Systematic review of the Australian ‘bush coconut’ genus Cystococcus (Hemiptera: Eriococcidae) uncovers a new species from Queensland
Thomas L. Semple A E , Penny J. Gullan B , Christopher J. Hodgson C , Nate B. Hardy D and Lyn G. Cook AA The University of Queensland, School of Biological Sciences, Brisbane, Qld 4072, Australia.
B Division of Evolution, Ecology & Genetics, The Research School of Biology, The Australian National University, Acton, ACT 2601, Australia.
C Department of Biodiversity and Biological Systematics, The National Museum of Wales, Cathays Park, Cardiff, CF10 3NP, Wales, UK.
D Department of Entomology and Plant Pathology, Auburn University, Auburn, AL 36849, USA.
E Corresponding author. Email: thomas.semple@uqconnect.edu.au
Invertebrate Systematics 29(3) 287-312 https://doi.org/10.1071/IS14061
Submitted: 1 December 2014 Accepted: 14 February 2015 Published: 30 June 2015
Journal Compilation © CSIRO Publishing 2015 Open Access CC BY-NC-ND
Abstract
Australia houses some unusual biota (insects included), much of which is undescribed. Cystococcus Fuller (Hemiptera : Sternorrhyncha : Coccoidea : Eriococcidae) currently comprises two species, both of which induce galls exclusively on bloodwoods (Myrtaceae: Corymbia Hill & Johnson). These insects display sexual dichronism, whereby females give birth first to sons and then to daughters. Wingless first-instar females cling to their winged adult brothers and are carried out of the maternal gall when the males fly to find mates – a behaviour called intersexual phoresy. Here, we use data from two gene regions, as well as morphology and host-use of the insects, to assess the status of a previously undescribed species. We describe this newly recognised species as Cystococcus campanidorsalis, sp. nov. Semple, Cook & Hodgson, redescribe the two existing species – C. echiniformis Fuller and C. pomiformis (Froggatt), designate a lectotype for C. echiniformis, and provide the first descriptions of adult males, and nymphal males and females for the genus. We have also reinterpreted a key morphological character of the adult females. This paper provides a foundation for further work on the genus, which is widespread across northern Australia and could prove to be useful for studies on biogeography and bloodwood ecosystems.
urn:lsid:zoobank.org:pub:3A9DC645-0CBC-48B0-8BD3-5ACC0E2130D1
Additional keywords: Bloodwood apple, Corymbia trachyphloia, dimorphism, eucalypt, phoresy.
Introduction
Gall-inducing scale insects (Hemiptera : Coccoidea) in the genus Cystococcus Fuller are obligatory parasites on red bloodwoods in the genus Corymbia Hill & Johnson (Gullan and Cockburn 1986; Gullan et al. 2005). The sub-spherical galls vary in size within and between species, with the largest belonging to Cystococcus pomiformis (Froggatt) (Froggatt 1893), at up to 90 mm in diameter (Austin et al. 2004). Commonly known as either ‘bloodwood apples’ or ‘bush coconuts’, they consist of a hard outer layer with a soft, white, fleshy layer lining the cavity that houses the adult female (Gullan and Cockburn 1986). The Australian Aborigines used the galls as a food source, eating the fleshy interior of the gall and the insects themselves (Froggatt 1893). Although Aboriginal tribes across central and northern Australia had several names for bush coconuts, such as ‘Ballabbi’ and ‘Durdunga’, it was thought by Fuller (1899) that they all referred to galls induced by one species – Cystococcus echiniformis Fuller (Fuller 1897). This is unlikely, but the Aboriginal names probably do all refer to galls induced by females of Cystococcus. In addition to providing food for people, the insects of Cystococcus species and the galls they induce are a food source for birds and other insects (e.g. moths; Turner 1942). Furthermore, the adult females are parasitised by wasps and flies (T. L. Semple and L. G. Cook, pers. obs.), and the galls provide shelter for arthropods such as tree crickets, ants and spiders (Froggatt 1893; Fuller 1899; Austin et al. 2004; T. L. Semple and L. G. Cook, pers. obs.).
Currently, C. pomiformis and C. echiniformis are the only described species of Cystococcus, and are found across large areas of northern Australia (Bowman et al. 2010). Froggatt (1893) described C. pomiformis from galls collected at Torrens’ Creek in north Queensland and in the Barrier Range, near King’s Sound in Western Australia, but he placed the species in the genus Brachyscelis Schrader (now known as Apiomorpha Rübsaamen). Fuller (1897) later erected the genus Cystococcus for the species C. echiniformis, which he described from material collected by R. Helms in the east Kimberley. Subsequently, Froggatt (1921) transferred B. pomiformis to Cystococcus. Gullan and Cockburn (1986) referred to a possible third species of Cystococcus, but provided no information on these specimens.
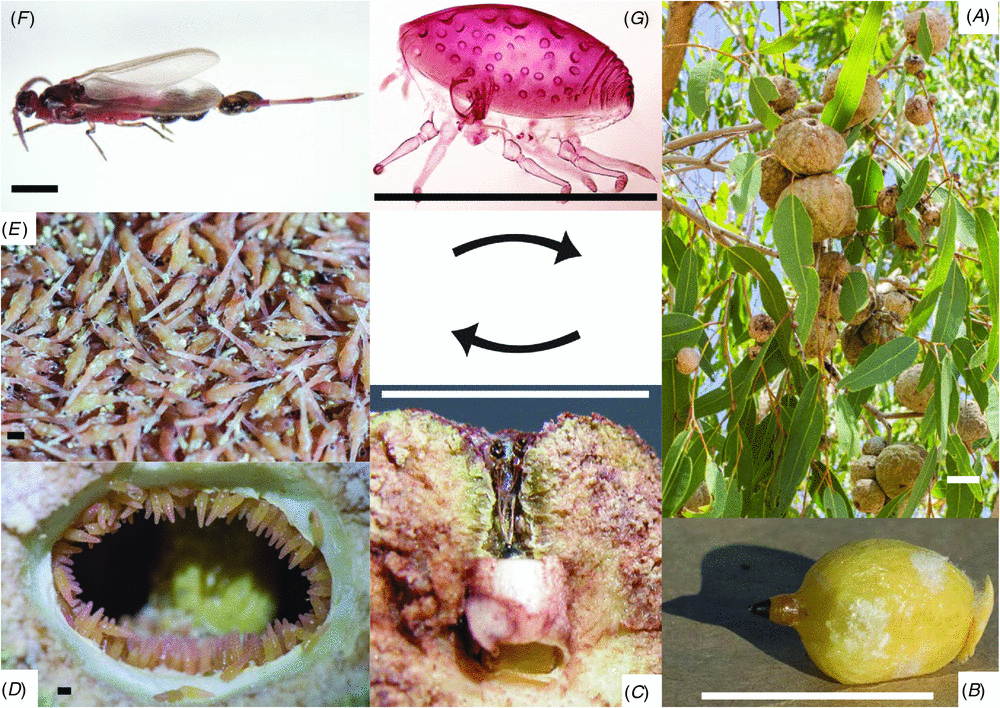
The life cycle and dispersal mechanism of Cystococcus are unusual and may be unique among insects. Females of Cystococcus feed, reproduce and spend the entirety of their adult lives inside their galls. They lack eyes, an anus, antennae and legs. They are soft-bodied except for a few patches of sclerotization, including a ‘button’ used to plug the gall entrance (Fuller 1897). Adult males have extremely elongate abdomens, which are likely an adaptation for mating through the gall entrance (Gullan et al. 2005), and perhaps also for intersexual phoresy (see below). Adult females control the sex allocation of their offspring, exhibited through sexual dichronism (Gullan and Cockburn 1986). A mother first gives birth to all male offspring, which develop entirely within the maternal gall. These males feed on the fleshy lining (nutritive tissue) of the gall and develop through two nymphal instars and two pupal stages, before moulting to winged adults (Gullan and Cockburn 1986). While the males are pupating, their mother produces daughters, which develop to the first instar inside the gall cavity (Gullan and Cockburn 1986) or inside their mother (T. L. Semple, pers. obs.). This co-mingling of adult males and their first-instar sisters allows for intersexual phoresy: the first-instar females grasp onto their brothers’ elongate abdomens and are carried from the maternal gall as those males fly in search of mates (Grant 1965; Gullan and Cockburn 1986) (Fig. 1).
Cystococcus has been used as an exemplar of biogeographic and ecological processes in Australia (Fuller 1899; Gullan and Cockburn 1986; Bowman et al. 2010; Ladiges et al. 2010), yet the genus has never been revised taxonomically. Here, we describe a new species of Cystococcus from Queensland, C. campanidorsalis, sp. nov., and redescribe C. echiniformis and C. pomiformis based on molecular data, the morphology of adult females and adult males, and host use. We provide the first descriptions and taxonomic illustrations of adult males, as well as nymphal stages of female and male Cystococcus.
Materials and methods
Species concept
Here, we recognise species as biologically distinct units, reproductively isolated from other such entities (e.g. biological species concept; Mayr 1942). We use multiple sources of data, such as DNA sequences, morphology of different life history stages, and host associations, to assess evidence of barriers to gene flow. In this way, our species concept also corresponds to that of an independently evolving gene pool (‘genotypic cluster’; Mallet 1995).
Specimens and taxonomy
Specimens of Cystococcus were obtained from across their known range and from around south-east Queensland (Table S1, available as Supplementary material online). We also examined specimens held in Australian and overseas museums (see below). Galls were opened by cutting away a segment of the side wall with secateurs or a knife, which allowed inspection of the contents. Where present, adult females were carefully removed by pushing the sclerotized button inwards from the gall opening and cutting away a small piece of gall tissue from around the mouthparts (tissue still attached in Fig. 1B). The lightly sclerotized disc around the mouthparts was often stuck to the gall tissue but was removed easily, with less chance of damage, after soaking during slide mounting. The cuticle of adult females is extremely fragile and is easily damaged during removal. For molecular work, the body contents, including ovaries and eggs, were usually removed and stored separately as another source for DNA extraction and to allow penetration of ethanol into the body cavity. Males of all developmental stages and first-instar females also were collected from galls when present and preserved separately from the adult females. Almost all specimens examined for the descriptions have an associated gall, which is housed in the same institution as the adult female insect. Thus galls are not listed in the ‘Material examined’ sections.
All specimens collected in remote locations were removed from their galls in the field, stored in 100% ethanol and refrigerated below 4°C for transport back to the laboratory. Each collection made by TLS and LGC was assigned a unique identifier (e.g. TLS001) to allow tracking of all material derived from that tree, including insects, galls, plant material used for host identification, DNA and all other preserved forms of these (i.e. slide-mounted, ethanol-preserved and frozen specimens).
Type specimens of C. campanidorsalis, sp. nov. will be deposited in the Queensland Museum (QM), Brisbane, Qld, Australia, as per collection permit requirements, and some paratypes will be deposited in the Australian National Insect Collection (ANIC), CSIRO Ecosystem Sciences, Canberra, ACT, Australia. We have registered the new name published in this paper with the Official Registry of Zoological Nomenclature (ZooBank) and cite the life science identifier (LSID) after the heading for the new name. Each LSID is a globally unique identifier for the nomenclatural act of naming a new taxon. DNA and frozen specimens will be maintained at The University of Queensland for the immediate future unless storage facilities become available at state or national institutions. Insect and gall material from collections made by PJG are housed in the ANIC. PJG also examined and measured specimens from the following institutions: Agricultural Scientific Collections Unit, Orange Agricultural Institute, New South Wales, Australia (ASCU); The Natural History Museum, London, UK (BMNH); South Australian Museum, Adelaide, South Australia (SAM); the United States National Collection of Coccoidea of the National Museum of Natural History (USNM), housed at the United States Department of Agriculture (USDA), Beltsville, Maryland, USA; Western Australian Museum, Perth, Western Australia (WAM).
The International Code of Zoological Nomenclature (ICZN 1999) requires lectotypes designated after 1999 to ‘contain an express statement of deliberate designation’ (amended article 74.7.3). We use the statement ‘we here designate’ to satisfy this requirement. A lectotype has been designated for C. echiniformis because this name lacks a primary type specimen and an unambiguous syntype has been identified. The purpose is to provide stability of nomenclature, and designation is done in a revisionary context in accordance with the amended recommendation 74G of article 74.7.3.
Slide-mounted specimens listed for the material examined are referred to by number of individuals and slides, for example, 2/5 refers to five specimens on two slides. For C. echiniformis and C. pomiformis, the lists of specimens examined are for sequenced specimens only, but many more adult females and galls were available in museum collections; thus, the descriptions of the galls of these two species include many specimens additional to those for which collection data are listed. The data for unlisted specimens is available upon request to PJG.
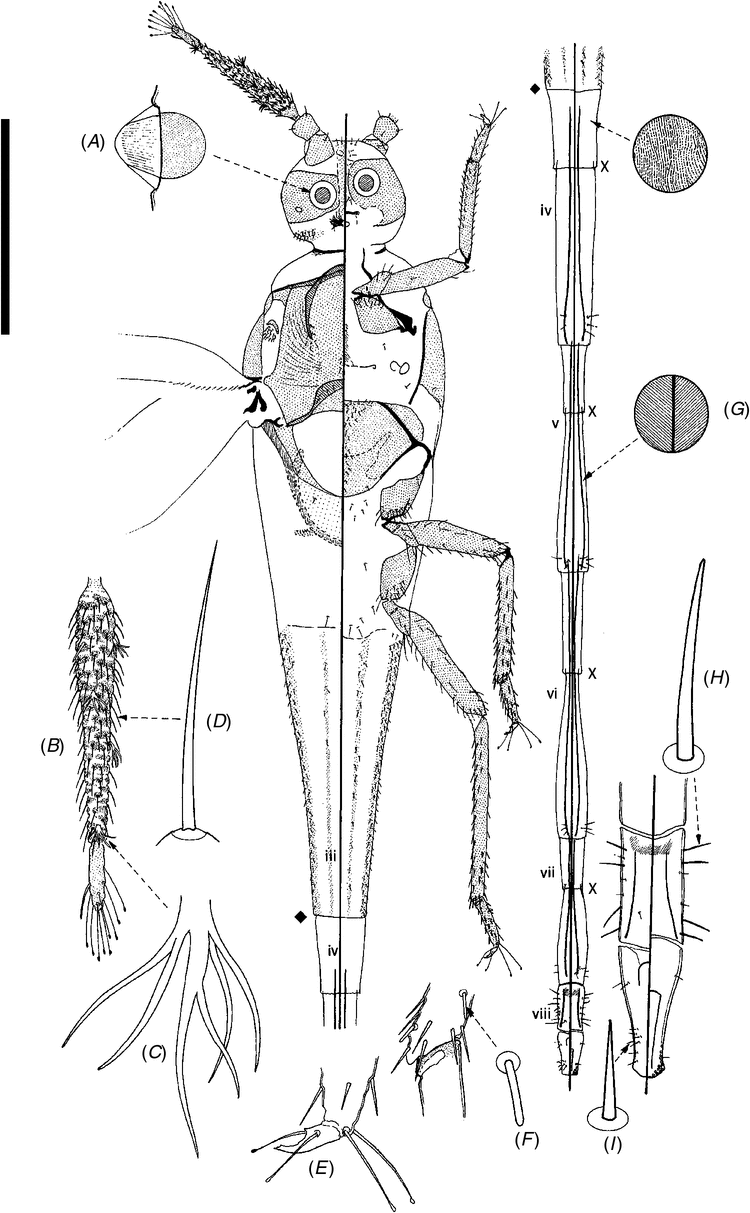
Measurements were made using an ocular micrometre in the eyepiece of a compound or dissecting microscope. Body lengths and widths are maximum values, and tibiotarsal lengths of the legs of nymphs exclude the claw. In the taxonomic illustrations of nymphs and adult males, the main figure is a composite with the dorsum on the left and the venter on right. For adult males, vignettes of the more important structures are enlarged (not to scale) around the margin. For nymphs and the adult female, the draft illustrations were prepared with a drawing tube and then scanned and edited using the Adobe programs Photoshop CS and Illustrator CS.
Molecular data
DNA extraction of whole female cuticles was performed using a cetyltrimethylammonium bromide (CTAB) method in which the cuticle was incubated overnight at 55°C in CTAB buffer with 10–20 μL of proteinase K added. A chloroform wash with gentle rocking followed by centrifugation was used to separate the DNA in the aqueous layer from the organic layer and tissue debris. The DNA was precipitated from the aqueous layer using 100% isopropanol, then cleaned using two washes with 80% ethanol. Extractions of small volumes of tissue (e.g. males or parts of ovaries) were carried out with a Bioline ‘Isolate 2’ DNA extraction kit (cat. no. BIO-52067) or a Qiagen DNeasy blood and tissue kit (cat. no. 69506) following the manufacturer’s instructions.
Extracted DNA was amplified using polymerase chain reaction (PCR) and checked using agarose gel electrophoresis. Gene regions used for analysis were the 5ʹ region of 18S (small subunit nuclear rDNA, SSU rDNA) and the ‘DNA barcode’ region of COI (mitochondrial cytochrome c oxidase 1). Park et al.’s (2010) scale insect COI primer combination (PCO_F1 (Park et al. 2010) and HCO (Folmer et al. 1994)) was effective for many specimens of C. pomiformis and C. echiniformis, but yielded only poorly amplified or no PCR product for most specimens of C. campanidorsalis, sp. nov., so new primers were designed as follows. Consensus sequences for the two specimens of C. campanidorsalis, sp. nov. that successfully amplified during the first round of PCR were aligned with sequences from C. pomiformis and C. echiniformis. Conserved regions near the 5ʹ and 3ʹ ends were chosen for potential priming sites. Cross-binding compatibility, secondary structure and melting temperatures were considered in primer design using Geneious ver.6.1.7 (Biomatters: www.geneious.com), and compatible pairs synthesised by IDT (http://sg.idtdna.com). Details of all primers and PCR programs used are listed in Table 1. Successfully amplified DNA was purified of unincorporated primers and dNTPs using Exonuclease 1 and Antarctic Phosphatase (New England Biolabs), then sequenced by Macrogen (Republic of Korea) using Sanger sequencing.

|
DNA sequences were checked for non-target DNA contamination (such as parasitoids and fungi) using BLAST (megablast or discontiguous megablast search: http://blast.ncbi.nlm.nih.gov), then aligned and manually edited using Geneious ver.6.1.7. PAUP* (Swofford 2003) was used to check overall base frequencies and for base composition bias among taxa for individual COI codon positions, as frequency differences between taxa violate the assumptions of most available tree estimation methods. Phylogenetic trees were estimated using maximum parsimony (MP) and maximum likelihood (ML), as these two methods estimate phylogenetic relationships according to different models and assumptions about the process of DNA evolution (Sleator 2011). Therefore, congruence between methods (when present) offers the strongest support from the data for relationships. Several species of Ascelis Schrader, which is closely related to Cystococcus (Cook and Gullan 2004), were used as outgroups. Diagnostic nucleotide changes were identified by using PAUP* to find synapomorphies for relevant clades (nucleotide changes with a consistency index equal to 1, i.e. no homoplasy).
Base composition bias was observed among species in the 3rd codon positions of COI, so MP and ML analyses were performed with and without 3rd codon positions to determine whether this bias was a confounding source of apparent divergence between species. A neighbour-joining (NJ) tree was also estimated using the LogDet transformation in PAUP*, as this method reduces the effect of grouping taxa with homoplasiously similar base frequencies (Lockhart et al. 1994).
Maximum parsimony
An heuristic search with 1000 random addition starting sequences was carried out in PAUP* for each gene region, with the 10 most parsimonious trees retained from each. These saved trees were then used for a second heuristic search, which was allowed to run to completion or until ~500 000 MP trees had been reached. A strict consensus tree was calculated from the resulting MP trees, and a bootstrap (BS) analysis performed using a fast-heuristic search with 1000 pseudoreplicates to calculate support values for each branch.
Maximum likelihood
Analyses were run with RAxML (Stamatakis 2006) using a generalised time reversible (GTR) model. The program uses per-site rate categories (GTR+CAT) and estimates model parameters based on the input data. We ran RAxML on the CIPRES Science Gateway (www.phylo.org) for faster processing, with a 1000 pseudoreplicate BS analysis used to calculate branch support values.
Morphology
After DNA extraction, adult females, males and immature stages were slide-mounted for morphological examination and for use as morphological vouchers for DNA sequences. Scale insect taxonomy is traditionally based on cuticular features of adult females, such as minute pores and setae, which are visible only under a compound microscope after clearing and staining. Adult males were also mounted and examined so that, as the dispersing adult life stage, they could be identified if ever collected outside the maternal gall.
Adult females were mounted in Canada balsam using Gullan’s adaptation of the method described by Kozarzhevskaya (1968). Briefly, cuticles were cleared of contents in 10% potassium hydroxide (KOH) solution, stained in acid fuchsin, dehydrated in a series of ethanol and isopropyl alcohol baths and cleared in xylene before mounting in Canada balsam on slides. In order to prepare specimens under coverslips with a low enough profile for compound microscopy, the sclerotized button of recently mounted specimens was removed from the membranous cuticle after staining. The buttons were glued with Canada balsam to the slide, beside the coverslip. Specimens in good condition were flattened lengthways, with the mouthparts at one end and the button (removed) at the other, as this is how they flatten naturally and how existing specimens were mounted. Damaged or small females were cut along one side and ‘butterflied’ on the slide to allow clearer viewing of pores and setae and distinction between dorsal and ventral surfaces. Adult males and immature stages prepared by CJH and TLS were mounted using a modification of the method described in Ben-Dov and Hodgson (1997). Briefly, cuticles were cleared of contents in 10% KOH, rinsed in 2% detergent water, stained in very dilute acid fuchsin, then dehydrated in ethanol, cleared in xylene and mounted in Canada balsam. Immature stages and adult males prepared by PJG were mounted according to the method described above for adult females, except that some nymphs were mounted in Stroyan’s mountant (Upton and Mantle 2010), either directly (i.e. alive) or from ethanol after thorough washing in several changes of water.
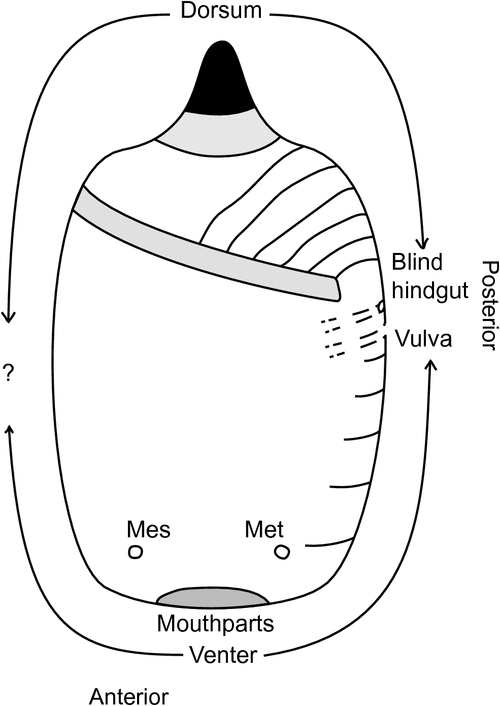
Interpretation of the anatomical position of the sclerotized ‘anal button’ needed revision because previous descriptions have broadly described its location as posterior abdominal or caudal (Fuller 1897), and interpretation of positioning has been difficult because abdominal segmentation is barely visible in C. pomiformis and C. echiniformis. There are few external features to help locate segments ventrally, other than the two pairs of spiracles and the vulva, and there are no clear landmarks for dorsal segmentation. Because internal structures cannot be discerned after the insect’s soft tissue is macerated with KOH, some females of C. campanidorsalis, sp. nov., which has more defined segmentation than the other two species, were dissected before mounting in an attempt to locate the gut and ovaries, and where they attach to the cuticle. The anus is blind-ended (it does not open externally) but its location, along with that of the vulva, was expected to assist with interpretation of segmentation and the position of the sclerotized button.
Adult and first-instar females were prepared for scanning electron microscopy (SEM) after preservation and storage in 80% ethanol. Each specimen was dehydrated in a graded ethanol series, de-waxed in xylene, rehydrated through a graded ethanol series into distilled water, post-fixed in 1% aqueous osmium tetroxide, washed in distilled water and sonicated briefly to remove any black precipitate, critical point dried, glued onto a metal stub with nail varnish and coated with gold palladium under vacuum. Specimens were then examined and photographed using a Cambridge S360 scanning electron microscope.
Results and discussion
Molecular data
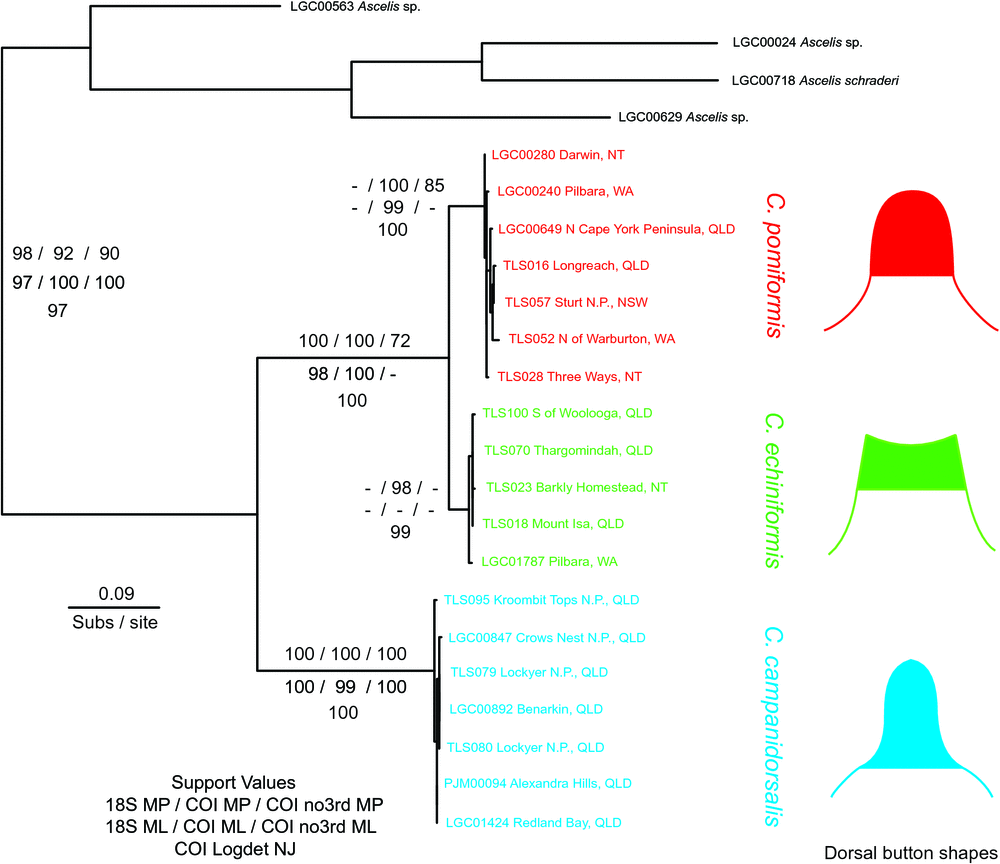
Both methods used for phylogeny estimation (MP and ML), and both gene regions (including COI without 3rd codon positions), provided strong support for the monophyly of C. campanidorsalis, sp. nov. (BS > 95; Fig. 2). Cystococcus campanidorsalis, sp. nov. was estimated to be sister to the other two species of Cystococcus in all analyses (BS > 70), except for the ML analysis of COI without 3rd codon positions, in which C. campanidorsalis, sp. nov. appeared nested within C. pomiformis. Relationships between C. echiniformis and C. pomiformis were not as clearly resolved; however, a sister relationship appears most likely, as shown by those analyses of COI that recovered support for reciprocal monophyly between the two species (Fig. 2). The lack of support recovered from 18S analyses (BS < 70) was likely due to the small amount of variation between C. echiniformis and C. pomiformis in the less variable 18S gene region.
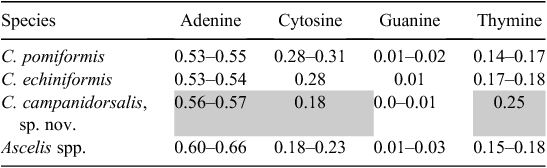
Twenty-three collections were sequenced and analysed for both COI and 18S, including four specimens of Ascelis spp. (outgroups) and 19 specimens of Cystococcus spp., sampled from across their known and newly discovered distribution (GenBank accession numbers: 18S: KP729354–729373; COI: KP729331–729353). For 18S, the final sequence alignment consisted of 611 base pairs (bp), with 51 variable sites and 38 parsimony informative sites. The alignment for COI consisted of 507 bp, with 183 variable sites and 129 parsimony informative sites. Across the full datasets, base frequencies were equal for 18S but not for COI. In COI, overall nucleotide frequency means were: A = 0.43, T = 0.22, G = 0.06, C = 0.29, showing an unequal adenine to guanine ratio (i.e. a strong AT bias). The bias was greatest in 3rd codon positions, with an average AT proportion of 0.75. In addition, there was base composition bias among taxa (non-stationarity) at 3rd codon positions of COI (χ2, P < 0.001), particularly between C. campanidorsalis and the other species of Cystococcus (Table 2). This bias between taxa could act as a confounding factor in phylogenetic analyses, exaggerating the apparent molecular separation of C. campanidorsalis, sp. nov. from the two other species, because most tree estimation methods assume stationarity of base composition.
|
Morphology
Physical characteristics of adult females of Cystococcus are minimal, including no eyes, antennae, legs or wings, and there is minimal sclerotization other than the button. Dissection and slide mounting of adult females of C. campanidorsalis, sp. nov. provided previously unknown information about their specific anatomy. Abdominal segmentation on the dorsal surface is not visible in C. echiniformis and C. pomiformis, but is defined in C. campanidorsalis, sp. nov. by light sclerotization between segments and a transverse row of setae and pore plates on each segment. Dissection revealed the abdominal, cuticular attachment points of the hindgut and oviduct, and confirmed the hindgut to be blind-ended with no anal opening. The oviduct appears attached to the cuticle seven or eight abdominal segments anterior to the dorsal button, on what appears to be the venter in slide-mounted specimens. That is, dorsal abdominal segments ~III through IX appear ventral and anterior to the button. This would place the button dorsally, on abdominal segment(s) II and/or III (Fig. 3).
|
Initial (and longstanding) misconceptions about this genus described the sclerotized button as caudal or posterior on the abdomen (see Fuller 1897; and Hardy et al. 2011). Froggatt (1921: 156) went so far as describing it as ‘analogous with the more distinct tails of Apiomorpha and Ascelis’. In eriococcids (which include Cystococcus and Ascelis) the vulva is typically found on or between abdominal segments VII and VIII (Williams 1985; Gullan and Jones 1989). However, in Apiomorpha the vulva appears to have been displaced anteriorly by at least one segment (Gullan and Jones 1989). Having confirmed the location of the blind-ended hindgut and vulva of Cystococcus, we can confidently revise the location of the button as dorsal, on abdominal segment II and/or III (Fig. 3). The use of a sclerotized dorsum to plug gall openings is not unique to Cystococcus, as it appears in other eriococcid scale insects, including Opisthoscelis Schrader (Hardy and Gullan 2010), Bystracoccus Hodgson (Hodgson et al. 2013) and Madarococcus (Hardy et al. 2008), among others. Along with Cystococcus, the closely related genus Ascelis is thought to plug the gall opening with the sclerotized caudal area of its abdomen (Gullan et al. 2005). This likely requires revision because, like Cystococcus, the abdominal segmentation of females of Ascelis is not clearly defined and the genus appears very similar morphologically to Cystococcus.
Although little is known about nutrient uptake and waste production in Cystococcus, the length of their feeding stylets can help us to make inferences about feeding and to explain the lack of a functional anus. Beardsley (1984) observed that gall-inhabiting scale insects had much shorter stylets than their non-gall-inducing relatives. Indeed, the stylets of Cystococcus are very short (<0.6 mm long), making the gall lining (typically at least as thick as the outer wall, >1.5 mm thick) the only tissue available for feeding. Within the Coccoidea, it is the phloem-feeding groups that are best known to produce excessive volumes of sugary excrement or ‘honeydew’ (Gullan and Kosztarab 1997). Thus, if females of Cystococcus do not feed on phloem, as evidenced by their short stylets, then the small amount of waste produced from feeding in the special nutritive tissue might be stored and/or recycled.
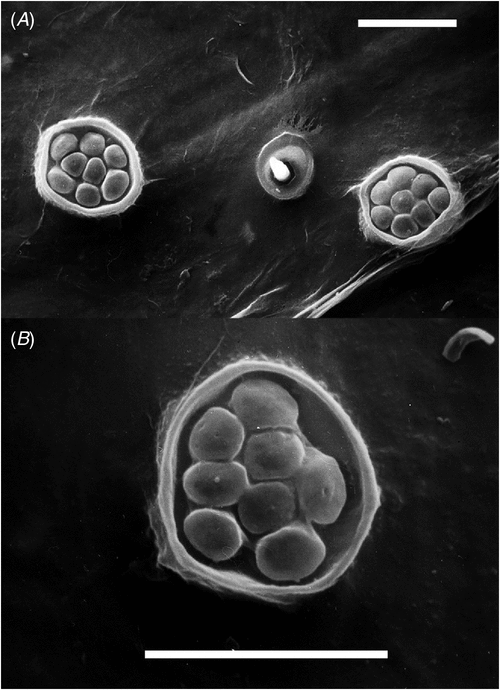
Another unusual morphological characteristic of Cystococcus is the absence of loculate pores on any instar, and the presence of ‘pore plates’ on the dorsal derm of second-instar males and the ventral derm of adult females. On adult females, pore plates are numerous surrounding the spiracles and on several segments of the abdominal venter. Scanning electron microscopy (Fig. 4) shows each plate of the adult female to be composed of several rounded tubercles, the so-called ‘pores’, clustered together and surrounded by a rim of sclerotized cuticle to form a plate. There are no loculi (i.e. holes) and any exudation must be secreted across the cuticle. These pore plates appear to produce white powdery wax on live adult females. The spiracles lack this type of powdery wax but exude long (perhaps up to 400 µm), silvery filaments.
|
Species delimitation
Cystococcus campanidorsalis, sp. nov. was identified as a member of the genus Cystococcus by the morphology of males and females, and its induction of woody galls on stems of bloodwoods (Corymbia spp.). Using DNA sequence data, morphology and host use, we provide evidence for a lack of gene flow between C. campanidorsalis, sp. nov. and other species of Cystococcus. We interpret this as equivalent to reproductive isolation under Mayr’s (1942) biological species concept, and therefore determine C. campanidorsalis, sp. nov. to be a distinct species. Cystococcus campanidorsalis, sp. nov. is currently known to exist in near-sympatry with C. echiniformis and to not co-occur with C. pomiformis, and so reproductive isolation cannot be inferred directly from divergence in sympatry.
The reciprocal monophyly of C. campanidorsalis, sp. nov. and the other species of Cystococcus indicates that there has been no recent gene flow between these two clades (Fig. 2). This was supported by all analyses except for one – ML analysis of COI with 3rd codon positions removed. In the absence of 3rd codon positions, only a few synapomorphic nucleotide sites were identified for the two relevant clades. However, the same relationship was recovered in our analysis using the LogDet method from Lockhart et al. (1994), used to correct for base composition bias among taxa, with 3rd codon positions included.
Although the ranges of C. campanidorsalis, sp. nov. and C. echiniformis overlap, the adult females and males of these two species are easily distinguishable. In addition, Cystococcus campanidorsalis, sp. nov. has been collected only from Corymbia trachyphloia, a bloodwood species from which C. echiniformis has not been collected. Corymbia trachyphloia is considered to be a brown bloodwood (section Apteria) and is the sole occupant of a section of corymbias nested within the red bloodwoods (Co. sect. Rufaria) (Parra-O et al. 2009). Cystococcus pomiformis and C. echiniformis have been collected from numerous other species within Co. sect. Rufaria.
We also examined specimens of adult females and adult males from three collections that PJG had recognised previously as a new species (Gullan and Cockburn 1986). All specimens were from the Northern Territory from either Gunn Point (north of Darwin) or the Coburg Peninsula in Arnhem Land, and the host of one collection was recorded as Corymbia bleeseri. The galls are 14–20 mm in height, 17–31 mm in diameter with a wall 1–3 mm thick, and most closely resemble the galls of C. echiniformis. However, adult males and adult females of this putative new species most closely resemble those of C. pomiformis, including in the shape of the dorsal button of the female. All specimens were collected in the 1970s and 1980s and no tissue is available for DNA analysis. Fresh samples are required for molecular study to determine whether these populations represent a fourth species or a geographic or host-related variant of C. pomiformis.
Taxonomy
Genus Cystococcus Fuller
This genus was considered to be a junior synonym of Ascelis by Cockerell (1902), Fernald (1903) and Hoy (1963), but Gullan and Cockburn (1986) and Gullan et al. (2005) treated the two genera as distinct. Cystococcus is distributed broadly across northern Australia at latitudes less than 28° south (data from this study), whereas Ascelis has been collected mostly from south-east Australia (mainly New South Wales) (Miller et al. 2014). Like Cystococcus, most currently recognised species of Ascelis have been collected from Corymbia, especially C. gummifera (formerly Eucalyptus corymbosa, as listed in Miller et al. (2014)). Galls of Cystococcus are always on the stems, whereas those of Ascelis are on leaves (Froggatt 1921).
Remarks
The most commonly found form of Cystococcus (adult female in gall) usually can be identified to species level without opening the gall, or even removing it from the tree. The shape of the sclerotized dorsal button (which can be seen from outside the gall) is usually sufficient to identify individuals in the field. Adult males can be difficult to distinguish, due to minimal differences among species and variation within species, especially in the absence of good quality slide-mounted specimens. Molecular data or adult female morphology are much more reliable for identification (when available).
Key to species of Cystococcus based on adult females (Fig. 5)|
1. Sclerotized dorsal button convex-ended |
|
2. Dorsal button ranging from dome-shaped to bluntly conical; pore plates in cluster around vulva, not in clearly separated transverse bands |
|
1. Antennal pedicel without fleshy setae; antennae with some digitate antennal bristles; scutum with scutal setae in two narrow bands; posterior abdominal segments (except sometimes segment VIII) and penial sheath with few fleshy setae |
|
2. Antennal flagellum with numerous broad, fleshy setae |
Species descriptions
Cystococcus campanidorsalis, sp. nov. Semple, Cook & Hodgson
Material examined
Remarks
Females of C. campanidorsalis have dorsal buttons most closely resembling those of C. pomiformis, but flaring out at the base in a bell shape (Fig. 5). These two species also can be distinguished by the pattern of pore plates on the venter of adult females, anterior to the vulva. Cystococcus campanidorsalis has clear, transverse bands of pore plates, in contrast to the unpatterned clustering on C. pomiformis and C. echiniformis (Fig. 5). Due to the small number of discernible differences between adult females of Cystococcus species, only one whole female illustration is included in this paper. Adult males of C. campanidorsalis can be distinguished from those of C. pomiformis and C. echiniformis by the presence of numerous broad, fleshy setae on the antennal pedicel and the absence of digitate bristles on the flagellum (Fig. 6).
Distribution and host plants
Known from south-east Queensland, north to 24°S and west to 151°E. Only known host tree is Corymbia trachyphloia.
Etymology
The name campanidorsalis comes from the bell-shaped (campana = bell in Latin), sclerotized dorsal button, and also describes the location of this button as being dorsal rather than caudal.
Cystococcus echiniformis Fuller
Fuller’s (1897) original description of this species is very brief, but later he (Fuller 1899) provided a more detailed description accompanied by line drawings of the adult female and its gall. The only insect specimen with label data that clearly match collection information in Fuller (1897, 1899) is in the Brain collection (#438) in the USNM (examined by PJG). The specimen is incomplete and split between two slides: one has just a piece of cuticle with two spiracles and the other only the apex of the abdomen. The basal width of the abdominal button is 1.1 mm and it is concave-ended. The slide label data are: ‘Cystococcus/echiniformis/cuticle’ and ‘Cystococcus/echiniformis/apex of abdomen’, and both slides have ‘[On Eucalyptus tesselaris/E. Kimberly [sic]. Australia/R. Helms Coll.]/438’. We here designate the remains of this adult female as the lectotype.
There is also a gall of C. echiniformis in the USNM (also examined by PJG), but it has a 6 mm diameter hole in the side wall, no gall contents and no locality or collector data. The box label is ‘Ascelis echiniformis (Full.)/TYPE/Ckll. Coll.’ and thus there is no evidence that this gall is associated with the remains of the adult female in the Brain collection.
There also are two galls (one complete and one half) in the SAM that clearly are part of Fuller’s original material as they have locality data of East Kimberley, Western Australia. The galls were received at the SAM in July 1897, which is before Fuller’s formal naming of the species as C. echiniformis in August 1897, and the names on the labels with the galls are ‘Cystococcus Fuller (n.g.)/Eucalypti, Fuller, nov. sp.’, and ‘Cystococcus n.g./Eucalypti n.sp. Fuller’, and ‘Cystococcus nov. gen./Eucalypti n.sp. Fuller m.s.’ (there are three labels with the two galls). Thus, Fuller must have been planning to call his species ‘Cystococcus eucalypti’, but changed the species name before publication. There are no insects associated with these SAM galls.
Material examined
Material examined
Australia: Queensland, Northern Territory and Western Australia, on Corymbia terminalis (Myrtaceae), (ID: LGC01787, TLS002, TLS004, TLS005, TLS006, TLS008, TLS018, TLS023, TLS025, TLS043, TLS070) (ANIC: 11/11 ♀).
Mounted material. Body up to 13 mm long and 13 mm wide. Sclerotized button 1.1–1.6 mm diameter at base, 0.3–0.7 mm long, shaped like a volcanic caldera rim, concave at the end (Fig. 5); located dorsally, assumed to be on anterior abdominal segments (similar to C. campanidorsalis), but exact location unknown due to lack of visible dorsal abdominal segmentation. Spiracles 100–200 μm diameter. Mouthparts of older individuals surrounded by sclerotized derm disc 1.35–2.25 mm diameter. Stylets 275–400 μm long, but often lost along with supporting aliform extensions when female removed from gall tissue.
Dorsum. Majority of cuticle with sparsely scattered, short hs, each 10.0–17.5 μm long. Long hs present on abdomen, each 12.5–27.5 μm long, posterior to dorsal button.
Venter. Majority of cuticle with sparsely scattered, short hs (each 7.5–15 μm long), and pore plates (each 5.0–27.5 μm diameter) with 4–46 pores (each 2–3 μm diameter), in median, posterior half of venter. Some very faint, transverse bands of sclerotization medially, in between mouthparts and vulva, apparently separating abdominal segments. Pore plates and hs also clustered densely around spiracles, these pore plates each 7.5–22.5 μm in diameter with 4–40 pores (each 2–3 μm diameter).
Remarks
Females of C. echiniformis are most easily distinguished by the short, concave-ended dorsal button, which contrasts with the convex buttons of C. pomiformis and C. campanidorsalis. Found in sympatry with C. pomiformis, the galls of C. echiniformis lack the depression around the apical orifice typical of C. pomiformis. However, the shape of the dorsal button is a more reliable characteristic (when the adult female is present).
Distribution and host plants
Known from north Western Australia to 23°S, the Northern Territory as far south as 22°S, and Queensland. Host trees include Corymbia cliftoniana, Co. collina, Co. deserticola, Co. dichromophloia, Co. drysdalensis, Co. erythrophloia, Co. hamersleyana, Co. intermedia and Co. terminalis (only records with positive identifications included).
Cystococcus pomiformis (Froggatt)
In the original description of this species, Froggatt (1893: 367) listed two localities: ‘Torrens Creek, N.Q., on E. sp. (– Chisholm); Barrier Range, King’s Sound, N.W.A., on E. sp. (W. W. Froggatt)’. He also said that there was only a single very large gall specimen from the north Queensland locality (Torrens Creek, near Charters Towers), and that ‘Only one gall contained the remains of a female; the anal segments appear to be robust and dark coloured’. Froggatt must have been referring to the sclerotized abdominal button of the adult female and it is most likely that this was not retained as it has not been found in Froggatt’s collection, which is split between ANIC and ASCU. Froggatt (1893) did not designate a type. However, Froggatt (1921: 157) clearly made a subsequent type designation: ‘The type specimen came from North Queensland, and was described on the gall and the remains of a female coccid as a Brachyscelis; ...’. Froggatt’s collection has two large cut-open galls in each of ANIC and ASCU (four large galls in total; examined by PJG), but the label associated with each lot of galls refers to both northern Western Australia and northern Queensland and it seems that the galls may have been used for display purposes with the associated data probably referring to the distribution known at the time of display and not to the collection site of the galls. Thus we cannot identify the one large gall from Torrens Creek in north Queensland that Froggatt (1921) designated as the type. However, based on Froggatt’s (1893, 1921) descriptions of the galls, there is no doubt as to species identity.
A slide of an adult female in the ANIC with a printed label saying ‘HOLOTYPE’ and two handwritten labels (‘1921/Cystococcus pomiformis Frg/Hardly full grown/Loc. Broome WA/Coll. L.J. Newman’ and ‘Type. Drawn’) has no type status and the type label is clearly an erroneous subsequent addition.
Material examined
Material examined
Australia: Queensland, Northern Territory and Western Australia, on Corymbia spp. (Myrtaceae), (ID: TLS007, TLS016, TLS024, TLS026, TLS028, TLS031, TLS034, TLS035, TLS037, TLS041, TLS045, TLS052) (ANIC: 12/12 ♀).
Mounted material. Body up to 25 mm long and 12 mm wide. Sclerotized button 1.0–2.4 mm diameter at base, 0.9–1.9 mm long, roughly dome-shaped, ranging from broad and round-ended (Fig. 5) to angular and pointed at end, located dorsally, probably on anterior abdominal segments (similar to C. campanidorsalis), but exact location unknown due to lack of visible dorsal abdominal segmentation. Spiracles 170–260 μm diameter. Mouthparts of older individuals surrounded by sclerotized derm disc 0.9–3.4 mm diameter. Stylets 325–600 μm long, but often lost along with supporting aliform expansions when female removed from gall tissue.
Dorsum. Majority of cuticle with sparsely scattered, short hs, each 12.5–20 μm long. Long hs, each 37.5–55 μm long, present on abdomen posterior to dorsal button.
Venter. Majority of cuticle with sparsely scattered, short hs, each 10–15 μm long. Slightly longer hs, 12.5–17.5 μm long, and pore plates each 7.5–27.5 μm diameter with 3–36 pores (each 2–3 μm diameter), densely clumped in median, posterior half of venter. Some faint, transverse bands of sclerotization medially, between mouthparts and vulva, presumably separating abdominal segments. Pore plates and setae also clustered densely around spiracles, these pore plates each 7.5–22.5 μm in diameter with 3–26 pores (each 2–3 μm diameter).
Remarks
Adult females of C. pomiformis have a convex button, varying from broad and dome-shaped to pointy and conical, but easily distinguishable from those of C. echiniformis (concave ended) and C. campanidorsalis (bell-shaped).
Distribution and host plants
Known from north Western Australia to 26°S, the Northern Territory, west Queensland to 148°E (all latitudes) and Sturt National Park in far-north-west New South Wales. Host trees include Corymbia chippendalei, Co. clarksoniana, Co. foelscheana, Co. greeniana, Co. lenziana, Co. polycarpa, Co. ptychocarpa and Co. terminalis (only records with positive identifications included).
Acknowledgements
This project was funded by grants to TLS and LGC from the Australian Government’s Australian Biodiversity Resources Study (ABRS) National Taxonomy Capacity Building and Research Grant Programs respectively, and by the Australian Research Council Discovery Project grants to LGC. Collections were made under permits from the relevant state authorities. We thank the curators of the entomological collections of the Natural History Museum in London, the Orange Agricultural Institute in New South Wales, the South Australian Museum in Adelaide and the Western Australian Museum in Perth for providing PJG with access to specimens in collections under their care. We also thank Dug Miller and Debra Creel for sending information on Cystococcus specimens held in the USNM collection housed at the USDA, USA, and Peter Hudson for sending information on Cystococcus galls in the SAM. Robert Hoare kindly commented on formation of the new name. Aimorn Stewart assisted with preparation of specimens for SEM. We would also like to thank Mike Crisp and Penny Mills for identifying host trees and collecting specimens, and the Cook Laboratory for technical support and encouragement throughout the Honours project that led to this publication. CJH gratefully acknowledges the research facilities provided by the National Museum of Wales.
References
Austin, A. D., Gullan, P. J., Hales, D. F., Taylor, G. S., Yeates, D. K., Cassis, G., Fletcher, M. J., La Salle, J., Lawrence, J. F., McQuillan, P. B., Mound, L. A., and Bickel, D. J. (2004). Insects ‘Down Under’ – diversity, endemism and evolution of the Australian insect fauna: examples from select orders. Australian Journal of Entomology 43, 216–234.| Insects ‘Down Under’ – diversity, endemism and evolution of the Australian insect fauna: examples from select orders.Crossref | GoogleScholarGoogle Scholar |
Beardsley, J. W. (1984). Gall-forming Coccoidea. In ‘Biology of Gall Insects’. (Ed. T. N. Ananthakrishnan.) p. 362. (Oxford and IBH: New Delhi.)
Ben-Dov, Y., and Hodgson, C. J. (1997). 1.4.1 Collecting and mounting. In ‘Soft Scale Insects – Their Biology, Natural Enemies and Control. Vol. 7A’. (Eds Y. Ben-Dov and C. J. Hodgson.) pp. 389–395. (Elsevier: Amsterdam and New York.)
Bowman, D. M. J. S., Isagi, Y., Joseph, L., McBride, J., Nelson, G., Ladiges, P. Y., O’Brien, E., Brown, G. K., Brown, J. R., Braby, M. F., Cook, L. G., Crisp, M. D., Ford, F., Haberle, S., and Hughes, J. (2010). Biogeography of the Australian monsoon tropics. Journal of Biogeography 37, 201–216.
| Biogeography of the Australian monsoon tropics.Crossref | GoogleScholarGoogle Scholar |
Cockerell, T. D. A. (1896). A check list of the Coccidae. Bulletin of the Illinois State Laboratory of Natural History 4, 318–339.
Cockerell, T. D. A. (1902). The nomenclature of the Coccidae. The Entomologist 35, 114.
Cook, L. G., and Gullan, P. J. (2004). The gall‐inducing habit has evolved multiple times among the eriococcid scale insects (Sternorrhyncha: Coccoidea: Eriococcidae). Biological Journal of the Linnean Society. Linnean Society of London 83, 441–452.
| The gall‐inducing habit has evolved multiple times among the eriococcid scale insects (Sternorrhyncha: Coccoidea: Eriococcidae).Crossref | GoogleScholarGoogle Scholar |
Fernald, M. E. (1903). A catalogue of the Coccidae of the world. Bulletin of the Hatch Experiment Station of the Massachusetts Agricultural College 88, 1–360.
Folmer, O., Black, M., Hoeh, W., Lutz, R., and Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3, 294–299.
| 1:CAS:528:DyaK2MXjt12gtLs%3D&md5=c84f72a2d796a7fee91c4e2e01175599CAS | 7881515PubMed |
Froggatt, W. W. (1893). Notes on the family Brachyscelidae, with some account of their parasites, and descriptions of new species, Part I. Proceedings of the Linnean Society of New South Wales 7, 353–372.
Froggatt, W. W. (1921). A descriptive catalogue of the scale insects (‘Coccidae’) of Australia, Part II. Science Bulletin. Department of Agriculture, New South Wales 18, 1–159.
Fuller, C. (1897). Some Coccidae of Western Australia. Journal of the Western Australia Bureau of Agriculture 4, 1344–1346.
Fuller, C. (1899). XIV. Notes and descriptions of some species of Western Australian Coccidae. The Transactions of the Entomological Society of London 1899, 435–473.
Grant, P. (1965). Dispersion of Cystococcus pomiformis (Frogg.). Journal of the Entomological Society of Queensland 4, 68.
Gullan, P. J., and Cockburn, A. (1986). Sexual dichronism and intersexual phoresy in gall-forming coccoids. Oecologia 68, 632–634.
| Sexual dichronism and intersexual phoresy in gall-forming coccoids.Crossref | GoogleScholarGoogle Scholar |
Gullan, P. J., and Jones, M. G. (1989). A new species of gall-forming coccoid (Insecta: Homoptera: Eriococcidae) from Western Australia. Records of the Western Australian Museum 14, 321–329.
Gullan, P. J., and Kosztarab, M. (1997). Adaptations in scale insects. Annual Review of Entomology 42, 23–50.
| Adaptations in scale insects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjvFShtQ%3D%3D&md5=7f40a1630b5957aa4a9f09ea132a338cCAS | 15012306PubMed |
Gullan, P. J., Miller, D. R., and Cook, L. G. (2005). Gall-inducing scale insects (Hemiptera: Sternorrhyncha: Coccoidea). In ‘Biology, Ecology and Evolution of Gall-Inducing Arthropods. Vol. 1’. (Eds A. Raman, C. W. Schaefer and T. M. Withers.) pp. 159–229. (Science Publishers: New Hampshire.)
Hardy, N. B., and Gullan, P. J. (2010). Australian gall-inducing scale insects on Eucalyptus: revision of Opisthoscelis Schrader (Coccoidea, Eriococcidae) and descriptions of a new genus and nine new species. ZooKeys 58, 1–74.
| Australian gall-inducing scale insects on Eucalyptus: revision of Opisthoscelis Schrader (Coccoidea, Eriococcidae) and descriptions of a new genus and nine new species.Crossref | GoogleScholarGoogle Scholar | 21594191PubMed |
Hardy, N. B., Gullan, P. J., Henderson, R. C., and Cook, L. G. (2008). Relationships among felt scale insects (Hemiptera: Coccoidea: Eriococcidae) of southern beech, Nothofagus (Nothofagaceae), with the first descriptions of Australian species of the Nothofagus-feeding genus Madarococcus Hoy. Invertebrate Systematics 22, 365–405.
| Relationships among felt scale insects (Hemiptera: Coccoidea: Eriococcidae) of southern beech, Nothofagus (Nothofagaceae), with the first descriptions of Australian species of the Nothofagus-feeding genus Madarococcus Hoy.Crossref | GoogleScholarGoogle Scholar |
Hardy, N. B., Beardsley, J. W., and Gullan, P. J. (2011). Uncovering diversity of Australian Eucalyptus‐constrained felt scales (Hemiptera: Coccoidea: Eriococcidae). Systematic Entomology 36, 497–528.
| Uncovering diversity of Australian Eucalyptus‐constrained felt scales (Hemiptera: Coccoidea: Eriococcidae).Crossref | GoogleScholarGoogle Scholar |
Hodgson, C. J., Isaias, R. M. S., and Oliveira, D. C. (2013). A new gall-inducing genus and species of Eriococcidae (Hemiptera: Sternorrhyncha: Coccoidea) on Sapindaceae from Brazil. Zootaxa 3734, 317–330.
| A new gall-inducing genus and species of Eriococcidae (Hemiptera: Sternorrhyncha: Coccoidea) on Sapindaceae from Brazil.Crossref | GoogleScholarGoogle Scholar |
Hoy, J. M. (1963). A catalogue of the Eriococcidae (Homoptera: Coccoidea) of the world. New Zealand Department of Scientific and Industrial Research Bulletin 150, 1–260.
International Commission on Zoological Nomenclature (1999). International Code of Zoological Nomenclature, 4th edition. (The International Trust for Zoological Nomenclature, c/- The Natural History Museum: London.)
Kozarzhevskaya, E. F. (1968). Techniques for preparing slides for coccoid (Homoptera: Coccoidea) determination. Entomological Review 47, 146–149.
Ladiges, P. Y., Evans, B. K., and Saint, R. B. (2010). Living in communities. In ‘Biology: an Australian Focus’. p. 1068. (McGraw-Hill: North Ryde, NSW.)
Lindinger, L. (1957). Ein weiterer beitrag zur synonymie der Cocciden. Beiträge zur Entomologie. Berlin 7, 543–553.
Lockhart, P. J., Steel, M. A., Hendy, M. D., and Penny, D. (1994). Recovering evolutionary trees under a more realistic model of sequence evolution. Molecular Biology and Evolution 11, 605–612.
| 1:CAS:528:DyaK2cXlsFaks7c%3D&md5=71b5482ef985294d240c4f74ff21e208CAS | 19391266PubMed |
Mallet, J. (1995). A species definition for the modern synthesis. Trends in Ecology & Evolution 10, 294–299.
| A species definition for the modern synthesis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M7itFaksA%3D%3D&md5=e6985b6cd16e8ac6e0495641493ab6b2CAS |
Mayr, E. (1942). ‘Systematics and the Origin of the Species from the Viewpoint of a Zoologist.’ (Columbia University Press: New York.)
Miller, D. R., Denno, B. D., and Gimpel, M. E. (2014). ScaleNet, Eriococcidae. Available at http://www.sel.barc.usda.gov/scalenet/scalenet.htm (Accessed 8 November 2014).
Park, D.-S., Suh, S.-J., Oh, H.-W., and Hebert, P. D. N. (2010). Recovery of the mitochondrial COI barcode region in diverse Hexapoda through tRNA-based primers. BMC Genomics 11, 423.
| Recovery of the mitochondrial COI barcode region in diverse Hexapoda through tRNA-based primers.Crossref | GoogleScholarGoogle Scholar | 20615258PubMed |
Parra-O, C., Bayly, M. J., Drinnan, A., Udovicic, F., and Ladiges, P. (2009). Phylogeny, major clades and infrageneric classification of Corymbia (Myrtaceae), based on nuclear ribosomal DNA and morphology. Australian Systematic Botany 22, 384–399.
| Phylogeny, major clades and infrageneric classification of Corymbia (Myrtaceae), based on nuclear ribosomal DNA and morphology.Crossref | GoogleScholarGoogle Scholar |
Sleator, R. D. (2011). Phylogenetics. Archives of Microbiology 193, 235–239.
| Phylogenetics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjtFahurY%3D&md5=c42f257243bd22b5d5d2f3bdb40c0a88CAS | 21249334PubMed |
Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690.
| RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFKlsbfI&md5=df50a1f20f2be0cbcb0dff3c135f1b17CAS | 16928733PubMed |
Swofford, D. L. (2003). ‘PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods).’ (Sinauer Associates: Sunderland, MA.)
Tautz, D., Hancock, J. M., Webb, D. A., Tautz, C., and Dowl, G. A. (1988). Complete sequences of the rRNA genes of Drosophila melanogaster. Molecular Biology and Evolution 5, 366–376.
| 1:CAS:528:DyaL1MXht12msLs%3D&md5=94d6aa57e77874b83c68090174cb7cc7CAS | 3136294PubMed |
Turner, A. J. (1942). Fragmenta lepidopterologica. Proceedings of the Royal Society of Queensland 53, 61–96.
Upton, M. S., and Mantle, B. L. (2010). ‘Methods for Collecting, Preserving and Studying Insects and Other Terrestrial Arthropods, 5th edn.’ (The Australian Entomological Society Miscellaneous Publication no. 3: Canberra.)
von Dohlen, C. D., and Moran, N. A. (1995). Molecular phylogeny of the Homoptera: a paraphyletic taxon. Journal of Molecular Evolution 41, 211–223.
| 1:CAS:528:DyaK2MXntVSlt7g%3D&md5=6f4f4d16b996fee57b835dee2a4486feCAS | 7666451PubMed |
Williams, D. J. (1985). The British and some other European Eriococcidae (Homoptera: Coccoidea). Bulletin of the British Museum (Natural History). Entomology Series 51, 347–393.