Is the Australian subterranean fauna uniquely diverse?
Michelle T. Guzik A G , Andrew D. Austin A , Steven J. B. Cooper A B , Mark S. Harvey C , William F. Humphreys C , Tessa Bradford A , Stefan M. Eberhard D , Rachael A. King A B , Remko Leys B E , Kate A. Muirhead A and Moya Tomlinson FA Australian Centre for Evolutionary Biology and Biodiversity, The University of Adelaide, SA 5005, Australia.
B South Australian Museum, North Terrace, Adelaide, SA 5000, Australia.
C Western Australian Museum, Collections and Research Centre, Locked Bag 49, Welshpool DC, WA 6986, Australia.
D Subterranean Ecology Pty Ltd, 8/37 Cedric St, Stirling, WA 6021, Australia.
E School of Biological Sciences, Flinders University, SA 5042, Australia.
F Department of Environment and Resource Management, GPO Box 2454, Brisbane, Qld 4001, Australia.
G Corresponding author. Email: michelle.guzik@adelaide.edu.au
Invertebrate Systematics 24(5) 407-418 https://doi.org/10.1071/IS10038
Submitted: 5 November 2010 Accepted: 8 January 2011 Published: 4 March 2011
Abstract
Australia was historically considered a poor prospect for subterranean fauna but, in reality, the continent holds a great variety of subterranean habitats, with associated faunas, found both in karst and non-karst environments. This paper critically examines the diversity of subterranean fauna in several key regions for the mostly arid western half of Australia. We aimed to document levels of species richness for major taxon groups and examine the degree of uniqueness of the fauna. We also wanted to compare the composition of these ecosystems, and their origins, with other regions of subterranean diversity world-wide. Using information on the number of ‘described’ and ‘known’ invertebrate species (recognised based on morphological and/or molecular data), we predict that the total subterranean fauna for the western half of the continent is 4140 species, of which ~10% is described and 9% is ‘known’ but not yet described. The stygofauna, water beetles, ostracods and copepods have the largest number of described species, while arachnids dominate the described troglofauna. Conversely, copepods, water beetles and isopods are the poorest known groups with less than 20% described species, while hexapods (comprising mostly Collembola, Coleoptera, Blattodea and Hemiptera) are the least known of the troglofauna. Compared with other regions of the world, we consider the Australian subterranean fauna to be unique in its diversity compared with the northern hemisphere for three key reasons: the range and diversity of subterranean habitats is both extensive and novel; direct faunal links to ancient Pangaea and Gondwana are evident, emphasising their early biogeographic history; and Miocene aridification, rather than Pleistocene post-ice age driven diversification events (as is predicted in the northern hemisphere), are likely to have dominated Australia’s subterranean speciation explosion. Finally, we predict that the geologically younger, although more poorly studied, eastern half of the Australian continent is unlikely to be as diverse as the western half, except for stygofauna in porous media. Furthermore, based on similar geology, palaeogeography and tectonic history to that seen in the western parts of Australia, southern Africa, parts of South America and India may also yield similar subterranean biodiversity to that described here.
Introduction
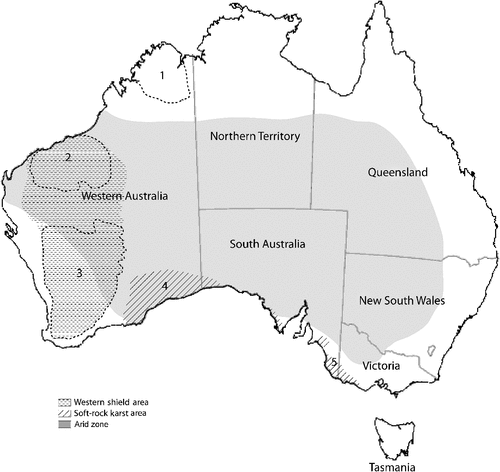
The subterranean fauna of Australia has recently revealed numerous higher taxa (classes, orders and families) not previously recorded from the southern hemisphere, as well as living representatives of lineages previously known only as fossils (see Humphreys 2008 for summary). Obligate subterranean lineages remain trapped in situ and are consequently potent subjects to test biogeographical and evolutionary hypotheses. This is especially the case for the numerous higher taxa of Crustacea that are found solely represented as obligate subterranean fauna. Here we present current estimates of newly described or identified subterranean taxa from several key regions in the western half of Australia, particularly in the arid zone (see Fig. 1). We also make projections on possible total subterranean biodiversity in this area, in particular to assess whether the diversity of the Australian subterranean fauna is notably high compared to that found elsewhere. Our aim is to document the scale of this biodiversity to encourage further exploration of these and other regions of the continent. We also make predictions about other locations in the world that reflect similar geomorphology to that seen in Australia’s arid region, and represent potential new sites for subterranean fauna.
|
Globally, the northern hemisphere dominates as a region of subterranean biodiversity hotspots, in particular temperate mid-latitude locations (Culver et al. 2006; Stoch and Galassi 2010) such as the Balkan Peninsula, the USA (Culver and Sket 2000), Mexico (Reddell 1981) and, most recently, caves of south-east Asia (Deharveng 2005). Subterranean faunal diversity is generally concentrated in karst and pseudokarst areas (Juberthie and Decu 1994; Culver et al. 2001; Christman et al. 2005; Deharveng 2005). Even within this geomorphological constraint (i.e. it is patchily distributed, Fong and Culver 1994; Culver et al. 2004) the density of caves and the sampling intensity by generations of researchers in these regions have provided biologists with the opportunity to efficiently document caves and their fauna (Christman and Culver 2001; Culver et al. 2004; Zagmajster et al. 2008). Consequently, knowledge of these regions and their biodiversity, phylogeography and functional ecology are well progressed (Gibert et al. 1994; Wilkens et al. 2000; Culver and White 2004).
As recently as 16 years ago, Humphreys (1994) advocated a knowledge gap of subterranean fauna in Australia, especially in areas other than the lava tubes of tropical north Queensland, which are a noted troglofauna hotspot (Culver and Sket 2000). Considered depauperate of karstic habitat and having widespread aridity, historically, Australia was considered a poor prospect for subterranean fauna. Australia’s arid Pleistocene climatic history was deemed to lack key climate history events, such as Pleistocene glaciations (Moore 1964; Hamilton-Smith 1967; Barr 1973), which were considered drivers of subterranean biodiversity in the northern hemisphere (Peck 1980; Boutin 1994). This excludes Tasmania (Derbyshire 1972), which is richly endowed with karst and caves and was subject to Pleistocene glaciations. These hypotheses, coupled with a paucity of research on the southern hemisphere subterranean biota over the last two centuries, have led to a prolonged lag in discovery of Australia’s obligate subterranean species. Historically, the most sampled cave regions in Australia were south-eastern South Australia (SA), New South Wales (NSW), Tasmania, and the Nullarbor Plain (spanning parts of SA and Western Australia (WA)). It is now appreciated that Australia holds a great variety of subterranean habitats, with associated invertebrate faunas, found both in karst and non-karst environments (Eberhard and Humphreys 2003). Notable differences between Australia and other regions of the world are the absence of urodele amphibians and stenasellid isopods, and a scarcity of carabid beetles and cave fishes.
Focussed research on subterranean fauna from karst regions in WA comprising troglobionts (Humphreys et al. 1989) and stygobionts soon after (Humphreys and Adams 1991), substantially advanced our knowledge of subterranean fauna in Australia. Further, the discovery of rich subterranean faunas in non-karstic substrates 20 years ago, also led to a rapid expansion in the discovery and documentation of subterranean faunal diversity. Increasingly, regulations requiring the inclusion of subterranean fauna during the environmental review process for major resource projects in WA by the Environmental Protection Agency (EPA) (EPA 2003) have accelerated the discovery of new species, based on either morphology or genetic differences, or both. Coupled with these environmental impact assessments (EIA) is an increased interest in groundwater biology research (Humphreys 2006, 2009; Boulton 2009). Government and privately funded research in the past five years has primarily focussed on the fauna of the Yilgarn and Pilbara regions of WA and aquifers in SA, in particular utilising boreholes and drill holes installed for exploration and exploitation of water, minerals and monitoring of groundwater levels and salinity, rather than fauna. This work has revealed a diverse subterranean fauna inhabiting both aquatic and terrestrial habitats found in a wide range of substrates, including alluvium, calcretes, fractured rock, karst in soft and hard rock, pisolites and pseudokarst in lava and sandstone (Poore and Humphreys 1998, 2003; Finston and Johnson 2004; Eberhard et al. 2005; Harvey et al. 2008; Humphreys 2008; Eberhard et al. 2009). Many of the resultant data are unavailable publicly during the EIA process but many become public after formal environmental approvals occur.
In an era when human induced extinction rates are high (Pimm et al. 1995), biodiversity estimates are a vital tool for identifying knowledge gaps for the purpose of prioritising research effort and funding resources, and also developing conservation policies (Brooks et al. 2006). Estimates of invertebrate species richness in Australia are typically centred around terrestrial arthropods (e.g. Yeates et al. 2003). Published studies suggest Australia’s subterranean fauna is diverse, especially the Pilbara region with 78 described species of stygofauna (Eberhard et al. 2005) and an estimated 500–550 undescribed species (Eberhard et al. 2009). However, a firm estimate of total species diversity is constrained by the generally sparse geographical coverage and the inevitable lag in taxonomic descriptions. Our position here is that, historically, Australia’s subterranean fauna have been vastly underestimated. Given the short duration of research targeting this field in Australia, it is not possible to realistically attempt an estimate of subterranean faunal diversity for the whole continent. Rather, we concentrate on several areas in the western half of the Australian continent that are better studied; we summarise the number of described and recognised species from morphological and molecular studies, and then project, based on the collective experience of the specialists currently working on these faunas, the likely species richness of broad taxonomic groups. The areas that were included and assessed in this study from north-west to east include: (1) the Kimberley and (2) Pilbara regions of north-western WA; (3) the Yilgarn region of WA; (4) the Nullarbor region; and (5) SA, including the Eyre Peninsula, Flinders Ranges, and the south-east (Fig. 1). We also note that a diverse stygofauna has recently been identified from alluvial aquifers in eastern Australia (Hancock and Boulton 2008; Tomlinson 2009; Camacho and Hancock 2010), but this region requires considerably more intensive surveying and taxonomic work to obtain reliable estimates of faunal diversity.
Methodology for estimating subterranean faunal diversity
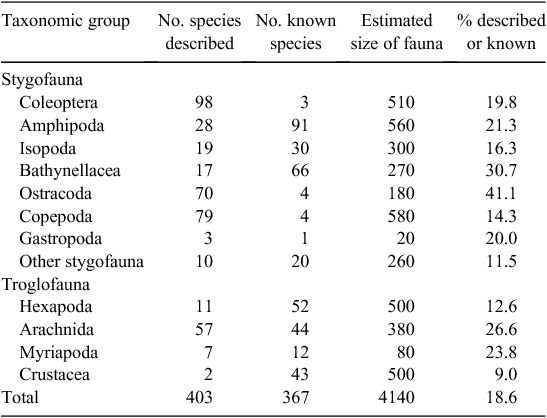
The criteria employed to identify species richness in subterranean habitats of Australia’s west (see Table 1 for data) were 5-fold.
|
(1) We surveyed the relevant literature for formally ‘described’ species, mostly from the last 10–20 years, which has been the most productive time for exploration and description of subterranean taxa (see references in Table 2 for a representative summary of this literature).
|
(2) Extensive surveys of key regions were carried out primarily by teams represented by three of the authors of this study (SA: Leys; Pilbara and Nullarbor: Eberhard; Yilgarn and Kimberley: Humphreys). The areas most comprehensively sampled were the Western Shield, a single long-emergent (since the Paleozoic) landmass comprising the Yilgarn and Pilbara cratons and associated orogens, southern SA, and the northern Carnarvon Basin. These surveys were conducted using a variety of access points, mostly boreholes drilled for water extraction, groundwater monitoring and mineral exploration, but also pastoral wells and caves, where present. In general, even in the better surveyed regions, sampling density was low. For example, in the only regional survey of the Pilbara region (Eberhard et al. 2009), sample density was 0.0022 km2 (one site every 460 km2) in an area of ~220 000 km2. In the Yilgarn, sampling has largely been restricted to groundwater calcretes, which are highly prospective for subterranean fauna, whereas other habitats have been found to be largely devoid of subterranean fauna.
(3) Identification of morphologically distinct species (morphospecies) beyond family or genus using current descriptions and keys was not always possible. Therefore we canvassed numbers of species from taxonomic experts (listed in Table 2) to identify probable new morphospecies. These experts used their prior knowledge to assign likely species.
(4) Molecular data have proven a major innovation in delineation of new species, both cryptic and otherwise (Juan et al. 2010). Hence, in situations in which it was uncertain whether there were distinct species present, genetic methods were used to estimate ‘known’ but undescribed species from recent collections and molecular studies. Such situations arose when geographically isolated populations were observed, but morphological differences were not immediately recognisable, or in situations in which expertise was unavailable or sample volumes were too large. In these cases, the mtDNA cytochrome c oxidase subunit I gene (cox1) was primarily used for assessing the presence of new genetic lineages.
Criteria for discriminating whether genetic lineages for cox1 were likely to be species here are as follows. (1) Reciprocally monophyletic lineages with >90% posterior probability support, and the position of these lineages within the broader phylogeny, were considered. (2) On the basis of total evidence, geographically discrete lineages that complied with all other criteria listed here and were also known to be spatially isolated were included. Isolation could be geographical distance and/or barriers or geological barriers. This criterion was crucial in situations in which percentage genetic divergence might have been low and provided insights into the possible mechanisms for species divergences. In particular, it was shown in several studies that major geographic barriers inhibit geneflow between regions, i.e. tributaries (Pilbara amphipods Finston and Johnson 2004; Finston et al. 2007, 2009) or geology of calcrete aquifers (Cooper et al. 2007, 2008; Guzik et al. 2008). (3) Genetically divergent lineages were conservatively ≥16% for pairwise distances based on a Kimura 2-parameter (Kimura 1980) model (i.e. between ‘species’ lineages, Lefébure et al. 2006). In some cases, where genetic lineages satisfied all of the other criteria (i.e. genetically monophyletic and geographically isolated), then lower divergences were considered. The justification for allowing lower divergences is that, in cases of recent speciation events, divergences as low as 11% have been observed in morphologically distinct but sympatric species (Bradford et al. 2010; R. A. King, unpubl. data). These findings have been observed in other crustaceans, particularly amphipods and parabathynellids (Cooper et al. 2007, 2008; Guzik et al. 2008; K. M. Abrams, unpubl. data). Where possible, evidence from a second marker was also taken into account to strengthen the hypothesis of distinct species. Genes such as 16S rRNA (mtDNA) and 28S rRNA (nuclear DNA) were used to supplement the cox1 data for parabathynellids and amphipods (R. Leys, unpubl. data).
An example of how these criteria were implemented is as follows. Using DNA alone ~90 new crustacean ‘lineages’ were identified from published studies (e.g. up to 21 Pilbara amphipods (Finston and Johnson 2004; Finston et al. 2007, 2009), 22 Yilgarn amphipods (Cooper et al. 2007), 1 anchialine shrimp (Page et al. 2008), 24 aquatic isopods (Cooper et al. 2008) and 17 parabathynellids (Guzik et al. 2008)). Each of these studies also demonstrated geographic isolation for each of the lineages (as above) confirming our species concept using a combined approach as exemplified by Harvey et al. (2008) where both morphological and molecular data reinforced conclusions. Finally, some unpublished molecular work by Eberhard, Leys and Abrams, generated largely for EIA datasets, were also assessed using the same criteria as that for published work.
(5) In order to provide an estimate of the potential size of the fauna for different taxonomic groups and the percentage that was currently ‘described’ or ‘known’ we extrapolated from the existing surveys. This extrapolation was carried out in different ways for different regions, for which we give two examples. First, in the Pilbara region it is likely that most of the landscape provides potential habitat for troglofauna and stygofauna and, hence, it is difficult to assign sampled/unsampled area estimates based on points (bores or caves). In this case, extrapolation of richness estimates was based on accumulation curves as outlined by Eberhard et al. (2009). Second, in the Yilgarn region, since subterranean taxa are restricted to calcretes, and each sampled calcrete was found to have a unique fauna, extrapolation of the data from sampled to unsampled calcretes was warranted. Of 200 major calcretes in the Yilgarn region ~50 (25%) have been surveyed allowing extrapolation based on the average number of described plus known species in different calcretes.
Australia’s subterranean fauna: a biodiversity hotspot
Here, we estimate 4140 species for subterranean systems in Australia’s western half (Table 1), many of which are restricted to arid and semiarid regions. Based on this figure, over 80% of the likely fauna remain undiscovered, a figure that is not surprising given that large tracts of potentially suitable habitat remain unexplored. ‘Described’ species represent slightly more taxa (403) than those ‘known’, but not described (367). In particular, beetles, ostracods and copepods have the largest number of described species for the stygofauna, while arachnids dominate the described troglofauna. This situation largely reflects the current taxonomic effort by specialists. While other potentially diverse groups have not been investigated in detail, either because of a lack of attention by existing specialists or a general lack of expertise for specific groups, they are still likely to represent significant diversity. For the 367 undescribed taxa, the majority represent geographically isolated monophyletic lineages, based on molecular studies, reflecting long-term isolated populations that are likely to be equivalent to distinct species, especially for crustaceans, such as parabathynellids (Guzik et al. 2008), amphipods (Finston and Johnson 2004; Cooper et al. 2007; Finston et al. 2007) and isopods (Cooper et al. 2008). Based on the data presented in Table 1, we predict for the stygofauna that copepods, isopods and beetles are the most poorly known groups, with less than 20% described species, followed by gastropods and amphipods, and for the troglofauna, hexapods (comprising mostly Collembola, Coleoptera, Blattodea and Hemiptera) are the least known relative to the number predicted. The beetles are interesting here because, despite rigorous taxonomic work on this group, the majority of newly discovered taxa remain undescribed or undiscovered.
Much of the subterranean faunal diversity has been identified from the Yilgarn and Pilbara regions (Fig. 2), largely due to the sustained research efforts of several groups over the last decade, in addition to the numerous EIAs fuelled by Australia’s mineral exploration boom (Eberhard et al. 2009). Geologically, the Pilbara and Yilgarn cratons of WA comprise the Western Shield, an area that has been continually emergent since the Proterozoic (Humphreys 1999, 2001) (Fig. 1). Suggestive of an ancient and remnant fauna, the aquifers of the Pilbara and Yilgarn contain an extraordinarily diverse stygofauna (Humphreys 2006) that largely appear unrelated to each other. Alternatively, troglofauna are better known in the Pilbara, with extensive sampling of fractured rock and pisolites associated with mining surveys revealing high faunal diversity. In the Yilgarn, troglofauna are comparatively poorly sampled but diversity is expected to be high especially in karstic calcretes. It is likely that our species richness values are considerably underestimated in both the Yilgarn and Pilbara but we consider it useful to provide an estimate based on the current state of knowledge and the overall conclusion that the western half of Australia represents a hotspot for subterranean faunal diversity. A survey of SA aquifers (2007–10) by Leys revealed stygobitic species in more than 200 localities across the Flinders Ranges (fractured rocks, springs and alluvia), Eyre Peninsula (limestone), Lofty Ranges (fractured rocks, springs and alluvia) and the south-east (limestone). The subterranean faunal diversity in SA appears to be lower than that of WA, however numerous taxa are yet to be worked through (e.g. Ostracoda, Gastropoda (Hydrobiidae), Turbellaria and Oligochaeta).
|
Australia-wide projections
Our estimate of 4140 species in the western half of Australia is a substantially higher figure than that postulated by Humphreys (2008). In that study, 560 stygofauna species were estimated from the Western Shield and this area comprises ~50% of the area examined in this study, thus clearly representing an underestimate of species richness based on the data presented here. Just for the Pilbara region, which represents an even smaller area of the Western Shield, Eberhard et al. (2009) estimated 500–550 undescribed species using species accumulation curves. Our results show that much of the subterranean taxa in the western half of Australia remain undiscovered and the potential for new species discovery is extremely high. In the event of broader investigations of Australia’s subterranean regions, besides caves and karst, several specific areas of Australia would benefit from a targeted approach. In particular, research on four alluvial systems in eastern Australia has uncovered a substantial fauna (Hancock and Boulton 2008; Tomlinson 2009; Camacho and Hancock 2010) indicating that a rich stygofauna occurs in eastern alluvial habitats. In particular, different river catchments have revealed distinct faunas offering a tantalising insight into potential diversity in this region. Arid regions of the Northern Territory and central Queensland, particularly in limestone areas, are also likely to harbour rich stygofaunas similar to those of the Yilgarn in WA. Additional taxa are likely to be found in SA, particular in springs and alluvia of less studied areas such as the Yorke Peninsula, southern Flinders Ranges and the Lofty Ranges. Temperate south-eastern Australia has already revealed significant diversity of subterranean fauna, predominantly collected from limestone caves (Hunt 1990; Eberhard et al. 1991; Eberhard 1996; Thurgate et al. 2001a, 2001b; Ponder et al. 2005; Rix et al. 2008) suggesting that Tasmania, Victoria and southern NSW would benefit from additional sampling effort in non-limestone terrains. In particular the Great Dividing Range and surrounds would be of interest.
The predicted origins of this diversity
Australia represents an ancient landscape and some of the subterranean habitats that we focus on here have survived throughout the formation and dissolution of Pangaea and the subsequent fragmentation of Gondwana. Indeed, some of the oldest known cave soils are found at Jenolan Caves, NSW, and have been dated to the Devonian 375 million years ago (Mya; Osborne et al. 2006), and in the Kimberley, caves were formed from ancient Devonian reefs beneath the Permian ice sheet (Playford 2009). The full breadth of subterranean ecosystems exists in Australia, in contrast to other parts of the world where only one or two ecosystem types are typically found. Australia has a variety of water types including anchialine, saline and freshwater, as well as better known subterranean types such as karst and pseudokarst, alluvial, and fractured rock. These ecosystems provide links to other global regions and reflect a vicariant relictual fauna, especially the apparent ‘Tethyan connections’ of anchialine fauna of epicontinental regions (e.g. the highly charismatic remipede species Lasionectes exleyi Yager and Humphreys 1996). Also providing links are isolated seamounts (Namiotko et al. 2004; Humphreys 2008) and Gondwanan lineages (Poore and Humphreys 1998) although the Tethyan origin of some anchialine faunal elements may be uncertain (Karanovic and Eberhard 2009). As discussed elsewhere (Humphreys 2008), subterranean ecosystems may be very persistent through geological time and many lineages probably have ancient origins (Cho et al. 2006b; Wilson 2008).
In the Yilgarn and Pilbara regions a myriad of short-range endemic species, including both stygobitic (Taiti and Humphreys 2001; Leys et al. 2003; Leys and Watts 2008; Page et al. 2008; Guzik et al. 2009; Bradford et al. 2010) and troglobitic (Humphreys and Adams 2001; Harvey et al. 2008) taxa have been identified. Much of this diversity is likely to have resulted from vicariance associated with the aridification of the Australian continent after the late Miocene (Byrne et al. 2008), which led to biotic isolation of calcretes and other subterranean habitats (e.g. pisolitic iron ore mesas in the Pilbara). Colonisation of these habitats by multiple unrelated surface species has also contributed to the high levels of diversity (Leys et al. 2003; Cooper et al. 2008; Guzik et al. 2008). Further, in situ speciation within aquifers is also considered a plausible source of species diversity, particularly in the Yilgarn (Guzik et al. 2009; Juan et al. 2010) and Pilbara (Finston et al. 2009). Abiotic heterogeneity within habitats (i.e. salinity clines, temperature variation and water level fluctuations) has been noted as possible sources of ecological variation and niche partitioning.
What is found in the rest of the world?
Regional assessments of the diversity of subterranean faunas have predominantly been conducted in the best studied locations, particularly North America and Europe. In the USA, 973 obligate subterranean species and subspecies were recorded by Culver et al. (2000), comprising 673 terrestrial species and 269 aquatic species. More than 650 stygobitic species have been recorded from the longest and most intensively researched region, the Balkan Peninsula, where the first stygal animal was described in 1768, and from where 975 species of troglofauna have been recorded (Sket et al. 2004). Slovenia, a key cave region in Europe, has 114 known stygobitic species (Culver and White 2004), while six other European countries (Belgium, France, Italy, Portugal, Slovenia, Spain (Malard et al. 2009; Michel et al. 2009)) have recorded 1059 stygobitic taxa with no more than 80 species from any one karst region. Most of these taxa are considered remnants of the Pleistocene, during which time cave populations were colonised during interglacial cycles, and isolated during glacial periods (Peck 1984; Peck and Christiansen 1990; Culver et al. 2006). However, this is likely not the sole source of species origins with pre-Pleistocene processes being well recognised (Hedin 1997; Buhay and Crandall 2005; Buhay et al. 2007). Culver et al. (2006) predicted that other regions of interest for cave fauna in the northern hemisphere are likely to include the Eurasian continent including Georgia and Kyrgyzstan. Alternatively, the southern hemisphere subterranean fauna are well documented for New Zealand, where 102 described species are known from groundwater habitats, particular Hydracarina (70 species) and crustacean groups such as Amphipoda (four species), Isopoda (four species), and Syncarida (seven species) (Scarsbrook et al. 2003). South and Central America have also been recognised to maintain novel cave fauna but which are under threat from deforestation. In particular, Brazil (Trajano 2000), Ecuador (Peck 1990), Mexico (Desutter-Grandcolas 1993), and several Caribbean islands (Peck 1974, 1999) have also yielded new cave fauna.
Possible subterranean biodiversity hotspots elsewhere in the world
Based on geology we expect that Africa and India may yield similar subterranean biodiversity hotspots to those described here for Australia. There are established links with Australia for some stygal lineages from India (Phreatoicidea (Wilson 2008); Atopobathynella (Cho et al. 2006b)), Africa (Phreatoicidea (Wilson and Keable 1999)), and more widely with Gondwana (Candoninae (Karanovic 2004, 2005a, 2005b); Spelaeogriphacea (Poore and Humphreys 1998, 2003)). Further these Gondwanan links between the major continents (e.g. the ‘cosmopolitan’ Bathynellacea (Lopretto and Morrone 1998)) are likely to be an indicator of new regions of subterranean faunal significance. To date, Africa remains largely unexplored, apart from the Mediterranean north coast and Atlas Mountains. While Botswana (Modisi 1983) and Namibia (Irish 1991; Christelis and Struckmeier 2001) are considered possible locations that may harbour an undocumented diversity of stygofauna, southern Africa as a whole is a likely subterranean hotspot, as similar geology, karst and calcrete aquifers to those observed in WA exist there (Pickford et al. 1999). South Africa has the endemic subterranean amphipod family Sternophysingidae (Tasaki 2006), within the globally distributed superfamily Crangonyctoidea (Holsinger 1992) and the order Spelaeogriphacea (Sharratt et al. 2000). The Spelaeogriphacea are only known from two other locations in the world (Brazil and Australia), indicating a shared Gondwanan distribution (Jaume 2008). In South America, the best characterised caves are in central Brazil and include the Serra do Ramalho karst area in Bahia state, well known for its populations of the troglomorphic catfish Rhamdia enfurnada Bichuette & Trajano, 2005 (e.g. Mattox et al. 2008), and Minas Gerais state, which is well known for its troglobitic invertebrate fauna (Ferriera and Horta 2001; Souza and Ferreira 2010). Future work would benefit from assessment of the geology and current literature of these continents as indicators of possible new areas of rich biodiversity.
Conclusion
Here we identify the western part of the Australian continent as a region of extremely rich biodiversity for subterranean fauna with a projected 4140 stygobitic and troglobitic species; a significant subterranean fauna is also likely to occur across the eastern part of the continent, but considerable survey work is required to estimate the diversity of this fauna. Compared with other regions of the world, we consider the Australian subterranean fauna to be unique in its diversity for three key reasons: (1) the range and diversity of subterranean habitats where fauna have been discovered are both extensive and novel compared with the northern hemisphere; (2) direct faunal links to Gondwana are found in Australia’s west, emphasising its early biogeographic history; and (3) tertiary events, particularly developing aridity in the late Miocene/Pliocene (14–2 Mya), appear to have dominated the diversification of Australia’s subterranean fauna, unlike much of the northern hemisphere (Stoch and Galassi 2010), where the fauna was not greatly modified during Pleistocene glaciations.
Order of authorship
MTG, ADA, SJBC, MSH and WFH all contributed to writing the manuscript and collating the taxonomic, geographical and species richness data. The remaining authors, listed in alphabetical order, contributed data and ideas during a workshop in Darwin in 2009 (see ‘Acknowledgements’) and during the writing of the manuscript. Images were kindly contributed by SME.
Acknowledgements
Much of the research that underpins the data presented in this review was funded by the Australian Research Council (ARC) Discovery and Linkage grants DP0663675, DP0770979, LP0669062, LP0776478, LP0669062 and LP100200494, and the Australian Biological Resources Study. The discussions that led to this review and collation of an early version of the species diversity data occurred at a workshop held in Darwin in September 2009 funded through a Working Group on The Diversity and Evolution of Troglobitic and Groundwater Ecosystems, which is a part of the ARC Research Network (RN0457921): Discovering the Past and Present to Shape the Future: Networking Environmental Sciences for Understanding and Managing Australian Biodiversity (Environmental Futures Network). Finally, we would like to thank numerous colleagues for their help, support and discussions on the evolution and diversity of subterranean animals. Thanks also to two anonymous reviewers and associate editor Gonzalo Giribet who provided detailed comments that helped to improve an earlier version of this article.
References
Barr, T. C. (1973). Refugees of the ice age. Natural History 26, 26–35.Barranco, P., and Harvey, M. S. (2008). The first indigenous palpigrade from Australia: a new species of Eukoenenia (Palpigradi: Eukoeneniidae). Invertebrate Systematics 22, 227–233.
| The first indigenous palpigrade from Australia: a new species of Eukoenenia (Palpigradi: Eukoeneniidae).Crossref | GoogleScholarGoogle Scholar |
Bichuette, M. E., and Trajano, E. (2005). A new cave species of Rhamdia (Siluriformes: Heptapteridae) from Serra do Ramalho, northeastern Brazil, with notes on ecology and behavior. Neotropical Ichthyology 3, 587–595.
| A new cave species of Rhamdia (Siluriformes: Heptapteridae) from Serra do Ramalho, northeastern Brazil, with notes on ecology and behavior.Crossref | GoogleScholarGoogle Scholar |
Boulton, A. J. (2009). Recent progress in the conservation of groundwaters and their dependent ecosystems. Aquatic Conservation. Marine and Freshwater Ecosystems 19, 731–735.
| Recent progress in the conservation of groundwaters and their dependent ecosystems. Aquatic Conservation.Crossref | GoogleScholarGoogle Scholar |
Boutin, C. (1994). Stygobiology and historical geology: the age of Fuerteventura (Canary Islands) as inferred from its present stygofauna. Bulletin de la Société Géologique de France 165, 273–285.
Bradbury, J. H. (1999). The systematics and distribution of Australian freshwater amphipods: a review. In ‘Proceedings of the Fourth International Crustacean Congress, Amsterdam, The Netherlands’. (Eds F. R. Schram and J. C. von Vaupel Klein.) pp. 533–540. (Brill: Leiden.)
Bradbury, J. H., and Eberhard, S. (2000). A new stygobiont melitid amphipod from the Nullarbor Plain. Records of the Western Australian Museum 20, 39–50.
Bradbury, J. H., and Williams, W. D. (1997a). Amphipod (Crustacea) diversity in underground waters in Australia: an Aladdin’s Cave. Memoirs of Museum Victoria 56, 513–519.
Bradbury, J. H., and Williams, W. D. (1997b). The amphipod (Crustacea) stygofauna of Australia: description of new taxa (Melitidae, Neoniphargidae, Paramelitidae), and a synopsis of known species. Records of the Australian Museum 49, 249–341.
| The amphipod (Crustacea) stygofauna of Australia: description of new taxa (Melitidae, Neoniphargidae, Paramelitidae), and a synopsis of known species.Crossref | GoogleScholarGoogle Scholar |
Bradford, T., Adams, M., Humphreys, W. F., Austin, A. D., and Cooper, S. J. B. (2010). DNA barcoding of stygofauna uncovers cryptic amphipod diversity in a calcrete aquifer in Western Australia’s arid zone. Molecular Ecology Resources 10, 41–50.
| DNA barcoding of stygofauna uncovers cryptic amphipod diversity in a calcrete aquifer in Western Australia’s arid zone.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1ans7k%3D&md5=ad9b543ceacbacf9d3013968b102e199CAS |
Brooks, T. M., Mittermeier, R. A., da Fonseca, G. A. B., Gerlach, J., Hoffmann, M., Lamoreux, J. F., Mittermeier, C. G., Pilgrim, J. D., and Rodrigues, A. S. L. (2006). Global biodiversity conservation priorities. Science 313, 58–61.
| Global biodiversity conservation priorities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmsFWisLg%3D&md5=973630903d639765adc2cb8d8662533cCAS | 16825561PubMed |
Bruce, N. L. (2008). New species and a new genus of Cirolanidae (Isopod: Cymothoida: Crustacea) from groundwater in calcretes in the Pilbarra [sic.], northern Western Australia. Zootaxa 1823, 51–64.
Bruce, N. L., and Humphreys, W. F. (1993). Haptolana pholeta, sp. nov., the first subterranean flabelliferan isopod crustacean (Cirolanidae) from Australia. Invertebrate Taxonomy 7, 875–884.
| Haptolana pholeta, sp. nov., the first subterranean flabelliferan isopod crustacean (Cirolanidae) from Australia.Crossref | GoogleScholarGoogle Scholar |
Buhay, J. E., and Crandall, K. A. (2005). Subterranean phylogeography of freshwater crayfishes shows extensive gene flow and surprisingly large population sizes. Molecular Ecology 14, 4259–4273.
| Subterranean phylogeography of freshwater crayfishes shows extensive gene flow and surprisingly large population sizes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhvFahsA%3D%3D&md5=abea11642e695f3d190ddc60a95fe17cCAS | 16313591PubMed |
Buhay, J. E., Moni, G., Mann, N., and Crandall, K. A. (2007). Molecular taxonomy in the dark: evolutionary history, phylogeography, and diversity of cave crayfish in the subgenus Aviticambarus, genus Cambarus. Molecular Phylogenetics and Evolution 42, 435–448.
| Molecular taxonomy in the dark: evolutionary history, phylogeography, and diversity of cave crayfish in the subgenus Aviticambarus, genus Cambarus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1CgsbjF&md5=bab17c0ac2274affbb5aca6553bad794CAS | 16971141PubMed |
Burger, M., Harvey, M. S., and Stevens, N. (2010). A new species of blind subterranean Tetrablemma (Araneae: Tetrablemmidae) from Australia. The Journal of Arachnology 38, 146–149.
| A new species of blind subterranean Tetrablemma (Araneae: Tetrablemmidae) from Australia.Crossref | GoogleScholarGoogle Scholar |
Byrne, M., Yeates, D. K., Joseph, L., Kearney, M., Bowler, J., Williams, M. A., Cooper, S. J. B., Donnellan, S. C., Keogh, J. S., Leys, R., Melville, J., Murphy, D. J., Porch, N., and Wyrwoll, K. H. (2008). Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota. Molecular Ecology 17, 4398–4417.
| Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1cjhvFGruw%3D%3D&md5=2bb4d789a88b0bd02263023fa38a4f00CAS | 18761619PubMed |
Camacho, A. I., and Hancock, P. J. (2010). A new genus of Parabathynellidae (Crustacea: Bathynellacea) in New South Wales, Australia. Journal of Natural History 44, 1081–1094.
| A new genus of Parabathynellidae (Crustacea: Bathynellacea) in New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Cho, J.-L. (2005). A primitive representative of the Parabathynellidae (Bathynellacea, Syncarida) from the Yilgarn Craton of Western Australia. Journal of Natural History 39, 3423–3433.
| A primitive representative of the Parabathynellidae (Bathynellacea, Syncarida) from the Yilgarn Craton of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Cho, J.-L., and Humphreys, W. F. (2010). Ten new species of the genus Brevisomabathynella Cho, Park and Ranga Reddy, 2006 (Malacostraca, Bathynellacea, Parabathynellidae) from Western Australia. Journal of Natural History 44, 993–1079.
| Ten new species of the genus Brevisomabathynella Cho, Park and Ranga Reddy, 2006 (Malacostraca, Bathynellacea, Parabathynellidae) from Western Australia.Crossref | GoogleScholarGoogle Scholar |
Cho, J.-L., Park, J.-G., and Humphreys, W. F. (2005). A new genus and six new species of the Parabathynellidae (Bathynellacea, Syncarida) from the Kimberley Region, Western Australia. Journal of Natural History 39, 2225–2255.
Cho, J.-L., Park, J.-G., and Ranga Reddy, Y. (2006a). Brevisomabathynella gen. nov. with two new species from Western Australia (Bathynellacea, Syncarida): the first definitive evidence of predation in Parabathynellidae. Zootaxa 1247, 25–42.
Cho, J.-L., Humphreys, W. F., and Lee, S.-D. (2006b). Phylogenetic relationships within the genus Atopobathynella Schminke (Bathynellacea: Parabathynellidae). Invertebrate Systematics 20, 9–41.
| Phylogenetic relationships within the genus Atopobathynella Schminke (Bathynellacea: Parabathynellidae).Crossref | GoogleScholarGoogle Scholar |
Christelis, G., and Struckmeier, W. (2001). ‘Groundwater in Namibia: An Explanation to the Hydrogeological Map.’ (Ministry of Agriculture Water and Rural Development: Windhoek, Namibia.)
Christman, M. C., and Culver, D. C. (2001). The relationship between cave biodiversity and available habitat. Journal of Biogeography 2, 367–380.
Christman, M. C., Culver, D. C., Madden, M. K., and White, D. (2005). Patterns of endemism of the eastern North American cave fauna. Journal of Biogeography 32, 1441–1452.
| Patterns of endemism of the eastern North American cave fauna.Crossref | GoogleScholarGoogle Scholar |
Cooper, S. J. B., Bradbury, J. H., Saint, K. M., Leys, R., Austin, A. D., and Humphreys, W. F. (2007). Subterranean archipelago in the Australian arid zone: mitochondrial DNA phylogeography of amphipods from central Western Australia. Molecular Ecology 16, 1533–1544.
| Subterranean archipelago in the Australian arid zone: mitochondrial DNA phylogeography of amphipods from central Western Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXlt1Gmu7w%3D&md5=c62979b56b10accce0e87007daf35d47CAS | 17391274PubMed |
Cooper, S. J. B., Saint, K. M., Taiti, S., Austin, A. D., and Humphreys, W. F. (2008). Subterranean archipelago: mitochondrial DNA phylogeography of stygobitic isopods (Oniscidea: Haloniscus) from the Yilgarn region of Western Australia. Invertebrate Systematics 22, 195–203.
| Subterranean archipelago: mitochondrial DNA phylogeography of stygobitic isopods (Oniscidea: Haloniscus) from the Yilgarn region of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Culver, D. C., and Sket, B. (2000). Hotspots of subterranean biodiversity in caves and wells. Journal of Caves and Karst Studies 62, 11–17.
Culver, D. C., and White, W. B. (Eds) (2004). ‘Encyclopedia of Caves.’ (Elsevier Academic Press: Amsterdam.)
Culver, D. C., Master, L. L., Christman, M. C., and Hobbs, H. H. (2000). Obligate cave fauna of the 48 contiguous United States. Conservation Biology 14, 386–401.
| Obligate cave fauna of the 48 contiguous United States.Crossref | GoogleScholarGoogle Scholar |
Culver, D. C., Deharveng, L., Gibert, J., and Sasowsky, I. D. (Eds) (2001). ‘Mapping Subterranean Biodiversity: Cartographie de la Biodiversitè Souterraine.’ Special publication 6. (Karst Water Institute: Labaoratoire Souterraine: Moulis, France.)
Culver, D. C., Christman, M. C., Šereg, I., Trontelj, P., and Sket, B. (2004). The location of terrestrial species-rich caves in a cave-rich area. Subterranean Biology 2, 27–32.
Culver, D. C., Deharveng, L., Bedos, A., Lewis, J., Madden, M., Reddell, J. R., Sket, B., Trontelj, P., and White, D. (2006). The mid-latitude biodiversity ridge in terrestrial cave fauna. Ecography 29, 120–128.
| The mid-latitude biodiversity ridge in terrestrial cave fauna.Crossref | GoogleScholarGoogle Scholar |
Deharveng, L. (2005). Diversity patterns in the tropics. In ‘Encyclopedia of Caves’. (Eds D. C. Culver and W. B. White.) pp. 166–170. (Elsevier/Academic Press: Burlington, MA.)
Derbyshire, E. (1972). Pleistocene glaciation of QF Tasmania: review and speculations. Australian Geographical Studies 10, 79–94.
| Pleistocene glaciation of QF Tasmania: review and speculations.Crossref | GoogleScholarGoogle Scholar |
Desutter-Grandcolas, L. (1993). The cricket fauna of chiapanecan caves (Mexico): systematics, phylogeny and the evolution of troglobitic life (Orthoptera, Grylloidea, Phalangopsidae, Luzarinae). International Journal of Speleology 22, 1–82.
Eberhard, S. M. (1996). Tasmanian cave fauna. In ‘Encyclopedia Biospeologica Tome III’. (Eds C. Juberthie and V. Decu.) pp. 2093–2103. (Societe Internationale de Biospeleologie: Moulis (C. N. R. S.), France and Bucharest (Academia Románă), Romania.)
Eberhard, S. M., and Humphreys, W. F. (2003). The crawling, creeping and swimming life of caves. In ‘Beneath the Surface’. (Eds B. Finlayson and E. Hamilton-Smith.) pp. 127–147. (University of New South Wales Press: Sydney.)
Eberhard, S. M., Richardson, A. M., and Swain, R. (1991). The invertebrate cave fauna of Tasmania. Report to the National Estate Office, Canberra.
Eberhard, S. M., Halse, S. A., and Humphreys, W. F. (2005). Stygofauna in the Pilbara region, north-west Western Australia: a systematic review. Journal of the Royal Society of Western Australia 88, 167–176.
Eberhard, S. M., Halse, S. A., Williams, M. R., Scanlon, M. D., Cocking, J., and Barron, H. J. (2009). Exploring the relationship between sampling efficiency and short-range endemism for groundwater fauna in the Pilbara region, Western Australia. Freshwater Biology 54, 885–901.
| Exploring the relationship between sampling efficiency and short-range endemism for groundwater fauna in the Pilbara region, Western Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltVOqurg%3D&md5=eae0a443b652843e1a7fff5dd170905fCAS |
Edgecombe, G. D. (2005). A troglomorphic species of the centipede Cryptops (Trigonocryptops) (Chilopoda: Scolopendromorpha) from Western Australia. Records of the Western Australian Museum 22, 315–323.
Edward, K. L., and Harvey, M. S. (2008). Short-range endemism in hypogean environments: the pseudoscorpion genera Tyrannochthonius and Lagynochthonius (Pseudoscorpiones: Chthoniidae) in the semiarid zone of Western Australia. Invertebrate Systematics 22, 259–293.
| Short-range endemism in hypogean environments: the pseudoscorpion genera Tyrannochthonius and Lagynochthonius (Pseudoscorpiones: Chthoniidae) in the semiarid zone of Western Australia.Crossref | GoogleScholarGoogle Scholar |
EPA (2003). Consideration of subterranean fauna in groundwater and caves during environmental impact assessment in Western Australia. Environmental Protection Authority, Perth.
Ferriera, R. L., and Horta, L. C. S. (2001). Natural and human impacts on invertebrate communities in Brazilian caves. Revista Brasileira de Biologia 61, 7–17.
Finston, T. L., and Johnson, M. S. (2004). Geographic patterns of genetic diversity in subterranean amphipods of the Pilbara, Western Australia. Marine and Freshwater Research 55, 619–628.
| Geographic patterns of genetic diversity in subterranean amphipods of the Pilbara, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Finston, T. L., Johnson, M. S., Humphreys, W. F., Eberhard, S., and Halse, S. (2007). Cryptic speciation in two widespread subterranean amphipod genera reflects historical drainage patterns in an ancient landscape. Molecular Ecology 16, 355–365.
| Cryptic speciation in two widespread subterranean amphipod genera reflects historical drainage patterns in an ancient landscape.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXks1yqsrw%3D&md5=7ae0ebf698f547f269e44511a7cafa14CAS | 17217350PubMed |
Finston, T. L., Francis, C. J., and Johnson, M. S. (2009). Biogeography of the stygobitic isopod Pygolabis (Malacostraca: Tainisopidae) in the Pilbara, Western Australia: evidence for multiple colonisations of the groundwater. Molecular Phylogenetics and Evolution 52, 448–460.
| Biogeography of the stygobitic isopod Pygolabis (Malacostraca: Tainisopidae) in the Pilbara, Western Australia: evidence for multiple colonisations of the groundwater.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXms12hsbs%3D&md5=392fdb21f04929e5edda5b08b965cd48CAS | 19303454PubMed |
Fong, D. W., and Culver, D. C. (1994). Fine scale biogeographic differences in the crustacean fauna of a cave system in West Virginia, USA. Hydrobiologia 287, 29–37.
| Fine scale biogeographic differences in the crustacean fauna of a cave system in West Virginia, USA.Crossref | GoogleScholarGoogle Scholar |
Gibert, J., Danielopol, D. L., and Stanford, J. A. (1994). ‘Groundwater Ecology.’ (Academic Press: London.)
Guzik, M. T., Abrams, K. M., Cooper, S. J. B., Humphreys, W. F., and Cho, J.-L. (2008). Phylogeography of the ancient Parabathynellidae (Crustacea: Bathynellacea) from the Yilgarn region of Western Australia. Invertebrate Systematics 22, 205–216.
| Phylogeography of the ancient Parabathynellidae (Crustacea: Bathynellacea) from the Yilgarn region of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Guzik, M. T., Cooper, S. J. B., Humphreys, W. F., and Austin, A. D. (2009). Fine-scale comparative phylogeography of a sympatric sister species triplet of subterranean diving beetles from a single calcrete aquifer in Western Australia. Molecular Ecology 18, 3683–3698.
| Fine-scale comparative phylogeography of a sympatric sister species triplet of subterranean diving beetles from a single calcrete aquifer in Western Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1WhtrbM&md5=9b2439f26b60a797a271311fa9e129beCAS | 19674311PubMed |
Hamilton-Smith, E. (1967). The arthropoda of Australian caves. Journal of the Australian Entomological Society 6, 103–118.
| The arthropoda of Australian caves.Crossref | GoogleScholarGoogle Scholar |
Hancock, P. J., and Boulton, A. J. (2008). Stygofauna biodiversity and endemism in four alluvial aquifers in eastern Australia. Invertebrate Systematics 22, 117–126.
| Stygofauna biodiversity and endemism in four alluvial aquifers in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Harvey, M. S. (1998). Unusual new water mites (Acari: Hydracarina) from Australia, part 1. Records of the Western Australian Museum 19, 91–106.
Harvey, M. S. (2001). New cave-dwelling schizomids (Schizomida: Hubbardiidae) from Australia. Records of the Western Australian Museum 64, 171–185.
Harvey, M. S., and Edward, K. L. (2007). A review of the pseudoscorpion genus Ideoblothrus (Pseudoscorpiones, Syarinidae) from western and northern Australia. Journal of Natural History 41, 445–472.
| A review of the pseudoscorpion genus Ideoblothrus (Pseudoscorpiones, Syarinidae) from western and northern Australia.Crossref | GoogleScholarGoogle Scholar |
Harvey, M. S., and Humphreys, W. F. (1995). Notes on the genus Draculoides Harvey (Schizomida: Hubbardiidae), with the description of a new troglobitic species. Records of the Western Australian Museum 52, 183–189.
Harvey, M. S., and Leng, M. C. (2008a). Further observations on Ideoblothrus (Pseudoscorpiones: Syarinidae) from subterranean environments in Australia. Records of the Western Australian Museum 24, 379–386.
Harvey, M. S., and Leng, M. C. (2008b). The first troglomorphic pseudoscorpion of the family Olpiidae (Pseudoscorpiones), with remarks on the composition of the family. Records of the Western Australian Museum 24, 387–394.
Harvey, M. S., and Volschenk, E. S. (2007). The systematics of the Gondwanan pseudoscorpion family Hyidae (Pseudoscorpiones: Neobisioidea): new data and a revised phylogenetic hypothesis. Invertebrate Systematics 21, 365–406.
| The systematics of the Gondwanan pseudoscorpion family Hyidae (Pseudoscorpiones: Neobisioidea): new data and a revised phylogenetic hypothesis.Crossref | GoogleScholarGoogle Scholar |
Harvey, M. S., Berry, O., Edward, K. L., and Humphreys, G. (2008). Molecular and morphological systematics of hypogean schizomids (Schizomida: Hubbardiidae) in semiarid Australia. Invertebrate Systematics 22, 167–194.
| Molecular and morphological systematics of hypogean schizomids (Schizomida: Hubbardiidae) in semiarid Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlslajsr8%3D&md5=21cec7432d015799786af7b7a053f51aCAS |
Hedin, M. C. (1997). Speciational history in a diverse clade of habitat-specialized spiders (Araneae: Nesticidae: Nesticus): inferences from geographic-based sampling. Evolution 51, 1929–1945.
| Speciational history in a diverse clade of habitat-specialized spiders (Araneae: Nesticidae: Nesticus): inferences from geographic-based sampling.Crossref | GoogleScholarGoogle Scholar |
Holsinger, J. R. (1992). Sternophysingidae, a new family of subterranean amphipods (Gammaridea: Crangonyctoidea) from South Africa, with description of Sternophysinx calceola, new species, and comments on phylogenetic and biogeographic relationships. Journal of Crustacean Biology 12, 111–124.
| Sternophysingidae, a new family of subterranean amphipods (Gammaridea: Crangonyctoidea) from South Africa, with description of Sternophysinx calceola, new species, and comments on phylogenetic and biogeographic relationships.Crossref | GoogleScholarGoogle Scholar |
Humphreys, W. F. (1999). Relict stygofaunas living in sea salt, karst and calcrete habitats in arid northwestern Australia contain many ancient lineages. In ‘The Other 99%: The Conservation and Biodiversity of Invertebrates’. (Eds W. Ponder and D. Lunney.) pp. 219–227. (Transactions of the Royal Zoological Society of New South Wales: Mosman.)
Humphreys, W. F. (2001). Groundwater calcrete aquifers in the Australian arid zone: the context to an unfolding plethora of stygal biodiversity. Records of the Western Australian Museum 64, 63–83.
Humphreys, W. F. (2006). Aquifers: the ultimate groundwater dependent ecosystems. Australian Journal of Botany 54, 115–132.
| Aquifers: the ultimate groundwater dependent ecosystems.Crossref | GoogleScholarGoogle Scholar |
Humphreys, W. F. (2008). Rising from down under: developments in subterranean biodiversity in Australia from a groundwater fauna perspective. Invertebrate Systematics 22, 85–101.
| Rising from down under: developments in subterranean biodiversity in Australia from a groundwater fauna perspective.Crossref | GoogleScholarGoogle Scholar |
Humphreys, W. F. (2009). Hydrogeology and groundwater ecology: does each inform the other? Hydrogeology 17, 5–21.
| 1:CAS:528:DC%2BD1MXhtF2ntro%3D&md5=2c467cbd5d5186873cc0db1fe7d920a7CAS |
Humphreys, W. F., and Adams, M. (1991). The subterranean aquatic fauna of the North West Cape peninsula, Western Australia. Records of the Western Australian Museum 15, 383–411.
Humphreys, W. F., and Adams, M. (2001). Allozyme variation in the troglobitic millipede Stygiochiropus communis (Diplopoda: Paradoxosomatidae) from arid tropical Cape Range, northwestern Australia: population structure and implications for the management of the region. Records of the Western Australian Museum 64, 15–36.
Humphreys, W. F. and the Heritage Council of Western Australia (1994). The subterranean fauna of the Cape Range coastal plain, northwestern Australia. (Heritage Council of Western Australia: East Perth.)
Humphreys, W. F., Adams, M., and Vine, B. (1989). The biology of Schizomus vinei (Chelicerata: Schizomida) in the caves of Cape Range, Western Australia. Journal of Zoology 217, 177–201.
| The biology of Schizomus vinei (Chelicerata: Schizomida) in the caves of Cape Range, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Hunt, G. S. (1990). Hickmanoxyomma, a new genus of cavernicolous harvestmen from Tasmania (Opiliones: Triaenonychidae). Records of the Australian Museum 42, 45–68.
| Hickmanoxyomma, a new genus of cavernicolous harvestmen from Tasmania (Opiliones: Triaenonychidae).Crossref | GoogleScholarGoogle Scholar |
Irish, J. (1991). Conservation aspects of karst waters in Namibia. Madoqua 17, 141–146.
Jaume, D. (2008). Global diversity of spelaeogriphaceans and thermosbaenaceans (Crustacea: Spelaeogriphacea and Thermosbaenacea) in freshwater. Hydrobiologia 595, 219–224.
| Global diversity of spelaeogriphaceans and thermosbaenaceans (Crustacea: Spelaeogriphacea and Thermosbaenacea) in freshwater.Crossref | GoogleScholarGoogle Scholar |
Jaume, D., and Humphreys, W. F. (2001). A new genus of epacteriscid calanoid copepod from an anchialine sinkhole in northwestern Australia. Journal of Crustacean Biology 21, 157–169.
| A new genus of epacteriscid calanoid copepod from an anchialine sinkhole in northwestern Australia.Crossref | GoogleScholarGoogle Scholar |
Jaume, D., Boxshall, G. A., and Humphreys, W. F. (2001). New stygobiont copepods (Calanoida: Misophrioida) from Bundera sinkhole, an anchialine cenote on north-western Australia. Zoological Journal of the Linnean Society, London 133, 1–24.
| New stygobiont copepods (Calanoida: Misophrioida) from Bundera sinkhole, an anchialine cenote on north-western Australia.Crossref | GoogleScholarGoogle Scholar |
Juan, C., Guzik, M. T., Jaume, D., and Cooper, S. J. B. (2010). Evolution in caves: Darwin’s ‘wrecks of ancient life’ in the molecular era. Molecular Ecology 19, 3865–3880.
| Evolution in caves: Darwin’s ‘wrecks of ancient life’ in the molecular era.Crossref | GoogleScholarGoogle Scholar | 20637049PubMed |
Juberthie, C., and Decu, V. (Eds) (1994). ‘Encyclopedia Biospeleologica. Vol. 1.’ (Societe Internationale de Biospeleologie: Moulis (C. N. R. S.), France and Bucharest (Academia Románă), Romania.)
Karanovic, T. (2003). First representative of the genus Allocyclops Kiefer, 1932 (Crustacea, Copepoda, Cyclopoida) from Australian subterranean waters. Annales de Limnologie 39, 141–149.
| First representative of the genus Allocyclops Kiefer, 1932 (Crustacea, Copepoda, Cyclopoida) from Australian subterranean waters.Crossref | GoogleScholarGoogle Scholar |
Karanovic, I. (2003a). Towards a revision of Candoninae (Crustacea, Ostracoda): description of two new genera from Australian ground-waters. Species Diversity 8, 353–383.
Karanovic, I. (2003b). A new genus of Candoninae (Crustacea, Ostracoda, Candonidae) from the subterranean waters of southwestern Western Australia. Records of the Western Australian Museum 21, 315–332.
Karanovic, I. (2004). Towards a revision of Candoninae (Crustacea, Ostracoda): on the genus Candonopsis Vavra, with description of new taxa. Subterranean Biology 2, 91–108.
Karanovic, T. (2004a). Subterranean Copepoda from arid Western Australia. Crustaceana Monographs 3, 1–366.
Karanovic, T. (2004b). The genus Metacyclops Kiefer in Australia (Crustacea: Copepoda: Cyclopoida), with description of two new species. Records of the Western Australian Museum 22, 193–212.
Karanovic, T. (2005). Two new subterranean Parastenocarididae (Crustacea, Copepoda, Harpacticoida) from Western Australia. Records of the Western Australian Museum 22, 353–374.
Karanovic, I. (2005a). Towards a revision of Candoninae (Crustacea, Ostracoda): Australian representatives of the subfamily, with description of three new genera and seven new species. New Zealand Journal of Marine and Freshwater Research 39, 29–75.
| Towards a revision of Candoninae (Crustacea, Ostracoda): Australian representatives of the subfamily, with description of three new genera and seven new species.Crossref | GoogleScholarGoogle Scholar |
Karanovic, I. (2005b). A new Candoninae genus (Crustacea: Ostracoda) from subterranean waters of Queensland with a cladistic analysis of the tribe Candonopsini. Memoirs of the Queensland Museum 50, 303–319.
Karanovic, T. (2006). Subterranean copepods (Crustacea, Copepoda) from the Pilbara region in Western Australia. Records of the Western Australian Museum 70, 1–239.
Karanovic, I. (2007). Candoninae Ostracodes from the Pilbara Region in Western Australia. Crustaceana Monographs 7, 1–432.
Karanovic, T., and Eberhard, S. M. (2009). Second representative of the order Misophrioida (Crustacea, Copepoda) from Australia challenges the hypothesis of the Tethyan origin of some anchialine faunas. Zootaxa 2059, 51–68.
Karanovic, I., and Marmonier, P. (2002). On the genus Candonopsis (Crustacea: Ostracoda: Candoninae) in Australia, with key to the world recent species. Annales de Limnologie 38, 199–240.
| On the genus Candonopsis (Crustacea: Ostracoda: Candoninae) in Australia, with key to the world recent species.Crossref | GoogleScholarGoogle Scholar |
Karanovic, I., and Marmonier, P. (2003). Three new genera and nine new species of the subfamily Candoninae (Crustacea, Ostracoda, Podocopida) from the Pilbara Region (Western Australia). Beaufortia 53, 1–51.
Karanovic, T., and Pesce, G. L. (2002). Copepods from ground waters of Western Australia, VII. Nitokra humphreysi sp. nov. (Crustacea: Copepoda: Harpacticoida). Hydrobiologia 470, 5–12.
| Copepods from ground waters of Western Australia, VII. Nitokra humphreysi sp. nov. (Crustacea: Copepoda: Harpacticoida).Crossref | GoogleScholarGoogle Scholar |
Karanovic, T., Pesce, L., and Humphreys, W. F. (2001). Copepods from ground waters of Western Australia, V. Phyllopodopsyllus wellsi n. sp. (Crustacea: Copepoda: Harpacticoida), with a key to world species. Records of the Western Australian Museum 20, 333–344.
Kimura, M. (1980). A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution 16, 111–120.
| A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXmtFSktg%3D%3D&md5=ab391328d4905b4419c219e3a81e46f1CAS | 7463489PubMed |
Koch, M. (2009). Biodiversity of the two-pronged bristletails (Diplura) in Western Australia as revealed from recent mining projects. EPA-Report 1361 (Appendix 3k).
Lefébure, T., Douady, C. J., Gouy, M., and Gibert, J. (2006). Relationship between morphological taxonomy and molecular divergence within Crustacea: proposal of a molecular threshold to help species delimitation. Molecular Phylogenetics and Evolution 40, 435–447.
| Relationship between morphological taxonomy and molecular divergence within Crustacea: proposal of a molecular threshold to help species delimitation.Crossref | GoogleScholarGoogle Scholar | 16647275PubMed |
Leys, R., and Watts, C. H. S. (2008). Systematics and evolution of the Australian subterranean hydroporine diving beetles (Dytiscidae), with notes on Carabhydrus. Invertebrate Systematics 22, 217–225.
| Systematics and evolution of the Australian subterranean hydroporine diving beetles (Dytiscidae), with notes on Carabhydrus.Crossref | GoogleScholarGoogle Scholar |
Leys, R., and Watts, C. H. S. (2010). Paroster extraordinarius sp. nov., a new groundwater diving beetle from the Flinders Ranges, with notes on other diving beetles from gravels in South Australia (Coleoptera: Dytiscidae). Australian Journal of Entomology 49, 66–72.
| Paroster extraordinarius sp. nov., a new groundwater diving beetle from the Flinders Ranges, with notes on other diving beetles from gravels in South Australia (Coleoptera: Dytiscidae).Crossref | GoogleScholarGoogle Scholar |
Leys, R., Watts, C. H. S., Cooper, S. J. B., and Humphreys, W. F. (2003). Evolution of subterranean diving beetles (Coleoptera: Dytiscidae: Hydroporini, Bidessini) in the arid zone of Australia. Evolution 57, 2819–2834.
| 14761060PubMed |
Lopretto, E. C., and Morrone, J. J. (1998). Anaspidacea, Bathynellacea (Crustacea, Syncarida), generalised tracks, and the biogeographical relationships of South America. Zoologica Scripta 27, 311–318.
| Anaspidacea, Bathynellacea (Crustacea, Syncarida), generalised tracks, and the biogeographical relationships of South America.Crossref | GoogleScholarGoogle Scholar |
Malard, F., Boutin, C., Camacho, A. I., Ferreira, D., Michel, G., Sket, B., and Stoch, F. (2009). Diversity patterns of stygobiotic crustaceans across multiple spatial scales in Europe. Freshwater Biology 54, 756–776.
| Diversity patterns of stygobiotic crustaceans across multiple spatial scales in Europe.Crossref | GoogleScholarGoogle Scholar |
Mattox, G. M. T., Bichuette, M. E., Secutti, S., and Trajano, E. (2008). Surface and subterranean ichthyofauna in the Serra do Ramalho karst area, northeastern Brazil, with updated lists of Brazilian troglobitic and troglophilic fishes. Biota Neotropica 8, 145–152.
| Surface and subterranean ichthyofauna in the Serra do Ramalho karst area, northeastern Brazil, with updated lists of Brazilian troglobitic and troglophilic fishes.Crossref | GoogleScholarGoogle Scholar |
Michel, G., Malard, F., Deharveng, L., Di Lorenzo, T., Sket, B., and De Broyer, C. (2009). Reserve selection for conserving groundwater biodiversity. Freshwater Biology 54, 861–876.
| Reserve selection for conserving groundwater biodiversity.Crossref | GoogleScholarGoogle Scholar |
Modisi, M. P. (1983). The carbonate resources of Botswana. Botswana Department of Geological Survey Mineral Resources, Report 6, Gaberone.
Moore, B. P. (1964). Present-day cave beetle fauna of Australia: a pointer to past climatic change. Helictite 3, 3–9.
Namiotko, T., Wouters, K., Danielopol, D. L., and Humphreys, W. F. (2004). On the origin and evolution of a new anchialine stygobitic Microceratina species (Crustacea, Ostracoda) from Christmas Island (Indian Ocean). Journal of Micropalaeontology 23, 49–59.
| On the origin and evolution of a new anchialine stygobitic Microceratina species (Crustacea, Ostracoda) from Christmas Island (Indian Ocean).Crossref | GoogleScholarGoogle Scholar |
Osborne, R. A. L., Zwingmann, H., Pogson, R. E., and Colchester, D. M. (2006). Carboniferous clay deposits from Jenolan Caves, New South Wales: implications for timing of speleogenesis and regional geology. Australian Journal of Earth Sciences 53, 377–405.
| Carboniferous clay deposits from Jenolan Caves, New South Wales: implications for timing of speleogenesis and regional geology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFKlsrjP&md5=6b7eead30c48df561bd9e0098ed3ad03CAS |
Page, T. J., Humphreys, W. F., and Hughes, J. M. (2008). Shrimps down under: evolutionary relationships of subterranean crustaceans from Western Australia (Decapoda: Atyidae: Stygiocaris). PLoS ONE 3, e1618.
| Shrimps down under: evolutionary relationships of subterranean crustaceans from Western Australia (Decapoda: Atyidae: Stygiocaris).Crossref | GoogleScholarGoogle Scholar | 18286175PubMed |
Peck, S. B. (1974). The invertebrate fauna of tropical American caves. Part II: Puerto Rico, an ecological and zoogeographic analysis. Biotropica 6, 14–31.
| The invertebrate fauna of tropical American caves. Part II: Puerto Rico, an ecological and zoogeographic analysis.Crossref | GoogleScholarGoogle Scholar |
Peck, S. B. (1980). Climatic change and the evolution of cave invertebrates in the Grand Canyon, Arizona. The NSS Bulletin 42, 53–60.
Peck, S. B. (1984). The distribution and evolution of cavernicolous Ptomaphagus beetles in the southeastern United States (Coleoptera; Leiodidae; Cholevinae) with new species and records. Canadian Journal of Zoology 62, 730–740.
| The distribution and evolution of cavernicolous Ptomaphagus beetles in the southeastern United States (Coleoptera; Leiodidae; Cholevinae) with new species and records.Crossref | GoogleScholarGoogle Scholar |
Peck, S. B. (1990). Eyeless arthropods of the Galapagos Islands, Ecuador: composition and origin of the cryptozoic fauna of a young, tropical, oceanic archipelago. Biotropica 22, 366–381.
| Eyeless arthropods of the Galapagos Islands, Ecuador: composition and origin of the cryptozoic fauna of a young, tropical, oceanic archipelago.Crossref | GoogleScholarGoogle Scholar |
Peck, S. B. (1999). Historical biogeography of Jamaica: evidence from cave invertebrates. Canadian Journal of Zoology 77, 368–380.
| Historical biogeography of Jamaica: evidence from cave invertebrates.Crossref | GoogleScholarGoogle Scholar |
Peck, S. B., and Christiansen, K. (1990). Evolution and zoogeography of the invertebrate cave faunas of the Driftless Area of the Upper Mississippi River Valley of Iowa, Minnesota, Wisconsin, and Illinois, USA. Canadian Journal of Zoology 68, 73–88.
| Evolution and zoogeography of the invertebrate cave faunas of the Driftless Area of the Upper Mississippi River Valley of Iowa, Minnesota, Wisconsin, and Illinois, USA.Crossref | GoogleScholarGoogle Scholar |
Pesce, G. L., and De Laurentiis, P. (1996). Copepods from ground waters of Western Australia, III. Diacyclops humphreysi n. sp. and comments on the Diacyclops crassicaudis-complex (Copepoda, Cyclopidae). Crustaceana 69, 524–531.
| Copepods from ground waters of Western Australia, III. Diacyclops humphreysi n. sp. and comments on the Diacyclops crassicaudis-complex (Copepoda, Cyclopidae).Crossref | GoogleScholarGoogle Scholar |
Pesce, G. L., De Laurentiis, P., and Humphreys, W. F. (1996a). Copepods from ground waters of Western Australia, I. The genera Metacyclops, Mesocyclops, Microcyclops and Apocyclops (Crustacea: Copepoda: Cyclopidae). Records of the Western Australian Museum 18, 67–76.
Pesce, G. L., De Laurentiis, P., and Humphreys, W. F. (1996b). Copepods from ground waters of Australia, II. The genus Halicyclops (Crustacea: Copepoda: Cyclopidae). Records of the Western Australian Museum 18, 77–85.
Pickford, M., Eisenmann, V., and Senut, B. (1999). Timing of landscape development and calcrete genesis in northern Namaqualand, South Africa. South African Journal of Science 95, 357–359.
Pimm, S. L., Russell, G. J., Gittleman, J. L., and Brooks, T. M. (1995). The future of biodiversity. Science 269, 347–350.
| The future of biodiversity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXntVyit7s%3D&md5=8f8048d5620350d29e6d3a3463a0ed99CAS | 17841251PubMed |
Platnick, N. I. (2008). A new subterranean ground spider genus from Western Australia (Araneae: Trochanteriidae). Invertebrate Systematics 22, 295–299.
| A new subterranean ground spider genus from Western Australia (Araneae: Trochanteriidae).Crossref | GoogleScholarGoogle Scholar |
Playford, G. (2009). Devonian reef complexes of the Canning Basin, Western Australia: review of Devonian palynology, Canning Basin. Geological Survey of Western Australia Bulletin 145, 441–444.
Ponder, W. F., Hershler, R., and Jenkins, B. (1989). An endemic radiation of hydrobiid snails from artesian springs in northern South Australia: their taxonomy, physiology, distribution and anatomy. Malacologia 31, 1–140.
Ponder, W. F., Clark, S. A., Eberhard, S. M., and Studdert, J. (2005). A remarkable radiation of hydrobiids in the caves and streams at Precipitous Bluff, south west Tasmania (Mollusca: Caenogastropoda: Hydrobiidae). Zootaxa 1074, 3–66.
Poore, G. C. B., and Humphreys, W. F. (1998). First record of Spelaeogriphacea from Australasia: a new genus and species from an aquifer in the arid Pilbara of Western Australia. Crustaceana 71, 721–742.
| First record of Spelaeogriphacea from Australasia: a new genus and species from an aquifer in the arid Pilbara of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Poore, G. C. B., and Humphreys, W. F. (2003). Second species of Mangkurtu (Spelaeogriphacea) from north-western Australia. Records of the Western Australian Museum 22, 67–74.
Reddell, J. R. (1981). A review of the cavernicole fauna of Mexico, Guatemala and Belize. Texas Memorial Museum Bulletin 27, 1–327.
Rix, M. G., Harvey, M. S., and Roberts, J. D. (2008). Molecular phylogenetics of the spider family Micropholcommatidae (Arachnida: Araneae) using nuclear rRNA genes (18S and 28S). Molecular Phylogenetics and Evolution 46, 1031–1048.
| Molecular phylogenetics of the spider family Micropholcommatidae (Arachnida: Araneae) using nuclear rRNA genes (18S and 28S).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjtlGrsr0%3D&md5=1a33e275c84cd6016cd48a2d43803c80CAS | 18162409PubMed |
Scarsbrook, M. R., Fenwick, G. D., Duggan, I. C., and Haase, M. (2003). A guide to the groundwater invertebrates of New Zealand. NIWA Science and Technology Series 51, 59.
Sharratt, N. J., Picker, M. D., and Samways, M. J. (2000). The invertebrate fauna of the sandstone caves of the Cape Peninsula (South Africa): patterns of endemism and conservation priorities. Biodiversity and Conservation 9, 107–143.
| The invertebrate fauna of the sandstone caves of the Cape Peninsula (South Africa): patterns of endemism and conservation priorities.Crossref | GoogleScholarGoogle Scholar |
Sket, B., Paragamian, K., and Trontelj, P. (2004). A census of the obligate subterranean fauna of the Balkan Peninsula. In ‘Balkan Biodiversity’. (Ed. H. I. Griffith.) pp. 309–322. (Kluwer Academic Publishers: Dordrecht.)
Souza, M. F. V. R., and Ferreira, R. L. (2010). Eukoenenia (Palpigradi: Eukoeneniidae) in Brazilian caves with the first troglobiotic palpigrade from South America. The Journal of Arachnology 38, 415–424.
| Eukoenenia (Palpigradi: Eukoeneniidae) in Brazilian caves with the first troglobiotic palpigrade from South America.Crossref | GoogleScholarGoogle Scholar |
Stoch, S., and Galassi, D. M. P. (2010). Stygobiotic crustacean species richness: a question of numbers, a matter of scale. Hydrobiologia 653, 217–234.
| Stygobiotic crustacean species richness: a question of numbers, a matter of scale.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXovV2ntbc%3D&md5=d8c8e86f5ad8e0c669e5f9cca481807dCAS |
Taiti, S., and Humphreys, W. F. (2001). New aquatic Oniscidea (Crustacea, Isopoda) from groundwater calcretes of Western Australia. Records of the Western Australian Museum 64, 63–83.
Tasaki, S. (2006). The presence of stygobitic macroinvertebrates in karstic aquifers: a case study in the Cradle of Humankind World Heritage Site. Master of Science Thesis, University of Johannesburg, South Africa.
Thurgate, M. E., Gough, J. S., Spate, A., and Eberhard, S. M. (2001a). Subterranean biodiversity in New South Wales: from rags to riches. Records of the Western Australian Museum 64, 37–48.
Thurgate, M. E., Gough, J. S., Clarke, A. K., Serov, P., and Spate, A. (2001b). Stygofauna diversity and distribution in eastern Australian caves and karst areas. Records of the Western Australian Museum 64, 49–62.
Tomlinson, M. (2009). A framework for determining the environmental water requirements of alluvial aquifer ecosystems. Ph.D. Thesis, University of New England, Armidale.
Trajano, E. (2000). Cave faunas in the Atlantic tropical rain forest: composition, ecology, and conservation. Biotropica 32, 882–893.
Volschenk, E. S., and Prendini, L. (2008). Aops oncodactylus, gen. et sp. nov., the first troglobitic urodacid (Urodacidae: Scorpiones), with a re-assessment of cavernicolous, troglobitic and troglomorphic scorpions. Invertebrate Systematics 22, 235–257.
| Aops oncodactylus, gen. et sp. nov., the first troglobitic urodacid (Urodacidae: Scorpiones), with a re-assessment of cavernicolous, troglobitic and troglomorphic scorpions.Crossref | GoogleScholarGoogle Scholar |
Watts, C. H. S., and Humphreys, W. F. (2003). Twenty-five new Dytiscidae (Coleoptera) of the genera Tjirtudessus Watts & Humphreys, Nirripirti Watts & Humphreys and Bidessodes Regimbart, from underground waters in Australia. Records of the South Australian Museum 36, 135–187.
Watts, C. H. S., and Humphreys, W. F. (2009). Fourteen new Dytiscidae (Coleoptera) of the genera Limbodessus Guignot, Paroster Sharp and Exocelina Broun, from underground waters in Australia. Transactions of the Royal Society of South Australia 133, 62–107.
Wilkens, H., Culver, D. C., and Humphreys, W. F. (Eds) (2000). ‘Ecosystems of the World: Subterranean Ecosystems.’ (Elsevier: Amsterdam.)
Wilson, G. D. F. (2001). Australian groundwater-dependent isopod crustaceans. Records of the Western Australian Museum 62, 239–240.
Wilson, G. D. F. (2003). A new genus of Tainisopidae fam. nov. (Crustacea: Isopoda) from the Pilbara, Western Australia. Zootaxa 245, 1–20.
Wilson, G. D. F. (2008). Gondwanan groundwater: subterranean connections of Australian phreatoicidean isopods (Crustacea) to India and New Zealand. Invertebrate Systematics 22, 301–310.
| Gondwanan groundwater: subterranean connections of Australian phreatoicidean isopods (Crustacea) to India and New Zealand.Crossref | GoogleScholarGoogle Scholar |
Wilson, G. D. F., and Johnson, R. T. (1999). Ancient endemism among freshwater isopods (Crustacea, Phreatoicidea). In ‘The Other 99%: The Conservation and Biodiversity of Invertebrates’. (Eds W. Ponder and D. Lunney.) pp. 264–268. (Transactions of the Royal Zoological Society of New South Wales: Mosman.)
Wilson, G. D. F., and Keable, S. J. (1999). A new genus of phreatoicidean isopod (Crustacea) from the north Kimberley region, Western Australia. Zoological Journal of the Linnean Society, London 126, 51–79.
| A new genus of phreatoicidean isopod (Crustacea) from the north Kimberley region, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Wilson, G. D. F., and Ponder, W. F. (1992). Extraordinary new subterranean isopods (Peracarida: Crustacea) from the Kimberley region, Western Australia. Records of the Australian Museum 44, 279–298.
| Extraordinary new subterranean isopods (Peracarida: Crustacea) from the Kimberley region, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Yager, J., and Humphreys, W. F. (1996). Lasionectes exleyi, sp. nov., the first remipede crustacean recorded from Australia and the Indian Ocean, with a key to the world species. Invertebrate Systematics 10, 171–187.
| Lasionectes exleyi, sp. nov., the first remipede crustacean recorded from Australia and the Indian Ocean, with a key to the world species.Crossref | GoogleScholarGoogle Scholar |
Yeates, D. K., Harvey, M. S. D., and Austin, A. D. (2003). New estimates for terrestrial arthropod species-richness in Australia. Proceedings of the Royal Society of South Australia 7, 231–241.
Zagmajster, M., Culver, D. C., and Sket, B. (2008). Species richness patterns of obligate subterranean beetles (Insecta: Coleoptera) in a global biodiversity hotspot – effect of scale and sampling intensity. Diversity & Distributions 14, 95–105.
| Species richness patterns of obligate subterranean beetles (Insecta: Coleoptera) in a global biodiversity hotspot – effect of scale and sampling intensity.Crossref | GoogleScholarGoogle Scholar |


