experiment; this was done in order to keep this parameter constant. After the addition of the Provasoli stock solution to the seawater, the medium was autoclaved. Typical oxidative stress conditions were set as 2 mM exogenous H2O2, which has been applied in other physiological studies.[27] This was obtained by adding the appropriate amount of H2O2 to each individual Petri dish that was grown under oxidative stress every time the medium was changed. The totAs in the media was measured to be 0.8 µg L–1, and plates grown under arsenic-enriched conditions were spiked with 10 mg L–1 AsV solution, resulting in a final totAs concentration of ~2.8 µg L–1. Only St3 was grown with added arsenic to keep the number of samples down owing to time-consuming measurements. Both concentrations are environmentally reasonable because the concentration of arsenic in the ocean most often ranges from 1 to 2 µg L–1.[28] Experimental parameters are summarised in Table 1.

|
The media were changed every 2 weeks, the cultures starting in small Petri dishes (55 mm) and later on being transferred to bigger Petri dishes (90 mm). The cultures were grown using the experimental parameters for 5 months in triplicates.
Sample preparation
Sequential extraction for speciation
Lipid soluble (LS) extraction. Ectocarpales (field-collected, 0.2–1 g) were used depending on available material and extracted in MeOH/DCM (1 : 2, 5–15 mL) and left overnight. A second extraction was performed and the combined supernatants evaporated to dryness. The samples were then dissolved in MeOH and concentrated to 200–1000 µL depending on starting material (LS fraction). Hexane extraction: the dried seaweed residue was then extracted in hexane (2 × 5–15 mL), supernatants were combined and evaporated, and the sample prepared for totAs measurement (1 mL HNO3 added and left overnight, 2 mL H2O2 added before microwaving and samples diluted to 20 mL).
Water soluble (WS) extraction. The dried residue was further extracted with water (5–15 mL, depending on the original starting material) and left overnight. The supernatant was removed and kept refrigerated until measurement (WS fraction).
Residue digestion. The residue was prepared for totAs digestion (using 1 mL HNO3 and 2 mL H2O2) (RS fraction).
Total arsenic. TotAs in the Ectocarpales was prepared by weighing the dry material (0.01–0.1 g depending on availability), which was left overnight in HNO3 (0.2–1 mL). H2O2 (0.4–2 mL) was added before microwave digestion (CEM MARS microwave, program: ramp to 50 °C; hold 5 min; ramp to 75 °C; hold 5 min; ramp to 95 °C; hold 30 min) and the samples were diluted (5–20 mL) after the microwave digestion. For totAs measurements of the different fractions: 1 mL of the water fraction was transferred to a 15-mL tube, 100 µL of MeOH and 200 µL of HNO3 were added and diluted to 10 mL ready for totAs measurement (standards also prepared in MeOH and HNO3). For the LS fraction, 50 µL of the concentrated MeOH fraction was transferred to 15-mL tubes, 0.2 mL HNO3 was added and 0.4 mL H2O2 added before microwave digestion. The samples were diluted to 5 mL.
The sample amount obtained from the cultures was much smaller (~10 mg per replicate) than for the field-collected Ectocarpales and the same procedure as above was scaled down proportionally. The MeOH/DCM fraction was concentrated down to ~150 µL MeOH and 80-µL injections were used for the high performance liquid chromatography (HPLC)–ICP-MS–electrospray ionisation mass spectrometry (ESI-MS). The remainder of the sample was prepared for totAs measurement.
Glassware was used for extractions using organic solvents, which could result in inorganic arsenic impurities in the samples, but this should not affect the arsenolipid quantification owing to different elution times of the species.
Transmission electron microscopy (TEM)
When observing the cultures, it was evident that a physiological difference could be noted, even by eye. To test whether a physiological difference in the cell membrane could be noted if for example AsLps were used in the membrane, TEM imaging was used to observe morphological features.
Preparation
Ectocarpus cultures were viewed under a light microscope (Zeiss Axio imager D2 inverted microscope with Zeiss Axiocam MRC) and somatic cells were isolated from senescent cells. Isolated cells were then processed through an optimised schedule kindly provided by Ingo Maier (Ecoscope, Amtzell, Germany). Briefly, specimens with 1 mL of culture media were fixed by the addition of 286 µL of fixing buffer (0.4 M sodium cacodylate, 0.1 M EDTA, pH 7.4, 1 % caffeine) and 143 µL of 25 % gluteraldehyde. These were incubated at room temperature for 2 h and processed through three washes of wash buffer (0.4 M sodium cacodylate, 0.1 M EDTA, pH 7.4) for 15 min each and post-stained with 1 % OsO4 in OsO4 buffer (106.7 mM sodium cacodylate) for 2 h before a single 15-min wash with OsO4 buffer. The specimens were then stained with 1 % uranyl acetate (in H2O) for 1 h at room temperature and dehydrated in a 10 % acetone series (10–60 %, 10 min each step; 70–100 %, 15 min each step, before two overnight washes in 100 % acetone). Spurr’s resin was then infiltrated into the samples through incubation, first with 1 : 1 acetone to Spurr’s resin, then 2 × 100 % Spurr’s resin for 1 day each. The specimens were then transferred to fresh Spurr’s resin and polymerised at 60–70 °C overnight. Sections were then cut using a diamond knife (with a Leica EM UC6 Ultramicrotome), placed on copper grids and post-stained again using uranyl acetate and lead citrate before being imaged using a TEM (Jeol, JEM-1400-Plus).
Image processing
Organelle sizes, presented in the electron micrographs, were measured using the freehand selection tool in ImageJ (http://imagej.net/Downloads, accessed 12 May 2015) and the percentage content was calculated by dividing the organelle area by the total cell area. In instances of plasmolysis during fixation, the cell size was calculated from the retracted cytoplasmic boundary. At least 20 cells were measured in each treatment. A one-way ANOVA with a least significant difference (LSD) post-hoc, to test the difference between the means of the treatments, was performed and box plots created using SPSS (http://www-01.ibm.com/software/uk/analytics/spss/, accessed 12 May 2015).
Instrumental set-up
The ICP-MS was optimised for optimal sensitivity and stability on As on a day-to-day basis. All speciation was carried out on an Agilent 1100 HPLC system connected directly to the ICP-MS. The instrumental operating parameters are summarised in Table S1.
Total arsenic
The Agilent triple quadrupole ICP-MS 8800 (ICP-QQQ) was used for arsenic detection. Total arsenic measurements were carried out in no-gas and O2 mode. In O2 mode, arsenic was measured indirectly as 75As16O+ on m/z 91. There was no significant difference between the different modes, and totAs concentrations reported are based on no-gas measurements. Method blanks were run with each batch of samples for totAs and quantification was performed with matrix-matched solutions of the calibration standard. To monitor the accuracy and performance of the ICP-MS on a daily basis, the reference material SPS-WW2 was prepared in matrix-matched solutions and analysed, yielding a concentration of 0.48 mg kg–1 As (±0.02 s.d.) (n = 9) compared with the certified value of 0.500 ± 0.003 mg kg–1.
Water-soluble (WS) As
For speciation of water-soluble arsenic species, a PRPX-100 Hamilton anion-exchange column (10 μm, 4.6 × 250 mm) with a flow rate of 1 mL min–1 was used with a 20 mM ammonium carbonate mobile phase. The measurements were performed in O2 mode. Rh was used as internal standard for the speciation and the totAs measurements. HPLC-ICP-MS/ESIMS measurement for a selected sample was performed to identify the arsenosugars present, because a similar arsenosugar profile was found for all brown algae species measured. Hydride generation (HG)-ICP-MS was used, where the HG is added as a selective step to determine iAs in the gaseous phase while organically bound As remains in the solution. This instrumental setup is described in detail in Musil et al.[29]
Lipid soluble (LS) As
HPLC-ICP-MS/ESIMS was used for the speciation of AsLps. A reverse-phase column (Agilent Eclipse, XBD-C18, 4.8 × 150 mm) with a gradient of water and methanol both containing 0.1 % formic acid, was used for the speciation of AsLps. The eluent was split post column: 25 % to the triple-quad Agilent 8800 (QQQ) and 75 % to the ESIMS (LTQ Orbitrap Discovery; Thermo Scientific). The QQQ was used in organic mode, with platinum cones and 6 % oxygen. The signal was optimised at m/z 75 to give a maximum response for As. To compensate for the changes in the response because of the varying carbon content of the gradient, a response factor (100 µg L–1 DMA in 10 µg L–1 Ge) was used before the measurement of samples, as previously described.[30] This makes it possible to quantify and identify species without species-specific standards. DMA was used as external standard for the quantification of the arsenic species and Ge m/z 74 (10 µg L–1) was used as internal standard. The quantification of the AsLps found with speciation analysis was compared with the mass balance determined by measuring the totAs concentration in every fraction of the sequential extraction, giving better confidence in the quantification (Tables S3–S4). The results showed that the field-collected Ectocarpus (both from Aberdeen and Reykjavik), Elachista and Pylaiella had a column recovery of 111 % ± 18 (85–138 %), whereas the column recovery for the cultures had a wider range (40–110 %). The lower column recovery was attributed to the small amount of sample material available, i.e. AsLps of very low concentrations would elute undetected. This theory was supported by the data from Ectocarpus grown under enriched arsenic conditions as the column recovery was higher (87 % ± 19). The quantification values for the cultures give good indication of the concentrations and can confidently be used for comparison between the different treatments. There is no certified reference material for AsLps and the filamentous brown alga Pylaiella was measured on each day of AsLp speciation to observe differences in retention times and quantification (Fig. S11).
SigmaStat (SigmaPlot 12, Systat Software, Inc., San Jose, CA, USA) was used for the statistical evaluation of the arsenolipid and arsenosugar profiles using either quantities or percentages. The different conditions were compared with control conditions with a one-way ANOVA, multiple comparisons versus control group (Holm–Sidak method).
Results and discussion
Growth of Ectocarpus cultures under different environmental conditions
The oxidative stress (OS) samples were visually different from the others because the Ectocarpus grew in compact balls as a response to stress compared with the tufts the Ectocarpus formed under all other conditions (Fig. S3). Under the microscope, it could be seen that the filaments in the OS series were generally aligned in one direction (Fig. S3b), unlike the non-organised alignment for the other culture conditions. There was a considerable amount of senescent and dead cell material for all conditions, ranging from 20 to 90 %, with the highest amount of dead cells in the Ectocarpus culture with 2 mM exogenous H2O2 and the lowest amount under control conditions (Table S2). St1 (E. crouaniorum) seemed better suited for growing in cultures and under the reduced nutrient conditions because the live cells were more dispersed throughout the algae and sporangia were visible whereas for the other strains, the live cells were clustered in the middle of each tuft, with no sporangia occurring.
Total arsenic in the Ectocarpales and cultures
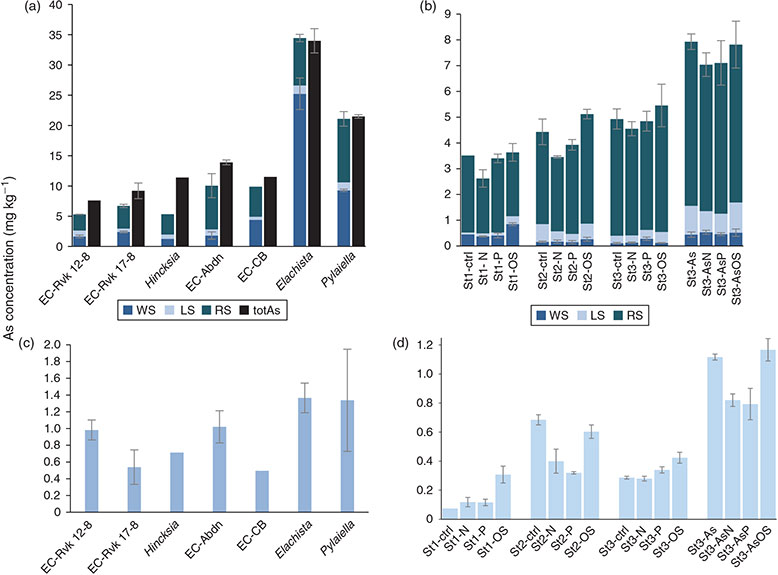
The sum of totAs of each fraction for the field-collected Ectocarpus ranged from 5 to 12 mg kg–1, where the Icelandic Ectocarpus spanned the lower range (5–7 mg kg–1), and the Scottish Ectocarpus had double the concentration (10–12 mg kg–1). The Elachista sp. (32 mg kg–1) and Pylaiella littoralis (20 mg kg–1) had higher arsenic concentrations, and closer to previously reported total arsenic concentrations in brown algae, which commonly range between 20 and 100 mg kg–1.[31–33] The sum of the fractions is in good agreement with the total arsenic in the algae (Fig. 2).
The three cultured Ectocarpus strains accumulated the same amount of arsenic (3–5 mg kg–1), whereas the strain exposed to higher arsenic concentrations showed a significantly higher total arsenic concentration (7–8 mg kg–1), Fig. 2. Both arsenic concentrations in media were close to the concentration found in the environment, and the arsenic concentrations for the cultures are comparable with those observed for the field-collected Ectocarpus. The different environmental conditions, however, had little effect on the totAs concentrations found, which is in agreement with previous studies showing that totAs in phytoplankton algae, the unicellular Dunaliella tertiolecta and the diatom Phaeodactylum tricornutum, were not significantly different when grown under different phosphate conditions.[9,10] The sample material obtained from the Ectocarpus cultures was too small (~10 mg) to measure the total arsenic in addition to the sequential extraction performed.
A large fraction (50–70 %) of the arsenic in the field-collected Ectocarpus was non-extractable and still bound in the residue (RS), Fig. 2. This was also seen, to a lesser extent, for Pylaiella (50 % RS As), whereas most of the arsenic was extractable as LS and WS for Elachista. The difference in these extraction pattern was reproducible for the three brown algae. This indicates a physiological difference in how the arsenic was bound or differences in the cell-wall structure rendering breaking up of cells more difficult in the cases of Ectocarpus and Pylaiella. For the Ectocarpus cultures, the proportion of residual arsenic was even higher (70–90 %), Fig. 2. This could be due to the high amount of dead cell material as the cytosolic arsenic (e.g. the AsSugars) would be released into the media once the cell breaks open, skewing the percentages of measured arsenicals. Similarly, a study of decomposing algae suggested that the amount of non-extractable (recalcitrant) arsenic increased with time.[34] This would agree with the fact that algae with a higher proportion of dead cell material appeared to have higher amounts of non-extractable arsenic.
Arsenolipids in Ectocarpales collected in their natural habitat
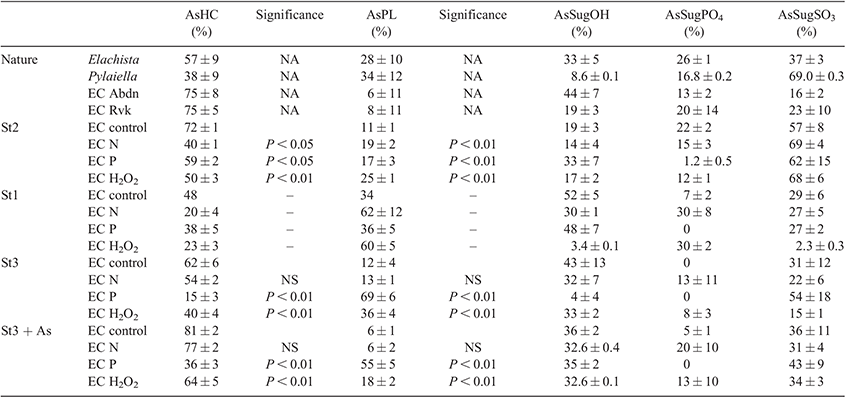
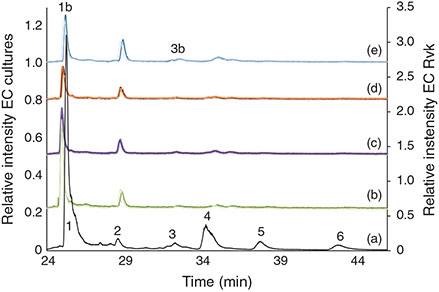
The hexane fraction of the Ectocarpales had negligible As concentrations and was not considered further for AsLp speciation analysis (Table S3). A significant portion was, however, found in the LS fraction (MeOH portion of the MeOH/DCM extraction), which was measured with HPLC-ICP-MS/ESIMS for determination of the AsLps. Fig. 3 depicts the individual arsenolipid profile for each of the Ectocarpales found in nature (n = 2, measured on the same day). These profiles were similar even for separate measurement days of different replicates.
Identification
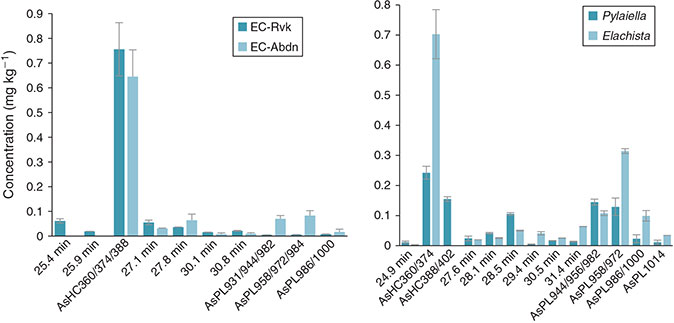
Identification of the AsLps was based on a comparison of the As signal from the ICP-MS and m/z data from the parallel ESIMS analysis. In Fig. 3, it can be clearly noted that AsHCs dominate. For the Ectocarpus and the Elachista samples, this peak consists almost exclusively of AsHC360 according to the ESIMS data (Figs S8a and S9b). It was the only AsLp that could be identified for all samples and in high enough concentrations that the MS-MS data was always available (Table S14). For Pylaiella, this peak was a double peak and seemed to equally consist of at least four main AsHCs; these AsHCs were found in the other samples, but in lower quantities (Fig. S10b).
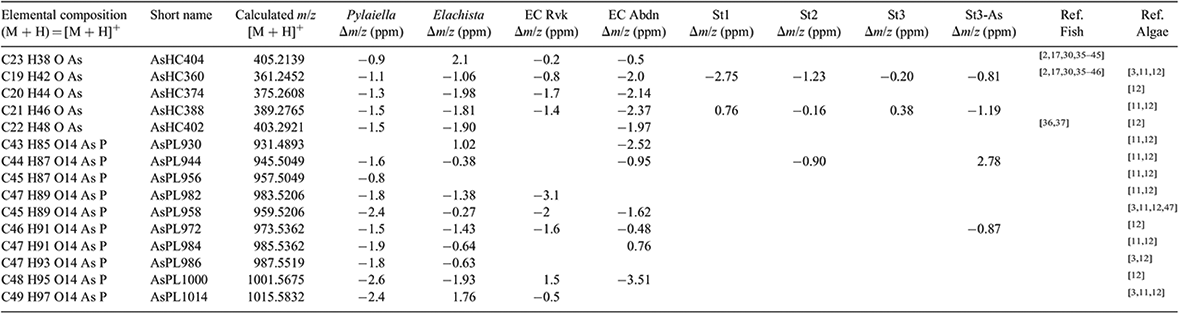
AsHCs have been reported in a range of seafood samples and also in seaweed. AsPLs have exclusively been reported in seaweed whereas the AsFAs have almost only been identified in seafood samples, although two AsFAs were reported in brown alga.[12] Four AsHCs were identified here (Table 2); the main AsHC360 is commonly found both in fish and algae, whereas AsHC374 and AsHC388 (found in field-collected Ectocarpus and cultures) have only been identified before in algae.[11,12] The ten AsPLs identified here have been reported in previous studies (Table 2).
Quantification
The identified AsHCs accounted for 36 % ± 8 (n = 5) of the total AsLp concentration (sum of all AsLp peaks) for Pylaiella, 57 % ± 9 (n = 3) for Elachista, 75 % ± 5 (n = 6) for Ectocarpus from Reykjavík and 76 % ± 7 (n = 6) for Ectocarpus from Aberdeen, Table 2 (detailed quantification for each species is given in Supplementary Tables S8–S12). The AsHC360 dominates in the Ectocarpus, with the other species present as minor species, including several AsPLs.
Arsenolipids in Ectocarpus cultures
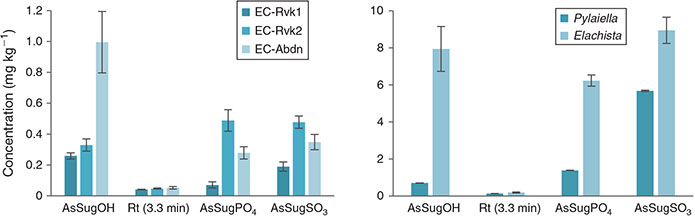
The sum of AsLps in the Ectocarpus (EC) cultures (Fig. 2) was in the same range as in field-collected Ectocarpales. In general, the sums of AsLps for each strain under different environmental conditions are similar, with the exception that the St1 (E. crouaniorum) grown under oxidative stress shows double the totAsLp concentration compared with the other conditions. Growing St3 (E. siliculosus) under enriched arsenic conditions led to 2–3× higher concentrations of AsLps and AsSugars.
The reproducibility of the arsenolipid profile for EC triplicates was very good when taking into account both the biological variability, because each replicate was grown in a separate Petri dish, and the small sample amount available for the experiments (Fig. 4). This resulted in a high precision for the quantification where the relative standard deviation (RSD) was <20 % on average for the main peaks (Table S5).
|
Identification of AsLps
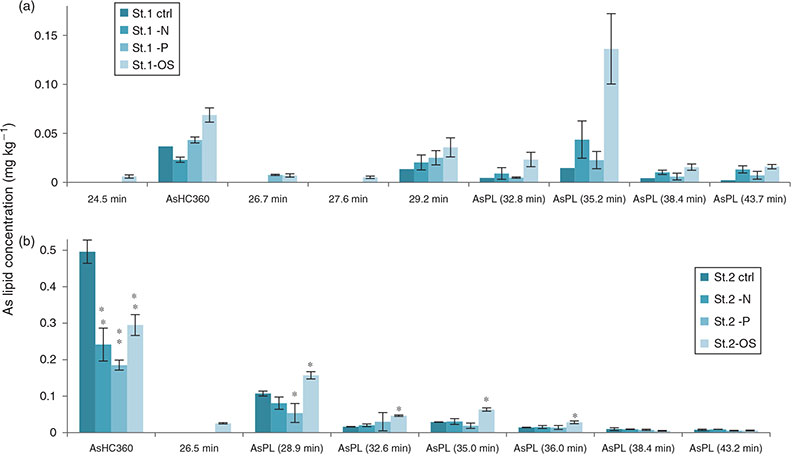
The main AsLp present, AsHC360, was identified by retention time Rt and ESIMS as well as MS-MS fragmentation pattern (Fig. S6). Because of the small amount of sample available, no other MS-MS was available. AsHC388 was found in almost all the cultures (Table S6). Both of these AsHCs were found in the field-collected Ectocarpales. Because of the small amount of sample available, it was difficult to identify the less-concentrated AsPLs. AsPL944 was found in St2 and St3, and AsPL972 under low phosphate conditions of arsenic-enriched St3 (Tables 2, S7). The identification of less-concentrated AsLps in the cultures was additionally attempted by comparison of retention times with the field-collected Ectocarpus and other algae but was not fully conclusive (Fig. 4). The peaks were, however, assigned as AsPLs or ‘unknown’ depending on elution pattern comparison and As-specific detection. Because of the similarities of AsLps found in Ectocarpus, Pylaiella and Elachista, all filamentous brown algae, and the identification in the cultures of the same main AsHC as found in the field-collected Ectocarpus, it can be assumed that similar AsPLs will be present in the cultures. The lipid profile differed between the different environmental conditions as well as between the three strains. Only the cultures grown under the low-nitrate conditions generally did not differ significantly from the controls.
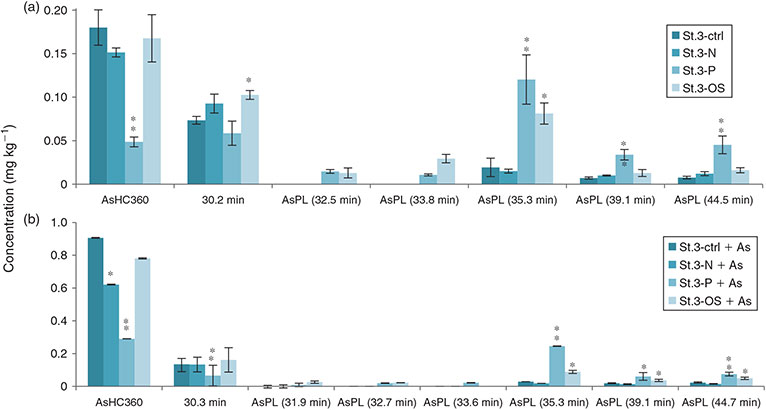
Oxidative stress
The cultures grown under oxidative stress had statistically significant higher concentrations for AsPLs compared with control samples, a pattern noticed for all three strains (Figs 5, 6). Seaweeds are constantly exposed to high UV radiation and radical formation, which could cause lipid peroxidation. The treatment with H2O2 not only caused significant physiological changes, but also an increase in AsLp synthesis. Whether the introduction of a redox-active element into a lipid environment as AsPL can be interpreted as a radical-scavenging mechanism, because trivalent and pentavalent arsenic are interconvertible at physiological conditions, remains to be studied in more detail.
|
Effect of arsenic on AsLp distribution
Higher As concentration in the media led to higher concentrations of AsLps (Fig. 6). For St2 (E. fasciculatus) and St3 (E. siliculosus), 60–80 % of the AsLps were AsHCs (Table 3), which is similar to the EC in nature (75 % ± 8). The AsHCs in the St1 (E. crouaniorum) control sample account for just under 50 % of the AsLp concentration, which was the sample with the lowest concentrations of AsLps. Conversely, the St3+As (E. siliculosus) had the highest amount of AsLps and the highest percentage of AsHCs in the control cultures. Therefore, it appears, for Ectocarpus, that at low concentrations of AsLps, the arsenic spreads fairly equally between all the AsLps, whereas excess arsenic results in the formation of additional AsHCs, mainly in the form of AsHC360 (Figs 5, 6).
It was observed that for both St3 (E. siliculosus) and St2 (E. fasciculatus), oxidative stress and low phosphate treatments differed from the control (statistically significant, at least P < 0.05, Table 3) for both AsPLs and AsHCs. These findings underline the effect the different stress conditions have on the arsenolipid profile. St1 (E. crouaniorum) had only one replicate for the control conditions, and hence it was not possible to evaluate if any statistical differences existed.
Low-phosphate conditions
For strain St3 (E. siliculosus), AsHC360 was the main AsLp peak for all conditions but under low-phosphate conditions, a shift occurred from AsHC360 to an AsPL. The AsHC360 peak was only ~1/3 compared with the other conditions, whereas the AsLp peaks are more concentrated, with an approximately four-fold increase. This could be due to mechanisms activating more efficient phosphate transporters under low-phosphate conditions, and because of the similarities between arsenate and phosphate, additional arsenate is taken up, leading to an increase of the AsPLs. The pattern of higher amounts of AsPLs in strain St3 under low-phosphate conditions can be clearly noted (Table 3).
Under low-phosphate conditions, strain St3 (E. siliculosus) increases the production of AsPLs but decreases AsHC production. This is different from the increase of AsLps under oxidative stress conditions, where this increase in AsLps represents a higher total AsLp concentration compared with control conditions. Here, for ECs grown under low-phosphate conditions, there is no increase in the total AsLp concentration but rather a shift in the lipids from one type of AsLp to another, i.e. from AsHCs to AsPLs.
Arsenosugars in Ectocarpales collected in their natural habitats
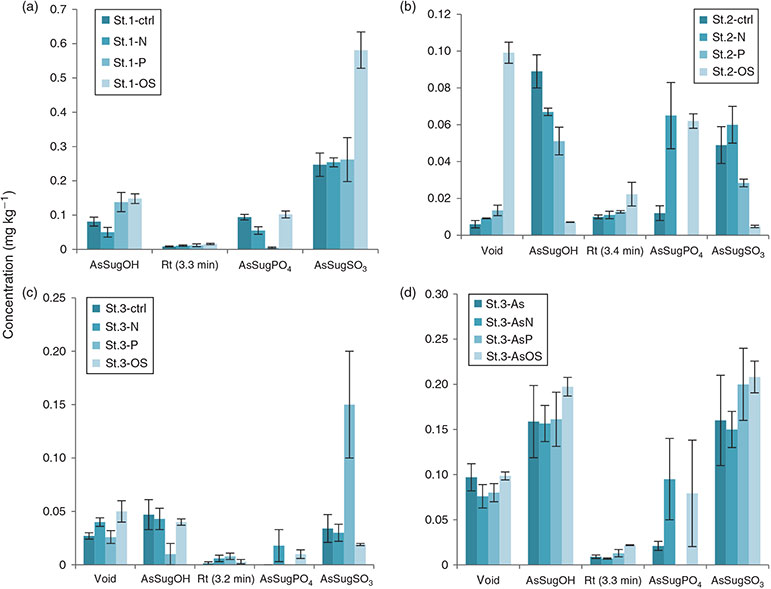
All water fractions were measured with HPLC-ICP-MS, and a Pylaiella sample studied with HPLC coupled simultaneously to ESIMS and ICP-MS to identify the main AsSugars. A similar pattern was found for all samples and the other AsSugars were identified by retention times of the ESIMS-identified peaks of the Pylaiella sample. The peak at Rt 14 min was iAs, which was confirmed both with spiking experiments as well as analyses with HG-ICP-MS (not shown). The small unidentified peak at Rt ~3.3 min is thought to be DMA based on earlier studies on seaweed using the same method (Fig. S12).[48] This was a minor peak and its identification was not pursued further. Quantification and the AsSugar profile in the Ectocarpales can be seen in Fig. 7.
Arsenosugars in Ectocarpus cultures
The arsenosugars in the Ectocarpus cultures were mainly found to be the same as in the Ectocarpales in nature. AsSugarOH, AsSugarSO3, AsSugarPO4 and DMA were identified in all cultures (Fig. 7).
The St1 (E. crouaniorum) culture under oxidative stress conditions yielded the highest totAs in the water fraction owing to an increase in AsSugarSO3 (Fig. 8a). However, for St2 (E. fasciculatus), the opposite pattern was noted, where AsSugarSO3 was found at very low concentrations in the H2O2 culture compared with the other three conditions (Fig. 8b). A noticeable pattern was seen for AsSugarPO4, which was present in all the strains in nature and in all cultures except in the low-phosphate EC cultures. AsSugarPO4, the only AsSugar that contains phosphate, is fairly abundant (13–20 % of AsSugars for EC in nature, ranging from 5 to 30 % of AsSugars for the cultures but constituting only 0–1 % of total AsSugars under low-phosphate conditions). Ectocarpus may have conserved the phosphate for other purposes.
The AsSugar concentrations in the cultures are lower than in field-collected Ectocarpus, apart from strain St1 (Fig. 8a), which had similar concentrations. This strain coped best with growing in cultures, because its live cells were more dispersed throughout the culture compared with the other strains where the live cells were clustered in the middle.
Changes in morphological structures dependent on environmental conditions
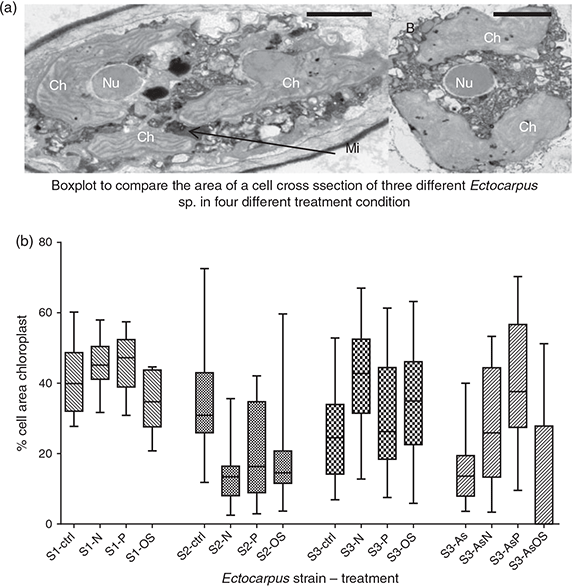
To obtain information about the morphological structure of the Ectocarpus under the different environmental conditions applied, the Ectocarpus cultures were prepared and analysed using TEM microscopy (Fig. 9a). This was done in order to see if the AsLps could have an effect on morphological structure, i.e. whether the incorporation of AsLps into the cell membrane would lead to a physical difference in the cell membrane or the mitochondria noticeable by TEM. This was not found to be the case as no sign of different cell membrane structure or difference in mitochondria was observed. However, there was an effect on the chloroplast size. St3 (E. siliculosus) had a significantly larger chloroplast size in the cell for low-nitrate and oxidative stress conditions (Table S16). Under the enriched arsenic conditions, the chloroplasts were larger for both low-nitrate and low-phosphate conditions when compared with control.
|
St2 (E. fasciculatus), however, demonstrated a completely different response with regard to chloroplast size, where the three treatments showed a reduction in chloroplast size compared with control conditions, although only statistically significant for low-nitrate conditions (P = 0.004) (Fig. 9b). This could be a different response to stress, or possibly the cultures could not cope as well with stress and the reduced chloroplast size indicates cell death. St1 (E. crouaniorum) showed no statistical difference for chloroplast size for the three different treatments compared with control conditions. This could be due to the fact that St1 (E. crouaniorum) coped better at growing under reduced nutrient conditions compared with the other two strains. No other organelles showed a noticeable visible change in size when comparing culture conditions.
Conclusions
Filamentous algae from nature and grown in laboratory cultures showed a similar distribution with regard to AsHCs and AsPLs, although some distinctive differences indicate genetic or habitat influence. AsHC360 was identified in all algae samples.
Owing to the similarity of arsenate to phosphate, it was expected that algae under low-phosphate conditions might use non-phosphate-containing AsLps such as AsHCs to replace phospholipids to conserve phosphate for ATP. Here, we demonstrated the opposite, where no increase in AsHC was observed but instead an increase in AsPLs, suggesting that a deficiency in phosphate has a direct positive effect on the biosynthesis of AsPLs. It might be hypothesised that under low-phosphate conditions, the cell membrane transporters for phosphate transport more arsenate to the mitochondria, were most of the phosphate is needed. The higher arsenate concentration at the site of phospholipid synthesis would then result in a higher AsPL concentration at low-phosphate status. The absence of AsSugPO4 confirms this and suggests that if phosphate is still bound to any organoarsenicals, it is preferentially bound as AsPL. Whether AsSugPO4 is a degradation product of AsPLs or an intermediate needs further investigation.
If the arsenic concentration is increased, then the excess arsenic is transformed into AsHCs, mainly AsHC360. Whether this could be an indication of this polar lipid being used to replace phospholipids or some kind of detoxification needs to be studied in more detail. The synthesis of AsHCs or AsPLs is not only dependent on nutritional status and oxidative stress, but may also be dose-dependent as shown in the two concentrations of arsenic tested.
It was observed that the different treatments resulted in morphological change, in particular to chloroplast size, most likely linked to stress. This was supported by the absence of changes in the strain that coped best with stress. The stress conditions also affected the AsLp profiles. Whether these morphological and AsLp changes could be related to each other needs to be addressed in a further study. The present findings with Ectocarpus sp. show that there appear to be species- or strain-specific traits affecting markers of stress and arsenolipid profiles.
Supplementary material
Contained in the Supplementary material are: a table of instrumental parameters applied in the study; microscopic imaging of cultures and field-collected algae; a figure depicting the visible difference from oxidative stress compared with control conditions; ahylogenetic tree of the three cultured strains and data on the observed senescence of the cultures; all quantification data, including tables with mass balance of all algae; tables with the quantification of AsLps and AsSugars of all the algae; ESIMS data (graphs) and tables with identified peaks (MS data); information on fragmentation patterns for cultures and field-collected Ectocarpales (MS-MS data); a figure showing Ectocarpus sample subjected to acid and base hydrolysis; and TEM imaging data.
Acknowledgements
We thank Gillian Milne, of the Aberdeen Microscopy Facility, University of Aberdeen, for help in preparing and viewing the samples through TEM and Ingo Maier, from the Universität Konstanz, for kindly providing us with an optimised processing schedule for the fixation of Ectocarpus for TEM. We also express our gratitude to Dawn Shewring for her help with algal culturing. Á. H. Pétursdóttir thanks the Icelandic research fund (grant reference 130542–051), the SORSAS award and The College of Physical Sciences at Aberdeen University for financial support. F. C. Küpper also received funding from the MASTS (The Marine Alliance for Science and Technology for Scotland) pooling initiative and their support is gratefully acknowledged. MASTS is funded by the Scottish Funding Council (grant reference HR09011) and contributing institutions.
References
[1] Á. H. Pétursdóttir, Inorganic and Lipophilic Arsenic in Different Food Commodities with Emphasis on Seafood 2014, Ph.D. thesis, University of Aberdeen, Aberdeen, UK.[2] V. Sele, J. J. Sloth, B. Holmelid, S. Valdersnes, K. Skov, H. Amlund, Arsenic-containing fatty acids and hydrocarbons in marine oils – determination using reversed-phase HPLC-ICP-MS and HPLC-qTOE-MS. Talanta 2014, 121, 89.
| Arsenic-containing fatty acids and hydrocarbons in marine oils – determination using reversed-phase HPLC-ICP-MS and HPLC-qTOE-MS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXjs1Kru7Y%3D&md5=73219b79c56210048a879cfc3f7143dcCAS | 24607114PubMed |
[3] R. A. Glabonjat, G. Raber, K. B. Jensen, J. Ehgartner, K. A. Francesconi, Quantification of arsenolipids in the certified reference material NMIJ 7405-a (Hijiki) using HPLC/mass spectrometry after chemical derivatization. Anal. Chem. 2014, 86, 10282.
| Quantification of arsenolipids in the certified reference material NMIJ 7405-a (Hijiki) using HPLC/mass spectrometry after chemical derivatization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsFOrurnK&md5=01db9bc7664f42d167c5fa8ccf204bf8CAS | 25241916PubMed |
[4] J. Alexander, D. Benford, A. Boobis, S. Ceccatelli, J.-P. Cravedi, A. Di Domenico, D. Doerge, E. Dogliotti, L. Edler, P. Farmer, M. Filipič, J. Fink-Gremmels, P. Fürst, T. Guerin, H. K. Knutsen, M. Machala, A. Mutti, J. Schlatter, R. van Leeuwen, P. Verger, Scientific opinion on arsenic in food. EFSA J. 2009, 7, 1351.
| Scientific opinion on arsenic in food.Crossref | GoogleScholarGoogle Scholar |
[5] Inorganic arsenic in seaweed and certain fish. NSW/FA/CP043/1102 2010 (NSW Food Authority: Sydney, NSW).
[6] J. Borak, H. D. Hosgood, Seafood arsenic: implications for human risk assessment. Regul. Toxicol. Pharmacol. 2007, 47, 204.
| Seafood arsenic: implications for human risk assessment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsFOjt78%3D&md5=e4a6039d0a0ac36fa4352379796dcd07CAS | 17092619PubMed |
[7] C. I. Ullrich-Eberius, A. Sanz, A. J. Novacky, Evaluation of arsenate-associated and vanadate-associated changes of electrical membrane-potential and phosphate transport in Lemna gibba G1. J. Exp. Bot. 1989, 40, 119.
| Evaluation of arsenate-associated and vanadate-associated changes of electrical membrane-potential and phosphate transport in Lemna gibba G1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXitV2gu70%3D&md5=cf86610651aed3f1d04558dc98b5d242CAS |
[8] F. L. Hellweger, K. J. Farley, U. Lall, D. M. Di Toro, Greedy algae reduce arsenate. Limnol. Oceanogr. 2003, 48, 2275.
| 1:CAS:528:DC%2BD3sXpvFagtrY%3D&md5=7fe1243697e0515773e04b606dc14156CAS |
[9] E. G. Duncan, W. A. Maher, S. D. Foster, F. Krikowa, The influence of arsenate and phosphate exposure on arsenic uptake, metabolism and species formation in the marine phytoplankton Dunaliella tertiolecta. Mar. Chem. 2013, 157, 78.
| The influence of arsenate and phosphate exposure on arsenic uptake, metabolism and species formation in the marine phytoplankton Dunaliella tertiolecta.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvVWksbzI&md5=c8fd668b8762bf50ab9605db9885905bCAS |
[10] S. Foster, D. Thomson, W. Maher, Uptake and metabolism of arsenate by anexic cultures of the microalgae Dunaliella tertiolecta and Phaeodactylum tricornutum. Mar. Chem. 2008, 108, 172.
| Uptake and metabolism of arsenate by anexic cultures of the microalgae Dunaliella tertiolecta and Phaeodactylum tricornutum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnvVyrtQ%3D%3D&md5=336a5860645e704a0942df74fc9c7c95CAS |
[11] S. García-Salgado, G. Raber, R. Raml, C. Magnes, K. A. Francesconi, Arsenosugar phospholipids and arsenic hydrocarbons in two species of brown macroalgae. Environ. Chem. 2012, 9, 63.
| Arsenosugar phospholipids and arsenic hydrocarbons in two species of brown macroalgae.Crossref | GoogleScholarGoogle Scholar |
[12] A. Raab, C. Newcombe, D. Pitton, R. Ebel, J. Feldmann, Comprehensive analysis of lipophilic arsenic species in a brown alga (Saccharina latissima). Anal. Chem. 2013, 85, 2817.
| Comprehensive analysis of lipophilic arsenic species in a brown alga (Saccharina latissima).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXit1ehsr0%3D&md5=325414c75c20e013339d1d903a22979eCAS | 23394220PubMed |
[13] B. A. S. Van Mooy, H. F. Fredricks, B. E. Pedler, S. T. Dyhrman, D. M. Karl, M. Koblizek, M. W. Lomas, T. J. Mincer, L. R. Moore, T. Moutin, M. S. Rappe, E. A. Webb, Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature 2009, 458, 69.
| Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1ehtbY%3D&md5=2d0bd6132922e798b4185521d8fd8abeCAS |
[14] R. V. Cooney, R. O. Mumma, A. A. Benson, Arsoniumphospholipid in algae. Proc. Natl. Acad. Sci. USA 1978, 75, 4262.
| Arsoniumphospholipid in algae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXhvFShtQ%3D%3D&md5=e424ee224ed4d498a9998f63074c468bCAS | 16592562PubMed |
[15] K. A. Francesconi, Arsenic species in seafood: origin and human health implications. Pure Appl. Chem. 2010, 82, 373.
| Arsenic species in seafood: origin and human health implications.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXivVShsLg%3D&md5=6fc30a530c87f2132f688c65afd68274CAS |
[16] J. S. Edmonds, K. A. Francesconi, Transformations of arsenic in the marine environment. Experientia 1987, 43, 553.
| Transformations of arsenic in the marine environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXltFaqtrc%3D&md5=f06dda7dfc9b15c098fc88132cfd1215CAS | 3556209PubMed |
[17] M. S. Taleshi, K. B. Jensen, G. Raber, J. S. Edmonds, H. Gunnlaugsdottir, K. A. Francesconi, Arsenic-containing hydrocarbons: natural compounds in oil from the fish capelin, Mallotus villosus. Chem. Commun. 2008, 4706.
| Arsenic-containing hydrocarbons: natural compounds in oil from the fish capelin, Mallotus villosus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtF2mtr7I&md5=c1d9e99c114830365358e711c69de41bCAS |
[18] V. Sele, J. J. Sloth, A. K. Lundebye, E. H. Larsen, M. H. G. Berntssen, H. Amlund, Arsenolipids in marine oils and fats: a review of occurrence, chemistry and future research needs. Food Chem. 2012, 133, 618.
| Arsenolipids in marine oils and fats: a review of occurrence, chemistry and future research needs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XivV2msb0%3D&md5=86bf6a94809223d8a068cf66bc34e9adCAS |
[19] S. Meyer, M. Matissek, S. M. Mueller, M. S. Taleshi, F. Ebert, K. A. Francesconi, T. Schwerdtle, In vitro toxicological characterisation of three arsenic-containing hydrocarbons. Metallomics 2014, 6, 1023.
| In vitro toxicological characterisation of three arsenic-containing hydrocarbons.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmslyksLg%3D&md5=50e30bbe602284328eb8ca31ab6ee979CAS | 24718560PubMed |
[20] B. Charrier, S. M. Coelho, A. Le Bail, T. Tonon, G. Michel, P. Potin, B. Kloareg, C. Boyen, A. F. Peters, J. M. Cock, Development and physiology of the brown alga Ectocarpus siliculosus: two centuries of research. New Phytol. 2008, 177, 319.
| Development and physiology of the brown alga Ectocarpus siliculosus: two centuries of research.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhvFGltbY%3D&md5=7d52054e0d2b30cc680328ed2b502d1cCAS | 18181960PubMed |
[21] A. F. Peters, D. Marie, D. Scornet, B. Kloareg, J. M. Cock, Proposal of Ectocarpus siliculosus (Ectocarpales, Phaeophyceae) as a model organism for brown algal genetics and genomics. J. Phycol. 2004, 40, 1079.
| Proposal of Ectocarpus siliculosus (Ectocarpales, Phaeophyceae) as a model organism for brown algal genetics and genomics.Crossref | GoogleScholarGoogle Scholar |
[22] D. G. Müller, C. M. M. Gachon, F. C. Küpper, Axenic clonal cultures of filamentous brown algae: initiation and maintenance. Cah. Biol. Mar. 2008, 49, 59.
[23] J. M. Cock, L. Sterck, P. Rouzé, D. Scornet, A. E. Allen, G. Amoutzias, V. Anthouard, F. Artiguenave, J.-M. Aury, J. H. Badger, B. Beszteri, K. Billiau, E. Bonnet, J. H. Bothwell, C. Bowler, C. Boyen, C. Brownlee, C. J. Carrano, B. Charrier, G. Y. Cho, S. M. Coelho, J. Collen, E. Corre, C. Da Silva, L. Delage, N. Delaroque, S. M. Dittami, S. Doulbeau, M. Elias, G. Farnham, C. M. M. Gachon, B. Gschloessl, S. Heesch, K. Jabbari, C. Jubin, H. Kawai, K. Kimura, B. Kloareg, F. C. Kuepper, D. Lang, A. Le Bail, C. Leblanc, P. Lerouge, M. Lohr, P. J. Lopez, C. Martens, F. Maumus, G. Michel, D. Miranda-Saavedra, J. Morales, H. Moreau, T. Motomura, C. Nagasato, C. A. Napoli, D. R. Nelson, P. Nyvall-Collen, A. F. Peters, C. Pommier, P. Potin, J. Poulain, H. Quesneville, B. Read, S. A. Rensing, A. Ritter, S. Rousvoal, M. Samanta, G. Samson, D. C. Schroeder, B. Segurens, M. Strittmatter, T. Tonon, J. W. Tregear, K. Valentin, P. von Dassow, T. Yamagishi, Y. Van de Peer, P. Wincker, The Ectocarpus genome and the independent evolution of multicellularity in the brown algae. Nature 2010, 465, 617.
| The Ectocarpus genome and the independent evolution of multicellularity in the brown algae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmvFWlu7c%3D&md5=60569d836ff090c5bc2b9c1ba6686199CAS | 20520714PubMed |
[24] R. C. Starr, J. A. Zeikus, UTEX – the culture collection of algae at the University of Texas at Austin. J. Phycol. 1987, 23, 1.
[25] H. C. Bold, M. J. Wynne (Eds), Introduction to the Algae: Structure and Reproduction 1985 (Prentice-Hall: Englewood Cliffs, NJ, USA).
[26] M. D. Patey, M. J. A. Rijkenberg, P. J. Statham, M. C. Stinchcombe, E. P. Achterberg, M. Mowlem, Determination of nitrate and phosphate in seawater at nanomolar concentrations. TrAC – Trend. Anal. Chem. 2008, 27, 169.
| Determination of nitrate and phosphate in seawater at nanomolar concentrations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjsVSitLw%3D&md5=b30f9f5376f3cd66902d5d9b6e82c269CAS |
[27] F. C. Küpper, L. J. Carpenter, G. B. McFiggans, C. J. Palmer, T. J. Waite, E. M. Boneberg, S. Woitsch, M. Weiller, R. Abela, D. Grolimund, P. Potin, A. Butler, G. W. Luther, P. M. H. Kroneck, W. Meyer-Klaucke, M. C. Feiters, Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry. Proc. Natl. Acad. Sci. USA 2008, 105, 6954.
| Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry.Crossref | GoogleScholarGoogle Scholar | 18458346PubMed |
[28] P. L. Smedley, D. G. Kinniburgh, A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517.
| A review of the source, behaviour and distribution of arsenic in natural waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhvVSmur0%3D&md5=36c2895b8fea47d7e3c145f44bf012daCAS |
[29] S. Musil, A. H. Petursdottir, A. Raab, H. Gunnlaugsdottir, E. Krupp, J. Feldmann, Speciation without chromatography using selective hydride generation: inorganic arsenic in rice and samples of marine origin. Anal. Chem. 2014, 86, 993.
| Speciation without chromatography using selective hydride generation: inorganic arsenic in rice and samples of marine origin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvFKhtr%2FE&md5=af43e65b5980de8c4db33ee38e621312CAS | 24354293PubMed |
[30] K. O. Amayo, A. Petursdottir, C. Newcombe, H. Gunnlaugsdottir, A. Raab, E. M. Krupp, J. Feldmann, Identification and quantification of arsenolipids using reversed-phase HPLC coupled simultaneously to high-resolution ICPMS and high-resolution electrospray MS without species-specific standards. Anal. Chem. 2011, 83, 3589.
| Identification and quantification of arsenolipids using reversed-phase HPLC coupled simultaneously to high-resolution ICPMS and high-resolution electrospray MS without species-specific standards.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXksFaktL4%3D&md5=532d21d376def6287d9c40ffd1c55575CAS | 21446761PubMed |
[31] J. S. Edmonds, K. A. Francesconi, Arsenic in seafoods – human health aspects and regulations. Mar. Pollut. Bull. 1993, 26, 665.
| Arsenic in seafoods – human health aspects and regulations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXhvV2qsb8%3D&md5=d19263608a1941075c4e6fbd15f60c66CAS |
[32] O. Díaz, Y. Tapia, O. Munoz, R. Montoro, D. Velez, C. Almela, Total and inorganic arsenic concentrations in different species of economically important algae harvested from coastal zones of Chile. Food Chem. Toxicol. 2012, 50, 744.
| Total and inorganic arsenic concentrations in different species of economically important algae harvested from coastal zones of Chile.Crossref | GoogleScholarGoogle Scholar | 22138359PubMed |
[33] C. Almela, M. J. Clemente, D. Velez, R. Montoro, Total arsenic, inorganic arsenic, lead and cadmium contents in edible seaweed sold in Spain. Food Chem. Toxicol. 2006, 44, 1901.
| Total arsenic, inorganic arsenic, lead and cadmium contents in edible seaweed sold in Spain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XpvFGms70%3D&md5=fc1291e2cf260247bff26ea315ced8d5CAS | 16901603PubMed |
[34] J. Navratilova, G. Raber, S. J. Fisher, K. A. Francesconi, Arsenic cycling in marine systems: degradation of arsenosugars to arsenate in decomposing algae, and preliminary evidence for the formation of recalcitrant arsenic. Environ. Chem. 2011, 8, 44.
| Arsenic cycling in marine systems: degradation of arsenosugars to arsenate in decomposing algae, and preliminary evidence for the formation of recalcitrant arsenic.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjs1GlsLc%3D&md5=1eaf1c1822c0a44201b740cbb2d35d2bCAS |
[35] M. S. Taleshi, G. Raber, J. S. Edmonds, K. B. Jensen, K. A. Francesconi, Arsenolipids in oil from blue whiting Micromesistius poutassou – evidence for arsenic-containing esters. Sci. Rep. 2014, 4, 7492.
| Arsenolipids in oil from blue whiting Micromesistius poutassou – evidence for arsenic-containing esters.Crossref | GoogleScholarGoogle Scholar | 25502848PubMed |
[36] U. Arroyo-Abad, S. Lischka, C. Piechotta, J. Mattusch, T. Reemtsma, Determination and identification of hydrophilic and hydrophobic arsenic species in methanol extract of fresh cod liver by RP-HPLC with simultaneous ICP-MS and ESI-Q-TOF-MS detection. Food Chem. 2013, 141, 3093.
| Determination and identification of hydrophilic and hydrophobic arsenic species in methanol extract of fresh cod liver by RP-HPLC with simultaneous ICP-MS and ESI-Q-TOF-MS detection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFWkurjO&md5=9a2b4486382bcd78147156bdf5d7680eCAS | 23871064PubMed |
[37] U. Arroyo-Abad, J. Mattusch, T. Reemtsma, C. Piechotta, Arsenolipids in commercial canned cod liver: an occurrence and distribution study. Eur. J. Lipid Sci. Technol. 2014, 116, 1381.
| Arsenolipids in commercial canned cod liver: an occurrence and distribution study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtVCqu7jI&md5=4be02b178ba6449431e22e84b7b4d6f5CAS |
[38] V. Sele, H. Amlund, M. H. G. Berntssen, J. A. Berntsen, K. Skov, J. J. Sloth, Detection of arsenic-containing hydrocarbons in a range of commercial fish oils by GC-ICPMS analysis. Anal. Bioanal. Chem. 2013, 405, 5179.
| Detection of arsenic-containing hydrocarbons in a range of commercial fish oils by GC-ICPMS analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXms1WlsLk%3D&md5=9bc482a4d340df07de18d38405b79cccCAS | 23620370PubMed |
[39] S. Lischka, U. Arroyo-Abad, J. Mattusch, A. Kuehn, C. Piechotta, The high diversity of arsenolipids in herring fillet (Clupea harengus). Talanta 2013, 110, 144.
| The high diversity of arsenolipids in herring fillet (Clupea harengus).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXksFeisL0%3D&md5=c9a22204eadd1b44917fc5d8c4405a88CAS | 23618187PubMed |
[40] K. O. Amayo, A. Raab, E. M. Krupp, H. Gunnlaugsdottir, J. Feldmann, Novel identification of arsenolipids using chemical derivatizations in conjunction with RP-HPLC-ICPMS/ESMS. Anal. Chem. 2013, 85, 9321.
| Novel identification of arsenolipids using chemical derivatizations in conjunction with RP-HPLC-ICPMS/ESMS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtlChs7vK&md5=c0a48fc3b85a0f2a3991a9bcda703bd0CAS | 23984920PubMed |
[41] A. Rumpler, J. S. Edmonds, M. Katsu, K. B. Jensen, W. Goessler, G. Raber, H. Gunnlaugsdottir, K. A. Francesconi, Arsenic-containing long-chain fatty acids in cod-liver oil: a result of biosynthetic infidelity? Angew. Chem. Int. Ed. 2008, 47, 2665.
| Arsenic-containing long-chain fatty acids in cod-liver oil: a result of biosynthetic infidelity?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXkvFejsL4%3D&md5=2aa5c6b69565bd290223aa10d11a3a49CAS |
[42] M. S. Taleshi, J. S. Edmonds, W. Goessler, M. J. Ruiz-Chancho, G. Raber, K. B. Jensen, K. A. Francesconi, Arsenic-containing lipids are natural constituents of sashimi tuna. Environ. Sci. Technol. 2010, 44, 1478.
| Arsenic-containing lipids are natural constituents of sashimi tuna.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptlymtg%3D%3D&md5=85d714a1c6c03b7b0fda76c7dcb4fd6fCAS | 20099809PubMed |
[43] M. J. Ruiz-Chancho, M. S. Taleshi, W. Goessler, K. A. Francesconi, A method for screening arsenolipids in fish oils by HPLC-ICPMS. J. Anal. At. Spectrom. 2012, 27, 501.
| A method for screening arsenolipids in fish oils by HPLC-ICPMS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XisVKjtbs%3D&md5=927b4b0a674de1039b6e90d207ba7ef9CAS |
[44] U. Arroyo-Abad, J. Mattusch, S. Mothes, M. Moeder, R. Wennrich, M. P. Elizalde-Gonzalez, F. M. Matysik, Detection of arsenic-containing hydrocarbons in canned cod liver tissue. Talanta 2010, 82, 38.
| Detection of arsenic-containing hydrocarbons in canned cod liver tissue.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXntVyktbo%3D&md5=f925b5100ba31bf68ff50e60df07558dCAS | 20685432PubMed |
[45] G. Raber, S. Khoomrung, M. S. Taleshi, J. S. Edmonds, K. A. Francesconi, Identification of arsenolipids with GC/MS. Talanta 2009, 78, 1215.
| Identification of arsenolipids with GC/MS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjtVSnsL8%3D&md5=c47539f95d177fc076b1577e9bb85ff9CAS | 19269497PubMed |
[46] K. O. Amayo, A. Raab, E. M. Krupp, J. Feldmann, Identification of arsenolipids and their degradation products in cod-liver oil. Talanta 2014, 118, 217.
| Identification of arsenolipids and their degradation products in cod-liver oil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvFWrsbnF&md5=40dc5f4fb5c37035e4e09c8fc65901f5CAS | 24274291PubMed |
[47] M. Morita, Y. Shibata, Isolation and identification of arsenolipid from a brown alga, Undaria pinnatifida (wakame). Chemosphere 1988, 17, 1147.
| Isolation and identification of arsenolipid from a brown alga, Undaria pinnatifida (wakame).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXkvVOlt78%3D&md5=80c1d36d94a060c4d6563890105630c8CAS |
[48] H. Castlehouse, C. Smith, A. Raab, C. Deacon, A. A. Meharg, J. Feldmann, Biotransformation and accumulation of arsenic in soil amended with seaweed. Environ. Sci. Technol. 2003, 37, 951.
| Biotransformation and accumulation of arsenic in soil amended with seaweed.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXnslyltA%3D%3D&md5=59985a66fdb97d80a90fd04fcad6ea69CAS | 12666926PubMed |