Systematics and biology of the aberrant intertidal parasitoid wasp Echthrodesis lamorali Masner (Hymenoptera : Platygastridae s.l.): a parasitoid of spider eggs
Simon van Noort A B H , Lubomir Masner C , Ovidiu Popovici D , Alejandro A. Valerio E , Charuwat Taekul E , Norman F. Johnson E , Nicholas P. Murphy F and Andrew D. Austin GA Department of Natural History, Iziko South African Museum, PO Box 61, Cape Town 8000, South Africa.
B Department of Biological Sciences, University of Cape Town, Private Bag, Rondebosch 7701, South Africa.
C Research Associate, Agriculture and Agri-Food Canada. Research Branch. K.W. Neatby Building, Ottawa, Ontario K1A OC6, Canada.
D Facultatea de Biologie, Universitatea Alexandru Ioan Cuza IASI, Bulevardul Carol I, Nr.11, 700506, IASI, Romania.
E Department of Evolution, Ecology, and Organismal Biology, The Ohio State University, 1315 Kinnear Road, Columbus, OH 43212, USA.
F Department of Genetics, School of Molecular Science, La Trobe University, Bundoora, Vic. 3086, Australia.
G Australian Centre for Evolutionary Biology & Biodiversity, School of Earth and Environmental Sciences, The University of Adelaide, SA 5005, Australia.
H Corresponding author. Email: svannoort@iziko.org.za
Invertebrate Systematics 28(1) 1-16 https://doi.org/10.1071/IS13015
Submitted: 11 April 2013 Accepted: 12 November 2013 Published: 20 March 2014
Abstract
The platygastroid wasp Echthrodesis lamorali has been of considerable interest since its description in 1968, primarily because of its highly modified, densely pilose, wingless body, its distribution and unusual biology. The species is endemic to the Cape Peninsula, South Africa, where it is an endoparasitoid of eggs of the marine spiders Desis formidabilis (Desidae) and Amaurobioides africanus (Anyphaenidae) in the intertidal region. Although a highly aberrant monospecific genus, the phylogenetic relationships of Echthrodesis are confused, in part due to convergence in body form across numerous unrelated platygastroid genera. We used sequence data from the nuclear 28S rRNA and 18S rDNA genes, and the mitochondrial cytochrome oxidase 1 (CO1) gene, to determine the phylogenetic affinities of E. lamorali. We present a revised taxonomic description for the genus and species, as well as new morphological information on the structure of its mouthparts and ovipositor system. Phylogenetic analyses of molecular data place E. lamorali within one of two independent clades of platygastroid wasps that use spider eggs as hosts. Echthrodesis is sister to a group of three genera: Neobaeus (New Zealand; host unconfirmed); Mirobaeoides (Australia; spider eggs); and Embidobia (near cosmopolitan; embiid eggs). Details on the biology, behaviour and morphological adaptations of E. lamorali are provided.
Additional keywords: morphology, phylogeny, Scelioninae, taxonomy.
Introduction
The platygastroid wasp Echthrodesis lamorali Masner is an endoparasitoid of the egg stage of two intertidal spiders, Desis formidabilis (O.P. Cambridge) (Desidae) and Amaurobioides africanus Hewitt (Anyphaenidae). It is remarkable in that the species is adapted to a marine environment, becoming completely submersed by sea water and exposed to intensive wave action in the intertidal zone at each high tide (Lamoral 1968; Masner 1968). Presumably due to the exclusive association with this habitat, E. lamorali has become highly modified and adapted for life in rocky areas of the intertidal zone. Morphological adaptations include extensive wing reduction in both sexes (Carey et al. 2006), a compact body form that probably facilitates movement in very confined spaces and a covering of dense setae (Figs 5, 6), hypothesised to trap air during daily immersion in sea water (Masner 1968). The first two of these morphological traits are likely to be reductional synapomorphies that are exhibited by several genera of Platygastroidea that parasitise spider eggs (e.g. Baeus Haliday, Mirobaeus Dodd, Mirobaeoides Dodd, Neobaeus Austin), and are thought to facilitate entry through the silk walls of spider egg sacs (Austin 1988a; Austin et al. 2005; Stevens and Austin 2007).
On first description, Echthrodesis was hypothesised to be closely related to Embidobia Ashmead, Mirobaeus and Mirobaeoides (Masner 1968). This relationship was later formalised by Masner and Dessart (1972), who united them within the tribe Embidobiini along with other putatively related genera (Endecascelio Masner and Dessart, Palaeogryon Masner, Embioctonus Masner) (Masner 1976, 1980). Echthrodesis differs from these genera in the remarkable pilosity of its body, and from Embidobia, Palaeogryon and Embioctonus in its host use as these three genera parasitise eggs of Embiidina whereas Mirobaeus and Mirobaeoides (like Echthrodesis) parasitise spider eggs. Galloway and Austin (1984) postulated that the production of silk by such different host groups provided a possible evolutionary link in host exploitation for this tribe.
Following analysis of several morphological characters, including the ovipositor system, and host-use data (Austin 1986,1988a; Austin and Field 1997), all platygastroid genera parasitising spider eggs were placed in the single nominal tribe Baeini s.l., whereas previously they had been distributed across three tribes (i.e. Embidobiini, Baeini and Idrini; sensu Masner 1976). At the same time, based on the morphology of the mandible and its host associations, Echthrodesis was removed from the Embidobiini and placed in the Baeini (Austin and Field 1997). Subsequently, a morphological phylogenetic analysis of the Baeini s.l. placed Echthrodesis as sister to a terminal clade comprising Apobaeus Masner, Baeus, Mirobaeoides and Neobaeus (Iqbal and Austin 2000); however, this study did not include any of the embiid egg parasitoids from the Embidobiini.
Somewhat surprisingly, recent molecular phylogenetic appraisals of platygastroid genera using multiple genes (Carey et al. 2006; Murphy et al. 2007) have revealed the spider egg parasitoids (i.e. Baeini) to be polyphyletic, with Baeus, Ceratobaeus Ashmead, Hickmanella Austin, Idris Foerster and Odontacolus Kieffer forming a monophyletic group, and Mirobaeoides and Neobaeus together forming an independent clade that is sister to Embidobia. This relationship supports a possible common origin for parasitising silk-encased host eggs, and two independent origins for parasitising spider eggs within the superfamily, as previously inferred (Masner (1968, 1976; Masner and Dessart 1972). However, an important question in platygastroid systematics remains; what are the evolutionary affinities of Echthrodesis? Is it related to the genera in the Baeus clade or the Embidobia–Mirobaeoides–Neobaeus clade, or does it represent a third independent lineage of platygastroids that exploits spider eggs as hosts?
Here we use sequence data from three genes, the nuclear 28S rRNA and 18S rDNA genes, and the mitochondrial cytochrome oxidase 1 (CO1) gene, to determine the affinities of E. lamorali with other lineages of playgastroid wasps. We also present a revised taxonomy for the genus and species, new comparative morphological information on the structure of its mouthparts and ovipositor system, and new field observations of its behaviour and biology in association with one of its host intertidal spiders, D. formidabilis, on the Cape Peninsula (South Africa).
Materials and methods
Field collections, observations and imaging
Egg sacs of Desis formidabilis were collected from the type locality of E. lamorali, ‘The Island’ at Kommetjie, 26 km south of Cape Town on the Atlantic side of the Cape Peninsula (34°8′24.33″S, 18°19′15.22″E) during July and August 2009 and May and August 2011. Visual searching in perceived optimal areas of the intertidal zone located spider colonies inhabiting limpet shells secured to the undersides of rocks or lodged in rocky crevices. Emergence and subsequent behaviour of E. lamorali was observed under a Wild M5A dissecting microscope in the laboratory. Photography of living specimens was undertaken with a Nikon D80 and a Nikkor 105-mm macro lens. Images of live and preserved specimens were acquired using the EntoVision multiple-focus imaging system. This system comprises a Leica M16 microscope with an attached JVC KY-75U 3-CCD digital video camera. Cartograph 5.6.0 was used to merge an image series into a single in-focus image. Lighting was achieved using techniques summarised in Buffington et al. (2005), Buffington and Gates (2009) and Kerr et al. (2009). Images included in this paper are available on WaspWeb (http://www.waspweb.org), and are archived at Morphbank (http://www.morphbank.net) and in Specimage (http://specimage.osu.edu) (the image database at The Ohio State University).
Specimen preparation
For mouthpart and genitalic dissections, the head and metasoma from both male and female specimens were boiled in 10% lactophenol for 30 min, followed by a distilled-water wash, and then dissected using tweezers (Dumont no. 5) and a no. 2 entomological pin with a sharp tip. The maxillo-labial complex, male aedeagus, female ovipositor and terminal tergites and sternites were extracted. These structures were placed in a micro-concavity slide in 10% NaOH for 30 min, followed by glacial acetic acid for 10 min (maxillo-labial complex, ovipositor and aedeagus) or 30 min (terminal tergites and sternites). Structures were transferred to 70% ethanol (30 min) followed by 96% ethanol (30 min) and clove oil (30 min), then mounted in Canada Balsam. Structures used for SEM examination were dried using hexamethyldisilazane. Microscopic slides were examined using a Euromex GE 3045 microscope and drawings were made using a Reichart drawing tube.
Terms and abbreviations
Morphological terminology generally follows Masner (1980) and Mikó et al. (2007), with terms for ovipositor structures following Austin and Field (1997), those for the maxillo-labial complex as labelled in Fig. 1, and those for male genitalia after Johnson (1984). Standard abbreviations are used for the following morphological features: antennomeres (A1, A2, etc); metasomal tergites (T1, T2, etc); metasomal sternites (S1, S2, etc).

|
Anatomical terms used in the descriptions are linked to concepts in the Hymenoptera Anatomy Ontology (HAO; Yoder et al. 2010, Hymenoptera Anatomy Portal (http://portal.hymao.org, accessed on 25 March 2010)) (Table S1, available in the Supplementary Material). Identifiers in the format HAO_XXXXXXX represent concepts in the HAO and are provided to enable readers to confirm their understanding of the concepts being referenced. The identifier can also be used as a URI (universal resource identifier) by appending the identifier to ‘http://purl.obolibrary.org/obo/’ (e.g. http://purl.obolibrary.org/obo/HAO_0000124). URLs in the format http://purl.org/net/hao/HAO_0123456 resolve to the HAO’s community-based resource that includes additional images, notes and other metadata.
The external hyperlinks are explicitly cited in the endnotes so that users of the printed version of the paper have access to the same resources, insofar as the external information conforms to standards developed and maintained through the organisation Biodiversity Information Standards (Taxonomic Database Working Group). Taxonomic names, where appropriate, have been registered with Zoobank (http://www.zoobank.org).
The following abbreviations are used for collections: Canadian National Collection of Insects, Ottawa (CNCI); C.A. Triplehorn Insect Collection, Ohio State University, Columbus (OSUC); Iziko South African Museum (SAMC); KwaZulu–Natal Museum, Pietermaritzburg (NMSA); Waite Insect and Nematode Collection, University of Adelaide, Adelaide (WINC).
Project information
This work is a product of the Platygastroidea Planetary Biodiversity Inventory, funded by the USA National Science Foundation (N.F. Johnson and A.D. Austin). One of the primary objectives of this project is to use biodiversity informatics tools to accelerate the taxonomic process and to make real-time collaboration possible within the community of researchers with appropriate expertise. Details on the data associated with specimens can be accessed at the following link: hol.osu.edu, and entering the identifier (e.g. OSUC 497717) in the form [urn:lsid:biosci.ohio-state.edu:osuc_occurrences:[specimen ID] where the spaces in the specimen ID are replaced by two underscores (__), i.e. urn:lsid:biosci.ohio-state.edu:osuc_occurrences:OSUC__497717.]
Taxon sampling and design of the phylogenetic analyses
To determine the likely relationships of Echthrodesis, two analyses were undertaken. In analysis 1, sequence data for the CO1 and 28S rDNA genes were employed for a comprehensive sampling of 22 species (representing seven genera and including E. lamorali) of platygastroids known to parasitise spider eggs, plus an additional 10 playgastroid species representing 10 genera associated with a range of insect hosts (Table 1). Of the 22 species that parasitise spider eggs, nine were newly sequenced while the remaining came from either Carey et al. (2006) or Murphy et al. (2007) (Table 1). Two platygastrids, Piestopleura sp. and Synopeas sp., were used as outgroups based on the results of Murphy et al. (2007).

|
To further test the relationships of Echthrodesis, the Bayesian analysis presented in Murphy et al. (2007) was repeated with sequences of Echthrodesis added to the dataset (Analysis 2). This analysis employed sequence data for three genes (CO1, 28S rDNA, 18S rDNA; see below for details) and had the advantage of placing Echthrodesis among a far greater sampling of taxa across the whole superfamily (86 species representing 59 genera). However, this analysis included only six taxa (including Echthrodesis) known to parasitise spider eggs.
DNA extraction, amplification and sequencing
Specimens were processed in the Austin laboratory at The University of Adelaide (UA specimen codes in Table 1) or in the Johnson laboratory at Ohio State University (OSUC specimen codes in Table 1). For the UA specimens, genomic DNA was extracted from ethanol-preserved tissue using the Puregene® DNA Purification Kit (Gentra Systems Inc.). For the OSUC material, non-destructive DNA extraction was performed using the DNeasy extraction protocol (Qiagen Inc., cat. no. 69506) as modified by C. D. Zhu and J. S. Noyes (unpubl. data): in brief, individual specimens were initially softened in 70% ethanol at room temperature for 2 h; vortexing in step 2 of the published protocol was modified by mixing the reaction gently and incubating at 55°C for 24 h with 40 µL of proteinase K; the mixture was then stored at −20°C for 24 h; the intact specimen was then removed from the tube and prepared for standard mounting; the reaction was incubated for 10 min at 70°C after addition of Buffer AL; then 200 µL of cold ethanol (96–100%) was added to the supernatant; finally, the final suspension of DNA from the column membrane (Step 7) was performed with two washes of Buffer AE previously warmed to 55–70°C.
Partial sequences were obtained from a ~600 base pairs (bp) product obtained by PCR amplification of the mitochondrial protein coding CO1 gene using the primers C1-J-1718 and C1-N-2329 (Simon et al. 1994). PCR amplification of an ~800 bp segment of the nuclear 28S rDNA gene, spanning the D2 and D3 segments, was implemented using the primers D2-3665 F and D3-4413 R (Gillespie et al., 2005). Amplification of an ~800 bp segment of the nuclear 18S rDNA gene for E. lamorali only was implemented using the primers 18Sai and 18Sbi (Whiting et al. 1997).
PCR amplifications were carried out in 25 μL containing PCR buffer, 0.2 mm of each dNTP, 0.4 μM of each primer, 2 mm MgCl2, 0.5 units of AmpliTaq Gold® DNA Polymerase (Applied Biosystems Inc.) and 25–100 ng of genomic DNA. Thermocycling conditions were: an initial denaturation step of 95°C for 5 min, followed by 35 cycles of 95°C for 30 s, an annealing temperature of 50°C for 30s, and an extension temperature of 72°C for 30 s. This was then followed by an additional extension of 72°C for 3 min. PCR products were purified using the Ultraclean™ PCR Clean-up™ Kit (MOBIO Laboratories Inc.). Sequencing reactions were performed using ABI Big Dye Terminator Chemistry and fragments were resolved on an ABI 3700 sequencer.
Sequence alignment and phylogenetic reconstruction
Sequence alignment of the three genes was undertaken following Murphy et al. (2007). The CO1 gene was aligned by eye, as few insertion/deletion events were present. The amino acid sequence was translated as a test for the presence of nuclear paralogues, e.g. stop codons. Alignment of both nuclear RNA genes (28S and 18S rRNA) was undertaken using Clustal X (Thompson et al. 1997) and employing several gap opening/gap extension schemes (gap to change costs 1 : 1 1 : 2 1 : 5 1:10). Regions of uncertain alignment that varied markedly between different alignment schemes were deleted (Gatesy et al. 1993), and a single alignment of each gene was used for subsequent analyses.
Maximum parsimony (MP) analyses were performed in PAUP* 4.0b10 (Swofford 2002) using the heuristic search algorithm with 100 random sequence addition replicates to help eliminate bias from taxon ordering in the datasets. Gaps were treated as missing data and characters were weighted equally. Confidence in the MP trees was assessed from 1000 non-parametric bootstrap pseudo-replications.
Bayesian phylogenetic analyses were performed for each gene alignment separately and for concatenated data using MrBayes 3.1.2 (Huelsenbeck and Ronquist 2001). The appropriate model of evolution was chosen by the Akaike information criterion using Modeltest (Posada and Crandall 1998) on all data partitions separately (28S rRNA, 18S rRNA and the separate codon positions of CO1). The optimal partitioning strategy was examined using Bayes factor analysis (Brandley et al. 2005) comparing the harmonic means of the log-likelihoods of different possible partition combinations, for example: no partitions; three partitions (18S + 28S + CO1); five partitions (18S + 28S + CO1 1st codon pos + CO1 2nd codon pos + CO1 3rd codon pos). The optimal partitioning chosen for ‘Analysis 1’ was the partitioning of 28S + CO1 1st codon pos + CO1 2nd codon pos (with 3rd codon pos removed), whilst the partitioning for ‘Analysis 2’ was similar, with the addition of an 18S partition. MrBayes analyses were run across four chains for five million generations sampling every 100 generations, and stationarity was determined from an examination of log-likelihoods and model parameters. Trees recovered before stationarity were discarded and Bayesian posterior probabilities of each bipartition, representing the percentage of times each node was recovered, were calculated from the remaining trees. Multiple runs were performed to assess that parameters were not considerably different at stationarity based on alternate prior probabilities.
Molecular phylogenetic results
Analysis 1
Sequences for CO1 and 28S rDNA were generated for all taxa. The CO1 alignment consisted of 538 bp and contained 233 parsimony-informative sites. Mirobaeoides contained an insertion of one codon but all other taxa had uniform sequence lengths. The 28S rRNA alignment consisted of 720 bp and had 288 parsimony-informative sites. A comparison of the topologies and Bayesian support between the gene regions suggested no major phylogenetic incongruence (trees not shown). Given this, the sequence data for the two genes were concatenated for further analysis.
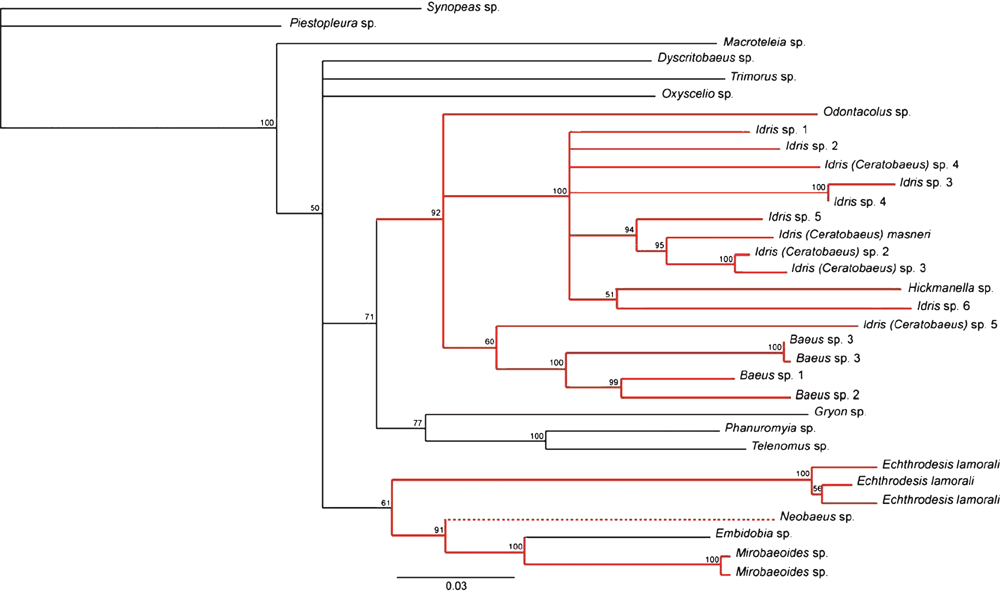
The 1258 bp combined CO1 and 28S rRNA alignment had 521 parsimony-informative sites when the ambiguous regions of 28S rRNA and the third codon position of CO1 were excluded. The Bayesian tree for the concatenated data (Fig. 2) resolves two separate clades associated with parasitising spider eggs; one comprising the genera Odontacolus, Idris, Hickmanella and Baeus with high Bayesian support (92%), and a second clade comprising Echthrodesis, Neobaeus and Mirobaeoides with low Bayesian support (61%). Embidobia is contained within the latter clade and is sister to Mirobaeoides (100%). The MP tree (not shown) was generally less resolved compared with the Bayesian tree but the general pattern was the same with regard to resolving the same two separate spider-egg parasitising clades and the position of Echthrodesis and Embidobia.
|
Analysis 2
The Bayesian re-analysis of the Murphy et al. (2007) three-gene dataset with the inclusion of E. lamorali estimated an almost identical set of relationships as the original study (see Supplementary Fig. 1). As in analysis 1, two independent clades associated with spider eggs were resolved, with Echthrodesis being placed in the second clade as sister to Mirobaeoides (Bayesian support 96%), and Embidobia sister to these two genera (67%). Neobaeus was not included in this re-analysis of the 2007 data.
Biology and behaviour
Host spider
Desis formidabilis (Fig. 3B–D) inhabits the bolder-strewn intertidal zone (Fig. 3A) and lives inside limpet shells, sometimes communally, with up to five (but more commonly one or two) individuals inhabiting a single shell. Eggs are laid within a silken compartmentalised wedge-shaped structure that lines the inside edge of the shell (Fig. 3A, B). Each compartment comprises a purse-like structure that is sealed from other compartments, but not all of these contain eggs. Each egg sac within a shell consists of five or six compartments, each containing different stages of development from newly oviposited eggs (Fig. 4B), through spider embryos to recently emerged spiderlings (1st instar) and older spiders (2nd and, possibly, 3rd instars), which stay inside the sealed compartment. Spider egg batches in individual compartments comprise 8–19 eggs.
Wasp development and emergence
A single wasp develops and pupates within each host egg, as is the norm for platygastroids (Austin et al. 2005), although there are some exceptions such as Platygaster zosine Walker, which is polyembryonic (Austin et al. 2005), and Telenomus monilicornis Ashmead, T. dendrolimi Matsumura and T. fariai Lima, which are gregarious (5–10 wasps emerging from one host egg) (Johnson 1984). During wasp development, the off-white, circular host eggs change shape and colour such that just before wasp emergence they have become more ovoid, brown in colour and papery. Adult wasps chew their way out of their host egg, with males usually emerging slightly before females.
Mating
Male wasps actively ‘wrestle’ each other for an opportunity to mate with a female. As many as three males may be involved in a tussle over a female that is chewing her way out of her host egg. The male actively antennates the female on her head and mesosoma and touches her with his mandibles, appearing to almost bite her behind the head. The male straddles the front portion of the female that is protruding from the host egg and may help to chew the exit hole allowing the female to escape. Once she is free, the male mounts her from behind, antennating and appearing to bite her head and pronotum. The latter action may involve mandibular gland secretion. Soon after, the male moves backwards such that his metasoma is overlapping the female’s metasoma. He may briefly clean his aedeagus with his back legs before inserting it into the female in a downwards and forward movement of the metasoma. During copulation the male clasps the female’s metasoma with his fore and mid legs. The act of copulation lasts for 7–9 s, after which the male immediately dismounts and loses interest in the female. Other males may subsequently attempt to copulate with her, but have not been observed to be successful. Males often mount other males, straddling them from behind and repeatedly antennating as they do with females, occasionally attempting to insert their aedeagus.
Oviposition
Wasps chew a small circular exit hole in the silken egg sac to escape, with often more than one exit hole being produced (Fig. 4A), sometimes adjacent to each other. Female wasps proceed to search for other egg sac compartments within the same limpet-shell retreat. They chew a circular entrance hole, just large enough for them to fit through, in the side wall of a compartment containing early-stage host eggs. Once inside the compartment, the female antennates the surface of the eggs (Fig. 4B) and, if the eggs are acceptable, proceeds to oviposit into each egg individually (Fig. 4C, D). Up to three females have been observed ovipositing inside a single egg compartment, with each having chewed its own entrance hole in the side of the silken sac. Host-egg mortality within a parasitised egg batch was 100% (n = 7). The sex ratio of hatching wasps is skewed, being highly female-biased with only 15 males reared from 118 parasitised eggs.
Taxonomy
Echthrodesis Masner
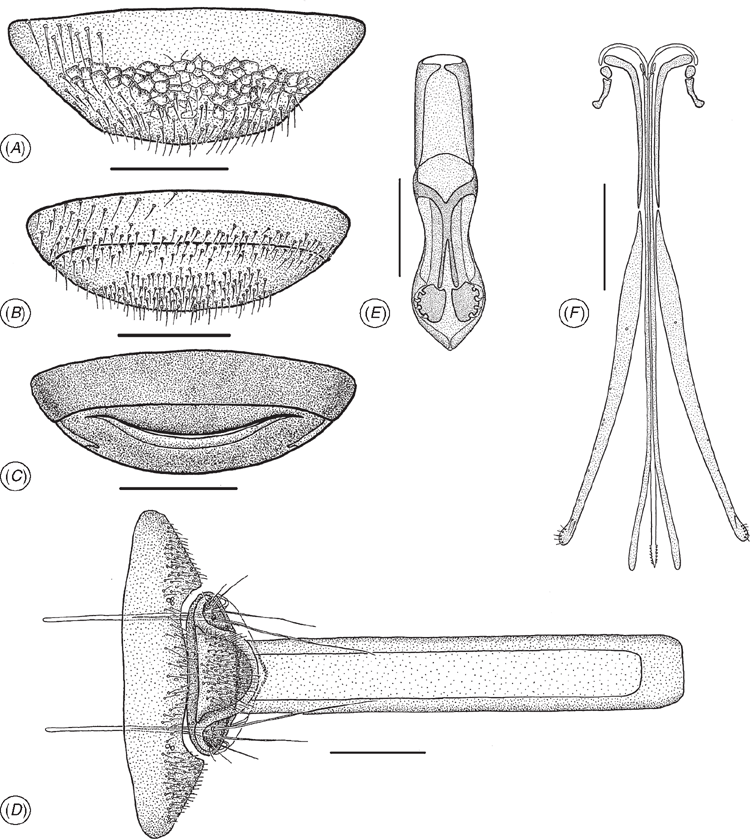
(Figs 1, 5A–D, 6A–D, 7C, 8A–F, 9D–I)

|

|

|
Diagnosis
Among genera in the tribes Embidobiini and Baeini, Echthrodesis shares with Endecascelio a labial palp comprising two sclerites and the presence of a conspicuous principal carina on the stipes that is distolaterally angled. Echthrodesis is easily separated from Endecascelio and all genera in the Embidobiini and Baeini by the dense pilosity of its body (although Neobaeus is moderately hairy on the dorsal surface). It differs from Neobaeus and Baeus in having narrow laterotergites attached to the sternites (rather than wide and free laterotergites). In addition, Echthrodesis differs from Neobaeus, Mirobaeus and Mirobaeoides by having a rounded (rather than a carinate) vertex and hirsute eyes, and from Embidobia by the 4–2 palpal formula, having the metasoma broadly and closely abutted to the mesosoma (i.e. sessile in female, sub-sessile in male), and a basally narrowed and longitudinally costate T1. Like Baeus, Neobaeus and Mirobaeoides, Echthrodesis has T2 of the metasoma as the longest tergite, whereas in Mirobaeus and virtually all baeine genera T3 is the longest (N.B. in Baeus and Mirobaeoides T1 is often vertical and hidden by the posterior mesosoma so that the first visible tergite ( = T2) appears to be the longest and should not be mistaken for T1).
Description
Head. Occipital carina complete. Frons flat to weakly convex. Ocular ocellar line (the shortest distance between the inner orbit of the eye and the outer margin of the lateral ocellus; OOL) > ocellar diameter. Submedian carina on frons absent. Orbital carina on frons absent. Central keel on frons present, short. Lower portion of central keel simple. Torulus orientated anterolaterally to lateral. Fan-like striae arising from anterior mandibular articulation. Malar sulcus present. Setation of compound eye present, very short. Genae densely setose.
Mouthparts (Fig. 1). Labrum internal, hidden beneath clypeus. Mandibles tridentate. Mandibular dentition transversely orientated. Maxillo-labial complex (MLC): maxillae (mx) comprising a well developed cardo (cd), more weakly sclerotised than stipes (st). Shape of cardo: distance between proximal lateral projection (plpc) and distal mesal projection (dmpc) almost equal to distance between proximal mesal projection (pmpc) and distal lateral projection (dlpc). Posterior stipital sclerite (pss) massive, well developed, with median or subapical area broader than basal area. Posterior stipital sclerite devoid of sensilla, except for one or two mechano-sensory hairs (msh), situated near point of insertion of lacinial lever (lacl). Proximomedial stipital flange (pmf) present. Distomedial stipital flange (dmf) present. Principal carina of stipes distolaterally angled (lsh). Ventral dististipital process (vdp) conspicuous. Segmentation of maxillary palps (mpalp) heteronomous. All four sclerites of the maxillary palp with mesal and lateral sides approximately symmetrical, 4th sclerite the longest, ~2.8–3 × as long as wide. Maxillary palpal sclerite sensilla: 1st sclerite (1mp) hairless, with apical large campaniform sensillum (csens); 2nd sclerite (2mp) almost glabrous with 1 or 2 trichoid sensilla in apical half; 3rd sclerite (3mp) covered with relatively short trichoid sensilla, distributed in 1 or 2 transverse rows; 4th sclerite (4mp) covered with numerous trichoid sensilla; apex with two strong trichoid sensilla. Lacinia (lac) concealed. Velum (vlm) with fringed distal edge. Lateral side of galeo-lacinial complex with same degree of sclerotisation as rest of galeo-lacinial complex. Basal galeal sclerite (bgs) postioned sub-apically on galeo-lacinial complex, surrounding lateral side of proximolateral galeal sclerite (pgs). Basal galeal sclerite (bgs) with straight internal edge. Dorsal lobe of basal galeal sclerite with one or two hairs. Proximolateral galeal sclerite (pgs) glabrous. Distal end of proximolateral galeal sclerite (pgs) with two or more setae in one row. Proximolateral (pgs) and distolateral galeal sclerites (dgs) separated by a membranous structure. Distolateral galeal sclerite with setae in a single row. Distolateral galeal sclerite well developed, broader than high. Galeal comb (gc) present. Galeal coeloconicum sensillum (cfs) present on distolateral galeal sclerite (dgs). Postmentum (psm) of normal size. Lateral faces of prementum not continuous posteriorly. Premental carina (premc) visible as a narrow stripe on lateral sides of ventral premental area (vprem). Ventral, premental area (vprem) diamond-shaped. Two symmetrical campaniform premental sensilla (csens) under distolateral premental incisions (dlpi). Trichoid sensilla on ventral premental area absent. Distolateral premental incisions conspicuous. Labial palp (lpalp) present. Segmentation of labial palps heteronomous. Labial palp comprising two sclerites, first sclerite obviously longer than second. Mechano-sensory hairs (msh) on proximal part of 1st sclerite of labial palp (1lp) present. Glossa dorsally with basiconic sensilla (gbs). Ventral side of glossa (gls) glabrous. Glossal styloconic sensilla absent. Four glossal basiconic sensilla (gbs).
Antenna. 11 female antennomeres. Radicle positioned parallel to A1. A1 more-or-less cylindrical. A3 of female distinctly shorter than A2. Apical antennomeres of female widened to form antennal clava. Claval formula: 1-2-2-1. 12 male antennomeres. Fifth male antennomere bearing tyloids.
Mesosoma. Transverse pronotal carina absent. Posterolateral edge of pronotum in dorsal view bifid, fitting around tegula. Vertical epomial carina absent. Netrion absent. Flexion of anterior margin of mesoscutum absent, mesoscutum abutting pronotum anteriorly. Skaphion absent. Notauli absent. Mesoscutellum semielliptical, strongly transverse. Apical spines of mesoscutellum absent. Metascutellum absent. Propodeal metasomal depression extending anteriorly to abut metanotum. Lateral propodeal projection absent. Mesopleural pit absent. Posterodorsal margin of mesopleuron rounded. Mesosomal foamy plates absent. Single mid and hind tibial spur. Pretarsal claws with laminate ridges basally. Tegula present. Female apterous; male micropterous with no tubular veins.
Metasoma. Female with 7 terga and 6 sterna. Male with 8 terga and 7 sterna. Metasoma sessile, closely abutting mesosoma. Laterotergites present and narrow. Laterosternites present. T2 slightly longest, T1 and T3 subequal in length. Dorsal setal fields absent. Basal crenulae absent. Apical margin of apical tergum of male evenly arcuate. Anterior margin of S1 straight to weakly concave. Felt fields absent. Apical sternite of male strongly broadened, width 4 × length. Transverse apodeme present in middle of apical sternite of male. Medial longitudinal apodeme of apical sternite of male absent. Apical sternite of female 2.6 × wider than long. Medial longitudinal apodeme of apical female sternite absent. Cerci flat, with 4–5 pairs of bristles, 2 pairs elongate, 2.6 × longer than T6.
Male genitalia. Basal ring aedeagus 0.33 × total aedeagal length, 0.5 × length of aedeagovolsellar shaft. Aedeagal apodemes merged in basal half, distinct apically. Digitus volsellaris apically with four concavities in a single row, three with short digital teeth.
Female ovipositor system. Ceratobaeus-type. Ovipositor elongate, 0.65–0.70 × length of metasoma. Proximal arms slender, short, 0.05 × ovipositor length. Gonoplacs elongate, 0.7 × ovipositor length. Second gonocoxa 0.5 × gonoplac length. Proximal part of ventral membranous plate present. Tubular A9 nearly 3 × combined length of T6–T8. Lateral apodemes on T7+T8 short, less than 3 × length of T6.
Morphological comparison of specific characters
There are several characters that are likely to be informative for inferring phylogenetic relationships among tribes and genera of platygastroid wasps (Austin and Field 1997), but these still need assessment within the framework of a comprehensive phylogenetic analysis. The following character systems are likely to be informative.
Female ovipositor system
The most striking difference between Echthrodesis and other genera comprising the Embidobiini and Baeini is the absence of a medial apodeme on the apical sternite (Fig. 8A) and, to a lesser degree, the shortened lateral apodemes (Fig. 8D). The absence of a sternal medial apodeme is known for only nine genera of platygastroids. Other characters, such as the length of the proximal arms, gonoplacs and tubular A9 (Fig. 8D, F) all need to be assessed across a wider array of taxa as they are likely to show some intergeneric variation, as is known to occur among Idris spp. (A. D. Austin, unpubl. data).
Mouthparts
The maxillo-labial complex (MLC) of Echthrodesis is strikingly different to that of other members of the Baeini and Embidobiini (Fig. 7A–F), in part because of the palpal formula (4 : 2 in Echthrodesis; 3 : 2 in Endecascelio; 2 : 1 in Embidobia, Mirobaeoides, Mirobaeus, Idris, Embioctonus and Palaeogryon; 1 : 1 in Baeus). Although labial palps have one sclerite in Embidobia, Mirobaeoides, Mirobaeus, Idris and Baeus, these genera can be divided into two groups on the basis of the shape/development of the labial palps: Baeus and Idris have the labial palps reduced/atrophied and without a mechano-sensory hair on their proximal part, while Embidobia, Mirobaeoides and Mirobaeus have long labial palps and a mechano-sensory hair present on the proximal part. A mechano-sensory hair on the proximal part of the first sclerite of the labial palp also occurs in Echthrodesis and suggests the possibility of movement of the labial palps. Its absence may be correlated with a reduction of the length of labial palps and very probably a reduction in movement of this sensory structure. The distal edge of the velum also differs among these genera; it is fringed in Echthrodesis, Embidobia, Mirobaeoides and Mirobaeus, but a fringe is lacking in Idris and Baeus. The postmentum is visible in Echthrodesis, Embidobia and Idris, but is not visible and is probably absent in Mirobaeus, Mirobaeoides and Baeus. Characteristic for all these genera is the absence of trichoid premental sensilla. In Echthrodesis and Idris, the prementum has two symmetrically campaniform sensilla under the distolateral premental incision, while in Embidobia, Mirobaeus, Mirobaeoides and Baeus these sensilla are absent. The glossa has three basiconic sensilla in Mirobaeus and Mirobaeoides, and four in Echthrodesis, Embidobia, Idris and Baeus.
Other potentially useful characters
Most notable are the form of the tarsi and tarsal claws (Fig. 9 comparing Baeus and Echthrodesis) and the male genitalia (Fig. 8E). However, the morphology of these characters has been documented for very few genera, and it will be important to first assess interspecific differences, as is known for the male genitalia of Telenomus (Johnson 1984), before their importance at generic level can be assessed.
Echthrodesis lamorali Masner
Material examined
Description (modified from Masner 1968)
Body length. 1.1–1.5 mm.
Colour. Generally dark brown to black. Antennae, mandibles and femora dark brown. Tarsi and tibiae yellow–brown. Ventral metasoma chestnut brown. Silver pilosity covering body.
Head. Head wider than long (28 : 19). Head 1.22 × wider than mesosoma. Sculpturing scaly–reticulate except for smooth patch around short keel above antennal insertions.
Mesosoma. With same scaly–reticulate sculpturing as head. Shorter than long (16 : 22). Mesoscutellum narrow, length to width (3 : 20). Mesopleuron and metapleuron surface bare and shining.
Metasoma. Moderately broad, 1.67 × longer than wide. Dorsal sculpturing same as on the head and mesosoma, but reticulation more prominent on first three tergites. T1 length to width (11 : 28). T2 length to width (15 : 30). T3 length to width (10 : 28). T4-T6 progressively shorter than T3.
Distribution
Only known from the type locality at Kommetjie, Cape Peninsula, South Africa.
Hosts
Reared from eggs of the intertidal spiders Desis formidabilis (Desidae) and Amaurobioides africanus (Anyphaenidae). These two spider species commonly commandeer old limpet shells and secure these to rocks or other shells trapped in the boulder-strewn intertidal zone. They exhibit ecological zonation, with D. formidabilis inhabiting the lower intertidal zone while A. africanus inhabits the upper intertidal zone (Lamoral 1968; Fig. 3A).
Discussion
Evolutionary relationships
The previously published molecular studies on platygastroid relationships (Carey et al. 2006; Murphy et al. 2007) and the new analyses presented here indicate that there are at least two independent clades of platygastroid wasps that exploit spider eggs as hosts. The first clade is by far the most species rich as Idris s.l. comprises many hundreds of undescribed species, while the genera in the second clade comprises no more than 50 or so species (A. D. Austin, unpubl. data). Echthrodesis belongs to the smaller of the two clades that parasitise spider eggs, suggesting a common origin of association with silk-encased host eggs for Mirobaeoides, Neobaeus and Echthrodesis. The position of Embidobia nested with this clade suggests a potential host-switching event from spider to embiid eggs, lending some credence to the hypothesis that searching for silk as a host substrate may have facilitated this switch (Galloway and Austin 1984). However, the sister genus to Echthrodesis will remain elusive until material becomes available for sequencing of several rare genera that putatively belong to this clade, i.e. Endecascelio, Palaeogryon and Embioctonus. Similarly, sequence data are also required for the micropterous genus Mirobaeus, which has variously been placed in the Embidoniini (Masner and Dessart 1972; Masner 1976) or the Baeini s.l. (Austin 1988a; Austin and Field 1997).
A comprehensive phylogenetic analysis of morphological data for platygastroid genera is yet to be undertaken, so independent support for the placement of Echthrodesis in a clade with Neobaeus, Mirabaoides and Embidobia is not available. However, such a treatment employing morphology is fraught with the complexity of assessing the degree and extent of homoplasy, given that many genera in the Baeini and Embidobiini display loss or reduction of characters (Austin et al. 2005). Underlying historical exposure to similar ecological and environmental parameters has also driven widespread convergent evolution in body form across a diverse range of platygastroid genera, and these will cloud any assessment of phylogenetic relationships based on morphology. Reduction or loss of wings and evolution of a fusiform body shape have clearly arisen independently several times in different lineages of platygastroid wasps (Austin et al. 2005). This is well illustrated by the polyphyly of the genera that parasitise spider eggs, with two clades having independently evolved similar morphologies that appear to facilitate their parasitism of silk-encased eggs (Murphy et al. 2007). A fusiform body, where the metasoma is broadly abutted to the mesosoma, is commonly associated with cryptic habitats such as leaf litter dwelling and is evident in other unrelated lineages, e.g. Parabaeus Kieffer (Sceliotrachelinae), Platyscelidris Szabó and Encyrtoscelio Dodd (Scelioninae) (Austin et al. 2005).
Mouthparts and ovipositor system
Associated with the mouthparts, the maxillary and labial palpal formula also displays likely convergent reduction in segmentation, confounding character polarity assessment. The tribe Embidobiini is characterised by a large range in the palpal formula, with Echthrodesis having a palpal formula of 4 : 2, whereas most members of the Baeini and Embidobiini possess a palpal formula of 2 : 1, except Endecascelio where it is 3 : 2 and Baeus where it is reduced to 1 : 1.
The absence of a median apodeme in the last metasomal sternite (Fig. 8A) is characteristic of a generic group with a Scelio-type ovipositor, the only known exception being Aradophagus (Austin and Field 1997). Among the genera with a Ceratobaeus-type ovipositor, only eight genera other than Echthrodesis are characterised by a lack of the median apodeme in the last metasomal sternite: Nixonia Masner, Sparasion Latreille, Sceliomorpha Ashmead, Habroteleia Kieffer, Fusicornia Risbec, Mantibaria Kirby, Neuroscelio Dodd and Teleas Latreille (Austin and Field 1997), all of which are only distantly related to Echthrodesis. The median apodeme is one of three points for the attachment of the muscles responsible for the extension and retraction of the ovipositor from the metasoma (Austin and Field 1997). The absence of this apodeme means that ovipositor movement must be achieved in these genera only by the muscles attached to the paired lateral apodemes. The complex configuration of the 2nd gonapophyses assembly present in Echthrodesis is characteristic of genera possessing the Scelio-type ovipositor system, with the exception of Doddiella Kieffer (Austin and Field 1997). The majority of genera possessing the Ceratobaeus-type ovipositor system have the second gonapophyses assembly simple, with the exception of Nixonia, Sparasion, Sceliomorpha, Fusicornia, Echthrodesis, Anteris Foerster, Opisthacantha Ashmead and Parascelio Dodd. In the first five of these genera, the presence of a complex 2nd gonapophyses assembly is associated with absence of the medial apodeme, although the significance of this is unclear.
Morphological adaptations
A diverse range of morphological and physiological adaptations have evolved in adult insects to successfully colonise aquatic environments. These include, for example, reduction or loss of wings (Nondula et al. 2004) and the development of breathing siphons and various kinds of physical gills (Hinton 1976); the latter being present in D. fomidibalis (Lamoral 1968). However, very few parasitic Hymenoptera are fully aquatic and able to immerse completely to reach submerged hosts. Some notable exceptions are the platygastroid genera Tiphodytes Bradley (Spence 1986) and Thoron Haliday (Masner 1972; Johnson and Masner 2004) that parasitise the eggs of Gerridae and Nepidae respectively, the mymarid Caraphractus cinctus Walker that parasitises the eggs of Dytiscidae (Jackson 1966), and the ichneumonid Agriotypus gracilis Waterson that is ectoparasitic on caddisfly larvae (Aoyagi and Ishii 1991).
In the case of Echthrodesis, a likely morphological adaptation includes a covering of dense setae over the body, hypothesised to form a thin plastron of air on submergence (Masner 1968). However, E. lamorali might rarely or never be physically immersed in water due to the air trapped within the egg sac and spider retreat inside limpet shells, which might provide sufficient air during submersion at high tide. Spider egg sacs and silk retreats play a critical role in preventing desiccation of eggs and juvenile spiders (Austin 1985, 1988b; Hieber 1992) and, hence, are likely to be sufficiently hydrophobic to keep water out. With the asynchronous development of egg clutches within a single egg sac, Echthrodesis can complete its life cycle protected inside a single spider retreat and only needs to expose itself to the external environment when it needs to locate new hosts. The host spider population is localised at Kommetjie, Cape Peninsula, and occurs in reasonably high densities such that dispersal to reach new host egg sacs might require movement over only tens of centimetres. Dispersal is likely to occur under favourable conditions at low tide, with the wasps being capable of jumping a short distance (a centimetre or so) when disturbed.
Wing reduction and a fusiform body is likely to facilitate entry to silken egg sacs, as proposed for Baeus and Mirobaeoides (Austin 1986; Stevens and Austin 2007), as well as being an adaptation in some genera to burrowing through litter or soil (Austin et al. 2005). The platygastroid genera that parasitise spider eggs exhibit different oviposition strategies, with some ovipositing from the outside of the egg sac (e.g. Idris, Ceratobaeus), whereas others burrow through the egg sac walls to reach the eggs within (e.g. Baeus, Echthrodesis, Mirobaeoides). Some of the latter possess highly modified tarsal claws that facilitate access through the sticky silk (Austin 1985). Males of Echthrodesis have the specialised body form of females with extremely reduced wings (although they are larger than in the female), a sub-fusiform body and sub-sessile metasoma. The extreme microptery of males and females of E. lamorali is, in addition to facilitating host egg sac silk circumvention in females, probably selectively driven by the need to stay in the localised area where the spider population is concentrated. Wings would be a distinct disadvantage, increasing the chance of being blown out to sea on the wind-swept Cape Peninsula. Such lack of sexual dimorphism, where males have the same modified body form as females, is associated with the genetic expression of effective winglessness in platygastroid wasps restricted to precarious habitats such as small oceanic islands, e.g. the baeine Mirabaeoides pecki (Austin), an endemic to Lord Howe Island (Austin 1988a, 1995).
Biology
The general biology and behaviour of Echthrodesis is similar to that documented for other platygastroids that parasitise spider eggs (Vachon 1955; Valerio 1971; Bradoo 1972; Austin 1984c; Cobb and Cobb 2004). However, some aspects of the biology of Echthrodesis appear to be unique. Since the parasitism rate observed was 100% in each egg batch, it seems likely that once a fecund female has gained access to a sealed egg compartment, she has sufficient eggs to lay in all the host eggs present. Complete mortality of spider eggs has previously not been documented for platygastroids (e.g. Austin 1984c, 1988b) and might be a function of Echthrodesis having several clutches with a small number of eggs. The highly biased female sex ratio suggests local mate competition and high inbreeding, which is not uncommon among platygastroid wasps (Austin et al. 2005). Thus, the multiple females attacking eggs in a chamber might all be sisters. Further investigations are required to establish the degree and nature of competition for oviposition resources and whether a female wasp can discern if an egg has been previously parasitised or not, as only a single parasitoid develops in each spider egg. Within an egg sac there are several empty compartments that are sealed and these might act to mislead this parasitoid.
Acknowledgements
Specimens were collected under permission granted by Cape Nature to SvN (permit numbers: AAA004-00092-0035 and AAA007-00324-0035). We thank István Miko (Pennsylvania State University) for formulating the URI table linking anatomical terms used in the descriptions to concepts in the Hymenoptera Anatomy Ontology project. This material is based upon work supported by the National Science Foundation under grant No. DEB-0614764 to NFJ and ADA, and South African National Research Foundation grants GUN 61497 and GUN 79004 to SvN.
References
Aoyagi, M., and Ishii, M. (1991). Host acceptance behavior of the aquatic wasp, Agriotypus gracilis (Hymenoptera: Ichneumonidae) toward the caddisfly host Goera japonica (Trichoptera: Limnephilidae). Journal of Ethology 9, 113–119.| Host acceptance behavior of the aquatic wasp, Agriotypus gracilis (Hymenoptera: Ichneumonidae) toward the caddisfly host Goera japonica (Trichoptera: Limnephilidae).Crossref | GoogleScholarGoogle Scholar |
Austin, A. D. (1984c). The fecundity, development and host relationship of Ceratobaeus spp. (Hymenoptera, Scelionidae), parasites of spider eggs. Ecological Entomology 9, 125–138.
| The fecundity, development and host relationship of Ceratobaeus spp. (Hymenoptera, Scelionidae), parasites of spider eggs.Crossref | GoogleScholarGoogle Scholar |
Austin, A. D. (1985). The function of spider egg sacs in relation to parasitoids and predators, with special reference to the Australian fauna. Journal of Natural History 19, 359–376.
| The function of spider egg sacs in relation to parasitoids and predators, with special reference to the Australian fauna.Crossref | GoogleScholarGoogle Scholar |
Austin, A. D. (1986). A taxonomic revision of the genus Mirobaeoides Dodd (Hymenoptera, Scelionidae). Australian Journal of Zoology 34, 315–337.
| A taxonomic revision of the genus Mirobaeoides Dodd (Hymenoptera, Scelionidae).Crossref | GoogleScholarGoogle Scholar |
Austin, A. D. (1988a). A new genus of baeine wasp (Hymenoptera: Scelionidae) from New Zealand associated with moss. New Zealand Journal of Zoology 15, 173–183.
| A new genus of baeine wasp (Hymenoptera: Scelionidae) from New Zealand associated with moss.Crossref | GoogleScholarGoogle Scholar |
Austin, A. D. (1988b). Guarding behaviour, eggmass shape and the eggsac in Clubiona robusta L. Koch (Araneae: Clubionidae). In ‘Australian Arachnology’. (Eds A. D. Austin and N. W. Heather.) pp. 87–95. (Australian Entomological Society: Brisbane.)
Austin, A. D. (1995). New species of scelionid wasps (Hymenoptera: Scelionidae: Baeini) from Western Australia, parasitic on spider eggs. Records of the Western Australian Museum 52, 253–263.
Austin, A. D., and Field, S. A. (1997). The ovipositor system of scelionid and platygastrid wasps (Hymenoptera, Platygastoidea), comparative morphology and phylogenetic implications. Invertebrate Taxonomy 11, 1–87.
| The ovipositor system of scelionid and platygastrid wasps (Hymenoptera, Platygastoidea), comparative morphology and phylogenetic implications.Crossref | GoogleScholarGoogle Scholar |
Austin, A. D., Johnson, N. F., and Dowton, M. (2005). Systematics, evolution and biology of scelionid and platygastrid wasps. Annual Review of Entomology 50, 553–582.
| Systematics, evolution and biology of scelionid and platygastrid wasps.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFOqtb0%3D&md5=5a84cac56531c429ed8ff3031410856eCAS | 15450001PubMed |
Bradoo, B. L. (1972). Life history and bionomics of Idris sp. (Scelionidae: Hymenoptera), egg parasite of Uloborus, a commensal on the web of the social spider Stegodyphus sarasinorum Karsch. Zoologischer Anzeiger 188, 43–52.
Brandley, M. C., Schmitz, A., and Reeder, T. W. (2005). Partitioned bayesian analyses, partition choice, and the phylogenetic relationships of scincid lizards. Systematic Biology 54, 373–390.
| Partitioned bayesian analyses, partition choice, and the phylogenetic relationships of scincid lizards.Crossref | GoogleScholarGoogle Scholar | 16012105PubMed |
Buffington, M. L., and Gates, M. (2009). Advanced imaging techniques II, using a compound microscope for photographing point–mount specimens. American Entomologist 54, 222–224.
Buffington, M. L., Burks, R., and Mcneil, L. (2005). Advanced techniques for imaging microhymenoptera. American Entomologist 51, 50–54.
Carey, D., Murphy, N. P., and Austin, A. D. (2006). Molecular phylogenetics and the evolution of wing reduction in a tribe of parasitoid wasps (Hymenoptera: Scelionidae: Baeini). Invertebrate Systematics 20, 489–501.
| Molecular phylogenetics and the evolution of wing reduction in a tribe of parasitoid wasps (Hymenoptera: Scelionidae: Baeini).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XoslGlt78%3D&md5=6d01f914bff59bd8ee4b565318a7ca70CAS |
Cobb, L. M., and Cobb, V. A. (2004). Occurrence of parasitoid wasps, Baeus sp. and Gelis sp. in the egg sacs of the wolf spiders Pardosa moesta and Pardosa sternalis (Araneae, Lycosidae) in southeastern Idaho. Canadian Field Naturalist 118, 122–123.
Galloway, I. D., and Austin, A. D. (1984). Revision of the Scelioninae (Hymenoptera, Scelionidae) of Australia. Australian Journal of Zoology Supplementary Series 32, 1–138.
| Revision of the Scelioninae (Hymenoptera, Scelionidae) of Australia.Crossref | GoogleScholarGoogle Scholar |
Gatesy, J., Desalle, R., and Wheeler, W. (1993). Alignment ambiguous nucleotide sites and exclusion of systematic data. Molecular Phylogenetics and Evolution 2, 152–157.
| Alignment ambiguous nucleotide sites and exclusion of systematic data.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXktVCntr0%3D&md5=76d64166172a804935fbea3537e0d1afCAS | 8025721PubMed |
Gillespie, J. J., Munro, J. B., Heraty, J. M., Yoder, M. J., Owen, A. K., and Carmichael, A. E. (2005). A secondary structural model of the 28S rRNA expansion segments D2 and D3 for chalcidoid wasps (Hymenoptera: Chalcidoidea). Molecular Biology and Evolution 22, 1593–1608.
| A secondary structural model of the 28S rRNA expansion segments D2 and D3 for chalcidoid wasps (Hymenoptera: Chalcidoidea).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlsFWhsb8%3D&md5=383e67ad9fc0ebb82c2fed74ad5bc60bCAS | 15843598PubMed |
Hieber, C. S. (1992). The role of spider cocoons in controlling desiccation. Oecologia 89, 442–448.
Hinton, H. E. (1976). Respiratory adaptations of marine insects. In ‘Marine Insects’. (Ed. L. Cheng.) pp. 43–78. (North–Holland Publishing Company: Amsterdam.)
Huelsenbeck, J. P., and Ronquist, F. (2001). MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755.
| 1:STN:280:DC%2BD3MvotV2isw%3D%3D&md5=ceab3d44bc74e85c441d4509c0328141CAS | 11524383PubMed |
Iqbal, M., and Austin, A. D. (2000). A preliminary phylogeny for the Baeini (Hymenoptera: Scelionidae): endoparasitoids of spider eggs. In ‘The Hymenoptera: Evolution, Biodiversity and Biological Control’. (Eds A. D. Austin and M. Dowton.) pp. 178–191. (CSIRO Publishing: Melbourne.)
Jackson, D. J. (1966). Observations on the biology of Caraphractus cinctus Walker (Hymenoptera: Mymaridae), a parasitoid of the eggs of Dytiscidae (Coleoptera). Transactions of the Royal Entomological Society (London) 118, 23–49.
| Observations on the biology of Caraphractus cinctus Walker (Hymenoptera: Mymaridae), a parasitoid of the eggs of Dytiscidae (Coleoptera).Crossref | GoogleScholarGoogle Scholar |
Johnson, N. F. (1984). Systematics of Nearctic Telenomus: classification and revisions of the podisi and phymatae species groups (Hymenoptera: Scelionidae). Bulletin of the Ohio Biological Survey 6, 1–113.
Johnson, N. F. (1992). Catalog of world Proctotrupoidea excluding Platygastridae. Memoirs of the American Entomological Institute 51, 1–825.
Johnson, N. F., and Masner, L. (2004). The genus Thoron Haliday (Hymenoptera: Scelionidae), egg-parasitoids of waterscorpions (Hemiptera: Nepidae), with a key to world species. American Museum Novitates 3452, 1–16.
| The genus Thoron Haliday (Hymenoptera: Scelionidae), egg-parasitoids of waterscorpions (Hemiptera: Nepidae), with a key to world species.Crossref | GoogleScholarGoogle Scholar |
Kerr, P., Fisher, E., and Buffington, M. L. (2009). Dome lighting for insect imaging under a microscope. American Entomologist 54, 198–200.
Lamoral, B. H. (1968). On the ecology and habitat adaptations of two intertidal spiders, Desis formidabilis (O.P. Cambridge) and Amaurobioides africanus Hewitt, at “The Island” (Kommetjie, Cape Peninsula), with notes on the occurrence of two other spiders. Annals of the Natal Museum 20, 151–193.
Masner, L. (1968). A new scelionid wasp from the intertidal zone of South Africa (Hymenoptera, Scelionidae). Annals of the Natal Museum 20, 195–198.
Masner, L. (1972). The classification and interrelationships of Thoronini (Hymenoptera: Proctotrupoidea, Scelionidae). Canadian Entomologist 104, 833–849.
| The classification and interrelationships of Thoronini (Hymenoptera: Proctotrupoidea, Scelionidae).Crossref | GoogleScholarGoogle Scholar |
Masner, L. (1976). Revisionary notes and keys to world genera of Scelionidae (Hymenoptera: Proctotrupoidea). Memoirs of the Entomological Society of Canada 108, 1–87.
| Revisionary notes and keys to world genera of Scelionidae (Hymenoptera: Proctotrupoidea).Crossref | GoogleScholarGoogle Scholar |
Masner, L. (1980). Key to genera of Scelionidae of the Holarctic region, with descriptions of new genera and species (Hymenoptera: Proctotrupoidea). Memoirs of the Entomological Society of Canada 112, 1–54.
| Key to genera of Scelionidae of the Holarctic region, with descriptions of new genera and species (Hymenoptera: Proctotrupoidea).Crossref | GoogleScholarGoogle Scholar |
Masner, L., and Dessart, P. (1972). Notes on Embidobiini (Scelionidae: Hymenoptera with description of a new genus. Canadian Entomologist 104, 505–510.
| Notes on Embidobiini (Scelionidae: Hymenoptera with description of a new genus.Crossref | GoogleScholarGoogle Scholar |
Mikó, I., Vilhelmsen, L., Johnson, N. F., Masner, L., and Pénzes, Z. (2007). Skeletomusculature of Scelionidae (Hymenoptera: Platygastroidea): head and mesosoma. Zootaxa 1571, 1–78.
Murphy, N. P., Carey, D., Castro, L. R., Dowton, M., and Austin, A. D. (2007). Phylogeny of the platygastroid wasps (Hymenoptera) based on sequences from the 18S rRNA, 28S rRNA and CO1 genes: implications for the evolution of the ovipositor system and host relationships. Biological Journal of the Linnean Society. Linnean Society of London 91, 653–669.
| Phylogeny of the platygastroid wasps (Hymenoptera) based on sequences from the 18S rRNA, 28S rRNA and CO1 genes: implications for the evolution of the ovipositor system and host relationships.Crossref | GoogleScholarGoogle Scholar |
Nondula, N., Marshall, D. J., Baxter, R., Sinclair, B. J., and Chown, S. L. (2004). Life history and osmoregulatory ability of Telmatogeton amphibius (Diptera, Chironomidae) at Marion Island. Polar Biology 27, 629–635.
| Life history and osmoregulatory ability of Telmatogeton amphibius (Diptera, Chironomidae) at Marion Island.Crossref | GoogleScholarGoogle Scholar |
Posada, D., and Crandall, K. A. (1998). Modeltest: testing the model of DNA substitution. Bioinformatics 14, 817–818.
| Modeltest: testing the model of DNA substitution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXktlCltw%3D%3D&md5=787c5224a9b3b4a919b481f4db32fc35CAS | 9918953PubMed |
Simon, C., Frati, F., Beckenbach, A., Crespi, B., Liu, H., and Flook, P. (1994). Evolution, weighting and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Annals of the Entomological Society of America 87, 651–701.
| 1:CAS:528:DyaK2MXis1Wiu7g%3D&md5=d5c73c20640b01d4cb1718c8d993d994CAS |
Spence, J. R. (1986). Interactions between the scelionid egg parasitoid Tiphodytes gerriphagus (Hymenoptera) and its gerrid hosts (Heteroptera). Canadian Journal of Zoology 64, 2728–2738.
| Interactions between the scelionid egg parasitoid Tiphodytes gerriphagus (Hymenoptera) and its gerrid hosts (Heteroptera).Crossref | GoogleScholarGoogle Scholar |
Stevens, N. B., and Austin, A. D. (2007). Systematics, distribution and biology of the Australian ‘micro-flea’ wasps, Baeus spp. (Hymenoptera: Scelionidae): parasitoids of spider eggs. Zootaxa 1499, 1–45.
Swofford, D. L. (2002). ‘PAUP* Phylogenetic Analysis Using Parsimony (* and other methods), Version 4.’ (Sinauer: Sunderland, MA.)
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., and Higgens, D. G. (1997). The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25, 4876–4882.
| The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXntFyntQ%3D%3D&md5=d7b0c32bbecdcace41b4f3e499261c0eCAS | 9396791PubMed |
Vachon, M. (1955). contribution a l’étude de la biologie de l’hyménoptère Baeus semilunum (Hal.) parasite des oeufs d’araignées. Annales de la Société Entomologique de France 124, 141–146.
Valerio, C. E. (1971). Parasitismo en huevus de araña Achaearanea tepidariorum (Koch) (Araneae: Theridiidae) en Costa Rica. Revista de Biologia Tropical 18, 99–106.
Whiting, M. F., Carpenter, J. M., Wheeler, Q. D., and Wheeler, W. C. (1997). The Strepsiptera problem: phylogeny of the holometabolous insect orders inferred from 18S and 28S ribosomal DNA sequences and morphology. Systematic Biology 46, 1–68.
| 1:STN:280:DC%2BD383js1yqtQ%3D%3D&md5=86df74a7ce3b490b4da8ad4ca25d6b71CAS | 11975347PubMed |
Yoder, M. J., Mikó, I., Seltmann, K. C., Bertone, M. A., and Deans, A. R. (2010). A gross anatomy ontology for Hymenoptera. PLoS ONE 5, e15991.
| A gross anatomy ontology for Hymenoptera.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXltVaqsg%3D%3D&md5=9fbc4644de2a42fe40b781c7ef147992CAS | 21209921PubMed |